Abstract
It is only in the last 20 years that many of the original ideas on artificial cells are being increasingly applied and extended by researchers around the world. Artificial cell has now evolved into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology and other areas. More futuristic research includes nanorobot, nanocomputer, multimodal locomotion delivery robot and others. This review starts with a general overview followed by specific examples in more details.
Idea of artificial cells
The very first humble “artificial cells” reported by Chang in 1957 [Citation1] is not to reproduce biological cells, but to use available basic knowledge to prepare a very simple system for possible uses in medicine and other areas. It is only in the last 20 years that many of the original ideas on artificial cells are being increasingly applied and extended by researchers around the world. This is because many of the original ideas [Citation2–7] were reported years before the modern era of nanotechnology, regenerative medicine, blood substitutes, biotechnology, gene therapy, stem cell therapy, cell therapy and other areas. Thus, following his 2005 review on “therapeutic applications of polymeric artificial cells” in Nature Review Drug Discovery [Citation8], a timeline shows that the author has made 20 of the 23 major discoveries in related areas up to that time. However, since that time, other groups are making rapid and exciting progress and numerous discoveries.
Each major progress in other areas has led to stepwise progress in artificial cells. First, there is the coming of age of polymer chemistry and biomaterial. Then there is the recognition of the importance and developments in biotechnology. Then there is the progress in molecular biology and genomics. All these have contributed to a quantum leap in the area of artificial cells. One can expect that there will be important future progress in other areas, for example, artificial intelligence and nanorobots, that will contribute to unlimited progress by increasing number of groups worldwide in the area of artificial cells.
This author predicted in his 1972 monograph on Artificial Cells [Citation6] that “Artificial Cell is not a specific physical entity. It is an idea involving the preparation of artificial structures of cellular dimensions for possible replacement or supplement of deficient cell functions. It is clear that different approaches can be used to demonstrate this idea”. This prediction is already out of date since the idea of artificial cells has progressed way beyond this 1972 prediction () [Citation9–12]. There are unlimited possibilities in variations for the artificial cell membranes and contents (. Artificial cells can now be of macro, micro, nano and molecular dimensions. Each of these has unlimited variations in configurations. Each configuration resulted in a new terminology that makes the field rather confusing to newcomers (. One hopes that the many arbitrary subdivisions of “artificial cells” under the guise of different names can come together! When this takes place, the result of the pooling of talents, specialized know-how in this very interdisciplinary and international area will lead to progress beyond anyone’s imagination.
Figure 1. Upper (from left to right): Basic idea of artificial cells that led to different types of early artificial cells. Lower: Present status of artificial cells with unlimited variations in contents, membrane material and dimensions. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 1. Upper (from left to right): Basic idea of artificial cells that led to different types of early artificial cells. Lower: Present status of artificial cells with unlimited variations in contents, membrane material and dimensions. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/384813f5-2124-4293-826f-68e0f2bfbc58/ianb_a_1577885_f0001_c.jpg)
Figure 2. Artificial Cell dimensions: macro, micro, nano and soluble nanobiotechnologic. Examples of variations in configurations with new terminologies for each extension.
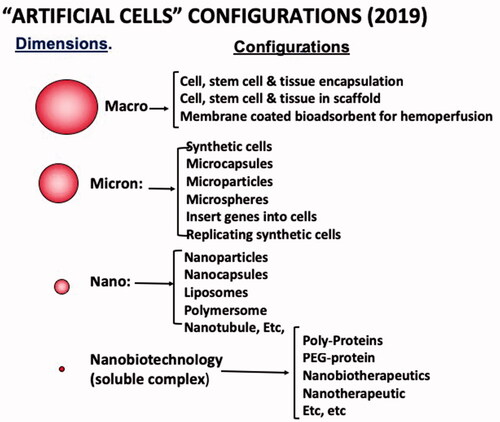
Figure 3. Examples of potential uses of the idea of Artificial Cells based on the above variations in configuration. Updates from Chang [Citation9,Citation10] with copyright permission.
![Figure 3. Examples of potential uses of the idea of Artificial Cells based on the above variations in configuration. Updates from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/89c110fd-1f89-4b89-ad7c-280b9a2b47de/ianb_a_1577885_f0003_b.jpg)
There are so many possibilities and this area is so interdisciplinary that this author decides to concentrate on innovative ideas, then to interest others with the specialized background to extend and develop these for clinical and practical uses. In addition, many groups around the world have started their own innovative approaches and efforts. Nevertheless, we have only touched the surface of the enormous potential of the extension, innovations and uses of artificial cells (). Space only allows for a general overview follows by some examples of the different configurations and their applications. More up to date details will be available elsewhere [Citation12].
Basic methods
This review cannot cover all the important methods of the preparation of the numerous configurations of artificial cells. Instead, we shall first look at the historical basic approaches () to be followed later in more details using specific examples.
Micro and nano dimension
The basic principle is to use emulsion followed by the use of physical or chemical methods to form a membrane around each microdroplet [Citation1–2]. The diameter is determined by the diameter of the emulsified micro or nano dimension droplets. Extensive novel emulsion methods developed around the world are now available for use. This principle has since been extended using modified physical or chemical methods for the preparation of microscopic or nano dimension artificial cells that are also called microcapsules, nanocapsules, liposomes, microparticles, nanoparticles, polymersomes, etc.
Macro dimension
The drop method for the preparation of large artificial cells [Citation1] has now been extended and modified using modified physical or chemical methods for cell/stem cell/tissue encapsulation. This will be described in more details later.
Crosslinking of proteins
The original basic method [Citation2,Citation7] of the use of bifunctional agents to assemble and crosslink haemoglobin (Hb) into PolyHaemoglobin. has been extended into many other areas of nanobiotechnology and nanobiotherapeutics. This will be described in more details later.
Conjugation of protein
The original basic method of conjugating haemoglobin to polymer [Citation2] has evolved into the conjugation of haemoglobin to soluble dextran or soluble (PEG) polyethylene glycol. Pegylation of proteins (PEG-protein) is now a popular approach in biotherapeutics.
Examples of routes of administration
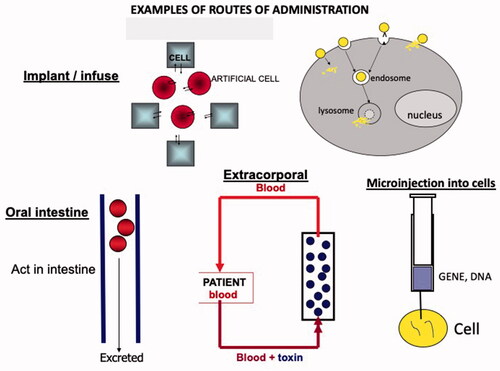
contains examples of possible routes of administration for the function of artificial cells in the body. Generally speaking, regulatory agencies are less worried about the use of artificial cells that are not implanted or injected into the body. We, therefore, started with artificial cells that are not implanted but act in a device for the extracorporeal route. This has resulted in the early approval of the use of artificial cells in patients way back in 1980. This is in the form of a haemoperfusion device.
