Abstract
Sleep disorders are associated with an elevated risk of developing various diseases. Herbal supplementation has been shown to alleviate sleep disorders such as insomnia. Here, the effect on sleep quality of an herbal supplement was evaluated subjectively. The supplement primarily contained lavender essential oil, as well as L-theanine and extracts of lemon balm, passion flower, and chamomile. Fifty-one healthy adults with self-reported symptoms of insomnia, divided into placebo and treatment groups, completed a 6-week crossover study (1-week washout, 2-week intervention, 1-week washout, and 2-week intervention, sequentially). Participants were asked to fill out the Leeds Sleep Evaluation Questionnaire (LSEQ) survey weekly and keep a sleep diary daily, which were used to assess their sleep quality. Compared to the placebo group, the active group (participants who received the supplement) showed significant improvements in overall LSEQ scores (p < 0.01) and several individual LSEQ measures. No significant differences in data from the sleep diaries were observed. Age, body mass index, gender, and ethnicity did not significantly interact with any dependent variable. No serious adverse event was reported. The lavender oil containing supplement was effective in improving several subjective sleep parameters in participants with mild to moderate insomnia.
Lack of sleep is a global burden leading to increased risk of diseases. More and more people are looking for relief beyond prescription drugs or over-the-counter sleep aids. In this clinical study, we tested a proprietary supplement (dōTERRA Serenity™ Restful Complex Softgels) containing lavender essential oil, L-theanine, lemon balm, passion flower, and chamomile, for its effect on sleep quality, using sleep quality questionnaires and sleep diaries. Fifty-one adults completed the 6-week crossover study. The study found that the Serenity supplement improved some sleep parameters for those with mild to moderate sleep problems. This study adds to our knowledge of the health effects of a complex, lavender essential oil based natural product on sleep quality. This study, along with others, suggests that complete natural products offer unique and effective alternatives to sleep aids or drugs.
Competing Interest
Nicole Stevens, Jeff Dorsett, Alex DaBell, Xuesheng Han, and Tory L. Parker are employees of dōTERRA, the supplier of the study product. Dennis L. Eggett is a professor of statistics at Brigham Young University.
1. Introduction
Nutrition, exercise, and sleep are considered the three pillars of health. Sleep disorders resulting in a lack of sleep is associated with an elevated risk of developing various diseases, a reduced quality of life, and negative socioeconomic consequences (Rao, Ozeki, & Juneja, Citation2015). Lack of sleep may result in individuals turning to prescription drugs or other over-the-counter (OTC) sleep aids for relief. Unfortunately, intake of these types of sleep aids is often accompanied by side effects. New effective complex herbal products for improving sleep quality are in demand, as many consumers prefer natural alternatives where the origin of the product is more apparent. However, these products need to be validated for their safety and efficacy before use.
An important goal of improving sleep quality is to achieve anxiolysis, which is considered a requirement for high-quality sleep (Rao et al., Citation2015). One natural compound that has shown promise as a sleep aid is the essential oil of lavender (Lavandula angustifolia) (Kasper et al., Citation2014; Uehleke, Schaper, Dienel, Schlaefke, & Stange, Citation2012; Woelk & Schläfke, Citation2010). Lavender essential oil has been well researched as an anxiolytic agent (Bradley, Brown, Chu, & Lea, Citation2009; Imanishi et al., Citation2009; Kasper et al., Citation2014). This could be attributed to its rich content of linalool and linalyl acetate, which are compounds that have well-known relaxing properties (Koto et al., Citation2006; Linck et al., Citation2009). It can be inhaled (Lillehei, Halcón, Savik, & Reis, Citation2015), applied topically (Hongratanaworakit, Citation2011), or ingested (Dimpfel, Pischel, & Lehnfeld, Citation2004) to promote relaxation and sleep.
