?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lung tumor is the first malignant tumor with the highest mortality, but only no more than one-third of patients can be treated by surgical resection. Microwave ablation (MWA) has become a new adjuvant therapeutic mean for lung tumors because of its low trauma, short treatment time, large ablation volume and wide application range. However, the treatment parameters of MWA, such as input power and ablation time, still depend on the doctors’ experience, which leads to the ineffectiveness of MWA. Therefore, the accurate modeling of temperature distribution of lung tumor MWA has become a significant technical problem to be solved. Recent research was devoted to personalized characterization of lung tumor parameters, finite element analysis of temperature distribution in MWA and accurate ablation effect evaluation. In this paper, a review of the recently obtained results and data will be presented and discussed.
1. Introduction
Lung tumor is one of the most serious threats to human health. In 2020, primary lung tumor is the second most common malignancy worldwide with approximately 2.2 million new cases and 1.8 million deaths [Citation1,Citation2]. Surgical resection is still the main method for the treatment of lung tumors. But due to the lack of typical clinical symptoms of lung tumors, many patients have reached advanced stage at the time of diagnosis and are unable to undergo surgical resection [Citation3].
Some adjuvant treatments are gradually emerging for the treatment of lung tumors, including radiotherapy, chemotherapy and thermal ablation. Imaged-guided thermal ablation techniques, including radiofrequency ablation (RFA), microwave ablation (MWA) and cryoablation have been widely used in the treatment of inoperable lung tumor [Citation4]. Other techniques, including laser ablation and irreversible electroporation (IRE), are not widely used in lung ablation due to lack of clinical data [Citation5]. Compared with other thermal ablation methods, cryoablation has the disadvantages of long therapeutic period, increased bleeding risk and complex preparation process. It is still controversial regarding cryoablation’s superiority over RFA and MWA [Citation6,Citation7]. RFA is easily affected by the heat sink effect of peripheral blood vessels [Citation8,Citation9] and tissue carbonization [Citation10,Citation11], and the electric field distribution is not easy to be uniformly controlled, resulting in incomplete ablation [Citation12]. Furthermore, it is seen from the past researches that the RFA can be employed only if the tumor size is less than 3 mm. In some cases, the skin burning is also a major disadvantage of the RFA [Citation10,Citation13]. MWA is not easily affected by heat sink effect [Citation14,Citation15] and has the advantages of minimal trauma, good tolerance, repeatability, large ablation area, fast heating speed and less damage to the surrounding normal tissue, so it has attracted more and more attention in the clinical treatment of lung tumors [Citation16,Citation17].
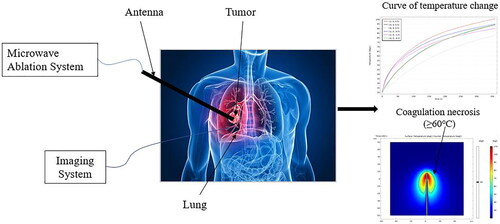
At the same time, MWA antenna can achieve deeper penetration [Citation18], higher specific absorption rate (SAR), faster damage rate in high impedance tissue (such as lung) and can reach a higher temperature in a short time, which makes MWA of lung tumors more efficient [Citation19]. Therefore, MWA has become a better way to treat unresectable lung tumors, and its ablation process is shown in . The doctor inserts the microwave antenna into the lung tumor by means of some imaging equipment and sets the microwave power and ablation time according to the size and shape of the tumor. The high-frequency electromagnetic field emitted by the antenna produces local high temperature to cause coagulative necrosis of tumor cells. After the ablation, the imaging equipment is used to detect whether the conformal treatment is achieved.
However, it will take time before MWA treatment based on simulation modeling to mature enough to be accepted for clinical applications. In particular, the expected temperature distributions cannot be accurately predicted and thus the most suitable MWA treatment planning cannot be set down. Only relying on doctors’ experience may require multiple ablations and aggravate pains of the patient. Temperature simulation has become a very important tool for predicting ablation results, which can assist doctors to input appropriate treatment parameters to achieve conformal ablation. This paper intends to provide a review about the modeling of MWA temperature distribution of lung tumors in recent years, including exact characterization of individual tissue parameters, simulation modeling technology of lung tumor MWA, experimental verification and clinical evaluation of MWA simulation accuracy, uncertainties and technical challenges in the simulation of MWA temperature distribution of lung tumors.
2. Methodology
2.1. Search strategy
Two investigators independently examined Scopus and PubMed online database for a comprehensive literature search (up to January 2023). A total of 1393 articles were collected, in which 890 were selected using the query on Scopus and 503 on PubMed. Following the elimination of duplicates, the relevant experimental studies were identified. In order to collect the most relevant articles, we identified keywords and divided them into four areas of interest: lung tumor, microwave ablation, temperature distribution and simulation modeling. The time limit and language restriction were not used. To try to expand our search, references lists of the retrieved articles were also screened to identify additional studies [Citation20].
2.2. Study selection
The articles founded by our search were filtered. In this systematic review, the main screening criteria were the original papers reporting the simulation model and ex vivo experiments of lung tumor MWA. The main inclusion criteria were:
Timeliness. The references should be based on recent papers.
Relevance. The references should be closely related to the review topic.
Representativeness. The references should include representative literatures at home and abroad and should not be pointlessly piled up in large numbers.
Reliability. The references must be real and reliable, complete description items, easy to search and verify, avoid wrong citations.
Authority. Authority is reflected in the author of the reference and published journal. Citing the work of authoritative authors will make the research more valuable.
Exclusion criteria were:
articles not within the field of interest of this review;
articles that were too long ago;
research focusing on the details of clinical surgery;
articles that were not in the English language;
articles that could not be found for their full text.
Two researchers independently reviewed the titles and abstracts of the retrieved articles by the above criteria. The four authors then independently reviewed the full-text of the remaining articles to determine their final inclusion.
3. Characterization of tissue parameters of lung parenchyma and lung tumors
The tissue electrical properties (electrical conductivity/ permittivity/
) directly affect the absorption of electromagnetic energy that produces heat, while thermal properties (thermal conductivity/
density/
specific heat capacity/
) and perfusion (blood perfusion/
) affect heat transfer in the tissue. Therefore, the setting of biological tissue parameters is of critical importance in the simulation modeling of MWA [Citation21]. The precise characterization of tissue parameters is conducive to improve the simulation accuracy. Many researchers have previously demonstrated the impact of uncertainty in tissue properties and their temperature dependency on MWA model outcomes. They have also carried out in-depth exploration on the parameters setting of the lung tissue. Furthermore, the effect of respiratory movement on tissue characteristics is also taken into account to increase the accuracy of simulation results.
3.1. Constant tissue parameters
In order to simplify the calculation, some research teams set biological tissue parameters to fixed values in the simulation process. Instead of tumor tissue, lung parameters are often used in lung MWA simulation. However, the dielectric properties of malignant tissues were 10–20% larger than those of normal tissues [Citation22]. The electrical and thermal properties of lung tissue (tissue type) involved in model simulation and ex vivo experiments (condition) are summarized, as shown in .
