ABSTRACT
Alzheimer’s disease (AD) is a neurodegenerative disorder that is clinically characterized by deteriorating cognitive function. Quercetin (Q), a bioflavonoid, has been reported to slow down AD progression. Q at a dose of 50 mg/kg-1 shows an important therapeutic effect in aluminum chloride (AlCl3)-induced Alzheimer’s disease, which has been previously published by us. Here, this study aimed to highlight the neuroprotective effect of quercetin on hallmark genes in AlCl3-induced Alzheimer’s disease in rats. Wistar male rats were subjected to a vehicle group, AlCl3 group, and co-administration with AlCl3 + Q50 for 60 sequential days. Behavioral tests and qPCR were performed to assess the efficacy of Q. The co-administration of quercetin (50 mg kg-1) has a significant effect on memory deficits. Furthermore, AlCl3 + Q50 group resulted in significantly decreased amyloid precursor protein levels (APP), β-amyloid converting enzyme 1 (BACE1), and presenilin I (PSEN1) and increased the expression of ADAM17 in the hippocampus tissue compared to AlCl3 group (p < 0.05). The current study showed that the quercetin’s neuroprotective properties may involve its ability to target the most significant Alzheimer’s disease-related genes and slow the progression of cognitive impairment.
Introduction
The prevalence of dementia worldwide is approximated at up to 36 million and is projected to reach 66 million by 2030 and 115 million by 2050, and also around two-thirds of these patients live in developing countries [Citation1]. Alzheimer’s disease (AD) is the most prevalent form of dementia in older patients, and it is distinguished by a gradual deterioration in cognitive ability, mostly beginning with memory impairment [Citation2]. From a neuropathological point of view, AD is distinguished by the accumulation of extracellular beta-amyloid (A) plaques, the rapid exhibition of intracellular tau pathology, the synaptic connections lack in certain brain regions and severe oxidative stress. Environmental factors are associated with an increased risk of Alzheimer’s disease, including exposure to aluminum [Citation3]. According to epidemiological research and animal studies, Al deposition in the hippocampus induces aberrant-amyloid deposition and neuronal apoptosis by stimulating the amyloid formation and neuroinflammation, leading to hippocampal-dependent learning and impairment of memory ability [Citation4,Citation5].
Amyloid precursor protein (APP) is a large protein that can have up to 771 amino acids. Non-amyloid products and/or amyloid derivatives of APP are formed by proteolytic action of secretases (α, β and γ) [Citation6]. In the amyloidogenic pathway, soluble amyloid precursor protein β (sAPβ) and C-terminal 99 fragment (CTFβ) were produced from APP by β-secretase cleavage and followed by γ-secretase to generate Aβ peptides [Citation7,Citation8]. Furthermore, the accumulation of these amyloid peptides results in the formation of amyloid-beta fibrils. On the other hand, the non-amyloidogenic pathway, α-secretase cleaves APP to generate the soluble amyloid precursor protein α (sAPP α) and the C83 fragment (CTFα). Then, the sequential degradation of sAPPα by γ-secretase occurred to produce non-amyloidogenic Aβ 17–43 peptides, known as protein 3 (P3) [Citation9].
Quercetin (3, 5, 7, 3′, 4′-pentahydroxyflavone) is a flavonoid compound that can be found in many different medicinal plants. By scavenging reactive oxygen species (ROS), quercetin has an apparent antioxidant Activity [Citation10]. It also has anticancer, antiviral anti-inflammatory properties [Citation11,Citation12]. Many studies demonstrated that quercetin has anti-amyloidogenic properties as well as neuroprotective effects against neurological disease [Citation13,Citation14].
The aim of this study is to investigate quercetin’s neuroprotective effect in AlCl3-induced Alzheimer’s disease by assessing the cognitive function and regulating amyloid and non-amyloid gene pathways.
