ABSTRACT
Conventional vaccines to combat COVID-19 through different approaches are at various stages of development. The complexity of COVID-19 such as the potential mutations of the virus leading to antigenic drift and the uncertainty on the duration of the immunity induced by the vaccine have hampered the efforts to control the COVID-19 pandemic. Thus, we suggest an alternative interim treatment strategy based on biological response modifier glucans such as the Aureobasidium pullulans AFO-202-derived β-glucan, which has been reported to induce trained immunity, akin to that induced by the Bacille Calmette-Guérin vaccine, by epigenetic modifications at the central level in the bone marrow. These β-glucans act as pathogen-associated molecular patterns, activating mucosal immunity by binding with specific pathogen recognition receptors such as dectin-1 and inducing both the adaptive and innate immunity by reaching distant lymphoid organs. β-Glucans have also been used as immune adjuvants for vaccines such as the influenza vaccine. Therefore, until a conventional vaccine is widely available, an orally consumable vaccine adjuvant that acts like biosimilars, termed as the wide-spectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach, with well-reported safety is worth in-depth investigation and can be considered for a clinical trial.
Background
The COVID-19 pandemic is wreaking havoc on the lives of billions of people worldwide, with unprecedented consequences and implications. COVID-19 biology and pathology are very complex, posing a big challenge in clinical and drug management. Hence, researchers across the globe are creating strategies for developing drugs, antibodies, vaccines, and other therapies to fight the causative virus, SARS-CoV-2.Citation1 According to the World Health Organization (WHO) [last accessed December 19, 2020], there are 202 companies and universities worldwide working on a SARS-CoV-2 vaccine.Citation2 The vaccines mRNA-1273 (Moderna), Ad5-nCoV (CanSino Biologicals), INO-4800 (Inovio, Inc.), LV-SMENP-DC, Pathogen-specific aAPC (ShinzenGeno-Immune Medical Institute), and ChAdOx1 (University of Oxford) have entered the phase I/II clinical trials.Citation2,Citation3 Authorisation/approval has been obtained in some countries such as China, United Arab Emirates, and Russia for six vaccines: CoronaVac by Sinova in China; Inactivated vaccine by Wuhan Institute of Biological Products in China and National Pharmaceutical Group (Sinopharm) in China, BBIBP-CorV by Sinopharm in China, United Arab Emirates, and Bahrain; Sputnik V, Non-replicating viral vector by Gamaleya Research Institute, Acellena Contract Drug Research and Development in Russia; EpiVacCorona, Peptide vaccine by Federal Budgetary Research Institution State Research Center of Virology and Biotechnology in Russia; and BNT162b2, mRNA-based vaccine by Pfizer, BioNTech, and Fosun Pharma in UK, Bahrain, Canada, Mexico, USA, Singapore, Oman, Saudi Arabia, and Kuwait.Citation4 As of July 2020, there were over 158 vaccine candidates, 135 of which were in the preclinical or exploratory stage of development,Citation5 virtually all concentrating on inducing neutralizing antibodies (nAbs) against the spike (S) protein on the virus surface.Citation1,Citation6
Vaccine approaches conventionally use live attenuated viruses, inactivated virus proteins, polysaccharide-conjugated subunits, virus-like particles, nucleic acid (DNA and RNA), viral vectors, and recombinant proteins. The vaccine’s ability to induce cellular immunity (other than B-cell-produced antibodies) has been indicated as necessary for a rational vaccine design, because nAb responses wane rapidly.Citation1 Furthermore, the coronavirus genome is highly prone to mutations that can lead to genetic drift and escape immune recognition. In fact, several variants that can cause drifts have already been identified.Citation7 Undesired immunopotentiation in the form of eosinophilic infiltration or increased infectivity has limited the exploration of some of the COVID-19 vaccine candidates and is currently a challenge in vaccine biology.Citation7
An ideal vaccine should meet all or most of the following criteria:
It should offer wide-spectrum protection across various substrains and novel variants that are emerging or may emerge later
It should possess characteristics such as minimal undesired immunopotentiation
It should be suitable for stockpiling for adult healthcare workers and for adults > 60 years old or for whom has underlying diabetes or hypertensionCitation8
It should induce long-lasting effective immunity in all vaccinated subjects across ages
It should be safe, stable, and easily available and administrable
Given the above criteria, we evaluated the suitability of β-glucans as they have been reported to exert several beneficial effects on human and animal health.Citation9
β-glucans and immunity
Trained immunity (TRIM) induction is a promising defense strategy against COVID-19.Citation10 The widely known Bacille Calmette-Guérin (BCG) vaccine induces TRIM, protects against severe forms of tuberculosis (TB) caused by Mycobacterium tuberculosis, with limited effect against pulmonary tuberculosis, and confers nonspecific protective properties against unrelated infections and mortality.Citation10 BCG’s nonspecific protection is T-cell and B-cell independent and is mediated by the functional and epigenetic reprogramming of innate immune cells such as monocytes, macrophages, and natural killer (NK) cells. This protection is called TRIM. TRIM has been reported to be induced by stimulants like β-glucans, LPS, or the BCG vaccine.Citation11 We focused on how β-glucans could serve as inducers of TRIM in the context of a wide-spectrum vaccine adjuvant approach to COVID-19.
