Abstract
Phenibut (β − phenyl − γ−aminobutyric acid) is licensed for use as a medicine in countries outside of the European Union (EU), but has also been sold as a “food supplement” from online shops to the general public in the EU. We present a case of phenibut use in a 25-year-old female undergoing alcohol and drug addiction treatment. She reported using phenibut, which she had purchased readily over the internet as a “food supplement.” Our clinical laboratory located in a hospital in the same region received urine samples for analysis which confirmed ingestion of phenibut. Identifying and responding to new psychoactive substances (NPS) emerging on the drug markets poses a challenge to clinical and forensic drug testing. A comprehensive laboratory analysis approach can identify the use of multiple NPS, including those used as medicines, offering beneficial opportunities for drug treatment services and clinical laboratories to work together.
Introduction
The growing number of new psychoactive substances (NPS) poses a challenge to public health and drug regulation in the European Union (EU) [Citation1,Citation2]. Drugs found on the NPS drug markets which have legitimate uses as medicines in countries outside of the EU complicate the situation. These include the anxiolytic drug phenibut (β − phenyl − γ−aminobutyric acid). Phenibut is licensed to treat alcohol dependence in Russia, but has also been detected in the EU where it is sold as a “food supplement” [Citation3].
Amongst EU countries, phenibut was first detected as an NPS in Sweden and reported for the first time in 2012 to the EU Early Warning System operated by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [Citation2]. Phenibut is used for its psychoactive effects, as a nootropic (“smart drug”) and to improve sleep [Citation3,Citation4]. Reported symptoms of phenibut withdrawal are both somatic (such as insomnia, convulsions and tachycardia) and psychological (such as craving and anxiety) [Citation3]. According to cases reported to the Dutch Poisons Information Center patients ingested between 1 and 10 grams of phenibut daily and presented with a range of adverse events including seizures and loss of consciousness [Citation5]. Reported symptoms of acute toxicity to phenibut overdose include agitation and reduced state of consciousness that may require supportive care and intubation [Citation6,Citation7].
This article presents a case report of phenibut ingestion, analytically confirmed, to highlight the presence of NPS in Denmark and discuss the benefits of a comprehensive approach to drug testing.
Case
A female patient, aged 25 years, attending alcohol and drug addiction treatment in a service in the North Denmark Region, reported use of phenibut to members of staff (initially reported in 2018). She purchased the phenibut product online, where it was sold as a “food supplement.” Disclosure of phenibut use occurred voluntarily as part of the assessment completed by the staff member in charge of treatment. The unfamiliarity of phenibut and its associated health and social risks (phenibut was new to the staff member) prompted further investigation. As part of this investigation we were contacted at the clinical laboratory, North Denmark Regional Hospital for possible confirmation of phenibut exposure and for help interpreting the results. After including phenibut in our drug testing programme conducted by liquid chromatography and tandem mass spectrometry (LC-MS/MS), we confirmed its presence in two urine samples obtained from the patient and delivered to our laboratory for testing. The first sample was positive for phenibut and alprazolam; the second for phenibut, oxazepam and gamma-hydroxybutyrate (GHB). This information was fed back to the drug treatment service and used for diagnostic and care planning illustrating the collaborative partnerships between hospital and addiction treatment in the region.
Discussion
To the best of our knowledge, this is the first published case report of confirmed phenibut use in Denmark. Publication as well as notification of NPS to the EU Early Warning System ensures that potential threats from new drugs are detected, assessed and responded to. Active substances used in medicines (such as phenibut) should also be reported.
The presence of a large number of NPS creates challenges for alcohol and drug addiction treatment that raise concerns for public health. The patient described here provided two urine samples testing positive for phenibut, GHB and benzodiazepines. Högberg et al. reported phenibut use in a patient also with concomitant consumption of benzodiazepines and a history of opioid addiction [Citation4]. LC-MS/MS testing at our laboratory for multiple drugs enabled staff members in the drug treatment service to establish substance intake and obtain information about unfamiliar NPS used to improve treatment plans.
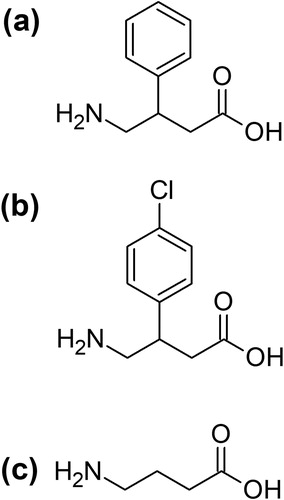
Others have reported that comorbid dependence on phenibut and benzodiazepines have successfully been treated with baclofen (also a GABA receptor agonist) () [Citation3]. Unambiguous identification of phenibut, achieved through partnerships between drug treatment services and clinical laboratories that offer comprehensive drug screening, can guide the decision to initiate the prescribing of substitution therapy, confirm treatment compliance, and monitor further use of phenibut [Citation8].
Figure 1. Structures of (a) phenibut (β − phenyl − γ−aminobutyric acid); (b) baclofen (β−(4-chlorophenyl)−γ − aminobutyric acid); and (c) γ − aminobutyric acid (GABA).
