Abstract
Objective
To evaluate the Milano-Torino staging (MiToS) and King’s staging systems as potential outcome measures for clinical trials in amyotrophic lateral sclerosis (ALS) by assessing these outcomes in FORTITUDE-ALS.
Methods
This was a post hoc analysis of the phase 2b FORTITUDE-ALS trial (NCT03160898), a double-blind, randomized, dose-ranging, placebo-controlled, parallel-group study of reldesemtiv in patients with ALS. The treatment period was 12 weeks, with a follow-up assessment at week 16. Patients were retrospectively classified into MiToS and King’s stages. Outcomes were the mean time maintaining baseline stage and risk of progression from the baseline stage to a later stage.
Results
The full analysis set consisted of 456 patients randomized 3:1 (reldesemtiv n = 342, placebo n = 114) who received at least one dose of double-blind study drug and had at least one post-baseline assessment. At baseline, MiToS and King’s stages were balanced between the reldesemtiv and placebo groups: >99% of patients were in MiToS stage 0 or 1 and King’s stage 1, 2 or 3. Time of maintaining the baseline stage was similar in both groups, for each staging system. The two staging systems exhibited considerably disparate results for risk of progression from baseline to a later stage: hazard ratio (HR) = 0.62 (95% confidence interval [CI] 0.38, 0.99) for MiToS and HR = 0.96 (95% CI 0.63, 1.44) for King’s.
Conclusion
This exploratory analysis showed the feasibility of MiToS and King’s staging as potential outcome measures in ALS. Additional studies of these staging systems are needed to further explore their utility in ALS clinical trials.
Introduction
The Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) is the most widely used measure for functional assessment of patients with ALS and is frequently used as the primary endpoint in randomized clinical trials (Citation1). The ALSFRS-R is based on 12 items rated on a 0–4-point scale assessing bulbar function, fine and gross motor skills, and respiratory functions (Citation1). However, assessing a patient’s level of independence or level of function requires evaluation of individual items from the ALSFRS-R, rather than simply the total score. In clinical trials, this makes it difficult to quantify the impact of a therapeutic intervention on late-stage disease changes, quality of life, and economic outcomes (Citation2).
Consequently, methods for ALS staging based on clinical milestones have been introduced. These can enhance communication between clinicians and patients, as well as provide important information on ALS outcomes. Staging determinations are primarily derived from evaluating components of the ALSFRS-R rather than the total score. In clinical practice, these staging criteria may assist with determining disease severity, prognosis and treatment options; in addition, they may have potential to provide an objective measure for ALS progression in clinical trials (Citation3,Citation4).
Of the staging systems developed, the Milano-Torino staging (MiToS) system and the King’s College system are the most commonly used (Citation3,Citation4). MiToS is entirely derived from the ALSFRS-R and characterizes the functional burden of the disease as defined by loss of autonomy in four key domains: walking/self-care, swallowing, communicating and breathing (Citation3). The number of domains impaired determines the MiToS stage: stage 0–4 being 0–4 domains impaired, with stage 5 being death. Functional loss is determined directly through scores of 0 and 1 on specific items from the ALSFRS-R, with the exception of item 12 (respiratory insufficiency) in which a score of 2 or lower is deemed functional loss. King’s staging is based on disease burden as measured by clinical milestones and considers how many of the three anatomical regions are involved (bulbar, upper and lower limbs; Stage 1 for one region involved, Stage 2 for two and Stage 3 for three) and the need for gastrostomy and noninvasive ventilation (stages 4A and 4B, respectively) (Citation4). King’s staging is not based solely on ALSFRS-R scores but can be estimated from them using a published mapping algorithm; estimated stages show approximately 92% correlation with the actual clinical stage (Citation5). Movement into a subsequent King’s stage occurs with the first appearance of a sign or symptom of either upper or lower motor neuron dysfunction in an anatomical region. It has been suggested that MiToS can better detect differences later in the disease course, whereas King’s better detects differences earlier (Citation6). These differences might support the use of both staging systems, as they may provide complementary information (Citation6,Citation7).