Table 1. Summary of characteristic parameters of the lung tissue.
3.2. Dynamic tissue parameters
In the MWA process, the increasing temperature of the lung tissue and tumor will have an impact on the thermophysical and electrical parameters. The changes of these parameters will significantly affect the modeling accuracy. Therefore, it is still a challenging work to precisely derive dynamic tissue parameters. Gabriel et al. [Citation42] calculated the permittivity and conductivity of lung tissue under the action of 2450 MHz electromagnetic wave using fourth-order Cole–Cole model. The model is described as follows:
(1)
(1)
where
is the dielectric increment of living tissue at the nth relaxation time,
and
are the static permittivity and the permittivity when the frequency tends to infinity, respectively, and
is the relaxation time.
Tehrani et al. [Citation43] proposed an extended model of tissue electrical conductivity and permittivity varying with temperature during MWA and pointed out that the temperature dependence of the thermal conductivity () and blood perfusion rate (
) of biological tissue is based on linear equations, which provided a reference for scholars to perform simulation of MWA temperature distribution based on dynamic biological tissue characteristic parameters. These dynamic tissue parameters included thermal and dielectric properties are conducive to derive more accurate simulation results and are described as follows:
(2)
(2)
(3)
(3)
(4)
(4)
(5)
(5)
where
is the temperature of lung tissue,
are constants of permittivity(
),
are constants of electrical conductivity(
),
and
are the base-line thermal conductivity, the change in
due to temperature and the baseline temperature at which
respectively.
Singh et al. [Citation37] considered that the electrical conductivity of tissue increased linearly with temperature (2% per °C). A non-linear piecewise decreasing model for blood perfusion was proposed. They are expressed as follows:
(6)
(6)
(7)
(7)
where
and
are the baseline electrical conductivity and baseline blood perfusion rate for the lung tissue, respectively, and
is the induced thermal damage. They also pointed out that in EquationEquation (5)
(5)
(5) ,
Choi et al. [Citation44] considered that
Bianchi et al. [Citation45] summarized the specific heat of lung adenocarcinoma cells at different temperatures, i.e. 3640 J/(kg·K) at 37 °C, 3880 J/(kg·K) at 43 °C, 3850 J/(kg·K) at 50 °C and 3790 J/(kg·K) at 60 °C.
Bonello et al. [Citation46] studied the temperature dependence of dielectric properties of ex vivo sheep lung tissue in the temperature range of 25 °C to 90 °C. It was found that the permittivity and electrical conductivity decreased linearly with the increase of temperature, which is contrary to the study of Singh et al. [Citation37]. And the temperature coefficients under 2.45 GHz are −0.87 and −1.21, respectively. Bianchi et al. [Citation47] studied the characterization of the temperature dependence of the thermal properties of lung tissue from room temperature (21 °C) to over 90 °C. They found that the thermal diffusivity () and
of the lung tissue increased exponentially with temperature, while the volumetric heat capacity (
) changed less noticeable, which was described by a linear equation:
(8)
(8)
(9)
(9)
(10)
(10)
Furthermore, since the characteristic parameters of lung varied with the respiratory process, Radmilovi et al. [Citation23] studied the change trend of electrical conductivity and permittivity of inspiratory and expiratory lungs at different microwave frequencies. It was found that the relative permittivity decreased while the electrical conductivity increased with the increase of frequency. The electrical conductivity changed a little at frequencies of lower than 1 GHz. But at higher frequencies, the increase of electrical conductivity is relatively large. The relative permittivity and electrical conductivity of the lung during deflation are about twice as large as those when inflated. Yang et al. [Citation26] studied the temperature distribution of lung tissue during MWA under blocking ventilation and normal ventilation, respectively. The constant physical parameters were used to simulate the conditions of blocking ventilation, and the sinusoidal function was used to express the physical parameters under periodically normal ventilation conditions. The results showed that a larger ablation area can be produced using the dynamic physical parameters, but the reverse heating effect of microwave antenna will also be more significant, thus resulting in greater damage to surrounding normal tissue.
In order to propose personalized treatments for different patients, Tacprasartsit et al. [Citation48] supplemented the radiomics features on the traditional biological heat transfer model. Nine regression convolution neural networks (CNN) specially trained for each feature are used to evaluate nine different characteristics of the tumor. The results are used as radiological features, and then the physical characteristics of the patients’ specific tumor and lung tissue are obtained according to a certain transformation model. In this way, personalized treatment parameters can be obtained, but this technique requires the measurement knowledge of tissue characteristics related to medical imaging data. It is still challenging to determine the relationship between radiological features and tissue parameters.
In the selection of tissue parameters, the simulation model using the tissue characteristics under exhalation condition was more accurate. Some studies have also found that the ablation area obtained by using dynamic tissue parameters is larger, but there is a problem of obvious backward heating effect. To date, the static tissue parameters which do not change with temperature are widely used in the study, and the dynamic tissue parameters of lung tissue are still in the exploratory stage.
4. Simulation modeling technology of lung tumor MWA
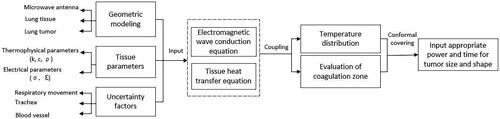
Due to the lack of typical clinical data, computer simulation has become an important tool for predicting the temperature distribution in MWA procedure. The workflow diagram is shown in , and some of the key technologies are summarized in this section.
4.1. Geometric modeling
The geometric modeling of MWA includes three parts: microwave antenna modeling, normal lung tissue modeling and lung tumor modeling. The geometric models conforming to the true anatomical structure will produce more real simulation results.
4.1.1. Construction of microwave antenna model
Depending on therapeutic organ and the size and shape of the tumor, a specific microwave antenna is pierced directly to the tumor site for emitting microwave energy. Microwave antenna is usually composed of inner conductor, insulating medium, outer conductor and catheter. Phairoh et al. [Citation27] designed a tip-open coaxial antenna with a microwave frequency of 2450 MHz to obtain spherical energy deposition. More researchers use ring-open coaxial antennas to provide symmetrical ring-coil heating zones [Citation49]. The microwave antenna made of coaxial cable is commonly used, and a 1 mm-wide slot is cut on the outer conductor to emit electromagnetic waves into the tissue. At the same time, the antenna is encapsulated in the PTFE catheter to prevent the microwave antenna from adhering to the dry ablation tissue. In addition, a circulate water cooling system needs to be added inside the antenna to avoid unnecessary thermal damage to patients [Citation16]. Habert et al. [Citation49] used full antenna water cooling technology, choke coil design at the front end of the antenna, and AntiphaseTM technology to prevent heat from spreading backward, and to reduce the central temperature of the ablation zone and the energy transmission loss. Different from the needle-shaped rigid microwave antennas used in traditional percutaneous ablation, Pfannenstiel et al. [Citation18] developed a new type of flexible microwave transmitter, which was transmitted to targeted tumor through bronchoscope. This equipment can enhance the accuracy of transmitter placement, improve ablation effect, reduce the risk of complications such as pneumothorax and treat targets that can’ t be reached by a common antenna.
