ABSTRACT
In recent years, ecstasy has become a very popular recreational drug. To date, very little data is available regarding its toxic side effects. Specifically, reports relating ecstasy use to the clinical diagnosis of leukoencephalopathy are quite rare. In this report, we present an interesting case of a 33-year-old female with a recent history of ecstasy abuse who presented with delirium and cognitive impairment. In addition, we review published case reports that explore the connection between MDMA and leukoencephalopathy.
Introduction
A 33-year-old Caucasian female patient presented to an inpatient psychiatric hospital with fluctuating levels of orientation, disorganized behaviour, and apathy. Regarding orientation, she was noted to be particularly frustrated by her inability to recall simple information such as the date. She was not able to remember recent events and described “missing pieces” in her memory. According to her, this was the first time she had experienced such symptoms. Apart from cognitive impairment, initial neurological examination was unrevealing. She had previously been diagnosed with bipolar disorder and had given birth to a healthy child eight weeks prior to her admission. Upon initial interview, the patient reported a past history of opiate abuse and recent use of methylenedioxymethamphetamine (MDMA).
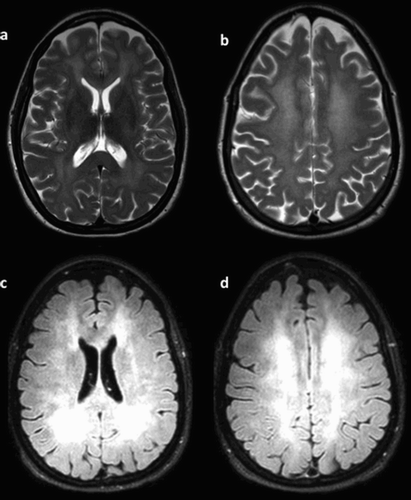
The attending clinicians considered hypotheses of delirium and/or puerperal psychosis and conducted a comprehensive workup in the inpatient setting. Her lab results, which included a complete blood count, metabolic panel, lipid profile, thyroid function tests, and serum toxicological screen, were all unrevealing. A magnetic resonance imaging (MRI) of the brain () showed diffuse, extensive confluent T2 hyper-intensity of the periventricular deep and subcortical white matter. There was sparing of subcortical U-fibres with associated patchy areas of abnormal restricted diffusion/cytotoxic oedema, predominantly along the deep and subcortical white matter. There was also signal abnormality that involved the bilateral caudate heads and lentiform nucleus. To a lesser extent, there was also involvement of the bilateral thalami. Subtle T2 hyper-intensity involving the left insular cortex and left amygdala was noted. Based on these findings, the diagnosis of delirium due to toxic leukoencephalopathy was strongly suspected. The patient was sent to an inpatient neurology setting for further investigation and management.
Figure 1. Magnetic resonance imaging of the brain showing diffuse white matter changes. (a) T2-weighted diffuse hyper-intensity involving the midbrain, insula, and basal ganglia bilaterally. (b) Bilateral symmetric reduced diffusion in periventricular white matter as seen on diffusion-weighted imaging (DWI). (c,d) FLAIR extent was graded severe.
On the neurology service, the diagnosis of toxic encephalopathy was confirmed. An EEG showed normal findings for the patient’s age. A comprehensive serological panel was sent. This included Hepatitis A, Hepatitis C, HIV, and an autoantibody workup (AChR ganglionic neuronal antibody, AGNA-1, Amphiphysin Antibody, ANNA-1,-2,-3, Calcium channel antibody N- and P/Q- types, CRMP-5 antibody, Neuronal K-channel antibody, Paraneoplastic antibody, PCA-1 and -2, ACHr binding antibody, striated muscle antibody). The results all came back negative. In addition, cerebrospinal fluid analysis which included a virology profile (VDRL, CMV, EBV, VZV, HIV, HSV1, HSV2, Enterovirus, Cryptococcus, Streptococcus pneumonia) was also unremarkable. After a few days, the patient’s condition remained unchanged. She continued to demonstrate apathy and cognitive impairment. She was discharged from the hospital and will likely require lifelong assisted-living.