Figure 4. Upper left: Original (Chang 1957) emulsion method of preparing micro-dimension artificial cells. Since extended to physical or chemical methods for microscopic and nanodimension artificial cells. Lower left:. Original (Chang 1957) drop method for the preparation of large artificial cells. This has been now been extended and modified for cell/stem cell encapsulation. Upper right: Basic method (Chang 1964 Science) of bifunctional agents to assemble and crosslink hemoglobin (Hb) into PolyHb that has evolved into the preparation of soluble polyhemoglobin and other biotherapeutics. Lower right: Basic method of conjugating hemoglobin to polymer (Chang 1964 Science) that has evolved into the use of other polymers like the Pegylation (PEG-protein) Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 4. Upper left: Original (Chang 1957) emulsion method of preparing micro-dimension artificial cells. Since extended to physical or chemical methods for microscopic and nanodimension artificial cells. Lower left:. Original (Chang 1957) drop method for the preparation of large artificial cells. This has been now been extended and modified for cell/stem cell encapsulation. Upper right: Basic method (Chang 1964 Science) of bifunctional agents to assemble and crosslink hemoglobin (Hb) into PolyHb that has evolved into the preparation of soluble polyhemoglobin and other biotherapeutics. Lower right: Basic method of conjugating hemoglobin to polymer (Chang 1964 Science) that has evolved into the use of other polymers like the Pegylation (PEG-protein) Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/0ab8ed16-ebad-498e-bb86-86cd0195ee86/ianb_a_1577885_f0004_c.jpg)
Total surface area and mass transfer
It is common knowledge that for the same volume of particles the smaller the particles, the larger would be the total surface area. It is also known that the theoretical diffusion across a membrane is proportional to the total surface area and inversely proportional to its membrane thickness. However, my 1966 analysis of the implication of combining all these factors for artificial cells of micro dimension is way beyond expectation [Citation5]. shows an updated analysis [Citation11] of the theoretical mass transfer of a fixed volume of 0.01 μm membrane thickness artificial cells with different diameters. This is compared to an artificial kidney (haemodialysis) machine with a mass transfer of 1. The mass transfer increases with decreasing diameter of artificial cells so that at the micro diameter range it can increase to 100 times that of an artificial kidney. At the nano diameter range, this can increase to an amazing 1000 times above that of an artificial kidney. Thus, artificial cells of different diameter containing different bioactive material can become efficient micro/nano dialyzer/bioreactor with unlimited possibilities ().
Figure 5. Contains examples of possible routes of administration for the function of artificial cells in the body. Generally speaking, regulatory agencies are less worry about the use of artificial cells that are not implanted or injected into the body. We therefore started with artificial cells that are not implanted but act in a device for the extracorporeal route. This has resulted in the early approval of the use of artificial cells in patients way back in 1980. This is in the form of a hemoperfusion device.
Haemoperfusion
Based on this analysis, 70 g of 90 micron diameter adsorbent artificial cells are retained inside a small container by screens at either end. The sorbent artificial cells remove toxins or drugs from the blood of patients perfusing through the column. The membrane of the artificial cells prevents the adsorbent from being released into the body and also prevents the adsorbent from damaging the blood cells (). This results in a cup size miniaturized haemoperfusion device with a hundred times the efficiency of a haemodialysis (artificial kidney), the size of a washing machine ().
The author starts the study on the use of artificial cells containing adsorbents for haemoperfusion. This included personally carried out scaled up, animal testing and clinical trials with patients. He shows the safety and effectiveness for using this first in animals and then in patients. shows the result of one of the many patient trials the author has carried out [Citation13]. This is a suicidal patient who ingests 3 times the lethal dose of a sleeping pill, methyprylon. Five hours of standard haemodialysis treatment cannot lower the drug level and the patient remains comatose, hypotensive with cardiac arrests. When the author starts haemoperfusion treatment the plasma methryprylon level decreases rapidly in 2 h and the patient is no longer comatose nor hypotensive and shortly recovers completely.
Figure 6. Left: Theoretical mass transfer of 5ml 0.01 um membrane thickness artificial cells with different diameters. This is compared an artificial kidney machine with a mass transfer of 1. Upper middle: Thus, artificial cells containing bioactive material can become efficient micro/nano dialyser/bioreactor. Right: 70 grams 90 micron diameter adsorbent artificial cells retained inside a small container by screens at either end. Its small size is compared to an artificial kidney. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 6. Left: Theoretical mass transfer of 5ml 0.01 um membrane thickness artificial cells with different diameters. This is compared an artificial kidney machine with a mass transfer of 1. Upper middle: Thus, artificial cells containing bioactive material can become efficient micro/nano dialyser/bioreactor. Right: 70 grams 90 micron diameter adsorbent artificial cells retained inside a small container by screens at either end. Its small size is compared to an artificial kidney. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/5f3a59b2-8a7d-4914-8cd6-cf0867a80bc8/ianb_a_1577885_f0006_c.jpg)
Following this first case, similar results are obtained in a number of other patients [Citation13]. He has also shown its effectiveness as partial support in patients for kidney failure and liver failure to remove toxic molecules. These results have led to FDA approval for routine clinical uses. Haemoperfusion is now an accepted routine clinical use for the treatment of patients with suicidal or accidental overdose of some medications around the world. A 2017 book [Citation11] by specialists around the world shows that the approach is being used extensively around the world, especially in countries where these can be manufactured with affordable costs.
What is more exciting is that extensive modifications and extension into many other uses including the use of specific bioadsorbents, immunosorbents. Furthermore, surface properties of artificial cell membranes can be varied by [Citation1] incorporation of negative or positive charge [Citation2]; incorporation of albumin to increase blood compatibility [Citation3]; incorporation of antigens to bind antibodies or antibodies to bind antigen [Citation4]; incorporation of polysaccharides like heparin or polyethylene glycol (PEG) to increase biocompatibility [Citation9–11,Citation14–19] (. This has led to systems for the specific removal of endotoxins, for the treatment of immunological diseases like Lupus and for the removal of unwanted cells. This is now such a large area with numerous publications that please refer to the book for more details [Citation12].
Blood substitutes
Unlike the use of artificial cells in a haemoperfusion device that is outside the body, this is an example where large volumes artificial cells have to be infused intravenously into the body. Thus, even though this is a very important and urgent life-saving method, it needs more time before regulatory approval.
Why blood substitutes?
The following is from a recent editorial by the author [Citation20]. Under normal circumstances, donor blood red blood cells (RBC) is the best replacement for blood. However:
Natural epidemics (e.g. HIV, Ebola etc) or man-made epidemics (terrorism, war, etc) can result in contaminated donor blood or disqualified disease contact donors. Unlike RBC, blood substitutes can be sterilized.
Heart attack and stroke are usually caused by obstruction of arterial blood vessels. Unlike RBC particles, blood substitute is a solution and in animal studies, it can more easily perfuse through obstructed vessels to reach the heart and brain.
Severe blood loss from accidents, disasters or war may require urgent blood transfusion that cannot wait for transportation to the hospital for blood group testing. Unlike RBC, blood substitutes do not have blood groups and can be given on the spot ().