The effects of phenibut withdrawal appear similar to those associated with benzodiazepine withdrawal [Citation3,Citation4]. A similar situation exists in treating drug overdoses where CNS depression caused by phenibut can result in clinical effects that are similar to those of opioid overdose. In one case report published by Sankary et al., a patient who was taken to the Emergency Department after phenibut ingestion was treated with intravenous naloxone although naloxone is an opioid receptor antagonist with no effect in the treatment of phenibut overdose (a GABA receptor agonist) [Citation9]. Waiting for the results of LC-MS/MS testing, which has a turnaround time of approximately 2 days in our laboratory, should not lead to unnecessary delays in the initiation of emergency care. Rather, results from LC-MS/MS analysis can improve diagnosis and follow-up care. At referral to addiction treatment, if deemed necessary and appropriate, accurate reports of ingested substances can be used during the initial assessment and to establish treatment plans. By contrast, relying on reports of patients who have ingested “food supplements” obtained from the internet presents a problem given the discrepancies between the ingredients declared on the label and the actual chemical contents and quantities (including in “food supplements” containing phenibut) [Citation10].
Phenibut is promoted for stress reduction and as a mood enhancer according to product declarations [Citation7]. Printed materials in the self-help genre can be ordered online, which describe how “to take phenibut safely” () [Citation11]. While such information is useful in understanding the marketing of NPS, detection using LC-MS/MS analysis plays a crucial role in responding in a timely and appropriate manner to threats from new drugs appearing on the market. In the current circumstances of availability and use of a variety of NPS, LC-MS/MS analysis – unlike most immunoassays – will simultaneously test for many drugs to improve treatment by correctly identifying the ingested substances. Compared to immunoassays, LC-MS/MS has lower limits of detection, lower costs of screening per specimen, and identifies a greater number of substances [Citation12].
Figure 2. Authors’ copy of the book “Phenibut: Your ultimate guide to unlocking your social side & more with this powerful pill” from 2014 (no author). Original photograph.

However, phenibut and many other NPS are not generally included in routine clinical drug testing programs in Denmark. Further, the widespread use of immunoassays and practices such as only forwarding samples to a laboratory for confirmatory analysis when pre-screening with immunoassays are positive hinder detection of NPS [Citation13]. After including phenibut in our multi-methods for routine drug testing, we have detected positive samples from a patient in primary care and one in prison suggesting its use across different groups of people taking drugs. Without a continuous update of drug testing methods many NPS are likely to remain undetected, which makes it difficult to fully characterise the NPS situation in Denmark.
In summary, a comprehensive laboratory analysis approach can identify the use of multiple NPS including those used as medicines. Conversely, the use of immunoassays represents a traditional and yet obsolete approach that is no longer adequate nor safe for the public in terms of dealing with the current challenges in the NPS landscape [Citation13].
Disclosure statement
Andreas Kimergård was appointed to the list of experts for 2020–2023 used by the EU drug agency, the EMCDDA, for the assessment of risks posed by NPS. No other conflicts of interest to declare.
Additional information
Funding
References
- Regulation (EU) 2017/2101 of the European Parliament and of the Council of 15 November 2017 amending Regulation (EC) No 1920/2006 as regards information exchange on, and an early warning system and risk assessment procedure for, new psychoactive substances. 2017. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2101
- Europol & European Monitoring Centre for Drugs and Drug Addiction. New drugs in Europe, 2012 EMCDDA–Europol 2012 annual report on the implementation of Council Decision 2005/387/JHA. Luxembourg: Publications Office; 2013.
- Coenen NCB, Dijkstra BAG, Batalla A, et al. Detoxification of a patient with comorbid dependence on phenibut and benzodiazepines by tapering with baclofen: case report. J Clin Psychopharmacol. 2019;39(5):511–514.
- Högberg L, Szabó I, Ruusa J. Psychotic symptoms during phenibut (beta-phenyl-gamma-aminobutyric acid) withdrawal. J Subst Use. 2013;18(4):335–338.
- Koppen A, van Riel A, Roelen C, et al. Reports of phenibut usage to the Dutch Poisons Information Center (DPIC). Clin Toxicol. 2015;53:716–717.
- Downes MA, Berling IL, Mostafa A, et al. Acute behavioural disturbance associated with phenibut purchased via an internet supplier. Clin Toxicol (Phila)). 2015;53(7):636–638.
- Wong A, Little M, Caldicott D, et al. Analytically confirmed recreational use of phenibut (β-phenyl-γ-aminobutyric acid) bought over the internet. Clin Toxicol (Phila)). 2015;53(7):783–784.
- Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health; 2017.
- Sankary S, Canino P, Jackson J. Phenibut overdose. Am J Emerg Med. 2017;35(3):516.e1–516.e2.
- Cohen PA, Sharfstein J, Kamugisha A, et al. Analysis of ingredients of supplements in the National Institutes of Health supplement database marketed as containing a novel alternative to anabolic steroids. JAMA Netw Open. 2020;3(4):e202818.
- Get Ahead. Phenibut: your ultimate guide to unlocking your social side & more with this powerful pill; 2014.
- Kahl KW, Seither JZ, Reidy LJ. LC-MS-MS vs ELISA: validation of a comprehensive urine toxicology screen by LC-MS-MS and a comparison of 100 forensic specimens. J Anal Toxicol. 2019;43(9):734–745.
- Breindahl T, Hindersson P, Leutscher PDC, et al. New psychoactive substances require a paradigm shift in drug testing in Denmark. Ugeskr Laeger. 2018;180:V07170564.