β-glucans and TRIM
β-Glucans are a heterogeneous group of polysaccharides abundant in the cell walls of yeasts, bacteria, and fungi that reportedly induce TRIM. β-Glucans induce epigenetic reprogramming in innate immune cells, leading to cellular activation, augmented cytokine production, and changes in metabolic function that shift cellular metabolism from oxidative phosphorylation to glucose fermentation mediated by the Akt/mammalian target of rapamycin (mTOR)/hypoxia-inducible factor 1α (HIF1α) pathway,Citation12 thus effectively inducing TRIM. Epigenetic alterations such as histone methylation and acetylation lead to the positive regulation of gene expression. When such epigenetically “trained” cells have contact with heterologous secondary stimuli, they are programmed to produce a more robust immune response.Citation10,Citation11 The cells are reportedly not peripherally trained, but β-glucans can impact the bone marrow (BM) and lead to a lasting TRIM phenotype.
Arms of the immunity stimulated by β-glucans and implications in COVID-19
Administering intraperitoneal β-glucans specifically expand Lin−Sca1+ cKit+ (LSKs) and multipotent myeloid progenitor 3 (MPP3)-expressing hematopoietic stem cells (HSC) in the BM, and such trained HSCs generate a “central” memory.Citation12,Citation13 Epigenetic modifications driven by β-glucans are rapidly activated by secondary infections or stimuli such as viruses and may serve as a potent strategy for vaccines against COVID-19.Citation8 β-Glucans act as pathogen-associated molecular patterns (PAMPs), because they are present in the cell wall of several pathogenic yeasts and bacteria and are involved in microorganism recognition and clearance by the human immune system. Upon reaching the intestine, β-glucans are internalized by intestinal epithelial and/or M cells and presented to immune cells within the Peyer’s patches. β-Glucan particles can also reach distant lymphoid organs via the blood or lymph. In the Peyer’s patches, β-glucan particles are recognized by the ligation of specific pathogen recognition receptors (PRRs) such as toll-like (TLR) and C-type lectin-like receptors. Among C-type lectin-like receptors, dectin-1 is the most-studied receptor that binds β-glucan from various sources. Dectin-1 is expressed on the surface of monocytes, macrophages, neutrophils, dendritic cells, and T lymphocytes, which are all activated by β-glucan binding. This binding leads to a number of cellular responses via the modulation of inflammasome and transcription factor activation, which results in the production of cytokines, chemokines, and reactive oxygen species. β-Glucans stimulate NK cell cytotoxic activity as part of the innate immune response by binding directly to the NKp30 activating receptor.Citation13,Citation14 β-Glucan innate immunity targets are monocytes, macrophages, dendritic cells, and NK cells. β-Glucans induce the antimicrobial activity of mononuclear cells and neutrophils as well.Citation13 Regarding T cells, β-glucans help in CD4 + T-cell immunomodulation, allowing them to infiltrate tumors and thereby inhibit tumor growth.Citation15 Orally administered β-glucans can reach the spleen and lymph nodes and significantly reduce tumor burdens by activating DCs, expanding and activating antigen-specific CD4 and CD8 T cells, and inducing IFN-γ production.Citation16 β-Glucans also induce B lymphocytes to produce antibodies. Short-term supplementation with β-glucans improves the levels of salivary immunoglobulins (sIgM, sIgG, and sIgA).Citation16 Orally administered β-glucans significantly stabilize IgG1 levels, maintaining anti-infectious immunity. Thus, all aspects of the immune system are activated and modulated by β-glucans, making them worth considering as an ideal vaccine that produces long-lasting effective immunity, is broadly protective, is effective in all vaccinated subjects across ages, and is stable and easily administrable.Citation17,Citation18
β-glucan supplementation for COVID-19