Figure 7. Upper left Present status of clinical uses of Hemoperfusion (from Chang et al , 2017 Book on hemoperfusion). Upper right: Example of a sleeping pill overdose suicidal patient (Chang et al 1973). Standard dialysis (AK) is not effective but hemoperfusion (HP) quickly lowered the plasma level and rapid recovery. Lower: Variations in the surface properties of artificial cell membranes. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 7. Upper left Present status of clinical uses of Hemoperfusion (from Chang et al , 2017 Book on hemoperfusion). Upper right: Example of a sleeping pill overdose suicidal patient (Chang et al 1973). Standard dialysis (AK) is not effective but hemoperfusion (HP) quickly lowered the plasma level and rapid recovery. Lower: Variations in the surface properties of artificial cell membranes. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/81630650-e864-4b8e-aedf-c3aad013107c/ianb_a_1577885_f0007_c.jpg)
Red blood cells have to be stored in refrigeration for up to 42 days thus difficult to transport and store in disaster and frontline. Blood substitutes can be stored at room temperature for 1 year, compared to RBC of 1 day at room temperature.
In very severe haemorrhagic shock there is usually a safety window of 60 min for blood replacement, beyond which there could be problems related to irreversible shock. Animal study shows that one type of blood substitutes with enhanced RBC enzymes can prolong the time.
What is the present status around the world?
After the first report of artificial red blood cells in 1964 [Citation2], people felt that blood substitute is a simple matter that could be quickly developed when needed. Thus blood substitute research was put aside and only the other areas of artificial cells were extensively developed around the world for other widespread uses. When AIDS arrived in 1989, there were no blood substitutes and many patients were infected with H.I.V. contaminated donor blood. It is only then that intense R&D on blood substitutes was belatedly carried out around the world [Citation20–35]. It was found out too late that blood substitute requires the same long-term research as in any other medical research for cancer and other diseases. Thus, the present status is as follows ():
Oxygen carriers (HBOCs)
Red blood cells have 3 major functions (1): transport oxygen from the lung to the tissue (2), remove damaging oxygen radicals and (3) carry carbon dioxide CO2 from the tissue to the lung to be removed. The urgency of H.I.V. in donor blood necessitates the development of the simplest system in the shortest time. The most extensive clinical trials were based on polyhaemoglobin (PolyHb) developed by Biopure (Hemapure: bovine PolyHb) [Citation24] and Northfield (human PolyHb) [Citation21a] using the basic principle of glutaraldehyde cross-linked haemoglobin first reported by Chang [Citation7] (. This has no blood groups and can be pasteurized to remove infective agents and can be stored at room temperature for more than 1 year. Large-scale clinical trials have been carried out including using human PolyHb in the ambulance without the need for typing or cross-matching [Citation21a]. Greenburg, Jahr and others have carried out clinical trials using Hemapure: bovine PolyHb [Citation23,Citation24]. This has been approved for routine clinical use in South Africa to avoid the use of H.I.V contaminated donor blood [Citation24]. Other ongoing research includes the use of other sources of haemoglobin by Chen’s groups with porcine Hb [Citation21b], Yang’s group with Placental Hb [Citation22], and Bulow’s group and others with recombinant Hb [Citation23].
Oxygen carriers + removal of oxygen radicals
Arterial obstruction can result in stroke and heart attack. Red blood cells, being 7 to 8 microns in diameter, have difficulty flowing through partially obstructed vessels to supply the needed oxygen. PolyHb, being a solution, can perfuse through to supply the needed oxygen. However, reperfusion with an oxygen carrier can release damaging oxygen radicals (.
Figure 8. Upper: comparing red blood cell substitutes to red blood cells. Lower Left: Artificial red blood cells of microscopic dimensions that can reversibly “crenate” in hypertonic solution. Lower Middle: Nano Artificial cells red blood of 80 nanometer mean diameters with Polyethylene-polylactide membrane. Lower right: 4 types of soluble nanobiotherapeutic complexes. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 8. Upper: comparing red blood cell substitutes to red blood cells. Lower Left: Artificial red blood cells of microscopic dimensions that can reversibly “crenate” in hypertonic solution. Lower Middle: Nano Artificial cells red blood of 80 nanometer mean diameters with Polyethylene-polylactide membrane. Lower right: 4 types of soluble nanobiotherapeutic complexes. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/7fd1b5d0-7fd9-4042-8ab0-96e22017859a/ianb_a_1577885_f0008_c.jpg)
D’Agnillo and Chang have prepared a soluble complex of Polyhaemoglobin containing antioxidant enzymes to remove oxygen radicals (PolyHb-SOD-CAT) [Citation25]. It has the dual function of an oxygen carrier that can also remove oxygen radicals (. After 90 min of combined haemorrhagic shock and brain ischemia in rats, reinfusion of PolyHb-SOD-CAT did not cause brain edema () [Citation26]. On the other hand, PolyHb or a solution contains free Hb, SOD and CAT causes significant increases in brain edema.
Ischemic small intestine releases damaging oxygen radicals when reperfused with PolyHb. However, PolyHb-SOD-CAT reperfusion does not increase oxygen radical release (. This is important during intestinal surgery or organ storage for transplantation.
The work of Hsia’s group using conjugated haemoglobin containing synthetic antioxidants (PNPH) is another way to solve the problem [Citation27]. Another example is that of Alayash’s group based on haptoglobin [Citation28] Others included those of Simoni, Zal and other groups [Citation20,Citation23]
All 3 RBC functions (carries oxygen + removes oxygen radicals + carries CO2)
Other conditions as in sustained severe haemorrhagic shock may require all three RBC functions. We have designed a novel soluble nanobiotechnological complex (PolyHb-SOD-CAT-CA) [Citation29] (. It not only has all 3 RBC functions, but it can have enhancement of all 3 RBC functions by increasing the concentrations of RBC enzymes in the complex [Citation29]. These RBC enzymes can be extracted from RBC inexpensively [Citation30]. This complex has no blood groups. The lyophilized preparation can be heat pasteurized at 68 F for 2 h [Citation31]. This can be important if there is a need to inactivate H.I.V. virus, Ebola virus, and other infective organisms. Unlike about 1 day for RBC at room temperature, this lyophilized preparation can be stored in room temperature for 320 days. Our result in a 90 min haemorrhagic shock animal model with 2/3 blood volume loss () [Citation29] shows that it is superior to whole blood in the following ways: lowering of elevated intracellular pCO2, recovery of ST elevation, tropronin levels, lowering of elevated lactate, histology of the heart and intestine. Long term study of bovine PolyHb-SOD-CAT-CA in rats shows safety and lack of immunological problems after 4 weekly 5% blood volume infusion followed by 30% volume exchange transfusion [Citation32]. This includes the measurement of histamine and tryptase that show no anaphylactic reaction (. Haemoglobin has very low antigenicity. Bovine PolyHb itself shows no immunological problems in patients [Citation23,Citation24]. For PolyHb-SOD-CAT-CA the small fraction of enzymes are nanoencapsulated inside the large excess of haemoglobin molecules [Citation36] ().
