ABSTRACT
Endophytes, as microorganisms widely present in plants, have an important role in plant growth and development. Abiotic stresses are very essential influence on plant growth and development. Endophytes in host plants are diverse, however, beneficial endophytes are used to make plants resistant to abiotic stresses. This review focuses on studying the regulatory roles of different endophytes under abiotic stresses, and explained the special pathway and related mechanism of endophytes under heavy metal stress, such as cadmium, manganese and zinc stress. How do the dominant endophytes respond to salt and heat stress and affect plant physiological characteristics? In addition, we also summarized the potential and application of endophytes in reducing the toxicity of plant pathogens, promoting crop growth, biomedicine and ecological restoration, and other aspects, to provide reference for further in-depth research on the mechanism of action of plant endophytes under abiotic stresses and effective utilization of endophytes.
Introduction
Plant endophytes are microorganisms that reside in plant organs but not harm the hosts of colonized plants, and also occur in asymptomatic diseases caused by fungal and bacterial infections of host plants [Citation1]. The concept of plant endophytes was first introduced by Bary in 1886 as microorganisms that colonize plant tissues and establish a harmonious relationship with the host plant. According to the classification of their role on the host plant, plant endophytes include symbiotic microorganisms that have evolved with a mutually beneficial, and neutral association with the host, and even pathogenic microorganisms that have been latent in the host plant for some time [Citation2]. In general, endophytes affect their hosts by colonizing plants, and this colonization usually occurs in two ways: vertically on the plant or horizontally from the soil into the host. While most endophytes enter the host from the plant roots, they may subsequently appear anywhere in the plant by infecting adjacent tissues, including stems, leaves, flowers, seeds, etc [Citation3]. In addition, most endophytes have a biphasic life cycle, which refers to alternating life activities between the plant and soil environments [Citation4]. The metabolic activity and functional diversity of plant endophytes enable them to exert additional beneficial effects on plant growth while establishing symbiotic relationships that contribute to nitrogen fixation and phosphorus solubilization. In additional, endophytes stimulate the production of essential plant hormones, including indoleacetic acid (IAA), cytokinin, and gibberellic acids (GA), and are beneficial in promoting plant growth [Citation5].
Changes in external extreme environments can alter the normal growth and metabolism of plants, and endophytes play an important role in regulating plant responses to stress and promoting plant growth. The presence of endophytes not only affects the host plant, but may also affect the stability of its surrounding ecosystem [Citation6]. Therefore, it is necessary to explore the effects of endophytes on plants under different stress conditions [Citation7]. It is known that abiotic stress mainly comes from the influence of environmental factors that are harmful to plant growth and development, such as salt stress, heavy metal contamination, and high-temperature stress [Citation8]. The effects of heavy metal and salt stress can persist for a long time in the soil, disrupting plant physiological processes, including nutrient uptake and photosynthesis, harming plant growth. Abiotic stress can also cause irreversible effects such as leaf wilting, plant dwarfing, and slow development, which ultimately harms human and animal health through the food chain [Citation9]. In order to alleviate abiotic stress, plants have their own physiological regulatory mechanisms to resist stress. For example, plants resist salt and heat stress by secreting the l-aminocyclopropane-l-carboxylic acid (ACC), maintaining Na+/K+ balance, and enhancing antioxidant defenses [Citation10]. The studies reported that the use of native dark septate endophytic (DSE) endophytic fungi enhanced the establishment of mine plants while reducing the toxic effects of mine waste on the ecosystem [Citation11]. Further, the seed endophytic fungus (FZT214) significantly improved the growth of host plants at flowering and fruiting stages under cadmium (Cd) stress [Citation12]. The endophytic fungus Exopiala sp. LHL08 isolated from cucumber roots appeared to confer tolerance to salinity and drought stress in rice [Citation13]. Additionally, an endophytic fungus, Curvularia protuberate, conferred thermotolerance to tomato and panicum after colonization [Citation14]. The role of endophytes under abiotic stresses has been studied previously, but the factors of abiotic stresses in nature are constantly changing, the endophyte species in plants, their adaptation and response to host plants and the environment stress, as well as the interrelationships among the endophytes, host plants, and abiotic stress are constantly being updated. Therefore, this review focuses on studying the regulatory roles of different endophytes under different abiotic stresses, especially the special regulatory pathways and mechanisms of endophytes in plants under heavy metals cadmium, manganese stresses. Moreover, the regulatory roles of dominant endophytes in plants under salt stress and heat stress are elucidated, and the potential prospects of plant endophytes are summarised in order to provide references for practical applications in various field.