Reldesemtiv is a selective, small-molecule, fast skeletal muscle troponin activator, currently in clinical trials in ALS (Citation8,Citation9). In a randomized, double-blind, phase 2b study (FORTITUDE-ALS), patients with ALS received one of three reldesemtiv doses or placebo for 12 weeks. In this trial, reldesemtiv was shown to be well tolerated, with nausea and fatigue being the most common adverse events (Citation9). In the primary efficacy analyses, the difference between reldesemtiv and placebo was not statistically significant (p = 0.11). Similarly, the differences between groups in the key secondary endpoint, ALSFRS-R, were not statistically significant. However, a post hoc analysis combining all doses of reldesemtiv compared with placebo demonstrated a statistically significant difference favoring reldesemtiv in the change in the ALSFRS-R total score from baseline to week 12 (p = 0.01) (Citation9). Overall, the investigators concluded that the effect of reldesemtiv in patients with ALS warranted further evaluation in longer studies. Consequently, a phase 3 trial, COURAGE-ALS, is under way (Citation10).
Previously published studies have shown the value of MiToS and King’s staging in assessing treatment efficacy and their potential utility in clinical trials as outcome measures, although evidence reported to date is still limited. To our knowledge, MiToS has been used as primary endpoint in a 6-month phase 2 clinical trial of guanabenz in patients with ALS (Citation11), and the effect of riluzole on survival based on clinical milestones in ALS has been evaluated using King’s staging (Citation12). Al-Chalabi et al. conducted a post hoc analysis of edaravone using phase 3 trial data that showed the utility of MiToS and King’s staging as endpoints in ALS clinical trials (Citation7). The main purpose of the current study was to further evaluate the value of MiToS and King’s staging as potential outcome measures in ALS by assessing these outcomes in FORTITUDE-ALS.
Materials and methods
Data for the current analysis came from the double-blind, randomized, placebo-controlled, phase 2b FORTITUDE-ALS trial (ClinicalTrials.gov, NCT03160898). In this trial, eligible patients with ALS were randomized 1:1:1:1 to receive either reldesemtiv oral tablets 150, 300 or 450 mg or placebo, dosed twice daily, for 12 weeks, with a 4-week follow-up after the last treatment dose. Details about patients randomized in FORTITUDE-ALS have been published (Citation9). The trial obtained all institutional review board approvals before enrollment and all patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
In FORTITUDE-ALS, the primary and the key secondary efficacy endpoints were the change from baseline to week 12 in percent predicted slow vital capacity (SVC) and the ALSFRS-R, respectively. Both SVC and ALSFRS-R were assessed at screening, day 1 and weeks 1, 2, 4, 8 and 12 with a follow-up assessment at week 16. For this post hoc analysis, patients were retrospectively classified into MiToS and King’s stages at each time point based on their ALSFRS-R scores. The MiToS stage was determined directly from ALSFRS-R scores, whereas the King’s stage was determined using the algorithm previously described (Citation5). Outcomes assessed were (Citation1) mean time remaining in the baseline stage, and (Citation2) time to progression from the baseline stage to a later stage (any decline of one or more stages).
Analyses were based on the full analysis set (FAS), which included all randomized patients who received any study drug and had a baseline assessment as well as at least one post-baseline efficacy measurement. Data from all three reldesemtiv dose groups were pooled and compared with placebo. All analyses included data up to week 12 (the end of the double-blind treatment period).
Mean time remaining in the baseline stage was compared using a two-sample t-test. The time to progression was compared using Kaplan–Meier time-to-event estimates and a Cox proportional-hazards model (Citation13,Citation14). We defined progression as a transition from baseline stage to a later MiToS (or King’s) stage during the treatment period of the trial. In this analysis, patients who discontinued before progressing to a later stage were censored. Cox regression was performed using a univariate analysis (only treatment assignment) and multivariate analysis (treatment assignment and baseline covariates). Baseline characteristics of age, sex, time since ALS symptom onset, time since ALS diagnosis, ALSFRS-R, MiToS or King’s stage (depending on which staging method was being analysed), riluzole and edaravone use and creatinine value were included as covariates in the multivariate Cox regression (Citation15). For the purpose of analysis, p-values ≤0.05 were considered statistically significant, although all analyses were exploratory.
Results
In FORTITUDE-ALS, the FAS included 456 patients who received either reldesemtiv (n = 342) or placebo (n = 114). The baseline demographic and clinical characteristics of patients were well balanced across the treatment arms ().
Table 1 Baseline characteristics.
At baseline, >99% of patients were in MiToS stage 0 or 1 () and in King’s stage 1, 2 or 3 (). The baseline MiToS and King’s stages were balanced across the treatment arms (reldesemtiv and placebo).
Table 2 MiToS stage at baseline.
Table 3 King’s stage at baseline.