Materials and methods
Drugs and chemicals
Aluminum chloride (AlCl3) hydrate (Cat. # 229393) and solid quercetin ≥95% (Cat. # Q4951, HPLC) were obtained from Sigma-Aldrich Chemicals Co., St. Louis, USA.
Animals
Male Wistar rats (8 weeks old) weighing 140 ± 10 g were obtained from the animal house of National Research Centre, Giza, Egypt. Prior to the start of the study, the animals were boarded in polypropylene cages and acclimated to specific pathogen-free settings for 3 days. The conditions of temperature (24 ± 1°C) and humidity level (55–65%) in a 12 h light/dark cycle were applied for rats. A typical rodent diet (17.48% protein, 6.85% fat, 62.99% carbohydrate, 4.08% ash and 2.16% minerals and vitamins) was given to the rats along with unlimited access to water. Animal handling procedures were carried out in accordance with the National Institutes of Health’s guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and the ethical standards of the Ethical Committee of Medical Research of the National Research Centre, with approval number ‘18,144’, Giza, Egypt.
Experimental design
Following the acclimation phase, the rats (n = 32) were weighed and equally distributed into three groups, with eight rats in each group
Group I: the normal control (NC) group was received vehicle (saline) through intraperitoneal route (IP).
Group II: The AD group intraperitoneally injected with AlCl3 dissolved in saline (50 mg/kg bwt) to induce the AD model [Citation15] for 60 days.
Group III: Q50 group orally administered with Q dissolved in saline by gastric intubation (50 mg/kg bwt).
Group IV: AlCl3+ Q50 group orally co-administered with 50 mg Q/kg bwt dissolved in saline by gastric intubation (1 h prior to AlCl3) and intraperitoneally injected with AlCl3 (50 mg/kg bwt) as group II for 60 days.
Behavioral assessment
During the final week of the study, the behavioral evaluations were conducted.
Y-maze spontaneous alternation test
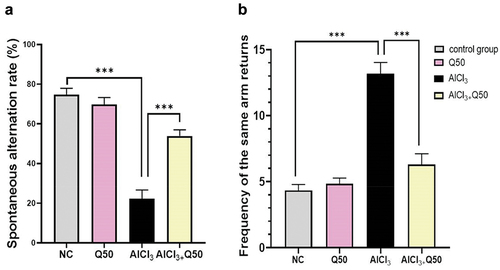
The purpose of the Y-maze spontaneous alternation test was to assess the short-term memory [Citation16,Citation17]. The Y-maze was constructed of painted wood with three horizontal arms, which were 120 degrees apart and marked with the letters start (A), familiar (B) and novel (C). The dimensions of every arm were 45 cm length × 15 cm width × 30 cm height. There were two trials separated by an intertrial break in the Y-maze test. 70% v/v ethanol was added after every test to clean the maze. Every rat was positioned in the starting (A) arm and permitted to investigate it as well as the (B) arm, while the (C) arm was sealed off during the first trial exposure of 5 minutes. The 2nd trial was carrying out after 1-hour intertrial break, and the (C) arm’s block was eliminated. Every rat was then returned to the arm (A) and given 5 minutes to explore novelty (C) vs. familiarity (B) in all three arms. The arm entry pattern was monitored. When all four of the rat’s limbs were fully placed into the arm, a complete arm entry was achieved. Each rat’s session was recorded on camera for later evaluation. The same arm returns were observed, the number of total arm entries to A, B and C was noted and recorded, and the number of alternations (consecutive entries into the arms on overlapping triads like CBA, ABC, CBA, etc.) was determined to calculate the spontaneous alternation rate percentage utilizing the following formula:
Spontaneous alternation rate (%) = [(number of alternations)/(total arm entries−2)] × 100.
Sample collection
The entire brain was quickly separated on a glass plate, cleaned with saline solution and dried on filter paper. Following that, each brain was split in half sagittal. The hippocampi from the right and left halves of the brain were kept in RNA later and then stored at −80°C till applied for quantitative real-time PCR (qRT-PCR) gene expression analysis.
