Abstract
Consumer’s concern on health through diet has been increased in recent time and majority of the population are trying to be healthy by consuming proper diet. Cereals, pseudo cereals, and pulses are getting appreciation from the consumers and the nutritionists because of their treasure of nutrition and taste. In recent times, the term “super grain” is became popular and fewer cereals and pseudo cereals are joined under this group. Different dieticians are recommending the grains such as Oats, Freekeh, Quinoa, Kamut, Teff, Faro, Spelt, Amaranth, Sorghum and Millets for their nutritional and health benefits. Among these, quinoa is world famous, got much appreciation from the consumers. Some of the cereals like teff are endemic to limited countries or part of the globe even though they are nutritionally rich but received very poor appreciation from the consumers. Teff is cultivating in Ethiopia and believed to be a rich source of nutrients, but the consumption and awareness of the teff is limited as compared to the quinoa. The major objective of this review is to compare the structural, nutritional and anti-nutritional properties of the teff and quinoa along with their applications.
PUBLIC INTEREST STATEMENT
Quinoa is considered as the top super grain and is widely studied as the healthiest pseudo-cereal. This is having the highest demand and appreciation from the consumers and available for high cost. Now, in the research community there is a huge discussion stated for the other super grains and many experts suggested that teff is the next super grain and articles readily available in famous editorials like The Guardian, BBC (British Broadcasting Corporation), Readers Digest, etc. In the case of teff, cultivation and consumption are limited to some parts of the world. The price of the teff is very low compared to quinoa and people also not aware about the nutritional quality of the teff. This paper is planned to review the differences between the quinoa and teff in structural, nutritional, anti-nutritional properties. This document will give the clarity about both crops for the researchers, which will help and encourage.
1. Introduction
Individuals always concern for ways to extend both their life span and quality of life. Longevity gets through a complex phenomenon, because many dietary, behavioral, socio-demographic and environmental factors influence aging and life-expectancy (Chrysohoou & Christodoulos, Citation2013).
Most deaths in low- and middle-income nations are now due to chronic disease. Mathers and Dejan (Citation2006) reported that worldwide mortality from chronic age-associated disease will projected to 66% in the next decade. According to United Nation Organization (Citation2015), core vision of the post 2015 development agenda is a ‘‘healthy life for all” in a world where everyone consumes food that is ‘‘affordable and nutritious”.
Several Sustainable Development Goals are intended to support consumer choice and boosted nutrition by promoting agricultural productivity among small-scale producers and supporting links between local and global markets. Healthy and balanced nutrition is crucial for everyone; it can obtained from various food sources like fruits, vegetables, whole grains, and protein source (Gul, Singh, & Rifat, Citation2016). Our body needs foods to develop, replace and repair cells and tissues, and also provide energy to keep our body warm, routine exercise and protect our body from potential disease causing microbes. By consuming balanced nutrition, people can avoid several health problems, such as hypertension, diabetes, coronary heart disease, gallbladder disease, certain cancers, dyslipidemia, stroke, osteoarthritis, and sleep apnea (Mir, Charanjit, & Sukhcharn, Citation2018).
Cereals are the staples, major source of carbohydrates, and energy and dietary pillar for the people on the globe. Most of the cereals also appreciated for fair amount of dietary fiber as well as several trace minerals, vitamins, and phyto-chemicals (Poutanen, Sozer, & Valle, Citation2014). Wholegrain foods have long been recognized as an essential part of a healthy diet, and many researchers appreciated whole grains foods as the source of nutrition (Fardet, Citation2010; Slavin, Citation2003). Different medical and epidemiological studies steadily reported that higher consumptions of whole grains are sturdily connected with reduced risk of acute and chronic diseases, including type 2 diabetes, cardiovascular disease and certain cancers, namely colorectal cancer (Aune et al., Citation2016; Geng, Alisa, Frank, & Qi, Citation2016; Hongyu et al., Citation2015).
In the case of the cereals, some are widely available like maize, wheat, rice, etc. Some are endemic to the some parts of the county or some regions like teff and quinoa. Both teff and quinoa cultivation and utilization traditionally limited to the some parts of the world, but recent times due to the proven health beneficial effects cultivation and utilization of quinoa and teff has practicing other than the place of origin (Poutanen et al., Citation2014). Both the crops cultivation is opened the new channels and reaching to the different parts of the world than cultivation regions. People are much known about the quinoa and it is considered as one of the costly crop. In the case of the teff now people are getting aware particularly in western world. Teff prices are low as compared quinoa . Both these crops are considered as the gluten free and super grains. Recent times, a discussion already started in the scientific media as teff is the next super grain to replace Quinoa (Goodyer, Citation2015). Thus, this review is planned to give the full insight for the readers with the major objective to review and compare the structure, nutritional, antioxidant, and anti-nutritional properties of both the crops along with their food applications.
2. Distribution and production of quinoa and teff
Quinoa is a pseudo-cereal and belongs to the family Chenopodiacea, endemic in all countries of the Andean region from Colombia to northern Argentina and southern Chile. Andean region has considered as the centre of origin and been cultivated for more than 7000 years (Pearsall, Citation1992). Teff is an ancient agricultural crop of highlands of Ethiopia and belongs to poaceae family. Teff has been cultivated in the Horn of Africa for at least 2,000 years, Ethiopia is known as the center of origin (Mengesha, Citation1966) and domestication for teff (Bultosa & Taylor, Citation2004a). Now, in contemporary world people and food markets have considered that both quinoa and teff are ancient and “super grains”.
Since the last two decades the quinoa has gained worldwide attention because of its ability to grow in various stress conditions like soil salinity, acidity, drought, frost, etc. (Bhargava, Sudhir, & Deepak, Citation2006; Jacobsen, Mujica, & Ortiz, Citation2003; Jensen et al., Citation2000; Rockwell, Marjorie, Jennifer, Laura, & Caroline, Citation2014; Vacher, Citation1998). Teff is cultivated successfully in a wide range of agro ecological conditions, such as marginal water logged soils to drought conditions.
Until recently, quinoa cultivation was restricted in some regions of South America (Bhargava et al., Citation2006). Quinoa cultivation is spreading from origin to the different countries as of now it is cultivating in more than 70 countries. Quinoa cultivation has been reported in France, England, Sweden, Scandinavia, Denmark, Holland, Italy, United States, Canada, Kenya and India (FAO, Citation2011). Teff cultivation has dated back to ancient times in Ethiopia, it has a wider distribution in high altitude and rainfall regions of central, eastern and southern Africa. In contrast to the quinoa, teff cultivation has bounded to some parts of the world, the United States of America, South Africa, Australia, India, Kenya, Eritrea, Djibouti, south-eastern Sudan and Netherlands (Curtis, Entsminger, Cowee, Davison, & Harris, Citation2008). Both the crops are cultivating for the purpose of food and feed.
FAO intended to focus world attention on the role that quinoa can play in providing food security and nutrition, and in the eradication of poverty. Due to the drought stability and nutritional quality, FAO considers quinoa as a perfect food (FAO, Citation2011) and quinoa cultivation and consumption in different parts of world has started. To encourage the quinoa production and utilization, FAO named and considered year 2013 was the “International year of quinoa”. FAO announced that quinoa is one of the crops destined to offer food security in the twenty-first century (Jacobsen et al., Citation2003).
3. Common usage and nutritional composition of quinoa and teff
There has been increasing interest in both quinoa and teff due to its perceived greater nutritional quality compared to other grains. Quinoa is used primarily in the same custom as wheat and rice and other grains. It is grounded into flour to prepare breads, cakes, and fermented drinks (Bhargava et al., Citation2006; Hitomi et al., Citation2002). The pre-Colombian Andean people used quinoa as a staple food component. Also it helps to replace the protein from animal source in their diet (Koziol, Citation1992). Teff accounts for about two-third of the daily protein intake in the diet of Ethiopian population. It also accounts for close to 26% of the annual crops and 31% of the cereals. It is commonly grounded into flour to make the popular pancake-like thin spongy Ethiopian bread called Injera (Asfaw & Gatahun, Citation2000). It is also used to make porridge (muk), local alcoholic drinks called tela and katikalla, as well as cakes and sweet dry unleavened bread (kita) (National Research Council, Citation1996; Seyfu, Citation1997). Recent studies reported the possibility of different non-traditional products from teff (Table ).
Table 1. Different types of the products developed from the teff
Researchers are highly interested in quinoa due to its carbohydrates (77.6%), protein (12.9%), a balanced amino-acid spectrum of high lysine and methionine contents, lipids (6.5%), and rich in dietary fiber (Atwell, Patrick, Johnson, & Glass, Citation1983; Gross et al., Citation1989; Hitomi et al., Citation2002; Ruales & Nair, Citation1994). Quinoa is also rich in mineral nutrients (3.0%), especially the K, Ca, Mg, P, and Fe contents which are much higher than those of conventional cereals (Calderelli et al., Citation2016; Konishi, Shigeru, Hideki, & Masao, Citation2004). Similarly, Teff grains are nutritionally well packed containing 9.4–13.3% protein with an excellent balance in essential amino acids including leucine, valine, proline, alanine and glutamic and aspartic acids being the major, 73% starch present in whole kernel stored as polygonal starch granules in the endosperm section of the grain (Bultosa & Taylor, Citation2004a), 2.6–3.0 % ash, and 2.0–3.1% lipid (Bultosa & Taylor, Citation2004a; Gebremariam, Martin, & Thomas, Citation2012) with rich source of Fe, Ca, Zn, Mg than other cereal grains (Abebe et al., Citation2007).
In addition, both quinoa and teff have recently been receiving global attention particularly as a “healthy food” due to the absence of gluten and gluten-like proteins, making it suitable for celiac disease patients (Spaenij-Dekking, Yvonne, & Frits, Citation2005). These super grains are currently emerging as healthy alternatives to gluten-containing grains in the gluten-free diet. Kupper (Citation2005) and Ogungbenle (Citation2003) reported that quinoa is easy to digest and is a complete food due to the gluten free, well-balanced set of essential amino acids with good source of proteins and minerals. Likewise, Teff has dietary advantages such as slow-release of carbohydrate constituents that are useful for diabetic patients (Bultosa & Taylor, Citation2004a). Recently, the use of teff in food systems is gaining popularity as both a naturally gluten-free alternative to wheat products and a nutrient-rich ingredient in the baby food industry (Curtis et al., Citation2008; Hopman et al., Citation2008). Rich source of the fiber is the other common factor in both of the seeds which makes them dietary choice for the entire world.
Teff contains substantial levels of Vitamins A and C, as well as niacin, and their amount is generally increased by the yeast fermentation process involved in the production of injera (National Research Council, Citation1996). Forsido, Vasantha, and Tess (Citation2013) reported antioxidant properties of teff indicate that it could be used for producing healthy food products. Similarly, the whole seeds of quinoa contain a large variety of antioxidant compounds, such as carotenoids (Eberhardt, Lee, & Liu, Citation2000), vitamin C, and flavonoids (De Simone, Dini, Pizza, Saturnino, & Schettino, Citation1990; Dini, Gian, & Antonio, Citation2004), which are protective against a variety of diseases, particularly cancer, allergy, inflammatory diseases and may reduce the risk of cardiovascular diseases. Consumers desire to try natural, different and ethnic foods, as well as interest in functional foods. Both of these seeds are an excellent example of functional food, defined as lowering the risk of various diseases and exerting health-promoting effects (Repo-Carrasco, Clara, & Jacobsen, Citation2003; Vega et al., Citation2010).
Both the quinoa and teff become attractive since last few decades. Quinoa has been evaluated as a food with excellent nutritional characteristics by the National Research Council and the National Aeronautics and Space Administration (NASA) (Schlick & David, Citation1993) and has been noted as a new foodstuff in the world. Similarly, teff is a very attractive cereal in the Western world since it is a gluten-free grain encompassing highly appreciated nutritional advantages. Teff has attracted much interest in the international market (Spaenij-Dekking et al., Citation2005) because it is a gluten-free food crop grown predominantly by small holders. It is given a high market value because it is in high demand, meaning that farmers earn more from growing teff than growing other staple crops (Araya, Keesstra, & Stroosnijder, Citation2010). During the last decade, teff prices have increased between 30 and 36%, depending on the type of teff (red, white or mixed). However, in the production year 2009, the price of teff increased dramatically by up to 35% with respect to the market prices recorded in 2008. Statistical reports reported that between year 1992 and 2010, the cultivated area and total production of quinoa in the main producer countries of Bolivia, Peru and Ecuador almost doubled and tripled, respectively, because of the world demand. Different researchers reported clear difference in composition of the quinoa and teff depends on the variety, agro ecological and geographical location of the growth.