Mean time at the baseline stage
When looking at the mean time at baseline stage for each staging system, the analysis was not able to detect any differences between groups ().
Table 4 Days of maintaining the baseline MiToS or King’s stage in the FORTITUDE-ALS trial.
Time to progression (at least one stage decline): MiToS
During the 12-week double-blind treatment period, 17.8% (95% confidence interval [CI] 13.8%, 21.9%) of the reldesemtiv group progressed from baseline to a later stage, compared with 23.7% (95% CI 15.9%, 31.5%) of the placebo group. Results of the Kaplan–Meier analysis using MiToS showed patients in the reldesemtiv group appeared to experience lower risk of progression compared with placebo, although the difference did not meet the threshold for statistical significance (log-rank test, p = 0.051) ().
Figure 1 Time to decline of ≥1 MiToS stage from baseline. p-Value from log-rank test at week 12 (end of the double-blind treatment period).
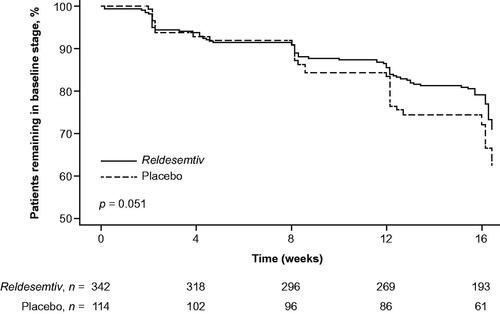
Using Cox regression, after controlling for baseline covariates, the risk of progression to a later MiToS stage was lower with reldesemtiv versus placebo (adjusted hazard ratio [HR] 0.62, 95% CI 0.38, 0.99, p = 0.047). The unadjusted HR of reldesemtiv versus placebo was 0.65 (95% CI 0.41, 1.03, p = 0.065) ().
Table 5 Progression from baseline to a later stage using MiToS or King’s staging.
Time to progression (at least one stage decline): King’s staging
The percentage of patients who progressed from baseline to a later stage during the 12-week treatment period was 28.9% (95% CI 24.1%, 33.8%) for reldesemtiv and 28.1% (95% CI 19.8%, 36.3%) for placebo. Using the Kaplan–Meier approach, a difference between the groups in time to progression was not shown using King’s staging (log-rank test, p = 0.849; ).
Figure 2 Time to decline of ≥1 King’s stage from baseline. p-Value from log-rank test at week 12 (end of the double-blind treatment period).
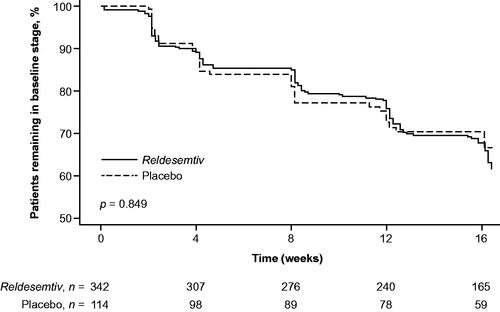
In Cox regression analyses, the risk of disease progression according to King’s staging also did not show a difference between the reldesemtiv and placebo groups (unadjusted HR 0.95, 95% CI 0.63, 1.42; adjusted HR 0.96, 95% CI 0.63, 1.44) ().
Discussion
In these analyses, we evaluated MiToS and King’s staging as potential tools to measure treatment effect in clinical trials. Our aim was to determine whether these staging systems could be used as a companion to the change in the total ALSFRS-R score, which is well established as a clinical trial outcome by regulatory authorities (Citation16). Use of systems that demarcate meaningful differences between stages could complement the ALSFRS-R by quantifying the impacts of a therapeutic intervention.
The clinical trial used as a data source for our analysis was a randomized, placebo-controlled trial of reldesemtiv, with a relatively short treatment duration of 12 weeks. In evaluating disease progression in this trial, MiToS was able to show a difference between the reldesemtiv and placebo groups, whereas a difference in time to progression was not detected using King’s staging. However, in view of the short trial period, the marginal statistical significance of the MiToS data and the wide confidence interval of the HR estimates, the interpretation of these results and any conclusions should be made with caution. The wide confidence intervals around the estimates of time to disease progression may result from the noticeable differences for patients in MiToS stage 2 at baseline. As shown in , a few patients in the reldesemtiv group with MiToS baseline stage 2 have much shorter time remaining at stage 2 before progression (mean 22.7 days) than that of the majority in the reldesemtiv group, while the patient in the placebo group with MiToS baseline stage 2 had much longer time remaining at stage 2 before progression (84 days) than that of the majority in the placebo group. This leads to a wider range of variation when combining patients with all baseline stages for the analysis of time to progression ().