Other plant extracts have also shown promise for sleep enhancement. L-theanine, a non-protein amino acid occurring naturally in green tea leaves (Camellia sinensis), has demonstrated relaxing properties and may be useful as a sleep aid (Jang et al., Citation2012; Lyon, Kapoor, & Juneja, Citation2011). Importantly, a study (Ozeki, Juneja, & Shirakawa, Citation2008) has shown that L-theanine does not promote daytime drowsiness, which is a desirable characteristic of sleep products. Other botanical ingredients, including lemon balm (Melissa officinalis), passion flower (Passiflora incarnata), and chamomile (Matricaria recutita) have long been used for their relaxing properties (Ngan & Conduit, Citation2011; Zick, Wright, Sen, & Arnedt, Citation2011).
In the present study, we evaluated an oral supplement (dōTERRA Serenity™ Restful Complex Softgels) containing lavender essential oil, L-theanine, lemon balm, passion flower, and chamomile for its effect on sleep quality. Our objective was to assess how Serenity affects sleep quality in healthy adults with mild to moderate sleep impairment using the Leeds Sleep Evaluation Questionnaire (LSEQ) (Shahid, Wilkinson, Marcu, & Shapiro, Citation2011) and sleep diaries. We hypothesized that there would be a significant positive improvement in the measured sleep parameters based on the existing individual ingredient research.
2. Materials and methods
2.1. Participants
The study subjects were recruited from among dōTERRA (Pleasant Grove, UT, USA) employees and their family members. They included both male and female participants between 18 and 55 years old. Potential subjects gave informed consent and were screened using Insomnia Severity Index (ISI) scores. Subjects scoring less than 8 (representing little or no sleep disturbance) were excluded from the study, whereas those scoring 8 or higher were asked to provide a detailed medical history. Other exclusion criteria included diagnosed clinical sleep disorders; intake of prescription medications affecting sleep; pregnancy or breastfeeding; lifestyle habits that modify the sleep-wake cycle (e.g. night shift); known allergy to any of the components of the study product; and unwillingness to stop taking OTC or herbal medications affecting sleep. Sixty-two participants were initially enrolled in the study.
2.2. Study products
The study product (dōTERRA Serenity™ Restful Complex Softgels) was provided by dōTERRA International. Each Serenity softgel contains 80 mg of lavender essential oil, 50 mg L-theanine, and 25 mg of a blend of lemon balm, passion flower, and chamomile (Table S1). A typical chemical composition of lavender essential oil is presented in Table S2. The placebo product was a softgel containing soybean oil. To mask color differences, the placebo and study products were made opaque by soaking them in a dye mixture (12.47 g maltodextrin, 10 g AmeriColor soft gel paste 119 Red, 8 g AmeriColor soft gel paste 103 Sky Blue, 180 g isopropyl alcohol, 500 mL water, and 1 g lavender oil, so that the placebo capsules smelled like the study products). The softgels were soaked in the dye mixture for 30 s, drained, and then spread in a single layer to dry overnight. A second coat of dye was applied by repeating the soaking, draining, and drying process. The softgels were stored in airtight plastic bags after they were completely dried.
2.3. Study design
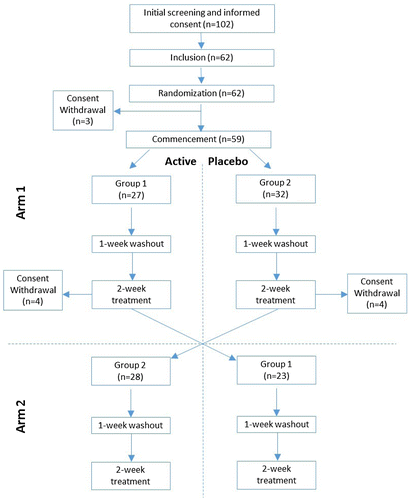
The study was reviewed and approved by an Institutional Review Board before commencement. A randomized, double-blind, placebo-controlled, crossover design was used. The participants were randomized into two groups (Figure ) and assigned an identification number. For each arm of the two-arm crossover design, participants first underwent a one-week washout during which no study products or sleep aids of any kind were used. This was followed by intake of a study product (either the active supplement or placebo) for two weeks. Participants were instructed to swallow 2 softgels every night, 30 min before retiring to bed. After another one-week washout period, the participants took the other study product for two weeks following the same regimen. Both participants and researchers were blinded to the study products during each arm of the study.