3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy or Molly, an exceptionally popular synthetic amphetamine-like drug that alters mood and perception. Its physiologic effects overlap those of stimulants and hallucinogens. MDMA can produce feelings of increased energy, pleasure, emotional warmth, and distorted perceptions of time and sensation [Citation1].
Although MDMA is often regarded as a safe drug by its users, reports of persistent psychosis are increasing. This outcome is being documented even with sporadic or isolated occasional use of the substance [Citation2,Citation3]. MDMA has two primary mechanisms of action. First, it causes serotonin “5-hyroxytryptamine: 5-HT” surge in the synapses of the brain. This leads to central serotonin depletion, and it takes several days for restoration of normal levels. Because of this, users often experience a protracted period of depression and low energy after using the drug [Citation4]. Second, MDMA leads to dopamine and noradrenaline release. The stimulant properties of the drug can be attributed to this change.
Toxic leukoencephalopathy is a structural alteration of the white matter caused by drugs. In this condition, insults can appear to be relatively symmetric on MRI. Characteristic distribution includes symmetrical involvement of the posterior limbs of the internal capsules that extends inferiorly into the pontine corticospinal tracts and superiorly into the peri-rolandic subcortical white matter. Also found is a symmetrical butterfly wing pattern of involvement of the cerebellar white matter and sparing of adjacent grey matter structures. Notably, there is sparing of subcortical U-fibres. Causes of such insults include, but are not limited to, chemotherapy, immunosuppressive therapy, select antimicrobial medications, carbon monoxide, and drugs of abuse [Citation5].
Here, we report a case that presented to the psychiatric ward with delirium and cognitive decline due to toxic encephalopathy. The patient’s condition was eventually attributed to MDMA abuse.
Discussion
This case presented a diagnostic challenge. The patient had a long-standing history of bipolar disorder and was in the puerperal period. However, her unique presentation of fluctuating cognition, inability to care for self, and memory gaps made it difficult to attribute her condition solely to her psychiatric diagnosis and drug abuse history. In addition, symptoms related to drug intoxication would be expected to remit in the hospital setting due to abstinence. In this case, the report of recent “Molly” abuse provided a hint to a possible underlying pathophysiological cause for her presentation.
Studies linking the development of leukoencephalopathy with MDMA use are sparsely reported in the literature. Originally named “Methylsafrylamin,” MDMA was first developed as a side product in 1912 by Merck’s German chemist Köllisch. He was trying to discover a substance that would control abnormal bleeding by enhancing clotting [Citation6]. The compound was abandoned at the time in favour of others. However, reports of the substance re-emerged in the 1950s. In 1952, Dr Albert van Schoor, the first to conduct a toxicological experiment using flies, reported an interesting outcome related to MDMA exposure. He noted that flies laid in the supine position and later died. In 1954, the US Army conducted animal studies on five species using MDMA at the University of Michigan [Citation7]. In 1959, Dr Wolfgang Fruhstorfer introduced MDMA as a novel stimulant. The first official scientific paper on MDMA synthesis was published in 1960 [Citation8], and the year 1970 marked its first known appearance as a street drug in Chicago. In 1976, Dr Alexander Shulgin popularized its usage as a human stimulant [Citation9,Citation10]. Officials in California noted the emergence of “Ecstasy” as MDMA’s first street name in 1984. The following year, it became a schedule I controlled substance in the United States and banned in most other countries [Citation11].
As early as 1975, the phenomenon of “Toxic Ecstasy” in young men was proposed. This was the first instance linking delirium and MDMA use [Citation12]. The first report of toxic leukoencephalopathy that developed after ecstasy use was documented in 1997. The authors described a 19-year-old man who presented to the ER with loss of consciousness and respiratory insufficiency after using ecstasy for the first time. A computed tomography of his head revealed multiple hypodense lesions in the white matter. One month later, he developed a vegetative state and his MRI revealed diffuse bilateral extensive hypodense lesions of the white matter [Citation13]. In 1999, an Italian research group reported delirium in three young adults after recreational ecstasy ingestion, but this study did not report brain image findings because the cases resolved rapidly [Citation14] (). In 2012, Sacks and colleagues reported a case of 26-year-old male patient who presented with unconsciousness after an overdose with “Europa = 2C-E,” an MDMA-related drug. His brain MRI showed diffuse findings suggestive of toxic leukoencephalopathy [Citation15]. In 2014, Narang and colleagues reported a case of a 22-year-old male patient who was first diagnosed with meningoencephalitis, but later developed upper motor neuron signs with pupillary dilatation. These findings were then confirmed by MRI to be toxic leukoencephalopathy associated with MDMA intake [Citation16]. In 2015, Ginat presented the neuroimaging of a 17-year-old boy who presented with headaches and alerted mental status after “Molly” ingestion. His MRI showed diffuse leukoencephalopathy affecting the midbrain, insula and basal ganglia bilaterally, and a lumbar puncture showed mild lymphocytosis and high protein levels [Citation17].
