 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The demand for new materials and technologies that are able to minimize the environmental impact generated by the disposal of materials from fossil sources has increased significantly in the past years. As these materials normally include gelatin, a high cost ingredient, our aim was to find a more effective solution through the development of films based on protein isolate sourced from Nile tilapias. Thickness, water solubility, water vapor permeability (WVP), tensile strength, elongation, color attributes, and opacity were evaluated. Casting technique was used to obtain the films, using different combinations of protein isolate, gelatin, and plasticizers (glycerol or sorbitol) in an experimental design 23. The films showed good continuity and handling. Surface was free from bubbles and cracks. Key results observed: thickness from 0.04 to 0.09 mm, solubility in water from 4.11 to 28.72%, WVP from 7.12 to 16.36 g mm d−1 KPa−1 m−2, tensile strength from 12.43 to 155.57 MPa, and elongation from 2.14 to 125.33%. Gelatin protein films were promising from the point of view of mechanical properties, visual appearance and easy handling, as well as low WVP and low solubility in water. However, the replacement of gelatin in the formulations implicated in films with reduced tensile strengths.
KEYWORDS:
Introduction
The demand for new materials and technologies capable of minimizing damage to the environment has increased significantly in the past years. Research around the world has been growing with the interest in reducing and replacing the use of materials that are difficult to degrade [Citation1,Citation2]. Biodegradable films obtained from natural polymers, e.g. polysaccharides and proteins are included in this context, showing potential application in the food and pharmaceutical industries [Citation3,Citation4]. Within the protein group, gelatin generates interest because of the high availability, the fact that it is produced at relatively low cost and also for having excellent functional and filmogenic properties [Citation5]. Thus, it has been widely used for the development of films by the method of “casting”.
In general, gelatin-based films have good mechanical resistance but have a low barrier to water vapor. On the other hand, due to the hydrophilic characteristics of gelatin, the material shows limitations related to their poor stability in the face of climate changes and high solubility in water. These films have high susceptibility to environmental conditions, impacting their application as packaging [Citation6]. In an attempt to improve these characteristics, several alternatives have been studied, such as: chemical and enzymatic modification of gelatin [Citation7]; use of mixtures of plasticizers with different degrees of hydrophilicity [Citation8,Citation9]; and incorporation of lipids [Citation10] or other biomaterials.
The plasticizers are adhered to the film matrix, which generates an increase in the free space between the polymeric chains, causing a decrease in intermolecular forces, increasing flexibility and handling, with a reduction in possible discontinuities and brittle regions due to the ease of these molecules to bind between polysaccharide chains by breaking hydrogen bonds [Citation11]. Plasticizers must be compatible with biopolymers, e.g. glycerol and sorbitol have properties that interact with the film chains, increasing molecular mobility and flexibility, although they can increase their hydrophilicity and water vapor permeability [Citation12]. These films cannot be considered as edible, due to the process of solubilization of proteins, since the HCl and NaOH used are not edible, but can constitute biodegradable materials. The production of films based on biopolymers or forming blends with biopolymers has been encouraged because they are produced with raw materials from renewable sources [Citation2,Citation13].
Thus, the aim of this work was to develop films based on Nile tilapia (Oreochromis niloticus) protein isolate obtained from residues and blend this biopolymer with gelatin, aiming to reduce the concentration of this high-cost component of the formulations.
