Abstract
Development of nanoparticles (NPs) as a part of cancer therapeutics has given rise to a new field of research – cancer nanomedicine. In comparison to traditional anti-cancer drugs, NPs provide a targeted approach which prevents undesirable effects. In this communication, we have reviewed the role of gold and silver NPs (AgNPs) in the cancer nanomedicine. The preparation of gold NPs (AuNPs) and AgNPs can be grouped into three categories – physical, chemical and biological. Among the three approaches, the biological approach is growing and receiving more attention due to its safe and effective production. In this review, we have discussed important methods for synthesis of gold and AgNPs followed by techniques employed in characterization of their physicochemical properties, such as UV–visible spectroscopy, electron microscopy (TEM and SEM) and size and surface analysis (DLS). The mechanism of formation of these NPs in an aqueous medium through various stages – reduction, nucleation and growth has also been reviewed briefly. Finally, we conclude our review with the application of these NPs as anti-cancer agents and numerous mechanisms by which they render cancer cell toxicity.
Introduction
Although nanoparticles (NPs) are associated with modern day science and technology, they have been known to be implemented in art and sculptures even before the 4th Century AD. The main reason for their use in ancient times was their optical properties, for example, a 20 nm gold nanoparticle imparts a magnificent wine colour. The artisans from the medieval ages were the first nanotechnologists who incorporated gold and silver NPs (AgNPs) in the stained glasses found now in Old Church’s infrastructure. These NPs were of various shapes and sizes differing in optical properties and in the colour that they transmit, other examples include – the Lycurgus Cup from 4th century AD and Deruta ceramicists from the Renaissance period (1450–1600 AD). Before the middle ages begun, a colloidal solution of gold was used for its healing power in China and Egypt against various diseases like – heart ailments, epilepsy, tumours and dysentery [Citation1].
In 1857, Michael Faraday delivered Bakerian Lecture of the Royal Society on “Experimental Relations of Gold (and other Metals) to Light” [Citation2]. It was the first descriptive study of interaction of light and gold (other metals) at nanoscale which influenced the conception of the nanosciences [Citation3]. Modern-day nanoscience took birth in a lecture “There’s plenty of room at the bottom” by physicists Richard Feyman on 29 December 1959 at California Institute of Technology [Citation4]. More than a century ago, Paul Ehrlich, a German scientist inspired the idea of NPs as a method for improving the drug therapy and to achieve targeted delivery. The delivery systems he formulated were called Zauberkugeln or magic bullets [Citation5]. In 1960s, Professor Peter Paul Speiser and his group carried out extensive formulations of NPs, nanocapsules for targeted drug delivery systems and vaccines [Citation5,Citation6].
The concept of nanotechnology includes the study and application of the particles measured at the scale of one billionth of a meter [Citation1]. The particles termed as NPs can be clusters of ions, atoms or molecules within a specified size range of 1–100 nm [Citation7]. Although the size can vary according to the field of application, for example NPs synthesized for the purpose of drug delivery are generally greater than 100 nm as to accommodate good amount of drug to be delivered [Citation8]. Similarly, the ideal size of the NPs for treatment of cancer is 70–200 nm as the fenestrations in the endothelium in a developing tumour is about 200–780 nm [Citation9]. NPs can be of various shapes – rods, triangle, round, octahedral, polyhedral, etc. along with different surface properties. NPs can not only be engineered in laboratory but also do exist in nature composed of organic and inorganic compounds as a result of volcanic eruptions, microbial processes, wildfires and plants – phytoremediation [Citation10]. In laboratory, they can be synthesized using different methods – physical, chemical and biological with ample number of sources – chemical agents, polymers, proteins, plants, microbes, etc. NPs can be classified according to their type – polymeric, metallic, magnetic, carbon nanotubes, liposomes, dendrimer and quantum dots [Citation11]. These NPs possess unique optical, magnetic, electronic and catalytic properties, different from their micro and macro-counterparts. Comprehensive utilization of various sources and methods result in synthesis of a number of different NPs products that can be employed in – fabrication of biosensors and electrochemical sensors [Citation12], wastewater treatment [Citation13], forensic investigation [Citation14], currency, hologram printing [Citation15] and the most important biological and medicinal applications [Citation16]. Consequently, many advantages and numerous applications of NPs in various fields have led to unregulated growth and development in the field of nanotechnology, causing “nanopollution” now expanding more than ever. As the saying goes “with great power comes great responsibility” – we need to lay down rules and regulation to assess the impact of the synthesized NPs on human health and their environment. In this review, we will focus on the synthesis of two noble metal NPs – silver and gold by various physical, chemical and most important biological methods. Further, we discuss the methods to characterize their physicochemical properties through UV–visible spectroscopy, size and surface charge analysis, TEM and SEM. Finally, we bestow upon the mechanisms because of which these metallic NPs have found successful application as anti-cancer agents.
Synthesis
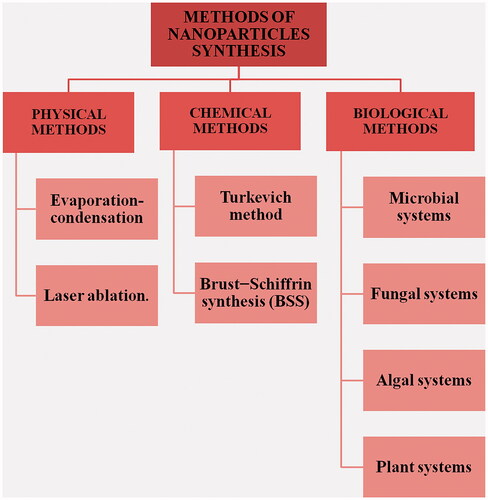
Broadly, the synthesis can be categorized as bottom-up and top-down methods. The bottom-up means building up of the NPs from molecules or atoms whereas top-down methods involve the disintegration of the bulk materials into smaller particles which eventually results in NPs [Citation17]. However, it is easier to divide the routes of synthesis of metallic NPs into – physical, chemical and biological ().