2.4. Data collection
The participants filled out a validated LSEQ prior to the study and at the end of each week during the 6-week study period. The LSEQ comprises ten self-rated questions, graded using a 100 mm analogue line, concerned with aspects of night sleep and morning behavior. Weekly LSEQ assessments were conducted and compared to those completed prior to the study. On each night of the study, the participants filled out a diary for the evaluation of total time in bed, sleep time (minutes), exercise (minutes), caffeine intake (occurrence), alcohol intake (occurrence), number and duration of night wakings, and sleep latency (time to fall asleep). Sleep efficiency was later calculated as follows: Sleep efficiency = Total sleep time (min)/Total time in bed (min).
Participants were also surveyed for ISI scores both before and after each of the two study arms. ISI has seven questions. The seven answers are added up to get a total score, which indicates the sleep difficulty of the participant.
2.5. Data analysis
The statistical analysis performed was a mixed model analysis of covariance using repeated measures from each subject across the weeks of the study and subject blocking. A mixed model is preferred when measuring repeated measurements or longitudinal data because it explicitly demonstrates correlation between variables and provides a correct statistical inference (Cornu et al., Citation2010). The dependent variables were LSEQ mean, total sleep time, number and duration of night wakings, sleep latency, and sleep efficiency. The independent variables were participant age, body mass index (BMI), ethnicity, and gender. The dependent and independent variables included a covariate of the initial week’s measure of the LSEQ to adjust for the initial state of each subject. Post hoc, F-tests (presented in the text with degrees of freedom as subscripts, along with p-values), and t-tests were performed to assess differences between means. A pseudo Bonferroni correction was performed to adjust for the multiple comparisons being made. Thus, we used a p-value of less than 0.01 for statistical significance. For each dependent variable, the residuals were checked for normality, equal variance, and outliers. All the assumptions were met. The statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA).
3. Results
Of the 62 intially recruited, 3 dropped out before the study began. Fifty-nine participants were randomized for the study; fifty-one individuals completed the study. Of those who completed the study, 31 were female (61%) and 20 were male (39%) (Table ). The mean age of the participants was 28 years (range 19–55). Compliance with treatment was 98.4% during arm 1 (three missing data points) and 97.9% during arm 2 (one missing data point) of the study. Before the study began, 63% of the participants had subclinical insomnia (ISI score, 8–14), 33% had moderate insomnia (ISI score, 15–21), and 4% had severe insomnia (ISI score, ≥ 22).
Table 1. Demographic information of study participants
3.1. LSEQ analysis
The means of the overall LSEQ scores from the two-week treatment period during each arm of the study showed significant differences between the active and placebo treatments (F1, 144 = 7.36, p = 0.008) (Table ). The mean score for the participants who took the active product was 7.1 points higher than that for those who took the placebo. However, the interaction between week of the study and treatment was not significant (p = 0.72).
Table 2. Summary of Leeds Sleep Evaluation Questionnaire (LSEQ) data
The mean LSEQ scores for all participants during the second washout period were significantly higher than those for all participants during the first washout period (F1, 46 = 13.18, p = 0.001), which shows an increase in score over time, possibly owing to familiarity with the LSEQ or placebo effect due to participation in the trial. However, this effect was balanced by the crossover design. By tracking participants after the crossover, mean LSEQ scores were the same following the second washout period for participants previously in the active and placebo groups during arm 1 of the study.
A subcategory statistical analysis was also performed (Table ) on the responses to the LSEQ. The results from the analysis of Subcategory 1 (questions 1–3, Getting to Sleep) and Subcategory 2 (questions 4–5, Quality of Sleep) did not show significant differences in effects between the active product and the placebo. However, significant differences between the effects of the active product and those of the placebo were observed from analysis of Subcategory 3 (questions 6–7, Awakening Following Sleep). Subcategory 3 showed mean scores that were at least 10 points higher for participants who took the active product. Considering individual questions on the LSEQ, it was noted that for questions 1, 6, and 10, there were significant differences in the responses between the active and placebo groups (Table ).