The diversity of endophytes in host plant
Endophytes are potential sources of microbial biochemical molecules in plants that can enter accessible plant tissues, with most endophytes entering and establishing themselves through the root zone of the plant, and some entering the interior of the plant through leaf stomata, others enter the plant vascular tissues from injured tissues and form biofilms that adhere to different parts of the plant such as xylem and phloem [Citation15]. Entry into plant tissues by these means is defined as horizontal transmission. The process of the survival and establishment of some endophytes within the host is dynamic. For example, yeasts in citrus plants can migrate from roots to leaves through the vascular system of plant [Citation16]. In addition, the endophytes enter the host tissue, which may not immediately affect the host, but when the environment changes to the benefit of the organism or when the state of the host changes, the endophytes evolve in concert with the phytoplasma and thus form a more harmonious symbiotic relationship [Citation17]. However, Most endophytes can reproduce sexually by producing ascospores, and endophytes colonize mature seeds in such a way that they can spread vertically to the next generation of plant tissues. An example is E. festucae, which is capable of effective vertical dispersal through the seeds of host plants [Citation18].
There are a large number and variety of endophytes in any part of the plant [Citation19]. At present, the colony morphology of endophytes obtained from pure culture has been determined by an optical microscope and digital camera, and the parameters such as colony color, texture, conidia size, and mycelium length were obtained [Citation17]. The diversity of endophytes is measured by the diversity of endophytic species, the diversity of distribution sites, the diversity of endophytic host species, and some other factors. It has also been reported that the season and cultivar were the main factors influencing the abundance and diversity of endophytes in different olive tree species, while the diversity of endophytes in the same olive tree differed somewhat within different regions [Citation20]. The Ensifer spp. endophyte in soybean was dominant in alkaline soils, and Bradyrhizobium spp. Endophyte of those was dominant in acidic soils [Citation21]. Harrison et al.. (2020) analyzed more than 600 studies on the diversity of endophytes in different plant tissue types [Citation19], and found that the most abundant tissues in woody plants were stems, while roots were the most abundant tissues in graminaceous plants. Additionally, pollution and urbanization also had an impact on the diversity of endophytes. The diversity of endophytic actinomycetes was also quite different in arid, aquatic and saline environments [Citation22]. Further, there were differences in the diversity of endophytic bacteria in different growth stages of plants, for example, the variety of endophytic bacteria isolated during the dormant stage of Lycium ruthenicum was greater than that of the flowering stage [Citation23].
According to the classification scheme of the organisms, endophytes mainly include fungi, bacteria, and actinobacteria living in plants [Citation24]. Previous study have shown that fungal endophytes have been isolated from virtually all plant types [Citation25]. In addition, endophytes discovered in darnel ryegrass were the earliest known record of the appearance of endophytic fungi [Citation26]. Hyde and Soytong (2008) divided the endophytes fungi into clavicipitaceous and nonclavicipitaceous types [Citation27], Epichloë and Neotyphodium are predominantly isolated from clavicipitaceous fungi, and the latter were mostly isolated from Ascomycota, Basidiomycota, and Mucormycota [Citation28]. Studies have shown that nonclavicipitaceous fungi were subdivided into three categories, the fungi that colonize the aerial part and the inter-rhizosphere, the fungi that were distributed in the tropics and occurred in the plant leaves, and the DSE that only colonize the host root septa containing melanin [Citation29]. However, Mundt (1976) recorded the first isolation of endophytic bacteria from the ovules and seeds of 27 plants [Citation30]. Endophytic bacteria were divided into obligate endophytes that are strictly dependent on the host plants and facultative endophytic bacteria that grow alternately between the host and soil [Citation31]. Liu et al..(2017) reported that endophytes were mainly divided into Bacteroidetes, Firmicutes, and Proteobacteria [Citation32]. As a kind of endophytes, Endophytic actinomycetes exist widely in various tissues and organs of plants. Lechevalier (1978) reported the actinomyces genus found in the grass blade of the liden river in New Jersey [Citation33], which may be the earliest recorded isolated endophytic actinomyces. Studies have shown that endophytic actinobacteria are abundant in roots, occur moderately in the stems, and have been found in the least numbers in the leaves [Citation34]. Thus, endophytic actinomycetes are widely distributed in the soil; then it is easier to contact the roots of plants. Endophytic actinomycetes are mainly Streptomyces and non-Streptomyces, and non-Streptomyces is classified as a rare group because it is uncommon [Citation35]. More endophytes have been isolated from different plants as shown in .
Table 1. Host plants and their associated endophytes.
Regulation effects of endophytes on host plants
Endophytes are constantly exposed to biological and abiotic stresses in the natural environment, which have both positive and negative regulation effects on host plants. When plants are inoculated with beneficial endophytes can promote the growth of leaves and roots of host plants, and the interaction between endophytes and host plants is not static during the process of co-evolution. For example, the inactivation of noxA, a single gene encoding NADPH oxidase, in the symbiotic endophyte fungi Epichloë festucae was sufficient for a shift from mutualistic to parasitic interaction in the ryegrass Lolium perenne [Citation46].
