Abstract
The decrease in cancer mortality indicates an improvement in cancer treatment and management. One strategy that has been a focus in cancer treatment is development of drug delivery systems (DDS). Lipid-based nanoparticles (e.g. liposomes or micelles) has been used in current DDS as vehicles to transport active molecules. Extracellular vesicles (EVs), a new player in DDS, consist of lipid and thus, can be categorized as lipid-based nanoparticles. EVs are derived from cells and harbour various targeting molecules from their origin cells. Therefore, EVs are not foreign to the host immune system and may be more effective and efficient than other synthetic nanoparticles to target solid tumours with a minimum adverse effect, providing an exciting alternative for lipid-based DDS. Active molecules can be loaded into EV endogenously by exposing cells with active molecules to generate EVs carrying these molecules, or exogenous loading using physical or chemical methods. In this review, we summarise the recent developments of EV-based DDS where the choice of donor cells, drug cargo, loading methods, and administration routes are discussed. Further, consideration of the bioavailability and biodistribution of EVs, as well as current challenges concerning the potential biosafety issue and standardized up-scale production of EVs are highlighted.
PUBLIC INTEREST STATEMENT
Extracellular vesicles (EVs) are nano-sized vesicles naturally derived from the cell. Their natural properties such as protein and lipid profiles are similar to its mother cell. These characteristics are advantageous when EVs are used as drug nanocarriers because EVs may be less susceptible to body’s own immune system allowing to circulate longer without causing side effects until the tumor is reached. To enhance EV-based drug delivery, EV surface could be decorated with specific proteins/molecules. This review will give the reader insights on current developments of EV-based Drug delivery system (DDS) and also their potentials for cancer treatment. Current challenges concerning the time span, distribution, and safety issues of the EVs in the body, as well as up-scaling EV production for drug delivery purposes are also discussed.
1. Introduction
Cancers are still a major concern worldwide, with an estimated 1.7 million new cases diagnosed each year in the United States and predicted 600,000 cases of mortality in 2018 (https://seer.cancer.gov/statfacts/html/common.html). Generally, there is an improvement in cancer diagnosis and therapy as indicated by an overall decrease in cancer mortality; around 1.4% in women and children and 1.7% among men from 2006 to 2015. While the observed decrease in mortality is encouraging, there is still a major need for improvement in cancer diagnosis and therapy. Advancing treatment strategies for cancers can be obtained through the development of new drugs with better specificity and efficacy; improving treatment regimes through biomarker detection, or improvement of the drug delivery system (DDS).
DDS plays an important role to improve the efficacy and safety of drugs. Whether a drug is given nasally, orally, systemically or through other routes, once it is in the circulation and reaches the affected site, drugs need to cross tissue surrounding the cancerous tissue. This tumour microenvironment is usually hypoxic and has a high interstitial fluid pressure, providing a physical barrier reducing the rate of absorption of drugs in targeted tumour cells. This issue has been prominent for solid tumour due to the lack of functional lymphatic vessels and the hypervascular permeability (Jain & Stylianopoulos, Citation2010; Otranto et al., Citation2012). It is also important to ensure efficient clearance from the circulation which will minimise the side-effects after therapy. Thus, choosing the right DDS strategy which is aimed to achieve an effective concentration for the highest possible curative benefit for patients while minimising cytotoxicity is important.
Targeted nanoparticles-based DDS, conceptualised since the 1980s, are considered to be smarter DDS due to their specificity to bind to a target organelle and allow the deliverance of the active compound in response to specific molecular signals (Ding, Tong, Feng, & Fu, Citation2016; Nelson et al., Citation2015; Xin, Yin, Zhao, Meng, & Luo, Citation2017). In this concept, nanoparticle as a vehicle or carrier can be used to attach or encapsulate active molecule for improved delivery to recipient cells. The vehicle/carrier can be inorganic (e.g. gold or magnetic nanoparticles) or organic (lipid-based such as liposome and micelles) materials. It has been demonstrated that the attachment/encapsulation of drug to nanoparticles improves its stability and biocompatibility during transport in the circulation, increase the solubility and bioavailability of hydrophobic drugs, and reduce the cytotoxicity for the surrounding healthy tissues [review in (Pattni, Chupin, & Torchilin, Citation2015). The lipid-based nanoparticles (liposomes and micelles) also can be loaded with inorganic nanoparticles such as gold or magnetic nanoparticles. These properties prompted an expansion in the utilisation of inorganic nanoparticles for many applications in medicine, including therapeutic function, tumour imaging and importantly drug delivery (Patra, Das, Fraceto, Campos, & Rodriguez-Torres, Citation2018).
While organic and inorganic nanoparticles have been developed and shown to improve the DDS, it is clear that there are still some disadvantages in using nanocarriers for cancer treatment. A considerable issue is toxicity, as a result of the chemical and physical feature of nanoparticles and the reaction to the host immune system (Sukhanova et al., Citation2018; Wu & Tang, Citation2018). As cancers can continuously adapt towards treatments, development of DDS to target specific cancer and to treat different type or stage of cancers is needed. The extracellular vesicles (EVs) as a new player in DDS may have the potential to overcome the barriers created by the tumour microenvironment, as well as overcome the issues of toxicity. EVs are naturally derived from the cell and their natural properties such as protein and lipid profiles are similar to its source cell (Soekmadji, Russell, & Nelson, Citation2013; Yuana, Sturk, & Nieuwland, Citation2013). These characteristics are advantageous as EVs are shown to be less susceptible to host immune system; allowing to circulate longer without causing adverse effects until the targeted area is reached. Similar to other nanoparticles, EV can be manipulated to deliver drugs or other inorganic particles. Two major strategies for loading strategy for EVs will be discussed further in this review. To enhance EV uptake in targeted cells, EV surface could be decorated with tissue-specific antibodies or active molecules such as homing peptides (Antes et al., Citation2018). Other potentials for targeting EVs to the recipient cancer cells will also be highlighted in the separate section in this review.
2. Terminology and biogenesis of EVs
Cells generate and release EVs to the extracellular space due to several factors such as growth factor and hormone stimuli, drug/toxin, and stress (D. S. Choi, Kim, Kim, & Gho, Citation2015; Soekmadji et al., Citation2016, Citation2013). Up to date, while it is still debatable on the number of EV subtypes, the most characterised EVs are categorised as exosomes (small EVs), microvesicles, and apoptotic vesicles (Bobrie, Colombo, Krumeich, Raposo, & Thery, Citation2012; D. S. Choi, Kim, Kim, & Gho, Citation2013; Raposo & Stoorvogel, Citation2013). Small EVs with a diameter around 30–150 nm and formed through intracellular budding at the multivesicular bodies (MVBs) are referred as exosomes (D. S. Choi et al., Citation2015; Yuana et al., Citation2013; Thery et al., Citation2018). Exosomes are enriched in sphingomyelin, cholesterol and glycophospholipid (Chaput et al., Citation2004; Denzer, Kleijmeer, Heijnen, Stoorvogel, & Geuze, Citation2000; Subra, Laulagnier, Perret, & Record, Citation2007; Valadi et al., Citation2007). The biogenesis of exosomes is mediated by the protein complexes Endosomal Sorting Complex Required for Transport (ESCRT, also reviewed in: Gruenberg & Stenmark, Citation2004; Schmidt & Teis, Citation2012). Additionally, studies have also shown that interaction between alix, syndecan and syntenin can regulate the endosomal membrane budding for exosome formation (Baietti et al., Citation2012; Hurley & Odorizzi, Citation2012) or by the action of lipid, such as sphingolipid ceramide in an oligodendroglial cell line (Trajkovic et al., Citation2008). The secretion of exosomes is shown to be regulated by Rab27A GTPase (Ostrowski et al., Citation2010).
Microvesicles or ectosomes are formed at the plasma membrane and enriched in phosphatidylserine with diameter from 100 nm up to 1 μm (Lima, Chammas, Monteiro, Moreira, & Barcinski, Citation2009). The formation of microvesicles at the plasma membrane involves phospholipid distribution and the cytoskeletal actin/myosin complex and the ADP-ribosylation factor 6 (ARF6) (Muralidharan-Chari et al., Citation2009; Muralidharan-Chari, Clancy, Sedgwick, & D’Souza-Schorey, Citation2010). The apoptotic vesicles are also a class of vesicles which are shed from plasma membrane of eukaryotic cell type during apoptosis. They contain DNA, histones, organelles and surface markers, allowing them to be recognised by phagocytic cells (Nunez, Sancho-Martinez, Novoa, & Lopez-Hernandez, Citation2010; Thery et al., Citation2001). Other newly characterized large vesicles shed from the plasma membrane, the oncosomes, are formed by tumourigenic cells and can be identified by the presence of CK18. The biogenesis of oncosome is stimulated by EGFR activation and overexpression of Akt1 (Di Vizio et al., Citation2012). Oncosomes are enriched in enzymes involved in glucose, glutamine and amino acid metabolisms (Di Vizio et al., Citation2012; Minciacchi et al., Citation2015).
Despite various methods and markers used, it is still a challenge to detect or isolate the subtypes of EVs in biological fluids (Ramirez, Amorim, Gadelha, & Milic, Citation2018). The characteristics of one subtype may overlap with others, thus it is difficult to obtain relatively pure preparations and to characterize one particular subtype properly. The International Society for Extracellular Vesicles (ISEV) proposed Minimal Information for Studies of Extracellular Vesicles (“MISEV”) guidelines in 2014 to streamline the EV research by means of defining the EVs and their functions (Lotvall et al., Citation2014). They updated this guideline in 2018 with more focus on defining the subsets of EV and specific EV-associated functional activities (Thery et al., Citation2018).
In cancer, EVs can mediate cell-cell communication through their transfer from the tumour to the tumour-microenvironment. This transfer can stimulate angiogenesis, remodelling of the matrix and regulate immune and therapy responses (Hartjes & Mytnyk, Citation2019; Soekmadji et al., Citation2013; Valadi et al., Citation2007). Recently, the EVs released from tumours have become a popular diagnostic marker. In many studies circulating EVs and their contents have shown to be a potential biomarker for many cancer types, including breast cancer (Moon et al., Citation2016), prostate cancer (McKiernan et al., Citation2016; Soekmadji, Rockstroh, Ramm, Nelson, & Russell, Citation2017), colorectal cancer (Li et al., Citation2017), pancreatic cancer (Allenson et al., Citation2017), and brain cancer (Osti et al., Citation2019). Furthermore, EVs also have been indicated to serve as biomarkers for diseases of various organs including, liver, lung, kidney, central nervous system (CNS), and arteries (Barile & Vassalli, Citation2017). In the clinical trials of cancer patients, promising results on EVs have also been reported, especially the use of EVs as immunoregulator to boost anti-tumor immunity in cancer patients (Besse et al., Citation2016). In this review, we will discuss the recent advance of EVs as vehicles for smarter DDS. We will also discuss the opportunities and pitfalls of EVs-based DDS for cancer treatment.