Long-lasting immunity is currently a big challenge in COVID-19-affected patients.Citation17 β-Glucans can produce long-lasting TRIM against a wide range of pathogens.Citation18 Furthermore, β-glucans are safe for consumption at all ages, and they fall under the FDA’s generally recognized as safe category.Citation19 β-Glucans are stable and can be consumed continuously as a food supplement.Citation9 Many types of β-glucans exist, but yeast- and mushroom-derived β-glucans exert stronger immunomodulatory effects than do other types of β-glucans.Citation20 Oral β-glucans have been thoroughly described as prophylactic supplements to boost immune responses and to abrogate COVID-19 symptoms via their TRIM actions.Citation10 Though SARS-CoV-2 is predominantly considered a virus that affects the respiratory system, the viral host receptor ACE2 appears in the cytoplasm of gastrointestinal epithelial cells, with the viral nucleocapsid protein appearing in the cytoplasm of rectal, duodenal, and gastric epithelial cells, suggesting that the intestine may be relevant in the pathogenesis of COVID-19 and maybe a possible route of infection.Citation21 β-Glucans with immune effects on the intestine may therefore be an advantageous supplementation strategy for COVID-19 therapy. Gut-dysbiosis is also a key element in determining infection-related diseases. β-Glucans can also modulate the gut bacteria, improving immune response.Citation22 β-Glucan supplements decrease the incidence of upper respiratory tract infections in randomized control trials.Citation23Citation24Citation25Citation26–Citation27 A β-glucan extract from the edible shiitake mushroom Lentinus edodes has recently been reported to show differential in vitro immunomodulatory and pulmonary cytoprotective effects and may be indicated for COVID-19 immunotherapy.Citation27 The study compared two types of Lentinan extracts that differentially reduced cytokine-induced NF-κB activation in human alveolar epithelial A549 cells and attenuated pro-inflammatory cytokine production (TNF-α, IL-8, IL-2, IL-6, and IL-26), as well as TGF-β and IL-10 secretion. The study suggested that β-glucans delivered as a tailored cocktail might fit future nutraceutical-based intervention for COVID-19. The study also mentioned a major drawback: maintaining functional bioactivity and increasing the β-glucan yield required less harmful extraction processes without enzyme and harsh chemical usage.Citation27 This extraction process is crucial for using β-glucans against COVID-19.Citation28
AFO-202 β-glucan
We herein describe the β-glucan derived from the black yeast Aureobasidium pullulans AFO-202 strain that is uniquely secreted as an exopolysaccharide. Therefore, it does not need any kind of extraction-to-purification procedure, resulting in highly pure β-glucan with significant bio-functional activity.Citation28 The AFO-202 β-glucan can induce various positive immune responses relevant to COVID-19. It decreased levels of IL-6, which is commonly the most elevated cytokine in a COVID-19 cytokine storm, the main mechanism leading to organ damage and mortality. It enhances IFN-γ and sFAS levels. It is associated with increased production of IL8, which causes the activation, migration, and chemotaxis of cytotoxic neutrophils. It decreases CCL2 and CXCL10 levels, inhibiting the chemoattraction of monocytes/macrophages, T cells, NK cells, and dendritic cells. The prevention of chemoattraction modulates immune responses. It also increased IL-7 production, leading to mature T-cell survival and development. Activating CD8+ (cytotoxic T cells), CD4+ (mainly Th1 cells), and Treg cells helps balance the regulatory immune response. Activation of B cells by AFO-202 β-glucan results in the production of virus-specific antibodies.Citation28,Citation29 AFO-202 β-glucan enhances NK cell activity against Leishmania amazonensis infection.Citation30 AFO-202 β-glucan is also present in the inner wall of Candida albicans, making it a strong PAMP candidate with a significant chance of recognition by PRRs. AFO-202 β-glucan has been consumed since 1996, when the Japanese Regulatory Authority approved it as a food additive. It has been established as safe and efficacious in elderly patients.Citation31 