 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Spices are generally consumed because of the taste and flavor they add to food. Some are also consumed because of their medicinal properties. We herein report on the nutrient and antinutrient compositions of five Ghanaian spices namely Xylopia aethiopica, Piper guineense, Monodora myristica, Aframomum melegueta and Parkia biglobosa. Nutritional composition was assessed by proximate analysis, minerals by atomic absorption spectrophotometry while titrimetric methods were utilized in vitamin C and antinutrients analysis. P. biglobosa was rich in proteins (38.60%) and had highest moisture content (32.79%). The highest levels of ash, fiber, fat and carbohydrates were observed P. guineense (11.90%), A. melegueta (31.12%), M. myristica (31.01%) and X. aethiopica (50.1%) respectively. Calorific values for all spices were between 243 and 402 kcal. Calcium, magnesium, potassium and iron were the most abundant minerals with levels ranging from 2.67 to 5,381.88 mg/kg. Zinc, copper and manganese were present in trace amounts. Vitamin C levels ranged from 3.3 to 18.4 mg/100 g. Phytates were present at generally higher levels than oxalates. P. biglobosa and X. aethiopica contained the highest concentration of oxalates and phytates respectively. The results indicate that these spices are good sources of valuable nutrients. However, the high levels of antinutrients implies consumption in moderation and good processing before eating is important.
Public Interest Statement
Global consumption of spices is well documented. Apart from the oomph or kick they give to food, they impart flavor and taste as well. They are useful sources of medicinally important compounds. This work looked at the presence of useful nutrients and antinutrients (chemicals that prevent absorption of other nutrients) in five commonly consumed spices in Ghana. Results from our study confirmed that these spices were rich sources of proteins, fats, carbohydrates, minerals and vitamin C were found in all spices. The antinutrients, phytates and oxalates, were also present. Antinutrients are undesirable because they reduce the bioavailability of essential minerals like calcium, zinc and iron. However, since spices are consumed usually in minute quantities, the effects of these antinutrients will be minimal. It is important that spices are well washed and cooked before consumption to reduce the levels of antinutrients.
Competing Interests
The authors declare no competing interest.
1. Introduction
Spices and aromatic herbs are an essential component of human nutrition and have a place in the cultures of various parts of the world. In Africa, Latin America, Asia and the Mediterranean, spices and herbs of various kinds are ubiquitous in the diet of the indigenes. Spices have been employed since time immemorial as preservatives, flavor enhancers and colorants. They have also been used for their appetite stimulation and gustatory perfection capabilities (Dziezak, Citation1989). Many traditional herbal formulations have spices as their base. Numerous in vitro and in vivo investigations have shown the ability of various spices to function as antioxidants, digestive stimulants, and hypolipidemics (Fernandez-Lopez et al., Citation2003; Viuda-Martos, Ruiz-Navajas, Fernández-López, & Pérez-Álvarez, Citation2010). Some of these spices have also shown antimicrobial, anti-inflammatory, antiviral, and antitumor properties (Shin, Masuda, & Naohide, Citation2004; Thomson et al., Citation2002; Viuda-Martos et al., Citation2010). Because of their potential use as functional foods, spices have been studied extensively by the chemical, pharmaceutical and food industries (Viuda-Martos et al., Citation2010).
Spices possess essential nutrients (minerals and vitamins inclusive) required for growth of the body and its maintenance and may therefore help alleviate the effects of some of these nutrient deficiencies. Even though they constitute only 4–6% of the human body, minerals are critical in the body where they serve as structural components of tissues and function in cellular and basal metabolism and water and acid-base balance (Özcan, Citation2004). Optimal intake of minerals such as sodium, potassium, magnesium, calcium, manganese, copper and zinc could potentially reduce risk factors associated with cardiovascular and other diseases (Sanchez-Castillo et al., Citation1998). Major minerals (required in amounts greater than 100 mg per day) include calcium, phosphorus, magnesium, sulfur, potassium, chloride and sodium whereas trace minerals such as zinc, iron, silicon, manganese, copper, fluoride, iodine and chromium are essential in much smaller amounts, less than 100 mg per day, and make up less than 0.01% of bodyweight (Macrae, Robinson, & Sadler, Citation1993; Özcan, Citation2004).