4.1.2. Construction of the lung tissue model
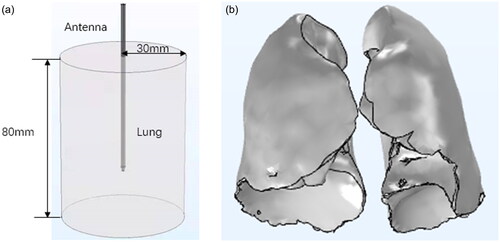
Because it is usually assumed that the lung is isotropic uniform tissue, an ideal cylindrical model is constructed. Additionally, the electromagnetic energy emitted by the microwave antenna is an axisymmetric region, so the cylindrical model can be simplified to a rectangle model, which is solved in a two-dimensional coordinate frame. A model of idealized lung tissue combined with a microwave antenna is shown in .
In order to construct a more realistic lung model, Yang et al. [Citation26] divided respiratory process into ten stages. They reconstructed lung tissue models according to the respiratory stage, in which 0 represented the end of inspiration and 50% or 60% represented the end of expiratory. The model of lung tissue after reconstruction is shown in .
4.1.3. Construction of the lung tumor model
Many scholars assumed that the tumor was spherical in MWA simulation, in this case it can be simplified into a two-dimensional model to simplify the calculation, as shown in .
Figure 4. Lung tumor model. (a) Whole lung tissue-level model. (b) Traditional spherical tumor model. (c) Real tumor model based on CT slice.
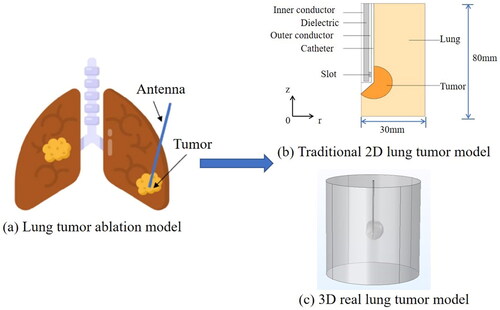
In order to obtain a more accurate tumor model, some researchers used CT slices of the tumor [Citation50] to reconstruct real lung tumor model, as shown in . The reconstructed lung tumor model was introduced into the Freeform tactile design system for smooth processing to make simulation results more accurate. Because there are trachea and bronchus in lung tissue, some tissue parameters such as thermal conductivity, electrical conductivity and density will change with the gas volume in the tissue. Tian et al. [Citation51] placed a bronchus around the microwave antenna based on the traditional model and set its airflow to 3.0 L/min, which is the same as human respiratory rate. Liu et al. [Citation28] took the structure of trachea into consideration to simulate the ablation process in a more real and complex anatomical environment. And five different tumor models were generated, mainly including the following: tumors near the main airway, tumors located in the deep lung, tumors attached to the bronchial wall and tumors adjacent to but not attached to the bronchial wall.
In the construction of geometric model, the simple cylindrical lung model is widely used, but the model involving human body structure (heart, trachea, blood vessels and so on) is few, which needs further investigation in the future.
4.2. Calculation of electromagnetic energy deposition
4.2.1. Electromagnetic wave conduction equation
In the simulation of lung tumor MWA, the electric field and magnetic field are time-varying TEM (transverse electromagnetic) waves. In two-dimensional axisymmetric cylindrical coordinates, the time-varying electric field and magnetic field are described as follows [Citation52].
Electric field:
(11)
(11)
(12)
(12)
(13)
(13)
where
is the longitudinal component of electric field,
is the traveling wave factor,
is the transverse component of distribution function of electric field,
is the wave impedance,
is the MWA input power,
is the outer radius of dielectric,
is the inner radius of dielectric,
is the angular frequency of microwave,
is the frequency of microwave,
is the propagation constant,
is the wavelength and
is the integral constant. According to Maxwell equation, the electromagnetic wave conduction in tissue is characterized by Helmholtz equation:
(14)
(14)
where
is the relative permittivity of lung tissue;
is the vacuum permittivity (
F/m);
is the electrical conductivity of lung tissue (S/m);
is the magnetic field intensity (A/m);
is the relative permeability (
) and
is the free space wave number (rad/m). The specific absorption rate (SAR) of lung tissue can be obtained by solving the distribution of electromagnetic field in lung tissue:
(15)
(15)
In order to improve the accuracy of temperature distribution simulation of MWA, an improved SAR method with thermal conductivity term can also be used [Citation52]. The calculated SAR is used as the heat source term of the model for simulation. Compared with the simulation result obtained by traditional electromagnetic coupling method, this SAR method simplifies simulation process, has better consistency with the experimental data and improves simulation accuracy. In general, the SAR near the emission slot is the maximum [Citation26]. However, the disadvantage of this method is that the SAR is a fixed value at a certain temperature and should be accurately measured.
4.2.2. Electromagnetic wave boundary condition setting
Aiming at the Helmholtz electromagnetic wave equation, Selmi et al. [Citation24] pointed out that the axis was a symmetry axis:
and
and there was continuity of the tangential component of the electrical field at the interface between the tissue and the tumor:
(16)
(16)
Avishek et al. [Citation34] adopted the first-order scattering boundary equation to reduce the boundary reflection and calculated the electric field.
(17)
(17)
(18)
(18)
The electric field distribution can be obtained by solving EquationEquation (14)(14)
(14) with the boundary conditions given by EquationEquations (17)
(17)
(17) and Equation(18)
(18)
(18) . According to the electric field derived from these equations, the temperature distribution can be obtained by solving the corresponding biological heat transfer equation.
4.3. Biological heat transfer technology
4.3.1. Tissue heat transfer equation
In the simulation of MWA temperature distribution, the heat transfer in biological tissue is calculated by biological heat transfer models. It is found that the temperature near the emission slot is the highest in general. With the increase of distance, the influence of the heat source becomes weaker and the rate of the temperature rise decreases. For the lung tissue, the commonly used models include Pennes equation and porous media model [Citation53,Citation54]. Compared with the traditional Pennes equation, the porous media model will produce the results closer to the experimental data, but at the same time, the solving process is more complicated [Citation54].
4.3.1.1. Pennes equation
where is the density of lung tissue (kg/m3);
is the density of blood (kg/m3);
is the specific heat capacity of lung tissue [J/(kg·K)];
is the specific heat capacity of blood [J/(kg·K)];
is the temperature of lung tissue (°C);
is the temperature of blood (°C);
is thermal conductivity [W/(m·K)];
is blood perfusion rate (s−1);
is the heat generated by new metabolism (W/m3);
is the heat generated by microwave generator (W/m3).