Physical method
The physical method includes two approaches for synthesis of metallic NPs – evaporation-condensation and laser ablation. Evaporation-condensation is a gas phase route that uses a horizontal tube furnace to produce NPs at atmospheric pressure. Within the centre of the tube, furnace is a boat carrying the metal source material for synthesis which is vaporized into the carrier gas [Citation18]. To this method, a change or modification in the reactor system controls the yield, concentration and the size of the NPs finally produced, for example indium NPs have been synthesized in great quantity using an aerosol generator [Citation19] and aluminium nanopowder have been synthesized by using an aerosol flow reactor [Citation20]. Moreover, AgNPs have been efficiently synthesized in high concentration by replacing the tube furnace with ceramic heater which overcomes various disadvantages of the tube furnaces [Citation21].
Irradiation of an area of the solid target material by a laser leads to its removal – this process is called laser ablation. NPs can be synthesized by laser ablation of a solid placed in a liquid medium. After irradiating with laser, the liquid will contain only the NPs of the target solid and no other ions, compounds, reducing agents, etc [Citation22]. Laser fluence, duration of irradiation, target solid, nature of liquid media influence the mechanism of ablation and characteristics of the metal NPs formed [Citation18]. Using this method, AgNPs [Citation23] and gold NPs (AuNPs) [Citation24] have been synthesized widely. Since there is no use of chemical reagents and the liquid medium used is basic with mild surfactants in some cases, the production of NPs using laser ablation is pure and uncontaminated, unlike chemical synthesis.
Chemical methods
Chemical methods of metal NPs include reduction of their metal salts. To these preparations, a stabilizing agent is usually added to avoid the aggregation of these NPs [Citation25]. The final product i.e. the shape, size and various other properties of the NP depends on type and strength of the reducing agent and the stabilizer employed in the method. Any number of metallic salts can be reduced to produce the corresponding NPs like – silver, gold, iron, zinc oxide, copper, cobalt, palladium, platinum, etc. Reduction of these salts can be achieved by various chemical agents – amino-boranes [Citation26], hydrazine [Citation27], oleylamine [Citation28], polyols, oxalic acid [Citation29], sugar [Citation30], citrate-the Turkevich method [Citation31] and sodium borohydride (NaBH4) Brust − Schiffrin synthesis (BSS) [Citation32]. However, gold and AgNPs are synthesized majorly through Turkevich method and NaBH4 BSS, respectively.
Turkevich method
In 1951, John Turkevich surveyed various chemicals for the preparation of AuNPs. Some of these were standard preparations using various reducing agents; these were – Bredig solution, Faraday solution, oxalic acid solution, hydroxylamine solution, acetylene solution, citrate solution and sodium citrate solution. In his original paper, he prepared both triangular and hexagonal with 10–50 nm diameter and spherical Au NPs with diameter of about 20 nm using 1% citric acid solution and 1% sodium citrate solution, respectively [Citation33]. This method of AuNPs synthesis using citrate solution is named after Turkevich even though the method was already devised in 1940 by Ernest and Lynn [Citation34]. The method employed by Turkevich gave a polydisperse solution of NPs; an improvised method was devised in a paper published by Frens [Citation35]. The procedure begins with boiling the gold hydrochlorate solution to about 100° and adding 1% sodium citrate to the boiling solution. A faint blue colour appears after a few seconds of citrate addition marking the nucleation process. As the boiling continues for few more seconds, the solution changes from blue to a deep wine red, indicating the formation of spherical AuNPs [Citation35]. The main factor affecting shape and size [Citation36] and reproducibility [Citation37] is the citrate ion concentration. Apart from gold, other noble metal NPs can also be synthesized using this method – silver, platinum or palladium [Citation38].
Brust–Schiffrin method
Brust–Schiffrin method produces monodisperse solutions with NPs of size less than 10 nm with a greater loading capacity. Moreover, it produces NPs stabilized with organic ligands mostly thiolate. Since it was first described in 1994 by Brust et al. for synthesis of AuNPs stabilized with thiolate ligand, it has been utilized to synthesize other metal NPs with different organic ligands [Citation39,Citation40]. Burst et al. prepared NPs of size 1–3 nm for which they used water-toluene system as a two-phase system to reduce hydrogen tetrachloroaurate (HAuCl4) using NaBH4 in the presence of stabilizing agent, alkanethiol which adheres to the surface of the particles [Citation40]. They also showed the synthesis of about 2 nm thiol coated AuNPs in single-phase (organic) system using p-mercaptophenol [Citation41]. The method generally involves three sequential steps: initially, tetraoctylammonium bromide, a phase transfer agents aids in transferring tetrahydrochloroaurate from aqueous to organic phase; alkanethiol reduce Au (III) to Au (I); in the final part addition of NaBH4 produces uniform monodispersed AuNPs formulation [Citation42]. It is also noteworthy to mention here that the mechanism that this method follows for synthesis is different from the general mechanism that the synthesis of NPs follows. Here, the process of nucleation is continuous rather than a sequential process of nucleation, then growth and so forth. This mechanism explains why Brust–Schiffrin method concludes in monodispersity; the continuous nucleation, growth and then capping by thiol limit the growth at a particular size and results in uniformity of size among the NPs [Citation39].