Figure 9. Upper right: Arterial obstruction can result in stroke and heart attack. Red blood cells cannot flow through. PolyHb, a solution, can perfuse through. (Upper left) PolyHb-SOD-CAT, a solution can perfuse through to supply oxygen and remove oxygen radicals Lower right: Unlike PolyHb, reinfusion of PolyHb- SOD-CAT does not cause brain edema in rat brain ischemia. Lower left: Unlike PolyHb, PolyHb-SOD-CAT reperfusion in ischemic small intestine does not releases damaging oxygen radicals. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 9. Upper right: Arterial obstruction can result in stroke and heart attack. Red blood cells cannot flow through. PolyHb, a solution, can perfuse through. (Upper left) PolyHb-SOD-CAT, a solution can perfuse through to supply oxygen and remove oxygen radicals Lower right: Unlike PolyHb, reinfusion of PolyHb- SOD-CAT does not cause brain edema in rat brain ischemia. Lower left: Unlike PolyHb, PolyHb-SOD-CAT reperfusion in ischemic small intestine does not releases damaging oxygen radicals. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/d8db5c5a-cd76-4694-8f29-2340a6e9bd47/ianb_a_1577885_f0009_c.jpg)
Nanodimension red blood cells
The original micro dimension artificial red blood cells are too large to survive in the circulation. Nanodimension artificial RBC is another way to solve this problem [Citation33–35]. Lipid membrane vesicles itself do not circulate well and the addition of PEG to the membrane to form a PEG-lipid-polymer membrane vesicle has increased the circulation time. At present, this approach at the late Tsuchida’s group [Citation35] is being continued by Sakai’s group [Citation34]. In our laboratory, we have been using an 80 nm mean diameter biodegradable PEG-Polylactide polymeric membrane nano RBC that contains all the RBC enzymes () [Citation33]. Both PEG-lipid and PEG-polylactide nano red blood cells can remain in the circulation longer than PolyHb or PolyHb-SOD-CAT-CA. However, they contain a substantial amount of nonfunctional lipid or a polymeric membrane. On the other hand, for soluble nanobiotherapeutic artificial RBC, PolyHb-SOD-CAT-CA, the “membrane” is functional in the form of oxygen-carrying haemoglobin (). Thus, each has its own advantage.
Figure 10. Upper left: Polyhemoglobin-catalase-superoxide dismutase-carbonic anhydrase can have up to 6 times red blood cell enzyme concentration. In a rat hemorrhagic shock model with 2/3 blood volume loss and 90 mins sustained shock the result is as follow: Upper right: significant faster lowering of the elevated tissue pCO2 and Lower Right: faster recovery of the ischemic heart Middle right: intestine having better histological finding. Lower left: Test for anaphylactic reaction: no significant increase in tryptase nor histamine. Above figures from Chang’s group [Citation29,Citation32,Citation36].
![Figure 10. Upper left: Polyhemoglobin-catalase-superoxide dismutase-carbonic anhydrase can have up to 6 times red blood cell enzyme concentration. In a rat hemorrhagic shock model with 2/3 blood volume loss and 90 mins sustained shock the result is as follow: Upper right: significant faster lowering of the elevated tissue pCO2 and Lower Right: faster recovery of the ischemic heart Middle right: intestine having better histological finding. Lower left: Test for anaphylactic reaction: no significant increase in tryptase nor histamine. Above figures from Chang’s group [Citation29,Citation32,Citation36].](/cms/asset/e223b771-23b8-4cf8-a50d-2718f0ad0848/ianb_a_1577885_f0010_c.jpg)
Future directions
The editorial [Citation20] concludes that international progress up to now shows that it is possible to tailor-make blood substitutes ranging from simple to complex. It is urgent to have these ready without again waiting until it is too late. We need to analyze the specific indications for 1,2,3 and 4 above. If a condition only needs oxygen, then there is no need to use a more complex one. On the other hand, it would be folly not to use a more complex one if indicated. We also need to intensify research on the many important ongoing research around the world. Examples include other novel approaches including novel crosslinkers; new sources of material from porcine, bovine, human cord RBC, recombinant, Arenicola marina; basic research on nitric oxide, oxidative stress, haptoglobin, the rate of oxygen supply; safety and efficacy analysis and many other areas
Drug delivery systems
Biodegradable polymeric membrane artificial cells
Polylactide is biodegraded in the body to lactic acid and then water and carbon dioxide () and is an F.D.A. approved material for medical implantation. Thus, in 1976 Chang reported the use of polylactide to prepare biodegradable membrane artificial cells containing enzymes, hormones, vaccines and other biologics [Citation37] (). Variations in the molecular weight of polylactides and thickness of the membrane and configurations can result in artificial cells that release insulin at a different rate (). This approach has been extended and developed extensively worldwide as drug delivery systems in the form of nanoparticles, polymersomes or nanocapsules [Citation37–41]. Bowerman et al. reported in 2016 that Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer [Citation40]. Ravanshad et al. in 2017 reported the use of nanoparticles in cancer detection by Raman scattering based techniques [Citation41]. Abed et al. reported in 2018 the use of Lysozyme and DNAse I loaded poly (D, L lactide-co-caprolactone) nanocapsules as an oral delivery system [Citation42].
Figure 11. Upper left: Nano artificial red blood cell (rbc) with biodegradable polymeric membrane and red blood cell enzymes. Upper middle: EM of PEG-PLA membrane nano artificial rbc with a mean diameter of 80 nanometer. Lower left: Circulation time of PEG-PLA membrane rbc in rats is 2x longer than Polyhemoglobin. Right: nano rbc contains substantial amount of nonfunctional lipid or polymer membrane. Soluble Hb nanoencapsulated nano rbc has functional oxygen carrying hemoglobin membrane. Updated from Chang [Citation9,Citation10,Citation13] with copyright permission.
![Figure 11. Upper left: Nano artificial red blood cell (rbc) with biodegradable polymeric membrane and red blood cell enzymes. Upper middle: EM of PEG-PLA membrane nano artificial rbc with a mean diameter of 80 nanometer. Lower left: Circulation time of PEG-PLA membrane rbc in rats is 2x longer than Polyhemoglobin. Right: nano rbc contains substantial amount of nonfunctional lipid or polymer membrane. Soluble Hb nanoencapsulated nano rbc has functional oxygen carrying hemoglobin membrane. Updated from Chang [Citation9,Citation10,Citation13] with copyright permission.](/cms/asset/1f301276-ec42-4a50-b782-48922ce0f824/ianb_a_1577885_f0011_c.jpg)
Bilayer lipid membrane artificial cells: liposomes
In 1965 Bangham reports the use of microspheres of onion-like concentric multilamellar lipid bilayers as membrane models in basic research [Citation43]. In 1968 Meuller and Rudin [Citation44] reported that they use Chang’s method [Citation2] to prepare single bilayer membrane vesicles. A McGill Ph.D. graduate, Gregoriadis, visits me before leaving for his postdoctoral fellowship in England. While there, he becomes the first person to start the use of liposomes as drug delivery systems [Citation45]. However, onion-like multi-lamellar liposomes limits the loading of water-soluble drugs. Thus, in 1976 Deamer and Bangham [Citation46] report the use of an “ether evaporation” method to form single bilayer lipid membrane vesicles. This “ether evaporation method” is an extension of the 1957 Chang method using ether for the preparation of artificial cells [Citation1,Citation2] (. These lipid-membrane artificial cells have since been extensively studied and used as drug delivery systems around the world [Citation47]. This is now a very successful approach for drug delivery. For the delivery of larger peptides, proteins and vaccines, the emphasis is using biodegradable polymeric system.