RNA extraction and the synthesis of cDNA
Using the Thermo Scientific GeneJET RNA Purification Kit, total RNA isolation from tissues (30 mg) was carried out. The ND-1000 Spectrophotometer (NanoDrop) was used to measure RNA spectrophotometrically. The Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific) was used to reverse transcribe the total purified RNA into cDNA in accordance with the manufacturer’s instructions.
qRT-PCR
The qRT-PCR was carried out in duplicate for each sample using Maxima SYBR Green qPCR Master Mix, Thermo Scientific. The reaction mixture was prepared in a total volume of 20 μL containing 10 μL of 2× SYBR Green Master Mix, 0.5 μL of each primer set for each gene and 4.5 μL of cDNA (100 ng/μL) and completed to 20 μL with water (nuclease-free). The housekeeping gene (GAPDH) was utilized to normalize each gene expression. The primer sequences for various genes (synthesized by Biosearch Technologies, USA) according to the previous study [Citation18] and illustrated in . PCR amplification was applied as the following protocol: 95°C for 10 min and 40 cycles of 95°C for 15 s, annealing for 30 s as appropriate for every primer set’s melting temperature, extension at 72°C for 10 s. RT‑qPCR was conducted using the 7500 Real‑Time PCR system (Applied Biosystems) in NRC lab. Relative expression levels of mRNA were analyzed using the 2−ΔΔCT formula.
Table 1. Specific primer sequences.
Statistical analysis
SPSS version 25 software was utilized for the analyses (SPSS, Chicago, IL). Data were presented as mean ± SEM. The statistical significance of group differences was determined using one-way analysis of variance (ANOVA), followed by Tukey’s test. For all data analysis, P < 0.05 was considered significant.
Results
Behavioral assessment
Y-maze spontaneous alternation test
displays the effect of quercetin co-administration at 50 mg kg-1 with AlCl3 on short-term memory in the rats of AlCl3-induced AD, utilizing the Y-maze spontaneous alternation test. As compared to NC rats, the AlCl3-induced AD rats had a substantial drop in spontaneous alternations and an increase in the frequency of returning to the same arm (P < 0.05), indicating a more serious deficiency in spatial recognition memory. On the other hand, we observed that the co-administration of AlCl3 with Q at 50 mg kg-1 bwt to AD rats (that induced with AlCl3) significantly elevated the spontaneous alternation rate and reduced the return of the same arm as compared to AlCl3-induced AD rats (P < 0.05), indicating to an enhancement in rats’ spatial recognition memory.
Protective effect of quercetin on Body weight
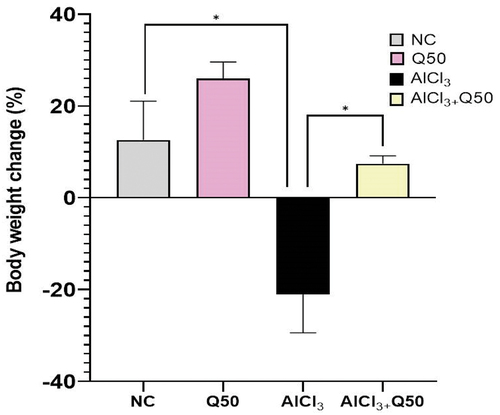
shows the protective effect of quercetin on changing body weight; we observed that a dramatic decrease in the percentage change of body weight in the AlCl3-induced AD rats compared with normal control. On the other hand, the co-administration of AlCl3 with quercetin at 50 mg kg-1 in the AlCl3-induced AD rats revealed a significant increase in the percentage change of body weight as compared to the AlCl3 AD-induced rat group (p < 0.05).