Biological methods
This approach forfeits the use of the many expensive and energetically unfavourable techniques that use harmful chemicals as reducing agents, surfactants and stabilizers making it a safer and better approach for the NPs synthesis. The biological approach or the “green” approach is relatively inexpensive, biocompatible, safer, non-toxic, mostly one-step synthesis method that employs biomolecules – proteins, polymers, carbohydrates (sugars), etc., from whole organism extract – fungi, bacteria, algae and plants as reducing agents for metal salts [Citation43] (). Green synthesis also produces pure NPs unlike the chemical methods wherein the produced NPs formulation is contaminated with the chemicals used for the process. Recently, antibody-mediated synthesis of metal NPs has also been successfully employed metal NPs synthesis and has been referred to as a biological method of synthesis [Citation44,Citation45]. Moreover, agriculture and industrial waste have also been used as sustainable approach for metallic NPs synthesis [Citation46,Citation47]. Generally, with green synthesis, the decisions which are supposed to be made are choosing of a medium, biological reducing agent and a choosing a stabilizing agent in some cases [Citation48]. One can control the synthesis, shape, size and properties of the NPs through green method by altering the pH, temperature, reaction time, pressure and the biological reducing agent [Citation49]. There are many different procedures for the biogenic NP synthesis that produces NPs with different shape, size and surface properties but the basic procedure is to incubate the metal salt solution with the biological agent in appropriate medium. The mechanism is similar as for other methods; the only difference is the reducing agents are part of the bacterial, fungal, viral or plant system. These reducing agents can be enzymes, reduced species, other biomolecules, phytochemicals or secondary metabolites as in plants –terpenoids, alkaloids, phenols, etc. Micro-organism can produce metal NPs either intracellularly or extracellularly; during extracellularly the AgNPs are formed due to the enzymes or proteins secreted by the micro-organism which reduced the metal salt whereas during the intracellular synthesis the metal NPs are synthesized by the enzymes or proteins present on the interior side of the cell wall [Citation50]. Of all the biological systems used, plant or algal systems are majorly used for the production of NPs as they are efficient, safer and less time-consuming. Whereas with the bacterial, viral or fungal mediated synthesis are less preferable due to the culturing of these microorganisms. In addition to culturing, these systems produce limited size and shapes of NPs at a slower rate. Alfalfa sprouts were the first reported plant system to synthesize metallic NPs (AgNPs) [Citation51]. A detailed mechanism in the case of plant-mediated NPs synthesis has been reported and divided into three phases: an activation phase when the phytochemicals reduce the salt and initiate the nucleation process following the growth phase by Ostwald’s ripening which then eventually terminates when the particle assumes the most energetically favourable shape [Citation52] ().
Figure 2. Mechanism of NPs formation by biological approach: reduction, growth and stabilization of NPs. M corresponds to Au or Ag.
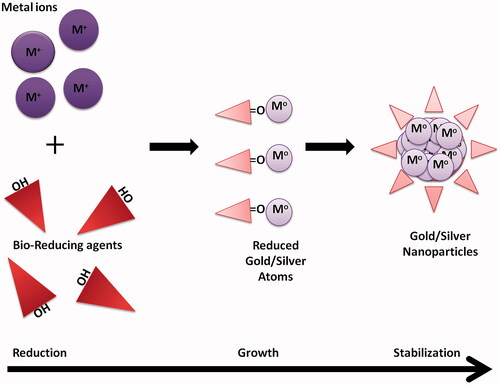
Table 1. Summary of gold and silver nanoparticles synthesized using biological agents.
Plant systems can either be used as a whole or parts of it can be used individually to synthesize NPs. Different plants produce NPs with different shape, size and properties; this variance is also seen in NPs synthesized using different parts/organs of the same plant i.e. NPs synthesized using a root and a seed of the same plant might give different types of NPs. The inference behind this observation corresponds to the molecular make-up of the cell of the particular plant part; type and quantity of biomolecule and secondary metabolite present in the cell define the size, shape and surface properties of the NPs synthesized. Even though plant-mediated synthesis holds advantages over conventional methods, complete understanding of the mechanism, control of size and shape, reproducibility of results and identification of a single reducing and capping agent (stabilizer) from the plant extract pose a challenge to the concept.
General mechanism of nanoparticles formation in solution
It is important to understand the mechanism of synthesis of NPs in the solution as this is affected by various parameters and conditions set during the reaction. A better understanding of the mechanism would lead to the optimization of the synthesis protocol to achieve NPs with particular size distribution and other associated – surface, optical, etc. properties. The mechanism includes about five different processes that take place in a sequential order – reduction, nucleation, growth, coarsening and agglomeration [Citation17,Citation53]. Following reduction of the metal salt, nucleation initiates. Nucleation in simplest terms is a process in which the atoms build up small clusters called nuclei which acts as a template for NPs formation. Over the time, with advancement in methods and techniques for study of NPs like- UV–visible spectroscopy, small angle X-ray scattering (SAXS) and liquid cell TEM, a number of theories have been proposed to explain the underlying mechanism of nucleation and growth of NPs in solution. The most pioneer theory is the classical theory of nucleation by Thanh et al. [Citation54] that explains the formation of new thermodynamic phase (a decrease in Gibbs free energy and an increase in entropy), crystal by elucidating the rate of nuclei formation by condensation of liquid from vapour phase. CNT explains only nucleation; different processes can be employed to explain the further growth of the nuclei into mature particles – mostly via Ostwald ripening [Citation55]. Nucleation can be both heterogeneous and homogenous; homogenous nucleation occurs spontaneously and randomly but supersaturation is its pre-requisite condition. Supersaturation is the initiation and growth of the soluble nucleating species which can be achieved by decomposition or reduction of soluble salts into metal atoms [Citation56]. On the other hand, heterogeneous nucleation occurs solitarily where solid surface contacts the liquid surface [Citation55]. The appropriate theory must explain and deduce the separation between the two phases – nucleation and growth; this is explained by the LaMer’s theory of burst nucleation and thus is the most applied theory to general mechanism of NPs synthesis [Citation55]. This theory states that during synthesis, the concentration increases rapidly above saturation for a short period of time followed by a burst of nucleation resulting in a number of small nuclei. A temporally separated phase arises when these established nuclei grow further decreasing the concentration levels way below the nucleation level [Citation57]. During the growth phase, a necessary decrease in surface energy results. Smaller particles have high surface to volume ratio and thus have a higher surface energy which makes them thermodynamically unstable, as these particles grow by monomer diffusion at the surface interface, the surface energy is decreased and the resulted larger particles are thermodynamically stable [Citation35,Citation36]. As diffusion continues, the particles grow; the particles will sustain only if their radius is above a critical value otherwise they dissolve in the solution again [Citation54]. With time the concentration of the monomers decrease which leads to formation of larger NPs at the expense of smaller NPs, i.e. the smaller NPs re-dissolve and contribute mass for the growth of larger particles. This process of formation of larger is termed is coarsening or Ostwald ripening [Citation58]. This is a phase transformation process that leads to an increase in size [Citation59] and broad particle size distribution in the solution. The size distribution is narrow in the initial growth phase when the concentration of the building material is abundant leading to monodispersity but broadens as the concentration decreases resulting in Ostwald’s ripening, increase in the average size and the polydispersity of the solution [Citation60]. Another consequence of this process is morphological changes as the small nuclei disappear and dissolve to form larger particles in the two-phase system [Citation61]. A mathematical approach to coarsening was devised individually by Lifshitz and Slyozov (1961) and Wagner (1961) now collectively known as the LSW theory [Citation62]. Perhaps, last, of all the processes, agglomeration is defined as a reversible phase wherein the weak physical forces like the Van der Waals forces hold the dispersed particles in the solution close together [Citation63]. It is driven by further decrease in the surface energy and more thermodynamic stable particles. The suspended particles in the solution can be aggregated too; there is a difference between agglomeration and aggregation – aggregation is irreversible and agglomeration is a reversible process. This part of the process is undesirable as it decreases the concentration of NPs in the solution, which is why additional steps have to be incorporated into the procedure to avoid agglomeration or aggregation of NPs. This step to avoid agglomeration or aggregation is referred to as stabilization of NPs and is necessary for their successful application in various fields.