AFO-202 β-glucan also helps to maintain blood glucose and lipid levels,Citation32,Citation33 addressing the high risk of comorbidities like diabetes and cardiac diseases in the pathogenesis of COVID-19. As fasting plasma glucose has been indicated as an independent predictor of the outcome at the time of admission in COVID-19 patients, the metabolic effects of AFO-202 β-glucan may have prophylactic potential in COVID-19.Citation34 In addition, AFO-202 β-glucan has been suggested to be beneficial in decreasing the risk of coagulopathy due to the presence of a dysregulated inflammatory system in COVID-19 patients, especially in vulnerable individuals based on race (Caucasians, African Americans and Hispanics), people with comorbidities, including diabetes, hypertension, and cardiovascular diseases, pregnant women.Citation35
AFO 202 β-glucan as a wide-spectrum immune effector
Oral vaccines generate immunity in gut-associated lymphoid tissue (GALT) that consists of lymph nodes, Peyer’s patches (containing 75% of B cells and 20% of T cells), and isolated lymphoid follicles in the gastrointestinal tract (GIT).Citation36 M cells transport the antigen in the vaccine across the mucosal barrier into Peyer’s patches, and the antigen is presented to T cells by antigen-presenting cells. CD4 + T cells are activated, supporting the germinal center development, B-cell affinity maturation, and class-switching to IgA, along with CD40/CD40 ligand interactions and cytokine secretion. The antigen-primed B cells migrate to distant effector regions, where they differentiate into plasma cells that secrete dimeric or polymeric IgA molecules. These molecules are transported into the intestinal lumen as secretory IgA (sIgA) that can prevent attachment and pathogen invasion, neutralize enterotoxins, and induce serum IgG via the vaccine, acting against mucosally and systemically invasive pathogens. Vaccines also activate cell-mediated immune responses against intracellular bacteria and viruses, along with antibody-dependent cellular cytotoxicity responses.Citation37,Citation38
Following intra-dermal vaccination, immune cells such as DCs, T lymphocytes, NK cells, macrophages, and mast cells present in the skin epithelium trigger the skin’s inflammation cascade, mainly via Langerhans cells (a specific DC subset that migrates into the lymph nodes following antigen capture and initiates the adaptive immune response). These cells are stimulated by PAMPs via an array of germline-encoded PRRs, including TLR and langerin (CD207). The skin’s resident mast cells induce the innate immune response in the skin by releasing granules containing inflammatory mediators.Citation39
The immune system triggers pathways of oral and intradermal vaccination depend on components of the reticuloendothelial system or the mononuclear phagocyte systemCitation39 employed to access the immune system. Oral vaccines start with mucosal-associated lymphoid tissue and GALT, while intra-dermal vaccines start with peripheral lymphoid tissues.
AFO-202 β-glucan as a vaccine adjuvant
β-Glucans have been suggested to be promising anti-infective vaccine adjuvants, as they alone can stimulate various immune reactions, including antibody production without any adverse reactions. β-Glucans have been employed as adjuvants to vaccines against Yersinia ruckeri.Citation39 β-Glucans as adjuvants have been found to enhance immunogenicity of hepatitis B vaccine, influenza vaccine, and vaccines against systemic aspergillosis and coccidioidomycosis. The AFO-202 β-glucan has been proven to be a potential immune adjuvant, because when it was administered with an avian influenza H5 subtype vaccine, it elicited strong immune responses with high hemagglutination inhibition titers and 10–20% ELISA seroconversion.Citation40
illustrates the mechanisms and pathways that differ between oral and intra-dermal vaccines, and how β-glucans interact with components involved in both the types of vaccines. provides the details on the various immune effector mechanisms and pathways through which β-glucans may be able to serve as vaccine adjuvants for COVID-19 therapy.