Table 1. Reported cases in literature of Ecstasy-induced leukoencephalopathy.
The mechanism by which ecstasy induces its toxic effect is not fully understood. However, there is a hypothesis that relates its potential to cause cerebral toxicity to the disruption of the thermoregulation mechanism with the excessive loss of sodium and inappropriate antidiuretic hormone release. Both of these changes lead to accumulation of water in brain tissue and cause epilepsy-like seizures, brainstem and cerebellum compression at the foramen magnum, and eventually fatal disruption in respiration. A second mechanism involving hypoxic injury to the cerebral white matter with sparing of the cortex is also hypothesized [Citation18].
In contrast to some other illicit substances, the long-term effects of the ecstasy are not well understood. Anecdotal evidence from users has reported extreme and long-lasting depression and/or cognitive impairment attributed to ecstasy. To date, there has been no scientific consensus regarding possible long-term damage to an MDMA user [Citation18].
The development of leukoencephalopathy has also been associated with other drugs of abuse. The inhalation of heroin was linked to the “chasing the dragon” syndrome. In this condition, toxic leukoencephalopathy presents with findings of demyelination that affect predominantly the occipital cortex, cerebellar, and brainstem regions [Citation19]. Cocaine-related toxic leukoencephalopathy has been also reported in the literature. Its toxic effect is mostly related to the substances used in adulteration. Three categories of cocaine-induced brain injury have been described. First, vascular pathology is seen due to ischaemia and haemorrhagic strokes. In addition, metabolic pathology is related to mitochondrial dysfunction that leads to widespread demyelination with vacuolar degeneration and axonal loss. Notably, these changes tend to have a different anatomical distribution than that seen with heroin. Finally, acute and recurrent white matter lesions can be seen. These are speculated to be caused by the adulterant levamisole, now commonly found in cocaine [Citation20–23]. Numerous other drugs of abuse have been associated with toxic leukoencephalopathy to a lesser extent. These include the psychoactive drug 2C-E “Europa” [Citation15], oxycodone [Citation24], and methadone [Citation25,Citation26].
In conclusion, toxic leukoencephalopathy is a serious pathological finding that should be considered in the differential diagnosis of patients that present with the recent onset of cognitive deficits and are known to have exposure to toxins or drugs of abuse.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gowing LR, Henry-Edwards SM, Irvine RJ, et al. The health effects of ecstasy: a literature review. Drug Alcohol Rev. 2002;21(1):53–63. doi: 10.1080/09595230220119363
- Kelly PAT. Does recreational ecstasy use cause long-term cognitive problems? West J Med. 2000;173:129–130. doi: 10.1136/ewjm.173.2.129
- Van Kampen J, Katz M. Persistent psychosis after a single ingestion of “ecstasy”. Psychosomatics. 2001;42(6):525–527. doi: 10.1176/appi.psy.42.6.525
- Ricaurte GA, McCann UD, Szabo Z, et al. Toxicodynamics and long-term toxicity of the recreational drug, 3, 4-methylenedioxymethamphetamine (MDMA, “Ecstasy”). Toxicol Lett. 2000;112–113:143–146. doi: 10.1016/S0378-4274(99)00216-7
- McKinney AM, Kieffer SA, Paylor RT, et al. Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol. 2009;193(1):192–206. doi: 10.2214/AJR.08.1176
- Freudenmann RW, Öxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction. 2006;101(9):1241–1245. doi: 10.1111/j.1360-0443.2006.01511.x
- Hardman HF, Haavik CO, Seevers MH. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicol Appl Pharmacol. 1973;25:299–309. doi: 10.1016/S0041-008X(73)80016-X
- Biniecki S, Krajewski E. Production of d,1-N-methyl-beta- (3,4-methylenedioxyphenyl)-isopropylamine and d,1-nmethyl-beta-(3,4-dimthoxyphenyl)-isopropylamine. Acta Polon Pharm. 1960;17:421–425.