Protective effect of quercetin on expression of APP and beta secretase (BACE1) genes
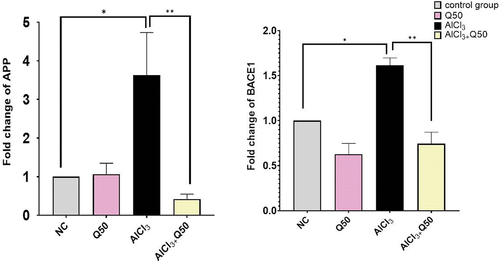
shows the protective effect of quercetin on the gene expression of APP and BACE1. We observed that the fold change for those genes was significantly increased by (3.63 ± 0.11, 1.61 ± 0.08), p < 0.05, respectively, in AlCl3-induced AD rats compared to the normal group. Regarding APP, the co-administration of AlCl3 with 50 mg kg-1 to the AlCl3-induced AD rats resulted in a significant decrease in fold change by (0.42 ± 0.13) fold compared to the AD-induced rat group (. On the other hand, the co-administration of AlCl3 with Q at 50 mg/kg bwt to the AlCl3-induced AD rats caused a significant decrease in fold change of BACE1 by (0.74 ± 0.13) compared to AD-induced rat group (.
Protective effect of quercetin on expression of gamma secretases (APH1&PSEN1) genes
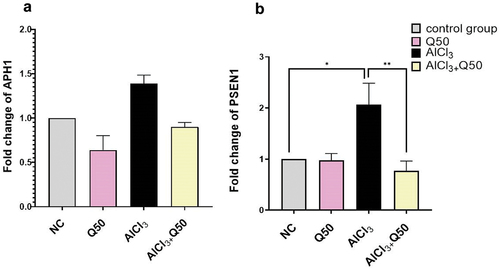
The gene expression of APH1 revealed insignificant up-regulation in the rats’ hippocampi in AlCl3-induced AD group as compared to the normal group. Furthermore, the co-administration of AlCl3 with Q at 50 mg kg-1 to the AlCl3-induced AD rat group reduced the expression level but this decrease did not reach a significant level compared to AlCl3 group (. As regards the gene expression of PSEN1, it showed a significant increase by fold change (2.07 ± 0.42) in the rat’s hippocampal region in AlCl3-induced AD group when compared to the normal group. Meanwhile, the co-administration of AlCl3 with Q 50 mg kg-1 to the AlCl3-induced AD rats caused a significant decrease in fold change by (0.77 ± 0.19), compared to the AD-induced rat group (.
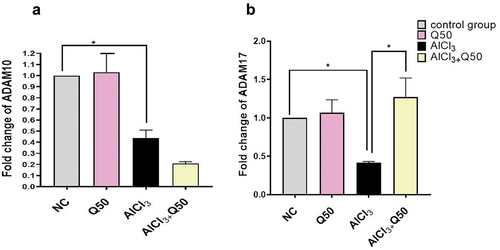
Protective effect of quercetin on expression of alpha secretases (ADAM10 & ADAM17) genes
As shown in , the expression level of ADAM10 was significantly down-regulated in the rats’ hippocampi in AlCl3 -induced AD group compared to the normal group. Besides, we observed that there were no significant changes in the expression level of ADAM10 by the co-administration of AlCl3 with Q 50 mg kg-1 to the AlCl3-induced AD rats compared to the AD-induced rat group. Concerning ADAM17, the qPCR revealed that the expression level was significantly decreased in the rat’s hippocampal region in AlCl3-induced AD group in comparison with the normal group. Also, the co-administration of AlCl3 with Q at 50 mg kg-1 resulted in a significant up-regulation fold change by (1.27 ± 0.249) p < 0.05 compared to the AD-induced rat group (.