The notable difference in terms of level of statistical significance for results of the different types of analyses is also worth further discussion. Results were compared using two methods that assess different aspects of the data; firstly, comparing the lengths of time remaining at baseline stage between the two groups (); secondly, presenting the ratios of event rates on stage changes between reldesemtiv and placebo groups (). As shown in , most of the MiToS stage changes did not occur until after Week 8. Therefore, it seems reasonable that the analyses would give different results, with a borderline significant difference in event rates (with separation in curves seen from Week 8) but no significant difference in duration of remaining at baseline stage (the separation did not occur early enough, or the trial duration is not long enough to capture the significant difference, if it exists, between reldesemtiv and placebo groups).
The apparent difference in results for MiToS and Kings’ staging observed in the current study reflects distinctions between the two staging systems, which capture different aspects of ALS progression that may manifest at different times—MiToS primarily assesses loss of function, while the King’s staging primarily assesses anatomical spread (Citation3,Citation4). There are several possible explanations why this study found a difference with MiToS but not with King’s staging. First, it may be that treatment affected functionality; this is in keeping the proposed mechanism of action of reldesemtiv, which affects muscle force (Citation8,Citation9). Second, the 12-week treatment duration may have contributed to this observed difference between the staging systems, as time to stage progression in early stages of the disease with the King’s system is typically longer (Citation6). Third, if study patients were already in advanced stages at baseline, it may have been too late for a difference to be detected according to King’s staging, while MiToS could still show progression in later stages. The latter seems unlikely, as baseline data showed the majority of patients to be in King’s stage 1 or 2. Lastly, King’s staging was derived indirectly from ALSFRS-R, using an algorithm with a published correlation of 0.92 (Citation5), so it remains possible that a different result may have been observed if King’s stages had been directly measured by the treating clinician.
In light of the differences, it is worth mentioning that a previously reported comparison of treatment outcomes using both MiToS and King’s staging also observed differences between the two systems. Al-Chalabi et al. applied the Kaplan–Meier method to data from the edaravone phase 3 study MC1186-19 and evaluated the clinical benefit of edaravone compared with placebo (Citation7). The study found no evidence that edaravone improved time to progression compared with placebo using MiToS (p = 0.308). The authors also found patients assigned to edaravone experienced longer time to progression in King’s staging compared with placebo, but the difference was not statistically significant (p = 0.103), and the study did not report HRs, so the precision around this estimate is unknown. Although we could speculate that some of the reasons for differences between staging systems discussed above might apply, comparison of the studies is somewhat limited as the two clinical trials, FORTITUDE-ALS and MC1186-19, had different treatment duration (12 weeks vs 24 weeks) as well as different inclusion criteria, as previously reported (Citation9,Citation17). Nevertheless, our study and the analysis of edaravone both suggest that these staging systems are feasible for assessing treatment effects. However, their utility as endpoints for clinical trials requires further investigation. It is notable that an ongoing phase 3 trial of AMX0035 in ALS is including both staging systems as secondary endpoints, and is anticipated to provide more data on their usefulness in a trial setting (Citation18).
A strength of the current analysis is use of data from a randomized, controlled clinical trial, in which ALSFRS-R data were collected prospectively. Nevertheless, the trial design posed limitations for this analysis, including the limited 12 weeks’ treatment duration, and exclusion of patients in later stages, as well as the inherent limitations of post hoc analyses. Consequently, these results should be considered exploratory. Future studies, ideally evaluating at least 24 weeks of treatment and direct collection of staging data, are warranted to confirm these findings.
In conclusion, this exploratory analysis showed the feasibility of MiToS and King’s staging as potential outcome measures in ALS. Additional studies of these staging systems are needed to further explore their utility in ALS clinical trials.
Author contributions
PG contributed to concept and study design. PG, JW and LM contributed to the statistical analysis. PG and PS conducted interpretation of data. PG drafted the manuscript, and all authors were involved in critical revision and review of the paper.
Acknowledgements
We wish to thank the participants of FORTITUDE-ALS and their families for their contributions to this clinical trial, the investigators of FORTITUDE-ALS, FORTITUDE-ALS study group and members of the Data Monitoring Committee and Steering Committee.