4.3.1.2. Heat transfer equation of porous media model
where represents the apparent increase of thermal conductivity in tissue caused by hemoperfusion in small vessels and
represents the total blood perfusion vector in the tissue. Other parameters are described above.
The construction of tissue heat transfer models is a key technology in MWA temperature distribution simulation. Different heat transfer models have their own advantages and disadvantages, as shown in .
Table 2. Comparison of advantages and disadvantages of heat transfer models.
Pennes equation is the most commonly used in the study, and it has many advantages [Citation55]. But it is oversimplified and ignores many problems. Thus, it has been further improved by many researchers. In the process of MWA, due to the rapid increase of temperature, the water will evaporate quickly and the carbonization will also occur. Therefore, some scholars have introduced the phase transition heat transfer analysis of tissue moisture when solving the heat transfer equation of biological tissue [Citation56]. Additionally, the direct contact between the liquid and the tissue will produce the heat source term. Therefore, adding the liquid heat transfer equation to the tissue heat transfer equation can achieve the goal of expanding ablation area. In order to build a more accurate simulation model, Truong et al. [Citation57] combined Bill’s law with the Pennes biological heat transfer equation to analyze the heat transfer in biological tissue. Tucci et al. [Citation54] added the effect of tissue vaporization to the Pennes equation, so that the term (ρc) is expressed as follows:
(21)
(21)
where
and
are density and specific heat of tissue at temperature below 100 °C (liquid phase),
and
are density and specific heat of tissue at temperature above 100 °C (gas phase),
is the product of water latent heat of vaporization and water density at 100 °C and
is the water content inside the lung tissue.
Because the lung tissue is filled with air, and the traditional cylindrical lung model does not take into account the effect of air, a porous media lung tissue model has been proposed. Coupled with the electromagnetic field of MWA, the flow field of air in lung tissue and the thermal field of porous media, the temperature distribution of lung tissue can be derived. The simulation results are closer to the in vitro experimental results. The ablation area produced by porous media model is 29% smaller and the maximum temperature is 36% lower [Citation54]. Wang et al. [Citation58] also pointed out that the increase of porosity will result in a more uniform temperature distribution, which may lead to more effective hyperthermia treatment.
In a word, the Pennes equation is widely used, but it is oversimplified. The porous media model is relatively closer to the experimental data [Citation59]. At the same time, the solution is relatively more complex.
4.3.2. Heat transfer boundary condition setting
In order to solve the biological heat transfer equation, it is necessary to set the boundary conditions. In general, the heat transfer boundary conditions are set as follows:
Z axis (see ) is a symmetry axis, expressed as shown in EquationEquation (22)
(22)
(22) . It describes that the heat flux is zero.
The insulation condition is suitable for the surrounding lung tissue. In other words, the heat flux through the surrounding wall is equal to zero, expressed as shown in EquationEquation (23)
(23)
(23) [Citation34].
The outer boundary adopts the Dirichlet condition of constant temperature (37 °C) [Citation28].
EquationEquation (23)(23)
(23) was further specified for each boundary by Selmi et al. [Citation24]:
(24)
(24)
They also supposed that the heat flux is continuous at the interface between the tissue and the tumor, i.e.
(25)
(25)
Convective boundary conditions are applied to the surface of external emitter to indicate water cooling:
(26)
(26)
where
is the outer normal vector, the convective heat transfer coefficient
is 1000 W·m−1K−1 and the cooling water temperature
is set to 15 °C. The value of h depends on the antenna. Its value and the
will be different in different studies.
4.4. Evaluation technique of thermal coagulation zone of lung tumors
Ablation coagulation zone is an important index to evaluate the thermal ablation effect. By comparing the coagulation area with the size of tumor, we can know whether the conformal coverage achieved. The common evaluation indexes of ablation coagulation zone include coagulation zone shape and coagulation zone volume.
4.4.1. Shape evaluation of coagulation zone
The ablation coagulation zone will increase with the increase of ablation frequency and ablation power, but beyond a certain range, only the longitudinal diameter of the coagulation zone will continue to increase with the increase of ablation power, thus forming an ellipsoidal shape. In addition, some researchers proposed the sphericity index (SI) [Citation49] to evaluate the coagulation zone shape. The sphericity of 1 corresponds to a perfect sphere, the sphericity less than 1 corresponds to a slender shape elongated along the antenna direction, and the sphericity greater than 1 corresponds to a coagulation zone extending in the direction of orthogonal to the antenna.
4.4.2. Volume evaluation of coagulation zone
The clinical goal of MWA is to heat the tumor zone to a cytotoxic temperature, while maximally sparing non-targeted tissue outside of this zone [Citation60]. Therefore, it is very important to accurately evaluate the extent of thermal coagulation zone. The volume of thermal coagulation zone depends on ablation power and time. The volume is usually evaluated by isotherm threshold (IT), Arrhenius model and thermal equivalent dose (TID) [Citation61].
With regard to IT method, the tissue is considered to be coagulative when the temperature exceeds a certain threshold. The usual thresholds involve 60 °C [Citation62], 54 °C [Citation63,Citation64] and 50 °C [Citation24].
Many researchers [Citation26,Citation48] also calculate the thermal damage volume based on Arrhenius model [Citation54], which is expressed as follows:
(27)
(27)
In addition, is used to indicate the degree of tissue damage:
(28)
(28)
The score of necrotic tissue is expressed by and its value is related to
(29)
(29)
where
indicates the degree of damage to the tissue,
indicates the reference temperature and
indicates the time that exceeds the reference time. The parameter
(=8.314 J/mol/K) represents the universal gas constant,
represents the frequency factor,
represents the reaction energy barrier,
represents the activation energy of irreversible damage reaction and
represents the fraction of necrotic tissue. For the lung tissue,
and
[Citation37], or
and
[Citation65], or
and
[Citation40], respectively. In the FEM model, the induced thermal damage value
corresponding to 63% probability of cell death (i.e.
), has been used as a critical threshold for calculating coagulation volume.
Liu et al. [Citation28] used the equivalent heating minutes at 43 °C (EM43°C) to calculate the equivalent thermal dose distribution and then evaluated the MWA effects by adding time intervals of different temperature exposures, as follows:
(30)
(30)
where
is the proportional constant of cell mortality dependent on temperature and
is the average temperature (°C) of the
time interval (minutes). This concept of thermal dose originates from the Arrhenius model of cell damage accumulation, which is a mature index to predict the thermal effect of tissue or the degree of injury, and is widely used in thermotherapy and thermal ablation monitoring and evaluation. A thermal dose of 120–240 min at 43 °C usually causes considerable tissue necrosis, but the sensitivity varies between tissue types [Citation66].
The advantages and disadvantages of these three thermal damage assessment techniques are shown in .
Table 3. Comparison of advantages and disadvantages of thermal damage assessment techniques.