Discussion
Environmental factors perform a dominant role in the development of AD [Citation19]. Aluminum (Al) is a neurotoxic substance that causes the development of neurofibrillary tangles and amyloid aggregates [Citation20]. Moreover, aluminum has shown adverse effects on neurological function through Aβ degradation in brain tissue, neuron death and Alzheimer’s-like symptoms [Citation21–23]. Consequently, learning and memory are important factors in diagnosing Alzheimer’s disease and evaluating its treatment [Citation24]. Quercetin is an important natural polyphenol that has beneficial effects on human health by scavenging ROS/metal chelation [Citation25]. The current study intends to investigate the molecular processes behind quercetin’s neuroprotective ability. Our results showed a significant reverse effect in spatial recognition memory in AlCl3-induced AD rats co-administered with 50 mg/kg bwt compared to AlCl3 group. Previous studies have recorded a similar finding of quercetin’s neuroprotective effects in an animal model of AD [Citation26,Citation27]. Furthermore, Jajin and Esmaeili [Citation13] assumed that nanoparticles of superparamagnetic iron oxide linked to quercetin (QT-SPIONs) in the AD model led to no formation of neurofibrillary tangles (NFTs) and increased regenerative changes. They found that the QT-SPION treatment had a significant reduction in escape time, a significant lengthening of time spent in the target area, as well as the number of crossed platelets in comparison to the AlCl3 group, and the identical results in that group were shown with the control group. Another study exhibited that quercetin caused significant improvement in cognition abnormality in Al-treated rats [Citation28].
In this study, the AlCl3-induced AD rats showed a significant decrease in percentage change of body weight compared to normal control. Abdel-Aal and Assi [Citation29] explained that AlCl3 injections for 60 days failed to gain weight compared to the control rats, which might result from lack of food intake desire. Co-administration of quercetin at 50 mg kg-1 to AlCl3 rats showed improvement in percentage change of the bodyweight. These findings are in accordance with Hassan and Abo El-Ela [Citation30] who found that significant weight growth by about 70% in the group of quercetin and about 50% when paired with imidacloprid (IMD), neurotoxic substance, considering weight gain in rats as an indicator of growth rate.
APP is a transmembrane glycoprotein that has a receptor-like structure which is crucial for neurite elongation, branching and sprouting [Citation31]. APP can pass through two different proteolytic pathways: the non-amyloidosis pathway, which inhibits Aβ formation and appears to be a protective pathway, along with the amyloidosis pathway, which results in Aβ generation [Citation32]. Aβ is produced through successive cleavage of APP by beta- and gamma-secretases. As a consequence, Aβ contents correlate with the expression of APP, beta and gamma-secretases [Citation33,Citation34]. In our study, the AlCl3-induced AD rats revealed significantly up-regulation of APP compared with normal group, which is the most important hallmark of AD [Citation35]. However, there is a significant down-regulation in expression level of APP in the co-administration of AlCl3 with Q 50 mg kg-1 to the AlCl3-induced AD rats. These results are in line with [Citation13], and they found that the expression level of APP decreased in the AlCl3 + QT-SPIONs group. Also, an in vitro study [Citation36] demonstrated that rutin and quercetin prevented the development of Aβ fibrils and disaggregated Aβ fibrils APP in Swedish mutation (APPswe) cells.
β-amyloid converting enzyme 1 (BACE1) is the major β-secretase [Citation37,Citation38]. Also, BACE1 is a first protein that cleavages APP forming Aβ in an amyloid pathway. Several studies have demonstrated that BACE1 could be a potential curative target. Mice that are BACE-1 knockout do not have severe phenotypic defects and do not develop measurable quantities of Aβ [Citation39,Citation40]. Our results showed that the level of BACE1 gene expression was significantly elevated in the hippocampal tissues of the AlCl3 group when compared to the normal control. On the other hand, a decrease in BACE1 gene expression was observed in the co-administration of AlCl3 with Q at 50 mg kg-1 to the AlCl3 -induced AD rat. The last findings are consistent with in vitro study by Shimmyo and Kihara [Citation41] who exhibited that quercetin decreases BACE1 activity in a cell-free system with an IC50 of 5.4 ± 0.5 µM, whereas, in a neural cell system, quercetin as well shown BACE1 decreasing activity with IC50 of 50 µM. Moreover, another study evaluated how quercetin affected old mice’s neurotoxicity caused by excessive cholesterol. They found that mice treated with quercetin showed a decrease in BACE1 expression [Citation42].