Table 1. Various immune effector mechanisms and pathways through which β-glucans may be able to serve as vaccine adjuvants for COVID-19 therapy
Figure 1. Schematic illustration describing (i) stepwise, the mechanisms of orally administered vaccines starting from Peyer’s patches of the gut to induce mucosal immunity and parenteral vaccines starting from immune cells of the skin to induce systemic immunity, (ii) ability of β-glucans to activate all aspects of the immune system including the central trained immunity (TRIM) of bone marrow and (iii) the strategic key advantages of β-glucans at five different stages and actions to play a role as vaccine adjuvant
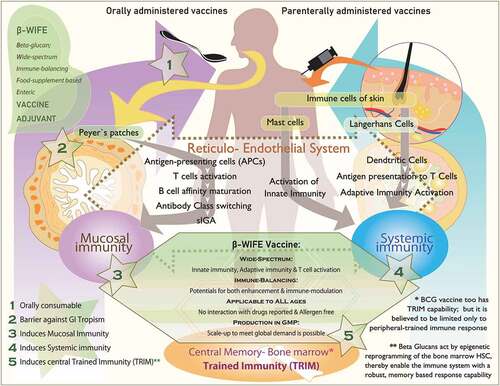
β-glucans as wide-spectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach to COVID-19
β-Glucans induce TRIM with epigenetic reprogramming in innate immune cells at the BM level, leading to a long-lasting central and peripheral TRIM.Citation9–11 Since β-glucans are recognized as PAMPs, they are recognized by the ligation of specific PRRs, such as TLR and C-type lectin-like receptors, which stimulate both innate immunity by targeting cells, including macrophages and NK cells, as well as adaptive immunity by expanding and activating antigen-specific CD4 and CD8 T cells and enabling B lymphocytes to produce antibodies.Citation15,Citation16,Citation28 β-Glucans also enhance mucosal immunity, employing the majority of the components of the reticuloendothelial system by inducing gut mucosal immunity, traveling to distant effector sites such as the spleen and lymph nodes.Citation39,Citation41 β-Glucans activate all aspects of the immune system,Citation9–13 resulting in a continuous, lasting immune response against various pathogens that can elicit specific antiviral immunity.Citation28 Above all, this β-glucan-based immune response is obtained through a simple oral food supplement administration, with a proven track record of safe consumption at all ages,Citation28 besides having been employed as vaccine adjuvants.Citation37,Citation38,Citation40 Thus, for COVID-19, we have termed this approach as a β-WIFE vaccine adjuvant approach.
Our group has recently initiated a pilot study in healthy volunteers (men aged between 40 and 60) on evaluation of biomarkers relevant to thrombogenicity, apart from immune enhancement and immune modulation with AFO-202 β-glucan and the interim results are encouraging. Based on these encouraging preliminary results, we plan to undertake a controlled study in COVID-19 patients whose general health conditions permit oral consumption.
Conclusion
Without definitive therapeutics for COVID-19, and although some vaccine candidates have been authorised/approved, significant hurdles still exist in identifying an ideal vaccine with a wide-spectrum activity and no side effects. Orally consumed β-glucans such as the AFO-202 β-glucan might be able to serve as a β-WIFE vaccine adjuvant approach to COVID-19. However, this approach needs extensive validation by multi-centric randomized clinical trials.
Disclosure of potential conflicts of interest
Author Samuel JK Abraham is a shareholder in GN Corporation, Japan which in turn is a shareholder in the manufacturing company of the AFO 202 Beta Glucan & also an inventor in several patents of relevance to the AFO 202 Beta Glucan.
Acknowledgments
The authors thank Prof. Gary Levy, (University of Toronto Transplant Institute, Multi Organ Transplant Program, University Health Network, Toronto, Canada) for his valuable guidance with the drafting of this manuscript. Mr. Takashi Onaka, (Sophy Inc, Kochi, Japan), for necessary technical clarifications. KR & JL are thankful to the National Science System (SNI) of National Secretariat for Science, Technology, and Innovation of Panama (SENACYT) for support.
References
- Burton DR, Walker LM. Rational vaccine design in the time of COVID-19. Cell Host Microbe. 2020;27:695–98.
- Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020 Oct 27;14(10):12522–37. doi:10.1021/acsnano.0c07197.
- COVID-19 vaccines. [accessed 2020 Dec 23]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
- Kaur SP, Gupta V. COVID-19 vaccine: A comprehensive status report. Virus Res. 2020;288:198114. doi:10.1016/j.virusres.2020.198114.
