ABSTRACT
Cell surface protrusions play central roles in the physiological function of a number of organ systems. Recent discoveries suggest that polarized cells in functionally diverse epithelia employ conserved cadherin-based adhesion complexes to shape, stabilize, and organize actin-based protrusions during differentiation. Below we discuss the implications of these findings for understanding human biology and disease, and highlight promising directions for future studies on this conserved mechanism for shaping the cell surface.
Epithelial sheets play critical roles in various aspects of human physiology, including nutrient transport out of the gut lumen, concentration of urine by kidney tubules, and sensing of physical forces in the inner ear. In each of these cases, polarized epithelial cells form continuous monolayers with morphologically and functionally distinct apical and basolateral surfaces. During differentiation, epithelial cells also assemble an apical “specialization”: an array of membrane protrusions that enables functional interactions with the luminal environment. This discussion is concerned with protrusions supported by parallel bundles of actin filaments, such as microvilli and stereocilia, which extend from the apical surface of transporting epithelial cells and mechanosensory hair cells, in the gut and inner ear, respectively. Because these structures are essential for the function of these organ systems, understanding how they are assembled and organized is a significant goal for cell biologists.
The brush border: A prototypical apical specialization
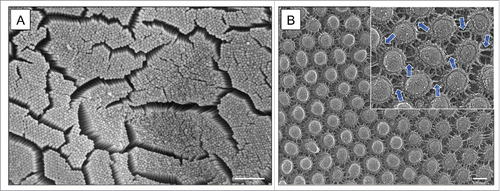
In the specific case of the intestinal epithelium where absorptive enterocytes are the most abundant cell type, the apical specialization is a collection of microvilli known as the “brush border.”Citation1,2 Here, the membrane supported by each microvillus is enriched in the molecular machinery that drives nutrient processing and transport. The gut epithelium also engages in host defense by preventing members of the luminal microbial community from crossing into peripheral tissues.Citation3-5 Microvilli support this function by releasing small vesicles laden with host defense factors into the luminal space.Citation6,7 To maximize functional efficiency, enterocytes build large numbers of microvilli to increase membrane surface area.Citation8 During differentiation the entire apical surface becomes covered with microvilli, with little free space remaining between adjacent protrusions. In viewing the brush border en face (i.e. top down) using scanning electron microscopy (EM)Citation1 (), one can appreciate the magnitude of the assembly problem. Each cell contains many hundreds of microvilli tightly packed in a hexagonal array, a pattern that reflects the maximum packing density for cylindrical protrusions. Microvilli in these arrays are also remarkably uniform in length with <5% variability between neighboring protrusions. Because the brush border forms the functional interface between our host tissues and the external environment of the gut lumen, proper formation of this organelle is essential for normal human health. Indeed, perturbations to the brush border have been linked to malabsorption symptoms in celiac disease, microvillus inclusion disease, and infections with enteric pathogens that target the apical surface (e.g. enteropathogenic or enterohemorrhagic E. coli).Citation9-12
Figure 1. (A) En face scanning electron microscopy of brush border microvilli extending from the surface of mouse duodenal enterocytes. (B) Deep-etch electron microscopy reveals a dense network of adhesion complexes connecting the tips of adjacent microvilli. Scale bars are 1 μm and 1 nm in A and B, respectively.