4. Structural properties
Teff grain is hull-less (naked) and comes in a range of colors from milky white to almost dark brown. The most common colors are white, creamy white, light brown, and dark brown (Tefera, Ayele, & Assefa, Citation1995). Similarly, Quinoa seed colors vary from white to grey or black, potentially having tones of yellow, rose, red and purple and violet, often with very colorful mixes. Black is dominant over red and yellow, white seed color.
Quinoa is flattened and circular-shaped seeds which may measure from 1.5 mm in diameter to 4 mm (about 350 seeds weigh 1 g) (Ruales & Baboo, Citation1993). The average length, width and thickness of quinoa seeds were 1.889, 1.885 and 0.98 mm, respectively. About 72% of the 1000 seeds sampled had sizes varying from 1.7 to 2.0 mm, whereas about 27% were of sizes greater than 2 mm. The mean equivalent diameter of seed varied from 1.4 to 1.6 mm. In case of teff, the teff kernel is extremely small in oval-shaped with size mean length ranging 0.61–1.17 mm and mean width ranging 0.13–0.59 mm, that gives an average thousand kernel weight of 0.264 g (Bultosa, Citation2007), 2500–3000 grains weighing about 1 g (Babatunde Obilana & Manyasa, Citation2002). In comparisons, with the quinoa, teff grains are very small and light in weight. The quinoa seed comprises several layers, e.g., pericarp, seed coat and perisperm (Risi & Galwey, Citation1984) from outside inwards (Figures and ) and may be conical, cylindrical or ellipsoidal, with saponins concentrated in the pericarp.
Figure 1. Scanning electron microscope (SEM) micrograph of section of quinoa grain. The notations in image are as follows: H: hypocotylradicle axis; C: cotyledons; F: funicle; P: perisperm; SC; seed coat; R: radicul tip (adapted from Arendt and Emanuele (Citation2013)).
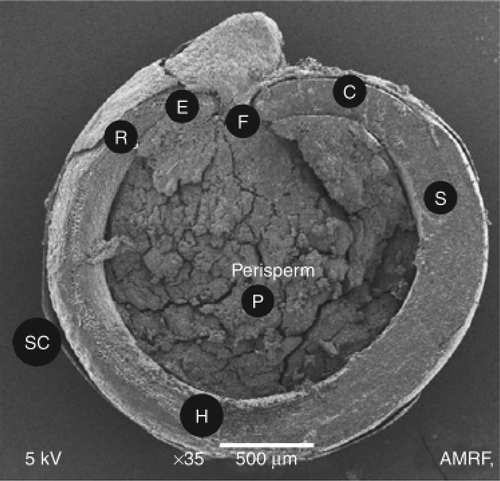
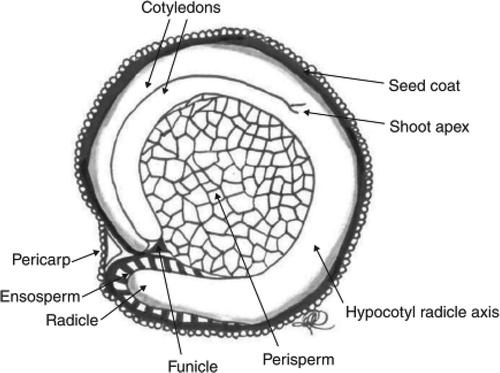
Figure 2. Median longitudinal section of the quinoa grain (adapted from Arendt and Emanuele (Citation2013)).
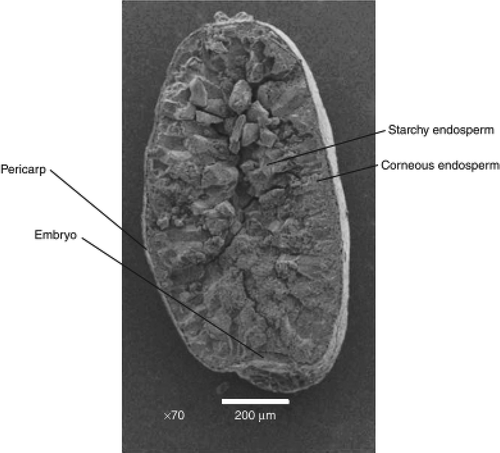
Compositional and nutritional evaluation of quinoa whole-grain flour and mill fractions (Gross et al., Citation1989) showed that the bran seed coat contained most of the sapogenins, protein, fat, fiber and ash. However, in case of teff grain outer thin and membranous structure of the kernel is termed pericarp, containing some starch granules, equivalent to the bran of wheat, beneath the cuticle toward the nuclear epidermis, teff grain is known to bear slime layer (slime layer to absorb and maintain moisture around the grain is implicated as a contributor to teff adaptive features to moisture stress) rich in pectins (Arendt & Emanuele, Citation2013).
In the inner surface of the pericarp, the mesocarp and endocarp are fused and appear as a single layer in teff grains. In this fused layer, some starch granules are present. Beneath the pericarp there is a seed coat or testa, in red teff varieties contains polyphenols or tannin and is responsible for the red color of the teff kernel. In some teff varieties, the testa is reported to contain tannins and is thus presumed to be thick. Next to the testa is the aleurone layer which is particularly rich in protein and lipid bodies. After the testa, the germ occupies a relatively large proportion of the grain and is rich in protein and lipids in teff grain (Figure ). The perisperm of quinoa seeds consisted of uniform, non- living, thin-walled cells full of starch grains which were angular in shape. Simple and compound starch grains occur in the same cells (Arendt & Emanuele, Citation2013).
Figure 3. Scanning electron microscope (SEM) longitudinal cross section of teff grain (reprinted from Arendt and Emanuele (Citation2013)).
Quinoa is referred as a pseudo-oilseed crop (Cusack, Citation1984) due to the exceptional balance between protein and fats. The perisperm, embryo and endosperm are the three areas containing food reserves in a quinoa seed (Prego, Sara, & Marisa, Citation1998). Protein and lipids are stored in the endosperm and embryo, and starch in the perisperm. In general, the quinoa seed is characterized by higher contents of protein and lipid and lower starch contents relative to the major cereals (wheat, barley, maize and rice). In the mature seed, the endosperm is present only in the micropylar region of the seed and consists of one to two cell-layered tissues surrounding the hypocotyl–radicle axis of the embryo.
Similarly, the endosperm is the largest component of the grain and consists of outer and inner layers in teff seeds. The outer layer is vitreous and contains most of the protein reserves of the endosperm and a few starch granules. The inner layer is mealy consisting mainly of thin-walled cells containing mostly starch granules with a few protein bodies. The endosperm represents the major component of the grain and, comprises an outer vitreous layer. This contains most of the protein of the kernel and a few starch granules and has an inner floury part which contains mainly starch granules with a few protein bodies. The embryo, which constitutes the living part of the teff kernel, occupies a relatively large proportion of the grain and is rich in protein and lipid bodies (Babatunde Obilana & Manyasa, Citation2002; Bultosa & Taylor, Citation2004a) few protein bodies.
5. Detailed nutritional compositions of quinoa and teff
5.1. Carbohydrates
Carbohydrates are typically granular forms of various shapes and sizes and the major constituent of plants sources (grains, seeds and tubers). Starch is the major source of physiological energy in the human diet and accordingly it is classified as available carbohydrate. The major components of quinoa are carbohydrates, making up 60–74 % of the dry matter (Koziol, Citation1992; Wright, Pike, Fairbanks, & Huber, Citation2002). Starch is about 58.1–64.2 % of the dry matter (Repo-Carrasco et al., Citation2003), of which around 10–21 % (depending on the variety) is amylose (Araujo-Farro, Podadera, Paulo, & Florencia, Citation2010; Lorenz & Coulter, Citation1991), located in the perispem of the quinoa seed which is lower than that present in wheat or maize, greater than some varieties of barley and similar to certain varieties of rice. Whereas, carbohydrates are the main constitute of teff caryopsis and also present in different tissues of the teff kernel. Among the carbohydrates, starch is the major component (73 % of the kernel dry weight) and is mainly concentrated in the endosperm (Bultosa & John, Citation2004b). According to the studies of Myoung, Ibáñez, and Shoemaker (Citation2007) and Werner et al. (Citation1999) quinoa starch has an average molar of 11.3 × 106 g mol−1, teff has reported 13.9 × 107 g mol−1 mass lower than waxy maize starch (17.4 × 106 g mol−1) or rice (0.52–1.96 × 108 g mol−1) and larger than wheat starch (5.5 × 106 g mol−1). Starch of quinoa is highly branched, with a minimum degree of polymerization of 4,600 glucose units, maximum of 161,000 and a weighted average of 70,000 (Werner et al., Citation1999). The length of the chain depends on the botanical source, ranging from 500 to 6,000 glucose units.
Quinoa starch have a polygonal form with a diameter of 0.4–2.0 µm, being smaller than those reported for maize (1–23 µm) and for wheat (2–40 µm) may be found as single entities or aggregates of spherical or elliptical composite structures. However, Starch granules in teff are conglomerates of many polygonal simple granules (Geremew, Alan, & John, Citation2002) and are very small (2–6 µm in diameter). However, they are larger in size compared to amaranth and quinoa and similar to rice starch granules (Geremew et al., Citation2002). As observed in other cereal grains, starch granules in teff are mainly composed of a branched fraction, amylopectin, and a linear fraction, amylose, which latter makes up 25–30 % of starch.
Tari, Uday, Rekha, and Pushpa (Citation2003) reported that the amylopectin in quinoa starch is 77.5%, in case of the teff the amylopectin reported around 83%. The fraction of amylopectin in both the grains is high and comparable to that of some rice varieties (Tukomane & Saiyavit, Citation2008). In quinoa, the amylopectin has a length distribution similar to waxy starch amylopectin, averaging 317 branching and average polymerization degrees of 6,700 glucose units per fraction. Quinoa amylopectin, as well as buckwheat and amaranth, contains a large number of short chains from 8 to 12 units and a small number of longer chains from 13 to 20 units in comparison with starches from other cereals (Abugoch, Nalda, Cristian, Jorge, & Monica, Citation2008; Tang, Katsumi, & Toshio, Citation2002). The gelatinization of quinoa starch occurs at a relatively low temperature, having been reported between 62.6 and 67°C, whereas gelatinization temperature of raw teff (68–80 °C) is similar to that of other tropical cereal starches like sorghum (67–81 °C), but occurs over a narrower temperature range than that of maize (60–79 °C) (Gebremariam et al., Citation2012). Quinoa starch gelatinizes at the temperatures less than amaranth starch and waxy barley, and little higher for starch from wheat, rice and barley. It has high maximum viscosity, higher water absorption capacity and greater swelling power, compared to starch from wheat and barley. The teff starch pasting temperature is similar to that of maize starch, but the cooking time for peak viscosity is longer. Peak, breakdown and setback viscosities are lower than those of maize starch (Bultosa and Taylor, Citation2004b). Due to the smooth, very small and uniform size of its granules, teff starch offers good functionality as a flavor and aroma carrier or fat replacer.
The rate of carbohydrate digestion of a food can be characterized by its glycemic index (GI) (Harris & Raymond, Citation2009). The GI of a food depends on endogenous factors of the food matrix such as starch susceptibility to α-amylase, protein and lipid content, and the macroscopic structure of the food (Fardet, Fanny, Delphine, Augustin, & Christian, Citation2006). Starch susceptibility to α-amylase depends on its structure, encapsulation, crystal structure, degree of gelatinization, the proportion of damaged granules as well as the retrogradation of the starch granules (Fardet et al., Citation2006). In vitro digestibility (α -amylase) of raw quinoa starch was reported at 22%, while that of autoclaved, cooked, and drum-dried samples was 32%, 45%, and 73%, respectively (Ruales & Nair, Citation1994). In comparison to wheat which has larger starch granules, the in vitro starch digestibility of teff was found to be significantly lower (Wolter, Anna, Emanuele, & Elke, Citation2013). In line with this, the predicted glycemic index of teff (74) was significantly lower than that of white wheat (100) but comparable to that of sorghum (72) and oats (71) but higher than quinoa (Wolter et al., Citation2013).