Material and methods
Obtaining of raw materials
Nile tilapia (Oreochromis niloticus) carcasses were obtained from a local fish processing plant (Dourados, MS, Brazil). The samples were packed in polyethylene plastic bags and transported under refrigeration to our laboratory. Mechanically separated meat (MSM) was produced from the Nile tilapia carcasses in 3 mm particle diameter using a meat bone separator (HT 250, High Tech, Brazil), operating at 6°C at the entrance and 10°C at the exit, 24 h after slaughter [Citation14]. The obtained MSM was homogenized with distilled water in a ratio of 1:9 (w/v) at 5°C for 5 min, using a propeller stirrer (Fisatom 713D). After homogenization, the protein was subjected to alkaline solubilization in an ultra-thermostatic water bath (Lauda, model Alpha RA-8) for 30 min, under constant stirring with a propeller stirrer (Fisatom 713D), using sodium hydroxide (1 N NaOH) as the alkalizing agent, and constant controlled temperature of 5°C. After this step, the mixture was centrifuged at 9,000 x g and 5°C (Nova Técnica, model MA 1815) for 15 min to separate the solubilized product into three phases: lipids, soluble proteins, and insoluble proteins, thus facilitating the removal of the supernatant. The intermediate phase resulting from centrifugation, corresponding to the soluble proteins, was separated and subjected to isoelectric precipitation, using hydrochloric acid (1 N HCl). The pH was adjusted to 5.8 at 5°C under constant stirring (Fisatom 713D) for 30 min. Another centrifugation was carried out at 9,000 x g and 5°C for 15 min to separate the precipitated fraction, thus facilitating the collection of the precipitate and obtaining the protein isolate. The fish protein isolate was dried in an air circulating oven (MA model 035) at 40°C for 12 h and then crushed in a double-bladed grinder. The protein isolate was stored in a hermetically sealed glass container at room temperature (23–25°C) and utilized throughout the course of this study [Citation15].
Obtaining and subjective evaluation of the polymeric biofilms
The films were produced by the casting technique as previously reported [Citation1]. A first filmogenic solution was obtained from Nile tilapia protein isolate, which was dispersed in 50 mL of distilled water and then maintained at 30°C under constant magnetic stirring (350 rpm) (Tecnal, model TE – 0851, São Paulo, Brazil) for 10 min to rehydration. Then, the pH of the dispersion solution was adjusted to 11 with the addition of 1 N NaOH using a bench potentiometer (Quimis model 400A, São Paulo, Brazil) with constant stirring for further 10 min. Thereafter, the temperature was raised to 80°C and added of the plasticizer (glycerol or sorbitol) previously solubilized homogenously in distilled water by using a homogenizer (Ultra-turrax IKA model T25, Campinas, Brazil) for 2 min. After complete dissolution of the protein isolate and the plasticizer, the film-forming solution was kept at constant magnetic stirring (350 rpm) for 20 more min and reserved. A second filmogenic solution was obtained from gelatin, which was dispersed in 50 mL of distilled water. After homogenization, the solution was taken to a thermostatic bath without stirring (TECNAL, model TE-054 MAG, São Paulo, Brazil) and heated to 80°C for 10 min. The solution was kept under constant manual agitation to avoid the formation of precipitates and to better homogenize the sample. The obtained solution was then gently added to the film-forming solution previously prepared in order to prevent the formation of bubbles.
For film production, 30 mL of the filmogenic solution was poured in the polycarbonate Petri dishes with a diameter of 15 cm and subjected to drying in a forced-air circulation oven in an incubator chamber with orbital agitation (Marconi, model MA420) at 25 ± 1°C for 96 h. Then, the films were stored for 48 h in desiccators maintained at 25 ± 2°C and relative humidity of 55 ± 1%, controlled using a saturated solution of calcium chloride, prior analyses. Nine formulations of films were prepared as described in .
Table 1. Subjective evaluation of the Nile tilapia protein isolate films
The parameters continuity (absence of rupture after drying), homogeneity (absence of insoluble particles, bubbles of air or opacity zone), and flexibility (handling without risk of rupture) were determined for the films using visual and tactile analyses [Citation16]. Films that did not have such characteristics were discarded.
Characterization of biopolymer films
Thickness
The film thickness was measured using a digital micrometer (Digimes, São Paulo, Brazil), resolution 0.0100 ± 0.0005 mm. The final value represented the average of 5 random measurements taken at different parts of the film.