Figure 12. Left: Biodegradable membrane artificial cells containing enzymes, hormones, vaccines and other biologicals (Chang, 1976). Variations result in the release of insulin at different rates. Extended now to many different configurations and dimensions. Right: Artificial cells containing magnetic material. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 12. Left: Biodegradable membrane artificial cells containing enzymes, hormones, vaccines and other biologicals (Chang, 1976). Variations result in the release of insulin at different rates. Extended now to many different configurations and dimensions. Right: Artificial cells containing magnetic material. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/d82f986b-7844-4200-bac6-72ddb02b98c0/ianb_a_1577885_f0012_c.jpg)
Targeting using surface ligands or magnetic properties and others
Back in the 1970, Chang’s group had investigated the incorporation of surface charges, polysaccharides and protein onto the surface of polymeric artificial cells () [Citation2,Citation6]. The most successful one is Davies of Enzon’s use of Polyethylene glycol (PEG). PEG has been incorporated to both types of nano artificial cells to result in longer circulation time. Further developments lead to the incorporation of antibodies onto the polymeric or lipid membrane of artificial cells (), to allow for targeting to cells with the corresponding antigens. Brennick, C. A. et al. in 2017 report the use of neoepitopes as cancer immunotherapy targets [Citation48]. Artificial cells containing biological materials and magnetic materials have been prepared by Chang in 1966 [Citation5] (). This way, external magnetic fields can direct their movement; remove or separate them from a mixture; retain them at specific site of action; stir or agitate them as in bioreactors, and other possibilities. This principle is now being used very extensively in bioreactors; in removing specific materials from a mixture as in diagnostics kits; in drug delivery systems; for locating radioactive material or chaemotherapeutic agents at the site of the tumour and other areas of application. A 2016 review by Karkan et al. on the use of magnetic nanoparticles for drug delivery is available [Citation49].
A more futuristic approach is Hu et al’s 2018 report in Nature of Small-scale soft-bodied robot with multimodal locomotion with potential for drug delivery [Citation50].
Enzyme and gene therapy
Enzymes inside artificial cells can act on external permeant substrates while avoiding protein sensitization, anaphylactic reaction, or antibody production with repeated injection [Citation2–4,Citation6,Citation8,Citation9] (. Chang’s groups has been investigating the use of artificial cells for enzyme therapy since 1964 [Citation2–10,Citation25,Citation26,Citation29–33,Citation51–55] ().
Figure 13. Left: Enzymes inside artificial cells, unlike those in free solution, do not have immunological problems. These can be in the form of membrane encapsulation, protein encapsulation or PEG covering of the enzyme molecule. Right: This approaching has been studied for a number of medical applications. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 13. Left: Enzymes inside artificial cells, unlike those in free solution, do not have immunological problems. These can be in the form of membrane encapsulation, protein encapsulation or PEG covering of the enzyme molecule. Right: This approaching has been studied for a number of medical applications. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/e34114fe-adcd-49f7-a779-974f4583e6b8/ianb_a_1577885_f0013_c.jpg)
Implanted urease artificial cells convert systemic urea into ammonia [Citation6]. Implanted artificial cells containing catalase replaces the defective enzyme in mice with a congenital defect in catalase, acatalasemia [Citation3]. Unlike the free catalase, there is no immunological problem with repeated injections [Citation51]. Implanted artificial cells containing asparaginase delay the onset and growth of lymphosarcoma in mice [Citation4]. This has been extended by other groups using PEG-asparaginase for the treatment of leukemia in patients [Citation56]. PolyHb-tyrosinase suppresses the growth of the skin cancer, melanoma [Citation54] Biodegradable PEG-PLA nano artificial cells containing PolyHb-tyrosinase are also effective [Citation9,Citation55]. These nanoparticles can also enter the melanoma cells to act internally [Citation55] (.
Some conditions like inborn errors of metabolism require administration throughout the life of the person. Instead of injections, orally administered artificial cells can act as they move down the intestine, then excreted without accumulation in the body (. For example, Chang found that oral artificial cells containing urease and ammonia adsorbent can lower the systemic urea level [Citation6]. This leads to NIH supported development by Kjellstrand’s group leading to clinical trials in patients [Citation58]. Our study shows that artificial cells containing xanthine oxidase lower the toxic systemic hypoxanthine levels in an infant with Lesch–Nyhan Disease [Citation52]. Bourget and Chang show that orally administered artificial cells containing phenylalanine ammonia lyase (PAL) lower the systemic phenylalanine levels in phenylketonuria (PKU) rats and improved the growth of the animals [Citation53]. Following my usual policy, I seek an expert, Scriver in Phenylketonuria [Citation59] and also a company to develop this for clinical use. They, in turn, collaborate with another company and develop an injectable PEG-PAL that has just been approved for use in patients [Citation57]. In order to avoid long term injection, they are now returning to look at doing this by oral administration [Citation60]. In the same way, our study shows that oral artificial cells containing tyrosinase when given orally lowers the systemic tyrosine level [Citation9]. Kaminsky et al. use argocytes containing enzyme nanoparticles to reduce toxic concentrations of arginine in the blood [Citation61]. Abed et al. reported in 2018 the use of Lysozyme and DNAse I loaded poly (D, L lactide-co-caprolactone) nanocapsules as an oral delivery system [Citation42].
Artificial cells containing biological cells
Present status
The first artificial cells containing intact biological cells were first reported by Chang in 1964 [Citation2] using the drop method. It was proposed that “protected from immunological process, encapsulated endocrine cells might survive and maintain an effective supply of hormone” () [Citation6]. He helps Connaught Laboratory to enclose islet in artificial cells for use in diabetes [Citation62]. This basic principle has been extensively developed around the world for cell therapy [Citation8,Citation9,Citation62–75]. Examples include artificial cells containing endocrine tissues, for instance, islets for diabetes. Another extensively investigated area is artificial cells containing genetically engineered cells for a number of clinical conditions.
Figure 14. Upper left: Cells inside artificial cells protected from outside. Lower: Cells can be bioencapsulated inside artificial cell or entrapped in scaffold of fibers or nanofibers Upper right: Bioencapsulation of islets, cells, genetically-engineered cells, microorganisms and stem cells. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 14. Upper left: Cells inside artificial cells protected from outside. Lower: Cells can be bioencapsulated inside artificial cell or entrapped in scaffold of fibers or nanofibers Upper right: Bioencapsulation of islets, cells, genetically-engineered cells, microorganisms and stem cells. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/12e7cac8-ea5d-4555-997a-ef31d8c0f6d4/ianb_a_1577885_f0014_c.jpg)
The result in animals has been promising. However, one implantation can only function for less than 1 year, and this is not practical for long-term illness like diabetes. Repeated injections would have retention problems.
There are at present 4 ways to solve this problem :
Figure 15. Artificial cells containing cells can only function for up to 1 year after implantation. This has been resolved by (1) biomaterial and method improvement (2) use in regenerative medicine that only need 3-4 months of function for example stem cells in liver regeneration. (3) Oral administration and (4) the use of biodegradable scaffold. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 15. Artificial cells containing cells can only function for up to 1 year after implantation. This has been resolved by (1) biomaterial and method improvement (2) use in regenerative medicine that only need 3-4 months of function for example stem cells in liver regeneration. (3) Oral administration and (4) the use of biodegradable scaffold. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/a57d38c3-6c74-4bd9-bda1-da00890ea7ac/ianb_a_1577885_f0015_c.jpg)
Improved biomaterials with better long-term biocompatibility and improvement in the method of preparation as shown in .