5.2. Dietary fiber
The dietary fiber in quinoa is mainly localized in the hull (seemingly seed coat and pericarp). Its total dietary fiber content varies between 2.0 and 2.2 % of the dry matter, matching the value reported for common grains and leguminous seeds (Varo, Laine, & Koivistoinen, Citation1983). In case of fiber content in teff is 3 % (dry base) particularly high and exceeds that of most other cereals, such as wheat (2%), rye (1.5%), rice (0.6–1.0%) and sorghum (0.6%) dietary fiber in dry basis (Gebremariam et al., Citation2012). Additionally, more than 80% of the quinoa fiber is insoluble type (Ranhotra, Gelroth, Glaser, Lorenz, & Johnson, Citation1993). Unlike soybean and peas, quinoa and teff grains are not a significant source of soluble fiber. The total dietary fiber is close to the value found in cereals (7 to 9.7% dry base), wherein the embryo contains higher levels than those in perisperm. The soluble fiber content is reported ranging from 1.3% to 6.1% (dry base). Quinoa still presents approximately 3% simple sugars, mostly maltose, followed by D-galactose and D-ribose, plus low levels of fructose and glucose (Abugoch & Lilian, Citation2009). Higher fiber content in teff is due to the fact that it is always consumed in the whole-grain form (bran and germ included), since it is impossible to perform any fractionation during the milling process due to the small size of teff grains (Bultosa & John, Citation2004b).
Dietary fiber has a number of beneficial effects related with its indigestibility in the small intestine. Therefore, the reported high content of quinoa fibers can improve digestibility by facilitating the absorption process of the other nutrients present in quinoa in the large intestine (Ogungbenle, Citation2003). The intake of gluten-free dietary fiber is considered inadequate, so, experts recommend a higher intake of whole grains rich in fiber, unlike grains and refined products in the diet of patients with celiac disease, relieving, at least partially, the deficit of fiber intake by that portion of the population (Alvarez-Jubete, Arendt, et al., Citation2010a).
5.3. Proteins and amino acids
Plants store proteins in the embryo to provide nutrients for growth and development (Herman & Larkins, Citation1999). In the plant foods, proteins stored in seeds are the source of the proteins consumed directly as food by humans (Shewry, Citation2009). Stored proteins provide building blocks for rapid growth upon seed and pollen germination (Herman & Larkins, Citation1999). The nutritional value of a food is determined by its protein quality, which depends directly on amino acid content, digestibility, influence of anti-nutritional factors, and the tryptophan to a large neutral amino acids ratio (Comai et al., Citation2007). Proteins are functional and structural units of tissues, enzymes, hormones and antibodies also involved in power supply and regulation of metabolic processes. Amino acids provide nitrogen as major mineral and small amounts of sulfur compounds to the body. In the form of lipoprotein, they are involved in the transport of different bio molecules (triglycerides, cholesterol, phospholipids and fat-soluble vitamins). High quality of the protein is the major problem population where majority of the diet is from animal source, so it is recommended to replace the animal diet with the protein rich plant sources. This strategy may useful for the prevalence of malnutrition by the contribution of essential amino acids in the diet.
Different researchers reported the mean protein content reported in quinoa seeds is 12–23%, The difference in variety and agro ecological conditions are the major factors for variation in protein (Abugoch et al., Citation2008; GonzáLez, Roldán, Gallardo, Escudero, & Prado, Citation1989; Hitomi et al., Citation2002; Karyotis, Noulas, & Mitsibonas, Citation2003; Koziol, Citation1992; Ruales & Nair, Citation1994). Compared to cereal grains, the total protein content of quinoa (16.3% dry base), in case of teff the protein content was reported that between 8.7 and 11 % with a mean of 10.4 % (Bultosa, Citation2007). Quinoa protein is higher than that of barley (11% dry base), rice (7.5% dry base), corn (13.4% dry base), and is comparable to that of wheat (15.4% dry base) (Abugoch et al., Citation2008). Thus, the protein content of the teff grain is comparable to that of other common cereals such as barley, wheat, maize and pearl millet and less than quinoa (Gebremariam et al., Citation2012). Protein is the second most abundant component in both quinoa and teff after starch.
Teff’s fractional protein composition suggests that glutelins (45%) and albumins (37%) are the major protein storages, while prolamins are a minor constituent (12 %) (Bekele, Roger, Arthur, & Peter, Citation1995; Tatham et al., Citation1996). In contrast, more recent studies report that prolamins are the major protein storages in teff (Adebowale, Abdul, Naushad, Mervyn, & John, Citation2011). The most abundant amino acids are glutamic acid, alanine, proline, aspartic acid, leucine and valine. Methionine, alanine and histidine contents are slightly higher than in most other cereals, but serine and glycine are lower (Adebowale et al., Citation2011). Teff combines a good content and balance of essential amino acids; however, as with many cereals, lysine represents its first limiting amino acid (Jansen, Di Maio, & Hause, Citation1962). The overall amino acid profile of teff can be regarded as being well-balanced. However, relative to cereal grains, quinoa proteins are particularly high in lysine, the limiting amino acid in most cereal grains. Their essential amino acid balance is excellent because of a wider amino acid range than in cereals and legumes with higher lysine (5.1–6.4%) and methionine (0.4–1%) contents (Ruales & Baboo, Citation1993). Quinoa proteins have higher histidine content than barley, soy wheat proteins, while the methionine, cystine content of quinoa similar to that of barley and soy, and lower than the amounts in wheat. Quinoa proteins have adequate levels of aromatic amino acids (phenylalanine and tyrosine) and similarly in histidine, isoleucine, threonine, phenylalanine, tyrosine, and valine contents. Another important feature of teff and quinoa is that they have no gluten (Hopman et al., Citation2008; Spaenij-Dekking et al., Citation2005) investigated the presence or absence of gluten in pepsin and trypsin digests of 14 teff varieties and reported that gluten not presented in teff.
Several authors have reported different values for protein fractions in cereals as a result of differences in the extraction conditions especially solvent used in the extraction (Chandna & Narender, Citation1990; Moroni, Iametti, Bonomi, Arendt, & Dal, Citation2010). A large proportion of the storage proteins in cereals contain disulfide bonds, so a reducing agent is necessary to efficiently extract these proteins (Bean & Lookhart, Citation2000; Taylor, John, Michael, & de Suretha, Citation2005)
5.4. Fats
Cereals are not the good source of fat, but as they are often consumed in high quantities, cereals can contribute a significant amount of essential fatty acids to the diet (Michaelsen et al., Citation2011). Fatty acids are potentially beneficial to growth, development and long-term health. Consequently, there has been a significant interest in recent years in their inclusion in diets. For instance, increased intake of n-3 fatty acids (α-linoleic acid) were found to reduce biological markers associated with cardio-vascular disease, cancer, inflammatory and autoimmune diseases among others (Simopoulos, Citation2001).
Oil content in quinoa ranges from 1.8% to 9.5%, with an average content of 5.0–7.2%, which is higher than that of maize (3–4%) lower than soybean (20.9% dry basis) (Oshodi, Ogungbenle, & Oladimeji, Citation1999; Ranhotra et al., Citation1993; Ryan, Galvin, O’connor, Maguire, & O’brien, Citation2007; Wood, Lawson, Daniel, Robison, & Andersen, Citation1993). In the case of Teff, grain contains lower levels of lipids (approximately 2.0–3.0% of total grain weight) and other cereals such as maize, oats (6.9 %), millet (4.2 %) and sorghum (3.4 %) as compared to quinoa.
In quinoa, saturated fatty acids make up approximately 11% of the total fatty acid in the seed and others lipids in quinoa contain high amounts of neutral lipids in all the seed fractions analyzed. Some researchers have characterized the fatty-acid composition of quinoa lipids as total saturated 19–12.3% (mainly palmitic acid), total monounsaturated 25–28.7% (mainly oleic acid), and total polyunsaturated 58.3% (mainly linoleic acid) (Oshodi et al., Citation1999; Ranhotra et al., Citation1993; Ryan et al., Citation2007; Wood et al., Citation1993). However, in teff grains are rich in unsaturated fatty acids (72.46 %), among which 39.91% were polyunsaturated and 20.06 % were saturated fatty acids (El-Alfy et al., Citation2012). The predominant saturated fatty acid in quinoa is palmitic (8.5 %).
As in most other cereal grains, oleic (32.41 %), linoleic (23.83 %) and palmitic (15.9 %) acids are the major fatty acids (El-Alfy et al., Citation2012). Linolenic acid levels are higher in teff than in maize, sorghum, and wheat (Bultosa & Taylor, Citation2004a). Similarly, linoleic acid (C18:2) is one of the most abundant polyunsaturated fatty acids (PUFA) identified in quinoa like in teff. PUFAs have several positive effects on cardiovascular disease (Abeywardena, Mc Lennan, & Charnock, Citation1991; Keys & Willis, Citation1966) and improved insulin sensitivity (Lovejoy, Citation1999). The oil fraction both quinoa and teff has high quality and is highly nutritious, based on the fact that it has a high degree of unsaturation.
Teff grains are rich in unsaturated fatty acids, predominantly oleic acid (32.4%) and linoleic acids (23.8%) (El-Alfy et al., Citation2012). Although a clear consensus has not been reached on the optimal ratio between LA (linoleic acid) and ALA (alfa-linoleic acid) fatty acids, the Codex standards for infant formula recommend a LA:ALA ratio in the range of 5–15 (Koletzko et al., Citation2005). The LA:ALA ratio of 7:1 for teff can be considered satisfactory and is comparable to legumes that are good sources of fatty acids.
Quinoa oil contains notably high concentration of squalene,where as amaranth reported high squalenes in pseudo-cereals. Squalene is used as antimicrobials (bactericide) and ingredients in pharmaceuticals, organic coloring materials, rubber and surface-active agents but squalienes not reported in teff. All fatty acids present in quinoa are well protected by the presence of vitamin E, which acts as a natural antioxidant (Ng, Alfred, Janice, & Martin, Citation2007).
Phytosterols are natural components with different biological activity (anti-inflammatory, antioxidant, and anti-carcinogenic), they present in plant cell membranes, rich in oils seeds and grains. Ryan et al. (Citation2007) reported that the phytosterols present in quinoa were 63.7 mg 100 g–1 of β-sitosterol, 15.6 mg 100 g-1 of campesterol and 3.2 mg 100 g-1 of stigmasterol, which are the most abundant sterols in plants. These levels are higher than in pumpkin seeds, barley and corn, but lower than in lentils, chickpeas and sesame seeds. The recommended dose is 0.8–1.0 g of equivalent phytosterol per day, including natural sources, and they are important components in dietary reduction of low-density lipoprotein (LDL) assisting in maintaining a healthy heart (Abugoch & Lilian, Citation2009; Ryan et al., Citation2007). In the case of the teff, identification and composition of phytosterols in teff remain to be systematically analyzed (Zhu, Citation2018) .
5.5. Minerals
Minerals unlike carbohydrates, lipids and proteins, are inorganic and cannot be produced by living beings with very important functions in the body. Low intake or reduction of bioavailability may generate imbalances in health and impairment of vital functions. Among the best known are calcium, phosphorus, iron, potassium, sulfur, sodium, magnesium, zinc, copper, selenium, and chromium (Vega et al., Citation2010).
Bultosa (Citation2007) reported that the ash content of 13 teff varieties ranged from 3.16 to 1.99%, whereas quinoa is 3.4%. Both these grains have the ash content greater than rice (0.5%), wheat (1.8%), and other cereals, whereas quinoa has slightly higher ash than the teff. In addition to providing protein and calories, teff is a good source of minerals, particularly Fe (16 mg 100 g−1), resulting in a mineral content approximately two to three times that of wheat, barley, and sorghum (Mengesha, Citation1966). However, quinoa was reported to contain the Fe content (13.2 mg 100 g−1) slightly lesser then the teff. Both of these can be considered as the rich source found in the Fe. Moreover, destruction of phytic acid by fermentation is known to contribute to high Fe availability in diets (Adams, Citation1990) and, additionally, fermented teff foods are the staples in the production areas. This explains the low frequency of anemia in the highlands of Ethiopia, where teff represents the first cereal crop (Gebremariam et al., Citation2012; Mengesha, Citation1966). Studies showed that teff consumers have higher level of hemoglobin in their blood than non-teff consumers, and they do not suffer from hookworm anemia even when infested; however, hookworm anemia develops in non-teff eaters if they are infested with hookworm. In Ethiopia, an absence of anemia seems to correlate with the levels of teff consumption and is presumed to be due to the grain’s high content of iron. In addition, according to the same studies, malaria is frequently found in the groups with lower hemoglobin levels (Molineaux & Biru, Citation1965; Tadesse, Citation1969).