In view of the fact that the Arrhenius damage model only considers two states of biological tissue (alive cells and dead cells), Tehrani et al. [Citation43] proposed a three-state cell death model to calculate the size of ablation coagulation zone. That is, the cell death process under the temperature gradient is described by coupling the ordinary differential equation, and the tissue is divided into three states: alive cells, vulnerable cells and dead cells. The expression is as follows:
(31)
(31)
where
and
are alive cells, vulnerable cells and dead cells, respectively. The positive rate constant
indicates the transition from alive to vulnerable state, while the reverse rate constant
represents the self-repair process from vulnerable state to fully functional resurrection state. Once beyond the critical point, the cell enters a state of death (
), after which the process is irreversible. Cell survival rate (
) was used to determine the size of lesion. The ablation results showed that the results of three-state cell death model were closer to the experimental data.
In the evaluation of coagulation zone, IT and Arrhenius model are widely used in the study because of its simplicity, intuition and wide range of application. The coagulation zone after lung tumor MWA is generally concentrated near the tip of the antenna and the emission slot, which is oval on the whole [Citation67]. And the coagulation zone of expiratory group is larger than that of inspiratory group [Citation12]. Therefore, in clinical treatment, one-lung ventilation [Citation26] (meaning that patients only use the lung on the non-operative side for ventilation) may be used for ablation surgery.
5. Experimental verification and clinical evaluation of simulation accuracy of lung tumor temperature distribution
5.1. Experimental verification of simulation model
In the MWA procedure, the choice of treatment parameters such as input power and duration is critical to ensuring the success of the procedure, because the improper parameter usage may lead to incomplete ablation or excessive ablation. Therefore, some researchers have carried out different explorations, including using simulation technology to simulate the ablation results of different parameter combinations and verifying these results by experiments. The aim is to provide some guidance for clinical application. Gao et al. [Citation16] studied the coagulation zones at three power levels (30 W, 40 W, 50 W) under different heating time (2 min, 4 min, 6 min). The measured indexes included the longitudinal diameter and transverse diameter of coagulation zone, as well as the slot temperature of microwave antenna. The experiment for each combination of power and time is repeated five times. Keangin et al. [Citation35] applied microwave power levels of 60 W, 80 W and 100 W to porcine lung tissue for 360 s. The ablation diameter was measured and the ablation volume was calculated.
shows the ablation results of different treatment parameter combinations (microwave power/ and ablation time/
) during lung tissue (tissue type) MWA in simulation and ex vivo experiments (condition). The evaluation indexes of the thermal damage zone include the long diameter (
) and short diameter (
) along the cross section of the ablation antenna, the volume (
) of the coagulation zone, the sphericity (
), the maximum temperature (
) reached at the end of the ablation and the coagulation threshold (
).
Table 4. Ablation results for different power and time combinations.
5.2. Clinical evaluation
The simplest geometric modeling is usually used in the model simulation, but in clinic, the lung tissue contains a substantial amount of air and blood vessels, and is adjacent to heart, so the ablation effect is affected by many factors. For the lung tumor with a diameter of 2–8.5 cm (average 4 cm), clinicians usually choose the ablation power of 60 to 70 W and the ablation time of 6–10 min, which are slightly higher than the ablation power and ablation time used in the ex vivo experiments [Citation16].
In the thermal ablation surgery, it is necessary to combine image guidance technology for real-time monitoring to achieve conformal ablation. In clinical application, there are mainly three kinds of image guidance techniques used in thermal ablation: ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). Ultrasound-guided and CT-guided ablation is performed in most cases, while MRI-guided ablation is rare. Shen et al. [Citation70] evaluated the safety, practicability and effectiveness of MRI-guided MWA in patients with the lung malignant tumor. The results showed that compared with CT, MRI was easier to identify whether ablation was complete or not. In addition, MRI could reduce or eliminate the artifact caused by respiration movement or heartbeat movement, could be performed without using contrast media and could avoid damage to pulmonary vessels. Thus, it may guide the antenna to puncture the lesion more accurately and avoid repeated operations to expose patients to ionizing radiation. The whole ablation process can be monitored in real time and the therapeutic effect can also be evaluated in real time.
6. Uncertainty factors in the simulation of MWA temperature distribution of lung tumors
Lungs are close to the heart, trachea, blood vessels and other structures, so the thermal ablation of lung tumor is affected by respiratory movement, the speed and direction of air flow in the bronchi and the heat sink effect of blood vessels.
6.1. The influence of respiratory movement on the simulation accuracy
The lung is filled with a large amount of air, which greatly affects the temperature distribution of MWA. Therefore, the accurate prediction and control of ablation zone are still very challenging. The volume of air in the lung changes with respiratory movement, so tissue parameters of the lung also change. Yang et al. [Citation26] performed three-dimensional reconstruction of lung models at different respiratory stages and studied the change trend of lung tissue parameters during respiration and the effect of these tissue parameters on MWA coagulation zone. During the ablation process, they divided the tissue parameters into dynamic and static to simulate respectively. The sinusoidal function was used to represent periodic physical parameters of lung tissue, and the fixed parameter value was used to represent a certain stage of the respiratory process, thus running through the whole respiratory process including end expiratory, end inspiratory and intermediate state. By setting different angular frequency values in sinusoidal functions, the effect of respiratory frequency on the temperature distribution in the ablation zone was also studied. The results showed that there was no significant difference between different respiratory frequencies on the damage zone. At the end of exhalation, the coagulation zone formed by the antenna was more concentrated in the tip and slot. The backward heating effect was weaker, and the damage to healthy tissue around the antenna was less. Using the dynamic physical parameters, it was found that the microwave coagulation zone was bigger obviously. However, the backward heating effect of the antenna was more significant, which would destroy a larger range of healthy tissue, and couldn’t effectively kill the tumor in the center of the puncture point.
6.2. The influence of patient-specific anatomical structure
6.2.1. The influence of pulmonary trachea on ablation results
Phairoh et al. [Citation27] and Sanpanich et al. [Citation71] studied the effect of endobronchial airflow on MWA by using a tip-open coaxial antenna and a slot-open coaxial antenna, respectively. They placed a catheter with airflow near the microwave antenna to represent a bronchus. Their results showed that in the absence of bronchi, the distribution of electromagnetic wave energy and the shape of coagulation zone were symmetrically spherical. The existence of airflow in the bronchus would affect the heating mode and the damaged zone would be affected by the thermal conductivity and electrical conductivity of air fluid, which resulted in the asymmetry of coagulation shape in the lung model. This study had some limitations because it used a simple lung model for simulation. Constructing a porous lung structure composed of many alveoli or air sacs and taking into account the rich capillary network around the lung would be the follow-up work.
Liu et al. [Citation28] explored the feasibility of constructing 3D lung models of specific patients in the lung tumor MWA simulation. Lung tumor models were attached to or not attached to the trachea, around the main bronchus or around the bronchioles located in the deep lung, respectively. The results showed that thermocoagulation effects were different for lung tumors under different locations.