Solubility in water
The solubility in water (Sw) was determined as described elsewhere [Citation17]. Triplicate disc samples with 2 cm diameter were extracted from the films. The initial dry matter of the samples was obtained by drying them in a forced-air circulation and renovation oven for 24 h at 105°C. After the first weighing, the samples were immersed in a recipient containing 50 mL distilled water and maintained under constant slow agitation at 50 rpm in an orbital shaker at 25°C for 24 h (Cientec, CT-712RNT). The swollen samples were then removed and dried in a forced-air circulation and renovation oven at 105°C up to constant weight to determine the final dry matter. The Sw of the film was represented by the total soluble material dissolved in water, calculated according to EquationEquation (1)(1)
(1) .
Where: Sw = solubility in water (%); mi = initial dry mass of the sample (g); mf = final dry mass of the sample (g).
Water vapor permeability
The water vapor permeability (WVP) was determined in triplicate according to the modified standard gravimetric method E-96 [Citation18]. The films were sealed in permeation aluminum cells with permeation area of 0.13 m2, containing calcium chloride and sealed with paraffin to ensure migration of moisture only through the exposed area of the bioplastic. The permeation cells were placed in desiccators kept at 25°C and 75% relative humidity. The amount of water vapor migrating through the film was determined from the gain in mass of the calcium chloride, measured every 24 h for 7 days [Citation19]. The effect of the air space between the region below the film and the surface of the calcium chloride of the test cells was not considered in the calculation [Citation20,Citation21]. The WVP was calculated according to Equation (7).
Where: WVP = water vapor permeability (g mm) (m2 d kPa)−1; ΔW = mass gain (g); L = initial film thickness (mm); t = storage time (days); A = exposed film area (m2); ΔP = partial pressure difference (kPa).
Tensile strength and elongation
The tensile strength and elongation at break were determined in triplicate using a TA-XT2 Texture Analyzer (SMS, Surrey, UK), operated according to the standard method ASTM D 882–83 [Citation22], as modified elsewhere [Citation23]. The films were cut into rectangles (8.0 cm long x 2.5 cm wide) and fixed to claws with initial distance to separation of 5.0 cm. Measurements were carried out uniaxially, stretching the sample in one direction. The velocity of the tests was fixed as 0.8 mm/s. Tensile strength was calculated by dividing the maximum force for film rupture by the cross-sectional area of the film (EquationEquation (3))(3)
(3) . Elongation in the rupture was determined by dividing the difference of the final distance coursed up to the rupture and initial distance of separation by the initial distance of separation multiplied by 100 (EquationEquation (4))
(4)
(4) .
Where: TS = tensile strength (MPa); Fm = maximum force at the moment of rupture (N); A = cross-sectional area of the film (m2).
Where: E = elongation (%); d0 = initial distance of separation between claws (cm); dr = distance to rupture (separation between the claws at the moment of rupture) (cm).
Color
The color of the films was determined by averaging three measurements, one at the center and the other two at the perimeter (edge distance) using a CR-400 colorimeter (Konica Minolta, Chroma Meter, Japan), operating at D65 (daylight).
The parameters a*, b*, and L* were determined in triplicate. L* indicates the luminosity and a* and b* the chromatographic coordinates. CIE-Lab scales were used to measure the color of the films, with L* ranging from 0 (black) to 100 (white), a* from green (−60) to red (+60) and b* from blue (−60) to yellow (+60) [Citation24]. The color difference in relation to the standard was determined by using EquationEquation (5)(5)
(5) [Citation25]:
Opacity
The opacity was determined using a CR-400 colorimeter (Konica Minolta, Chroma Meter, Japan) as reported elsewhere [Citation26]. The measurements were made in triplicate, in different film points aleatory, after calibration of the colorimeter with a white (Yw) and a black (Yb) background. The values for opacity were calculated according to EquationEquation (6)(6)
(6) [Citation25]:
where: Y= opacity of the film (%); Yb = opacity of the film against a black background; Yw = opacity of the film against a white background.