There was a significant interaction between a person’s Insomnia Sleep Index (ISI) score prior to the study and their response to the treatment (F1, 144 = 6.59, p = 0.0018). Participants with ISI scores between 15 and 21 (Category 2, representing clinical sleep disturbance, although none of the participants had been diagnosed with a clinical sleep disorder) showed better responses to the treatment than those with ISI scores between 8 and 14 did (Category 1, representing subclinical sleep disturbance) (p = 0.001, Table ). There was an insufficient number of participants with ISI scores greater than 21 (Category 3, representing severe clinical insomnia) to determine statistical significance in that category (Table ).
Table 3 Summary of Insomnia Sleep Index (ISI) scores and significance of interaction with treatment
3.2. Analysis of sleep diary data
No significant differences were found between the active and placebo groups for measures regarding total sleep, sleep latency, number of waking events, duration of waking events, or sleep efficiency (Table ). However, all the parameters evaluated from the sleep diaries showed higher scores when the participants were taking the active product than when they were taking the placebo, except waking duration, which was just slightly higher in the active group.
Table 4. Summary of data from the sleep diaries on a per night basis
3.3. Adverse events
Four participants (7.8%) in the active group and three participants (5.9%) in the placebo group reported minor adverse events including headache, acid reflux, dizziness, and feelings of anxiety. All reported adverse events were generally mild and resolved quickly without medical intervention. No serious adverse event was reported during the study or within the three months following the study.
4. Discussion
The present study shows that dōTERRA Serenity™ Restful Complex Softgels produced significant improvements in the participants’ subjective assessments of sleep, particularly those assessments involving ease of falling asleep, balance and coordination upon awakening, ease of awakening, and alertness following waking. The average increase in scores over time demonstrated familiarity with the LSEQ, though the results were consistent with a significant improvement in several measures.
Since several of these measures (subcategory 3 and questions 1 and 6) involved LSEQ scores that were 10 points higher for participants taking the active product, this could indicate a clinically significant effect of the active supplement, based on previous validations performed using the questionnaire (Zisapel & Nir, Citation2003).
The study provides an interesting insight into the potential beneficial effects of the supplement on sleep quality. The LSEQ was originally designed to assess subjective change in sleep parameters, including quality of sleep, getting to sleep, awakening events, and behavior following waking, in participants following a single night of treatment; however, it has been used extensively for assessing drug activity in studies ranging from one day to 24 weeks (Zisapel & Laudon, Citation2003). Zisapel and Nir (Citation2003) noted that the LSEQ was used in 83 studies involving various drugs to investigate self-reported sleep measures. The authors concluded that the LSEQ is a reliable and robust instrument for psychopharmacological evaluations. In addition, it provides meaningful and consistent measures for estimating the effectiveness of sleep modulators. In the current study, LSEQ measures provided a simple means of assessing subjective differences between the effects of an active botanical product and those of an inert placebo on sleep measures in healthy participants.
Lavender essential oil is composed primarily of linalyl acetate (30–40%) and linalool (30–40%). It contains many other minor components such as ocimene, lavandulyl acetate, terpinen-4-ol, and trans-caryophyllene (Table S1). While several studies have suggested that individual components of lavender essential oil have beneficial physiological activities (Brum, Elisabetsky, & Souza, Citation2001; Elisabetsky, Marschner, & Souza, Citation1995; Guimarães, Quintans, & Quintans-Júnior, Citation2013; Koto et al., Citation2006; Kuroda et al., Citation2005; Linck et al., Citation2009; Peana, Marzocco, Popolo, & Pinto, Citation2006; Peana et al., Citation2002), there is also some evidence that the whole oil has improved properties that maximize its therapeutic benefits (Barocelli et al., Citation2004).