Antinutrients such as phytates, oxalates, polyphenols, tannins and alkaloids present in spices (Nwinuka, Ibeh, & Ekeke, Citation2005; Ogunka-Nnoka & Mepba, Citation2008) interfere with the bioavailability of minerals and vitamins. For a mineral to be absorbed in the body, they are required to be in their ionic states. Phytates possess a high density of negative charges due to the presence of phosphate groups. As such, phytates form very stable complexes with calcium, manganese, iron, copper and zinc, resulting in precipitation and subsequent unavailability for intestinal uptake (Lopez, Leenhardt, Coudray, & Remesy, Citation2002). Vitamin C, in particular, helps iron absorption by making iron less susceptible to phytate complexation, thus increasing its bioavailability (Hallberg, Brune, & Rossander, Citation1989a; Lynch & Cook, Citation1980). Free oxalates released from food after ingestion binds to calcium to form insoluble calcium oxalates. This reduces the amount of calcium available for absorption in the intestinal lumen (Park, Citation2013; Savage & Mårtensson, Citation2010). Apart from markedly reducing the bioavailability of minerals in the body, oxalates also contribute significantly to kidney stones, with an estimated 75% of all kidney stones composed of calcium oxalate (Park, Citation2013; Tang, Larson-Meyer, & Liebman, Citation2008).
It has been estimated by UNICEF that as many as a third of the world’s people do not meet their physical and intellectual potential because of vitamin and mineral deficiencies. Iron deficiency impairs intellectual development in young children and is lowering national intelligence quotients (IQs) (Adamson, Citation2004). This coupled with the increasing incidence of malnutrition reports in various parts of the globe (FAO, Citation2014; Kumssa et al., Citation2015) means that every component of a human diet should contribute essential nutrients for the upkeep of the body. It is therefore important that the nutritional and anti-nutritional composition of every ingredient in a diet is properly evaluated.
In Ghana, spices are ever present in the diet of the indigenes. Xylopia aethiopica (Common name: “Hwentia”), Piper guineense (Common name: “Soro wisa”), Monodora myristica (Common names: “nutmeg”, “kotokorawa”, “awerewa”), Aframomum melegueta (Common name: “Fem wisa”) and Parkia biglobosa (Common name: African locust beans, “Dawadawa”) are spices used regularly in many Ghanaian delicacies. We herein report the proximate composition, mineral, vitamin C and antinutrient levels in X. aethiopica, P. guineense, M. myristica, A. melegueta and P. biglobosa.
Our results show that P. biglobosa is protein rich and hence its use as a meat replacement in the diets of some poor rural folks is justified. All spices had high levels of magnesium, calcium and potassium. Phytate and oxalate levels were generally high in all spices under investigation, with phytate levels always higher than oxalates, except in A. melegueta where a reverse trend was observed. P. biglobosa again had the highest vitamin C content. This shows that when properly harnessed, these spices could contribute significantly to the essential nutrient requirements of our diets. However, the high levels of antinutrients in these spices means that care has to be taken in preparing them so that important minerals in the dishes are not complexed and rendered unavailable for absorption. One such method to achieve reduction of antinutrient levels is cooking.
2. Materials and methods
2.1. Samples
All spices were purchased from the Kejetia and Asafo markets in Kumasi, Ghana. Samples were placed in labeled, dry plastic bags and transported to the laboratories of the Department of Chemistry, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi for analysis. Spice samples were authenticated at the Department of Pharmacognosy, KNUST before use. Samples were milled into fine powder before the various analysis were carried out.
2.2. Proximate composition
Proximate parameters (carbohydrate, fats, protein, moisture and ash) of the five spices were determined using the Association of Official Analytical Chemists (AOAC, Citation1990) method. Nitrogen content of the samples was determined by the micro-Kjedhal method. The nitrogen value obtained was multiplied by 6.25 to convert it to crude protein. The weight difference method was used to determine moisture and ash levels while crude fat of the spices were determined using the AOAC procedure with petroleum ether as solvent. The carbohydrate content was determined by calculation using the difference method(1)
(1)
The various proximate parameters were all reported in percentages (Akoto, Borquaye, Howard, & Konwuruk, Citation2015; AOAC, Citation2000)
2.3. Calorific value
The gross food energy values (kcal/100 g sample) of each spice sample was estimated using the Atwater factors for protein (4), fat (9) and carbohydrate (4) (Onyeike, Olungwe, & Uwakwe, Citation1995; Zou, Moughan, Awati, & Livesey, Citation2007). The equation is(2)
(2)
2.4. Minerals
Two grams of ash from the determination of ash content was dissolved in 50 mL of 5% hydrochloric acid (HCl). After complete dissolution of ash, the solution was filtered into a 100 mL volumetric flask and topped to the 100 mL mark with 5% hydrochloric acid (HCl). Digested samples were stored in plastic containers and stored at 4°C prior to analysis.