Statistical analysis
It was calculated the analysis of variance (ANOVA) prior the Tukey test, to determine the differences between the properties of the films in the range of 95% confidence using the Statistica® 6.0 (Stasoft, USA) software. The evaluations were performed from data obtained at least in triplicates and the results were presented by the mean ± standard deviation.
Results and discussion
Obtaining and subjective evaluation of the films
The subjective evaluation shows that the protein films with gelatin presented excellent continuity and handling. There was no difficulty in removing the films from the plates, maintaining their integrity. Regarding homogeneity, the films were shown intact, without cracks or breaks after the drying process. However, T1, T2, T3, T6, T7, and T8 treatments presented insoluble particles visible to the naked eye, indicating that there was no complete solubilization of the protein isolate, which was responsible for the marked yellow color observed in T1 and T6, which had the highest concentration of this component (). Nevertheless, all evaluated treatments obtained satisfactory results for the analyzed parameters, as observed in .
Figure 1. Nile tilapia protein isolate films
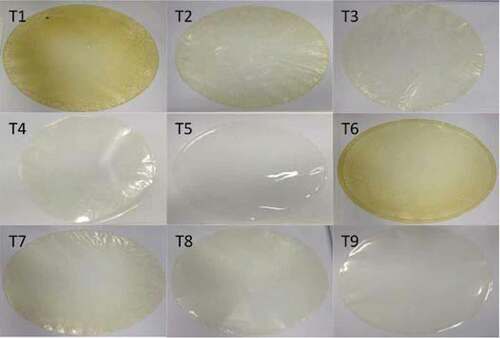
To establish a flexible and hydrophobic filmogenic solution, it is necessary to use plasticizers that are responsible for these characteristics. However, in low concentrations, they may cause an anti-plasticizer effect [Citation27] while that in high concentrations, a hydrophilic effect, i.e., in both situation they can cause an opposite effect to the desired one for films produced by the casting technique.
Characterization of biopolymer films
The results obtained for thickness, solubility in water, water vapor permeability, tensile strength, and elongation are shown in .
Table 2. Thickness (T), solubility in water (Sw), water vapor permeability (WVP), tensile strength (TS), and elongation (E) of the Nile tilapia protein isolate films
Thickness
The films showed a significant difference (p < 0.05) for thickness (), indicating that the composition influenced this property, even with a standardization of 30 mL of solution filmogenic per petri dish. The films with the greatest thickness (0.009 mm and 0.008 mm) were obtained in T1 (3.0 g protein isolate 100 mL−1 and 0.6 g glycerol 100 mL−1) and T6 (3.0 g protein isolate 100 mL−1, 0.6 g sorbitol 100 mL−1), respectively. These were the treatments with the highest concentration of protein isolate and plasticizers. The smallest thickness (0.004 mm) was observed in T4 (0.75 g protein isolate 100 mL−1, 2.25 g gelatin 100 mL−1, and 0.15 g glycerol 100 mL−1), T7 (2.25 g protein isolate 100 mL−1, 0.75 g gelatin 100 mL−1, and 0.45 g sorbitol 100 mL−1), and T9 (0.75 g protein isolate 100 mL−1, 2.25 g gelatin 100 mL−1, and 0.15 g sorbitol 100 mL−1).
The thickness can be influenced by the quantity and type of components of the polymeric matrix, preparation technique, type of solvent used, among other factors. It was reported elsewhere values of thickness ranging from 0.022 to 0.177 mm for bionanocomposite films based on pectin and cocoa pulp [Citation28], and from 0.015 to 0.024 mm and 0.012 to 0.046 mm for corn starch films plasticized with glycerol and triacetin, respectively [Citation29]. This variation was associated with a strong interaction between the starch molecule and the plasticizer. These values are lower than those found in our study, suggesting that the concentration of protein isolate used in the filmogenic solution does not provide such strong interaction.