Gamma-secretase is an intramembrane aspartyl protease which engaged in Alzheimer’s disease through the proteolysis of APP. Thus, gamma-secretase creates the pathogenic Aβ 1–42 peptide that causes amyloid plaques [Citation43,Citation44]. A protein called Presenilin I (PSEN1) belongs to the aspartic protease family and is involved in the control of intramembrane proteolysis [Citation8]. PSEN1 was believed to be a central, catalytic moiety of the gamma-secretase complex. PSEN1 was reported to be capable of cleaving substrates in the absence of Nicastrin (NCT), APH1 and presenilin enhancer-2 (PEN-2) in an activity assay performed in the liposomes [Citation45]. Therefore, PSEN1 is a candidate target gene in drug design against AD. In the current research, we evaluated the levels of PSEN1 and APH1, two different components of gamma-secretase, in the hippocampus of rat brains. Both PSEN1 and APH1 levels were elevated in the AlCl3-induced AD group in comparison with the normal group. Meanwhile, PSEN1 gene expression level was significantly decreased in co-administration of AlCl3 with Q 50 mg kg-1 to AlCl3-induced AD rat. Suggesting that polyphenols could act as an inhibitor of PSEN1, a study by Lakey-Beitia and Berrocal [Citation46] intended that polyphenols could occupy the active site of gamma-secretase (displacing the water molecule needed for catalysis by the enzyme), would inactivate the enzyme and decrease Aβ formation. On the other hand, Q at 50 mg kg-1 to AlCl3 -induced AD rats showed no significant effect on APH1gene expression compared to AlCl3 group.
Additionally, most information on APP’s non-amyloidogenic cleavage is yet unclear, mostly with relation to pharmacological approaches that could impact various signaling pathways. Three of the zinc-dependent transmembrane-dependent metalloproteinases, ADAM 10, ADAM 17 and ADAM 9, exhibit α-secretase activity [Citation47]. According to our research, a significant reduction in ADAM 10 and ADAM 17 was observed inside the hippocampus tissue in AlCl3 group compared to normal control. These results are similar with [Citation5]. In contrast, ADAM 17 was significantly increased in the co-administration of AlCl3 with Q at 50 mg kg-1 to the AlCl3-induced AD rats. On the other hand, the effect of quercetin did not show any significant changes to ADAM 10 in the co-administration of AlCl3 with Q 50 mg/kg bwt to the AlCl3 -induced AD rats. The last findings may explain the mechanism of quercetin to regulate ADAM17 activity. ADAM17 effects on other mediators directly or indirectly reduce Aβ. Previous studies demonstrated that ADAM17 can enhance pro-inflammatory cytokines of microglia, for instance tumor necrosis factor-α, fractalkine, and interleukin-8 (IL-8), and stimulate IL-1, IL −6, and Notch receptor that provoke phagocytosis leading to Aβ degradation [Citation48,Citation49]. In contrast to ADAM17, ADAM 10 directly mediated APP cleavage, in this study the targeting of quercetin was shown to be nonspecific to ADAM10. Suggesting that both enzymes, ADAM 10 and BACE1, compete with one another to cleave APP, thus, quercetin targeting BACE-1 activity may inhibit neurotoxic amyloid generation via the amyloid pathway.
Conclusion
Quercetin (50 mg kg-1) could be a promising neuroprotective natural compound against AD symptom development. Co-administration of quercetin with AlCl3 resulted in significant improvement of short memory. It showed a reduction in APP expression level, AD hallmark gene, and increases the expression level of ADAM 17 in the non-amyloidosis pathway. Therefore, future clinical trials of quercetin as neuroprotective agents could be evaluated as potentially preventing the development of early-stage AD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ricci G. Social aspects of dementia prevention from a worldwide to national perspective: a review on the international situation and the example of Italy. Behav Neurol. 2019;2019:8720904.