3. EV-based drug delivery system
Similar to the design of other lipid-based drug nanocarriers, the development of EV-based DDS is currently focused on improving the efficacy by altering their physical and biological properties to reach the target recipient cells and deliver their content. The choice of donor cells, strategies for drug loading into EVs, and modifications on EVs will be discussed below.
3.1. Choice of donor cells
The composition of EVs is largely determined by the physiological state of the donor cells. EVs carry proteins and lipids which will influence their targeting and docking modalities. However, possible oncogenic risks entailed by certain donor cells have been demonstrated, such as shown on mesenchymal stem cell (MSC)-derived EVs. MSCs are non-hematopoietic precursor cells that can be derived from various adult tissue with the ability to differentiate into several cell types (Eirin et al., Citation2018). Depending on the physiological state of donor cells, the cargo of the EV could also be oncogenic, immunogenic, or pathogenic for the target/recipient cells. The MSC-derived EVs are reported to support the proliferation of recipient cells by modulating their intracellular signalling pathways (Bruno et al., Citation2009, Citation2017; R. C. Lai et al., Citation2010). These pro-proliferative effects of MSC-EVs imply the possibility that MSC-EVs can induce growth in cancer cells, via production of growth factors or through the promotion of tumour vascularisation (Roccaro et al., Citation2013; W. Zhu et al., Citation2012). Contradictory reports have been shown as in some studies, where MSC-derived EVs display anti-tumourigenic effects (Bruno et al., Citation2013; Kalimuthu et al., Citation2016; Pascucci et al., Citation2014). As such, further studies are required to investigate in greater detail the reasons of these differences, which may be due to the source of MSC or the type of the recipient tumour (Del Fattore et al., Citation2015; Katsuda & Ochiya, Citation2015). Similar to MSC-EVs, tumour cells (TC)-derived EVs have been investigated as a potential source for cancer vaccines. However, in some studies TC-EVs can promote angiogenesis, metastasis, and immune suppression, demanding further investigations (Becker et al., Citation2016; Kitai et al., Citation2017; Soekmadji & Nelson, Citation2015).
Because most EVs display major histocompatibility complex (MHC) proteins on their surface and carry a wide variety of proteins derived from their cell source, one approach was attempted by utilising EVs from the autologous cell source for therapy, for example in the case of vaccination of cancer patients with EVs derived from autologous dendritic cell (DC) (Escudier et al., Citation2005; Gyorgy, Hung, Breakefield, & Leonard, Citation2015; Morse et al., Citation2005). However, vaccination of mice using TC-EVs derived from identical (syngeneic) or different (allogeneic) mice were to be equally effective. This may be due to the protection of tumours as the host dendritic cells (DCs) were able to recognise the EV-derived tumour antigen, independently of the MHC molecules of EVs (Robbins & Morelli, Citation2014; Wolfers et al., Citation2001).
Biofluids, such as blood, urine, and milk have also been considered as source of EV nanocarriers (Yuana et al., Citation2013). Red blood cells-derived EVs (RBC-EVs) have been studied as drug nanocarrier (Chang et al., Citation2010; Wang, Shi, Yang, & Huang, Citation2013). Since membrane vesiculation is a natural process of RBC aging during storage, the RBC can be used as a source cell of EVs. RBC-EV can be easily isolated and relatively stable (Sebaihi, Boeck, Yuana, Nieuwland, & Pétry, Citation2017). Other sources such as EVs from human milk, are shown to carry naturally immune relevant proteins which influence host immune responses (Admyre et al., Citation2007; Alsaweed, Lai, Hartmann, Geddes, & Kakulas, Citation2016). EVs from food products such as bovine/cow milk have also the potential to be developed as drug carriers as they are extremely stable under degrading conditions, including low pH, boiling and freezing (Munagala, Aqil, Jeyabalan, & Gupta, Citation2016; Pieters et al., Citation2015). It is, nevertheless, unclear whether bovine milk-derived EVs may confer a substantial cytotoxic risk for the host during continuous or longer exposure (Melnik & Schmitz, Citation2019; Somiya, Yoshioka, & Ochiya, Citation2018).
EVs are also secreted by prokaryotes (Gram-positive and Gram-negative bacteria, and archaea) and eukaryotes (protists, fungi, animals, and plants) (Biller et al., Citation2014; Deatherage & Cookson, Citation2012), as such these organisms can be utilised as EV donors. Although the mechanism of biogenesis and secretion of prokaryotes and non-mammalian EVs are not yet clear, these EVs comprise enzymes, nucleic acids, polysaccharides, pigments, and lipoproteins (Biller et al., Citation2014). Further, EV derived from bacteria can also deliver toxins, implicating its potential as nanocarrier to deliver toxic material (Brown, Wolf, Prados-Rosales, & Casadevall, Citation2015). Gram‐negative bacteria Neisseria meningitides derived EVs, which has also been referred as outer membrane vesicles (OMVs), are utilized for immunization to prevent invasive disease caused by Neisseria meningitidis serogroup B and has been marketed as a vaccine product (Bexsero, (van der Pol, Stork, & van der Ley, Citation2015). Nevertheless, bacteria or their derived products could be pathogenic. Thus, safety will be a primary concern. Attenuated bacterial strains with no substantial pathogenicity or toxicity may be employed in the development of cargos for vaccines or therapeutic agents for treating cancer in the future (Chen et al., Citation2010; Gujrati et al., Citation2014).
Non-mammalian eukaryotes such as (edible) plants and the non-pathogenic amoeba Dictyostelium discoideum are potential EV donors (Ju et al., Citation2013; Lavialle et al., Citation2009; Perez-Bermudez, Blesa, Soriano, & Marcilla, Citation2016; Tatischeff, Larquet, Falcon-Perez, Turpin, & Kruglik, Citation2012; Wang et al., Citation2014). EVs isolated from edible plant extracts also have drawn lots of attention as drug nanocarriers as it can overcome the safety and delivery requirements (Perez-Bermudez et al., Citation2016). EVs could be isolated from grape juice and pulp of grapefruit skin. These EVs can be taken up by intestinal macrophages and stem cells in murine models. The inherent biocompatibility and stability at wide ranges of pH values highlight the potential of EVs from the edible plants for further development of EV-based oral delivery system (Ju et al., Citation2013; Wang et al., Citation2014). Dictyostelium cells and their EVs are non-pathogenic providing great potential as a source of therapeutic EVs. Dictyostelium cells are particularly easy to grow in vitro and produce EVs resembling EVs derived from mammalian cells and could be loaded with an anti-tumoural compound hypericin (Lavialle et al., Citation2009). The therapeutic potential of EVs from various sources is summarized in .
Table 1. Therapeutic potential of EVs derived from various donor cells
3.2. EV loading strategies
Development of EV as nanocarrier for drug delivery relies heavily on loading a substantial amount of drug into EVs. To date, several approaches have been attempted such as transfection methods by electroporation, incubation of donor cells or EVs directly with the drug of interest, as well as manipulations on cells or EVs to improve the targeting, such as cellular metabolic labelling, and EVs covalent modification on their membrane (Armstrong, Holme, & Stevens, Citation2017; Johnsen et al., Citation2014). Some of these strategies have been tested for liposomes, even though not all drug loading strategies for liposomes are also feasible for EVs (van der Meel et al., Citation2014).
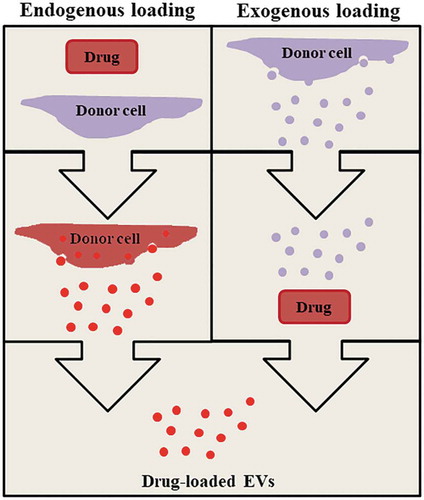
Two major strategies for loading EVs with drugs can be performed endogenously or exogenously (van der Meel et al., Citation2014). The endogenous loading strategy involves step-by-step approach to load drugs by manipulating the donor cells. As such the donor cells will utilise its natural mechanism to package drugs into EVs (Tang et al., Citation2012). This strategy utilized the natural mechanisms of some cells to secrete xenobiotic/cytotoxic molecules via EVs (Gong et al., Citation2012; Safaei et al., Citation2005). Donor cells loaded with drugs could be exposed to biological, mechanical, or chemical stimuli, such as serum starvation, heat, chemotherapeutic drugs, hypoxia, and ultraviolet light, or combination of those techniques, to induce the release of drug-loaded EVs (Chen, Guo, Yang, Zhu, & Cao, Citation2011; King, Michael, & Gleadle, Citation2012; Li et al., Citation2015; Wang et al., Citation2013). We recently showed that sonoporation using a combination of microbubbles (gas-filled bubbles) and ultrasound could be used to trigger the release of EVs from cancer cells in vitro (Yuana et al., Citation2017). The endogenous loading is normally a straight forward technique which requires very few manipulation steps (Figure ). However, this technique displays a few challenges such as packing sufficient amount of drug with less or minimum toxicity to donor cells. The optimisation can be performed by prolonged incubation time of drug with donor cells, to increase the non-specific binding between drugs with the cytoplasmic membrane of the donor cells. This approach has been useful especially for hydrophobic drugs, where the drug can be loaded into the cytosol with minimum entrapment of drugs in the endosomal compartment even though the entrapment of drugs in the endosome of donor cells could lead to lysosomal degradation, resulting in a decrease of the amount of drug packaged into secreted EVs (Lavialle et al., Citation2009).
The exogenous loading strategy relies on passive or active stimuli to load the drug of interest directly into EVs (Figure ). Using this technique, EVs from donor cells will be isolated and purified to obtain concentrated EVs with maximum purity. Drugs can be loaded by a simple step of incubation (passive drug loading) or by exposing EVs with active stimuli using electroporation, saponin treatment, freeze/thaw cycles, sonication, hypotonic dialysis, and/or extrusion. Passive drug loading has been used to load lipophilic molecules such as natural compounds curcumin and cucurbitacin-I, and synthetic chemodrugs doxorubicin and paclitaxel into EVs (Sun et al., Citation2010; Zhuang et al., Citation2011). Depending on the compound, incubation time may vary. For example, curcumin was efficiently loaded into EVs after only 5 minutes of incubation at 22 °C, whereas efficient loading of paclitaxel requires incubation for 1 hour at 22 °C (Saari et al., Citation2015; Sun et al., Citation2010; Zhuang et al., Citation2011). Currently, there is no systematic investigation which compare the required optimal temperature and incubation time, even though the hydrophobicity of active compounds has been shown to play a role in effective loading of drugs into EVs (Saari et al., Citation2015).