Solubility in water
The solubility in water (Sw) of films is an important analysis for determining their affinity with water, which is related to the Sw of the raw materials, considering that most of these films are made with hydrophilic materials [Citation30], as the protein isolate.
The solubility of the films varied from 4.11 to 28.72%, as shown in . This is a desirable characteristic for the films in this study if the interest is to apply them directly to the products. For these applications, the films must not be water-soluble to prevent damage to the product to be protected. Literature reports that when an edible film is exposed to water, the hydrogen bonds between the polymeric chains dissociate by competition with water molecules, which results in the deformation and dissolution of the film [Citation31].
The use of plasticizer, in particular glycerol, has a direct influence on the solubility of starch films, since it interacts with the film polymeric matrix, increasing the free space between the chains, promoting the entry of water in the film and, consequently, increasing its solubility [Citation32]. This fact can be observed in T1 (), in which there was an increase in Sw in the presence of high glycerol content.
However, the range of values for the Sw is highly variable, depending on the composition of the components of the film-forming matrix, e.g. it was reported elsewhere Sw varying from 16.8 to 52.9% in cassava starch films added of barbados cherry pulp and glycerol [Citation33], and from 18.75 to 51.35% for corn starch (waxy and native) films added of glycerol [Citation34].
Water vapor permeability
Water vapor permeability (WVP) is the rate of water vapor transmission through the unit area of the flat material, indicating how easily a material can be penetrated by water vapor [Citation35]. This analysis is essential to understand the behavior of polymeric films, since water is present both in the films and the products. Factors such as the polymer itself, the area exposed to permeation, and the thickness can affect the permeability of the film. Here the increase in thickness was directly proportional to the WVV, where a variation from 7.12 to 16.36 g mm kPa−1 day−1 m−2 was found ().
The increased spacing between the chains, due to the additional inclusion of molecules between the polymer chains can promote an increase in WVP through the film and, therefore, accelerate the transmission of water vapor. This fact was observed by other authors e.g. in biopolymeric films based on lipophilic corn starch and gelatin [Citation36], in gelatin and lauric acid films [Citation10], and in potato starch films [Citation37]. In all these cases, the increase in the thickness caused an increase in WVP of the films.
The increase in the concentration of glycerol induces an increase in the permeability to gases of hydrophilic films, when the glycerol binds to the molecules of the biopolymer, increases mobility, and decreases the density between its molecules, facilitating the transmission of gases through the material [Citation21].
Tensile strength and elongation
Tensile strength and elongation are important mechanical properties of a film. The first has the function of expressing the maximum force that the film supports during the tensile test, the second refers to its ability to stretch before breaking. The mechanical properties of the films closely depend on the formulation (biomolecules, solvents, plasticizers, pH adjusters) and the procedures used to produce them. The concentration of plasticizer in the formulation is an important factor capable of altering the mechanical properties of a film. Starch films without plasticizers are resistant and elastic and, as the plasticizer content is added, these materials become more deformable [Citation16]. Plasticizers act by decreasing intermolecular forces between the starch chains, causing a reduction in the glass transition temperature and, in general, the resistance drops with the increase in the concentration of the plasticizer [Citation26].
The analysis of the mechanical properties of the films showed values of tensile strength between 12.43 and 155.57 MPa (). The addition of gelatin caused an increase in tensile strength compared to the films without adding it. The highest resistance value was observed in T5 (3.0 g gelatin 100 mL−1), which contained the highest concentration of gelatin. Some authors reported values of tensile strength between 15.09 and 60.32 MPa in bionanocomposite films based on pectin and cocoa pulp [Citation28]. This fact was associated with the possible occupation of part of the empty spaces between the polymer chains by the nanostructures, causing an increase in resistance due to a greater compaction of the polymeric matrix.