Oral administration of artificial cells containing microorganisms: Garofalo & Chang in 1991 show the effectiveness of artificial cells containing microorganisms for the in vitro removal of serum cholesterol [Citation67]. In 1996, Prakash and Chang [Citation68] show that artificial cells containing genetically engineered E. coli DH5 cells given orally to kidney failure rats effectively lower the elevated blood urea level. Even though Reardon in his 2018 Nature paper [Citation69] supports the use of genetically modified bacteria in the fight against diseases, regulatory agencies are still hesitant about the use of genetic engineered microbes. In anticipation of this Chang’s group in 2003 [Citation70] use artificial cells containing modified lactobacilli, since lactobacilli are being safely used in Yogurt. This also avoids the use of genetically engineered microbe and allows the safer use for oral administration in a human. Prakash’s group has since carried out extensive research into the use of this approach for clinical use in patients [Citation71].
Use in regenerative medicine that only needs months of function, for example, the use of artificial cells containing bone marrow stem cells in liver regeneration. Liu and Chang [Citation72,Citation73] study this in rats using artificial cells containing bone marrow stem cells. When implanted into 90% hepatectomized rats, this increases the recovery of the rats to 100% vs 11% in the control group and 33% in the free bone marrow stem cells ().
The use of biodegradable scaffolds started by Langer, Sefton and other groups, this is now a very popular and exciting approach. Grant’s 2018 review [Citation75] shows that this is now a very promising and active area. Biodegradable scaffolds are prepared in the shape of specific tissue or organs. The cells are seeded into the scaffold and allow to grow in the scaffold until they reach the required shape and dimension and take over the biodegraded scaffold support.
Towards a completely artificial “BIOLOGICAL CELL”
Red blood cells are the simplest of all human cells. As described above, complete artificial red blood cells have already been successfully prepared. Researchers are now interested in doing this for the more complicated types of cells as discussed by Gopfrich et al. in 2018 [Citation76].
Multienzyme systems with cofactor recycling
Most enzymes in the body function as multienzyme systems with cofactor recycling. Gu and Chang [Citation77] have prepared artificial cells containing multienzyme system with cofactor recycling and show that they can be used to convert metabolic waste like urea and ammonia into essential amino acids (. The cofactor, NADH, can be retained inside the artificial cells in the form of NADH-dextran or by the use of lipid–polymer membrane. We have also included all the multienzyme system of red blood cells inside nanodimension artificial red blood cells [Citation33] ().
Figure 16. Upper: Artificial cells containing multienzyme systems with cofactor recycling can convert waste, urea and ammonia , into useful essential amino acids, Upper right: Artificial cells that contain liver cytosol and organelles like microsomes) Lower right: Reverse hemolysis to load red blood cells with drugs. Microinjection to introduce synthetic DNA into microbes. Updated from Chang [Citation9,Citation10] with copyright permission.
![Figure 16. Upper: Artificial cells containing multienzyme systems with cofactor recycling can convert waste, urea and ammonia , into useful essential amino acids, Upper right: Artificial cells that contain liver cytosol and organelles like microsomes) Lower right: Reverse hemolysis to load red blood cells with drugs. Microinjection to introduce synthetic DNA into microbes. Updated from Chang [Citation9,Citation10] with copyright permission.](/cms/asset/98a8ec57-6619-416d-92c5-029c55d53210/ianb_a_1577885_f0016_c.jpg)
Artificial cells with intracellular compartments
Biological cells contain organelles that allow for more effective compartmental function. We have prepared artificial cells with intracellular compartments [Citation6,Citation78] (. This can allow for more efficient stepwise enzymatic or other biological functions. This principle has been extended for possible use in therapy by Hosta-Rigau and Stadler [Citation79].
Artificial cells containing microsomes, cytosol, ribosome and polymerase
Yuan and Chang isolate microsomes and cytosol from rat liver and encapsulated into polymeric membrane artificial cells [Citation80]. 20NADPH-cytochrome C reductase and lactate dehydrogenate are used as the marker enzymes for respectively microsomes and cytosol and show retention of activities.
Monnard and Deamer [Citation81] prepare models for primitive cellular life by encapsulating T7 RNA polymerases and templates into lipid membrane artificial cells, lipid vesicles. They can synthesize an RNA transcript from the DNA template. This is a slow process because the lipid membrane has low permeability to the needed 4 nucleoside triphosphates. Oberholzer et al. encapsulate a complex polymerase system into liposomes and show that the PCR reaction could be carried out [Citation82]. The problem is again the low permeability of the lipid membrane to the needed substrates. They have also encapsulated ribosomes into liposomes and obtain some translation product. More permeable polymeric or lipid-polymer membranes may shove these permeability problems. In another study, Griffiths and Tawfik use compartmentalization to load the transcription/translation system in a water-in-oil emulsion. This way each gene can occupy a separate water emulsion to carry out its function. Artificial cells containing “subcellular compartments” can be another possible way of doing this [Citation6,Citation83] (.
Synthetic genome for replicating synthetic cells
After extensive research, in 2016 Venter’s group report in Science their successful preparation of a synthetic minimal bacterial genome [Citation84]. Instead of a synthetic membrane, by microinjection, they have ingeniously made use of the complete membrane of the microbe. By doing this, they are able to prepare synthetic replicating cells using their synthetic genome.
Nonmedical uses of artificial cells
There are many developments and uses of the principle of artificial cells for agriculture, bioreactors, cosmetics, food production and aquatic culture [Citation85].
Another area is the use of artificial cells in nanorobatics and nanocomputers that in 2004 becomes the European Commission sponsored Programmable Artificial Cell Evolution (PACE) and in 2008 becomes the European Centre for Living Technology [Citation86].
Future of artificial cells
The following prediction in Chang’s 1972 monograph on “Artificial Cells” is already out of date: “Artificial Cell is not a specific physical entity. It is an idea involving the preparation of artificial structures of cellular dimensions for possible replacement or supplement of deficient cell functions. It is clear that different approaches can be used to demonstrate this idea”. Artificial cells have now already progressed way beyond this 1972 prediction. Even then, we have only just touched the surface of the enormous potential of artificial cells. One hopes that the many arbitrary subdivisions of “artificial cells” under the guise of different names can come together! When this takes place, the result of the pooling of talents, specialized know-how in this very interdisciplinary and international area will lead to progress beyond anyone’s imagination [Citation87].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chang, TMS. Haemoglobin corpuscles. Report of a research project for Honours Physiology, Medical Library, McGill University. Also reprinted 1988 as part of “30th Anniversary in Artificial Red Blood Cells Research. Biomater Artif Cells Artif Organs 1957;16:1–9.
- Chang TMS. Semipermeable microcapsules. Science 1964;146:524–525.
- Chang TMS, Poznansky MJ. Semipermeable microcapsules containing catalase for enzyme replacement in acatalsaemic mice. Nature 1968;218:242–245.
- Chang TMS. The in vivo effects of semipermeable microcapsules containing L-asparaginase on 6C3HED lymphosarcoma. Nature 1971;229:117–118.
- Chang TMS. Semipermeable aqueous microcapsules “artificial cells”: with emphasis on experiments in an extracorporeal shunt system. Trans Am Soc Artif Intern Organs 1966;12:13–19.
- Chang TMS. Artificial cells. Springfield, IL: Charles C. Thomas; 1972.
- Chang TMS. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Commun. 1971;44:1531–1536.
- Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221–235.
- Chang TMS, editor. ARTIFICIAL CELLS: biotechnology, nanotechnology, blood Substitutes, regenerative medicine, bioencapsulation, cell/stem Cell Therapy. London: World Scientific Publisher/Imperial College Press; 2007. p. 435.