To increase the drug loading efficiency and overcome its hydrophobic characteristics, active loading of a drug into cells (endogenous) or EVs (exogenous) using a method such as electroporation seems to be most promising. The cell or EV membrane can be transiently permeabilized by electroporation to enhance the uptake of the drugs. This technique has also been applied for uploading small molecules including siRNA, small molecule drugs, and superparamagnetic iron oxide nanoparticles into cells or EVs (Armstrong et al., Citation2017; Fuhrmann, Serio, Mazo, Nair, & Stevens, Citation2015). However, electroporation can also trigger the aggregation of EVs which will inhibit the efficiency of drug loading (Hood, Scott, & Wickline, Citation2014). To minimise the aggregation of EVs, membrane stabilizers such as sucrose and trehalose can be added (Hood et al., Citation2014). Other methods have been tested for active drug loading such as treatment of EVs with detergent saponin, freeze/thaw cycles, sonication, hypotonic dialysis, and extrusion (Fuhrmann et al., Citation2015; Zhuang et al., Citation2011). However, these methods were reported to significantly increase the size of EVs, which may interfere with the uptake of EVs in the recipient cells (Fuhrmann et al., Citation2015). Among those techniques, high loading efficiency was achievable by sonication and extrusion, or membrane permeabilization with saponin (Fuhrmann et al., Citation2015; Haney et al., Citation2015). Modifying EVs before drug loading was done by fusing EVs with functionalized liposomes triggered by polyethylene glycol (PEG) to create “hybrid” EVs. In this way, EVs seemed to be efficiently enriched with exogenous lipophilic or hydrophilic compounds, while preserving their intrinsic content and biological properties (Piffoux, Silva, & Wilhelm, Citation2018).
Exogenous loading technique will require further purification of EVs to separate drug loaded EV from free drug and non-EV contaminants (protein/drug-protein aggregates). This could be achieved by using techniques such as size-exclusion chromatography, dialysis, and filtration (Gardiner et al., Citation2016). Although ultracentrifugation remains the most commonly used method for EV isolation, ultracentrifugation is not widely recommended technique to purify EVs for therapeutic purpose as it may co-isolate protein aggregates (Yuana et al., Citation2015). The presence of EV aggregates and non-EV contaminants in the isolated fraction would then interfere with the quantification of EVs, leading to underestimation or overestimation of the concentration of EVs. Furthermore, the purity of isolated drug-loaded EVs will be one of the deciding aspects to translate the use of EVs as therapeutic nanocarriers in clinical settings (Lener et al., Citation2015).
3.3. Targeting EVs to recipient cancer cells
One aspect for strategic and improved delivery of chemotherapeutic drugs is to ensure the accumulation the drugs specifically only at the tumour site to increase the potency and efficacy of the treatment. Although EVs carry proteins and lipids required for potential targeting and docking modalities, reports have shown that EVs can quickly disappear from the systemic circulation when injected intravenously (i.v.) in mice (Charoenviriyakul et al., Citation2017; Imai et al., Citation2015). EVs were rapidly cleared from the systemic circulation and accumulated mostly in the liver. They are also found in the spleen, GI-tract and lungs, and with a small percentage in the tumour indicating that the homing of circulating EVs may be limited by reticuloendothelial system (RES) present in these organs or by the role of macrophages in this process (H. Choi & Lee, Citation2016; Smyth et al., Citation2015). The clearance of EVs does not seem to depend on the source of the donor (Lener et al., Citation2015; Smyth et al., Citation2015). Therefore, modification strategies are needed to improve the delivery of EV nanocarrier (Lu et al., Citation2018)
The use of targeting peptide or protein on EVs has been tested. Introducing targeting peptide can be performed by engineering the donor cells to express the targeted peptide. The resulting EVs can be further isolated, purified, and loaded with the drug of interest. This technique has been shown successfully by modifying immature DCs to express lysosome-associated membrane glycoprotein 2b (Lamp2b) fused to αv integrin-specific iRGD peptide (CRGDKGPDC). These EVs were then loaded with doxorubicin via electroporation. The iRGD-EVs showed highly efficient targeting and delivery of doxorubicin to αv integrin-positive breast cancer cells in vitro. The same EVs were intravenously injected to deliver doxorubicin in BALB/c nude mice and able to reach tumour tissues, leading to inhibition of tumour growth without overt toxicity (Tian et al., Citation2014). Other targeting was achieved by engineering the Human embryonic kidney 293 (HEK 293) cells to express the transmembrane domain of platelet-derived growth factor receptor fused to a novel small peptide GE11 (sequence YHWYGYTPQNVI), which are able to target epidermal growth factor receptor (EGFR)-expressing breast cancer cells (Ohno et al., Citation2013). Other strategies involving the incorporation of folic acid has also been tested for EVs derived from bovine milk which improve the targeting of EVs to tumour cells (Munagala et al., Citation2016).
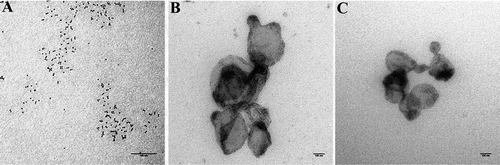
EVs could also be modified and applied for cancer theranostics such as shown in other nanoparticles. The use of superparamagnetic iron oxide nanoparticles (SPIONs) as EV cargo is promising for theranostic approach allowing simultaneous imaging and monitoring treatment of cancer cells (Santhosh & Ulrih, Citation2013). Due to their superparamagnetic behaviour, relatively nontoxic, and their nanosize (<20 nm), SPIONs would be suited as EV cargo (Jarockyte et al., Citation2016). Additionally, the surfaces of SPIONs can be easily conjugated with drugs to increase the concentration of drugs in EVs (Huang, Neoh, Xu, Kang, & Chiong, Citation2012). It has been shown that SPIONs with a size of 5 nm in diameter could be loaded into EVs by using electroporation in the presence of trehalose (Hood et al., Citation2014). In our preliminary work, SPIONs loaded into donor cells using sonoporation will then generate SPIONs containing-EVs (Figure ). Considering all promising aspects of SPIONs, this suggests that loading EVs with drug-conjugated SPIONs will enable us to track drug-loaded EVs with MRI and monitor the release of drugs in the recipient cells.
Figure 2. Generation of SPIONs containing EVs (Yuana Y, unpublished results). A. Exogenous loading were performed by growing cells in medium containing SPION and stimulating by sonoporation. B. EVs were harvested from conditioned medium containing SPIONs. C. Negative control EVs from cells grown without SPIONs and stimulated by sonoporation. Scale bars are 100 nm.
4. Delivery of drug-loaded EVs
Different from other synthetic drug nanocarriers, as EVs are naturally secreted by cells to mediate specific cell to cell communication and abundantly found in the systemic circulation, therapeutic EV nanocarrier were developed as it may have the advantage to avoid their removal by immune cells or to avoid hepatic clearance. However, results obtained from in vivo studies show that EVs are rapidly cleared from the circulation and minimally accumulate in the tumour (Choi & Lee, Citation2016; Smyth et al., Citation2015). Therefore, a better understanding of the biodistribution and the bioavailability of drug-loaded EVs in vivo is necessary. One important aspect that seems to play a major role in the biodistribution of EVs is the chosen administration route of drug-loaded EVs.
4.1. Biodistribution of drug-loaded EVs
Observation of the biodistribution of EVs can be a challenge as EVs can be as small as 20 nm, resulting in a high specificity and a high signal-to-noise ratio. As such, EVs can be labelled using a fluorescence-, bioluminescence-, radioactive-, or nanoparticle to track their route in vivo (Choi & Lee, Citation2016). Lipophilic dyes including FM, PKH, DiI, and DiR are commonly used dyes to label the plasma membrane of EVs as it has been shown to yield stable fluorescent signals for in vivo tracking of EVs. However, these dyes are not specific as they could also label lipoproteins which are present in plasma and co-isolate with EVs (Yuana, Levels, Grootemaat, Sturk, & Nieuwland, Citation2014). Non-specific binding of these dyes to other lipid molecules should be avoided by removing any unincorporated dye, reducing the impact of unspecific signals. Removal of dye contaminant can be achieved by washing steps using dialysis or size-exclusion chromatography. This is an important step as lipophilic dyes have a much longer half-life compared to the EVs, which could give false positive signals when EVs are tracked at longer time points. Tracking of EVs labelled with lipophilic dye in vivo was reported best during the first 24 hours as the overall EV biodistribution profile remained largely unchanged at the later time point (Wiklander et al., Citation2015). In vivo studies show that the highest accumulation of EVs labelled by lipophilic dyes was detected in the liver and spleen (Nordin et al., Citation2015; Smyth et al., Citation2015; Wiklander et al., Citation2015).
EV labelling could also be performed using membrane permeable chemical compounds, including the carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) and acetomethoxy derivate of calcein (calcein-AM) (Barteneva et al., Citation2013; Pospichalova et al., Citation2015). The CFDA-SE is a non-fluorescent dye and widely used for cellular tracking and monitoring cell proliferation (Parish, Citation1999; Quah & Parish, Citation2010). Viable cells can be labelled with CFDA-SE by diffusion. Once it enters the cells, intracellular esterase enzymes works by cleaving its acetate groups to produce a detectable fluorescent ester, carboxyfluorescein succinimidyl ester (CFSE) (Morales-Kastresana et al., Citation2017). The succinimidyl ester group will then react with primary amines, crosslinking the dye to intracellular proteins. Using endogenous loading strategy, CFDA-SE can be used to label donor cells and upon stimulation, cells can secrete EVs loaded with CFSE (Figure ).
Similar to CFDA-SE, calcein-AM is hydrolysed by esterase enzymes in the cytoplasm of viable cells to convert calcein-AM, a non-fluorescent label, resulting in a green fluorescent calcein. CFDA-SE would be more suitable than calcein AM for in vivo tracking of cells or EVs as the fluorescent CFSE leaks out of the cell/EVs comparatively less than calcein. In contrast to CFDA-SE, calcein does not bind to amine molecules of proteins in the cytoplasm and therefore, labelling may not be optimal if there is any disruption on the plasma membrane, such as by the presence of detergents or trypsinization. Any unincorporated CFDA-SE or calcein-AM dye should also be removed to avoid non-specific EV labelling. In contrast to lipophilic dye, CFDA-SE or calcein-AM dye can be used to differentiate between intact EVs and cell debris. CFDA-SE has been used to study the in vivo biodistribution of EVs derived from endothelial cells. These EVs were taken up by spleen cells within a few minutes and by liver and lung to a lesser extent (Al Faraj et al., Citation2012).