Teff also contains high levels of Ca, P, Cu, Zn, and Mg (Bultosa & Taylor, Citation2004a; Seyfu, Citation1997). Teff contains an excellent concentration (147 mg 100 g−1) of calcium and the level of this mineral in teff is by far higher than other cereals like maize (16 mg 100g−1), sorghum (5.8 mg 100g−1), wheat (39.5 mg 100g−1), and rice (23 mg 100g−1). Similarly, quinoa contains 148 mg/100 gm of the calcium which is similar to the teff. The zinc content of teff (6.7 mg 100g−1) is also higher than that of sorghum (1.7 mg 100g−1), wheat (1.7 mg 100g−1), rice (2.2 mg 100g−1), and wheat (1.7 mg 100g−1). However, Zn concentration of quinoa is reported to be 4 mg 100g−1. This is less than that of teff but greater than other cereal like sorghum, wheat, rice, and wheat (Navruz-Varli & Nevin, Citation2016).
The concentration of magnesium in both teff (184 mg 100g−1) and quinoa (362 mg 100g−1) is higher than that of wheat (103 mg 100g−1) and maize (142 mg 100g−1), but teff contains half concentration Mg than quinoa. Potassium reported in quinoa and teff is 1475 mg 100g−1 and 477 mg 100g−1 respectively. The quinoa has reported a higher amount of the potassium compared with teff and also wheat (478 mg 100g−1), maize (320 mg 100g−1), and rice (80 mg 100g−1). According to Alvarez-Jubete, Wijngaard, et al. (Citation2010a), calcium, magnesium, and iron are the main mineral deficiency in gluten-free products. In particular, the high calcium content of these seeds has great relevance for celiac individuals due to the well-known prevalence of osteopenia and osteoporosis among patients recently diagnosed with this disease quinoa and teff contains more iron than ordinary cereals; however, its availability may be affected to some extent by saponins and phytic acid present in the seeds. Both the teff and quinoa have minerals in bio-available forms. Iron, calcium, magnesium, and potassium are found in sufficient quantities in the quinoa grain for a balanced human diet (Bhargava et al., Citation2006; Repo-Carrasco et al., Citation2003; Vega et al., Citation2010).
5.6. Vitamins
Vitamin B1 (thiamin) was reported in teff (0.39 mg100g−1) and quinoa (0.38 mg100g−1). Both are almost the similar. Thiamin in the maize and wheat is similar with that of the teff and quinoa but rice (0.06 mg 100g−1) contains the very less amount of thiamine. The riboflavin (vitamin B2) in teff was reported to be about 0.27 mg100g−1 in quinoa 0.39 mg 100g−1 (Baye, Citation2014). Thus, quinoa has high concentrations of the Vitamin B2 as compared with teff. The riboflavin concentration was reported in the other common cereals like maize (0.10 mg 100g−1), rice (0.06 mg 100g−1), and wheat (0.17 mg 100g−1). In the case of niacin, teff (3.363 mg 100g−1) conations high concentration as compared with quinoa (0.70 mg 100g−1). Teff contains high Vitamin B3 compared to quinoa (maize: 1.80 mg 100 g−1 and rice: 1.92 mg 100g−1). However, wheat is a good source of the niacin (5.50 mg 100g−1), which is reported higher than all the staple cereals (Abugoch & Lilian, Citation2009; Alvarez-Jubete, Arendt, et al., Citation2010a).
Vitamin B6 is reported to be 0.482 mg100g−1 in teff and 0.49 mg100g−1 quinoa. Both concentrations are almost similar. However, the pyridoxine in rice (0.51 mg100g−1) is reported to be higher than teff and quinoa, but is less in wheat (0.41 mg100g−1) and oats (0.12 mg100g−1) (Abugoch & Lilian, Citation2009). In the case of Vitamin E, quinoa is reported to be reported high concentrations as 5.37 mg100g−1 than teff 0.08 mg100g−1. The Vitamin E in rice (1.20 mg 100g−1) and wheat (0.70 mg 100g−1) is less than the quinoa, but all are having the high concentration of tochopherols as compared with the teff. Vitamin A in teff is reported to be 9 mg100g−1 whereas in quinoa it is reported to be high as 14 mg100g−1 (Navruz-Varli & Nevin, Citation2016). Other cereals do not have Vitamin A in delectable level except the wheat. Wheat has the Vitamin A concentration similar to that of the teff (Baye, Citation2014).
6. Anti-oxidant properties of quinoa and teff
Antioxidants are famous due to the capacity of anti-oxidation chain reactions in different parts of the body. Vega et al. (Citation2010) reported the anti-oxidants usage as food preservative (it stops the rancidity, toxic products formation and maintains nutritional quality and shelf life). Recently, much attention has been focused on natural antioxidants, which reported the proven success in neural functions, reducing the risk of several degenerative diseases, cancer, cardiovascular disease and osteoporosis (Alvarez-Jubete, Mark, Elke, & Eimear, Citation2009; Vega et al., Citation2010; Yawadio, Hiroe, & Yotaro, Citation2008). Appropriate diets that include fruits, vegetables, whole grains, and pseudocereals may contribute to good health due to the rich source of anti-oxidants. Among these foods, cereals and pseudocereals play an important role (Calderelli et al., Citation2016; Shela et al., Citation2008).
Phenolic acids are the most important anti-oxidant agents and in quinoa have reported as 251.5 μg g−1, ferulic acid, 1.1 μg g−1, p-coumaric acid and 6.31 μg g−1 caffeic acids on a dry basis. Kotásková et al. (Citation2016) reported the different poly phenols in teff as ferulic acid is the major phenolic compound in teff and reported 160.0 μg g−1 as the free and 290.0 μg g−1 as the bounded form in the brown teff from USA. In the case of the total ferulic acid (bounded+ free), teff had shown high concentration than quinoa but there is a clear variability in the concentrations of this phenolic acid depending on the variety and origin of the teff. Some other phenolic compounds such as protocatechuic (25.5 μg g−1), Gentisic (15 μg g−1), vanillic (54.8 μg g−1), syringic (14.9 μg g−1), coumaric (36.9 μg g−1), and cinnamic (46 μg g−1) acids are also present in teff in considerable amounts (Blandino, Al-Aseeri, Pandiella, Cantero, & Webb, Citation2003). In the case of the quinoa, the major sources of phenolic acids are vanillic (523.92 μgg−1), coumaric (275 μg g−1), 3, 4-Dihydroxybenzoic acid (275 μg g−1), p-hydroxybenzoic acid (97 μg g−1), galic acid (320 μgg−1), and caffic acid (6.31 μgg−1). The quantitative and qualitative difference in the compositions of the phenolic acids between teff and quinoa was reported (Tang & Rong, Citation2017).
In the case of the phenolic content, Yawadio et al. (Citation2008) reported that quinoa sample from Japan had the highest phenolic content (148.0 mg g−1) of equivalent tannic acid, while the quinoa sample from Bolivia presented the lowest content (94.3 mg g−1). In the case of teff (15 mg g−1), which is much lower than the quinoa, there is still a possibility of the effect of varieties and agronomical conditions. These types of the studies are not yet studied to determine the effect of the variety and agronomical conditions on the total phenolic content (Hurrell & Ines, Citation2010). Red sorghum has the highest content of total polyphenols of flour (16.07 mg g−1), followed by barley (3.10 mg g−1), wheat (1.43 mg g−1), and white sorghum (8.1 mg g−1). Among the all the quinoa is the superior in the antioxidant activity.
7. Anti-nutritional properties of quinoa and teff
Anti-nutritional factors are the compounds present in a wide variety of plant foods. Anti-nutritional factors in food reduce nutritional value of food, interfering with digestibility, and absorption of nutrients. Anti-nutritional factors in quinoa reported are saponins, phytic acid, tannins, nitrates, oxalates, and trypsin inhibitors. These substances are present in higher concentrations in the outer layers of the grain. However, in th case of the teff only tannic and phytic acids are mostly reported. The quinoa grain has a natural bitter coating called saponin, soluble in methanol or water and has toxic properties (hemolysis). Some saponins form complexes with iron and zinc reducing their absorption (González et al., Citation1989; Jancurová, Lucia, & Alexander, Citation2009; Ruales & Baboo, Citation1993). The saponin content of 0.2–0.4 g kg−1 of dry matter in sweet genotypes and from 4.7 to 11.3 g kg−1 in bitter genotypes was reported. The utilization of quinoa limited due to the presence of saponins (which provides the bitter taste), to increase the sensory acceptability of quinoa many researchers are reported different methods to remove them. To overcome this saponins, sweet varieties were developed and for bitter varieties are reported to process by wet methods (strong washing in cold alkaline water), dry methods (heat treatment, extrusion, roasting, or mechanical abrasion) or a combination of both methods (Brady, Chi, Robert, Shengmin, & Mukund, Citation2007; Comai et al., Citation2007; Jancurová et al., Citation2009). Data on the Saponins in the teff were not available.
Phytic acid is capable of chelating bivalent minerals (Ca, Fe, Mg, Zn, and Cu), starch, protein and enzymes. It is mainly found in the peel of most cereals and legumes, in concentrations of 1%–3% dry matter, and also can be found in some fruits and vegetables (Jancurová et al., Citation2009; Ruales & Baboo, Citation1993). Values from 10.5 to 13.1 mg/g of phytic acid from five different varieties of quinoa were reported by Koziol (Citation1992), and values close to those were found in barley grains (9.7 to 11.6 mg g−1), corn (from 8.9 to 9.9 mg g-1), rice (8.9 mg g−1) and wheat (6.2 to 13.5 mg g−1). In case of teff 0.295 mg g−1, of phytate reported and the concentrations are far most less than that of the all the cereals (Mezgebo, Tefera, & Neela, Citation2018). The process of removing saponins from quinoa seeds by wet or dry methods and the food preparation methods like steeping, germination and fermentation were reported to reduce the phytic acid content in the grains. Similarly, the fermentation process (part of injera preparation) will reduce the phytic acid in teff (Fischer, Ines, Isabelle, Richard, & Leo, Citation2014). The degradation efficiency is higher in processes that promote the activation of phytase, such as fermentation and cooking (Khattab, Arntfield, & Nyachoti, Citation2009; Ruales & Baboo, Citation1993). In the case of tannin, cereals are the best source for it and 0.111 mg g−1was reported in the teff but data are not available for the quinoa.
The presence of trypsin inhibitor in the intestinal tract reduces the action of trypsin, which is responsible for the digestion of proteins, leading to increased enzyme production by the pancreas with resultant hypertrophy of this organ and reduction in growth. In human nutrition, such anti-nutritional factors have little consequence because they are thermo-labile and are usually destroyed in the normal conditions of domestic or industrial food preparation (Khattab et al., Citation2009). This fact demonstrates that inactivation of this anti-nutrient in quinoa can be obtained by techniques generally employed in domestic food preparation (Borges, Renata, Cláudia, Ludmilla, & Márcia, Citation2010). The concentration of protease inhibitors in quinoa seeds is <50 ppm, but these trypsin inhibiters are not reported in the teff.
Oxalate is often found in vegetables such as spinach, beets, chard, rhubarb, tomatoes, nuts and cocoa. High intake of oxalate in the diet influences the absorption of minerals and trace elements, playing a key role in hyperoxaluria, a risk factor for the formation of calcium oxalate stones in the kidneys, due to the ability of the oxalate to form insoluble complexes with divalent cations in the gastrointestinal tract (Jancurová et al., Citation2009). The highest oxalate content was found in leaves and stems of quinoa in leaves and stems in roots (258–1029 mg100g−1) and seeds (143–232 mg100g−1) (Jancurová et al., Citation2009). But oxalate-related data are not readily available for teff.
8. Value added products from quinoa and teff
Teff and quinoa both are the staple food crops in the production region. In the case of the teff, it is used to prepare the fermented flat pan bread called as injera. Injera is a staple in Ethiopia and famous dish in the different parts of the world. Teff is an ingredient in the different alcoholic beverages products. In the case of quinoa, the seed is used as the basic staple in the adult and children food. Along with the traditional usages, both crops are now using for the production of the different value-added products, as shown inTables and .
Table 2. Different types of the products developed from the Quinoa
9. Conclusions
Quinoa and teff are sharing some structural, composition properties and some differences same also reported. In the case of the basic nutritional compositions, these two are superior to the basic cereals like rice and maize. Researchers already identified that teff consumption is positively increasing the iron content and avoiding the Anemia in consumers. In the case of anti-oxidant studies, the quinoa is reported to have better properties than teff. Still there is a lot research need to be done in the case of the anti-nutritional and anti-oxidant properties of the teff. In the case of the food application of the both crops, it was evident from the literature that these both crops are using in baked products specially breads, pasta etc.. Already research studied the quinoa role in edible films and coatings but still this type of advanced research work is limited on teff. In conclusion, both the crops have the health beneficial components and can be used to different products preparations.
Competing Interests
The authors declare no competing interests.