The endophytes directly generate plant hormones, iron carriers, etc., which have growth-promoting effects on plants; indirectly generate hydrolases, alkaloids, organic acid antibiotics, and secondary metabolites to induce the expression of stress-resistance genes of the plants and compete with pathogenic bacteria for a nutrient niche to achieve the growth-promoting purpose [Citation47]. The endophytic fungus Epichloë enhanced the resistance of Buddleja officinalis to pathogens by affecting the synthesis of salicylic acid (SA) and activating the SA signaling pathway [Citation48]. The salt-tolerant bacillus isolated from Lily promoted the growth of plant by producing indoleacetic acid and ACC deaminase with strong phosphate-solubilizing activity [Citation49]. Moreover, Pseudomonas aeruginosa can induce disease resistance in peas and inhibit the root rot pathogen Fusarium oxysporum by increasing the content of ascorbate oxidase (AO), catalase (CAT), polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL) and peroxide (PO) in pea. Further, Pseudomonas prevents herbicide accumulation in plants by degrading the organochlorine herbicide, 2,4-dichlorophenoxyacetic acid (2,4-d) in peas [Citation50].
The symbiotic relationship between the endophyte and the host may also inhibit the growth of the host. A previous study demonstrated that endophytic fungi associated with roots, vascular tissue, and foliage may interfere with water uptake and transport, increase rates of foliar transpiration, and induce xylem embolism and tissue death [Citation51]. Colletotrichum musae and Fusarium moniliforme reduced the chlorophyll content in the corn body by damaging the electron transfer in the thylakoid membrane in the banana cells and inhibited plant growth [Citation52]. The research showed that endophytes may cause damage to the epidermis or interfere with stomatal closure when they penetrate the host epidermis [Citation53]. Endophytic fungi are pathogenic to their hosts by altering cell structure. For example, inoculation of three endophytic fungi, F. verticillioides, F. sacchari, and P. citrinum, isolated from healthy sweet corn tissues into maize reduced the diameter of the posterior xylem and bast ducts of maize plants. It also caused growth retardation, leaf necrosis, shriveling, and other disease symptoms, and increased susceptibility to infection with vascular pathogens [Citation54]. Fusarium fujikuroi isolated from Lilium lancifolium invaded plant bulb wounds causing stem wilt and host death [Citation55]. In addition, endophytes that act as growth promoters for one plant may indirectly inhibit the growth of neighboring or other plants when colonizing.
Response of plant endophytes to abiotic stress
The symbiotic relationship between endophytes and plants affects the physiological state of plants through a series of signalling and metabolic regulation [Citation56]. However, how this symbiotic relationship functions in the face of abiotic stresses from the external environment has become a research area of great interest. Endophytes may affect plants and their responses to abiotic stresses such as heavy metals, drought, salinity, or high temperature, even generate multiple responses through interactions with these stresses. For example, some of the ways in which endophytes respond to stress are also shown in .
Figure 1. The mechanisms by which endophytes mediate increased plant resistance under heavy metal stress, salt stress, and high temperature stress. For example, under high temperature stress, endophytes enhance the expression of heat shock protein 90, activate protein kinases YDA, MKK, MPK, and affect the phosphorylation of SPCH to regulate stomatal size [Citation57]. Under heavy metal stress, the endophytes promoted the secretion of organic small molecules and extracellular polymers from the root system to bind heavy metals and limit the influx of heavy metals into the cells [Citation58]. Under salt stress, endophytes promote the secretion of ABA, which binds to the PYL/PP108 receptor and to the protein phosphatase PP2C to form the PYL-ABA-PP2C complex, ultimately reducing ROS [Citation59].
![Figure 1. The mechanisms by which endophytes mediate increased plant resistance under heavy metal stress, salt stress, and high temperature stress. For example, under high temperature stress, endophytes enhance the expression of heat shock protein 90, activate protein kinases YDA, MKK, MPK, and affect the phosphorylation of SPCH to regulate stomatal size [Citation57]. Under heavy metal stress, the endophytes promoted the secretion of organic small molecules and extracellular polymers from the root system to bind heavy metals and limit the influx of heavy metals into the cells [Citation58]. Under salt stress, endophytes promote the secretion of ABA, which binds to the PYL/PP108 receptor and to the protein phosphatase PP2C to form the PYL-ABA-PP2C complex, ultimately reducing ROS [Citation59].](/cms/asset/d864426d-90e7-44db-87d6-78bc2206f6a2/tcsb_a_2323123_f0001_oc.jpg)
Heavy metal stress
Heavy metals stress is one of the challenges currently faced by abiotic stress. As heavy metals can combine with organic or inorganic ions in the soil to form more stable complexes, their accumulation and toxicity can not only reduce the number of microorganisms in the soil but also jeopardize the normal growth of plants [Citation60]. Plants are subjected to common heavy metal stresses include cadmium, chromium, copper, lead, and mercury.
