Abstract
Today, nano-medicine promotes new therapeutics and diagnostics tools, including sensing of biomolecules as a biosensor, cancer chemotherapy and drug or gene delivery. Because of small size and biocompatibility of gold nanoparticles (GNPs), they become a good candidate for biological application. Also, thanks to their biological and chemical properties, they can mimic function of some enzymes including super oxide dismutase (SOD), esterase, etc. Also, biomaterials and bioengineering have grown so fast since the last decade for many therapeutic applications such as tissue regeneration. Among these cutting edge technology, nanomaterials find the way to becoming a very powerful tool for using in many fields of researchers including biosensing, gene therapy and chemotherapy. In this review, we focused on some biological applications of GNPs in biology and medicine.
Introduction
Gold nanoparticles (GNPs): an overview
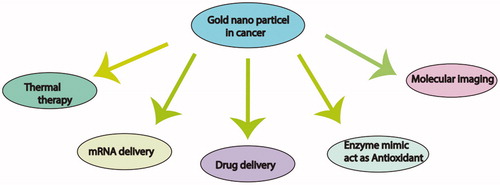
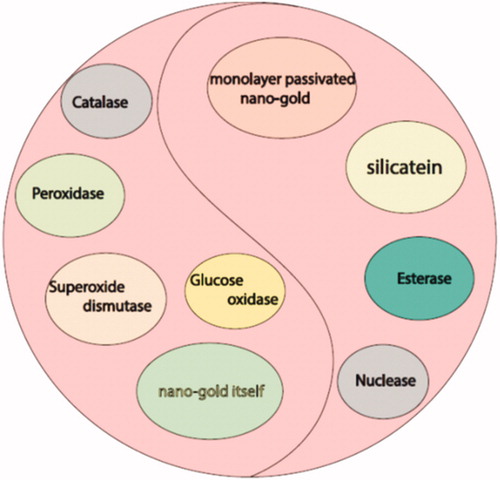
It is accepted that some metal-based nanoparticles (NPs) potentially mimic enzyme function [Citation1]. Among all NPs, GNPs are so believed because of enzymatic properties and their application in biology [Citation2]. Moreover, "hidden talents" of GNPs revealed as artificial enzymes, including ability of GNP to mimic the nuclease, esterase, silicatein, glucose oxidase, peroxidase, catalase and superoxide dismutase [Citation3] as well as GNPs with either positive or negative surface charges show peroxidase mimicking activity [Citation4]. These enzyme-like activities of GNPs are related to functional groups. In fact, GNPs enzyme-like activities help scientist to develop and design of biosensors, immunoassay, clinical studies, detection and photothermal activity of micro-organisms and cancer cells, targeted delivery of the drug, and as well as in bioimaging according to this potential () [Citation3–7]. GNPs are biocompatible because of special properties such as non-toxicity, facile synthesis, size and shape tenability [Citation7]. Besides, biological safety and user-friendly methods for constructing GNPs, they have no toxic material after degradation [Citation8]. In this manner, it is possible that GNPs combined to different parts of plants, fruits, microorganisms and biomolecules [Citation8]. For example, synthesis of GNPs from Curcuma longa root extract by ‘exploiting’ the reduction capabilities of varied phytochemicals present in was confirmed [Citation9].
GNPs enzymatic potential
GNPs have enzymatic properties itself via surface-bound ligands that derived them to catalytic reactions; it is a usual method to mimic catalytic activity in synthetic aspects. However, the GNP can also be designed as the catalytic component as various enzyme mimics [Citation10]. One of the enzymatic aspects of GNPs is to act as glucose oxidase mimic that the performance of GNPs is mentioned above ().