To enhance the in vivo tracking of EV, fluorescence or bioluminescence tagged transmembrane proteins found in EVs could provide more specific information than the use of dyes to label EVs. One example is using green fluorescent protein (GFP) to tag CD63, an exosome marker (Suetsugu et al., Citation2013). The use of bioluminescence reporter system, Gaussia luciferase (Gluc) has also been used to visualize EVs, particularly its mRNA cargo. This system provides a high signal to intensity ratio. The Gluc bioluminescence signal also rapidly decays to a background level, making it an ideal reporter to study temporal dynamics of EV uptake and suitable to study the translation of EV-delivered mRNAs in recipient cells (Lai et al., Citation2015). While there are strong advantages to use fluorescence or bioluminescence tags to label EVs, these procedures also have a few shortcomings. The preparation is a lengthy process as it requires genetically engineered donor cells. The yield of fluorescence-labelled EVs by the donor cells is relatively low. This system is also not suitable for in-depth tissue imaging, such as tracking of EV in the internal organs, as the fluorescence or luminescence signal is attenuated (Choi & Lee, Citation2016). Biodistribution analysis of intravenously administered GlucB (membrane-bound Gluc and biotin)-labelled EVs derived from HEK 293T cells revealed a short half-life of less than 30 min in vivo in most tissues and predominantly localized in the spleen followed by the liver, kidneys, and lung (Lai et al., Citation2014). EVs from B16-BL6 melanoma cells labelled with Gluc-lactadherin (Gluc-LA) are even more rapidly cleared from the blood circulation after systemic administration in mice where EV were distributed first to the liver and then to the lungs (Takahashi et al., Citation2013).
For clinical application and deep tissue imaging, radionuclide imaging or MRI would be a more suitable approach. These could be achieved by using 111In-oxine, (99m)Tc-hexamethylpropyleneamineoxime (HMPAO), iodine-125, or SPIONs to tag EVs (Al Faraj et al., Citation2012; Andriola Silva et al., Citation2013; Choi & Lee, Citation2016; Hood et al., Citation2014; Hwang Do et al., Citation2015; Morishita et al., Citation2015; Varga et al., Citation2016). The 99mTc-HMPAO has been used to label macrophage-derived exosome-mimetic nanovesicles (ENVs). The administration of these ENVs in mice showed high accumulation in the liver and spleen at 30 min, which was about 2–3 folds higher than the administration of 99mTc-HMPAO alone in mice (Hwang Do et al., Citation2015). These results show similarity with those obtained using the 99mTc-HMPAO to label RBC-EVs, showing accumulation of EVs mainly in liver and spleen (Varga et al., Citation2016). The use of SPION to label endothelial cells derived EV has been successful. These EVs, when administered to mice by intravenous injection, were accumulated mainly in the spleen (Al Faraj et al., Citation2012).
Up to date, the reason why EVs administered systemically are rapidly cleared from the circulation is not yet clearly understood. Some suggest that this characteristic is independent of the labelling method used. Most studies indicate that macrophages could play role in the clearance of EVs irrespective of the labelling methods (Imai et al., Citation2015; Matsumoto et al., Citation2017). Most studies reported that EVs are predominantly accumulated in the liver and spleen rich in macrophages (Table ). Studies have also shown that the clearance and tissue distribution of EVs could be influenced by the administration route of EVs (Wiklander et al., Citation2015), which will be discussed further in the section 4.3.
Figure 3. CFSE-containing cells stimulated with calcium ionophore (CaI) released EVs with CFSE (Yuana Y, unpublished results). A. 0.5 µM CFDA-SE were used to label cell using diffusion method. The CFDA-SE was cleaved by intracellular esterase enzymes to form an amine-reactive product, CFSE which can be detected by fluorescent microscopy. B. Negative control image (non-labelled cells). C. CFSE-labelled cells treated with 10 µM CaI have comparable CFSE signal to untreated CFSE-labelled cells as detected by flow cytometry. D. Detection of CD9-positive EVs using flow cytometry captured by CD9-antibody (AB) conjugated beads and stained with Alexa Fluor 647-tagged anti-CD9 AB. E. Detection of CD63-positive EVs using flow cytometry captured by CD63-antibody (AB) conjugated beads and stained with Alexa Fluor 647-tagged anti-CD63 AB. EVs were stained positive for CD9 or CD63 and CFSE as shown in the region Q2 (green rectangle) in D and E. E. Negative control (EVs captured with beads and unstained).
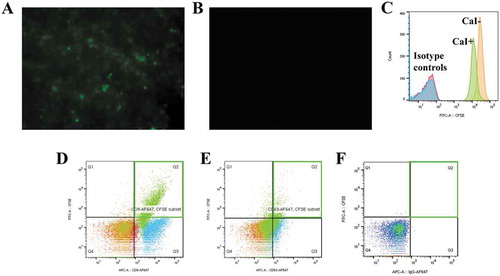
Table 2. Tissue distribution of EVs derived from different cell lines in mice model
4.2. Bioavailability of drug-loaded evs
Currently, a few mechanisms are proposed as the uptake mechanism of EVs. EVs were able to directly fuse with the plasma membrane of target cells or via endocytic pathways such as the clathrin-dependent or clathrin-independent endocytosis pathways (e.g. caveolin-mediated uptake, macropinocytosis, phagocytosis, and lipid raft–mediated internalization) (Mulcahy, Pink, & Carter, Citation2014). While understanding how EVs enter the target cells could help to improve the bioavailability of drug-loaded EVs, the uptake mechanisms do not provide complete control of the bioavailability and functionality of the drug as these characteristics are also affected by the accumulation and degradation of the drug in the lysosomal compartment of target cells. Furthermore, some stimulation may be necessary to trigger the release of EV cargo in vitro, such as shown by the release of VEGF cargo from EVs stimulated by low pH (Taraboletti et al., Citation2006). Another in vitro study also shows that disruption of EVs is required to release bioactive Fas Ligand (FasL) in EVs derived from epithelial ovarian cancer cells. Free FasL could then bind with Fas to induce apoptosis in target cells (Abrahams et al., Citation2003). Nevertheless, drugs encapsulated in EVs are more stable and are able to reach higher concentration in the systemic circulation than the free drug alone, thereby provide significant advantage for sustaining the release of drugs on the targeted cells in vivo (Manca, Giraud, & Zempleni, Citation2016; Sun et al., Citation2010; Zhuang et al., Citation2011).
4.3. Administration route of EVs
EVs can be administrated via different routes to reach the area of the tumour. Studies have shown that the route of injection influences the tissue distribution of drug-loaded EVs in vivo (Smyth et al., Citation2015; Wiklander et al., Citation2015). Intravenous (i.v.) injection is the most widely tested administration route for delivering drug-loaded EVs (Table ). However, in vivo clearance studies reveal that i.v.-injected EVs are rapidly cleared from the circulation with a surprisingly short estimated half-life of approximately 2 minutes (Lai et al., Citation2014; Morishita et al., Citation2015), comparable to i.v.-injected liposomes (Smyth et al., Citation2015). Due to the rapid clearance of EVs from the circulation, EVs administered using i.v. injection show minimal tumour accumulation. EVs were found mainly in the liver and spleen, presumably by the act of macrophages.
In contrast to systemic administration (i.v. injection), EVs can be administered locally by intraperitoneal (i.p.) and subcutaneous (s.c.) injections, resulting in a significantly lower EV accumulation in the liver and spleen (Wiklander et al., Citation2015). Recent advances show that intratumoural administration can deliver drug-loaded EVs at higher concentrations to target cells. When delivered intratumourally on subcutaneous tumour, EVs remain associated with tumours to a significantly greater extent than liposomes (Smyth et al., Citation2015). However, as intratumoural delivery requires access to the tumour site, this approach may be invasive for some organ-specific tumour and will be less desirable when repeated treatments are needed.
The intranasal administration route has been shown to be more effective than other administration routes to deliver the drug to the central nervous system, in particularly to circumvent the challenges of delivery across the blood-brain barrier (BBB). Intranasal delivered-EVs loaded with curcumin and cucurbitacin I were rapidly transported to the mouse brain. Using this route, curcumin loaded-EVs led to a significant reduction in the number of microglial cells. Cucurbitacin I-loaded EVs also successfully increased the rate of tumour apoptosis and showed a concomitant reduction in disease progression in these mice models (Zhuang et al., Citation2011). These reports support that locally administered EVs may perform better than systemic administration of drugs, and that the technique for administration should be chosen depending on the organ-specific tumour.
Therapeutic EVs can also be administered orally as shown by drug-loaded EVs derived from milk and plant extracts for cancer (Munagala et al., Citation2016; Wang et al., Citation2014). Oral administration reduces the accumulation of EVs in the liver in comparison with i.v. injection (Munagala et al., Citation2016). Improved strategy by tagging of EVs with folic acid for oral administration of EV therapeutic has been shown able to target lung tumour (Munagala et al., Citation2016). Development of oral administered EV therapeutics will have to consider their characteristic as they should remain stable in the gastrointestinal (GI) environment where EV will be exposed to low pH, GI mucus, and mucosal immune system (Ensign, Cone, & Hanes, Citation2012). EVs derived from bovine milk were found to overcome these challenges and stable under such degrading conditions (low pH, boiling and freezing) (Pieters et al., Citation2015). Some bovine milk derived-EVs have been shown to escape the intestinal mucosal barrier (Manca et al., Citation2016).
5. Towards EV-based therapies
The development of EV as drug nanocarriers will need to consider how to improve the targeting strategy, to maximise drug loading capacity in EVs, and to reach an economical large scale production of EVs. Up to date, FDA has approved approximately 50 nanoparticles to use in medicine including treatments for cancers (Bobo, Robinson, Islam, Thurecht, & Corrie, Citation2016). The development of EVs as drug nanocarrier is still at an early stage where some clinical trials were reported for DC or MSC-derived EVs (Lener et al., Citation2015). Strategies to increase the targeting and carrier potential of EVs are still being developed. One promising report has shown that the targeting of EV can be improved by the association of EVs with an AAV vector for gene therapy. This strategy was able to rescue partial hearing in mice by direct cochlear injection, showing that the chosen targeting strategy (i.e. ligand) and route of delivery (injection site) could improve the above-mentioned challenges of using EVs as drug nanocarrier (Gyorgy et al., Citation2017). Understanding of the biology of diseases to manipulate EVs to reach its target site and to avoid side effects, such as shown by MSC-derived EVs, is also important to avoid adverse effect such as inducing proliferation of target cancer cells (Roccaro et al., Citation2013; Zhu et al., Citation2012). Finally, to translate these findings into clinical applications, achieving the current safety requirements set by the FDA, European Medicines Agency (EMA) or other regulatory agencies in the respective countries is required. In this section, we will also review some attempts that have been made to increase the production of EVs in order to bring EVs as the new drug nanocarrier in future medicine.