Standards and samples were analyzed by flame atomic absorption spectrophotometry with a novAA 400P (Analytik Jena, Germany). Minerals were determined with their specific hollow cathode lamps at wavelengths specified by the manufacturer. Standards and reagent blanks were run at regular intervals to ensure consistent instrument performance. All samples were analyzed in triplicates.
2.5. Vitamin C
Vitamin C was determined by iodometry. About 2 g of sample was dissolved in 100 mL distilled water and stirred. The solution was filtered and used for the titrations. Freshly prepared ascorbic acid was used as the standard. 20 mL aliquots of the sample filtrates were transferred into an Erlenmeyer flask and 1 mL of starch indicator was added. The solutions were titrated against 5.0 mM Iodine solution. The endpoint of the titration was identified as the first permanent trace of a dark blue-black color due to the starch-iodine complex. The amount of iodine that reacted was calculated and the number of moles of ascorbic acid was determined using concordant titers and the reaction below:
where C6H8O6 is ascorbic acid (vitamin C) and C6H6O6 is dehydroascorbic acid. The concentration was reported as mg of ascorbic acid per 100 g of dry powder.
2.6. Anti-nutrients
2.6.1. Phytate
To 4 g of ground spice sample was added 100 mL of 2% HCl. The mixture was constantly shaken for 3 h and filtered. To 25 mL of the filtrate was added 5 mL of 0.3% ammonium thiocyanate (NH4SCN). Fifty milliliters of distilled water was added to afford the desired acidity. This solution was titrated against a 0.00195 g/mL ferric chloride solution. The end point was characterized by a persistent brownish yellow coloration. Phytate content was estimated from Equation (4) below;(4)
(4)
where t = titer value.
2.6.2. Oxalate
Oxalates were extracted with acid followed by titrimetric analysis. Briefly, to 1 g of the spice sample was added 75 mL of a 1.5 N sulfuric acid solution. The resultant mixture was carefully stirred for an hour followed by filtration. The filtrate (25 mL) was then warmed and titrated hot against 0.1 M potassium permanganate (KMNO4) solution to a faint pink color at the end point. The oxalate content was estimated using Equation (5)(5)
(5)
2.6.3. Estimation of relative mineral bioavailability
The molar ratios of phytate to zinc (Phy:Zn), phytate to iron (Phy:Fe), phytate to calcium (Phy:Ca) and that of oxalate to calcium (Oxa:Ca) were calculated to estimate the relative bioavailability of calcium, zinc and iron in the presence of antinutrients. These molar ratios also give an indication of the inhibitory effects of the antinutrients on the bioavailability of these minerals in the spices. Molar ratios were calculated using (6)(6)
(6)
The molar mass of phytate used was 660 g/mol and that of oxalate was 88 g/mol. The recommended critical values used in this work are (Phy:Ca) > 0.24 (Morris & Ellis, Citation1985), (Phy:Fe) > 1 (Hallberg, Brune, & Rossander, Citation1989b), (Phy:Zn) > 15 (Morris & Ellis, Citation1989), and (Ox:Ca) > 1 (Bhandari & Kawabata, Citation2004; Gargari, Mahboob, & Razavieh, Citation2007; Suma & Urooj, Citation2011).
2.7. Statistical analysis
Graphpad Prism for windows version 7.01 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. All analyses were carried out in 3 replicates. Mean and standard deviations (SD) of parameters determined were calculated for each spice sample and the results presented as mean ± SD. Data obtained were subjected to one way Analysis of Variance (ANOVA). Differences between means were tested using Tukey’s multiple comparison test at a significance level of p < 0.05.
3. Results
For any given spice, or food, its nutritional value is dependent on its nutrient composition as well as the levels of antinutrients or toxicants present. For the 5 spices evaluated, crude protein was highest in P. biglobosa. There were no significant differences (p < 0.05) in crude protein levels between M. myristica and A. melegueta as well as between X. aethiopica and P. guineense. Fat composition differed between all spices with M. myristica’s 31.01 ± 0.48 being the highest. A. melegueta had the highest amount of fiber with moisture highest in P. biglobosa. X. aethiopica’s 50.1% was the highest carbohydrate level recorded. Carbohydrates were estimated by difference. Proximate composition data have summarized in Table .