Stabilization
The stability of NPs in the solution is attributed to the adsorption of a dispersant layer around each particle in the solution. The thickness of the layer influences the stability of the NPs. A dispersant layer of appropriate thickness would result in stable particles as it would be able to successfully overcome the attractive forces among individual particles due to flow of excluded solvent between the two adsorbed layers on adjacent particles whereas a thin layer would fail to do so which results in agglomeration or aggregation of particles [Citation64]. Generally, there are two approaches to stabilize a metallic NP formulation: steric and electrostatic stabilization. As the name suggests, electrostatic stabilization is based on same charges on the surface of the particles which causes repulsion and prevents aggregation. Steric repulsion also referred to as polymeric stabilization uses mostly polymers as capping agents which when adsorbed on the surface of the particles inhibit the particles to reach the minimum distance for the Van der Waals forces to act [Citation56,Citation65]. A few examples of stabilizers are N,N-dimethylformamide (DMF) [Citation66], (4–(3-phenylpropyl)pyridine) [Citation67] and PVA (poly(vinyl alcohol)) and PVP (polyIJvinylpyrrolidone) [Citation55].
Characterization
NPs have specific physicochemical properties different from their bulk or micro or macro-sized counterparts. These properties are influenced specific application and use of these nano-products. Therefore, it is necessary to assess in order to control them for the desired application. Some of the very common properties are shape/morphology, size, surface potential, composition, structure and surface properties [Citation68]. Therefore, various characterization techniques have been employed which can be categorized into three categories in the review.
Characterization by UV–visible spectroscopy
Metallic NPs possess optical properties that are a product of the composition, size and morphology of the NPs. These optical properties are a result of a phenomenon called surface plasmon resonance (SPR). When a metal is irradiated with an electromagnetic wave in the UV or the visible region, the cumulative effect of free electrons oscillating coherently, give rise to plasmons. As the incident electromagnetic wave can’t penetrate deep enough to generate plasmons, only the surface electrons of the metal NPs generate plasmons, hence the name surface plasmons [Citation69]. Since the surface electrons absorb, excite and result in plasmons, they are responsible for a peak in the UV absorption spectra. However, gold and silver absorb in the visible range, therefore, the solution is coloured. The colour of the solution is also affected by the shape of the particle; triangular-shaped particles are mostly red, Pentagon is green whereas the blue is spherical. The size, shape, metal’s composition and concentration of the NPs influence the wavelength at which the absorbance peak appears and the absorbance [Citation70]. It can be concluded that NP composed of a particular metal, of a particular size and shape, will correspond to a specific absorption peak in the UV absorption spectrum at a constant NPs concentration. A change in shape or size or concentration produces a significant change in maximum absorption peak [Citation71]; an increase in the particle size leads to absorption at longer wavelength. Thus this technique can be used to determine the approximate size, shape and concentration of the NPs and also to follow the different stages of the NPS formation.
Characterization by scattering techniques
In a colloidal solution, particles exhibit random zig-zag movement in the medium which is termed as Brownian motion. When such a solution is irradiated with light, these suspended particles in Brownian motion scatter light at different angles which is analysed by dynamic light scattering (DLS). DLS is a basic analytical technique used to characterize NPs to determine their size and size distribution of particles in nanometre range. The light source uses its lasers of red-green wavelength with minimum noise and maximum coherence providing a stable incident light. DLS measures the autocorrelation coefficient or the time-dependent intensity – intensity correlation function G(τ) from the intensities of the scattering light. G(τ) can be used to reduce the linewidth distribution function G(T) which can be used to determine the diffusion coefficient and finally the diffusion coefficient along with Stokes–Einstein relation gives the hydrodynamic size of the NPs along with a size distribution [Citation72,Citation73]. Thus, DLS is a basic technique to determine the average size (hydrodynamic radius) and size distribution. With variations in size and its distribution, one can interpret if the NPs form aggregate. Along with size and size distribution, a Zetasizer (Malvern Instruments, Worcestershire, UK) extends the results with additional parameters like polydispersity index (PDI) which measure the uniformity of the solution; for monodisperse solutions PDI is less than 0.5, a value greater than this would indicate agglomeration/aggregation or NPs with a broad size distribution or a polydisperse formulation. By using a totally different principle and method, a Zetasizer can also measure the surface potential of the NPs as Zeta-potential in mV.