Cd is one of the most toxic heavy metals, which can have a half-life in the plant of up to 25–30 years [Citation61]. According to a survey, Cd pollution in cultivated land is as high as 7%, and even most of it is of severe pollution level [Citation62]. Given that Cd pollution exceeds the standard, it is not uncommon to cause damage to the growth of cultivated crops. The toxicity of Cd hinders the absorption and transportation of essential ions by plants, leading to nutrient deficiency. Cd2+ not only damaged chloroplast structure, but also possible to replace Ca2+ in the photosystem II (PSII) reaction center of plants, thereby inhibiting the photoresponse activity of PSII and reducing the photosynthetic capacity of plants, resulting in reduced biomass [Citation63]. Some endophytes promoted growth of plants in soils where heavy metals are accumulated because it was resistant to higher concentrations of heavy metals. For example, Alyssum montanum isolated in a waste mine had a high tolerance to Cd at 1300 μM [Citation64]. The accumulation of Cd by endophytic fungus CBRF59 isolated from rape grown in soil polluted by heavy metals was 65 mg g−1 higher than that of other Mucor genera [Citation65]. Endophytes isolated from plant in soils with heavy metal accumulation are more resistant to metals than those isolated from normal environments. For example, DSE isolated from Alnus nepalensis in metal tailings was more resistant to metal Cd than endophytes found in common wastelands [Citation66]. The endophytic fungi isolated from Solanum nigrum, which had been subjected to cadmium stress, showed higher resistance and accumulation ability compared to those not contaminated with cadmium [Citation67]. It may be that endophytes are able to adapt to the toxicity caused by heavy metal stress due to their long-term exposure to heavy metals polluted environments.
Plants surviving in heavy metal-rich Cd environments have developed multiple mechanisms to alleviate Cd stress and improve tolerance to Cd to enable them to survive in unfavorable environments. There are two main strategies: extracellular limitation of Cd uptake by plants and intracellular accumulation of Cd to optimize its distribution [Citation68]. The endophytes exert their influence on these two strategies and thus on Cd tolerance, as shown in . The cell wall is the first effective barrier against the entry of excess harmful metal ions into the cell, and the metal binding sites located in the cell wall are able to bind metal ions, preventing them from accumulating in heavy metal-sensitive sites within the cell and interfering with the normal functional activity of the cell [Citation70]. Polysaccharide components in the cell wall are the binding sites for heavy metal binding and adsorption. For example, the endophytic fungus Exophiala pisciphila increased the retention capacity of the cell wall for Cd by increasing the total sugar content of pectin (PE), hemicellulose (HE) in the cell wall of Zea mays L. under Cd stress [Citation71]. Arbuscular mycorrhizal fungi (AMF) increased the cell wall fixation of Cd by increasing the content of pectin, hemicellulose 1 (HC1), and lignin in the cell wall of rice roots and reduced the transport of Cd from the roots to the above the ground parts [Citation72]. It was also reported that endophytes could secrete amino acids, organic acids, and phenolic compounds [Citation38]. In order to prevent excessive cadmium ions from entering cells and impairing normal cellular functions, endophytes can promote or directly secrete chelates to bind to metal ions, and the formation of this complex can effectively limit the toxicity of cadmium ions [Citation73]. Glutathione (γ-glu-cys-gly), a tripeptide nonprotein thiol widely present in all cellular compartments, is considered a key compound for the mitigation of Cd stress in endophytes, exerting its role as a ligand peptide and an antioxidant. Glutathione is converted to phytochelatins (PCs) in reactions catalyzed by the enzyme phytochelatin synthase, and Cd entering plant roots is neutralized by chelation with PCs, forming PCs-Cd complexes that are transferred and stored in vesicles [Citation74]. This transfer from Cd-sensitive cell compartments to vesicles results in an optimal distribution of Cd between plant organs and cell compartments for Cd stress mitigation.
Figure 2. Pathways to improve plant tolerance to Cd through the endophytic fungi, arbuscular mycorrhizal fungi (AMF). Plant extracellular Cd-restriction -and intracellular Cd-accumulation to optimize their distribution. Extracellularly, AMF anchors Cd to the plant root and reduces the transfer of Cd to the shoot by increasing the polysaccharide content in the cell wall and thus improving its ability to bind Cd. Inside the cell, AMF enhances the synthesis of plant chelatins (PCs) by glutathione (GSH), allowing more Cd to form PCs-Cd complexes with PCs and be stored in organelles such as vacuoles. The top left illustrates plants that were not inoculated with endophytes, yet these endophytes exhibited a notable enhancement of plant growth in Cd compared to their inoculated counterparts. These endophytes are composed of endophytes (pink), endophytic bacteria (blue), and endophytic actinomycetes (yellow). Adapted from [Citation69].