The exact mechanisms underlying the anxiolytic and sleep-promoting activities of lavender essential oil are poorly understood. It has been suggested that it may influence the cholinergic system, which facilitates vagal nerve activity (Akhondzadeh et al., Citation2003). Linalool, one of the major components of lavender oil, may also exert effects by modulating muscarinic transmission (Peana et al., Citation2004). However, in vitro studies have suggested that the anxiolytic effect of lavender oil is not due to direct interaction with the cholinergic system (Atanassova-Shopova & Roussinov, Citation1969), and that other pathways are likely involved. Lavender oil and its components have shown several effects on hormones and neurotransmitter systems, such as the opioidergic system (Barocelli et al., Citation2004), the glutamatergic system involved in central nervous system excitation (Elisabetsky et al., Citation1995), and histaminergic pathways in the suprachiasmatic nucleus involved in regulating autonomic nervous system output and circadian rhythms (Tanida, Niijima, Shen, Nakamura, & Nagai, Citation2006).
The present study focused on the oral consumption of lavender oil and the other botanical ingredients, and their subsequent absorption. Several studies (Baldinger et al., Citation2015; Bradley et al., Citation2009; Kasper, Citation2013; Uehleke et al., Citation2012; Woelk & Schläfke, Citation2010) have shown that oral consumption of lavender oil produces physiological effects such as anxiolysis and sleep. It is important to note that individual physiology may contribute to the effectiveness of a lavender-based product. At least one study has suggested that lavender essential oil has differential effects on females compared to males (Bradley et al., Citation2009). Lavender oil has also been noted to produce paradoxical outcomes among certain age groups and in some mental states by causing sympathetic arousal rather than sedation (Bradley et al., Citation2009; Goel & Grasso, Citation2004; Goel, Kim, & Lao, Citation2005; Hawken, Fiol, & Blache, Citation2012). In our study, the females responded slightly better to the supplement than the males did; however, this difference was not statistically significant (F1, 144 = 1.91, p = 0.17), nor was the difference of 4.33 clinically meaningful.
As part of the post hoc analyses, we evaluated differences in response to the supplement between Caucasian and non-Caucasian individuals. We found that the non-Caucasian participants appeared to respond better to the study product (mean LSEQ scores were 4.1 points higher with F1, 144 = 1.65, p = 0.20) than the Caucasian participants did. Again, the observed differences were not statistically significant. We also found no significant differences in responses to the supplement among different age groups. Additionally, no significant interaction between BMI and responsiveness to the study product was observed.
The current study was relatively short (2-weeks of active treatment for each participant); therefore, it is unknown whether extended periods of treatment will improve the efficacy of the supplement. One study comparing a lavender oil supplement with the selective serotonin re-uptake inhibitor (SSRI) paroxetine showed increasingly significant differences between the two treatments after the second week of treatment (Kasper et al., Citation2014). SSRIs generally require several weeks of administration to achieve the required efficacy (Sinclair & Nutt, Citation2007). Since the effects of lavender essential oil may occur via similar or overlapping pathways as those of SSRIs—possibly via interaction with the 5-hydroxytryptamine system (Bradley et al., Citation2009)—it is conceivable that the positive results seen in this study will be potentiated over a longer treatment period. Additional research is needed to confirm this.
It is unknown why significant differences were observed in LSEQ scores but not in sleep diary measures. This may have been due to misunderstanding instructions on the form, inconsistencies in maintaining sleep diary records, and variability in reporting events. We expected to observe improvements in some of the objective measures of sleep improvement (such as increased sleep duration and efficiency, decreased sleep latency, and decreased waking events or duration) following the treatment; however, no significant differences were observed in the parameters between the active and placebo groups. Furthermore, since the measurements were positive in all aspects except waking duration of the sleep diary, this could indicate a trend toward significance. For future studies, we recommend the use of a more objective measure, such as an accelerometer or smart device, which may be used to obtain alternative measurements (van Hees et al., Citation2015; Tudor-Locke, Barreira, Schuna, Mire, & Katzmarzyk, Citation2014).