6. Safety and stability of drug-loaded EVs
In June 2014, FDA published a guideline for nanoparticles to be approved and comply with the safety regulation in the application for nanotechnology (https://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm#_ftn1). Considering that this field continues to develop rapidly, two points have been addressed which include the particle dimensions and dimension-dependent properties or phenomena for not only drug application but also including its use in food substances and cosmetic products. In this first point in the guideline, a nanoparticle is defined as a particle with approximately 1 nm to 100 nm. Particle dimension has been shown to contribute to the safety, performance and quality of a nanoparticle. Secondly, a particle up to 1 μm can also be considered as a nanoparticle under this guideline if it exhibits the properties which are attributable to its dimension(s). The EVs, with a size range from 20 nm up to 1 μm will then fall under the same regulation as other nanoparticles, even though it must be noted that currently the regulation for the use of nanoparticles in medicine is still being discussed (Tinkle et al., Citation2014).
Like nanoparticles, the utilization of EVs for therapeutic application demands a comprehensive toxicological assessment. Nonetheless, information regarding the toxicological aspects of using EVs as DDS is inadequate. Due to the heterogeneous components encompassing EVs, they have the capacity to generate an immune response or potentially toxic effects dependent on the origin of the donor cells (e.g allogenic, autologous and xenogeneic) and the target species in which the EVs are being introduced into (Ha, Yang, & Nadithe, Citation2016; Maji et al., Citation2017).
An in vitro investigation using mesenchymal stem cell and bovine milk-derived EVs (BM-EVs), has shown no significant genotoxic effects in response to the two EVs. However, significant collagen-induced platelet aggregation was observed in BM-EVs in a dose-dependent manner, as well as testing positive for endotoxins. The distinction has been suggested due to the possible variation in techniques for EV isolation, EV content or cross-species difference (Maji et al., Citation2017). This may be the case as a recent in vivo study using BM-EVs, purified using acid treatment and ultracentrifugation, observed no systemic toxicity or anaphylaxis after multiple injections over 14 days (Somiya et al., Citation2018). Furthermore, assessment of blood biochemistry and immune markers of C57BL/6 mice dosed with either wildtype or engineered HEK293T cell EVs (8.5 µg protein per dosage) via i.v and intraperitoneally for 3 weeks showed no signs of toxicity and no changes were observed in immune markers with wild type EVs (Zhu et al., Citation2017). Although these results sound promising, these in vitro and in vivo models do not investigate the effect over a more extended period or in a higher dosage. Therefore, this toxicology aspect needs to be assessed before EVs are administered clinically.
Phase I and II clinical trials demonstrated feasibility, safety and low toxicity of exosomes derived from tumor peptide-loaded DCs administered to patients suffering from metastatic melanoma or non-small cell lung cancer (NSCLC) (Pitt et al., Citation2016). In another phase II trial, it has also been reported that from twenty-two patients bearing inoperable NSCLC without tumor progression who have received IFN-γ-DC-derived exosomes, only one patient exhibited a grade three hepatotoxicity (Besse et al., Citation2016). Thus, DC-derived exosomes have minimal toxicity. In both clinical studies, however, the stability of these tumor peptide-loaded DCs was not addressed.
Some studies have been done using EVs alone and not on drug-loaded EVs, which may or may not give similar results. Furthermore, EV derived from different sources may behave differently under certain conditions. For example, bovine milk-derived EVs were shown to be extremely stable under degrading conditions (Pieters et al., Citation2015), while this may not be the same for MSC or tumour–derived EVs. It is also important to know whether long-term storage will affect the stability of EVs and the drug inside EVs. The most recent report has shown that the size of RBC-EVs was not affected during storage for a week at room temperature (Sebaihi et al., Citation2017). Another study has been shown that a single freeze-thaw cycle and storage up to 1 year do not change the characteristic of EVs derived from biological fluids (Yuana et al., Citation2015). The long-term stability of DC-derived exosomes after freezing, their high density in MHC peptide complexes that can be transferred to surrounding antigen presenting cells (APCs), and their potential to trigger NK cells through membrane-bound IL-15/IL-15Rα and NKG2D ligand exposure have been reported suggesting the potential of DC-derived exosomes for immunotherapy approaches (Viaud et al., Citation2009). Hence, over the last decade, DC-derived exosomes have been developed as clinical cell-free cancer vaccines (Lamparski et al., Citation2002; Viaud et al., Citation2009).
7. Up-scale production and quality control of drug-loaded EVs
Commercial production of nanomedicine will include additional steps to improve the targeting and stability of drugs. This process will naturally lead to higher cost than the production of free-drug analogues (Tinkle et al., Citation2014). Thus, EV as drug nanocarrier, similar to other synthetic nanoparticles such as liposomes must show a significant improvement in the clinical benefit. Preserving the quality of EVs for clinical trial as well as large scale manufacturing could be difficult. Positive results from preclinical trial mainly have been obtained through a small scale production of EVs, where potential error can be minimised. Manufacturing of EV in a large scale requires a selection of donor/source cells which are capable producing EV in a large quantity and this will require a strict Good Manufacturing Practice (GMP)-compliant production. In large-scale production, batch-to-batch variations are commonly found in the manufacturing process which can be caused by various parameters such as temperature or pH to culture donor cells which can alter the physical and chemical properties, or by the characteristic of the drug itself, as well as the contents and surface epitopes of EVs. Furthermore, the standardised serum-free medium or fetal bovine serum depleted from bovine vesicles to culture the donor cells for EV production will be necessary, to ensure the quality of EVs for clinical applications (Shelke, Lässer, Gho, & Lötvall, Citation2014). Finally, the properties and functionality of EVs should be kept the same during storage (Zonneveld et al., Citation2014). These challenges should be considered to obtain a large quantity of biologically functional EV as the future drug nanocarrier.
8. Concluding remarks
Current advances have shown that EVs have the potential for therapeutic application and as drug nanocarriers in the future. Advantages of using EVs as drug nanocarriers become more evident as EVs withstand degrading conditions and can be manipulated to increase the dosage of drugs and to enhance tumour targeting. Furthermore, EVs can be used in theranostic applications and deep tissue imaging. However, isolating pure EV population remains an issue. Hence, a more standardised approach to produce clinically stable drug-loaded EVs is needed. Understanding the mechanisms of EV uptake in particular cancer, as well as the development of the disease, are also crucial for the future development of EV-mediated DDS for cancers. Finally, naturally derived EVs from food products (milk/plants) which do not induce toxicity in cross-species application are a potential source that may help the delivery of drugs, reducing the adverse side effect for cancer patients.
Declaration of Interest
Authors declare no conflict of interest.
Acknowledgements
Yuana Yuana is supported by a grant from Focused Ultrasound Foundation. The authors wish to acknowledge Dr. Carolina Soekmadji (QIMR Berghofer Medical Research Institute) for insightful discussion and advice during the development of the manuscript. The authors also would like to thank Kim M. van der Wurff-Jacobs (Pharmaceutical Sciences, University of Utrecht) for flow cytometry measurement presented in Figure .
Additional information
Funding
Notes on contributors
Yuana Yuana
Dr. Yuana Yuana is a senior scientist with expertise in extracellular vesicles in diagnostic and drug delivery. Currently, she and her team work on the development of drug delivery strategies using extracellular vesicles and microbubbles-assisted ultrasound for non-invasive cancer treatment. The current review contains state-of-the-art strategies of the extracellular vesicles-based drug delivery system for cancer treatment.
References
- Abrahams, V. M., Straszewski, S. L., Kamsteeg, M., Hanczaruk, B., Schwartz, P. E., Rutherford, T. J., & Mor, G. (2003). Epithelial ovarian cancer cells secrete functional fas ligand. Cancer Research, 63(17), 5573.
- Admyre, C., Johansson, S. M., Qazi, K. R., Filen, J. J., Lahesmaa, R., Norman, M., … Gabrielsson, S. (2007). Exosomes with immune modulatory features are present in human breast milk. Journal of Immunology, 179(3), 1969–23. doi:10.4049/jimmunol.179.3.1969
- Al Faraj, A., Gazeau, F., Fau - Wilhelm, C., Wilhelm, C., Fau - Devue, C., Devue, C., … Rautou, P. E. (2012). Endothelial cell-derived microparticles loaded with iron oxide nanoparticles: Feasibility of MR imaging monitoring in mice. (1527-1315 (Electronic)). doi:10.1094/PDIS-11-11-0999-PDN
- Allenson, K., Castillo, J., San Lucas, F. A., Scelo, G., Kim, D. U., Bernard, V., … Alvarez, H. (2017). High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO, 28(4), 741–747. doi:10.1093/annonc/mdx004
- Alsaweed, M., Lai, C. T., Hartmann, P. E., Geddes, D. T., & Kakulas, F. (2016). Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Scientific Reports, 6, 20680. doi:10.1038/srep20680
- Andriola Silva, A. K., Di Corato, R., Pellegrino, T., Chat, S., Pugliese, G., Luciani, N., … Wilhelm, C. (2013). Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials. Nanoscale, 5(23), 11374–11384. doi:10.1039/C3NR01541F
- Antes, T. J., Middleton, R. C., Luther, K. M., Ijichi, T., Peck, K. A., Liu, W. J., … Marban, E. (2018). Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. Journal of Nanobiotechnology, 16(1), 61. doi:10.1186/s12951-018-0388-4
- Armstrong, J. P., Holme, M. N., & Stevens, M. M. (2017). Re-Engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano, 11, 69–83. doi:10.1021/acsnano.6b07607
- Baietti, M. F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., … David, G. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology, 14(7), 677–685. doi:10.1038/ncb2502
- Barile, L., & Vassalli, G. (2017). Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & Therapeutics, 174, 63–78. doi:10.1016/j.pharmthera.2017.02.020