![Figure 2. Pathways to improve plant tolerance to Cd through the endophytic fungi, arbuscular mycorrhizal fungi (AMF). Plant extracellular Cd-restriction -and intracellular Cd-accumulation to optimize their distribution. Extracellularly, AMF anchors Cd to the plant root and reduces the transfer of Cd to the shoot by increasing the polysaccharide content in the cell wall and thus improving its ability to bind Cd. Inside the cell, AMF enhances the synthesis of plant chelatins (PCs) by glutathione (GSH), allowing more Cd to form PCs-Cd complexes with PCs and be stored in organelles such as vacuoles. The top left illustrates plants that were not inoculated with endophytes, yet these endophytes exhibited a notable enhancement of plant growth in Cd compared to their inoculated counterparts. These endophytes are composed of endophytes (pink), endophytic bacteria (blue), and endophytic actinomycetes (yellow). Adapted from [Citation69].](/cms/asset/1e46c47c-ccdc-4d77-85e3-c17c9c7f65d1/tcsb_a_2323123_f0002_oc.jpg)
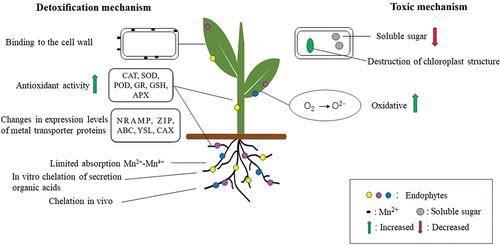
In addition to cadmium, mercury, and arsenic (As), which are common soil heavy metal contaminants, the heavy metal manganese (Mn) also deserves more attention. In agricultural production, long-term improper fertilization and natural soil acidification can convert previously the manganese into free manganese and increase its activity, which may greatly increase the chances for plants to uptake manganese [Citation75]. Mn is also one of the essential elements for plant growth and development, and plays an important physiological role in composing the structure of chloroplasts, acting as a catalyst for water photolysis and regulating redox potentials [Citation76]. However, when manganese level in the soil is too high, the plant in which it is located causes manganese toxicity, induced oxidative stress by elevating the levels of reactive oxygen species, which can severely affect the photosynthetic system by destroying the structure of the cysts and the photosynthetic electron transport chain, reducing photosynthetic efficiency, and ultimately leading to wilting and necrosis of the leaves [Citation77]. The growth and development of the plant’s primary and lateral roots are also strongly inhibited by manganese toxicity, with browning and breakage occurring as a result of prolonged exposure of roots to soils with high manganese concentrations [Citation78]. Actually, plants are not passive in the face of high manganese stress, because endophytes can help plants to resist manganese stress. For example, the endophytic Bacillus sp. AP10 significantly increased the expression of genes regulating the phenylpropane pathway in Arabidopsis thaliana, which may promote the scavenging of reactive oxygen species (ROS) and the accumulation of antioxidant flavonoids, improve Mn tolerance and possibly reduce Mn toxicity by enhancing abscisic acid (ABA) responsive gene expression and ABA biosynthesis [Citation79]. The Bacillus cereus strain WSE01 isolated from the stem of Myriophyllum verticillatum was able to produce indole-3-acetic acid (IAA) and iron carriers. Moreover, under 400 mg/L Mn stress, the Mn content of stems increased by 36.4% and that of leaves by 54.7% in the inoculated group. This study demonstrated that strain WSE01 has the potential to be used as a biocontrol agent against Mn pollution [Citation80]. The endophytic bacterium Bacillus megaterium 1Y31 significantly increased plant Mn resistance by producing IAA, iron carrier, and ACC deaminase, increasing the expression of photosynthesis- and energy production-related proteins, and decreasing the expression of proteins responsive to stress-related proteins, Furthermore, there was also an interesting finding that strain 1Y31 regulated the relative proportions of IAA and iron-carrier-producing endophytic bacteria in the root and leaves, conferring stronger Mn resistance to the plant [Citation81]. Therefore, we speculate that specific endophytes in plants can help improve their tolerance to heavy metals. From these examples, it is found that these endophytes help plants cope with Mn stress by increasing antioxidant activity and enhancing the uptake and translocation of Mn. The toxic effects of manganese on plants and the detoxification mechanisms of endophytes in response to manganese stress are shown in .
Figure 3. Toxic effects of manganese on plants and detoxification mechanisms of endophytes in response to manganese stress. Manganese stress harms plants by reducing soluble sugars, destroying chloroplast structure, producing excessive reactive oxygen species, and inhibiting root growth. Endophytes help plants alleviate Mn stress mainly by limiting Mn attachment to cell walls, regulating antioxidant system of plants and the transport of their metal transporter proteins, restricting the uptake of Mn ions by roots, and secreting organic acids for chelating in vitro.