Long term sleep disorders may lead to an increased risk of developing cardiovascular disease, diabetes, hypertension, chronic illness, reduced longevity and quality of life, and work-related injuries (Budhiraja, Roth, Hudgel, Budhiraja, & Drake, Citation2011; Buysse, Citation2013; Kripke, Garfinkel, Wingard, Klauber, & Marler, Citation2002; Roth, Citation2009). While the current study was a small-scale, short-term clinical trial, the results indicate that the herbal supplement containing lavender essential oil, L-theanine, lemon balm, passion flower, and chamomile might improve subjective sleep measures in healthy adults with moderate sleep problems. The study also showed that the supplement produced a low incidence of side effects and was well tolerated by participants. This herbal formulation containing lavender essential oil may serve as an effective and natural alternative to single component sleep aids or drugs.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/2331205X.2017.1380871.
Funding
This study was funded by dōTERRA International, LLC (Pleasant Grove, UT, USA).
OAMD_1380871_Supplementary_Material.doc
Download MS Word (43 KB)Additional information
Notes on contributors
Nicole Stevens
Our group at dōTERRA studies the health benefits of essential oils, particularly their efficacy, safety, and application. Our in vitro and clinical studies of essential oils utilize various experimental approaches, including analytical, biological, biochemical, and biomedical methodologies. We work closely with research institutes, universities, hospitals, and clinics to develop and validate quality products with therapeutic benefits. Our work is designed to further the understanding of the health benefits of essential oils and contribute to the literature. We believe that a deeper understanding of these health benefits will ultimately lead to the evaluation and use of essential oils as an adjunctive therapy for a variety of needs. We also explore the benefits of essential oils in conjunction with other dietary supplements and herbal ingredients, as well as within skin care models and applications.
References
- Akhondzadeh, S., Kashani, L., Fotouhi, A., Jarvandi, S., Mobaseri, M., Moin, M., & Taghizadeh, M. (2003). Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27, 123–127. doi:10.1016/S0278-5846(02)00342-1
- Atanassova-Shopova, S., & Roussinov, K. S. (1969). On certain central neurotropic effects of lavender essential oil. Izvestiia Na Instituta Po Fiziologiia, 13, 69–77.
- Baldinger, P., Höflich, A. S., Mitterhauser, M., Hahn, A., Rami-Mark, C., Spies, M., & Kasper, S. (2015). Effects of Silexan on the serotonin-1A receptor and microstructure of the human brain: A randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. The International Journal of Neuropsychopharmacology, 18, pyu063. doi:10.1093/ijnp/pyu063
- Barocelli, E., Calcina, F., Chiavarini, M., Impicciatore, M., Bruni, R., Bianchi, A., & Ballabeni, V. (2004). Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sciences, 76, 213–223. doi:10.1016/j.lfs.2004.08.008
- Bradley, B. F., Brown, S. L., Chu, S., & Lea, R. W. (2009). Effects of orally administered lavender essential oil on responses to anxiety-provoking film clips. Human Psychopharmacology, 24, 319–330. doi:10.1002/hup.1016
- Brum, L. F., Elisabetsky, E., & Souza, D. (2001). Effects of linalool on [(3)H]MK801 and [(3)H] muscimol binding in mouse cortical membranes. Phytotherapy Research, 15, 422–425. doi:10.1002/ptr.973
- Budhiraja, R., Roth, T., Hudgel, D. W., Budhiraja, P., & Drake, C. L. (2011). Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep, 34, 859–867. doi:10.5665/SLEEP.1114
- Buysse, D. J. (2013). Insomnia. Journal of the American Medical Association, 309, 706–716. doi:10.1001/jama.2013.193
- Cornu, C., Remontet, L., Noel-Baron, F., Nicolas, A., Feugier-Favier, N., Roy, P., & Kassaï, B. (2010). A dietary supplement to improve the quality of sleep: A randomized placebo controlled trial. BMC Complementary and Alternative Medicine, 10, 29. doi:10.1186/1472-6882-10-29
- Dimpfel, W., Pischel, I., & Lehnfeld, R. (2004). Effects of lozenge containing lavender oil, extracts from hops, lemon balm and oat on electrical brain activity of volunteers. European Journal of Medical Research, 9, 423–431.