- COVID-19 vaccine tracker. [ accessed 2020 Dec 23]. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker.
- Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: the current state of play. Paediatr Respir Rev. 2020;35:43–49. doi:10.1016/j.prrv.2020.06.010.
- Koyama T, Weeraratne D, Snowdon JL, Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens. 2020;9:324. doi:10.3390/pathogens9050324.
- Beverley PC. Immunology of vaccination. Br Med Bull. 2002;62:15–28. doi:10.1093/bmb/62.1.15.
- Vetvicka V, Vannucci L, Sima P, Richter J. Beta glucan: supplement or drug? From laboratory to clinical trials. Molecules. 2019;24:1251. doi:10.3390/molecules24071251.
- Geller A, Yan J. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Front Immunol. 2020;11:1782. doi:10.3389/fimmu.2020.01782.
- Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–71. doi:10.1016/j.celrep.2016.11.011.
- Del Cornò M, Gessani S, Conti L. Shaping the Innate Immune response by dietary glucans: any role in the control of cancer? Cancers (Basel). 2020;12(1):155. doi:10.3390/cancers12010155.
- Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230(1):38–50. doi:10.1111/j.1600-065X.2009.00793.x.
- Zou S, Duan B, Xu X. Inhibition of tumor growth by β-glucans through promoting CD4+ T cell immunomodulation and neutrophil-killing in mice. Carbohydr Polym. 2019;213:370–81. doi:10.1016/j.carbpol.2019.03.006.
- Li B, Cai Y, Qi C, Hansen R, Ding C, Mitchell TC, Yan J. Orally administered particulate beta-glucan modulates tumor-capturing dendritic cells and improves antitumor T-cell responses in cancer. Clin Cancer Res. 2010;16(21):5153–64. doi:10.1158/1078-0432.CCR-10-0820.
- Richter J, Kral V, Vetvicka V, Rajnohova DL, Fernandez-Botran R. Effect of β-glucan supplementation on levels of IgM, IgA, IgG and its subclasses IgG1, IgG2, IgG3, and IgG4 in cancer patients. Journal of Tumor. 2016;4(5–6):469–73. doi:10.17554/j..1819-6187.2016.04.98.
- Kirkcaldy RD, King BA, Brooks JT. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA. 2020;323(22):2245–46. doi:10.1001/jama.2020.7869.
- Petit J, Wiegertjes GF. Long-lived effects of administering β-glucans: indications for trained immunity in fish. Dev Comp Immunol. 2016;64:93–102. doi:10.1016/j.dci.2016.03.003.
- GRAS FDA. [accessed 2020 Nov 12]. https://www.betaglucan.org/fdagras/
- Bashir KMI, Choi JS. Clinical and physiological perspectives of β-glucans: the past, present, and future. Int J Mol Sci. 2017;18:1906. doi:10.3390/ijms18091906.
- Infusino F, Marazzato M, Mancone M, Fedele F, Mastroianni CM, Severino P, Ceccarelli G, Santinelli L, Cavarretta E, Marullo AG, et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: A scoping review. Nutrients. 2020;12:1718. doi:10.3390/nu12061718.
- Jayachandran M, Chen J, Chung SSM, Xu B. A critical review on the impacts of β-glucans on gut microbiota and human health. J Nutr Biochem. 2018;61:101–110
- Talbott SM, Talbott JA. Baker’s yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J Am Coll Nutr. 2012;31:295–300. doi:10.1080/07315724.2012.10720441.
- Dharsono T, Rudnicka K, Wilhelm M, Schoen C. Effects of yeast (1,3)-(1,6)-beta-glucan on severity of upper respiratory tract infections: a double-blind, randomized, placebo-controlled study in healthy subjects. J Am Coll Nutr. 2019;38:40–50. doi:10.1080/07315724.2018.1478339.
- Fuller R, Moore MV, Lewith G, Stuart BL, Ormiston RV, Fisk HL, Noakes PS, Calder PC. Yeast-derived β-1,3/1,6 glucan, upper respiratory tract infection and innate immunity in older adults. Nutrition. 2017;39-40:30–35. doi:10.1016/j.nut.2017.03.003.
- Jesenak M, Majtan J, Rennerova Z, Kyselovic J, Banovcin P, Hrubisko M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int Immunopharmacol. 2013;15:395–99. doi:10.1016/j.intimp.2012.11.020.