Characterization by microscopy
Since NPs are in the nanometre range they can be observed by imaging techniques, such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), scanning tunnelling microscopy (STM), etc.
Scanning electron microscopy (SEM)
It’s a surface imaging method that uses a beam of electrons to scan the surface of the NPs to produce results as signals corresponding to the details of atomic composition and other topographical details. SEM can achieve a resolution of equal to or less than 1 nm [Citation68]. A SEM is prepared for a sample of NPs to determine the shape, size, size distribution and number of particles [Citation74]. The SEM measurement uses dried sample which may shrink the NPs’ radius and thus can hamper the correct size analysis of the formulation of the NPs.
Transmission electron microscopy (TEM)
TEM necessarily produces the same results as SEM – morphology, size and size distribution but uses a very thin section of the sample through which an incident ray of electron beam is transmitted. The incident electron ray when interacts with the specimen may scatter or remain unscatter which are then focused by the electromagnetic lenses on to a screen as an image of varying densities corresponding to the densities of electrons [Citation68]. TEM is preferred over SEM as it provides better spatial resolution and possibility for additional analytical measurements [Citation75]. Like any other technique, TEM has its drawbacks; thin sample preparation is time-consuming and complicated and requirement of high vacuum. To make the NPs sample to bear the high vacuum they are stained with negative staining dyes like uranyl acetate [Citation76].
Anticancer application
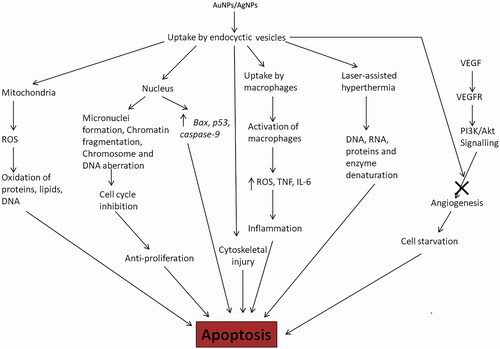
Gold and AgNPs have shown great capabilities and promising results in both cancer diagnostics and therapeutics. One must wonder what such improvements does the application of metal NPs provide over the conventional therapy; the drugs currently used for anti-cancer treatments are toxic to the body, producing side effects and unintended or untargeted effects on normal body physiology, development of drug resistance, quick drug metabolism and clearance from the patient’s body decreasing effective treatment time. Biological synthesis of metal NPs provides safe and effective therapeutic agents for cancer treatment. NPs formulated with different metals may show different properties and thus are expected show different mechanisms of toxicity against various cancer cells (). AuNPs and AgNPs can also be used in conjugation or combination with drugs or coated with a polymer to be used against cancer cells.
Figure 3. Major mechanisms followed by AuNPs and AgNPs to exert their anti-cancer properties: AuNPs and AgNPs are taken up by the cell by endocytosis, the vesicles are distributed in the cytoplasm and nucleus producing toxic effects leading to apoptosis or programmed cell death (ROS – reactive oxygen species, TNF – Tumour necrosis factor, VEGF – Vascular endothelial growth factor, VEGFR – Vascular endothelial growth factor receptor and IL-6 – interleukin-6).
Silver nanoparticles
AgNPs enter the mammalian cells as aggregates mostly through endocytosis and can also cross the blood-brain barrier due to their small size. Upon entering the cell in an endocytic vesicle, they are distributed to cytoplasm and nucleus through intracellular trafficking [Citation77]. Due to difference in their physicochemical properties, they may affect different cells through different cellular processes. In an attempt to analyse their toxicity against human health it was realized that they are toxic against both normal and cancer cells [Citation78]. Their anti-cancer property has been analysed in vitro against various types of cancer cells – human hepatoma cells [Citation79], lung cancer [Citation80], breast cancer [Citation81] and cervical carcinoma [Citation82]. They impart toxicity to cancer cells by decreasing mitochondrial function, reactive oxygen species (ROS) production, releasing lactate dehydrogenase (LDH), cell cycle deregulation, induction of apoptotic genes like Bax, formation of micronuclei, chromosome aberration and DNA damage [Citation83,Citation84]. The interaction between AgNPs and the immune system has also been elucidated in certain studies which state the AgNPs induce inflammation in the treated cell. Ingestion of AgNP by the macrophages is where the inflammatory response is initiated. These activated macrophages release ROS species, TNF-α, inflammatory cytokines and interleukins (IL-6) [Citation85]. Briefly, size dependent activity of the AgNPs has also been investigated; small sized AgNPs were more toxic and were more efficient in ROS production [Citation86,Citation87]. Apart from these cellular mechanisms AgNPs have also shown anti-angiogenic [Citation88] and anti-proliferative [Citation89] properties. In normal tissue cells, vascular endothelial growth factor (VEGF) binds to its receptor on endothelial cells to induce angiogenesis by activation of the PI3K/Akt signalling pathway. Ag-NPs are anti-angiogenic as they inhibit the phosphorylation of Akt by PI3K because of which the signalling pathway cannot complete, eventually terminating angiogenesis, starving the cell, depriving oxygen and killing the tumour cell [Citation88]. The anti-proliferative in cancer cells mediated by AgNPs is due to their ability to damage DNA, break chromosome – producing genomic instability, disruption of calcium (Ca2+) homeostasis which induced apoptosis, cell injury and cytoskeletal instability; cytoskeletal injury blocks cell cycle and division, promoting anti-proliferative activity of cancer cells [Citation89].