- Elisabetsky, E., Marschner, J., & Souza, D. O. (1995). Effects of Linalool on glutamatergic system in the rat cerebral cortex. Neurochemical Research, 20, 461–465. doi:10.1007/BF00973103
- Goel, N., & Grasso, D. J. (2004). Olfactory discrimination and transient mood change in young men and women: Variation by season, mood state, and time of day. Chronobiology International, 21, 691–719. doi:10.1081/CBI-200025989
- Goel, N., Kim, H., & Lao, R. P. (2005). An olfactory stimulus modifies nighttime sleep in young men and women. Chronobiology International, 22, 889–904. doi:10.1080/07420520500263276
- Guimarães, A. G., Quintans, J. S. S., & Quintans-Júnior, L. J. (2013). Monoterpenes with analgesic activity–a systematic review. Phytotherapy Research, 27, 1–15. doi:10.1002/ptr.4686
- Hawken, P. A. R., Fiol, C., & Blache, D. (2012). Genetic differences in temperament determine whether lavender oil alleviates or exacerbates anxiety in sheep. Physiology & Behavior, 105, 1117–1123. doi:10.1016/j.physbeh.2011.12.005
- van Hees, V. T., Sabia, S., Anderson, K. N., Denton, S. J., Oliver, J., Catt, M., & Singh-Manoux, A. (2015). A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One, 10, e0142533. doi:10.1371/journal.pone.0142533
- Hongratanaworakit, T. (2011). Aroma-therapeutic effects of massage blended essential oils on humans. Natural Products Communications, 6, 1199–1204.
- Imanishi, J., Kuriyama, H., Shigemori, I., Watanabe, S., Aihara, Y., Kita, M., & Fukui, K. (2009). Anxiolytic effect of aromatherapy massage in patients with breast cancer. Evidence-Based Complementary and Alternative Medicine, 6, 123–128. doi:10.1093/ecam/nem073
- Jang, H. S., Jung, J. Y., Jang, I. S., Jang, K. H., Kim, S. H., Ha, J. H., & Lee, M. G. (2012). L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharmacology, Biochemistry, and Behavior, 101, 217–221. doi:10.1016/j.pbb.2012.01.011
- Kasper, S. (2013). An orally administered lavandula oil preparation (Silexan) for anxiety disorder and related conditions: An evidence based review. International Journal of Psychiatry in Clinical Practice, 17, 15–22. doi:10.3109/13651501.2013.813555
- Kasper, S., Gastpar, M., Müller, W. E., Volz, H. P., Möller, H. J., Schläfke, S., & Dienel, A. (2014). Lavender oil preparation Silexan is effective in generalized anxiety disorder–a randomized, double-blind comparison to placebo and paroxetine. The International Journal of Neuropsychopharmacology, 17, 859–869. doi:10.1017/S1461145714000017
- Koto, R., Imamura, M., Watanabe, C., Obayashi, S., Shiraishi, M., Sasaki, Y., & Azuma, H. (2006). Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. Journal of Cardiovascular Pharmacology, 48, 850–856. doi:10.1097/01.fjc.0000238589.00365.42
- Kripke, D. F., Garfinkel, L., Wingard, D. L., Klauber, M. R., & Marler, M. R. (2002). Mortality associated with sleep duration and insomnia. Archives of General Psychiatry, 59, 131–136. doi:10.1001/archpsyc.59.2.131
- Kuroda, K., Inoue, N., Ito, Y., Kubota, K., Sugimoto, A., Kakuda, T., & Fushiki, T. (2005). Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. European Journal of Applied Physiology, 95, 107–114. doi:10.1007/s00421-005-1402-8
- Lillehei, A. S., Halcón, L. L., Savik, K., & Reis, R. (2015). Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: A randomized controlled trial. The Journal of Alternative and Complementary Medicine, 21, 430–438. doi:10.1089/acm.2014.0327
- Linck, V. M., da Silva, A. L., Figueiró, M., Piato, A. L., Herrmann, A. P., Dupont Birck, F., & Elisabetsky, E. (2009). Inhaled linalool-induced sedation in mice. Phytomedicine, 16, 303–307. doi:10.1016/j.phymed.2008.08.001
- Lyon, M. R., Kapoor, M. P., & Juneja, L. R. (2011). The effects of L-theanine (Suntheanine®) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): A randomized, double-blind, placebo-controlled clinical trial. Alternative Medicine Review, 16, 348–354.