Glucose oxidase (GOx) mimic
GOx is an oxide-reductase enzyme that catalyzes the oxidation of β-d-glucose to gluconic acid, by utilizing oxygen as an electron acceptor with simultaneous production of hydrogen peroxide (H2O−) [Citation10]. Notably, the GOx-like activities of Au-NPs have been for simple and reliable detection of DNAs [Citation11,Citation12]. Although other metal nanomaterials, such as Cu, Ag, Pd and Pt, are tested, but did not show significant oxidase-like activity under similar conditions [Citation13]. A non-enzymatic electrochemical method has been developed for the detection of glucose by using gold (Au) NPs self-assembled on a three-dimensional (3D) silicate network obtained by using sol–gel processes [Citation14]. As well as, GNPs showed that the Au NPs catalyze the oxidation of glucose at less positive potential (0.16 V) in phosphate-buffered solution (pH 9.2) and in the absence of any enzymes or redox mediators [Citation15,Citation16].
In addition to glucose sensing, nano-gold-aggregation (NGA) phenomenon has provided some other usages, such as solution-based immune-assays and DNA-hybridization assay [Citation17]. In NGA reaction, two macromolecules, such as antigen–antibody, should be bounded together [Citation18]. Notably, GNPs were used as a novel ultrasensitive multiplexed immunoassay method by combining alkaline phosphatase (ALP)-labelled antibody-functionalized GNPs (ALP-Ab/Au NPs) and GNP as an enzyme catalyzed in the presence of silver NPs at a disposable immune-sensor [Citation19,Citation20]. Also, Weijie et al. fabricated a self-catalyzed and self-limiting growth of glucose oxidase mimicking GNP. They showed that AuNP catalyzed glucose oxidation and produced H2O2. However, size-dependent activity decreases have a great impact to control enzymatic activity [Citation21]. Self-limiting or smart nanomaterial fabricates according to shape and size, and having fewer toxicity.
Peroxidase mimic
Previously, researchers proved that ferromagnetic NPs occupy the intrinsic peroxidase-like activity. Since then, many studies have been developed to design of various GNPs with a great potential of peroxidase-like activity. Peroxidases are a large family of enzymes that typically catalyze the oxidation of horseradish peroxidase (HRP). However, GNPs are optimized in pH =3 and in vitro condition, but not in biological condition and pH =7.4 [Citation22]. Glucose detection is a well-known biological activity of GNPs [Citation23]. Colorimetric detection of H2O2 and glucose was performed according to intrinsic peroxidase-like activity and catalyzes the oxidation of the peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB) by [Citation24]. GNPs as a peroxidase mimetic were first developed for the enzymatic spectrophotometric analysis of uric acid via detection H2O2 that was generated by uric acid [Citation25]. Notably, GNPs are a promising method for uric acid analysis in human serum [Citation23,Citation24]. A novel immunoassay technique designed thanks to peroxidase-like activity and GNPs for detecting of α-fetoprotein, by enhanced ultrasensitive chemiluminescence enzyme immunoassay for the determination of α-fetoprotein [Citation26]. The method was based on 4-(4′-iodo) phenyl-phenol (IPP) as a new potential signal enhancer and double-codified GNPs (DC-Au-NPs) that are labelled with HRP-conjugated anti-AFP which is used for further signal amplification [Citation27]. According to the protocol, antigen in the sample was captured by the immobilized primary antibody on the surface of magnetic beads and recognized by the second antibody labelled with DC-Au-NPs consequently [Citation28]. Also, it is possible to design peptides on GNPs to give nanomaterial with some chemical properties that are analogous to those of proteins [Citation29].
Superoxide dismutase (SOD) mimic and catalase mimic
SOD is an important antioxidant defence against free radicals. It catalyzes the dismutation of superoxide (O2−) into O2 and H2O2 [Citation30]. Also, catalase is an enzymatic group supporting the cell from oxidative damage by reactive oxygen species [Citation29]. Markedly, GNPs have received a great deal of interest because of their unique properties of optical and in biomedical applications [Citation31,Citation32]. There is growing evidence that GNPs can catalyze then rapid decomposition of hydrogen peroxide that is followed by the formation of hydroxyl radicals at lower pH and oxygen at higher pH. These results strongly demonstrated that GNPs can act as SOD catalase mimetic [Citation33,Citation34]. However, these challenges thank to chemical synthesis of NPs that are proposed in toxicology [Citation35]. In a study, these synthesized biocompatible GNPs (Tu-AuNPs) are used as an antioxidant against 1-methyl-2-phenyl pyridinium ion (MPP+) which induced cytotoxicity and cell death in PC-12 cells [Citation36,Citation37]. Incubation of PC-12 cells with Tu-AuNPs prevented MPP+ -induced loss in cell viability and enhanced LDH. In addition, reduction in the level of non-protein thiol, glutathione (GSH), activities of the antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST) as well as the increased MDA levels have also been found to be prevented by this NP in cancerous cells [Citation38,Citation39].