Acknowledgements
The authors are highly thankful to the Faculty of Chemical and Food Engineering, Institute of Technology, Bahir Dar University, Bahir Dar, Ethiopia, for encouragement and support to finish this review paper successfully.
Additional information
Funding
Notes on contributors
Neela Satheesh
Dr. Neela Satheesh is an associate professor in Faculty of Chemical and Food Engineering, Postharvest Technology Department, Institute of Technology, Bahir Dar University, Ethiopia. He obtained his PhD degree from JNTU (Jawaharlal Nehru Technological University) Anatapur, India. His research areas include Postharvest Management, Technology, Food Product Development and Food Quality and Safety, Processing and Handling of perishables and durables.
Solomon Workneh Fanta
Dr. ir. Solomon Workneh Fanta is an assistant professor in faculty of Chemical and Food Engineering, Postharvest Technology Department, Institute of Technology, Bahir Dar University, Ethiopia. He obtained his PhD degree from KU Leuven, Belgium, working in the area of Postharvest Technology, Development and modeling of Thermal and non-thermal storage structures for perishables and durables, Transportation phenomenon, Food Quality and Safety.
References
- Abebe, Y., Alemtsehay, B., Michael, H., Barbara, K., Karl, J. S., & Rosalind, B. (2007). Phytate, Zinc, Iron and Calcium content of selected raw and prepared foods consumed in rural sidama, southern Ethiopia, and implications for bioavailability. Journal of Food Composition and Analysis, 20, 161–168.
- Abeywardena, M. Y., Mc Lennan, P. L., & Charnock, J. S. (1991). Differential effects of dietary fish oil on Myocardial Prostaglandin I2 and Thromboxane A2 Production. American Journal of Physiology, 260, 379–385. doi:10.1152/ajpheart.1991.260.2.H379
- Abugoch, J., & Lilian, E. B. T. (2009). Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Advances in Food and Nutrition Research, 58, 1–31. doi:10.1016/S1043-4526(09)58001-1
- Abugoch, L., Cristián, T., Maria, C. V., Mehrdad, Y. P., & Díaz-Dosque, M. (2011). Characterization of quinoa protein-chitosan blend edible films. Food Hydrocolloids, 25, 879–886. doi:10.1016/j.foodhyd.2010.08.008
- Abugoch, L. E., Nalda, R., Cristian, A. T., Jorge, S., & Monica, R. (2008). Study of some physicochemical and functional properties of Quinoa (Chenopodium quinoa Willd) protein isolates. Journal of Agricultural and Food Chemistry, 56, 4745–4750. doi:10.1021/jf703689u
- Adams, M. R. (1990). Topical aspects of fermented foods. Trends in Food Science and Technology, 1, 140–144. doi:10.1016/0924-2244(90)90111-B
- Adebowale, A., Abdul, R. A., Naushad, M. E., Mervyn, B., & John, R. N. T. (2011). Fractionation and characterization of teff proteins. Journal of Cereal Science, 54, 380–386. doi:10.1016/j.jcs.2011.08.002
- Alaunyte, I., Valentina, S., Andrew, P., Paul, A., & Emma, D. (2012). Improving the quality of nutrient-rich teff (Eragrostis tef) breads by combination of enzymes in straight dough and sourdough breadmaking. Journal of Cereal Science, 55, 22–30. doi:10.1016/j.jcs.2011.09.005
- Alvarez-Jubete, L, Arendt, E. K, & Gallagher, E. (2010a). Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends in Food Science And Technology, 21, 106–113. doi: 10.1016/j.tifs.2009.10.014
- Alvarez-Jubete, L., Mark, A., Elke, K. A., & Eimear, G. (2009). Baking Properties and Microstructure of pseudocereal flours in gluten-free bread formulations. European Food Research and Technology, 230, 437–445. doi:10.1007/s00217-009-1184-z
- Alvarez-Jubete, L, Wijngaard, H, Arendt, E. K, & Gallagher, E. (2010b). Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chemistry, 119, 770–78. doi: 10.1016/j.foodchem.2009.07.032
- Araujo-Farro, P. C., Podadera, G., Paulo, J. A. S., & Florencia, C. M. (2010). Development of films based on quinoa (Chenopodium quinoa Willd) Starch. Carbohydrate Polymers, 81, 839–848. doi:10.1016/j.carbpol.2010.03.051
- Araya, A., Keesstra, S. D., & Stroosnijder, L. (2010). Simulating yield response to water of teff (Eragrostis tef) with FAO’s aquacrop model. Field Crops Research, 116, 196–204. doi:10.1016/j.fcr.2009.12.010
- Arendt, E., & Emanuele, Z. (2013). Cereal grains for the food and beverage industries (1st ed.). London: wood house publications. doi:10.1533/9780857098924.409
- Asfaw, Z., & Gatahun, A. (2000). Biology and human welfare: Domestication of plants by humans. In S. Demissew & S. Belayneh (Eds.), Biology (pp. 229–246). Addis Ababa, Ethiopia: Mega publishing.
- Ashenafi, M. (2006). A review on the microbiology of indigenous fermented foods and beverages of ethiopia. Ethiopian Journal of Biological Sciences, 5, 189–245. doi:10.4314/ejbs.v5i2.39036
- Atwell, W. A., Patrick, B. M., Johnson, L. A., & Glass, R. W. (1983). Charecterization of quinoa starch. Cereal Chemistry, 60, 9–11.
- Aune, D., Na, K., Edward, G., Lars, T. F., Paolo, B., Darren, C. G., … Teresa, N. (2016). Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. The Biomedical Journal, 353, 1–14. doi:10.1136/bmj.i2716
- Axel, C., Brid, B., Emanuele, Z., Ambrose, F., Aidan, C., & Elke, K. A. (2016). Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. International Journal of Food Microbiology, 239, 86–94. doi:10.1016/j.ijfoodmicro.2016.05.006
- Ayyash, M., Stuart, K. J., Shao-Quan, L., Aysha, A. M., & Aisha, A. (2018). Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented Lupin, Quinoa and wheat by bifidobacterium species: In-vitro investigations. LWT - Food Science and Technology, 95, 295–302. doi:10.1016/j.lwt.2018.04.099
- Babatunde Obilana, A., & Manyasa. (2002). Millets. In P. S. Belton & J. R. Taylor (Eds.), Pseudo-cereals and less common cereals (pp. 281–304). Berlin: Springer-Verlag.
- Baye, K. (2014). Synopsis : Teff : Nutrient composition and health benefits. Working paper. International Food Policy Research Inistitute.
- Baye, K., Claire, M. R., Christèle, I. V., Isabelle, R., & Jean, P. G. (2013). Influence of flour blend composition on fermentation kinetics and phytate hydrolysis of sourdough used to make injera. Food Chemistry, 138, 430–436. doi:10.1016/j.foodchem.2012.10.075
- Bean, S. R., & Lookhart, G. L. (2000). Electrophoresis of cereal storage proteins. Journal of Chromatography A, 881, 23–36. doi:10.1016/S0021-9673(99)01270-4
- Bekele, E., Roger, J. F., Arthur, S. T., & Peter, R. S. (1995). Heterogeneity and polymorphism of seed proteins in Tef (Eragrotis Tef). Hereditas, 122, 67–72. doi:10.1111/j.1601-5223.1995.00067.x
- Bhargava, A., Sudhir, S., & Deepak, O. (2006). Chenopodium quinoa — An India perspective. Industrial Crops and Products, 23, 73–87. doi:10.1016/j.indcrop.2005.04.002
- Blandino, A., Al-Aseeri, M. E., Pandiella, S. S., Cantero, D., & Webb, C. (2003). Cereal-based fermented foods and beverages. Food Research International, 36, 527–543. doi:10.1016/S0963-9969(03)00009-7
- Borges, J. T., Renata, C. B., Cláudia, D. P., Ludmilla, C. O., & Márcia, C. C. (2010). Características Físico-Químicas, Nutricionais e Formas de Consumo Da Quinoa (Chenopodium quinoa Willd.). Temas Agrarios, 15, 9–23. doi:10.21897/rta.v15i1.815
- Brady, K., Chi, T. H., Robert, T. R., Shengmin, S., & Mukund, V. K. (2007). Effects of processing on the nutraceutical profile of Quinoa. Food Chemistry, 100, 1209–1216. doi:10.1016/j.foodchem.2005.12.001
- Bultosa, G., & Taylor, J. R. N. (2004a). Teff. In C. Wrigley, H. Corke, & C. Walker (Eds.), Encyclopedia of Grain Science (pp. 20–36). Oxford, London: Elsevier.
- Bultosa, G. (2007). Physicochemical characteristics of grain and flour in 13 Tef [Eragrostis tef (Zucc .) Trotter] Grain Varieties. Journal of Applied Sciences Research, 3, 2042–2050.
- Bultosa, G., & John, R. N. T. (2004b). Paste and gel properties and in vitro digestibility of tef [Eragrostis tef (Zucc). Trotter]. Starch, 56, 20–28. doi:10.1002/star.200200191
- Burešová, I., Marián, T., Ján, M., Luděk, H., Oldřich, F., & Viera, Š. (2017). The comparison of the effect of added Amaranth, buckwheat, chickpea, corn, millet and quinoa flour on rice dough rheological characteristics, textural and sensory quality of bread. Journal of Cereal Science, 75, 158–164. doi:10.1016/j.jcs.2017.04.004
- Burešová, I., Stanislav, K., Petra, D., & Tomáš, S. (2014). The relationship between rheological characteristics of gluten-free dough and the quality of biologically leavened bread. Journal of Cereal Science, 60, 271–275. doi:10.1016/j.jcs.2014.07.001
- Calderelli, V. A. S., de Marta, T. B., Jesuí, V. V., & Graciette, M. (2010). Quinoa and Flaxseed: Potential ingredients in the production of bread with functional quality. Brazilian Archives of Biology and Technology, 53, 981–986. doi:10.1590/S1516-89132010000400029
- Calderelli, V. A. S., de Marta, T. B., Jesuí, V. V., Graciette, M., Antonio, M. M. F., Carlo, G. R., & Anna, L. (2016). Use of sourdough made with Quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Journal of Cereal Science, 37, 1–13. doi:10.1016/j.fm.2015.11.018
- Callejo, M. J., Benavente, E., Ezpeleta, J. I., Laguna, M. J., Carrillo, J. M., & Rodríguez-Quijano, M. (2016). Influence of teff variety and wheat flour strength on breadmaking properties of healthier teff-based breads. Journal of Cereal Science, 68(1), 38–45. doi:10.1016/j.jcs.2015.11.005
- Campo, E., Lis Del, A., Leyre, U., Rosa, O., & Ana Ferrer, M. (2016). Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with Teff Flour. Journal of Cereal Science, 67, 75–82. doi:10.1016/j.jcs.2015.09.010
- Caperuto, L., Amaya-Farfan, J., & Camargo, C. R. O. (2000). Performance of Quinoa (Chenopodium quinoa Willd) flour in the manufacture of gluten free spaghetti. Journal of Science of Food and Agriculture, 81, 95–101. doi:10.1002/1097-0010(20010101)81
- Chandna, M., & Narender, K. M. (1990). Characterization of pearl millet protein fractions. Phytochemistry, 29, 3395–3399. doi:10.1016/0031-9422(90)85245-B
- Chillo, S., Civica, V., Iannetti, M., Suriano, N., Mastromatteo, M., & Del Nobile, M. A. (2009). Properties of Quinoa and Oat spaghetti loaded with carboxymethylcellulose sodium salt and pregelatinized starch as structuring agents. Carbohydrate Polymers, 78, 932–937. doi:10.1016/j.carbpol.2009.07.013
- Chrysohoou, C., & Christodoulos, S. (2013). Longevity and diet. Myth or pragmatism? Maturitas, 76, 303–307. doi:10.1016/j.maturitas.2013.09.014
- Coleman, J., Abaye, A. O., William, B., & Wade, T. (2013). The suitability of teff flour in bread, layer cakes, cookies and biscuits. International Journal of Food Sciences and Nutrition, 64, 877–881. doi:10.3109/09637486.2013.800845
- Collar, C. (2016). Impact of visco-metric profile of composite dough matrices on starch digestibility and firming and retrogradation kinetics of breads thereof: Additive and interactive effects of non-Wheat flours. Journal of Cereal Science, 69, 32–39. doi:10.1016/j.jcs.2016.02.006
- Collar, C., Teresa, J., & Antonio, P. C. (2015). Significance of thermal transitions on starch digestibility and firming kinetics of restricted water mixed flour bread matrices. Carbohydrate Polymers, 122, 169–179. doi:10.1016/j.carbpol.2014.12.083
- Comai, S., Antonella, B., Lucia, B., Mirella, Z., Carlo, V. L. C., & Graziella, A. (2007). The content of proteic and nonproteic (Free and Protein-Bound) tryptophan in Quinoa and cereal flours. Food Chemistry, 100, 1350–1355. doi:10.1016/j.foodchem.2005.10.072
- Coulter, L., & Lorenz, K. (1991). Extruded corn grits-quinoa blends: I proximate compositio, nutritional properties and sensory evaluation. Journal of Food Processing and Preservation, 15, 231–242. doi:10.1111/j.1745-4549.1991.tb00169.x
- Curtis, K. R., Entsminger, J. S., Cowee, M. W., Davison, J., & Harris, T. R. (2008). Market potential for nevada teff products. Technical Report: UCED 2008/ 09-02. University Center for Economic Development, University of Nevada, Reno, NV.