Tian et al. [Citation51] studied the influences of bronchi on ablation results. When building the simulation model, they placed a bronchiole near the opening at the tip of coaxial antenna and set the airflow velocity to 3 L/min that matched the velocity of human body. They found that the airflow would take away part of the heat, thus forming a nonuniform temperature distribution.
Anai et al. [Citation72] conducted in vivo experiments to verify the effects of bronchi on ablation results. They compared ablation results between the experimental group (occlusion of unilateral main bronchi) and the control group (without any treatment) and found that the diameter and volume of coagulation zone in the experimental group were significantly larger than those of the control group.
6.2.2. The influence of blood vessels on ablation results
Wang et al. [Citation73] studied the effects of blood flow parameters (e.g. vessel diameter and vessel-antenna spacing) on the temperature distribution and coagulation volume of MWA. It was found that the coagulation area on the side of vessels was smaller than that on the other side, and the distance between the antenna and vessel had a more significant effect on the ablation result than the diameter of vessel. However, if the blood vessel was located outside the predicted coagulation zone, the ablation results would hardly be affected by the blood vessel. The blood temperature also affected the volume of coagulation zone, which was obvious near the blood vessel. The closer the blood temperature was to the body temperature (37 °C), the closer the volume of coagulation zone was to the actual measured value.
Vaidya et al. [Citation74] comprehensively considered vascular morphological parameters and the construction of ablation antennas and studied their effects on the thermal damage zone. In order to evaluate the effects of geometric parameters on thermal damage, they introduced three indicators: the average lesion boundary displacement the maximum Nusselt number
and the relative ablation volume
The results showed that the most important contribution to the directional effect measurement
was the parameter
(the vertical distance between the center of antenna slot and the vessel-antenna connection). The most important factor affecting
was the parameter
(blood vessel radius) and
decreased with the decrease of the distance between antenna and blood vessel. The directional effect of thermal damage occurred between 0.4 mm and 0.5 mm radius of blood vessels, and blood vessels were classified according to the condition under which the directional effect occurred. However, this study only considers the influence of a single blood vessel, does not consider parameters except the blood vessel radius and lacks the experimental verification of simulation conclusions.
Anai et al. [Citation72] studied the effects of blood flow on the thermal ablation coagulation zone by occluding the ipsilateral pulmonary artery of the tumor and set up the experimental group (occluded pulmonary artery) and the control group (without any treatment) for in vivo experiments. The results showed that the diameter and coagulation zone in the experimental group were larger than those in the control group. The limitation was that the experiment caused serious complications.
7. Discussion
MWA is a promising therapeutic technique in the treatment of malignant tumors. However, some therapeutic parameters cannot be accurately set before clinical ablation surgery. As a result, it will be useful to develop numerical tools that can correctly predict the MWA temperature distribution. In this paper, the simulation techniques of MWA for lung tumors in recent years were reviewed in order to provide some help for the future research.
Previous studies have shown that tissue properties, frequency, power and tip temperature significantly affect hyperthermia, especially MWA [Citation75–77]. The tissue thermophysical and electrical parameters change with the increase of temperature, which has a significant impact on the ablation effects [Citation23]. With the increase of blood perfusion rate, thermal conductivity and permittivity, the coagulation zone will decrease. The increase of electrical conductivity has a positive effect on the ablation results. When other parameters are kept constant, the coagulation zone will expand with the increase of frequency and power. However, in order to avoid damage to normal tissue, the use of low power for a long time is more reasonable [Citation16]. Compared with other parameters, the applied power, frequency and blood perfusion rate have relatively significant effects on the coagulation zone volume [Citation34]. It is worth noting that the coagulation zone under the dynamic tissue parameters is larger, but the backward heating effect is also more significant [Citation26]. Although increasing power and frequency may improve ablation efficiency, this approach may not be appropriate for all types of tumors. For tumors with small radius (r < 1 cm), increasing power has no significant effect on the ablation results. For larger tumors (r ≥ 1.5 cm), higher power will destroy more tumor cells. For slender tumors, the use of lower frequency and double-slot antennas will obtain better results, however, for spherical and oblate tumors, the use of higher frequency will produce better results [Citation43]. Therefore, the optimal ablation parameters such as frequency, power, time and antenna setting should be selected according to the shape and size of the tumor. Some researchers have also studied the relationship between ablation volumes and tip temperatures. The results showed that the higher tip temperature could improve MWA results [Citation78]. Additionally, in the process of simulation, the modeling based on the real anatomical structure of the human body [Citation28] will also make the prediction results more accurate.
Although many scholars have established various simulation models of lung tumors MWA and made certain achievements, there are still challenges in the simulation of temperature field: (1) Most of the models are based on the results of ex vivo porcine lung experiments or tissue phantom experiments and do not consider the individual differences. (2) The effects of the large blood vessels, the heart and other organs around lung tissue are ignored. In the reconstruction of the lung tissue model, these organs need to be reconstructed together to get a more realistic model [Citation28]. (3) Although some scholars have studied the relationship between biological tissue characteristic parameters and temperature, most microwave simulation models still use fixed values for biological tissue parameters. (4) The main problem of lung tumor MWA is local recurrence [Citation79], and pneumothorax is the most common serious complication [Citation80,Citation81] after percutaneous ablation. The main cause of pneumothorax seems to be associated with the insertion of the antenna [Citation82]. Therefore, some flexible MWA applicators are under development [Citation83,Citation84] and can offer the possibility of reducing the risk of pneumothorax.
The limitations of this review include the lack of in-depth understanding of treatment parameters for clinical application. Surgical path planning, another key technique for lung tumor MWA, has not been reviewed. In addition, the studies related to Hyperbolic equation and Weinbaum-Jiji equation are not involved in the comparison of heat transfer models. Because the achieved MWA results may be linked to the specific antenna, the summarized data are not necessarily general.
8. Conclusions
MWA has been proved to be a safe and feasible alternative to surgery with acceptable morbidity and mortality in medically inoperable patients with lung tumor. The model simulation techniques of lung tumor MWA in recent years were mainly reviewed in this paper. The challenges and future promising research directions associated with the MWA modeling have also been highlighted. Some of the key technologies, such as the selection of heat transfer equations, the characterization of biological tissue parameters varying with temperature, and the construction of internal structures of lung tissue including blood vessels and tumors still need to be further explored. Pennes biological heat transfer equation is the most widely used, but the ablation results obtained by the porous media heat transfer model are closer to the real situation. Although the relationship between biological tissue parameters and temperature has been studied, the biological tissue parameters in most microwave simulation models still choose fixed values. The construction of internal structures of lung tissue such as blood vessels and tumors for modeling and simulation analysis is still a problem worthy of in-depth study. The solution of these problems will provide some guidance for improving the prediction accuracy of MWA temperature distribution and coagulation zone of lung tumors.