Esterase mimic
An esterase is a hydrolase enzyme that ruptures esters into an acid and an alcohol in a chemical reaction in water. The reaction is called hydrolysis [Citation40]. According to evidence, the first example of peptide-functionalized GNPs is hydrolytically active against carboxylate esters. The active units are constituted by His-Phe-OH terminating thiols [Citation41,Citation42]. A highly sensitive and selective fluorescent assay for the detection of acetylcholine (ACh) was developed based on enzyme mimics of Au/Ag NPs [Citation43]. This mechanism involved is the following: reacting ACh with acetylcholinesterase (AChE) to form choline that is in turn oxidized by choline oxidase (ChOx) to produce betaine and H2O2, which reacts with Amplex UltraRed (AUR) in the presence of bimetallic NPs catalyst to form a fluorescent product [Citation44].
Other biological usages
Gene delivery
GNPs with synthetic microRNAs can enter cells without the aid of cationic co-carriers [Citation45]. MicroRNAs belong to non-coding RNA family and regulate multiple proteins in interactions with the 3 prime untranslated regions of the target messenger RNA and control cell behaviour at post-translational level [Citation46]. The GNPs–microRNA conjugation is a new tool for microRNA delivery and is candidates for the microRNA replacement delivery system [Citation47].
It is demonstrated that polyvalent DNA-functionalized GNPs (DNA-Au NPs) selectively enhance Ribonuclease H (RNase H) activity while inhibiting most biologically relevant nucleases. Then, high RNase H activity results in rapid mRNA degradation and general nuclease inhibition results in high biological stability [Citation48,Citation49]. Selective RNase H activity in the high DNA density of DNA-Au-NPs is responsible for this unusual behaviour [Citation50]. Also, polyvalent DNA-Au-NPs regulate gene expression as a new model for selectively controlling protein − NP interactions [Citation51]. The potential of a single molecular nanoconjugate is to intersect with all RNA pathways including gene-specific down-regulation such as siRNA and miRNA pathways [Citation52]. Gold-nano-beacons are capable of silencing gene expression and endogenous miRNAs, while yielding a quantifiable fluorescence signal directly proportional to the level of silencing [Citation53,Citation54]. Also, GNPs have a great role in tissue regeneration as a scaffold [Citation53]. Human mesenchymal stem cells (hMSCs), is critical for the development of effective cellular therapies for tissue engineering [Citation53]. Developing bioengineering as a multidisciplinary field of research helps scientists to fabricate different stem cells by GNPs. One of the main advantages of this method is the induction of immune tolerance [Citation54].
Drug delivery
Another application is cancer-targeted drug delivery. GNPs conjugated with chemotherapeutics drugs such as doxorubicin may overcome side effect of chemotherapy like nausea or cardiac toxicity [Citation55]. In 2009, gold nanoshells Aurora's took FDA approval in chemotherapy [Citation56]. However, other GNPs are still using in research [Citation57–60].
Immunoassays
Au nanorodes were employed for immunoassays. For example, sandwich ELIZA for IL-2. Integrin specific peptide was engineered by Au NP to mimic peroxidase mimics. This protein is used as a probe to detect cancer [Citation21]. Glucose have been detected by Au-based nanomaterial. According to evidence, AuNPs mimics glucose oxidase to detect glucose.
Anti-bacterial properties
AuNPs encapsulated within mesoporous silica demonstrated antibacterial activity. AuNPs have exhibited both oxidase and peroxidase mimicking activities and end material of reactions is reactive oxygen species. Antibacterial properties proved against both Gram-negative and Gram-positive bacteria [Citation61].