- Cusack, D. (1984). Quinoa: Grain of the Incas. Ecologist, 14, 21–31.
- D’ambrosio, T., Maria, L. A., Donato, P., Giuditta, D. S., & Giancarlo, C. (2017). Chemical, physical and sensorial characterization of fresh Quinoa Sprouts (Chenopodium quinoa Willd.) and effects of modified atmosphere packaging on quality during cold storage. Food Packaging and Shelf Life, 14, 52–58. doi:10.1016/j.fpsl.2017.08.003
- D’Silva, T. V., John, R. N. T., & Emmambux, M. N. (2011). Enhancement of the pasting properties of Teff and maize starches through wet-heat processing with added stearic acid. Journal of Cereal Science, 53, 192–197. doi:10.1016/j.jcs.2010.12.002
- De Simone, F., Dini, A., Pizza, C., Saturnino, P., & Schettino, O. (1990). Two flavonol glycosides from Chenopodium quinoa. Phytochemistry, 29, 3690–3692. doi:10.1016/0031-9422(90)85310-C
- Dini, I., Gian, C. T., & Antonio, D. (2004). Phenolic constituents of kancolla seeds. Food Chemistry, 84, 163–168. doi:10.1016/S0308-8146(03)00185-7
- Dogan, H., & Karwe, M. V. (2003). physicochemical properties of quinoa extrdates. Food Science Technology International, 9, 101–114. doi:10.1177/108201303033940
- Eberhardt, M. V., Lee, C. Y., & Liu, R. H. (2000). Antioxidant activity of fresh Apples. Nature, 405, 903–914. doi:10.1038/35016151
- El-Alfy, T. S., Shahira, M. E., Amani, A. S. (2012). Chemical and biological study of the seeds of Eragrostis Tef (Zucc.) Trotter. Natural Product Research, 26, 619–629.doi. 10.1080/14786419.2010.538924.
- Elgeti, D., Sebastian, D. N., Maike, F., Marina, B., Martin, H. L., Volker, H., … Thomas, B. (2014). Volume and texture improvement of gluten-free bread using quinoa white flour. Journal of Cereal Science, 59, 41–47. doi:10.1016/j.jcs.2013.10.010
- Esther, I. P., Vicente, M., & Monika, H. (2015). Bread with whole Quinoa flour and bifidobacterial phytases increases dietary mineral intake and bioavailability. LWT - Food Science and Technology, 21, 1398–1402. doi:10.1016/j.jiec.2014.06.013
- FAO. (2011). Quinoa: An ancient crop to contribute to world food security. Roam: Regional Office for Latin America and the Caribbean, Food and Agriculture Organization.
- Fardet, A. (2010). New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutrition Research Reviews, 23, 65–134. doi:10.1017/S0954422410000041
- Fardet, A., Fanny, L., Delphine, L., Augustin, S., & Christian, R. (2006). Parameters controlling the glycaemic response to breads. Nutrition Research Reviews, 19, 18–25. doi:10.1079/NRR2006118
- Fikreyesus, S., Zerihun, K., Amsalu, N., Nardos, Z., & Seife, B. (2011). protein rich extruded products from tef, corn and soy protein isolate blends. Ethiopian Journal of Applied Science and Technology, 2, 75–90.
- Fischer, M. M., Ines, M. E., Isabelle, A., Richard, F. H., & Leo, M. (2014). Phytic acid degrading lactic acid bacteria in Tef-Injera fermentation. International Journal of Food Microbiology, 190, 54–60. doi:10.1016/j.ijfoodmicro.2014.08.018
- Forsido, S. F., Vasantha, H. P. R., & Tess, A. (2013). Antioxidant capacity, total phenolics and nutritional content in selected ethiopian staple food ingredients. International Journal of Food Sciences and Nutrition, 64(915–920). doi:10.3109/09637486.2013.806448
- Föste, M., Mario, J., & Thomas, B. (2017). Structure stabilization in starch-quinoa bran doughs: The role of water availability and gelatinization. Carbohydrate Polymers, 174, 1018–1025. doi:10.1016/j.carbpol.2017.06.068
- Gebremariam, M. M., Ahmed, H., Martin, Z., & Thomas, B. (2015). investigation of fermentation conditions for Teff (Eragrostis tef) malt-wort by Lactobacillus amylolyticus. LWT - Food Science and Technology, 61, 164–171. doi:10.1016/j.lwt.2014.11.008
- Gebremariam, M. M., Kebede, A., Martin, Z., & Thomas, B. (2015). Studies on the mashing conditions of Teff (Eragrostis Tef) malt as a raw material for lactic acid-fermented gluten-free beverage. International Journal of Food Science and Technology, 50, 2032–2037. doi:10.1111/ijfs.12854
- Gebremariam, M. M., Martin, Z., & Thomas, B. (2012). Teff (Eragrostis tef) as a raw material for malting, brewing and manufacturing of gluten-free foods and beverages: A review. Journal of Food Science and Technology, 51, 2881–2895. doi:10.1007/s13197-012-0745-5
- Gebremariam, M. M., Martin, Z., & Thomas, B. (2013a). Effect of Teff (Eragrostis tef) variety and storage on malt quality attributes. Journal of the Institute of Brewing, 119, 64–70. doi:10.1002/jib.65
- Gebremariam, M. M., Martin, Z., & Thomas, B. (2013b). Thermal stability of starch degrading enzymes of Teff (Eragrostis tef) malt during isothermal mashing. Process Biochemistry, 48, 1928–1932. doi:10.1016/j.procbio.2013.08.019
- Geng, Z., Alisa, G., Frank, B. H., & Qi, S. (2016). Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis of prospective cohort studies. Circulation, 133, 2370–2380. doi:10.1016/j.coviro.2015.09.001.Human
- Geremew, B., Alan, N. H., & John, R. N. T. (2002). Physico-chemical characterization of grain tef [Eragrostis tef (Zucc.) Trotter] starch. Starch, 54, 461–468. doi:10.1002/1521-379X(200210)54:10<461::AID-STAR461>3.0.CO;2-U
- Ghebrehiwot, H. M., Hussein, A. S., Kevin, P. K., Mark, D. L., & Tafadzwanashe, M. (2016). Nutritional and sensory evaluation of injera prepared from Tef and Eragrostis Curvula (Schrad.) Nees. flours with sorghum blends. Frontiers in Plant Science, 7, 1–8. doi:10.3389/fpls.2016.01059
- Giménez, M. A., Drago, S. R., Bassett, M. N., Lobo, M. O., & Sammán, N. C. (2016). nutritional improvement of corn pasta-like product with broad bean (Vicia faba) and Quinoa (Chenopodium quinoa). Food Chemistry, 199, 150–156. doi:10.1016/j.foodchem.2015.11.065
- Giuberti, G., Antonio, G., Lucia, F., Paola, F., & Francesco, M. (2016). In vitro starch digestibility and quality attributes of gluten free ‘tagliatelle’ prepared with teff flour and increasing levels of a new developed bean cultivar. Starch, 68, 374–378. doi:10.1002/star.201500007
- González, J. A., Roldán, A., Gallardo, M., Escudero, T., & Prado, F. E. (1989). Quantitative determinations of chemical compounds with nutritional value from inca crops:Chenopodium quinoa (Quinoa). Plant Foods for Human Nutrition, 39, 331–337. doi:10.1007/BF01092070
- Goodyer, P. (2015). Is teff the new quinoa? the Sydney morning herald. Retrieved March 21, 2018 from SMH website: https://www.smh.com.au/lifestyle/health-and-wellness/is-teff-the-new-quinoa-20150620-ght7ho.html
- Griffith, L. D., Castell-Perez, M. E., & Griffith, M. E. (1998). Effects of blend and processing method on the nutritional quality of weaning foods made from select cereals and legumes. Cereal Chemistry, 75, 105–112. doi:10.1094/CCHEM.1998.75.1.105
- Gross, R., Koch, F., Malaga, I., de Miranda, A. F., Schoeneberger, H., & Trugo, L. C. (1989). chemical composition and protein quality of some local andean food sources. Food Chemistry, 34, 25–34. doi:10.1016/0308-8146(89)90030-7
- Gul, K., Singh, A. K., & Rifat, J. (2016). Nutraceuticals and functional foods: The foods for the future world. Critical Reviews in Food Science and Nutrition, 56, 2617–2627. doi:10.1080/10408398.2014.903384
- Hager, A. S., Anika, W., Fritz, J., Emanuele, Z., & Elke, K. A. (2012b). Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to Wheat flours. Journal of Cereal Science, 56, 239–247. doi:10.1016/j.jcs.2012.06.005
- Hager, A. S., & Elke, K. A. (2013). Influence of Hydroxypropylmethylcellulose (HPMC), Xanthan Gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocolloids, 32, 195–203. doi:10.1016/j.foodhyd.2012.12.021
- Hager, A. S., Fabian, L., Emanuele, Z., & Elke, K. A. (2012a). Development of gluten-free fresh egg pasta based on Oat and Teff flour. European Food Research and Technology, 235, 861–871. doi:10.1007/s00217-012-1813-9
- Hager, A. S., Michael, C., Jürgen, B., Emanuele, Z., & Elke, K. A. (2013). Starch Properties, Invitro digestibility and sensory evaluation of fresh egg pasta produced from Oat, Teff and Wheat flour. Journal of Cereal Science, 58, 156–163. doi:10.1016/j.jcs.2013.03.004
- Harris, P., & Raymond, J. G. (2009). Primer on dietary carbohydrates and utility of the glycemic index in equine nutrition. Veterinary Clinics of North America: Equine Practice, 25, 23–37. doi:10.1016/j.cveq.2009.01.006
- Hawa, A., Neela, S., & Kumela, D. (2018). Functional, physical and sensory properties of cookies prepared from okara, red teff and wheat flours. Croatian Journal of Food Sciencen and Technology, 10, 23–32. doi:10.17508/CJFST.2018.10.1.05
- Heiru, M. (2017). Effect of grain teff, Sorghum and Soybean blending ratio and processing condition on weaning food quality. Journal of Food Processing and Technology, 8, 8–12. doi:10.4172/2157-7110.1000659
- Helen, T. Z., & Mammo, M. (2014). The Significance of whole grain teff for improving nutrition: From injera to ready to eat porridge by using extrusion cooking technology. International Journal of African Development, 2, 40–56.