Gold nanoparticles
The AuNPs show anticancer activities resulting from different mechanisms and this difference in mechanism can be explained on the basis of different metal properties. The various ways in which AuNPs can be employed for anti-cancerous effects are photothermal, photodynamic, and anti-angiogenic and drug delivery [Citation90]. AuNPs like AgNPs are anti-angiogenic and inhibit the normal cell signalling and processes due to VEGF binding to VEGFR. Here too the AuNPs block the phosphorylation of the downstream molecules like Akt, ERK ½ in the PI3K/Akt signalling pathway [Citation91,Citation92]. In a fairly recent discovery, it was shown that AuNPs target cancer cells specifically. Once inside the cells, AuNPs target tumour suppressor genes and oncogenes to induce effective expression of caspase-9 which is an initiator caspase involved in apoptosis [Citation93]. In another study AuNPs targeted to the nucleus, promote cell-cycle arrest, cytokinesis inhibition – which then push the cell over to apoptosis [Citation94]. In many instances, AuNPs are used as a delivery system [Citation95] or in conjugation with the therapeutic molecule [Citation96] or maybe even gene to give significantly better toxicity against cancer cells. AuNPs due to their distinctive photo-optical properties have been successfully applied in both photothermal and photodynamic therapy. In photothermal therapy, AuNPs are used as a probe because of their strong SPR absorption in near-infrared regions leading to heating effects followed by irradiating with a non-ionizing source of energy like lasers. The application of laser to the AuNPs, the SPR band is converted to heat which causes hyperthermia eventually leading to cell necrosis [Citation90]. Photodynamic therapy is based on the use of a photosensitizer like 5-aminolevulinic acid (5-ALA) which when irradiated, is excited and reacts with molecular oxygen present in the cell to produce ROS which damages lipid, proteins, DNA, etc. and drives it to apoptosis or necrosis (cell death). AuNPs have been used in to deliver these photosensitizers specifically to the tumour cells [Citation97].
Gold-silver nanoparticles
AgNPs and AuNPs have been widely tested for their toxicity against different tumours and cancers both in vitro and in vivo. However, with growing knowledge in the field, researchers have been successful in producing alloys of these two noble metals. These nano-composites as they are referred to can be synthesized in both ways; Ag-coated Au and/or Au-coated colloidal particles. This can be simply done by reducing one metal salt on the already formed NPs formulation of the other, i.e. for synthesizing the Ag-coated Au colloids, chemically reduce silver salt on the AuNPs solution. Moreover, one can control and produce different compositions of these bimetallic NPs by controlling the amount of the salt to be deposited on the pre-formed colloids [Citation98]. These alloys also exhibit specific properties that are different from their respective NPs. They were shown to be substrates for surface-enhanced Raman spectroscopy (SERS) and, therefore, can be used to detect critical proteins and biomolecules in blood and other body fluids, detection of early cancer biomarkers, drug levels, etc. Therefore, they can be successfully employed in cancer detection [Citation99]. In another study, Au-Ag NPs were employed in differential colorimetric detection of different DNA targets in a single reaction system. This molecular nano diagnostic approach can be used for rapid and sensitive detection of cancer [Citation100]. These alloys also show therapeutic potential which makes them suitable theranostic agents [Citation101]. They curb the cytotoxicity that AgNPs exhibit and show increased plasmonic activity than pure AuNPs. Even though, they might not show increased toxicity against cancer cells in comparison to individual AgNPs as their mechanism is release of silver ions which are controlled in these alloys but increased plasmon than AuNPs might make them good anti-cancer agents as these alloys have demonstrated a better photothermal activity. However, further evaluation of such biomedical application is still to be performed.
Conclusion
The synthesis and utilization of metal NPs in arts and sculptures have been established to historical times. Soon enough variety of ways were devised to synthesize metal NPs broadly classified as physical, chemical and biological methods. In recent times, biological methods or green approach in case of plants has received immense attention due to its safe and uncontaminated products. AuNPs and AgNPs are applicable to many fields like wastewater treatment, sensor development, medicinal and therapeutics. These activities depend on their physicochemical properties – size, charge, shape, optical and electrical properties which are easily observed and characterized majorly through spectroscopy, electron microscopy and size and surface analysis. In this review, we have focused on their application as anti-cancer agents. AgNPs and AuNPs have been extensively testified as a part of various therapies in vitro and in vivo for their anticancer properties against different types of cancer. Since perhaps because the history marks the benefits of these noble metals they have been considerably researched upon for their biomedical applications. These NPs make promising anti-cancer agents because first, they are affective against drug-resistant tumour cells and it’s hard to develop resistance against them. Second, they help in targeted delivery and due to their nanometre size range are able to cross the fenestration in the blood capillaries and even the blood-brain barrier. Third, they can be formulated and targeted in a number of ways – conjugation, coatings, drug encapsulation, bimetallic application, etc. which even makes them a potential candidate for cancer-specific and patient-specific treatments. But the fight has not been fought yet, we are still milestones away from their effective application as anticancer agents.
Disclosure statement
Authors have no conflict of interest regarding the publication of the article.
Additional information
Funding
References
- Horikoshi S, Serpone N. Introduction to nanoparticles. Microwaves in nanoparticle synthesis: fundamentals and Applications. Hoboken (NJ): John Wiley & Sons; 2013. p. 1–24.
- Faraday M. The Bakerian lecture: experimental relations of gold (and other metals) to light. Philos Trans R Soc Lond. 1857;147:145–181.
- Edwards PP, Thomas JM. Gold in a metallic divided state—from faraday to present‐day nanoscience. Angew Chem Int Ed. 2007;46:5480–5486.
- Feynman RP. There's plenty of room at the bottom. Miniaturization. 1959;282–296.
- Kreuter J. Nanoparticles-a historical perspective. Int J Pharm. 2007;331:1–10.
- Singh M, Manikandan S, Kumaraguru A. Nanoparticles: a new technology with wide applications. Res J Nanosci Nanotechnol. 2011;1:1–11.
- Johnston RL, Wilcoxon JP. Metal nanoparticles and nanoalloys. Amsterdam (Netherlands): Elsevier; 2012.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133.
- Gaumet M, Vargas A, Gurny R, et al. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9.
- Heiligtag FJ, Niederberger M. The fascinating world of nanoparticle research. Mater Today. 2013;16:262–271.
- Bhatia S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. Natural polymer drug delivery systems. Berlin (Germany): Springer; 2016. p. 33–93.