Apart from Cd and Mn, the heavy metal stresses faced by plants are diverse. When plants are exposed to copper stress beyond their limits, their root systems are the first to be damaged and their water absorption capacity deteriorates, which is followed by drying out of the plant, crumpling and curling of the leaves, and a severe reduction in biomass [Citation82]. Excess zinc induces changes in antioxidant enzyme activities, cellular structure destruction and imbalance of nitrogen metabolism in plants, reduces relative growth rate and net photosynthesis, leading to leaf necrosis [Citation83]. In plants, the toxic effects of Cr are also evident in its ability to cause root damage, biomass reduction, plant height reduction, photosynthetic damage, leaf chlorosis, necrosis, and ultimately plant mortality [Citation84].
Research on endophytes helping plants to mitigate heavy metal stress has also been continuously updated. The study found that basidiomycete Sporobolomyces ruberrimus, after colonizing Arabidopsis thaliana, is able to convert iron into amorphous (oxy)hydroxides and phosphates and immobilize them as precipitates, thus limiting iron stress [Citation85]. Cladosporium spp. isolated from Polygonatum kingianum has a strong tolerance to As (V) or Cd (II) stress through increased antioxidant enzyme activity and antioxidant substance content, as well as immobilisation of metal ions in the mycelium [Citation86]. Synergistic interaction of the endophytic bacterium Rhizophagus irregularis with exogenous abscisic acid promotes the growth of Robinia pseudoacacia and enhances its resistance to Zn stress by altering the distribution of Zn and endogenous abscisic acid [Citation87]. Sphingomonas paucimobilis ZJSH1 is an endophytic bacterium isolated from the roots of Dendrobium officinale, identifying a variety of genes encoding active substances, such as hormones (IAA, SA, ABA, and Zeaxanthin), the phosphate cycle, antioxidant enzymes, and polysaccharides, which conferred to the strain the potential to withstand salt, drought, and cadmium stresses [Citation88]. Recent studies have found that AMF is able to alleviate thallium (TI) stress in soybeans. AMF increases the activity of enzymes involved in proline biosynthesis in soybeans, such as P5CS (pyrroline-5-carboxylate synthesis), P5CR (pyrroline-5-carboxylate reductase) and OAT (ornithine aminotransferase), and the synergistic effect of AMF with TI also promotes growth [Citation89]. Based on the ability of AMF to alleviate TI stress, the search for plant endophytes with the ability to accumulate TI is relevant for environmental remediation. In addition, the presence of one heavy metal stress is usually accompanied by the presence of other heavy metals. Therefore, there is currently a lack of comprehensive utilization research on endophytes in solving complex problem of various heavy metal pollution. Meanwhile, it is important to select appropriate endophytes to participate in the prevention and control of heavy metal stress, in order to avoid exacerbating the degree of stress caused by endophytes.
Salinity stress
Salinization of the soil is tremendously harmful to plant growth, specifically in terms of plant death and reduction of gross product. Plant roots are the first and most sensitive organs exposed to the saline environment. Under salinity stress, the osmotic pressure of root cells decreases, cell membranes undergo peroxidation, and the ability to absorb mineral elements and water decreases, that ultimately inhibit plant growth and development. Salinization also affects the dynamic balance of ions in plants [Citation56]. Excess Na+ in soils causes cell membrane K+ and Ca2+ efflux, and this reduced K+ and Ca2+ uptake leads to inhibition of normal cellular functions, instability of cell membranes, and inhibition of enzymatic activities [Citation90]. On the other hand, the most harmful effect of salinization on plants is the impact on photosynthesis. Salinization can increase the rate of chlorophyll degradation by chlorophyllase, and weaken photosynthesis, thus overall affecting plant growth. Furthermore, there is an elevated generation of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide radicals (O2-), singlet oxygen (O2), and hydroxyl ions (OH−), in chloroplasts and mitochondria due to salinization, which significantly exceed normal levels. These ROS with strong oxidative capacity can lead to plant tissue damage, DNA mutations, cell membrane rupture, as well as degradation of lipids, proteins, and photosynthetic pigments [Citation91].
Many studies have shown that endophytes can mitigate the damage caused by salinity stress by participating in osmoregulation, photosynthesis, improving plant nutrient uptake, and enhancing the antioxidant system [Citation92]. For example, B. circulans PK3–15 and PK3–109 could significantly increase plant fresh weight by 50% under salt stress [Citation93]. Further, Trichoderma longibrachiatum HL167 increased the K+/Na+ ratio of cowpea, thereby reducing the ionic toxicity of saline stress on cowpea [Citation94]. The endophytic bacterium Epichloë significantly enhanced the resistance of tall fescue to saline stress by increasing K+ accumulation while decreasing Na+ concentration [Citation95]. Another study found that Enterobacter sp. FN0603 significantly increased the salinity stress tolerance index by increasing inter-root soil nutrient content and enzyme activity and enhancing some important antioxidant enzyme activities [Citation96]. The endophytes of the genera Bacillus, Salmonella, Kushneria, and Micrococcus can improve plant tolerance to salt stress by inducing changes in plant antioxidant enzyme activities, such as ascorbate peroxidase (APX), CAT, and superoxide dismutase [Citation97]. Similarly, endophytes can also alleviate salt stress by regulating the expression of related genes in host plants, as shown in .