- Chang TMS, editor. Selected topics in nanomedicine. London: World Science Publisher/Imperial College Press; 2014.
- Chang E, Nicolaev T, Zheng, et al. editors. Haemoperfusion and plasma-perfusion and other clinical uses of general, biospecific, immune and leucocyte adsorbents. London: World Scientific Publisher/Imperial College Press; 2017. p. 1004 www.medicine.mcgill.ca/artcell/HPBk_Ch1.pdf
- Chang TMS. ARTIFICIAL CELLS: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, cell/stem cell therapy. London: World Scientific Publisher/Imperial College Press; 2019.
- Chang TMS, Coffey JF, Barre P, et al. Microcapsule artificial kidney: treatment of patients with acute drug intoxication. Can Med Assoc J. 1973;108:429–433.
- Chang TMS. Blood compatible coating of synthetic immunoadsorbents. Trans Am Soc Artif Intern Organs 1980;26:546–549.
- Bensinger W, Baker DA, Buckner CD, et al. Immunoadsorption for removal of A and B blood-group antibodies. N Engl J Med. 1981;314:160–162.
- Terman DS, Tavel T, Petty D, et al. Specific removal of antibody by extracorporeal circulation over antigen immobilized in collodion-charcoal. Clin Exp Immunol. 1977;28:180.
- Hakim RM, Milford E, Himmelfarb J, et al. Extracorporeal removal of anti-HLA antibodies in transplant candidates. Am J Kidney Dis. 1990;16:423.
- Chen J, Han W, Su R, et al. Non-ionic macroporous polystyrene adsorbents for removal of serum toxins in liver failure by haemoperfusion. Artif Cells Nanomed Biotechnol. 2017;45:174–183.
- Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B haemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452.
- (a) Chang TMS. Red blood cell replacement, or nanobiotherapeutics with enhanced red blood cell functions? Artif Cells Nanomed Biotechnol. 2015;43:145–147. (b) Winslow RM. editor. Blood substitutes. Amsterdam: Academic Press; 2006.
- (a) Moore EE, Moore FA, Fabian TC, et al. Human polymerized haemoglobin for the treatment of haemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. (b) Wang L, Liu F, Yan K, et al. Effects of resuscitation with polymerized porcine haemoglobin pPolyHb on haemodynamic stability and oxygen delivery in a rat model of haemorrhagic shock, Artificial Cells. Nanomed Biotechnol. 2017;45:51–57.
- Li Y, Yan D, Hao S, et al. Polymerized human placenta haemoglobin improves resuscitative efficacy of hydroxyethyl starch in a rat model of haemorrhagic shock. Artif Cells Nanomed Biotechnol. 2015;43:174–179.
- Kim HW, Greenburg AG, editors. Haemoglobin-based oxygen carriers as red cell substitutes and oxygen therapeutics. New York, London: Springer; 2014. p.738.
- Mer M, Hodgson E, Wallis L, et al. Haemoglobin glutamer-250 bovine in South Africa: consensus usage guidelines from clinician experts who have treated patients. Transfusion 2016;56:2631–2636.
- D’Agnillo F, Chang TMS. Polyhaemoglobin-superoxide dismutasecatalase as a blood substitute with antioxidant properties. Nat Biotechnol. 1998;16:667–671.
- Powanda D, Chang TMS. Cross-linked polyhaemoglobin-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cells Blood Substit Immobil Biotechnol. 2002;30:25–42.
- Ma L, Hsia CJC. Polynitroxylated Hb as a multifunctional therapeutic for critical care and transfusion medicine in selected topics in nanomedicine Singapore, London: World Science Publisher/Imperial College Press; 2013. p.169–194
- Jia YP, Alayash AI. Molecular basis of haptoglobin and haemoglobin complex formation and protection against oxidative stress and damage. In: Chang TMS, editor. Selected topics in nanomedicine, London: World Science Publisher/Imperial College Press; 2013.
- Bian Y, Chang TMS. A novel nanobiotherapeutical Poly-[haemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a 90 minutes sustained severe haemorrhagic shock rat model with 2/3 blood volume loss. Artificial Cells, Nanomed Biotechnol. 2015;43:1–9. http://www.medicine.mcgill.ca/artcell/2015bian&chang.pdf
- Guo C, Gynn M, Chang TMS. Extraction of Superoxide Dismutase, Catalase and Carbonic Anhydrase from stroma-free red blood cell haemolysate for the preparation of the nanobiotechnological complex of PolyHaemoglobin-Superoxide Dismutase-Catalase-Carbonic Anhydrase. Artificial Cells, Nanomed Biotechnol. 2015;43:157–162.
- Bian Y, Guo C, Chang TMS. Temperature stability of Poly-[haemoglobin-superoxide dismutase–catalase- carbonic anhydrase] in the form of a solution or in the lyophilized form during storage at −80 °C, 4 °C, 25 °C and 37 °C or pasteurization at 70 °C. Artificial Cells, Nanomed Biotechnol. 2016;44:41–47.
- Guo C, Chang TMS. Long term safety and immunological effects of a nanobiotherapeutic, bovine poly-[haemoglobin-catalase-superoxide dismutase-carbonic anhydrase], after four weekly 5% blood volume top-loading followed by a challenge of 30% exchange transfusion. Artificial Cells, Nanomed Biotechnol. 2018;46:1349–1363. www.artcell.mcgill.ca/safety_immune.pdf
- Chang TMS, Powanda D, Yu WP. Analysis of polyethyleneglycolpolylactide nano-dimension artificial red blood cells in maintaining systemic haemoglobin levels and prevention of methaemoglobin formation. Artif Cells Blood. Substit Biotechnol. 2003;31:231–248.
- Sakai H. 2013 Biocompatibility of a highly concentrated fluid of haemoglobin-vesiclesas a transfusion alternative. In: Chang TMS, editor. Selected topics in nanomedicine. London: World Science Publisher/Imperial College Press.
- Tsuchida E, Sakai H, Horinouchi H, et al. Haemoglobin-vesicles as a transfusion alternative. Artificial Cells, Blood Subst Biotechnol, an Int J. 2006;34:581–588.
- Chang TMS. Translational feasibility of soluble nanobiotherapeutics with enhanced red blood cell functions. Artificial Cells, Nanomed Biotechnol. 2017;45:671–676.
- Chang TMS. Biodegradable semipermeable microcapsules containing enzymes, hormones, vaccines, and other biologicals. J Bioeng. 1976;1:25–32.
- Zhang Y, Li Y, Budak G. Nanoparticles for imaging and therapy —functionalization, endocytosis and characterization. In: Chang TMS, editor. Selected topics in nanomedicine. London: World Science Publisher/Imperial College Press; 2013.
- Chandra R, Madan J, Singh P, et al. 2013 Noscapines: novel carrier systems to target the tumour cells. In: Chang TMS, editor. Selected topics in nanomedicine. London: World Science Publisher/Imperial College Press.
- Bowerman CJ, Byrne JD, Chu KS, et al. Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer. Nano Lett. 2017;17:242–248.
- Ravanshad R, et al. Application of nanoparticles in cancer detection by Raman scattering based techniques. Nano Rev Exper. 2017;9:1373551.
- Abu AOS, Chaw C, Williams L et al. Lysozyme and DNAse I loaded poly D, L lactide-co-caprolactone nanocapsules as an oral delivery system Nature Scientific Reports, 03 September; 2018.