- Barteneva, N. S., Fasler-Kan, E., Bernimoulin, M., Stern, J. N. H., Ponomarev, E. D., Duckett, L., & Vorobjev, I. A. (2013). Circulating microparticles: Square the circle. BMC Cell Biology, 14(1), 23. doi:10.1186/1471-2121-14-23
- Becker, A., Thakur, B. K., Weiss, J. M., Kim, H. S., Peinado, H., & Lyden, D. (2016). Extracellular vesicles in cancer: Cell-to-Cell mediators of metastasis. Cancer Cell, 30(6), 836–848. doi:10.1016/j.ccell.2016.10.009
- Besse, B., Charrier, M., Lapierre, V., Dansin, E., Lantz, O., Planchard, D., … Chaput, N. (2016). Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology, 5(4), e1071008. doi:10.1080/2162402x.2015.1071008
- Biller, S. J., Schubotz, F., Roggensack, S. E., Thompson, A. W., Summons, R. E., & Chisholm, S. W. (2014). Bacterial vesicles in marine ecosystems. Science, 343(6167), 183–186. doi:10.1126/science.1243457
- Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., & Corrie, S. R. (2016). Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharmaceutical Research, 33(10), 2373–2387. doi:10.1007/s11095-016-1958-5
- Bobrie, A., Colombo, M., Krumeich, S., Raposo, G., & Thery, C. (2012). Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. Journal of Extracell Vesicles, 1, 18397. doi:10.3402/jev.v1i0.18397
- Brown, L., Wolf, J. M., Prados-Rosales, R., & Casadevall, A. (2015). Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nature Reviews Microbiology, 13(10), 620–630. doi:10.1038/nrmicro3480
- Bruno, S., Collino, F., Deregibus, M. C., Grange, C., Tetta, C., & Camussi, G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells and Development, 22(5), 758–771. doi:10.1089/scd.2012.0304
- Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., … Camussi, G. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology : JASN, 20(5), 1053–1067. doi:10.1681/ASN.2008070798
- Bruno, S., Tapparo, M., Collino, F., Chiabotto, G., Deregibus, M. C., Soares Lindoso, R., … Camussi, G. (2017). Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Engineering Part A, 23(21–22), 1262–1273. doi:10.1089/ten.TEA.2017.0069
- Chang, M., Hsiao, J. K., Yao, M., Chien, L. Y., Hsu, S. C., Ko, B. S., … Huang, D. M. (2010). Homologous RBC-derived vesicles as ultrasmall carriers of iron oxide for magnetic resonance imaging of stem cells. Nanotechnology, 21(23), 235103. doi:10.1088/0957-4484/21/23/235103
- Chaput, N., Taieb, J., Schartz, N. E., Andre, F., Angevin, E., & Zitvogel, L. (2004). Exosome-based immunotherapy. Cancer Immunology, Immunotherapy : CII, 53(3), 234–239. doi:10.1007/s00262-003-0472-x
- Charoenviriyakul, C., Takahashi, Y., Morishita, M., Matsumoto, A., Nishikawa, M., & Takakura, Y. (2017). Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. European Journal of Pharmaceutical Sciences, 96, 316–322. doi:http://dx.doi.org/10.1016/j.ejps.2016.10.009
- Chen, D. J., Osterrieder, N., Metzger, S. M., Buckles, E., Doody, A. M., DeLisa, M. P., & Putnam, D. (2010). Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proceedings of the National Academy of Sciences of the United States of America, 107(7), 3099–3104. doi:10.1073/pnas.0805532107
- Chen, T., Guo, J., Yang, M., Zhu, X., & Cao, X. (2011). Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. Journal of Immunology, 186(4), 2219–2228. doi:10.4049/jimmunol.1002991
- Choi, D. S., Kim, D. K., Kim, Y. K., & Gho, Y. S. (2013). Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics, 13(10–11), 1554–1571. doi:10.1002/pmic.201200329
- Choi, D. S., Kim, D. K., Kim, Y. K., & Gho, Y. S. (2015). Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrometry Reviews, 34(4), 474–490. doi:10.1002/mas.21420
- Choi, H., & Lee, D. S. (2016). Illuminating the physiology of extracellular vesicles. Stem Cell Research & Therapy, 7, 55. doi:10.1186/s13287-016-0316-1
- Deatherage, B. L., & Cookson, B. T. (2012). Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infection and Immunity, 80(6), 1948–1957. doi:10.1128/iai.06014-11
- Del Fattore, A., Luciano, R., Saracino, R., Battafarano, G., Rizzo, C., Pascucci, L., … Muraca, M. (2015). Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opinion on Biological Therapy, 15(4), 495–504. doi:10.1517/14712598.2015.997706
- Denzer, K., Kleijmeer, M. J., Heijnen, H. F., Stoorvogel, W., & Geuze, H. J. (2000). Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. Journal of Cell Science, 113(Pt 19), 3365–3374.
- Di Vizio, D., Morello, M., Dudley, A. C., Schow, P. W., Adam, R. M., Morley, S., … Freeman, M. R. (2012). Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. The American Journal of Pathology, 181(5), 1573–1584. doi:10.1016/j.ajpath.2012.07.030
- Ding, C., Tong, L., Feng, J., & Fu, J. (2016). Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules, 21(12), 1715. doi:10.3390/molecules21121715
- Eirin, A., Zhu, X. Y., Jonnada, S., Lerman, A., van Wijnen, A. J., & Lerman, L. O. (2018). Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in Swine. Cell Transplantation, 27(7), 1080–1095. doi:10.1177/0963689718780942
- Ensign, L. M., Cone, R., & Hanes, J. (2012). Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Advanced Drug Delivery Reviews, 64(6), 557–570. doi:10.1016/j.addr.2011.12.009
- Escudier, B., Dorval, T., Chaput, N., Andre, F., Caby, M. P., Novault, S., … Zitvogel, L. (2005). Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. Journal of Translational Medicine, 3(1), 10. doi:10.1186/1479-5876-3-10
- Fuhrmann, G., Serio, A., Mazo, M., Nair, R., & Stevens, M. M. (2015). Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. Journal of Controlled Release : Official Journal of the Controlled Release Society, 205, 35–44. doi:10.1016/j.jconrel.2014.11.029
- Gardiner, C., Di Vizio, D., Sahoo, S., Thery, C., Witwer, K. W., Wauben, M., & Hill, A. F. (2016). Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. Journal of Extracellular Vesicles, 5, 32945. doi:10.3402/jev.v5.32945
- Gong, J., Jaiswal, R., Mathys, J. M., Combes, V., Grau, G. E., & Bebawy, M. (2012). Microparticles and their emerging role in cancer multidrug resistance. Cancer Treatment Reviews, 38(3), 226–234. doi:10.1016/j.ctrv.2011.06.005
- Gruenberg, J., & Stenmark, H. (2004). The biogenesis of multivesicular endosomes. Nature Reviews Molecular Cell Biology, 5(4), 317–323. doi:10.1038/nrm1360
- Gujrati, V., Kim, S., Kim, S. H., Min, J. J., Choy, H. E., Kim, S. C., & Jon, S. (2014). Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano, 8(2), 1525–1537. doi:10.1021/nn405724x
- Gyorgy, B., Hung, M. E., Breakefield, X. O., & Leonard, J. N. (2015). Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annual Review of Pharmacology and Toxicology, 55, 439–464. doi:10.1146/annurev-pharmtox-010814-124630
- Gyorgy, B., Sage, C., Indzhykulian, A. A., Scheffer, D. I., Brisson, A. R., Tan, S., … Maguire, C. A. (2017). Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Molecular Therapy : the Journal of the American Society of Gene Therapy, 25(2), 379–391. doi:10.1016/j.ymthe.2016.12.010
- Ha, D., Yang, N., & Nadithe, V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharmaceutica Sinica B, 6(4), 287–296. doi:10.1016/j.apsb.2016.02.001
- Haney, M. J., Klyachko, N. L., Zhao, Y., Gupta, R., Plotnikova, E. G., He, Z., … Batrakova, E. V. (2015). Exosomes as drug delivery vehicles for Parkinson’s disease therapy. Journal of Controlled Release, 207, 18–30. doi:http://dx.doi.org/10.1016/j.jconrel.2015.03.033
- Hartjes, T. A., & Mytnyk, S. (2019). Extracellular vesicle quantification and characterization: Common methods and emerging approaches. Bioengineering (Basel), 6(1). doi:10.3390/bioengineering6010007
- Hood, J. L., Scott, M. J., & Wickline, S. A. (2014). Maximizing exosome colloidal stability following electroporation. Analytical Biochemistry, 448, 41–49. doi:10.1016/j.ab.2013.12.001
- Huang, C., Neoh, K. G., Xu, L., Kang, E. T., & Chiong, E. (2012). Polymeric nanoparticles with encapsulated superparamagnetic iron oxide and conjugated cisplatin for potential bladder cancer therapy. Biomacromolecules, 13(8), 2513–2520. doi:10.1021/bm300739w
- Hurley, J. H., & Odorizzi, G. (2012). Get on the exosome bus with ALIX. Nature Cell Biology, 14(7), 654–655. doi:10.1038/ncb2530
- Hwang Do, W., Choi, H., Jang, S. C., Yoo, M. Y., Park, J. Y., Choi, N. E., … Lee, D. S. (2015). Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Scientific Reports, 5, 15636. doi:10.1038/srep15636
- Imai, T., Takahashi, Y., Nishikawa, M., Kato, K., Morishita, M., Yamashita, T., … Takakura, Y. (2015). Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. Journal of Extracellular Vesicles, 4, 26238. doi:10.3402/jev.v4.26238
- Jain, R. K., & Stylianopoulos, T. (2010). Delivering nanomedicine to solid tumors. Nature Reviews. Clinical Oncology, 7(11), 653–664. doi:10.1038/nrclinonc.2010.139
- Jarockyte, G., Daugelaite, E., Stasys, M., Statkute, U., Poderys, V., Tseng, T. C., & Rotomskis, R. (2016). Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. International Journal of Molecular Sciences, 17(8), 1193. doi:10.3390/ijms17081193
- Johnsen, K. B., Gudbergsson, J. M., Skov, M. N., Pilgaard, L., Moos, T., & Duroux, M. (2014). A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochimica Et Biophysica Acta, 1846(1), 75–87. doi:10.1016/j.bbcan.2014.04.005
- Ju, S., Mu, J., Dokland, T., Zhuang, X., Wang, Q., Jiang, H., … Zhang, H. G. (2013). Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Molecular Therapy : The Journal of the American Society of Gene Therapy, 21(7), 1345–1357. doi:10.1038/mt.2013.64
- Kalimuthu, S., Gangadaran, P., Li, X. J., Oh, J. M., Lee, H. W., Jeong, S. Y., … Ahn, B. C. (2016). In vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model. Scientific Reports, 6, 30418. doi:10.1038/srep30418
- Katsuda, T., & Ochiya, T. (2015). Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Research & Therapy, 6, 212. doi:10.1186/s13287-015-0214-y
- King, H. W., Michael, M. Z., & Gleadle, J. M. (2012). Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer, 12(1), 421. doi:10.1186/1471-2407-12-421
- Kitai, Y., Kawasaki, T., Sueyoshi, T., Kobiyama, K., Ishii, K. J., Zou, J., & Kawai, T. (2017). DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. Journal of Immunology, 198, 1649–1659. doi:10.4049/jimmunol.1601694
- Lai, C. P., Kim, E. Y., Badr, C. E., Weissleder, R., Mempel, T. R., Tannous, B. A., & Breakefield, X. O. (2015). Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nature Communications, 6, 7029. doi:10.1038/ncomms8029
- Lai, C. P., Mardini, O., Ericsson, M., Prabhakar, S., Maguire, C. A., Chen, J. W., … Breakefield, X. O. (2014). Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano, 8(1), 483–494. doi:10.1021/nn404945r
- Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S., Choo, A., Chen, T. S., … Lim, S. K. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4(3), 214–222. doi:10.1016/j.scr.2009.12.003
- Lamparski, H. G., Metha-Damani, A., Yao, J. Y., Patel, S., Hsu, D. H., Ruegg, C., & Le Pecq, J. B. (2002). Production and characterization of clinical grade exosomes derived from dendritic cells. Journal of Immunological Methods, 270(2), 211–226.