- Herman, E. M., & Larkins, B. A. (1999). Protein storage bodies and vacuoles. The Plant Cell, 11, 601–614. doi:10.1105/tpc.11.4.601
- Hitomi, A., Yi-Chun, C., Hanjun, T., Mayumi, S., Katsumi, W., & Oshio, M. (2002). Food components in fractions of quinoa seed. Food Science and Technology Research, 8, 80–84. doi:10.3136/fstr.8.80
- Hofmanová, T., Hrušková, M., & Švec, I. (2014). Evaluation of wheat/non-traditional flour composites. Czech Journal of Food Sciences, 32, 288–295. doi:10.17221/CJFS
- Hongyu, W., Alan, J. F., Qibin, Q., Rob, M. D., Laura, A. S., Eric, B. R., … Qi, S. (2015). Whole Grain intake and mortality: Two large prospective studies in U.S. Men and Women. JAMA International Medical Journal, 175, 373–384. doi:10.1016/j.coviro.2015.09.001.Human
- Hopman, E., Liesbeth, D, Marie, L. B, Maud, W, Walter, Z, Frits, K, & Schweizer, J. (2008). Tef in the diet of celiac patients in the Netherlands. Scandinavian Journal of Gastroenterology, 43, 277–282. doi:10.1080/00365520701714871
- Hurrell, R., & Ines, E. (2010). Iron bioavailability and dietary reference values. The American Journal of Clinical Nutrition, 91, 1461S–1467S. doi:10.3945/ajcn.2010.28674F.Am
- Jacobsen, S. E., Mujica, A., & Ortiz, R. (2003). The global potential for Quinoa and other andean crops. Food Reviews International, 19, 139–148. doi:10.1081/FRI-120018880
- Jancurová, M., Lucia, M., & Alexander, D. (2009). Quinoa – A review. Czech Journal of Food Sciencen, 27, 71–79. doi:10.17221/32/2008-CJFS
- Jansen, G. R., Di Maio, L. R., & Hause, N. L. (1962). Cereal proteins, amino acid composition and lysine supplementation of Teff. Journal of Agricultural and Food Chemistry, 10, 62–64. doi:10.1021/jf60119a021
- Jensen, C. R., Jacobsen, S. E., Andersen, M. N., Núñez, N., Andersen, S. D., Rasmussen, L., & Mogensen, V. O. (2000). Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. European Journal of Agronomy, 13, 11–25. doi:10.1016/S1161-0301(00)00055-1
- Kahlon, T. S., Roberto, J. A., & Mei, C. M. C. (2016). Sensory evaluation of gluten-free quinoa whole grain snacks. Heliyon, 2, 1–12. doi:10.1016/j.heliyon.2016.e00213
- Karyotis, T. C. I., Noulas, C., & Mitsibonas, T. (2003). Preliminary research on seed production and nutrient content for certain quinoa varieties in a saline-sodic soil. Journal of Agronomy and Crop Science, 189(402–8). doi:10.1046/j.0931-2250.2003.00063.x
- Keys, A., & Willis, P. R. (1966). Serum cholesterol response to changes in dietary lipids. The American Journal of Clinical Nutrition, 19, 175–181. doi:10.1093/ajcn/19.3.175
- Khattab, R. Y., Arntfield, S. D., & Nyachoti, C. M. (2009). Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT - Food Science and Technology, 42, 1107–1112. doi:10.1016/j.lwt.2009.02.008
- Koletzko, B., Susan, B., Geoff, C., Ulysses, F. N., Sarath, G., Olle, H., & Quak, S. H. 2005. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. Journal of Pediatric Gastroenterology and Nutrition, 41,584–599. PMID:16254515. doi: 10.1097/01.mpg.0000187817.38836.42
- Konishi, Y., Shigeru, H., Hideki, T., & Masao, W. (2004). Distribution of minerals in Quinoa (Chenopodium quinoa Willd.) seeds. Bioscience, Biotechnology, and Biochemistry, 68, 231–234. doi:10.1271/bbb.68.231
- Kotásková, E, Sumczynski, D, Mlček, J, & Valášek, P. (2016). Determination of free and bound phenolics using hplc-dad, antioxidant activity and in vitro digestibility of eragrostis tef. Journal of Food Composition and Analysis, 46, 15-21. doi: 10.1016/j.jfca.2015.11.001
- Kowalski, R. J., Medina-Meza, I. G., Thapa, B. B., Murphy, K. M., & Ganjyal, G. M. (2016). Extrusion processing characteristics of Quinoa (Chenopodium quinoa Willd.) Var. cherry vanilla. Journal of Cereal Science, 70, 91–98. doi:10.1016/j.jcs.2016.05.024
- Koziol, M. J. (1992). Chemical composition and nutritional evaluation of Quinoa (Chenopodium quinoa Willd.). Journal of Food Composition and Analysis, 5, 35–68. doi:10.1016/0889-1575(92)90006-6
- Kupper, C. (2005). Dietary guidelines and implementation for celiac disease. Gastroenterology, 128, S121–127.
- Lamacchia, C., Chillo, S., Lamparelli, S., Suriano, N., La Notte, E., & Del Nobile, M. A. (2010). Amaranth, Quinoa and Oat doughs: Mechanical and rheological behaviour, polymeric protein size distribution and extractability. Journal of Food Engineering, 96, 97–106. doi:10.1016/j.jfoodeng.2009.07.001
- Lorenz, K., & Coulter, L. (1991). Quinoa flour in baked products. Plant Foods for Human Nutrition, 41, 213–223. doi:10.1007/BF02196389
- Lorenz, K., Coulter, L., & Johnson, D. (1995). Functional and sensory characteristics of quinoa in foods. Developments in Food Science, 37, 1031–1041. doi:10.1016/S0167-4501(06)80216-5
- Lorusso, A., Michela, V., Marco, M., Rossana, C., Marco, G., & Carlo, G. R. (2017). Use of fermented Quinoa flour for pasta making and evaluation of the technological and nutritional features. LWT - Food Science and Technology, 78, 215–221. doi:10.1016/j.lwt.2016.12.046
- Lovejoy, J. C. (1999). Dietary fatty acids and insulin resistance. Current Atherosclerosis Reports, 1, 215–220. doi:10.1007/s11883-999-0035-5
- Machado, A., Natália, M., Caroline, J. S., Izabela, D. A., Elisa, C. M., & Helena, M. A. B. (2015). Addition of quinoa and amaranth flour in gluten-free breads: Temporal profile and instrumental analysis. LWT - Food Science and Technology, 62, 1011–1018. doi:10.1016/j.lwt.2015.02.029
- Manuel, G. C., Mancebo Picón, M. J., & Javier Gómez, P. (2015). Effect of flour properties on the quality characteristics of gluten free sugar-snap cookies. LWT - Food Science and Technology, 64, 264–269. doi:10.1016/j.lwt.2015.05.057
- Martin, J., Ramos, D., Jussi-Petteri, S., Kevin, C. D., & Ritva, S. (2015). Physical and sensory characteristics of corn-based extruded snacks kiwa flour containing amaranth, quinoa and kaniwa flour. LWT - Food Science and Technology, 64, 1047–1056. doi:10.1016/j.lwt.2015.07.011
- Mathers, C. D., & Dejan, L. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3, 2011–2030. doi:10.1371/journal.pmed.0030442
- Mengesha, M. H. (1966). Chemical composition of Teff (Eragrostis Tef) compared with that of wheat, barley and grain Sorghum. Economic Botany, 20, 268–273. doi:10.1007/BF02904277
- Mezgebo, K., Tefera, B., & Neela, S. (2018). Optimization of Red Teff flour, malted Soybean flour, and Papaya fruit powder blending ratios for better nutritional quality and sensory acceptability of Porridge. Food Science and Nutrition, 6, 891–903. doi:10.1002/fsn3.624
- Michaelsen, K. F., Kathryn, G. D., Ana, B. P. E., Mulia, N., Lotte, L., & Nanna, R. (2011). Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6-24 Months), and pregnant and lactating women. Maternal and Child Nutrition, 7, 124–140. doi:10.1111/j.1740-8709.2011.00302.x
- Mir, N. A., Charanjit, S. R., & Sukhcharn, S. (2018). Nutritional constituents of pseudo-cereals and their potential use in food systems: A review. Trends in Food Science and Technology, 75, 170–180. doi:10.1016/j.tifs.2018.03.016
- Mohammed, N. A., Ahmed, I. A. M., & Babiker, E. E. (2011). Nutritional evaluation of Sorghum flour (Sorghum bicolor L. Moench) during processing of Injera. World Academy of Science, Engineering and Technology, 5, 99–103.
- Molineaux, L., & Biru, M. (1965). Teff consumption, hookworm infestation, and hemoglobin levels: A preliminary report. Journal of Health, 51, 1–5.
- Moroni, A. V., Elke, K. A., & Dal, F. B. (2011). Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff Sourdoughs. Food Microbiology, 28, 497–502. doi:10.1016/j.fm.2010.10.016
- Moroni, A. V., Iametti, S., Bonomi, F., Arendt, E. K., & Dal, F. B. (2010). Solubility of proteins from non-gluten cereals: A comparative study on combinations of solubilising agents. Food Chemistry, 121, 1225–1230. doi:10.1016/j.foodchem.2010.02.009
- Myoung, P., Ibáñez, A. M., & Shoemaker, C. F. (2007). Rice starch molecular size and its relationship with amylose content. Starch, 59, 69–77. doi:10.1002/star.200600568
- National Research Council. (1996). Lost crops of Africa grains. Washington, DC: National Academy Press.
- Navruz-Varli, S., & Nevin, S. (2016). Nutritional and health benefits of quinoa (Chenopodium quinoa Willd). Journal of Cereal Science, 69, 371–376. doi:10.1016/j.jcs.2016.05.004
- Ng, S. C., Alfred, A., Janice, C., & Martin, O. (2007). Characterization of lipid oxidation products in quinoa (Chenopodium quinoa). Food Chemistry, 101, 185–192. doi:10.1016/j.foodchem.2006.01.016
- Ogungbenle, H. N. (2003). Nutritional evaluation and functional properties of quinoa (Chenopodium quinoa) Flour. International Journal of Food Sciences and Nutrition, 54, 153–158. doi:10.1080/0963748031000084106
- Oshodi, A. A., Ogungbenle, H. N., & Oladimeji, M. O. 1999. Chemical composition, nutritionally valuable minerals and functional properties of Benni seed (Sesamum radiatum), Pearl Millet (Pennisetum typhoides) and Quinoa (Chenopodium quinoa) flours. International Journal of Food Sciences and Nutrition, 50,325–331. PMID:10719563. doi: 10.1080/096374899101058
- Pagno, C. H., Tania, M. H. C., De Menezes, W., Edilson, V. B., Plinho, F. H., Carla, R. M., … Simone, H. F. (2015). Development of active biofilms of Quinoa (Chenopodium Quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chemistry, 173, 755–762. doi:10.1016/j.foodchem.2014.10.068
- Paśko, P., Henryk, B., Paweł, Z., Shela, G., Maria, F., & Zofia, Z. (2009). Anthocyanins, total polyphenols and antioxidant activity in Amaranth and Quinoa seeds and sprouts during their growth. Food Chemistry, 115, 994–998. doi:10.1016/j.foodchem.2009.01.037
- Pearsall, D. M. (1992). The origins of plant cultivation in South America. In C. W. Watson & P. J. Cowan (Eds.), The origins of agriculture (pp. 173–205). Washington, DC, USA: Smithsonian Institute Press.
- Pineli, L., Raquel, B. A., Renata, P. Z., Juliana, L. S., Guilherme, T. O., Caio, E., & Teixeira, S. (2015). Low glycemic index and increased protein content in a novel Quinoa milk. LWT - Food Science and Technology, 63, 1261–1267. doi:10.1016/j.lwt.2015.03.094
- Poutanen, K., Sozer, N., & Valle, G. D. (2014). How can technology help to deliver more of grain in cereal foods for a healthy diet? Journal of Cereal Science, 59, 327–336. doi:10.1016/j.jcs.2014.01.009
- Prego, I., Sara, M., & Marisa, O. (1998). Seed structure and localization of reserves in Chenopodium quinoa. Annals of Botany, 82, 481–488. doi:10.1006/anbo.1998.0704
- Ramos, J. M. D, Kirjoranta, S, Tenitz, S, Paavo, A. P, Serimaa, R, Lampi, A. M, & Jouppila, K. (2013). Use of Amaranth, Quinoa and Kañiwa in Extruded Corn-based Snacks, Journal of Cereal Science, 58, 59-67. doi: 10.1016/j.jcs.2013.04.003
- Ranhotra, G. S., Gelroth, J. A., Glaser, B. K., Lorenz, K. J., & Johnson, D. L. (1993). Composition and protein nutritional quality of quinoa. Cereal Chemistry, 70, 303–312.
- Regine, S., Julian, D., Veronika, O., Katerina, J., & Emmerich, B. (2007). Functional properties of gluten-free pasta produced from Amaranth, quinoa and buckwheat. Plant Foods for Human Nutrition, 65, 339–349. doi:10.1007/s11130-010-0194-0
- Renzetti, S., Fabio, D. B., & Elke, K. A. (2008). Microstructure, fundamental rheology and baking characteristics of batters and breads from different gluten-free flours treated with a microbial transglutaminase. Journal of Cereal Science, 48, 33–45. doi:10.1016/j.jcs.2007.07.011
- Repo-Carrasco, R., Clara, E., & Jacobsen, S. E. (2003). Nutritional value and use of the andean crops quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Reviews International, 19, 179–189. doi:10.1081/FRI-120018884
- Risi, C. J., & Galwey, N. W. (1984). Chenopodium Grains Of The Andes: Inca crops for modern agriculture. Advances in Applied Biology, 10, 145–216.