Although this discussion focuses on the form and function of the intestinal brush border, similar structures are assembled to fulfill a variety of functions in other epithelial tissues such as kidney, choroid plexus, lung, and gall bladder. In some cases, brush borders represent transient structural intermediates that provide a foundation for the assembly of more elaborate apical specializations during development and differentiation.Citation13
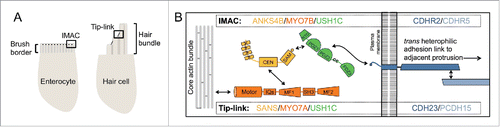
Intermicrovillar adhesion drives microvillar packing
Despite clear significance for human physiology, the molecular basis of tight microvillar packing in the brush border remained unclear for many years. However, recent discoveries implicate cadherin superfamily members in this process. We now know that microvilli are organized during brush border assembly by adhesion complexes that form between the tips of adjacent protrusionsCitation14 (). These “intermicrovillar adhesion complexes” (IMACs) are composed of CDHR2 (also known as protocadherin-24) and CDHR5 (also known as mucin-like protocadherin), which target to microvillar tips and interact to form a trans heterophilic complex ∼24–78 nm in length; CDHR2 also exhibits weak trans homophilic interactions. Knockdown of either CDHR2 or CDHR5 in epithelial model systems leads to grossly disheveled brush borders, with reduced microvillar packing density and increased variability in length. Tip targeting of the IMAC is essential for proper function; by positioning adhesion factors only at microvillar tips, cells ensure that only the tips are adherent and capable of interacting with each other. This simple mechanism provides an organizing principle that controls microvillar packing and may also regulate length uniformity in this system.
Getting the IMAC to the tips
Because microvilli are short (∼1 μm in length), diffusion of transmembrane cadherins in the plane of the membrane could provide the driving force for their movement toward the tips. However, diffusion alone cannot explain the high level of CDHR2 and CDHR5 enrichment at microvillar tips.Citation14 Cytoplasmic binding partners might also be involved in positioning the IMAC at the distal tips, as a variant of CDHR2 lacking the cytoplasmic tail is unable to enrich in these sites.Citation14 Other actin-based protrusions that accumulate specific factors at their tips have invoked myosin motors to drive transport to and enrichment at the barbed-ends of supporting actin bundles (e.g., myosin-10 in filopodia).Citation16-21 A myosin motor might play a similar role in microvilli as the cytoplasmic domains of CDHR2 and CDHR5 interact with the tandem MyTH4/FERM (MF) domain motor, myosin-7b (MYO7B).Citation14 CDHR2, CDHR5, and MYO7B also interact with the PDZ domain scaffolding protein, USH1C, which likely enhances the lifetime of interactions between the motor and its cargo (i.e., the protocadherins). Although the role of MYO7B in positioning the IMAC at microvillar tips has yet to be investigated directly, mice lacking USH1C exhibit clear mislocalization of MYO7B and defects in microvillar packing along the length of the small intestine and colon.Citation14 Moreover, mistargeting of the CDHR2 cytoplasmic tail deletion mutant (alluded to above) is rescued by fusion to USH1C.Citation14 Thus, interactions with cytoplasmic factors such as MYO7B and USH1C are critical for the tip localization of microvillar protocadherins.
The IMAC gets a new member
More recent studies revealed a fifth member of the IMAC: ankyrin repeat and sterile α motif domain containing 4B (ANKS4B).Citation15,22 ANKS4B was originally identified as a USH1C binding protein that is highly expressed in epithelial tissues.Citation23 Cell biological studies and biochemical characterization of ANKS4B binding to other IMAC components revealed new insights on the multivalent interactions that drive IMAC assembly, distal tip targeting, and intermicrovillar adhesion.Citation15 Knockdown of ANKS4B in epithelial cells led to a striking loss of all other IMAC components from microvillar tips; ANKS4B knockdown cells were essentially null for the entire IMAC. Not surprisingly, apical surface phenotypes associated with ANKS4B knockdown were among the most severe observed in IMAC studies to date, with a significant reduction in microvillar density, and near total loss of protrusion clustering and tip-tip adhesion. Careful biochemical analysis also revealed that MYO7B simultaneously interacts with ANKS4B and USH1C.Citation15 This is made possible by tandem MF domains in the MYO7B cargo binding tail; ANKS4B uses its CEN domain to interact specially with MF1, whereas USH1C interacts with the more C-terminal MF2 using an undefined mechanism.Citation22 In addition, the C-terminus of ANKS4B, which includes a type-I PDZ ligand, interacts directly with the first PDZ domain of USH1C with strikingly high affinity (∼nM range).Citation22 The resulting MYO7B-ANKS4B-USH1C tripartite complex can interact with the cytoplasmic domains of CDHR2 and CDHR5 using either USH1C or MYO7B. These experiments also revealed an ordered binding process, where ANKS4B/USH1C interactions precede binding to the tandem MF domains of MYO7B.Citation15 Given the high affinity of the ANKS4B/USH1C interaction, and the fact that this interaction is required for subsequent IMAC assembly, one might expect ANKS4B/USH1C binding to be tightly regulated during differentiation, although no studies to date have addressed this point. Interestingly, ANKS4B N-terminal ankyrin repeats targeted to brush border microvilli entirely independent of USH1C or MYO7B, suggesting interactions with an as-of-yet unidentified apical docking factor.