- Lavialle, F., Deshayes, S., Gonnet, F., Larquet, E., Kruglik, S. G., Boisset, N., … Tatischeff, I. (2009). Nanovesicles released by Dictyostelium cells: A potential carrier for drug delivery. International Journal of Pharmaceutics, 380(1–2), 206–215. doi:10.1016/j.ijpharm.2009.06.039
- Lener, T., Gimona, M., Aigner, L., Borger, V., Buzas, E., Camussi, G., … Giebel, B. (2015). Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. Journal of Extracellular Vesicles, 4, 30087. doi:10.3402/jev.v4.30087
- Li, J., Chen, Y., Guo, X., Zhou, L., Jia, Z., Peng, Z., … Ren, C. (2017). GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. Journal of Cellular and Molecular Medicine, 21(5), 838–847. doi:10.1111/jcmm.12941
- Li, J., Lee, Y., Johansson, H. J., Mager, I., Vader, P., Nordin, J. Z., … Andaloussi, S. E. (2015). Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. Journal of Extracellular Vesicles, 4, 26883. doi:10.3402/jev.v4.26883
- Lima, L. G., Chammas, R., Monteiro, R. Q., Moreira, M. E., & Barcinski, M. A. (2009). Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Letters, 283(2), 168–175. doi:10.1016/j.canlet.2009.03.041
- Lotvall, J., Hill, A. F., Hochberg, F., Buzas, E. I., Di Vizio, D., Gardiner, C., … Thery, C. (2014). Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International society for extracellular vesicles. Journal of Extracellular Vesicles, 3, 26913. doi:10.3402/jev.v3.26913
- Lu, M., Xing, H., Xun, Z., Yang, T., Zhao, X., Cai, C., … Ding, P. (2018). Functionalized extracellular vesicles as advanced therapeutic nanodelivery systems. European Journal of Pharmaceutical Sciences : Official Journal of the European Federation for Pharmaceutical Sciences, 121, 34–46. doi:10.1016/j.ejps.2018.05.001
- Maji, S., Yan, I. K., Parasramka, M., Mohankumar, S., Matsuda, A., & Patel, T. (2017). In vitro toxicology studies of extracellular vesicles. Journal of Applied Toxicology : JAT, 37(3), 310–318. doi:10.1002/jat.3362
- Manca, S., Giraud, D., & Zempleni, J. (2016). Bioavailability and biodistribution of fluorophore-labeled exosomes from cow’s milk after intravenous and oral administration in C57Bl/6J Mice. The FASEB Journal, 30(1 Supplement), 690–698.
- Matsumoto, A., Takahashi, Y., Nishikawa, M., Sano, K., Morishita, M., Charoenviriyakul, C., … Takakura, Y. (2017). Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. Journal of Pharmaceutical Sciences, 106(1), 168–175. doi:10.1016/j.xphs.2016.07.022
- McKiernan, J., Donovan, M. J., O’Neill, V., Bentink, S., Noerholm, M., Belzer, S., … Carroll, P. (2016). A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncology, 2(7), 882–889. doi:10.1001/jamaoncol.2016.0097
- Melnik, B. C., & Schmitz, G. (2019). Exosomes of pasteurized milk: Potential pathogens of Western diseases. Journal of Translational Medicine, 17(3), 1–33. doi:10.1186/s12967-018-1760-8
- Minciacchi, V. R., You, S., Spinelli, C., Morley, S., Zandian, M., Aspuria, P. J., … Di Vizio, D. (2015). Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget, 6(13), 11327–11341. doi:10.18632/oncotarget.3598
- Moon, P. G., Lee, J. E., Cho, Y. E., Lee, S. J., Jung, J. H., Chae, Y. S., … Baek, M. C. (2016). Identification of developmental endothelial locus-1 on circulating extracellular vesicles as a novel biomarker for early breast cancer detection. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research, 22(7), 1757–1766. doi:10.1158/1078-0432.ccr-15-0654
- Morales-Kastresana, A., Telford, B., Musich, T. A., McKinnon, K., Clayborne, C., Braig, Z., … Watson, D. C. (2017). Labeling extracellular vesicles for nanoscale flow cytometry. Scientific Reports,7(1), 1878. doi:10.1038/s41598-017-01731-2
- Morishita, M., Takahashi, Y., Nishikawa, M., Sano, K., Kato, K., Yamashita, T., … Takakura, Y. (2015). Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. Journal of Pharmaceutical Sciences, 104(2), 705–713. doi:10.1002/jps.24251
- Morse, M. A., Garst, J., Osada, T., Khan, S., Hobeika, A., Clay, T. M., … Lyerly, H. K. (2005). A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of Translational Medicine, 3(1), 9. doi:10.1186/1479-5876-3-9
- Mulcahy, L. A., Pink, R. C., & Carter, D. R. F. (2014). Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles, 3. doi:10.3402/jev.v3403.24641
- Munagala, R., Aqil, F., Jeyabalan, J., & Gupta, R. C. (2016). Bovine milk-derived exosomes for drug delivery. Cancer Letters, 371(1), 48–61. doi:10.1016/j.canlet.2015.10.020
- Muralidharan-Chari, V., Clancy, J., Plou, C., Romao, M., Chavrier, P., Raposo, G., & D’Souza-Schorey, C. (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current Biology: CB, 19(22), 1875–1885. doi:10.1016/j.cub.2009.09.059
- Muralidharan-Chari, V., Clancy, J. W., Sedgwick, A., & D’Souza-Schorey, C. (2010). Microvesicles: Mediators of extracellular communication during cancer progression. Journal of Cell Science, 123(Pt 10), 1603–1611. doi:10.1242/jcs.064386
- Nelson, A. G., Zhang, X., Ganapathi, U., Szekely, Z., Flexner, C. W., Owen, A., & Sinko, P. J. (2015). Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. Journal of Controlled Release : Official Journal of the Controlled Release Society, 219, 669–680. doi:10.1016/j.jconrel.2015.08.042
- Nordin, J. Z., Lee, Y., Vader, P., Mäger, I., Johansson, H. J., Heusermann, W., … Andaloussi, S. E. L. (2015). Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine: Nanotechnology, Biology and Medicine, 11(4), 879–883. doi:10.1016/j.nano.2015.01.003
- Nunez, R., Sancho-Martinez, S. M., Novoa, J. M., & Lopez-Hernandez, F. J. (2010). Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death and Differentiation, 17(11), 1665–1671. doi:10.1038/cdd.2010.96
- Ohno, S. I., Takanashi, M., Sudo, K., Ueda, S., Ishikawa, A., Matsuyama, N., … Kuroda, M. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Molecular Therapy, 21(1), 185–191. doi:10.1038/mt.2012.180
- Olaya-Abril, A., Prados-Rosales, R., McConnell, M. J., Martín-Peña, R., González-Reyes, J. A., Jiménez-Munguía, I., … Rodríguez-Ortega, M. J. (2014). Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. Journal of Proteomics, 106, 46–60. doi:10.1016/j.jprot.2014.04.023
- Osti, D., Del Bene, M., Rappa, G., Santos, M., Matafora, V., Richichi, C., … DiMeco, F. (2019). Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clinical Cancer Research, 25(1), 266–276. doi:10.1158/1078-0432.ccr-18-1941
- Ostrowski, M., Carmo, N. B., Krumeich, S., Fanget, I., Raposo, G., Savina, A., … Thery, C. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology, 12(1), sup pp 11–13, 19–30. doi:10.1038/ncb2000
- Otranto, M., Sarrazy, V., Bonte, F., Hinz, B., Gabbiani, G., & Desmouliere, A. (2012). The role of the myofibroblast in tumor stroma remodeling. Cell Adhesion & Migration, 6(3), 203–219. doi:10.4161/cam.20377
- Parish, C. R. (1999). Fluorescent dyes for lymphocyte migration and proliferation studies. Immunology and Cell Biology, 77(6), 499–508. doi:10.1046/j.1440-1711.1999.00877.x
- Pascucci, L., Cocce, V., Bonomi, A., Ami, D., Ceccarelli, P., Ciusani, E., … Pessina, A. (2014). Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. Journal of Controlled Release : Official Journal of the Controlled Release Society, 192, 262–270. doi:10.1016/j.jconrel.2014.07.042
- Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V. R., & Rodriguez-Torres, M. D. P. (2018). Nano based drug delivery systems: Recent developments and future prospects. 16, (1), 71. doi:10.1186/s12951-018-0392-8
- Pattni, B. S., Chupin, V. V., & Torchilin, V. P. (2015). New developments in liposomal drug delivery. Chemical Reviews, 115(19), 10938–10966. doi:10.1021/acs.chemrev.5b00046
- Perez-Bermudez, P., Blesa, J., Soriano, J. M., & Marcilla, A. (2016). Extracellular vesicles in food: Experimental evidence of their secretion in grape fruits. European Journal of Pharmaceutical Sciences : Official Journal of the European Federation for Pharmaceutical Sciences. doi:10.1016/j.ejps.2016.09.022
- Pieters, B. C., Arntz, O. J., Bennink, M. B., Broeren, M. G., van Caam, A. P., Koenders, M. I., … van de Loo, F. A. (2015). Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-beta. PloS One, 10(3), e0121123. doi:10.1371/journal.pone.0121123
- Piffoux, M., Silva, A. K. A., & Wilhelm, C. (2018). Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano, 12(7), 6830–6842. doi:10.1021/acsnano.8b02053
- Pitt, J. M., Andre, F., Amigorena, S., Soria, J. C., Eggermont, A., Kroemer, G., & Zitvogel, L. (2016). Dendritic cell-derived exosomes for cancer therapy. The Journal of Clinical Investigation, 126(4), 1224–1232. doi:10.1172/jci81137
- Pospichalova, V., Svoboda, J., Dave, Z., Kotrbova, A., Kaiser, K., Klemova, D., & Bryja, V. (2015). Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. Journal of Extracellular Vesicles; Vol 4 (2015) Incl Supplements, 25530. doi:10.3402/jev.v4.25530
- Quah, B. J. C., & Parish, C. R. (2010). The Use of Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) to monitor lymphocyte proliferation. Journal of Visualized Experiments, 44, e2259. doi:10.3791/2259
- Ramirez, M. I., Amorim, M. G., Gadelha, C., & Milic, I. (2018). Technical challenges of working with extracellular vesicles. Nanoscale, 10(3), 881–906. doi:10.1039/c7nr08360b
- Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. The Journal of Cell Biology, 200(4), 373–383. doi:10.1083/jcb.201211138
- Rivera, J., Cordero, R. J. B., Nakouzi, A. S., Frases, S., Nicola, A., & Casadevall, A. (2010). Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proceedings of the National Academy of Sciences, 107(44), 19002–19007. doi:10.1073/pnas.1008843107
- Robbins, P. D., & Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nature Reviews Immunology, 14(3), 195–208. doi:10.1038/nri3622