The elongation values obtained in this study varied between 2.14 and 125.33%, showing a significant difference (p < 0.05) between treatments (). A significant negative effect was observed for this parameter, where the addition of gelatin reduced the elongation of the films. Glycerol was the variable that most increased the elongation parameter when compared to sorbitol. Elongations ranging from 1.0 to 1.5% have been reported in other studies with soy protein films, but it was affected by the addition of phenolic acid [Citation38].
A high tensile strength is desired, but the elongation will depend on the application of the film, since to maintain its integrity and barrier properties, the film must allow the normal tension presented during its application, transportation, and handling [Citation17].
Color and opacity
The color and the opacity of the polymer result from the morphology or the chemical structure associated with the molecular mass of the material [Citation39]. Gelatin-based films are transparent and homogeneous, while the addition of some compounds may change the appearance of hydrocolloid films making them opaquer [Citation40,Citation41]. Thus, the values obtained for each film can only be considered good or bad depending on their application.
The results obtained for the color parameters (L*, a*, b*, ∆E*) of the films showed a significant difference at the level of 5% (). Regardless of the results of the statistical analysis, these films could be considered as highly colored (∆E* > 4) and opaque (Y > 2.5) [Citation9]. The luminosity varied from 83.68 to 93.94, indicating that they can be considered clear because the closer to 100, the more transparent the films are.
Table 3. Color attributes and opacity of the Nile tilapia protein isolate films
The films showed a yellowish color, as observed for b*, where the values were close to 28, and with a greenish tendency, as verified for a*. The latter may be associated with the presence of collagen that has its characteristic coloration.
The films showed opacity values that varied between 12.64 and 21.15 (). The lowest value was observed for the films containing only gelatin (T5: 3.0 g gelatin 100 mL−1). The addition of oils or derivatives promotes an increase in the diffraction of the light rays that pass through the film, thus producing greater opacity [Citation19]. The results obtained here were close to those reported elsewhere for films containing modified cassava starch, gelatin, and glycerol, whose average opacity was 14.5 [Citation42].
Conclusion
Homogeneous, continuous, and good handling films were successfully obtained by combining different components. Protein isolate was the variable that had the greatest influence on thickness, solubility, water vapor permeability, and elongation. The results for thickness showed that the protein isolate associated with gelatin influenced significantly, unlike the plasticizers used in the formulations. The increase in thickness was directly proportional to the permeability to water vapor permeability. The addition of gelatin caused an increase in tensile strength compared to the films without its addition. Glycerol had a direct influence on the solubility in water of the films due to its interaction with the polymeric matrix, increasing its solubility. Glycerol has also increased the elongation when compared to the sorbitol. The films showed light coloration tending to the yellowish. However, the replacement of gelatin from the formulations implicated in films with reduced tensile strengths.
Acknowledgments
The authors gratefully acknowledge the Brazilian research funding agencies CNPq, CAPES and FUNDECT for the financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Cintia Granzotti da Silva Scudeler
Cintia Granzotti da Silva Scudeler has PhD degree in Biotechnology and Biodiversity (Federal University of Grande Dourados - UFGD) and researchers at the Bioenginering Research Group (BIOENG) of the UFGD.
Thayná de Lima Costa
Thayná de Lima Costa has bachelor’s degree in Food Engineering (UFGD) and researchers at the Bioenginering Research Group (BIOENG) of the UFGD.
José Ignácio Velasco
José Ignácio Velasco is full Professor and the Coordinator of the Polyfunctional Polymeric Materials Research Group (Poly2) at the Polytechnic University of Catalonia (UPC).
Farayde Matta Fakhouri
Farayde Matta Fakhouri is Associate Professor at UFGD and member of Poly2 group (UPC).
Gustavo Graciano Fonseca
Gustavo Graciano Fonseca is Associate Professor at UFGD and Coordinator of the BIOENG research group.