- Murphy EJ, Masterson C, Rezoagli E, O’Toole D, Major I, Stack GD, Lynch M, Laffey JG, Rowan NJ. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects - Implications for coronavirus disease (COVID-19) immunotherapies. Sci Total Environ. 2020;732:139330. doi:10.1016/j.scitotenv.2020.139330.
- Ikewaki N, Fujii N, Onaka T, Ikewaki S, Inoko H. Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement. Microbiol Immunol. 2007;51:861–73. doi:10.1111/j.1348-0421.2007.tb03982.x.
- Rao KS, Suryaprakash V, Senthilkumar R, Preethy S, Katoh S, Ikewaki N, Abraham SJK. Role of immune dysregulation in increased mortality among a specific subset of COVID-19 patients and immune-enhancement strategies for combatting through nutritional supplements. Front Immunol. 2020;11:1548. doi:10.3389/fimmu.2020.01548.
- Yatawara L, Wickramasinghe S, Nagataki M, Takamoto M, Nomura H, Ikeue Y, Watanabe Y, Agatsuma T. Aureobasidium-derived soluble branched (1,3-1,6) beta-glucan (Sophy beta-glucan) enhances natural killer activity in Leishmania amazonensis-infected mice. Korean J Parasitol. 2009;47:345–51. doi:10.3347/kjp.2009.47.4.345.
- Miyamoto M. Effect of oral intake of black yeast beta-glucan on NK activity in the elderly and patients with cancer. Abstract presented at the 29th Annual Meeting of the Japanese Society of Venous and Enteral Nutrition. February 27-28, 2014: Pacifico Yokohama, Japan.
- Dedeepiya VD, Sivaraman G, Venkatesh AP, Preethy S, Abraham SJ. Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: preliminary findings from the study on three patients from India. Case Rep Med. 2012;2012:895370. doi:10.1155/2012/895370.
- Ganesh JS, Rao YY, Ravikumar R, Jayakrishnan GA, Iwasaki M, Preethy S, Abraham SJ. Beneficial effects of black yeast derived 1-3, 1-6 beta glucan-nichi glucan in a dyslipidemic individual of Indian origin–a case report. J Diet Suppl. 2014;11:1–6. doi:10.3109/19390211.2013.859211.
- Ikewaki N, Iwasaki M, Abraham S. Biological response modifier glucan through balancing of blood glucose may have a prophylactic potential in COVID-19 patients. J Diabetes Metab Disord. 2020. doi:10.1007/s40200-020-00664-4.
- Ikewaki N, Rao KS, Archibold AD, Iwasaki M, Senthilkumar R, Preethy S, Katoh S, Abraham S. Coagulopathy associated with COVID-19 – perspectives & preventive strategies using a biological response modifier glucan. Thromb J. 2020. doi:10.1186/s12959-020-00239-6.
- Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–48. doi:10.1111/j.1600-065X.2010.00970.x.
- Criscuolo E, Caputo V, Diotti RA, Sautto GA, Kirchenbaum GA, Clementi N. Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res. 2019;2019:8303648. doi:10.1155/2019/8303648.
- Vetvicka V, Vannucci L, Sima P. β-glucan as a new tool in vaccine development. Scand J Immunol. 2020;91:e12833. doi:10.1111/sji.12833.
- Reticuloendothelial system. In: Moreland LW, editor. Rheumatology and immunology therapy. Berlin (Heidelberg): Springer; 2004. Online ISBN: 978-3-540-29662-1 doi:10.1007/3-540-29662-X_2332
- Le T, Le T, Doan TH, Quyen D, Le KX, Pham V, Nagataki M, Nomura H, Ikeue Y, Watanabe Y, et al. The adjuvant effect of Sophy β-glucan to the antibody response in poultry immunized by the avian influenza A H5N1 and H5N2 vaccines. J Microbiol Biotechnol. 2011;21:405–11. doi:10.4014/jmb.1011.11024.
- Stier H, Ebbeskotte V, Gruenwald J. Immune-modulatory effects of dietary yeast beta-1,3/1,6-D-glucan. Nutr J. 2014;13:38. doi:10.1186/1475-2891-13-38.