Conclusions
All in all, because of many advantages that GNPs including small size, flexible synthesis and biocompatibility, they have wide applications in biology and medicine. However, they mimic some biological effects of functional proteins such as enzymes. Also, they are used in drug delivery and gene delivery system. Thanks to the fast growing of this technology, great varieties of GNPs synthesize in different shapes and size. It is useful for promoting NPS biological applications and it is necessary to continue research on nanomaterial safety in parallel.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Lin Y, Ren J, Qu X. Nano-gold as artificial enzymes: hidden talents. Adv Mater Weinheim. 2014;26:4200–4217.
- Jena BK, Raj CR. Enzyme-free amperometric sensing of glucose by using gold nanoparticles. Chemistry 2006;12:2702–2708.
- Liu J, Lu Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc. 2003;125:6642–6643.
- Jv Y, Li B, Cao R. Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. Chem Commun (Camb). 2010;46:8017–8019.
- Lai G, Yan F, Wu J, et al. Ultrasensitive multiplexed immunoassay with electrochemical stripping analysis of silver nanoparticles catalytically deposited by gold nanoparticles and enzymatic reaction. Anal Chem. 2011;83:2726–2732.
- Liu T, Zhao J, Zhang D, et al. Novel method to detect DNA methylation using gold nanoparticles coupled with enzyme-linkage reactions. Anal Chem. 2009;82:229–233.
- Sperling RA, Gil PR, Zhang F, et al. Biological applications of gold nanoparticles. Chem Soc Rev. 2008;37:1896–1908.
- Choi Y, Ho NH, Tung CH. Sensing phosphatase activity by using gold nanoparticles. Angew Chem Int Ed. 2007;46:707–709.
- Yang X-Y, Guo Y-S, Bi S, et al. Ultrasensitive enhanced chemiluminescence enzyme immunoassay for the determination of α-fetoprotein amplified by double-codified gold nanoparticles labels. Biosens Bioelectron. 2009;24:2707–2711.
- Sanz VC, Mena ML, González-Cortés A, et al. Development of a tyrosinase biosensor based on gold nanoparticles modified glassy carbon electrodes: application to the measurement of a bioelectrochemical polyphenols index in wines. Anal Chim Acta. 2005;528:1–8.
- Lévy R. Peptide-capped gold nanoparticles: towards artificial proteins. Chembiochem. 2006;7:1141–1145.
- Zeng S, Yong K-T, Roy I, et al. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011;6:491–506.
- Liang H, Zhang X-B, Lv Y, et al. Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy. Acc Chem Res. 2014;47:1891–1901.
- Londoño Ramírez H. Upconverting and plasmonic nanomaterials for targeted detection and treatment of bladder cancer. 2013.
- Nazir S, Hussain T, Ayub A, et al. Nanomaterials in combating cancer: therapeutic applications and developments. Nanomed: Nanotechnol Biol Med. 2014;10:19–34.
- Jain S, Hirst D, O'sullivan J. Gold nanoparticles as novel agents for cancer therapy. Brit J Radiol. 2014;5:101–113.
- Hainfeld JF, Smilowitz HM, O'Connor MJ, et al. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond) 2013;8:1601–1609.
- Liu GL, Yin Y, Kunchakarra S, et al. A nanoplasmonic molecular ruler for measuring nuclease activity and DNA footprinting. Nat Nanotech. 2006;1:47–52.
- Hao L, Patel PC, Alhasan AH, et al. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small 2011;7:3158–3162.
- Prigodich AE, Alhasan AH, Mirkin CA. Selective enhancement of nucleases by polyvalent DNA-functionalized gold nanoparticles. J Am Chem Soc. 2011;133:2120–2123.
- Gholizadeh-Ghaleh Aziz S, Gholizadeh-Ghaleh Aziz S, Akbarzadeh A. The potential of nanofibers in tissue engineering and stem cell therapy. Artif Cells Nanomed Biotechnol. 2016;44:1195–1200.