Parallels between IMACs and stereocilia tip-links – implications for function
One of the most interesting revelations from these recent studies of brush border assembly is the striking similarity between the IMAC and the “tip-link” complex that connects adjacent stereocilia found on the surface of inner ear mechanosensory hair cells (for review see refs.Citation24,25) (). Hair cells express cadherin-23 (CDH23, distantly related to CDHR2) and protocadherin-15 (PCDH15, distantly related to CDHR5), which form a heterophilic adhesion link between the side of one stereocilium and the tip of its shorter neighbor.Citation26 CDH23 forms the upper portion of the tip-link, where its cytoplasmic domain interacts directly with USH1C, which in turn interacts with the ANKS4B-like protein, SANS, and the class VII myosin, MYO7A.Citation27-33 PCDH15 forms the lower portion of the tip-link and is believed to interact with a mechanotransduction channel that allows hair cells to respond to physical forces.Citation34,35 Consistent with studies on the IMAC, formation of the tip-link complex also appears to be critical for hair bundle organization.Citation36 Thus, a conserved apical adhesion complex consisting of 2 cadherin family members, 2 scaffolding proteins, and a myosin motor, physically connects and organizes protrusions in apical specializations with entirely distinct morphologies and physiological functions (, ). Additional evidence for a conserved apical adhesion complex comes from studies in Drosophila, which suggest that a class VII myosin (crinkled) and a cadherin (Cad99c, ortholog of PCDH15) function together to regulate microvillar growth on the surface of follicle cells during oogenesis.Citation37,38
Table 1. Tip-link and IMAC components.
The simple transporting epithelium that lines the gut arose in evolution long before the more specialized sensory epithelium of the inner ear. With this in mind, it is not surprising that hair cell stereocilia emerge from a lawn of simple microvilli during differentiation.Citation39 Tip-link components probably arose through a series of gene duplications followed by structural adaptations that eventually allowed these molecules to fulfill hair cell-specific functions. The most obvious hair cell-specific function would be mechanotransduction, because there is currently no evidence indicating that the intestinal epithelium exhibits apical mechanosensory activity (It is worth noting, however, that this possibility has yet to be addressed with directed experimentation). Given similarities in IMAC and tip-link molecular architecture, it is interesting to consider functional requirements that may be common to brush borders and hair bundles. For example, the conserved apical adhesion complex might be employed by enterocytes and hair cells to resist physical forces that originate within the cell, such as membrane surface tension, which would otherwise promote the coalescence or fusion of closely spaced protrusions. These complexes might also be employed to resist external forces that impinge on the cell surface. While one can easily envision this being the case for hair cells, less is known about the physical forces that impact the brush border. Because the intestinal tract is enveloped in smooth muscle, which drives repeated peristaltic contractions, the brush border is likely subject to significant mechanical forces that would otherwise disrupt microvillar packing and function. Thus, one of the most probable fundamental roles for the conserved apical adhesion complex is imparting physical stability to ensembles of actin bundle-supported protrusions.