- Robin, F., Christinek, T., & Sathaporn, S. (2015). Properties of extruded whole grain cereals and pseudocereals flours. International Journal of Food Science and Technology, 50, 2152–2159. doi:10.1111/ijfs.12893
- Robledo, N., Paola, V., Luis, L., Mehrdad, Y. P., Cristian, T., & Lilian, A. (2018). Thymol nanoemulsions incorporated in quinoa protein/Chitosan edible films; Antifungal effect in cherry tomatoes. Food Chemistry, 246, 211–219. doi:10.1016/j.foodchem.2017.11.032
- Rockwell, P. L., Marjorie, A. K., Jennifer, S. A., Laura, J. T., & Caroline, L. S. (2014). Various-sourced pectin and polyethylene oxide electrospun fibers. Carbohydrate Polymers, 107, 110–118. doi:10.1016/j.carbpol.2014.02.026
- Ronda, F., Workineh, A., Sandra, P. Q., & Concha, C. (2015). Suitability of tef varieties in mixed wheat flour bread matrices: A physico-chemical and nutritional approach. Journal of Cereal Science, 64, 139–146. doi:10.1016/j.jcs.2015.05.009
- Ruales, J., & Baboo, M. N. (1993). Content of fat, vitamins and minerals in quinoa (Chenopodium Quinoa Willd) seeds. Food Chemistry, 48, 131–136. doi:10.1016/0308-8146(93)90047-J
- Ruales, J., & Nair, B. M. (1994). Properties of starch and dietary fibre in raw and processed quinoa (Chenopodium quinoa, Willd) Seeds. Plant Foods for Human Nutrition, 45, 223–246. doi:10.1007/BF01094092
- Ruales, J., Yolanda de, G., Patricio, L. J., & Baboo, M. N. (2002). The nutritional quality of an infant food from Quinoa and Its fffect on the plasma level of Insulin-like growth factor-1 (IGF-1) in undernourished children. International Journal of Food Sciences and Nutrition, 53, 143–154. doi:10.1080/09637480220132157
- Ryan, E., Galvin, K., O’connor, T. P., Maguire, A. R., & O’brien, N. M. (2007). Phytosterol, Squalene, Tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods for Human Nutrition, 62, 85–91. doi:10.1007/s11130-007-0046-8
- Sabrina, N. C., Bruno, M. C., & Ana Lúcia, B. P. (2014). Evaluation of the Effect of supplementing fermented milk with Quinoa flour on probiotic activity. Journal of Dairy Science, 97, 6027–6035. doi:10.3168/jds.2014-8197
- Samuel, M. (2015). Probiotic potential and nutritional importance of Teff. African Journal of Food, Agriculture, Nutritiona and Development, 15, 9964–9981.
- Schlick, G., & David, L. B. (1993). Quinoa: An emerging “New” Crop with Potential for CELSS. Moffett Field, CA: National Aeronautics and Space Administration, Ames Research Center.
- Schoenlechner, R., Jurackova, K., & Berghofer, E. (2005). Pasta production from the pseudocereals amaranth, quinoa and buckwheat. Using cereal science and technology for the benefit of consumers. Proceedings of the 12th International ICC Cereal and Bread Congress, p. 74–81. Harrogate, England, UK.
- Schumacher, A. B., Adriano, B., Tâmmila, V. K., Erna, V., De, J., & Fernanda, C. M. (2010). Chemical and sensory evaluation of dark chocolate with addition of Quinoa (Chenopodium quinoa Willd). Journal of Food Science and Technology, 47, 202–206. doi:10.1007/s13197-010-0029-x
- Sciammaro, L., Cristina, F., & Cecilia, P. (2018). Physicochemical and nutritional characterization of sweet snacks formulated with prosopis alba flour. LWT - Food Science and Technology, 93, 24–31. doi:10.1016/j.lwt.2018.03.019
- Seyfu, K. (1997). Teff. Eragrostis tef (Zucc.) Trotter, promoting the conservation and use of underutilized and neglected crops. Gatersleben, Rome: Institute of Plant Genetics and Crop Plant Research, International Plant Genetic Resources Institute.
- Shela, G., Lojek, A., Číž, M., Pawelzik, E., Delgado, L. E., Medina, O. J., … Goshev, I. (2008). Comparison of composition and antioxidant capacity of some cereals and Pseudocereals. International Journal of Food Science and Technology, 43, 629–637. doi:10.1111/j.1365-2621.2007.01498.x
- Shewry, P. R. (2009). Wheat. Journal of Experimental Botany, 60, 1537–1553. doi:10.1093/jxb/erp058
- Simopoulos, A. P. (2001). N− 3 fatty acids and human health: Defining strategies for public policy. Lipids, 36, S83–S89. PMID:11837998.
- Slavin, J. (2003). Why whole grains are protective: Biological Mechanisms. Proceedings of the Nutrition Society, 62, 129–134. doi:10.1079/PNS2002221
- Spaenij-Dekking, L., Yvonne, K. W., & Frits, K. (2005). The ethiopian cereal tef in celiac disease. The New England Journal of Medicine, 353, 1748–1749. doi:10.1056/NEJMc051492
- Starzyńska, J. A., Robert, D., Bożena, S., Barbara, M., & Agnieszka, W. (2016). Prolonged tempe-type fermentation in order to improve bioactive potential and nutritional parameters of Quinoa Seeds. Journal of Cereal Science, 71, 116–121. doi:10.1016/j.jcs.2016.08.001
- Stikic, R., Djordje, G., Mirjana, D., Biljana, V. R., Zorica, J., Dusanka, M. O., … Mirjana, M. (2012). Agronomical and nutritional ealuation of Quinoa Seeds (Chenopodium quinoa Willd.) as an ingredient in Bread Formulations. Journal of Cereal Science, 55, 132–138. doi:10.1016/j.jcs.2011.10.010
- Tadesse, E. (1969). Teff (Eragrostis tef): The cultivation, usage and some of the known diseases and insect pests, part I. Debre Zeit agricultural experiment station Bulletin No. 60. Dire Dawa, Ethiopia: Alemaya University of Agriculture.
- Tang, H., Katsumi, W., & Toshio, M. (2002). Characterization of storage starches from quinoa, barley and adzuki seeds. Carbohydrate Polymers, 49, 13–22. doi:10.1016/S0144-8617(01)00292-2
- Tang, Y., & Rong, T. (2017). Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial ofEffects: A Review. Molecular Nutrition and Food Research, 61, 1–16. doi:10.1002/mnfr.201600767
- Tari, T., . A., Uday, S. A., Rekha, S. S., & Pushpa, R. K. (2003). Starch-based spherical aggregates: Screening of small granule sized starches for entrapment of a model flavouring compound, Vanillin. Carbohydrate Polymers, 53, 45–51. doi:10.1016/S0144-8617(02)00293-X
- Tatham, A. S., Fido, R. J., Moore, C. M., Kasarda, D. D., Kuzmicky, D. D., Keen, J. N., & Shewry, P. R. (1996). Characterisation of the major prolamins of Tef (Eragrostis tef) and finger millet (Eleusine coracana). Journal of Cereal Science, 24, 65–71. doi:10.1006/jcrs.1996.0038
- Taylor, J., John, R. N. T., Michael, F. D., & de Suretha, K. (2005). Glacial acetic acid- A novel food-compatible solvent for kafirin extraction. Cereal Chemistry, 82, 485–487. doi:10.1094/CC-82-0485
- Tefera, H., Ayele, M., & Assefa, K. (1995). Improved varieties of tef (Eragrostis tef) in Ethiopia, releases of 1970–1995. Researches bulletin No 1. Debre Zeit, Ethiopia: Debre Zeit Agricultural Research Center, Alemaya University of Agriculture.
- Teklehaimanot, W. H., Kwaku, G. D., & Mohammed, N. E. (2013). Maize and teff starches modified with stearic acid as potential fat replacer in low calorie mayonnaise-type emulsions. Starch, 65, 773–781. doi:10.1002/star.201200244
- Temesgen, M. (2013). Effect of ambient temperature on the performance of a combined cycle power plant. Open Acess Scientific Reports, 2, 1–9. doi:10.4172/scientificreports
- Tukomane, T., & Saiyavit, V. (2008). Classification of rice starch amylose content from rheological changes of starch paste after cold recrystallization. Starch, 60, 292–297. doi:10.1002/star.200700672
- Turkut, G. M., Hulya, C., Seher, K., & Sebnem, T. (2016). Effect of quinoa flour on gluten-free bread batter rheology and bread quality. Journal of Cereal Science, 69, 174–181. doi:10.1016/j.jcs.2016.03.005
- United Nation Organization. (2015).Transforming our world: The 2030 Agenda for Sustainable Development, Resolution adopted by the General Assembly on 25 September 2015. UNO,Rome, Italy
- Vacher, J. J. (1998). Responses of two main andean crops, Quinoa (Chenopodium quinoa Willd) and Papa Amarga (Solanum juzepczukii Buk.) to drought on the bolivian altiplano: Significance of local adaptation. Agriculture, Ecosystems and Environment, 68, 99–108. doi:10.1016/S0167-8809(97)00140-0
- Valenzuela, C., Cristian, T., Luis, L., Andrea, B., Victor, E., & Lilian, A. (2015). Effect of edible quinoa protein-Chitosan based films on refrigerated strawberry (Fragaria × Ananassa) qauality. Journal of Biotechnology, 18, 406–411. doi:10.1016/j.ejbt.2015.09.001
- Valenzuela, C., Lilian, A., & Cristian, T. (2013). Quinoa protein-chitosan-sunflower oil edible film: Mechanical, barrier and structural properties. LWT - Food Science and Technology, 50, 531–537. doi:10.1016/j.lwt.2012.08.010
- Varo, P., Laine, R., & Koivistoinen, P. (1983). Effect of heat treatment of Dietary fiber: Interlaboratory study. Journal Association of Official Analytical Chemists, 66, 933–938.
- Vega, G., Margarita, A., Judith, M., Elsa, V., Luis, U., & Enrique, P. (2010). Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient andean grain: A review. Journal of the Science of Food and Agriculture, 90, 2541–2547. doi:10.1002/jsfa.4158
- Villegas, J. M., Brown, L., de Giori, G. S., & Hebert, E. M. (2016). Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT. Food Science and Technology, 67, 22–26. doi:10.1016/j.lwt.2015.11.027
- Werner, P., Mundigler, N., Kogler, A., Pelzl, B., Huber, A., & Wollendorfer, M. (1999). Molecular background of technological properties of selected starches. Starch, 51, 197–211. doi:doi:10.1002/(SICI)1521-379X(199906)51:6<197::AID-STAR197>3.0.CO;2-K
- Wolter, A, Anna, S. H, Emanuele, Z, & Elke, K. A. (2013). In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. Journal Of Cereal Science, 58, 431–436. doi: 10.1016/j.jcs.2013.09.003
- Wolter, A., Anna, S. H., Emanuele, Z., Michael, C., & Elke, K. A. (2014). Influence of dextran-producing weissella cibaria on baking properties and sensory profile of gluten free and wheat breads. International Journal of Food Microbiology, 172, 83–91. doi:10.1016/j.ijfoodmicro.2013.11.015
- Wolter, A., Hager, A. S., Zannini, E., Czerny, M., & Arendt, E.K. (2014b). Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads, European Food Research and Technology, 239, 1-12. doi:10.1007/s00217-014-2184-1
- Wolter, A., Hager, A. S., Zannini, E., Galle, S., Gänzle, M. G., Waters, D. M., & Arendt, E. K. (2014). Evaluation of exopolysaccharide Producing Weissella Cibaria MG1 strain for the production of sourdough from various flours. Food Microbiology, 37, 44–50. doi:10.1016/j.fm.2013.06.009
- Wood, S. G., Lawson, L. D., Daniel, J. F., Robison, L. R., & Andersen, W. R. (1993). Seed lipid content and fatty acid composition of three Quinoa cultivars. Journal of Food Composition and Analysis, 6, 41–44. doi:10.1016/j.jcs.2013.09.003
- Wright, K. H., Pike, O. A., Fairbanks, D. J., & Huber, C. S. (2002). Composition of atriplex hortensis, sweet and bitter chenopodium quinoa seeds. Journal of Food Science, 67, 1383–1385. doi:10.1111/j.1365-2621.2002.tb10294.x
- Yawadio, N. R., Hiroe, K., & Yotaro, K. (2008). Antioxidant activity of various extracts and fractions of chenopodium quinoa and amaranthus Spp. seeds. Food Chemistry, 106, 760–766. doi:10.1016/j.foodchem.2007.06.004
- Zannini, E., Stephanie, J., Kiran, L., & Elke, K. A. (2018). Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. International Journal of Food Microbiology, 268, 19–26. doi:10.1016/j.ijfoodmicro.2018.01.001
- Zhu, F. (2018). Chemical composition and food uses of teff (Eragrostis tef). Food Chemistry, 239, 402–415. doi:10.1016/j.foodchem.2017.06.101