Table 1. Proximate composition of five Ghanaian spices
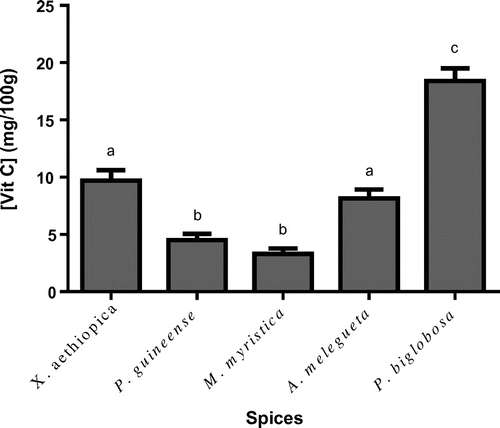
In terms of minerals, Ca, Mg, K and Fe were the most abundant in the spices (Table ). Cu, Zn and Mn were in trace amounts. Ca was present up to as high as 5,381 mg/kg in A. melegueta. Vitamin C (Figure ) ranged from 3.3 to 15.4 mg/100 g in spices tested. Highest vitamin C level was recorded in P. biglobosa. The spice with the lowest vitamin C level (3.3 mg/100 g) was M. myristica. The levels of vitamin C in A. melegueta and X. aethiopica were similar (p > 0.05). In X. aethiopica and P. guineense, there was no significant difference in vitamin C levels (p > 0.05).
Table 2. Concentrations of essential minerals in five Ghanaian spices
Figure 1. Levels of vitamin C in the various spices.
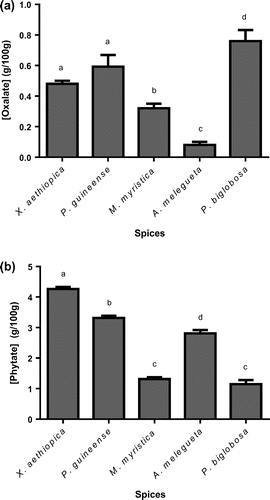
The antinutrients determined in this work were phytates and oxalates (Figure (a) and (b)). In general, phytate levels were much higher than oxalate levels. Phytate concentrations ranged between 1.5 and 4.27 g/100 g whereas oxalates were found at levels between 0.08 and 0.76 g/100 g. P. biglobosa had the highest and lowest levels of oxalates and phytates respectively. X. aethiopica was phytate rich, with a highest level of 4.27 g/100 g. The lowest oxalate concentration was detected in A. melegueta.
Figure 2. Levels of antinutrients, oxalate (a) and phytate (b) in the various spices.
Whereas several methods exist for evaluating bioavailable minerals in food or diet, phytate: mineral and oxalate: Ca are widely accepted as being generally good models for estimating fraction of minerals in a food available to an organism (Lee et al., Citation2013; Ma et al., Citation2007; Suma & Urooj, Citation2011). Due to the high levels of phytates in all spices, coupled with the relatively low levels of minerals, the phytate:zinc, phytate:calcium and phytate:iron molar ratios were generally high, much greater than the recommended critical values for any diet (Table ). All spices exceeded the critical values for phytate:iron and phytate:zinc. The phytate:calcium ratio for P. bibglibosa was 0.24, the same as the recommended critical value.
Table 3. Comparison of antinutrients to minerals molar ratio with recommended critical values
4. Discussion
In general, spices consist of different plant parts (seeds, leaves, roots etc.) utilized primarily for seasoning food. They are aromatic and may have bitter or sweet taste, have strong odors and sometimes pungent. In many parts of the world, spices are obtained from the wild and only few milligrams (about ½ a teaspoon) is added in foods. Nevertheless, they contribute substantial nutrients and antinutrients to dishes. The low moisture levels of the spices increases their shelf life as they are less susceptible to microbial damages. P. biglibosa, which is sometimes fermented for some Ghanaian dishes, had a much higher moisture content which permits its fermentation. The high crude protein in P. biglobosa validates its use as a protein substitute in dishes in some communities of northern Ghana. These communities are in the low economic brackets and hence utilize P. biglobosa to fill the protein gap in their diet as meat is expensive.