Discovery of the IMAC provides insight on the basis of human disease
Inactivating mutations in any of the components of the stereocilia tip-link complex (CDH23, PCDH15, USH1C, SANS, or MYO7A) impair mechanotransduction and have been linked to Type 1 Usher syndrome, characterized by profound loss of hearing, vestibular dysfunction, and retinitis pigmentosa with onset in the first decade of life.Citation40-45 Molecular mechanisms underpinning the various forms of Usher Syndrome have been the focus of extensive investigations for many years, and insights offered by these studies have illuminated our understanding of the mechanotransduction process.Citation46 Whereas most tip-link and IMAC components are expressed from different genes, USH1C represents the only component common to both complexes. Consistent with this, initial reports implicating USH1C in Type 1C Usher syndrome indicated that human patients present not only with perturbations to hearing and vision, but also inflammatory enteropathy and renal tubular malfunction.Citation41,47 At the time, the basis for gut and kidney phenotypes was not clear because the function of USH1C in these tissues was unknown. However, given that USH1C serves as a scaffolding factor for the IMAC, the gut and kidney symptoms are mostly likely explained by defects in brush border assembly in these organ systems. In support of this assertion, mice lacking USH1C (a model for human Type 1C Usher syndrome) exhibit significant defects in microvillar packing along the length of the small intestine and colon.Citation14 Because several forms of Type 1 Usher syndrome (USH1E, USH1H, and USH1K) have yet to be assigned to a specific gene, future studies might reveal additional IMAC components.
Parallels between the IMAC and tip-link complex have also allowed exploration of how specific mutations lead to disease in Usher syndrome patients. For example, the L48P substitution associated with Type 1G Usher syndrome impacts a conserved residue in the N-terminal ankyrin repeats of SANS,Citation48 although the functional effect of this mutation remained unclear. By expressing this mutation in the IMAC functional homolog, ANKS4B, in the context of the CACO-2BBE intestinal epithelial model system, investigators were able to determine that L48P disrupts targeting of this protein to the apical domain. Thus, in Type 1G Usher syndrome patients, L48P-SANS is likely deficient in stereocilia targeting; this would be expected to disrupt tip-link formation, leading to hair bundle disarray and a loss of mechanotransduction, similar to that observed in mice where SANS is deleted postnatally.Citation49
Questions for the future
A number of intriguing lines of investigation should be considered for future studies. At the molecular scale, the most pressing questions relate to complex architecture, formation, and positioning. In the IMAC, nearly all molecules interact with at least 2 other complex components, which begs the question: why so many interactions? Does this reflect a physical need for generating a high-tensile strength complex between extracellular cadherins and the intracellular actin cytoskeleton? How are specific interactions regulated, as alluded to in recent biochemical studies of IMAC complex formation?Citation15 What is the stoichiometry of molecules in these complexes, e.g. how many scaffolding molecules and motors interact with a single cadherin cytoplasmic domain? What are the molecular mechanisms that drive trans homophilic and heterophilic adhesion complex formation? Are cis interactions, like those observed in the stereocilia tip-link,Citation26 required for IMAC complex formation? Does IMAC localization depend on the motor activity of MYO7B? Another significant issue relates to parsing out shared and common functions for the 2 complexes, i.e. does mechanotransduction represent a shared primordial function in both gut and inner ear, or an adaptation specific to the inner ear? The recent identification of at least some of the components of the hair cell mechanotransduction channelCitation35,51 will hopefully facilitate experiments along these lines. In addition to the tip-localized IMAC, do microvilli also employ “lateral links” like those observed in the hair cell system during hair bundle differentiation?Citation50 Finally, proteomic investigations in both the gut and inner ear systems have already provided important clues on the biogenesis of brush borders and hair bundles.Citation52-54 As biochemical preparations of these organelles are refined, it will be interesting to see how the next generation of proteomic data illuminates our understanding of conserved apical adhesion complex function in normal physiology and dysfunction in human disease.