- Luo X, Morrin A, Killard AJ, et al. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18:319–326.
- Sadegh H, Ali GA, Gupta VK, et al. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J Nanostruct Chem. 2017;7:1–14.
- Chauhan V, Singh V, Tiwari A. Applications of nanotechnology in forensic investigation. Int J Life Sci Scienti Res. 2017;3:1047–1051.
- Poulose S, Panda T. Synthesis of silver nanoparticles for possible printing applications. Adv Sci Engng Med. 2016;8:954–959.
- Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:3.
- Pacioni NL, Borsarelli CD, Rey V, et al. Synthetic routes for the preparation of silver nanoparticles. Silver nanoparticle applications. Berlin (Germany): Springer; 2015. p. 13–46.
- El-Nour KMA, Eftaiha AA, Al-Warthan A, et al. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010;3:135–140.
- Singh Y, Javier JR, Ehrman SH, et al. Approaches to increasing yield in evaporation/condensation nanoparticle generation. J Aerosol Sci. 2002;33:1309–1325.
- Panda S, Pratsinis S. Modeling the synthesis of aluminum particles by evaporation-condensation in an aerosol flow reactor. Nanostruct Mater. 1995;5:755–767.
- Jung JH, Oh HC, Noh HS, et al. Metal nanoparticle generation using a small ceramic heater with a local heating area. J Aerosol Sci. 2006;37:1662–1670.
- Yang G. Laser ablation in liquids: principles and applications in the preparation of nanomaterials. Boca Raton (FL): CRC Press; 2012.
- Chen Y-H, Yeh C-S. Laser ablation method: use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf A. 2002;197:133–139.
- Sylvestre J-P, Poulin S, Kabashin AV, et al. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J Phys Chem B. 2004;108:16864–16869.
- Herizchi R, Abbasi E, Milani M, et al. Current methods for synthesis of gold nanoparticles. Artif Cells Blood Substit Biotechnol. 2016;44:596–602.
- Babu Kalidindi S, Sanyal U, Jagirdar BR. Chemical synthesis of metal nanoparticles using amine–boranes. ChemSusChem. 2011;4:317–324.
- Kahani S, Molaei H. Synthesis of nickel metal nanoparticles via a chemical reduction of nickel ammine and alkylamine complexes by hydrazine. J Iran Chem Soc. 2013;10:1263–1270.
- Mourdikoudis S, Liz-Marzán LM. Oleylamine in nanoparticle synthesis. Chem Mater. 2013;25:1465–1476.
- Preethi S, Anitha A, Arulmozhi M. A comparative analysis of the properties of zinc oxide (ZnO) nanoparticles synthesized by Hydrothermal and Sol-Gel methods. Indian J Sci Technol. 2016;9.
- Panigrahi S, Kundu S, Ghosh S, et al. General method of synthesis for metal nanoparticles. J Nanopart Res. 2004;6:411–414.
- Dobrowolska P, Krajewska A, Gajda-Rączka M, et al. Application of turkevich method for gold nanoparticles synthesis to fabrication of SiO2@ Au and TiO2@ Au core-shell nanostructures. Materials. 2015;8:2849–2862.
- Moyano DF, Duncan B, Rotello VM. Preparation of 2 nm gold nanoparticles for in vitro and in vivo applications. Methods Mol Biol. 2013;1025:3–8.
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.
- Ernest AH, Lynn JE. Experiments in colloid chemistry. New York and London: Mcgraw-Hill Book Company, Inc; 1940.
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–22.
- Pillai ZS, Kamat PV. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J Phys Chem B. 2004;108:945–951.
- Kettemann F, Birnbaum A, Witte S, et al. Missing piece of the mechanism of the turkevich method: the critical role of citrate protonation. Chem Mater. 2016;28:4072–4081.
- Kimling J, Maier M, Okenve B, et al. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707.
- Perala SRK, Kumar S. On the mechanism of metal nanoparticle synthesis in the Brust–Schiffrin method. Langmuir. 2013;29:9863–9873.
- Brust M, Walker M, Bethell D, et al. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun. 1994;0:801–802.
- Brust M, Fink J, Bethell D, et al. Synthesis and reactions of functionalised gold nanoparticles. J Chem Soc Chem Commun. 1995;0:1655–1656.
- Zhu L, Zhang C, Guo C, et al. New insight into intermediate precursors of Brust–Schiffrin gold nanoparticles synthesis. J Phys Chem C. 2013;117:11399–11404.
- Saif S, Tahir A, Chen Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials. 2016;6:209.
- Fayaz AM, Balaji K, Girilal M, et al. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6:103–109.
- Payne JN, Waghwani HK, Connor MG, et al. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front Microbiol. 2016;7:607.
- Adelere IA, Lateef A. A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev. 2016;5:567–587.
- Mishra A, Sardar M. Rapid biosynthesis of silver nanoparticles using sugarcane bagasse—an industrial waste. J Nanoeng Nanomanufact. 2013;3:217–219.
- El-Shishtawy RM, Asiri AM, Al-Otaibi MM. Synthesis and spectroscopic studies of stable aqueous dispersion of silver nanoparticles. Spectrochim Acta A. 2011;79:1505–1510.
- Patra JK, Baek K-H. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater. 2014;2014:219.
- Shedbalkar U, Singh R, Wadhwani S, et al. Microbial synthesis of gold nanoparticles: current status and future prospects. Adv Colloid Interface Sci. 2014;209:40–48.
- Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, et al. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361.
- Makarov V, Love A, Sinitsyna O, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. 2014;6:35–44.
- Haumesser P-H. Nucleation and growth of metals: from thin films to nanoparticles. Amsterdam (Netherlands): Elsevier; 2016.
- Thanh NT, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014;114:7610–7630.
- Polte J. Fundamental growth principles of colloidal metal nanoparticles–a new perspective. CrystEngComm. 2015;17:6809–6830.