The proximate data reported in this work is comparable to a similar study conducted by Abolaji and coworkers in Nigeria (Abolaji, Adebayo, & Odesanmi, Citation2007). In that work, carbohydrates were estimated at 55% while it was 50% in this work. The crude protein levels reported by Effiong, Ibia, and Udofia (Citation2009) were twice the levels obtained in this work (Effiong et al., Citation2009). In another work on X. aethiopica done in Cameroun (Abdou Bouba et al., Citation2012), crude protein levels were comparable to that for this work. However, the fat levels were about 5X higher. For A. melegueta and P. guineense, the various proximate parameters were generally in agreement with spices from Nigeria and Cameroun (Abdou Bouba et al., Citation2012; Effiong et al., Citation2009). The high fat observed in M. myristica was also observed in the Cameroun samples. The high protein content of P. biglobosa agrees with a number of similar studies elsewhere (Cook et al., Citation2000; Lockett, Calvert, & Grivetti, Citation2000). Differences in proximate compositions may be due to differences in soil characteristics and climatic conditions at the areas where they were cultivated. They may also be due to growth conditions, genetic variations and differences in analytical procedures (Guerrero, Martınez, & Isasa, Citation1998; Özcan, Citation2004; Özcan & Akgül, Citation1998). The range of calorific values reported in this work (243–402 kcal) is similar to those reported for other spices (Effiong et al., Citation2009; Nwinuka et al., Citation2005; Ogunka-Nnoka & Mepba, Citation2008).
All spices were good sources of minerals with the major minerals, calcium, magnesium and potassium, in high abundance. The minor minerals, zinc, manganese and copper were present in trace amounts. Minerals are important for a variety of reasons. They maintain proper body function and good health. Mineral deficiency leads to increased susceptibility to infectious diseases due to weakened immune systems. Trace elements are associated with decreased DNA damage, maintenance of immune functions, reduced lipid peroxidation and inhibition of malignant cell transformation. Their absence leads to numerous metabolic disorders such as phenylketonuria, alkaptonuria, galactosemia and Tay-Sachs disease (Rosen & American Society for Bone & Mineral Research, Citation2009). In general, the levels of minerals were lower than that of similar spices from Cameroun and Nigeria (Abdou Bouba et al., Citation2012; Abolaji et al., Citation2007; Effiong et al., Citation2009). The high levels of iron in P. biglobosa, A. melegueta and M. myristica probably indicates hematinic property and justifies their inclusion in some local blood tonic preparations to manage iron deficiencies. Solanum torvum is another fruit that is a usual feature of such blood tonics (Akoto et al., Citation2015).
Iron deficiency leads to anemia, a condition in which the level of iron in the human body is significantly reduced. This results in a decrease in healthy red blood cells level (Longo & Camaschella, Citation2015). Such a condition can present a severe complication with adverse consequences for expectant mothers (Breymann, Citation2015; Longo & Camaschella, Citation2015). Calcium deficiency leads to an increased probability of getting diseases such as osteoporosis, osteopenia and calcium deficiency disease (hypocalcemia) (Prasad, Citation2013). A deficiency in zinc results in delayed wound healing, increased rate of diarrhea, impaired immune function and some psychological disorders (Sandström, Citation1992). In general, the deficiency of these important minerals could be as a result of inadequate absorption, reduced dietary intake or increased loss of mineral in the body. Inadequate absorption on calcium, iron and zinc may be related to low bioavailability in the human body (Lönnerdal, Citation2000).
Antinutrients are known to chelate calcium, iron and zinc with strong binding affinities, and thus significantly reducing their bioavailability. The presence of high levels of phytates and oxalates in human diets are therefore undesirable. Zinc bioavailability is a function of animal protein in food (Sandström, Citation1992; Sandström & Cederblad, Citation1980). High calcium levels are known to reduce zinc bioavailability. Calcium’s inhibitory effect on zinc may be due to the formation of calcium-zinc-phytate complexes in the intestines (Lönnerdal, Citation2000). These complexes are usually insoluble. In Africa, consumption of animal protein are too low to significantly alter zinc bioavailability (Schönfeldt & Gibson, Citation2012; Speedy, Citation2003). The high levels of phytates and oxalates recorded from these spices are undesirable. The Phy:Zn, Phy:Ca and Phy:Fe molar ratios recorded were all higher than recommended threshold values (Hallberg et al., Citation1989b; Morris & Ellis, Citation1985, Citation1989). The Ox:Ca molar ratio was above the threshold in four out of the five spices evaluated. Despite the high levels of calcium in all spices, the corresponding high levels on oxalates and phytates recorded means that calcium bioavailability is highly compromised.