- Ding Y, Jiang Z, Saha K, et al. Gold nanoparticles for nucleic acid delivery. Mol Ther. 2014;22:1075.
- Derbyshire N, White SJ, Bunka DH, et al. Toggled RNA aptamers against aminoglycosides allowing facile detection of antibiotics using gold nanoparticle assays. Anal Chem. 2012;84:6595–6602.
- Alhasan AH, Kim DY, Daniel WL, et al. Scanometric microRNA array profiling of prostate cancer markers using spherical nucleic acid-gold nanoparticle conjugates. Anal Chem. 2012;84:4153–4160.
- Pan T, Song W, Gao H, et al. miR-29b-Loaded gold nanoparticles targeting to the endoplasmic reticulum for synergistic promotion of osteogenic differentiation. ACS Appl Mater Interfaces. 2016;8:19217–19227.
- Young SWS, Stenzel M, Jia-Lin Y. Nanoparticle-siRNA: a potential cancer therapy? Crit Rev Oncol Hematol. 2016;98:159–169.
- Kisailus D, Najarian M, Weaver JC, et al. Functionalized gold nanoparticles mimic catalytic activity of a polysiloxane‐synthesizing enzyme. Adv Mater. 2005;17:1234–1239.
- Pengo P, Polizzi S, Pasquato L, et al. Carboxylate-imidazole cooperativity in dipeptide-functionalized gold nanoparticles with esterase-like activity. J Am Chem Soc. 2005;127:1616–1617.
- Wang C-I, Chen W-T, Chang H-T. Enzyme mimics of Au/Ag nanoparticles for fluorescent detection of acetylcholine. Anal Chem. 2012;84:9706–9712.
- He W, Zhou Y-T, Wamer WG, et al. the Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013;34:765–773.
- Zhao H, Wang Z, Jiao X, et al. Uricase-based highly sensitive and selective spectrophotometric determination of uric acid using BSA-stabilized Au nanoclusters as an artificial enzyme. Spectroscopy Lett. 2012;45:511–519.
- He W, Wu X, Liu J, et al. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem Mater. 2010;22:2988–2994.
- Nellore J, Pauline C, Amarnath K, et al. Antioxidant effect of gold nanoparticles synthesized from Curcuma longa restrains 1-methyl-2-phenyl pyridinium ion induced stress in PC12 cells. J Nanoneurosci. 2012;2:63–74.
- Kumar V, Guleria P, Kumar V, et al. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci Total Environ. 2013;461:462–468.
- Liu J, Jiang X, Wang L, et al. Ferroxidase-like activity of Au nanorod/Pt nanodot structures and implications for cellular oxidative stress. Nano Res. 2015;8:4024–4037.
- Shotorbani BB, Alizadeh E, Salehi R, et al. Adhesion of mesenchymal stem cells to biomimetic polymers: a review. Mater Sci Eng C Mater Biol Appl. 2017;71:1192–1200.
- Daraee H, Eatemadi A, Abbasi E, et al. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:410–422.
- Nasrabadi HT, Abbasi E, Davaran S, et al. Bimetallic nanoparticles: preparation, properties, and biomedical applications. Artif Cells Nanomed Biotechnol. 2016;44:376–380.
- Ebrahimi E, Khandaghi AA, Valipour F, et al. In vitro study and characterization of doxorubicin-loaded magnetic nanoparticles modified with biodegradable copolymers. Artif Cells Nanomed Biotechnol. 2016;44:550–558.
- Majidi S, Zeinali Sehrig F, Samiei M, et al. Magnetic nanoparticles: applications in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol. 2016;44:1186–1193.
- Alizadeh-Ghodsi M, Zavari-Nematabad A, Hamishehkar H, et al. Design and development of PCR-free highly sensitive electrochemical assay for detection of telomerase activity using Nano-based (liposomal) signal amplification platform. Biosens Bioelectron. 2016;80:426–432.
- Sperling RA, García-Fernández L, Ojea-Jiménez I, et al. One-pot synthesis of cationic gold nanoparticles by differential reduction. Z Physik Chem. 2017;231:7–18.