Disclosure statement
PG, LM, SAR, JW and AAW own stock in and are employees of Cytokinetics, Incorporated. PS was an employee of Cytokinetics, Incorporated at the time of this study. AC serves on the advisory board for Biogen, Cytokinetics, Incorporated, Denali Pharma, Amylyx and Mitsubishi Tanabe. JAA has received research funding to their institution from Alexion, AZTherapies, Amylyx, Biogen, Cytokinetics, Orion, Novartis, MGH Foundation, Ra Pharma, Biohaven, Clene, and Prilenia and consulting fees from AL-S Pharma, Affinia, Amylyx, Apellis, Biogen, Cytokinetics, Denali, Orphazyme, Neurosense, Novartis, UCB, and Wave Life Sciences. AG served as a consultant for Alexion, AL-S Pharma, Biogen, Calico and Cytokinetics, Incorporated. CEJ has served as a member of data safety monitoring boards for Anelixis Therapeutics, Inc. and Mitsubishi Tanabe Pharma America. NL is a consultant for Cytokinetics, Incorporated. TMM is a consultant for Cytokinetics, Incorporated and Disarm Therapeutics; has licensing agreements with C2N Diagnostics and Ionis Pharmaceuticals; and serves on the advisory board and receives research support from Biogen. JMS received personal compensation from Avexis, Biogen, Brainstorm Cell Therapeutics, Cytokinetics, Incorporated, Mitsubishi Tanabe Pharma America, Neurosense, Orphazyme, Otsuka and Revalesio; and research support from Amylyx, Brainstorm Cell Therapeutics, Cytokinetics, Incorporated, Medicinova and Mitsubishi Tanabe Pharma America.
Data availability statement
Data reported herein are part of a sponsor-led clinical development program that is ongoing, and thus complete datasets for the trial will not be made available with this report.
Additional information
Funding
References
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
- Tramacere I, Dalla Bella E, Chiò A, Mora G, Filippini G, Lauria G, et al. The MITOS system predicts long-term survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:1180–5.
- Chiò A, Hammond ER, Mora G, Bonito V, Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:38–44.
- Roche JC, Rojas-Garcia R, Scott KM, Scotton W, Ellis CE, Burman R, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012;135:847–52.
- Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:279–84.
- Fang T, Al Khleifat A, Stahl DR, Lazo La Torre C, Murphy C, Uk-Mnd Lical S, et al. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:227–32.
- Al-Chalabi A, Chiò A, Merrill C, Oster G, Bornheimer R, Agnese W, et al. Clinical staging in amyotrophic lateral sclerosis: analysis of Edaravone Study 19. J Neurol Neurosurg Psychiatry. 2021;92:165–71.
- Andrews JA, Miller TM, Vijayakumar V, Stoltz R, James JK, Meng L, et al. CK-2127107 amplifies skeletal muscle response to nerve activation in humans. Muscle Nerve. 2018;57:729–34.
- Shefner JM, Andrews JA, Genge A, Jackson C, Lechtzin N, Miller TM, et al. A phase 2, double-blind, randomized, dose-ranging trial of reldesemtiv in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:287–99.
- ClinicalTrials.gov. A study to evaluate the efficacy and safety of reldesemtiv in patients with amyotrophic lateral sclerosis (ALS) (COURAGE-ALS). https://clinicaltrials.gov/ct2/show/NCT04944784. Accessed March 29, 2022.
- Dalla Bella E, Bersano E, Antonini G, Borghero G, Capasso M, Caponnetto C, et al. The unfolded protein response in amyotrophic later sclerosis: results of a phase 2 trial. Brain. 2021;144:2635–47.
- Fang T, Al Khleifat A, Meurgey JH, Jones A, Leigh PN, Bensimon G, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018;17:416–22.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JAMA 1958;53:457–81.
- Cox DR. Regression models and life‐tables. J R Stat Soc B. 1972;34:187–220.
- Chiò A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10:310–23.
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Biologics Evaluation and Research (CBER). Amyotrophic lateral sclerosis: developing drugs for treatment guidance for industry. 2019. Available at: https://www.fda.gov/media/130964/download. Accessed November 10, 2022.
- Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017;16:505–12.
- ClinicalTrials.gov. Phase III trial of AMX0035 for amyotrophic lateral sclerosis treatment (Phoenix). https://clinicaltrials.gov/ct2/show/NCT05021536. Accessed July 21, 2022.