- Bangham AD, Standish MM, Watkins JC, et al. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252.
- Mueller P, Rudin D. Resting and action potential in experimental bilayer lipid membranes. J Theor Biol. 1968;18:222.
- Gregoriadis G, editor. Drug carriers in biology and medicine. New York: Academic Press, Inc.; 1976.
- Deamer DW, Bangham AD. Large-volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976;443:629–634.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160.
- Brennick CA, George MM, Corwin WL, et al. Neoepitopes as cancer immunotherapy targets: key challenges and opportunities. Immunotherapy 2017;9:361–371.
- Karkan S, Mohammadhosseini M, Panahi Y, et al. Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artificial Cells, Nanomed Biotechnol. 2016;45:1–5.
- Hu, et al. Small-scale soft-bodied robot with multimodal locomotion. Nature 2018;554:81–85.
- Poznansky MJ, Chang TMS. Comparison of the enzyme kinetics and immunological properties of catalase immobilized by microencapsulation and catalase in free solution for enzyme replacement. Biochim Biophys Acta. 1974;334:103–115.
- Palmour RM, Goodyer P, Reade T, et al. Microencapsulated xanthine oxidase as experimental therapy in Lesch-Nyhan disease. Lancet 1989;2:687–688.
- Bourget L, Chang TMS. Phenylalanine ammonia-lyase immobilized in microcapsules for the depleture of phenylalanine in plasma in phenylketonuric rat model. Biochim Biophys Acta. 1986;883:432–438.
- Yu BL, Chang TMS. In vitro and in vivo effects of polyhaemoglobin-tyrosinase on murine B16F10 melanoma. Melanoma Res. 2004;14:197–202.
- Wang Y, Chang TMS. Biodegradable nanocapsules containing a nanobiotechnological complex for the in-vitro suppression of a melanoma cell line B16F10. J Nanosci: Curr Res. 2016;1:1–6. www.medicine.mcgill.ca/artcell/2016Wang&Chang.pdf
- Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with PEGylated. Asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: cancer and leukemia group B study 9511. Blood 2007;109:4164–4167.
- FDA, U.S.F.D.A. FDA approves a new treatment for PKU, a rare and serious genetic disease; 2018.
- Kjellstrand C, Borges H, Pru C, et al. On the clinical use of microencapsulated zirconium phosphate-urease for the treatment of chronic uremia. Trans Am Soc Artif Intern Org. 1981;27:24–30.
- Levy HL, Sarkissian CN, Scriver CR. 2018, Phenylalanine ammonia lyase PAL: from discovery to enzyme substitution therapy for phenylketonuria. Mol Genet Metabol. 2018;124:223–229.
- Sarkissian CN, Kang TS, Gámez A, et al. Evaluation of orally administered PEGylated phenylalanine ammonia lyase in mice for the treatment of Phenylketonuria. Mol Genet Metabol. 2011;104:249–254.
- Kaminsky YG, Kosenko EA. Argocytes containing enzyme nanoparticles reduce toxic concentrations of arginine in the blood. Bull Experiment Biol Med. 2012;153:406–408.
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980;210:908–909.
- Wong H, Chang TMS. Bioartificial liver: implanted artificial cells microencapsulated living hepatocytes increases survival of liver failure rats. Int J Artif Organs. 1986;9:335–336.
- Hunkeler D, Rajotte R, Grey D, et al. Bioartificial organ grafts: a view at the beginning of the third millennium. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:365–382.
- Rokstad A, Lacík I, de Vos P, et al. Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation. Adv Drug Del Rev. 2014;67–68:111–130.
- Wong H, Chang TM. A novel two step procedure for immobilizing living cells in microcapsules for improving xenograft survival. Biomater Artif Cells Immobilization Biotechnol. 1991;19:687–697.
- Garofalo FA, Chang TMS. Effects of mass transfer and reaction kinetics on serum cholesterol depletion rates of free and immobilized Pseudomonas-pictorum. Appl Biochem Biotechnol. 1991;27:75–91.
- Prakash S, Chang TMS. Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats. Nat Med. 1996;2:883–887.
- Reardon S. Genetically modified bacteria enlisted in fight against disease. Nature 2018;558:497–498.
- Chow KM, Liu ZC, Prakash S, et al. Free and microencapsulated Lactobacillus and effects of metabolic induction on Urea Removal. Artificial Cells, Blood Subst Biotechnol. 2003;4:425–434.
- Iqbal U, Westfall S, Prakash S. Novel microencapsulated probiotic blend for use in metabolic syndrome: design and in-vivo analysis. Artificial Cells, Nanomed Biotechnol. 2018;46:1–9.
- Liu ZC, Chang TMS. Transdifferentiation of bioencapsulated bone marrow cells into hepatocyte-like cells in the 90% hepatectomized rat model. Liver Transpl. 2006;12:566–572.
- Liu ZC, TMS Chang L. Artificial Cell microencapsulated stem cells in regenerative medicine. Tissue Eng Cell Ther Adv Exp Med Biol. 2010;670:68–79.
- Song W, Lu Y.-C, Frankel AS, et al. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep. 2015;5:16884.
- Grant R, Hay D, Callanan A. From scaffold to structure: the synthetic production of cell derived extracellular matrix for liver tissue engineering. Biomed Phys Eng Express. 2018;4:065015.
- Göpfrich K, Platzman I, Spatz JP. 2018, Mastering complexity: towards bottom-up construction of multifunctional eukaryotic synthetic cells. Trends in Biotechnol. 2018;36:938–951.
- Gu KF, Chang TMS. Production of essential L-branched-chained amino acids, in bioreactors containing artificial cells immobilized multienzyme systems and dextran-NAD+. Appl Biochem Biotechnol Bioeng. 1990;26:263–269.
- Chang TMS, Macintosh FC, Mason SG. Semipermeable aqueous microcapsules: I. Preparation and properties. Can J Physiol Pharmacol. 1966;44:115–128.
- Hosta-Rigau L, Stadler B. 2013 Subcompartmetalized systems toward therapeutic cell mimics. In: Chang TMS, editor. Selected topics in nanomedicine. London: World Science Publisher/Imperial College Press
- Yuan ZY, Chang TMS. Rat microsomes and cytosol immobilized by microencapsulation in artificial cells. Int J Artif Organs. 1986;9:63–68.
- Monnard PA, Deamer DW. Nutrient uptake by protocells: a liposome model system. Orig Life Evol Biosph. 2001;31:147–155.
- Oberholzer T, Albrizio M, Luisi PL. Polymerase chain reaction in liposomes. Chem Biol. 1995;2:677–682.
- Griffiths AD, Tawfik DS. Man-made enzymes-from design to in vitro compartmentalisation. Curr Opin Biotechnol. 2000;11:338–353.
- Hutchison CA, Chuang RY, Noskov VN, et al. Design and synthesis of a minimal bacterial genome. Science 2016;351:6280. PMID: 27013737
- Poncelet D, Neufeld R. 20th Bioencapsulation Conference. Present status of bioencapsulation; 2012.
- Bedau MA, McCaskill JS, Packard NH, Rasmussen S. Living technology: exploiting life’s principles in technology. Artificial Life. 2010;16:89–97.
- Chang TMS. 2019 Artificial cells, blood substitutes and nanomedicine. available from: www.medicine.mcgill.ca/artcell