Author contributions
Conceptualization, S.W. and H.G.; investigation, Y.H. and X.L.; resources, Z.C.; writing—original draft preparation, J.L. and J.W.; writing—review and editing, S.W. and H.G.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors would like to thank the anonymous reviewers for their constructive comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
- Alexander ES, Dupuy DE. Lung cancer ablation: technologies and techniques. Semin Intervent Radiol. 2013;30(2):141–150.
- Bartlett EC, Rahman S, Ridge CA. Percutaneous image-guided thermal ablation of lung cancer: what is the evidence?. Lung Cancer. 2023;176:14–23.
- Lin M, Eiken P, Blackmon S. Image guided thermal ablation in lung cancer treatment. J Thorac Dis. 2020;12(11):7039–7047.
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non–small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26(3):312–319.
- Jiang B, Mcclure MA, Chen T, et al. Efficacy and safety of thermal ablation of lung malignancies: a network meta-analysis. Ann Thorac Med. 2018;13(4):243–250.
- Steinke K, Haghighi KS, Wulf S, et al. Effect of vessel diameter on the creation of ovine lung radiofrequency lesions in vivo: preliminary results. J Surg Res. 2005;124(1):85–91.
- Gillams AR, Lees WR. Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol. 2008;18(4):672–677.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6):2967–2973.
- Brace CL, Hinshaw JL, Laeseke PF, et al. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705–711.
- Hu H, Nan Q, Tian Z, et al. Study on the microwave ablation effect of inflated porcine lung. Appl Sci. 2022;12:5916.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? . Curr Probl Diagn Radiol. 2009;38(3):135–143.
- Planché O, Teriitehau C, Boudabous S, et al. In vivo evaluation of lung microwave ablation in a porcine tumor mimic model. Cardiovasc Intervent Radiol. 2013;36(1):221–228.
- Crocetti L, Bozzi E, Faviana P, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010;33(4):818–827.
- Gao X, Tian Z, Cheng Y, et al. Experimental and numerical study of microwave ablation on ex-vivo porcine lung. Electromagn Biol Med. 2019;38(4):249–261.
- Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260(3):633–655.
- Pfannenstiel A, Keast T, Kramer S, et al. Flexible microwave ablation applicator for the treatment of pulmonary malignancies. In: Energy-based treatment of tissue and assessment IX. Vol. 10066. California, United States: SPIE; 2017. p. 189–201.
- Deshazer G, Merck D, Hagmann M, et al. Physical modeling of microwave ablation zone clinical margin variance. Med Phys. 2016;43(4):1764–1776.
- Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(1):37–44.
- Rossmann C, Haemmerich D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit Rev Biomed Eng. 2014;42(6):467–492.
- O'Rourke AP, Lazebnik M, Bertram JM, et al. Dielectric properties of human normal, malignant and cirrhotic liver tissue: in vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys Med Biol. 2007;52(15):4707–4719.
- Radmilović-Radjenović M, Sabo M, Prnova M, et al. Finite element analysis of the microwave ablation method for enhanced lung cancer treatment. Cancers. 2021;13:3500.
- Selmi M, Bin Dukhyil AA, Belmabrouk H. Numerical analysis of human cancer therapy using microwave ablation. Appl Sci. 2019;10:211.
- Neagu V. A study of microwave ablation antenna optimization. In: 2017 E-health and bioengineering conference (EHB). Sinaia, Romania: IEEE; 2017. p. 41–44.
- Yang D, Cao M. Effect of changes in lung physical properties on microwave ablation zone during respiration. Biomed Eng Lett. 2020;10(2):285–298.
- Phairoh C, Sanpanich A, Kajornpredanon Y, et al. Airflow effect on microwave ablation in lung model. In: 2015 8th biomedical engineering international conference (BMEiCON). Pattaya, Thailand: IEEE; 2015. p. 1–4.
- Liu D, Adams MS, Diederich CJ. Endobronchial high-intensity ultrasound for thermal therapy of pulmonary malignancies: simulations with patient-specific lung models. Int J Hyperthermia. 2019;36:1107–1120.
- Duck FA. Physical properties of tissues: a comprehensive reference book. England: Academic Press; 2013.
- Mcintosh RL, Anderson V. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys Rev Lett. 2010;5:129–151.
- Giering K, Lamprecht I, Minet O, et al. Determination of the specific heat capacity of healthy and tumorous human tissue. Thermochim Acta. 1995;251:199–205.
- Wu CH, Lindsey DC, Traber DL, et al. Measurement of bronchial blood flow with radioactive microspheres in awake sheep. J Appl Physiol (1985). 1988;65(3):1131–1139.
- Williams LR, Leggett RW. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas. 1989;10(3):187–217.
- Avishek S, Samataray S. Sensitivity analysis of critical parameters affecting the efficacy of microwave thermal ablation on lungs. In: Current advances in mechanical engineering. Singapore: Springer; 2021. p. 293–303.
- Keangin P, Manop P, Nonthakhamchan T, et al. Experimental study of microwave ablation in ex vivo tissues. In: IOP conference series: materials science and engineering. Vol. 501. England: IOP Publishing; 2019. p. 012038.
- Hasgall PA, Di Gennaro F, Baumgartner C, et al. IT’IS database for thermal and electromagnetic parameters of biological tissues. Version 4.1; 2022 [accessed 2022 Feb 22]. Available from: https://itis.swiss/virtual-population/tissue-properties/database
- Singh S, Repaka R. Numerical study to establish relationship between coagulation volume and target tip temperature during temperature-controlled radiofrequency ablation. Electromagn Biol Med. 2018;37(1):13–22.
- Zorbas G, Samaras T. Simulation of radiofrequency ablation in real human anatomy. Int J Hyperthermia. 2014;30(8):570–578.
- Singh S, Repaka R, Al‐Jumaily A. Sensitivity analysis of critical parameters affecting the efficacy of microwave ablation using Taguchi method. Int J RF Microw Comput‐Aided Eng. 2019;29:e21581.
- Sebek J, Taeprasartsit P, Wibowo H, et al. Microwave ablation of lung tumors: a probabilistic approach for simulation-based treatment planning. Med Phys. 2021;48(7):3991–4003.
- Hall SK, Ooi EH, Payne SJ. Cell death, perfusion and electrical parameters are critical in models of hepatic radiofrequency ablation. Int J Hyperthermia. 2015;31(5):538–550.
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys Med Biol. 1996;41(11):2271–2293.
- Tehrani MHH, Soltani M, Kashkooli FM, et al. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes-a computational approach. PLoS One. 2020;15(6):e0233219.
- Choi J, Morrissey M, Bischof JC. Thermal processing of biological tissue at high temperatures: impact of protein denaturation and water loss on the thermal properties of human and porcine liver in the range 25-80 °C. J Heat Transf. 2013;135:061302–061308.