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137.
- Bhattacharjee S, Zhao Y, Hill JM, et al. Aluminum and its potential contribution to Alzheimer’s disease (AD). Front Aging Neurosci. 2014;6:62.
- Al-Otaibi SS, Arafah MM, Sharma B, et al. Synergistic effect of quercetin and α-lipoic acid on aluminium chloride induced neurotoxicity in rats. J Toxicol. 2018;2018.
- Wang L, Hu J, Zhao Y, et al. Effects of aluminium on β-amyloid (1–42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem Res. 2014;39(7):1338–1345.
- Morishima-Kawashima M. Molecular mechanism of the intramembrane cleavage of the β-carboxyl terminal fragment of amyloid precursor protein by γ-secretase. Front Physiol. 2014;5:463.
- Venugopal C, Demos CM, Jagannatha Rao K, et al. Beta-secretase: structure, function, and evolution. CNS & Neurol Disord Drug Targets. 2008;7(3):278–294.
- Zhou Y, Suram A, Venugopal C, et al. Geranylgeranyl pyrophosphate stimulates γ-secretase to increase the generation of Aβ and APP-CTFγ. FASEB J. 2008;22(1):47–54.
- Tang BL. Alzheimer’s disease: channeling APP to non-amyloidogenic processing. Biochem Biophys Res Commun. 2005;331(2):375–378.
- Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009;8(4):397–409.
- Shafabakhsh R, Asemi Z. Quercetin: a natural compound for ovarian cancer treatment. J Ovarian Res. 2019;12(1):1–9.
- Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Current Opin Clin Nutr Metab Care. 2008;11(6):733–740.
- Jajin EA, Esmaeili A, Rahgozar S, et al. Quercetin-conjugated superparamagnetic iron oxide nanoparticles protect AlCl3-induced neurotoxicity in a Rat model of alzheimer’s disease via antioxidant genes, APP Gene, and miRNA-101. Front Neurosci. 2020;14:14.
- Du G, Zhao Z, Chen Y, et al. Quercetin attenuates neuronal autophagy and apoptosis in rat traumatic brain injury model via activation of PI3K/Akt signaling pathway. Neurol Res. 2016;38(11):1012–1019.
- Linardaki ZI, Orkoula MG, Kokkosis AG, et al. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol. 2013;52:163–170.
- Perusini JN, Cajigas SA, Cohensedgh O, et al. Optogenetic stimulation of dentate gyrus engrams restores memory in Alzheimer’s disease mice. Hippocampus. 2017;27(10):1110–1122.
- Van der Borght K, Havekes R, Bos T, et al. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121(2):324.
- Elfiky AM, Mahmoud AA, Elreedy HA, et al. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021 Nov 15;285:119964.
- Mir RH, Sawhney G, Pottoo FH, et al. Role of environmental pollutants in Alzheimer’s disease: a review. Environ Sci Pollut Res. 2020;27(36):1–19.
- Thenmozhi AJ, Raja TRW, Janakiraman U, et al. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res. 2015;40(4):767–776.
- Butterfield DA, Mattson MP. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol Dis. 2020;138:104795.
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian study of health and aging cohort. Neurotoxicology. 2021;83:157–165.
- Foley MH, Déjean G, Hemsworth GR, et al. A cell-surface GH9 endo-glucanase coordinates with surface glycan-binding proteins to mediate xyloglucan uptake in the gut symbiont Bacteroides ovatus. J Mol Biol. 2019;431(5):981–995.
- Hanko V, Apple AC, Alpert KI, et al. In vivo hippocampal subfield shape related to TDP-43, amyloid beta, and tau pathologies. Neurobiol Aging. 2019;74:171–181.
- Taïlé J, Arcambal A, Clerc P, et al. Medicinal plant polyphenols attenuate oxidative stress and improve inflammatory and vasoactive markers in cerebral endothelial cells during hyperglycemic condition. Antioxidants. 2020;9(7):573.