These spices must therefore be consumed in moderation. It is known that washing and cooking reduces antinutrient levels in food substances. Spices should therefore be well-washed and well-cooked before consumption to reduce oxalate and phytate levels. However, spices form only a small component of the entire human diet. Hence, mineral contributions from other food components in the diet will ameliorate the high phytate and oxalate content of the spices. Diets that are rich in spices should be consumed with care as high phytate and oxalate levels may render important minerals unavailable.
Some vitamins have been reported to aid the absorption of minerals even in high phytate-containing diets. Vitamin C aids in iron absorption (Hallberg et al., Citation1989a; Lynch & Cook, Citation1980) while vitamin D helps with calcium absorption. The presence of vitamin C in these spices are therefore beneficial in this regard. The high vitamin C level in P. biglobosa especially increases its appeal as a healthy additive to various diets.
5. Conclusion
Spices are important components of many African diets and contribute important nutrients to many diets. X. aethiopica, P. guineense, M. myristica, A. melegueta and P. biglobosa are rich sources of proteins, carbohydrates and minerals. Calcium, magnesium, potassium and iron were the most abundant minerals present in the spices. P. biglobosa was rich in vitamin C. High levels of phytates and oxalates were present in the spices. It is therefore important that spices are used in moderation and should be complemented with other food components rich in minerals and proteins.
Funding
The authors received no direct funding for this research.
Author contributions
LSB and GD conceived the study. All experiments were designed by LSB, GD, ENG and MKL. Samples were collected by NAAA and ENA. MKL, ENG, NAAA and ENA carried out all the experiments. Data analysis was by LSB, ENG and MKL. Manuscript was prepared by LSB, GD, MKL and ENG. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the Departments of Chemistry and Food Science and Technology of the Kwame Nkrumah University of Science and Technology, Kumasi, for the use of its facilities for this study. Mineral analysis was done at the KNUST Central Laboratory. We are also grateful to Dr Nathaniel Owusu Boadi of the Department of Chemistry for helpful discussions. Mr Ebenezer Asante Donkor of the KNUST Central Lab is also acknowledged for his technical support.
Additional information
Notes on contributors
Lawrence Sheringham Borquaye
Lawrence Sheringham Borquaye is a bioorganic chemist with research focus on science at the interface of chemistry and biology. He’s been exploring biologically active natural products from marine organisms, plants and microbes. Other research interests include the monitoring of pharmaceutical and personal care products in the environment and the characterization of essential oils from plants.
Godfred Darko
Godfred Darko’s research interests spans environmental, analytical and nano-chemistry. He currently runs the SHEATHE project (www.sheathe.org) which seeks to characterize xenobiotics on a national scale in Ghana.
Michael Konney Laryea
Michael Konney Laryea is a graduate student in the Borquaye Research Group working on the isolation of biologically active natural products from plants while Edward Ntim Gasu, a Research Assistant, is investigating the unique properties of antimicrobial peptides.
Nana Afia Abrafi Amponsah
Nana Afia Abrafi Amponsah and Eunice Nyarkoah Appiah are undergraduate researchers with interest in herbs, spices and other food additives. Visit us at www.borquayelab.com for detailed profiles of all authors.
References
- Abdou Bouba, A., Njintang, N. Y., Foyet, H. S., Scher, J., Montet, D., Moses, C., & Mbofung, C. M. (2012). Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food and Nutrition Sciences, 3, 423–432.10.4236/fns.2012.34061
- Abolaji, O. A., Adebayo, A. H., & Odesanmi, O. S. (2007). Nutritional qualities of three medicinal plant parts (Xylopia aethiopica, Blighia sapida and Parinari polyandra) commonly used by pregnant women in the Western Part of Nigeria. Pakistan Journal of Nutrition, 6, 665–668.10.3923/pjn.2007.665.668
- Adamson, P. (2004). Vitamin and mineral deficiency, a global report. Ottawa.
- Akoto, O., Borquaye, L. S., Howard, A. S., & Konwuruk, N. (2015). Nutritional and mineral composition of the fruits of Solanum torvum from Ghana. International Journal of Chemical and Biomolecular Science, 1, 222–226.
- AOAC (1990). Official methods of analysis (15th ed.). Washington, DC: Author.