Abbreviations
| IMAC | = | intermicrovillar adhesion complex |
| MF | = | MyTH4/FERM |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank members of the Tyska laboratory for helpful discussion.
Funding
This work was supported in part by National Institutes of Health Grants R01-DK075555 and R01-DK095811 (MJT).
References
- Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol 2014; 207(4):441-51; PMID:25422372; http://dx.doi.org/10.1083/jcb.201407015
- Delacour D, Salomon J, Robine S, Louvard D. Plasticity of the brush border - the yin and yang of intestinal homeostasis. Nat Rev Gastroenterol Hepatol 2016; 13(3):161-74; PMID:26837713; http://dx.doi.org/10.1038/nrgastro.2016.5
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31:107-33; PMID:334036; http://dx.doi.org/10.1146/annurev.mi.31.100177.000543
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101(44):15718-23; PMID:15505215; http://dx.doi.org/10.1073/pnas.0407076101
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005; 307(5717):1915-20; PMID:15790844; http://dx.doi.org/10.1126/science.1104816
- Shifrin DA, Jr., McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol 2012; 22(7):627-31; PMID:22386311; http://dx.doi.org/10.1016/j.cub.2012.02.022
- McConnell RE, Higginbotham JN, Shifrin DA, Jr., Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol 2009; 185(7):1285-98; PMID:19564407; http://dx.doi.org/10.1083/jcb.200902147
- Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol 2014; 49(6):681-9; PMID:24694282; http://dx.doi.org/10.3109/00365521.2014.898326
- Wilson W, Scott RB, Pinto A, Robertson MA. Intractable diarrhea in a newborn infant: microvillous inclusion disease. Can J Gastroenterol 2001; 15(1):61-4; PMID:11173328; http://dx.doi.org/10.1155/2001/743925
- Khubchandani SR, Vohra P, Chitale AR, Sidana P. Microvillous inclusion disease?an ultrastructural diagnosis: with a review of the literature. Ultrastruct Pathol 2011; 35(2):87-91; PMID:21299349; http://dx.doi.org/10.3109/01913123.2010.537438
- Vallance BA, Chan C, Robertson ML, Finlay BB. Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Can J Gastroenterol 2002; 16(11):771-8; PMID:12464970; http://dx.doi.org/10.1155/2002/410980
- Bailey DS, Freedman AR, Price SC, Chescoe D, Ciclitira PJ. Early biochemical responses of the small intestine of coeliac patients to wheat gluten. Gut 1989; 30(1):78-85; PMID:2563983; http://dx.doi.org/10.1136/gut.30.1.78
- Tilney LG, Cotanche DA, Tilney MS. Actin filaments, stereocilia and hair cells of the bird cochlea. VI. How the number and arrangement of stereocilia are determined. Development 1992; 116(1):213-26; PMID:1483389
- Crawley SW, Shifrin DA Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, et al. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 2014; 157(2):433-46; PMID:24725409; http://dx.doi.org/10.1016/j.cell.2014.01.067
- Crawley SW, Weck ML, Grega-Larson NE, Shifrin DA, Jr., Tyska MJ. ANKS4B Is Essential for Intermicrovillar Adhesion Complex Formation. Dev Cell 2016; 36(2):190-200; PMID:26812018; http://dx.doi.org/10.1016/j.devcel.2015.12.022
- Almagro S, Durmort C, Chervin-Pétinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P, et al. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol Cell Biol 2010; 30(7):1703-17; PMID:20123970; http://dx.doi.org/10.1128/MCB.01226-09
- Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol 2002; 4(3):246-50; PMID:11854753; http://dx.doi.org/10.1038/ncb762
- Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol 2007; 179(7):1569-82; PMID:18158328; http://dx.doi.org/10.1083/jcb.200704010
- Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem Biophys Res Commun 2004; 319(1):214-20; PMID:15158464; http://dx.doi.org/10.1016/j.bbrc.2004.04.167
- Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol 2004; 6(6):523-31; PMID:15156152; http://dx.doi.org/10.1038/ncb1136
- Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, Du QS, Mei L, Ding YQ, Xiong WC. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol 2007; 9(2):184-92; PMID:17237772; http://dx.doi.org/10.1038/ncb1535
- Li J, He Y, Lu Q, Zhang M. Mechanistic Basis of Organization of the Harmonin/USH1C-Mediated Brush Border Microvilli Tip-Link Complex. Dev Cell 2016; 36(2):179-89; PMID:26812017; http://dx.doi.org/10.1016/j.devcel.2015.12.020
- Johnston AM, Naselli G, Niwa H, Brodnicki T, Harrison LC, Gonez LJ. Harp (harmonin-interacting, ankyrin repeat-containing protein), a novel protein that interacts with harmonin in epithelial tissues. Genes Cells 2004; 9(10):967-82; PMID:15461667; http://dx.doi.org/10.1111/j.1365-2443.2004.00776.x
- Pan L, Zhang M. Structures of usher syndrome 1 proteins and their complexes. Physiology (Bethesda) 2012; 27(1):25-42; PMID:22311968; http://dx.doi.org/10.1152/physiol.00037.2011
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet 2004; 5(7):489-98; PMID:15211351; http://dx.doi.org/10.1038/nrg1377
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 2007; 449(7158):87-91; PMID:17805295; http://dx.doi.org/10.1038/nature06091
- Pan L, Yan J, Wu L, Zhang M. Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23. Proc Natl Acad Sci U S A 2009; 106(14):5575-80; PMID:19297620; http://dx.doi.org/10.1073/pnas.0901819106
- Wu L, Pan L, Zhang C, Zhang M. Large protein assemblies formed by multivalent interactions between cadherin23 and harmonin suggest a stable anchorage structure at the tip link of stereocilia. J Biol Chem 2012; 287(40):33460-71; PMID:22879593; http://dx.doi.org/10.1074/jbc.M112.378505
- Wu L, Pan L, Wei Z, Zhang M. Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo. Science 2011; 331(6018):757-60; PMID:21311020; http://dx.doi.org/10.1126/science.1198848
- Yan J, Pan L, Chen X, Wu L, Zhang M. The structure of the harmonin/sans complex reveals an unexpected interaction mode of the two Usher syndrome proteins. Proc Natl Acad Sci U S A 2010; 107(9):4040-5; PMID:20142502; http://dx.doi.org/10.1073/pnas.0911385107
- Bahloul A, Michel V, Hardelin JP, Nouaille S, Hoos S, Houdusse A, England P, Petit C. Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet 2010; 19(18):3557-65; PMID:20639393; http://dx.doi.org/10.1093/hmg/ddq271
- Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J 2002; 21(24):6689-99; PMID:12485990; http://dx.doi.org/10.1093/emboj/cdf689
- Kikkawa Y, Shitara H, Wakana S, Kohara Y, Takada T, Okamoto M, Taya C, Kamiya K, Yoshikawa Y, Tokano H, et al. Mutations in a new scaffold protein Sans cause deafness in Jackson shaker mice. Hum Mol Genet 2003; 12(5):453-61; PMID:12588793; http://dx.doi.org/10.1093/hmg/ddg042
- Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, Nicolson T. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci U S A 2014; 111(35):12907-12; PMID:25114259; http://dx.doi.org/10.1073/pnas.1402152111
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 2013; 79(3):504-15; PMID:23871232; http://dx.doi.org/10.1016/j.neuron.2013.06.019
- Lefevre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin JP, Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development 2008; 135(8):1427-37; PMID:18339676; http://dx.doi.org/10.1242/dev.012922
- D'Alterio C, Tran DD, Yeung MW, Hwang MS, Li MA, Arana CJ, Mulligan VK, Kubesh M, Sharma P, Chase M, et al. Drosophila melanogaster Cad99C, the orthologue of human Usher cadherin PCDH15, regulates the length of microvilli. J Cell Biol 2005; 171(3):549-58; PMID:16260500; http://dx.doi.org/10.1083/jcb.200507072