- Camargo PH, Rodrigues TS, da Silva AG, et al. Controlled synthesis: nucleation and growth in solution. Metallic nanostructures. Amsterdam (Netherlands): Springer; 2015. p. 49–74.
- Viswanatha R, Sarma D. Growth of nanocrystals in solution. Nanomaterials chemistry: recent developments and new directions. Weinheim (Germany): Wiley-VCH; 2007. p.139–70.
- Voorhees PW. The theory of Ostwald ripening. J Stat Phys. 1985;38:231–252.
- Tadros T. Ostwald ripening. Encyclopedia of colloid and interface science. Amsterdam (Netherlands): Springer; 2013. p. 820.
- Clark MD, Kumar SK, Owen JS, et al. Focusing nanocrystal size distributions via production control. Nano Lett. 2011;11:1976–1980.
- Baldan A. Review progress in Ostwald ripening theories and their applications to nickel-base superalloys Part I: Ostwald ripening theories. J Mater Sci. 2002;37:2171–2202.
- Niethammer B. Effective theories for Ostwald ripening. Analysis and stochastics of growth processes and interface models. Oxford (UK): Oxford University Press; 2008. p. 223.
- Sokolov SV, Tschulik K, Batchelor-McAuley C, et al. Reversible or not? Distinguishing agglomeration and aggregation at the nanoscale. Anal Chem. 2015;87:10033–10039.
- Studart AR, Amstad E, Gauckler LJ. Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir. 2007;23:1081–1090.
- Cushing BL, Kolesnichenko VL, O’Connor CJ. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev. 2004;104:3893–3946.
- Pastoriza-Santos I, Liz-Marzán LM. Formation and stabilization of silver nanoparticles through reduction by N, N-dimethylformamide. Langmuir. 1999;15:948–951.
- Favier I, Massou S, Teuma E, et al. A new and specific mode of stabilization of metallic nanoparticles. ChemComm. 2008;0:3296–3298.
- Lin P-C, Lin S, Wang PC, et al. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32:711–726.
- Jing H, Zhang L, Wang H. Geometrically tunable optical properties of metal nanoparticles. UV–VIS and photoluminescence spectroscopy for nanomaterials characterization. Amsterdam (Netherlands): Springer; 2013. p. 1–74.
- Joshi M, Bhattacharyya A, Ali SW. Characterization techniques for nanotechnology applications in textiles. Indian J Fibre Text Res. 2008;33:304–317.
- Liz-Marzán LM. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir. 2006;22:32–41.
- Zheng T, Bott S, Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl Mater Interfaces. 2016;8:21585–21594.
- Chu B, Liu T. Characterization of nanoparticles by scattering techniques. J Nanopart Res. 2000;2:29–41.
- Bootz A, Vogel V, Schubert D, et al. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 2004;57:369–375.
- Zhang X-F, Liu Z-G, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534.
- Pal SL, Jana U, Manna P, et al. Nanoparticle: an overview of preparation and characterization (2000–2010). J Appl Pharm Sci. 2011;1:228–234.
- Greulich C, Diendorf J, Simon T, et al. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomaterialia. 2011;7:347–354.
- Jeyaraj M, Sathishkumar G, Sivanandhan G, et al. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surf B Biointerfaces. 2013;106:86–92.
- Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ Sci Technol. 2009;43:6046–6051.
- Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85:743–750.
- Gurunathan S, Han JW, Eppakayala V, et al. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res Int. 2013;2013:535796.
- Vasanth K, Ilango K, MohanKumar R, et al. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces. 2014;117:354–359.
- Liu F, Mahmood M, Xu Y, et al. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci. 2015;9:115.
- Zhang X-F, Shen W, Gurunathan S. Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int J Mol Sci. 2016;17:1603.
- Park E-J, Yi J, Kim Y, et al. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol In Vitro. 2010;24:872–878.
- Ahmed KBR, Nagy AM, Brown RP, et al. Silver nanoparticles: significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol In Vitro. 2017;38:179–192.
- Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619.
- Gurunathan S, Lee K-J, Kalishwaralal K, et al. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30:6341–6350.
- Asharani P, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, et al. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28:237–259.
- Pan Y, Wu Q, Qin L, et al. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed Res Int. 2014;2014:418624.
- Kang S, Rho C, Cho W, et al. The anti‐angiogenic effects of gold nanoparticles on experimental choroidal neovascularization in mice. Acta Ophthalmol. 2016;94:6561–6567.
- Tiloke C, Phulukdaree A, Anand K, et al. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in A549 cells. J Cell Biochem. 2016;117:2302–2314.
- Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J Am Chem Soc. 2010;132:1517–1519.
- Brown SD, Nativo P, Smith J-A, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc. 2010;132:4678–4684.
- Abel EE, Poonga PRJ, Panicker SG. Characterization and in vitro studies on anticancer, antioxidant activity against colon cancer cell line of gold nanoparticles capped with cassia tora SM leaf extract. Appl Nanosci. 2016;6:121–129.
- Meyers JD, Cheng Y, Broome AM, et al. Peptide‐targeted gold nanoparticles for photodynamic therapy of brain cancer. Part Part Syst Charact. 2015;32:448–457.
- Rivas L, Sanchez-Cortes S, Garcia-Ramos J, et al. Mixed silver/gold colloids: a study of their formation, morphology, and surface-enhanced Raman activity. Langmuir. 2000;16:9722–9728.
- Lu L, Wang H, Zhou Y, et al. Seed-mediated growth of large, monodisperse core–shell gold–silver nanoparticles with Ag-like optical properties. ChemComm. 2002;0:144–145.
- Doria G, Larguinho M, Dias J, et al. Gold-silver-alloy nanoprobes for one-pot multiplex DNA detection. Nanotechnology. 2010;21:255101.
- Sotiriou GA, Etterlin GD, Spyrogianni A, et al. Plasmonic biocompatible silver-gold alloyed nanoparticles. Chem Commun (Camb). 2014;50:13559–13562.