- AOAC. (2000). Official methods of analysis of the AOAC International (18th ed.). Washington, DC: Author.
- Bhandari, M., & Kawabata, J. (2004). Assessment of antinutritional factors and bioavailability of calcium and zinc in wild yam (Dioscorea spp.) tubers of Nepal. Food Chemistry, 85, 281–287.10.1016/j.foodchem.2003.07.006
- Breymann, C. (2015). Iron deficiency anemia in pregnancy. Clinical Obstetrics and Gynecology, 38, 443–454.
- Cook, J. A., VanderJagt, D. J., Pastuszyn, A., Mounkaila, G., Glew, R. S., Millson, M., & Glew, R. H. (2000). Nutrient and chemical composition of 13 wild plant foods of Niger. Journal of Food Composition and Analysis, 13, 83–92.10.1006/jfca.1999.0843
- Dziezak, J. D. (1989). Innovation food trends spices. Journal of Food Technology, 43, 102–116.
- Effiong, G., Ibia, T., & Udofia, U. (2009). Nutritive and energy values of some wild fruit spices in south eastern Nigeria. Electronic Journal of Environmental, Agricultural & Food Chemistry, 8.
- FAO. (2014). The state of food insecurity in the World. Rome: Author.
- Fernandez-Lopez, J., Sevilla, L., Sayas-Barbera, E., Navarro, C., Marin, F., & Perez-Alvarez, J. A. (2003). Evaluation of the antioxidant potential of hyssop (Hyssopus officinalis L.) and Rosemary (Rosmarinus officinalis L.) extracts in cooked pork meat. Journal of Food Science, 68, 660–664.10.1111/jfds.2003.68.issue-2
- Gargari, B., Mahboob, S., & Razavieh, S. (2007). Content of phytic acid and its mole ratio to zinc in flour and breads consumed in Tabriz, Iran. Food Chemistry, 100, 1115–1119.10.1016/j.foodchem.2005.11.018
- Guerrero, J., Martınez, J., & Isasa, M. (1998). Mineral nutrient composition of edible wild plants. Journal of Food Composition and Analysis, 11, 322–328.10.1006/jfca.1998.0594
- Hallberg, L., Brune, M., & Rossander, L. (1989a). The role of vitamin C in iron absorption. International Journal for Vitamin and Nutrition Research Supplement, 30, 103–108.
- Hallberg, L., Brune, M., & Rossander, L. (1989b). Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. The American Journal of Clinical Nutrition, 49, 140–144.
- Kumssa, D. B., Joy, E. J. M., Ander, E. L., Watts, M. J., Young, S. D., Walker, S., & Broadley, M. R. (2015). Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Scientific Reports, 5, 193.10.1038/srep10974
- Lee, S. E., Talegawkar, S. A., Merialdi, M., Caulfield, L. E., Billah, M., & Islam, S. (2013). Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutrition, 16, 1340–1353.10.1017/S1368980012004417
- Lockett, C. T., Calvert, C. C., & Grivetti, L. E. (2000). Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. International Journal of Food Sciences and Nutrition, 51, 195–208.
- Longo, D., & Camaschella, C. (2015). Iron-deficiency anemia. New England Journal of Medicine, 372, 1832–1843.
- Lönnerdal, B. (2000). Dietary factors influencing zinc absorption. Journal of nutrition, 130, 1378S–1383S.
- Lopez, H. W., Leenhardt, F., Coudray, C., & Remesy, C. (2002). Minerals and phytic acid interactions : Is it a real problem for human nutrition ? International Journal of Food Science and Technology, 37, 727–739.10.1046/j.1365-2621.2002.00618.x
- Lynch, S. R., & Cook, J. D. (1980). Interaction of vitamin C and iron. Annals of the New York Academy of Sciences, 355, 32–44.10.1111/nyas.1980.355.issue-1
- Ma, G., Li, Y., Jin, Y., Zhai, F., Kok, F., & Yang, X. (2007). Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. European Journal of Clinical Nutrition, 61, 368–374.10.1038/sj.ejcn.1602513
- Macrae, R., Robinson, R., & Sadler, M. (1993). Encyclopaedia of food science, food technology and nutrition (5th ed.). San Diego, CA: Academic Press.