- Bianchi L, Cavarzan F, Ciampitti L, et al. Thermophysical and mechanical properties of biological tissues as a function of temperature: a systematic literature review. Int J Hyperthermia. 2022;39(1):297–340.
- Bonello J, Elahi MA, Porter E, et al. An investigation of the variation of dielectric properties of ovine lung tissue with temperature. Biomed Phys Eng Exp. 2019;5:045024.
- Bianchi L, Bontempi M, De Simone S, et al. Temperature dependence of thermal properties of ex vivo porcine heart and lung in hyperthermia and ablative temperature ranges. Ann Biomed Eng. 2023:1–18.
- Taeprasartsit P, Pathompatai C, Jusomjai K, et al. A personalized approach for microwave ablation treatment planning fusing radiomics and bioheat transfer modeling. In: Medical imaging 2020: image-guided procedures, robotic interventions, and modeling. Vol. 11315. Texas, United States: SPIE; 2020. p. 780–795.
- Habert P, Di Bisceglie M, Hak JF, et al. Percutaneous lung and liver CT-guided ablation on swine model using microwave ablation to determine ablation size for clinical practice. Int J Hyperthermia. 2021;38(1):1140–1148.
- Wang L, Zhang L, Wang Z. Transplanted pulmonary cancer model in experimental animals: recent progress in research. J Intervent Radiol. 2015;24(7):569–573.
- Tian Z, Cheng Y, Dong T, et al. Numerical study for lung microwave ablation in different thermal and electrical properties. In: World congress on medical physics and biomedical engineering 2018. Singapore: Springer; 2019. p. 563–566.
- Gao H, Wu S, Wang X, et al. Temperature simulation of microwave ablation based on improved specific absorption rate method compared to phantom measurements. Comput Assist Surg. 2017;22(sup1):9–17.
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122.
- Tucci C, Trujillo M, Berjano E, et al. Pennes’ bioheat equation vs. porous media approach in computer modeling of radiofrequency tumor ablation. Sci Rep. 2021;11(1):13.
- Valvano JW. Encyclopedia of medical devices and instrumentation. Bioheat Transf. 2006;1:188–197.
- Yang D, Converse MC, Mahvi DM, et al. Expanding the bioheat equation to include tissue internal water evaporation during heating. IEEE Trans Biomed Eng. 2007;54(8):1382–1388.
- Truong VG, Kim H, Park JS, et al. Multiple cylindrical interstitial laser ablations (CILAs) of porcine pancreas in ex vivo and in vivo models. Int J Hyperthermia. 2021;38(1):1313–1321.
- Wang K, Tavakkoli F, Wang S, et al. Analysis and analytical characterization of bioheat transfer during radiofrequency ablation. J Biomech. 2015;48(6):930–940.
- Mai X, Wu N, Nan Q, et al. Simulation study of microwave ablation of porous lung tissue. Appl Sci. 2023;13:625.
- Wolf FJ, Grand DJ, Machan JT, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879.
- Zhang B, Moser MAJ, Zhang EM, et al. A review of radiofrequency ablation: large target tissue necrosis and mathematical modelling. Phys Med. 2016;32(8):961–971.
- Vogl TJ, Nour-Eldin NEA, Hammerstingl RM, et al. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms-review article. Rofo. 2017;189(11):1055–1066.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–260.
- Sun Y, Cheng Z, Dong L, et al. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine livers. Eur J Radiol. 2012;81(3):553–557.
- Sebek J, Bortel R, Prakash P. Broadband lung dielectric properties over the ablative temperature range: experimental measurements and parametric models. Med Phys. 2019;46(10):4291–4303.
- Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia. 2005;21(8):745–753.
- Vogl TJ, Nour-Eldin NEA, Albrecht MH, et al. Thermal ablation of lung tumors: focus on microwave ablation. Rofo. 2017;189(9):828–843.
- Radmilović-Radjenović M, Radjenović D, Radjenović B. Finite element analysis of the effect of microwave ablation on the liver, lung, kidney, and bone malignant tissues. Europhys Lett. 2022;136:28001.
- Sebek J, Kramer S, Rocha R, et al. Bronchoscopically delivered microwave ablation in an in vivo porcine lung model. ERJ Open Research. 2020;6:00146–2020.
- Shen X, Chen T, Yang B, et al. Magnetic resonance imaging-guided microwave ablation for lung tumor: a case report. Quant Imaging Med Surg. 2021;11(6):2780–2784.
- Sanpanich A, Khongkhanon C, Kajornpredanon Y, et al. Thermal ablation for cancer treatment by using microwave energy in a simple lung model. In: The 7th 2014 biomedical engineering international conference. Fukuoka, Japan: IEEE; 2014. p. 1–4.
- Anai H, Uchida BT, Pavcnik D, et al. Effects of blood flow and/or ventilation restriction on radiofrequency coagulation size in the lung: an experimental study in swine. Cardiovasc Intervent Radiol. 2006;29(5):838–845.
- Wang J, Wu S, Wu Z, et al. Influences of blood flow parameters on temperature distribution during liver tumor microwave ablation. Front Biosci Landmark. 2021;26:504–516.
- Vaidya N, Baragona M, Lavezzo V, et al. Simulation study of the cooling effect of blood vessels and blood coagulation in hepatic radio-frequency ablation. Int J Hyperthermia. 2021;38(1):95–104.
- Wu X, Liu B, Xu B. Theoretical evaluation of high frequency microwave ablation applied in cancer therapy. Appl Therm Eng. 2016;107:501–507.
- Chiang J, Wang P, Brace CL. Computational modelling of microwave tumour ablations. Int J Hyperthermia. 2013;29(4):308–317.
- Selmi M, Bajahzar A, Belmabrouk H. Effects of target temperature on thermal damage during temperature-controlled MWA of liver tumor. Case Stud Therm Eng. 2022;31:101821.
- Wang X, Gao H, Wu S, et al. Numerical evaluation of ablation zone under different tip temperatures during radiofrequency ablation. Math Biosci Eng. 2019;16(4):2514–2531.
- Hinshaw JL, Lubner MG, Ziemlewicz TJ, et al. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation-what should you use and why? . Radiographics. 2014;34(5):1344–1362.
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6:s99.
- Izaaryene J, Cohen F, Souteyrand P, et al. Pathological effects of lung radiofrequency ablation that contribute to pneumothorax, using a porcine model. Int J Hyperthermia. 2017;33(7):713–716.
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241(1):275–283.
- Chaddha U, Hogarth DK, Murgu S. Bronchoscopic ablative therapies for malignant central airway obstruction and peripheral lung tumors. Ann Am Thorac Soc. 2019;16(10):1220–1229.
- Yuan HB, Wang XY, Sun JY, et al. Flexible bronchoscopy-guided microwave ablation in peripheral porcine lung: a new minimally-invasive ablation. Transl Lung Cancer Res. 2019;8:787.