- Wang D-M, S-Q L, W-L W, et al. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem Res. 2014;39(8):1533–1543.
- Falode JA, Akinmoladun AC, Olaleye MT, et al. Sausage tree (Kigelia africana) flavonoid extract is neuroprotective in AlCl3-induced experimental Alzheimer’s disease. Pathophysiology. 2017;24(4):251–259.
- Sharma DR, Wani WY, Sunkaria A, et al. Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotox Res. 2013 May;23(4):336–357.
- Abdel-Aal RA, Assi -A-A-A, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol. 2011;659(2–3):169–176.
- AmS H, El-Ela FI A, Abdel-Aziz AM. Investigating the potential protective effects of natural product quercetin against imidacloprid-induced biochemical toxicity and DNA damage in adults rats. Toxicol Rep. 2019 Jan 01;6:727–735.
- Young-Pearse TL, Chen AC, Chang R, et al. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3(1):1–14.
- Cam JA, Bu G. Modulation of β-amyloid precursor protein trafficking and processing by the low density lipoprotein receptor family. Mol Neurodegener. 2006;1(1):1–13.
- Hazarika I, Mukundan GK, Sundari SP, et al. The modulatory effect of Hydrocotyle sibthorpioides in attenuating the aluminium chloride induced neurotoxicity in rat brain. Adv Tradit Med 2021;22(1):1–13.
- Tamagno E, Guglielmotto M, Aragno M, et al. Oxidative stress activates a positive feedback between the γ‐and β‐secretase cleavages of the β‐amyloid precursor protein. J Neurochem. 2008;104(3):683–695.
- Ghammraoui B, Badano A, Ran C. Identification of amyloid plaques in the brain using an x-ray photon-counting strip detector. PLoS One. 2020;15(2):e0228720.
- Jiménez-Aliaga K, Bermejo-Bescós P, Benedí J, et al. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011 Dec 19;89(25–26):939–945.
- Stratman N, Mathews W, Buhl A, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. (Vol. 286), 1999 Oct.
- Yan R, Bienkowski M, Shuck M, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402(6761):533–537.
- Luo Y, Bolon B, Kahn S, et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci. 2001;4(3):231–232.
- Naushad M, Durairajan SSK, Bera AK, et al. Natural compounds with Anti-BACE1 activity as promising therapeutic drugs for treating alzheimer’s disease. Planta Med. 2019;85(17):1316–1325.
- Shimmyo Y, Kihara T, Akaike A, et al. Flavonols and flavones as BACE-1 inhibitors: structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim Biophys Acta. 2008;1780(5):819–825.
- Lu J, Dm W, Zheng Y, et al. Quercetin activates AMP‐activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol‐induced neurotoxicity. J Pathol. 2010;222(2):199–212.
- McCarthy AJ, Coleman-Vaughan C, McCarthy JV. Regulated intramembrane proteolysis: emergent role in cell signalling pathways. Biochem Soc Trans. 2017;45(6):1185–1202.
- Hass MR, Sato C, Kopan R, Zhao G. Presenilin: RIP and beyond. In Seminars in cell & developmental biology. Academic Press. 2009; p. 201–210.
- Servián-Morilla E, Robles-Lanuza E, Sánchez-Hidalgo AC, et al. Proteolytic processing of neurexins by presenilins sustains synaptic vesicle release. J Neurosci. 2018;38(4):901–917.
- Lakey-Beitia J, Berrocal R, Rao K, et al. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol Neurobiol. 2015;51(2):466–479.
- Tippmann F, Hundt J, Schneider A, et al. Up‐regulation of the α‐secretase ADAM10 by retinoic acid receptors and Acitretin. FASEB J. 2009;23(6):1643–1654.
- Qian M, Shen X, Wang H. The distinct role of ADAM17 in APP proteolysis and microglial activation related to Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(4):471–482.
- Garton KJ, Gough PJ, Blobel CP, et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001;276(41):37993–38001.