- Roccaro, A. M., Sacco, A., Maiso, P., Azab, A. K., Tai, Y. T., Reagan, M., … Ghobrial, I. M. (2013). BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. The Journal of Clinical Investigation, 123(4), 1542–1555. doi:10.1172/JCI66517
- Saari, H., Lázaro-Ibáñez, E., Viitala, T., Vuorimaa-Laukkanen, E., Siljander, P., & Yliperttula, M. (2015). Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. Journal of Controlled Release, 220(Part B), 727–737. doi:10.1016/j.jconrel.2015.09.031
- Safaei, R., Larson, B. J., Cheng, T. C., Gibson, M. A., Otani, S., Naerdemann, W., & Howell, S. B. (2005). Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Molecular Cancer Therapeutics, 4(10), 1595–1604. doi:10.1158/1535-7163.mct-05-0102
- Santhosh, P. B., & Ulrih, N. P. (2013). Multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Letters, 336(1), 8–17. doi:10.1016/j.canlet.2013.04.032
- Schmidt, O., & Teis, D. (2012). The ESCRT machinery. Current Biology: CB, 22(4), R116–120. doi:10.1016/j.cub.2012.01.028
- Sebaihi, N., Boeck, B. D., Yuana, Y., Nieuwland, R., & Pétry, J. (2017). Dimensional characterization of extracellular vesicles using atomic force microscopy. Measurement Science and Technology, 28(3), 034006. doi:10.1088/1361-6501/28/3/034006
- Shelke, G. V., Lässer, C., Gho, Y. S., & Lötvall, J. (2014). Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. Journal of Extracellular Vesicles, 3. doi:10.3402/jev.v3403.24783
- Smyth, T., Kullberg, M., Malik, N., Smith-Jones, P., Graner, M. W., & Anchordoquy, T. J. (2015). Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. Journal of Controlled Release, 199, 145–155. doi:http://dx.doi.org/10.1016/j.jconrel.2014.12.013
- Soekmadji, C., & Nelson, C. C. (2015). The emerging role of extracellular vesicle-mediated drug resistance in cancers: Implications in advanced prostate cancer. BioMed Research International, 454837. doi:10.1155/2015/454837
- Soekmadji, C., Riches, J. D., Russell, P. J., Ruelcke, J. E., McPherson, S., Wang, C., … Nelson, C. C. (2016). Modulation of paracrine signaling by CD9 positive small extracellular vesicles mediates cellular growth of androgen deprived prostate cancer. Oncotarget. doi:10.18632/oncotarget.11111
- Soekmadji, C., Rockstroh, A., Ramm, G. A., Nelson, C. C., & Russell, P. J. (2017). Extracellular vesicles in the adaptive process of prostate cancer during inhibition of androgen receptor signaling by enzalutamide. Proteomics,17, 23–24. doi:10.1002/pmic.201600427
- Soekmadji, C., Russell, P. J., & Nelson, C. C. (2013). Exosomes in prostate cancer: Putting together the pieces of a puzzle. Cancers, 5(4), 1522–1544. doi:10.3390/cancers5041522
- Somiya, M., Yoshioka, Y., & Ochiya, T. (2018). Biocompatibility of highly purified bovine milk-derived extracellular vesicles. Journal of Extracellular Vesicles, 7(1), 1440132. doi:10.1080/20013078.2018.1440132
- Subra, C., Laulagnier, K., Perret, B., & Record, M. (2007). Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie, 89(2), 205–212. doi:10.1016/j.biochi.2006.10.014
- Suetsugu, A., Honma, K., Saji, S., Moriwaki, H., Ochiya, T., & Hoffman, R. M. (2013). Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Advanced Drug Delivery Reviews, 65(3), 383–390. doi:http://dx.doi.org/10.1016/j.addr.2012.08.007
- Sukhanova, A., Bozrova, S., Sokolov, P., Berestovoy, M., Karaulov, A., & Nabiev, I. (2018). Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Research Letters, 13(1), 44. doi:10.1186/s11671-018-2457-x
- Sun, D., Zhuang, X., Xiang, X., Liu, Y., Zhang, S., Liu, C., … Zhang, H. G. (2010). A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy : The Journal of the American Society of Gene Therapy, 18(9), 1606–1614. doi:10.1038/mt.2010.105
- Takahashi, Y., Nishikawa, M., Shinotsuka, H., Matsui, Y., Ohara, S., Imai, T., & Takakura, Y. (2013). Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. Journal of Biotechnology, 165(2), 77–84. doi:10.1016/j.jbiotec.2013.03.013
- Tang, K., Zhang, Y., Zhang, H., Xu, P., Liu, J., Ma, J., … Huang, B. (2012). Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nature Communications, 3, 1282. doi:10.1038/ncomms2282
- Taraboletti, G., D’Ascenzo, S., Giusti, I., Marchetti, D., Borsotti, P., Millimaggi, D., … Dolo, V. (2006). Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia (New York, N.Y.), 8(2), 96–103. doi:10.1593/neo.05583
- Tatischeff, I., Larquet, E., Falcon-Perez, J. M., Turpin, P. Y., & Kruglik, S. G. (2012). Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. Journal of Extracell Vesicles, 1, 19179. doi:10.3402/jev.v1i0.19179
- Thery, C., Boussac, M., Veron, P., Ricciardi-Castagnoli, P., Raposo, G., Garin, J., & Amigorena, S. (2001). Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. Journal of Immunology (baltimore, Md. : 1950), 166(12), 7309–7318. doi:10.4049/jimmunol.166.12.7309
- Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., … Zuba-Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. doi:10.1080/20013078.2018.1535750
- Tian, Y., Li, S., Song, J., Ji, T., Zhu, M., Anderson, G. J., … Nie, G. (2014). A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials, 35(7), 2383–2390. doi:http://dx.doi.org/10.1016/j.biomaterials.2013.11.083
- Tinkle, S., McNeil, S. E., Muhlebach, S., Bawa, R., Borchard, G., Barenholz, Y. C., … Desai, N. (2014). Nanomedicines: Addressing the scientific and regulatory gap. Annals of the New York Academy of Sciences, 1313, 35–56. doi:10.1111/nyas.12403
- Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., … Simons, M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science, 319(5867), 1244–1247. doi:10.1126/science.1153124
- Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., & Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659. doi:10.1038/ncb1596
- van der Meel, R., Fens, M. H., Vader, P., van Solinge, W. W., Eniola-Adefeso, O., & Schiffelers, R. M. (2014). Extracellular vesicles as drug delivery systems: Lessons from the liposome field. Journal of Controlled Release : Official Journal of the Controlled Release Society, 195, 72–85. doi:10.1016/j.jconrel.2014.07.049
- van der Pol, L., Stork, M., & van der Ley, P. (2015). Outer membrane vesicles as platform vaccine technology. Biotechnology Journal, 10(11), 1689–1706. doi:10.1002/biot.201400395
- Varga, Z., Gyurko, I., Paloczi, K., Buzas, E. I., Horvath, I., Hegedus, N., … Szigeti, K. (2016). Radiolabeling of extracellular vesicles with (99m)Tc for quantitative in vivo imaging studies. Cancer Biotherapy & Radiopharmaceuticals, 31(5), 168–173. doi:10.1089/cbr.2016.2009
- Viaud, S., Terme, M., Flament, C., Taieb, J., Andre, F., Novault, S., … Chaput, N. (2009). Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Ralpha. PloS One, 4(3), e4942. doi:10.1371/journal.pone.0004942
- Wang, B., Zhuang, X., Deng, Z. B., Jiang, H., Mu, J., Wang, Q., … Zhang, H. G. (2014). Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Molecular Therapy : The Journal of the American Society of Gene Therapy, 22(3), 522–534. doi:10.1038/mt.2013.190
- Wang, L. Y., Shi, X. Y., Yang, C. S., & Huang, D. M. (2013). Versatile RBC-derived vesicles as nanoparticle vector of photosensitizers for photodynamic therapy. Nanoscale, 5(1), 416–421. doi:10.1039/c2nr32506c
- Wiklander, O. P. B., Nordin, J. Z., O’Loughlin, A., Gustafsson, Y., Corso, G., Mäger, I., … Andaloussi, S. E. L. (2015). Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles, 4. doi:10.3402/jev.v3404.26316
- Wolfers, J., Lozier, A., Raposo, G., Regnault, A., Thery, C., Masurier, C., … Zitvogel, L. (2001). Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Medicine, 7(3), 297–303. doi:10.1038/85438
- Wu, T., & Tang, M. (2018). Review of the effects of manufactured nanoparticles on mammalian target organs. Journal of Applied Toxicology : JAT, 38(1), 25–40. doi:10.1002/jat.3499
- Xin, Y., Yin, M., Zhao, L., Meng, F., & Luo, L. (2017). Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biology & Medicine, 14(3), 228–241. doi:10.20892/j.issn.2095-3941.2017.0052
- Yuana, Y., Boing, A. N., Grootemaat, A. E., van der Pol, E., Hau, C. M., Cizmar, P., … Nieuwland, R. (2015). Handling and storage of human body fluids for analysis of extracellular vesicles. Journal of Extracellular Vesicles, 4, 29260. doi:10.3402/jev.v4.29260
- Yuana, Y., Jiang, L., Lammertink, B. H. A., Vader, P., Deckers, R., Bos, C., … Moonen, C. T. (2017). Microbubbles-Assisted ultrasound triggers the release of extracellular vesicles. International Journal of Molecular Sciences, 18(8). doi:10.3390/ijms18081610
- Yuana, Y., Levels, J., Grootemaat, A., Sturk, A., & Nieuwland, R. (2014). Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. Journal of Extracell Vesicles, 3, 23262. doi:10.3402/jev.v3.23262
- Yuana, Y., Sturk, A., & Nieuwland, R. (2013). Extracellular vesicles in physiological and pathological conditions. Blood Reviews, 27(1), 31–39. doi:10.1016/j.blre.2012.12.002
- Zhu, W., Huang, L., Li, Y., Zhang, X., Gu, J., Yan, Y., … Xu, W. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Letters, 315(1), 28–37. doi:10.1016/j.canlet.2011.10.002
- Zhu, X., Badawi, M., Pomeroy, S., Sutaria, D. S., Xie, Z., Baek, A., … Phelps, M. A. (2017). Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. Journal of Extracellular Vesicles, 6(1), 1324730. doi:10.1080/20013078.2017.1324730
- Zhuang, X., Xiang, X., Grizzle, W., Sun, D., Zhang, S., Axtell, R. C., … Zhang, H.-G. (2011). Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Molecular Therapy, 19(10), 1769–1779. doi:10.1038/mt.2011.164
- Zonneveld, M. I., Brisson, A. R., van Herwijnen, M. J., Tan, S., van de Lest, C. H., Redegeld, F. A., … Nolte-’t Hoen, E. N. (2014). Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. Journal of Extracell Vesicles, 3. doi:10.3402/jev.v3.24215