Table 2. Effect of plant endophytes on salinity stress.
High-temperature stress
The global temperature increase has become one of the factors that have a significant impact on crop growth. The research indicated that temperatures worldwide may rise by a mean range of 2–5°C by the end of this century [Citation150]. When the ambient temperature is 10–15°C higher than the optimal growth temperature range for plants, this temperature constitutes heat stress [Citation105]. Heat stress can directly affect the fluidity of plant cell membranes, leading to protein denaturation in plants and indirectly affecting the uptake of various nutrients by plants. The increase in the duration of heat stress may cause irreversible changes in plant cells, causing plants to exhibit various signs of heat stress, such as wilting, leaf damage, fruit drop, and embolism [Citation106].
Endophytes can promote the growth of host plants under high-temperature stress conditions by increasing fresh weight, dry weight, chlorophyll content, and increasing root length, etc [Citation107]. The specific effect of endophytes on host plant against heat stress are shown in . A previous research showed the crude extract of the endophytic fungus Paecilomyces variotii on tomato plants subjected to long-term heat stress and significantly promoted the growth of plant by up-regulating the expression of antioxidant genes Fe-SOD, DAHR, and CAT1 [Citation114]. The endophytic Rhododendron simsii roots could improve the oxidative stress response of the plant by increasing the intracellular glutathione level, resulting in increased plant biomass [Citation115]. The endophyte Aspergillus japonicus EuR-26 improved plant biomass and other growth characteristics under heat stress at 40°C by regulating abscisic acid (ABA), catalase, and ascorbate oxidase activities in soybean and sunflower [Citation116]. In another study, inoculation of B. cereus SA1 on soybean plants under heat stress conditions showed an increase in chlorophyll a, chlorophyll b, carotenoids, and proteins in the plants, which significantly improved plant damage caused by high-temperature stress [Citation117].
Table 3. Role of endophytes in alleviating heat stress (↑: increase traits, ↓: decrease traits).
Other abiotic stresses
Although animals in nature can escape some threats through movement, most plants have to endure a variety of abiotic stresses such as nutrient stress, air pollution, and cold stress [Citation8]. When endophytes colonize plants, they can alleviate the effects of nutrient deficiency on plant growth and development [Citation118]. Endophytes can help produce plant hormones to stimulate the growth of plant cells in order to induce the release of nutrients by endophytes [Citation5]. For example, the macronutrient absorption of soybeans was improved after inoculation with the endophytic bacteria Galactomyces geotrichum, which secretes plant hormones [Citation77]. Cold stress is an abiotic stress that affects the normal growth and development of plants. Endophytes can reduce the damage of low temperatures to plants by regulating the levels of plant hormones, such as balancing the imbalance of ABA and gibberellin (GA) and sugar metabolism. The roles of some endophytes in host plants under other abiotic stresses are shown in .
Table 4. Role of endophytes in host plants under other abiotic stresses (↑: increase traits, ↓: decrease traits).
Potential applications
Research on plant endophytes focused on the development of valuable bioactive molecules produced by endophytes and exploring the possibility of endophytes being used as biocontrol [Citation127]. Endophytes can be used in agriculture, medicine, and many other industries. Due to their close interaction with the host plant, they share distinct characteristics with other microorganisms residing on the plant surface or in the interroot zone, which can be easily identified and exploited [Citation128].
Resistance to plant pathogens
Crop diseases caused by plant pathogens are one of the main causes of agricultural production loss, which in turn endanger global food security. The utilization of chemical pesticides is a perilous threat to both human health and the environment [Citation129], therefore, plant pathogens can be combated by biopesticides derived from plant endophytes or their biologically active natural products, which are classified as alkaloids, terpenoids, polyketides and other compounds based on their chemical structures [Citation130]. It was reported 132 antifungal metabolites isolated from plant endophytic fungal bodies with significant antifungal properties over the past 20 years [Citation131]. For instance, the species Burkholderia vietnamiensis isolated from wild Populus (poplar) showed strong in vitro resistance to highly virulent pathogens such as the oomycete, Rhizoctonia solani and Fusarium culmorum. Eleven isolates from Burkholderia vietnamiensis were found to be inhibitory to at least two plant pathogens [Citation132]. The endophytic fungus Xylaria sp. was isolated from guarana plant leaves, which produced cytochalasin-D that was fungicidal against the plant pathogen Colletotrichum gloeosporioides that causes anthracnose [Citation133]. Further, silver nanoparticles (AgNPs) synthesized by the endophytic fungus Cochliobolus lunatus could act as insecticides and effectively kill Anopheles stephensi (the malarial vector) [Citation134]. The endophytic bacterium B. phytofirmans PsJN has a direct antimicrobial effect, i.e. it forms biofilms on the surface of grapevine leaves that limit the growth of pathogens, in addition to stimulating the defense mechanism of grapevines [Citation135]. Moreover, the antifungal metabolites produced by the root endophyte Phialocephala sphareoides of Norway spruce act as root protectors to prevent root rot [Citation136]. More other effects of endophytes on plant pathogens are shown in .