References
- De Andrade CS, Fonseca GG, Mei LHI, et al. Development and characterization of multilayer films based on polyhydroxyalkanoates and hydrocolloids. J Appl Polym Sci. 2017;134:44458.
- Da Silva AO, Cortez-Vega WR, Prentice C, et al. Development and characterization of biopolymer films based on bocaiuva (Acromonia aculeata) flour. Int J Biol Macromol. 2020;155:1157–1168.
- Nur Hanani ZA, Roos YH, Kerry JP. Use and application of gelatin as potential biodegradable packaging materials for food products. Int J Biol Macromol. 2014;71:94–102.
- Rosseto M, Krein DDC, Balbé NP, et al. Starch‐gelatin film as an alternative to the use of plastics in agriculture - A review. J Sci Food Agric. 2019;99:6671–6679.
- Silva RS, Santos BMM, Fonseca GG, et al. Analysis of hybrid sorubim protein films incorporated with glycerol and clove essential oil for packaging applications. J Polym Environ. 2020;28:421–432.
- Bertuzzi MA, Vidaurre EFC, Armada M, et al. Water vapor permeability of edible starch based films. J Food Eng. 2007;80:972–978.
- Carvalho RA, Grosso CRF. Effect of thermal and enzymatic treatment on the properties of gelatin films. Ciên Tecnol Alim. 2006;26:495–501.
- Thomazine M, Carvalho RA, Sobral PJA. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J Food Sci. 2005;70:172–176.
- Vanin FM, Sobral PJA, Menegalli FC, et al. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005;19:899–907.
- Bertan LC, Tanada-Palmu PS, Sian ACC, et al. Effect of fatty acids and ‘Brazilian elemi’ on composite films based on gelati. Food Hydrocoll. 2005;19:73–82.
- Andreceutti C, Carvalho R, Galicia-Garcia T, et al. Gelatin-based films containing hydrophobic plasticizers and saponin from Yucca schidigera as the surfactant. Food Res Int. 2010;43:1710–1718.
- Vieira MGA, da Silva MA, Dos Santos LO, et al. Natural-based plasticizers and biopolymer films: A review. Eur Polym J. 2011;47:254–263.
- De Andrade CS, Nascimento VM, Cortez-Vega WR, et al. Exploiting cheese whey as co-substrate for polyhydroxyalkanoates synthesis from Burkholderia sacchari and as raw material for the development of biofilms. Waste Biom Valoriz. 2019;10:1609–1616.
- Cortez-Vega WR, Bagatini DC, Souza JTA, et al. Nanocomposite biofilms obtained from Whitemouth croaker (Micropogonias furnieri) protein isolate and Monmorilonite: evaluation of the physical, mechanical and barrier properties. Braz J Food Technol. 2013;16:90–98.
- Chevalier RC, Pizato S, De Lara JAF, et al. Obtaining protein isolate of tilapia (Oreochromis niloticus) and its application as coating in fresh‐cut melons. J Food Safety. 2018;38:e12496–2323.
- Mali S, Sakanaka LS, Yamashita F, et al. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr Polym. 2005;60:283–289.
- Gontard N, Duchez C, Cuq J, et al. Edible composite films of wheat gluten and lipids: water vapor permeability and other physical properties. Int J Food Sci Technol. 1994;29:39–50.
- ASTM. American Society for Testing and Materials. Standard methods of water vapor transmission of materials. In: ASTM annual book of ASTM standards. Método: E00996-00; 2000a. pp. 907–914.
- Pimentel T, Pizzuti L, Fakhouri FM, et al. Development of multilayer films obtained from epoxidized methyl esters, polyhydroxyalkanoates and their combinations. J Polym Environ. 2018;26:1661–1672.
- Gennadios A, Mchugh TH, Weller CL, et al. Edible coatings and films based on proteins. In: Krochta JM, Baldwin EA, Nisperos-Carriedo M, editors. Edible coatings and films to improve food quality. Lancaster USA: Technomic Publishing Co, Inc; 1994. p. 9.78–1–42003–198.