- Caetano FR, Felippe LB, Zarbin AJ, et al. Gold nanoparticles supported on multi-walled carbon nanotubes produced by the biphasic modified method and dopamine sensing application. Sensors Actuators B: Chem. 2017;243:43–50.
- Xia N, Wang X, Yu J, et al. Design of electrochemical biosensors with peptide probes as the receptors of targets and the inducers of gold nanoparticles assembly on the electrode surface. Sensors Actuators B: Chem. 2017;239:834–840.
- D'Agata R, Palladino P, Spoto G. Streptavidin-coated gold nanoparticles: critical role of oligonucleotides on stability and fractal aggregation. Beilstein J Nanotechnol. 2017;8:1–11.
- Sun B, Gou Y, Ma Y, et al. Investigate electrochemical immune sensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide. Biosens Bioelectron. 2017;88:55–62.
- Wu S, Li D, Wang J, et al. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sensors Actuators B: Chem. 2017;238:427–433.
- Cao Q, Liu X, Yuan K, et al. Gold nanoparticles decorated Ag (Cl, Br) micro-necklaces for efficient and stable SERS detection and visible-light photocatalytic degradation of Sudan I. Appl Catal B: Environ. 2017;201:607–616.
- Ribeiro M, Ferraz MP, Monteiro FJ, et al. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed: Nanotechnol Biol Med. 2017;13:231–239.
- Yu M, Lei B, Gao C, et al. Optimizing surface-engineered ultra-small gold nanoparticles for highly efficient miRNA delivery to enhance osteogenic differentiation of bone mesenchymal stromal cells. Nano Res. 2017;10:49–63.
- Gupta R, Rastogi PK, Ganesan V, et al. Gold nanoparticles decorated mesoporous silica microspheres: a proficient electrochemical sensing scaffold for hydrazine and nitrobenzene. Sensors Actuators B: Chem. 2017;239:970–978.
- Chauhan G, Chopra V, Tyagi A, et al. "Gold nanoparticles composite-folic acid conjugated graphene oxide nanohybrids" for targeted chemo-thermal cancer ablation: in vitro screening and in vivo studies. Eur J Pharm Sci. 2017;96:351–361.
- Dong Y-X, Cao J-T, Liu Y-M, et al. A novel immune sensing platform for highly sensitive prostate specific antigen detection based on dual-quenching of photocurrent from CdSe sensitized TiO 2 electrode by gold nanoparticles decorated polydopamine nanospheres. Biosens Bioelectron. 2017;91:246–252.
- Roya H, Elham A, Morteza M, et al. Current methods for synthesis of gold nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:596–602.
- Rawat KA, Bhamore JR, Singhal RK, et al. Microwave assisted synthesis of tyrosine protected gold nanoparticles for dual (colorimetric and fluorimetric) detection of spermine and spermidine in biological samples. Biosens Bioelectron. 2017;88:71–77.
- Guhrenz C, Wolf A, Adam M, et al. Tetrazole-stabilized gold nanoparticles for catalytic applications. Z Physik Chem. 2017;231:51–62.
- Taiebeh K, Leila K, Abolfazl A, et al. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol 2016;44:581–587.
- Yang X, Ouyang Y, Wu F, et al. Size-controllable preparation of gold nanoparticles loading on graphene sheets@ cerium oxide nanocomposites modified gold electrode for nonenzymatic hydrogen peroxide detection. Sensors Actuators B: Chem. 2017;238:40–47.
- Kim D-Y, Kim M, Shinde S, et al. Cytotoxicity and antibacterial assessment of gallic acid capped gold nanoparticles. Colloids Surf B Biointerfaces. 2017;149:162–167.
- Liang S, Hammond L, Xu B, et al. Hydroamination of alkynes with gold nanoparticles/Titania. Synfacts 2017;13:0113.
- Tao Y, Ju E, Ren J, et al. Bifunctionalized mesoporous silica‐supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater. 2015;27:1097–1104.