- Shulgin AT, Nichols DE. Characterization of three new psychotomimetics. In: Stillman RC, Willette RE, editor. The psychopharmacology of the hallucinogens. New York (NY): Pergamon Press; 1978. p. 74–83.
- Anderson GMIII, Braun G, Braun U, et al. Absolute configuration and psychotomimetic activity. “QuaSAR” research monograph. Natl Inst Drug Abuse. 1978;20:8–15.
- Chakraborty K, Neogi R, Basu D. Club drugs: review of the “rave” with a note of concern for the Indian scenario. Indian J Med Res. 2011;133(6):594–604.
- Bron B, Ruttenberg BA. Delirium and ecstasy. The phenomenon of toxic ecstasy in young men. Confin Psychiatr. 1975;18(2):61–72.
- Bertram M, Egelhoff T, Schwarz S, et al. Toxic leukencephalopathy following “ecstasy” ingestion. J Neurol. 1999 Jul;246(7):617–618. doi: 10.1007/s004150050416
- Alciati A, Scaramelli B, Fusi A, et al. Three cases of delirium after “ecstasy” ingestion. J Psychoactive Drugs. 1999;31(2):167–170. doi: 10.1080/02791072.1999.10471740
- Sacks J, Ray MJ, Williams S, et al. Fatal toxic leukoencephalopathy secondary to overdose of a new psychoactive designer drug 2C-E (“Europa”). Proc (Bayl Univ Med Cent). 2012;25(4):374–376. doi: 10.1080/08998280.2012.11928883
- Narang RK, Jadun CK, Carr B. A case of MDMA toxicity with unusual clinical and neuroradiological features 2CO1. J Intensive Care Soc. 2014;15(1):70–73. doi: 10.1177/175114371401500116
- Ginat DT. MRI of toxic leukoencephalopathy syndrome associated with methylenedioxymethamphetamine. Neurology. 2015;17;84(7):757. doi: 10.1212/WNL.0000000000001271
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;2;165(7):917–928.
- Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63(2):146–152. doi: 10.1016/j.crad.2007.07.021
- Gilbert JW, Xu N, Zhou W, et al. Clinical and MRI characteristics of levamisole-induced leukoencephalopathy in 16 patients. J Neuroimaging. 2011;21:e188. doi: 10.1111/j.1552-6569.2010.00466.x
- González-Duarte A, Williams R. Cocaine-induced recurrent leukoencephalopathy. Neuroradiol J. 2013;26(5):511–513. doi: 10.1177/197140091302600503
- Vosoughi R, Schmidt BJ. Multifocal leukoencephalopathy in cocaine users: a report of two cases and review of the literature. BMC Neurol. 2015;15:208. doi: 10.1186/s12883-015-0467-1
- AbdelRazek MA, Albrecht A, Han C, et al. A case of cocaine-induced toxic leukoencephalopathy With severe radiologic but mild clinical disease. Addict Disord Their Treat. 2017;16(2):59–63. doi: 10.1097/ADT.0000000000000102
- Odia MY, Jinka M, Ziai WC. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit Care. 2010;13(1):93–97. doi: 10.1007/s12028-010-9373-y
- Salgado RA, Jorens PG, Baar I, et al. Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. Am J Neuroradiol. 2010;31(3):565–566. doi: 10.3174/ajnr.A1889
- Cerase A, Leonini S, Bellini M, et al. Methadone-induced toxic leukoencephalopathy: diagnosis and follow-up by magnetic resonance imaging including diffusion-weighted imaging and apparent diffusion coefficient maps. J Neuroimaging. 2011;21(3):283–286. doi: 10.1111/j.1552-6569.2010.00530.x