- McHugh TH, Krochta JM. Sorbitol- vs glycerol-plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J Agric Food Chem. 1994;42:841–845.
- ASTM. American Society for Testing and Materials. Standard test methods for tensile properties on thin plastic sheeting. In: ASTM annual book of ASTM standarts. Método: D00882-00; 2000b. pp.160–168.
- Tanada-Palmu PS, Hélen H, Hyvönen L. Preparation, properties and applications of wheat gluten edible films. J Agric Food Sci. 2000;9:23–35.
- Kunte LA, Gennadios A, Cuppett SL, et al. Cast films from soy protein isolates and fractions. Cereal Chem. 1997;74:115–118.
- Hunterlab. The color management company. version 3.2. Universal software, Reston, 1997.
- Sobral PJA. Thickness effects of myofibrillar protein based edible films on their functional properties. Pesq Agropec Bras. 2000;35:1251–1259.
- Suppakul P, Chalernsook B, Ratisuthawat B, et al. Empirical modeling of moisture sorption characteristics and mechanical and barrier properties of cassava flour film and their relation to plasticizing - antiplasticizing effects. LWT - Food Sci Technol. 2013;50:290–297.
- Melo PTS, Aouada FA, Moura MR. Production of nanocomposite films of pectin based on cocoa puree with potential use as packaging for food. Quim Nova. 2017;40:247–251.
- Soares IFO, Fakhouri FM, Giraldi ALFM, et al. Síntese e caracterização de biofilme de amido plastificado com glicerol ou triacetina. Foco: Cad Est Pesq. 2014;7:79–98.
- Mali S, Grossmann MVE, Yamashita F. Starch films: production, properties and potential of utilization. Semina: Ciên Agrár. 2010;31:137–156.
- Kim SRB, Choi YG, Kim JY, et al. Improvement of water solubility and humidity stability of tapioca starch film by incorporating various gums. LWT - Food Sci Technol. 2015;64:475–482.
- Matta Junior MD, Sarmento SBS, CIGL S, et al. Barrier properties of films of pea starch associated with xanthan gum and glycerol. Polímeros. 2011;21:67–72.
- Faria MG, Fakhouri FM, Carvalho CWP, et al. Physicochemical characterization of edible starch films with barbados cherry (Malphigia emarginata D.C.). Quím Nova. 2012;35:546–552.
- Fakhouri FM, Martelli SM, Caon T, et al. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharv Biol Technol. 2015;109:57–64.
- ASTM- American Society for Testing and Materials. Standard Test Methods for Water Vapor Transmission of Materials: E 96-95. 1995. p. 10.
- Fakhouri FM, Fontes LCB, Innocentini-Mei LH, et al. Effect of fatty acid addition on the properties of biopolymer films based on lipophilic maize starch and gelatin. Starke. 2009;61:528–536.
- Jiang S, Liu C, Wang X, et al. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT - Food Sci Technol. 2016;69:251–257.
- Insaward A, Duangmal K, Mahawanich T. Mechanical, optical and barrier properties of soy protein film as affected by phenolic acid addition. J Agric Food Chem. 2015;63:9421–9426.
- Chen H. Functional properties and applications of edible films made of milk proteins. J Dairy Sci. 1995;78:2563–2583.
- Kamper SL, Fennema O. Water vapor permeability of an edible, fatty acid bilayer film. J Food Sci. 1984;49:1482–1485.
- Wang Y, Zhang R, Qin W, et al. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mat Design. 2020;185;108277. DOI:https://doi.org/10.1016/s0300-9629(76)80010-2
- Fakhouri FM, Martelli SM, Bertan LC, et al. Edible films made from blends of manioc starch and gelatin - Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT - Food Sci Technol. 2012;49:149–154.