- Ngan, A., & Conduit, R. (2011). A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phytotherapy Research, 25, 1153–1159. doi:10.1002/ptr.3400
- Ozeki, M., Juneja, L. R., & Shirakawa, S. (2008). A study of L-theanine and daytime drowsiness. Japanese Journal of Physiological Anthropology, 13, 9–15.
- Peana, A. T., D’Aquila, P. S., Panin, F., Serra, G., Pippia, P., & Moretti, M. D. L. (2002). Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine, 9, 721–726. doi:10.1078/094471102321621322
- Peana, A. T., De Montis, M. G., Nieddu, E., Spano, M. T., D’Aquila, P. S., & Pippia, P. (2004). Profile of spinal and supra-spinal antinociception of (-)-linalool. European Journal of Pharmacology, 485, 165–174. doi:10.1016/j.ejphar.2003.11.066
- Peana, A. T., Marzocco, S., Popolo, A., & Pinto, A. (2006). (-)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sciences, 78, 719–723. doi:10.1016/j.lfs.2005.05.065
- Rao, T. P., Ozeki, M., & Juneja, L. R. (2015). In search of a safe natural sleep aid. Journal of the American College Nutrition, 34, 436–447. doi:10.1080/07315724.2014.926153
- Roth, T. (2009). Sleep duration, insomnia and longevity. Sleep Medicine, 10, 1071–1072. doi:10.1016/j.sleep.2009.04.004
- Shahid, A., Wilkinson, K., Marcu, S., & Shapiro, C. M. (2011). Leeds sleep evaluation questionnaire (LSEQ). In A. Shahid, K. Wilkinson, S. Marcu, & C. M. Shapiro (Eds.), STOP, THAT and one hundred other sleep scales (pp. 211–213). New York: Springer. doi:10.1007/978-1-4419-9893-4_48
- Sinclair, L., & Nutt, D. (2007). Anxiolytics. Psychiatry, 6, 284–288. doi:10.1016/j.mppsy.2007.04.007
- Tanida, M., Niijima, A., Shen, J., Nakamura, T., & Nagai, K. (2006). Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neuroscience Letters, 398, 155–160. doi:10.1016/j.neulet.2005.12.076
- Tudor-Locke, C., Barreira, T. V., Schuna, J. M., Jr, Mire, E. F., & Katzmarzyk, P. T. (2014). Fully automated waist-worn accelerometer algorithm for detecting children’s sleep-period time separate from 24-h physical activity or sedentary behaviors. Applied Physiology, Nutrition, and Metabolism, 39, 53–57. doi:10.1139/apnm-2013-0173
- Uehleke, B., Schaper, S., Dienel, A., Schlaefke, S., & Stange, R. (2012). Phase II trial on the effects of Silexan in patients with neurasthenia, post-traumatic stress disorder or somatization disorder. Phytomedicine, 19, 665–671. doi:10.1016/j.phymed.2012.02.020
- Woelk, H., & Schläfke, S. (2010). A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine, 17, 94–99. doi:10.1016/j.phymed.2009.10.006
- Zick, S. M., Wright, B. D., Sen, A., & Arnedt, J. T. (2011). Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: A randomized placebo-controlled pilot study. BMC Complementary and Alternative Medicine, 11, 78. doi:10.1186/1472-6882-11-78
- Zisapel, N., & Laudon, M. (2003). Subjective assessment of the effects of CNS-active drugs on sleep by the Leeds sleep evaluation questionnaire: A review. Human Psychopharmacology, 18, 1–20. doi:10.1002/hup.455
- Zisapel, N., & Nir, T. (2003). Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale. Journal of Sleep Research, 12, 291–298. doi:10.1046/j.0962-1105.2003.00365