- Morris, E., & Ellis, R. (1985). Effect of phytate on adult men consuming nonvegetarian diets. In C. Kies (Ed.), Nutritional bioavailability of calcium (pp. 63–72). Washington, DC: American Chemical Society.10.1021/symposium
- Morris, E., & Ellis, R. (1989). Usefulness of the dietary phytic acid/zinc molar ratio as an index of zinc bioavailability to rats and humans. Biological Trace Element Research, 19, 107–117.10.1007/BF02925452
- Nwinuka, N. M., Ibeh, G. O., & Ekeke, G. (2005). Proximate composition and levels of some toxicants in four commonly consumed spices.
- Ogunka-Nnoka, C. U., & Mepba, H. D. (2008). Proximate composition and antinutrient contents of some common spices in Nigeria. The Open Food Science Journal, 2, 62–67.10.2174/1874256400802010062
- Onyeike, E., Olungwe, T., & Uwakwe, A. (1995). Effect of heat-treatment and defatting on the proximate composition of some Nigerian local soup thickeners. Food Chemistry, 53, 173–175.10.1016/0308-8146(95)90784-5
- Özcan, M. (2004). Mineral contents of some plants used as condiments in Turkey. Food Chemistry, 84, 437–440.
- Özcan, M., & Akgül, A. (1998). Influence of species, harvest date and size on composition of capers (Capparis spp.) flower buds. Molecular Nutrition & Food Research, 42, 102–105.
- Park, S. W. N. S. (2013). Bioavailability analysis of oxalate and mineral content in selected edible mushrooms. Journal of Nutritional Disorders & Therapy, 4, 1–6.
- Prasad, A. (Ed.). (2013). Essential and toxic element: Trace elements in human health and disease. Amsterdam: Elsevier.
- Rosen, C. J., & American Society for Bone and Mineral Research. (2009). Primer on the metabolic bone diseases and disorders of mineral metabolism. American Society for Bone and Mineral Research.
- Sanchez-Castillo, C., Dewey, P., Aguirre, A., Lara, J. J., Vaca, R., de la Barra, P. L., … James, W. P. T. (1998). The mineral content of Mexican fruits and vegetables. Journal of Food Composition and Analysis, 11, 340–356.10.1006/jfca.1998.0598
- Sandström, B. (1992). Dose dependence of zinc and manganese absorption in man. Proceedings of the Nutrition Society, 51, 211–218.10.1079/PNS19920031
- Sandström, B., & Cederblad, A. (1980). Zinc absorption from composite meals. II. Influence of the main protein source. The American Journal of Clinical Nutrition, 33, 1778–1783.
- Savage, G., & Mårtensson, L. (2010). Comparison of the estimates of the oxalate content of taro leaves and corms and a selection of Indian vegetables following hot water, hot acid and in vitro extraction methods. Journal of Food Composition and Analysis, 23, 13–117.10.1016/j.jfca.2009.07.001
- Schönfeldt, H. C., & Gibson, Hall N. (2012). Dietary protein quality and malnutrition in Africa. British Journal of Nutrition, 108, S69–S76.10.1017/S0007114512002553
- Shin, I., Masuda, H., & Naohide, K. (2004). Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. International Journal of Food Microbiology, 94, 255–261.10.1016/S0168-1605(03)00297-6
- Speedy, A. W. (2003). Global production and consumption of animal source foods. Journal of Nutrition, 133(11 Suppl 2), 4048S–4053S.
- Suma, F., & Urooj, A. (2011). Nutrients, antinutrients and bioaccessible mineral content (invitro) of pearl millet as influenced by milling. Journal of Food Science and Technology, 1–6.
- Tang, M., Larson-Meyer, D., & Liebman, M. (2008). Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. The American Journal of Clinical Nutrition, 87, 1262–1267.
- Thomson, M., Al-Qattan, K., Al-Sawan, S., Alnaqeeb, M., Khan, I., & Ali, M. (2002). The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins, Leukotrienes and Essential Fatty Acids, 67, 475–478.10.1054/plef.2002.0441
- Viuda-Martos, M., Ruiz-Navajas, Y., Fernández-López, J., & Pérez-Álvarez, J. A. (2010). Spices as functional foods. Critical Reviews in Food Science and Nutrition, 51, 13–28.10.1080/10408390903044271
- Zou, M., Moughan, P., Awati, A., & Livesey, G. (2007). Accuracy of the Atwater factors and related food energy conversion factors with low-fat, high-fiber diets when energy intake is reduced spontaneously. American Journal of Clinical Nutrition, 86, 1649–1656.