- Glowinski C, Liu RH, Chen X, Darabie A, Godt D. Myosin VIIA regulates microvillus morphogenesis and interacts with cadherin Cad99C in Drosophila oogenesis. J Cell Sci 2014; 127(Pt 22):4821-32; PMID:25236597; http://dx.doi.org/10.1242/jcs.099242
- Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol 1992; 8:257-74; PMID:1476800; http://dx.doi.org/10.1146/annurev.cb.08.110192.001353
- Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res 2006; 83(1):97-119; PMID:16545802; http://dx.doi.org/10.1016/j.exer.2005.11.010
- Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, Hussain K, Furth-Lavi J, Cosgrove KE, Shepherd RM, et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat Genet 2000; 26(1):56-60; PMID:10973248; http://dx.doi.org/10.1038/79178
- Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet 2000; 26(1):51-5; PMID:10973247; http://dx.doi.org/10.1038/79171
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 1995; 374(6517):60-1; PMID:7870171; http://dx.doi.org/10.1038/374060a0
- Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, Ness SL, Polomeno R, Ramesh A, Schloss M, Srisailpathy CR, et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 2001; 68(1):26-37; PMID:11090341; http://dx.doi.org/10.1086/316954
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Riazuddin S, Wilcox ER. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet 2001; 69(1):25-34; PMID:11398101; http://dx.doi.org/10.1086/321277
- Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta 2015; 1852(3):406-20; PMID:25481835; http://dx.doi.org/10.1016/j.bbadis.2014.11.020
- Hussain K, Bitner-Glindzicz M, Blaydon D, Lindley KJ, Thompson DA, Kriss T, Rajput K, Ramadan DG, Al-Mazidi Z, Cosgrove KE, et al. Infantile hyperinsulinism associated with enteropathy, deafness and renal tubulopathy: clinical manifestations of a syndrome caused by a contiguous gene deletion located on chromosome 11p. J Pediatr Endocrinol Metab 2004; 17(12):1613-21; PMID:15645695
- Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Lainé S, Delmaghani S, Adato A, Nadifi S, Zina ZB, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet 2003; 12(5):463-71; PMID:12588794; http://dx.doi.org/10.1093/hmg/ddg051
- Caberlotto E, Michel V, Foucher I, Bahloul A, Goodyear RJ, Pepermans E, Michalski N, Perfettini I, Alegria-Prévot O, Chardenoux S, et al. Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc Natl Acad Sci U S A 2011; 108(14):5825-30; PMID:21436032; http://dx.doi.org/10.1073/pnas.1017114108
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol 2005; 485(1):75-85; PMID:15776440; http://dx.doi.org/10.1002/cne.20513
- Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, Millis BA, Cui R, Nakanishi H, Fujikawa T, Kawashima Y, et al. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep 2015; 12(10):1606-17; PMID:26321635; http://dx.doi.org/10.1016/j.celrep.2015.07.058
- Shin JB, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, et al. Molecular architecture of the chick vestibular hair bundle. Nat Neurosci 2013; 16(3):365-74; PMID:23334578; http://dx.doi.org/10.1038/nn.3312
- Wilmarth PA, Krey JF, Shin JB, Choi D, David LL, Barr-Gillespie PG. Hair-bundle proteomes of avian and mammalian inner-ear utricles. Sci Data 2015; 2:150074; PMID:26645194; http://dx.doi.org/10.1038/sdata.2015.74
- McConnell RE, Benesh AE, Mao S, Tabb DL, Tyska MJ. Proteomic analysis of the enterocyte brush border. Am J Physiol Gastrointest Liver Physiol 2011; 300(5):G914-26; PMID:21330445; http://dx.doi.org/10.1152/ajpgi.00005.2011