Table 5. The effects of endophytes on the resistance to the pathogens.
Promoting growth of crops
Increasing evidence show that plant endophytes can promote crop growth. For example, Bacillus firmus J22N and Bacillus sp. REN51N produced IAA and ACC deaminases to promote peanut growth [Citation145]. The endophytic yeasts Aureobasidium pullulans, Candida zeylanoides, Hanseniaspora uvarum promoted the growth of fruits such as apples and pears by producing large amounts of IAA [Citation146]. Further, non-rhizobial endophytes (NREs) increased germination and chlorophyll levels in lentils by colonizing lentil roots and also caused root hair elongation, promoted lentil growth, and improved soil health [Citation147]. The endophytic bacterium Kosakonia radicincitans colonized in maize and increased the accumulation of mannitol, alginose, and galactose, increasing the yield by 50% [Citation148]. In another study, colonization of Chinese cabbage (Brassica campestris subsp. chinensis) by the endophytic fungus Piriformospora indica increased the fresh weight of cabbage seedlings by approximately twofold, promoted shoot and root development, and also stimulated root hair development [Citation149].
Environmental protection
Phytoremediation is considered to be the most effective and environmentally friendly way to restore the original soil conditions when contaminated by various environmental pollutants [Citation150]. Endophytic bacteria-assisted phytoremediation mitigates the toxicity of pollutants to plants (through natural colonization or plant genetics), enhances the removal of toxic compounds from the soil, and cleans the environment more effectively [Citation151]. It was found that endophytes degrade crude oil by secreting ligninolytic enzymes (manganese peroxidase, lignin peroxidase and laccase) and glycosyl hydrolases (xylanase and cellulase) [Citation152]. Inoculation with beneficial endophytes increased microbial community abundance and total soil nitrogen, organic carbon and pH in abandoned mine environments [Citation153]. The endophytic bacterium Alcaligenes faecalis (AF-1) isolated from maize colonised Lactuca sativa grown in Cr-contaminated soil significantly reduced aboveground Cr content while promoting the growth of Lactuca sativa, reducing Cr contamination of the soil [Citation154]. Inter-root inoculation of Phytolacca acinosa with Bacillus paramycoides increased soil cadmium uptake by 32–40% [Citation155]. Bacillus safensis isolated from Vigna radiata is able to significantly absorb nickel from electroplating industry wastewater [Citation156]. In addition, mycelium of endophytic fungi can be used as biosorbents to adsorb metal wastewater and may be a useful environmental strategy for metal cleaning industries [Citation134]. These studies have clearly shown that beneficial endophytes are often used to repair damaged environments by enhancing the antioxidant system of plants and promoting the secretion of biochemicals by plants to increase the activity and abundance of microorganisms in the environment. Therefore, we can choose various strategies to use endophytes to solve different environmental remediation problems.
Use as biomedicine
Endophytes have a high species diversity and adaptability to various environments, and the active substances isolated from them may not adversely affect human cells. This makes them one of the potential sources of new antibiotics and other drugs that have not yet been developed [Citation157]. Endophytic species of plants have antitumor, antiviral, and antibacterial effects through the direct secretion of medicinally important bioactive substances or by promoting their production by the host plant, and their metabolites [Citation158]. For example, non-targeted metabolism profiling results showed that the endophytic fungus HGUP191049 of prickly pear contains about 120 antimicrobial secondary metabolites with diverse structures and functions [Citation159]. In addition, the onychotoxin produced from Trichoderma vulgaris is an active ingredient in the treatment of leukemia [Citation160]. Interestingly, Penicillium chrysogenum MTCC5108, an endophytic fungus isolated from mangroves, is capable of producing non-ribosomal peptides and penicillin [Citation161]. According to another study, the metabolite APL-16-5 isolated from the plant endophytic fungus Aspergillus chrysogenum improved anti-influenza A virus (IAV) efficacy and specificity by modulating affinity for the E3 ligase TRIM25 and viral PA protein [Citation162]. The role of more endophytic secondary metabolites, as shown in .
Table 6. Role of secondary metabolites of endophytes.
Use as exogenous gene carriers
Plant endophytes are better exogenous gene carriers because this function enables plants to obtain beneficial traits while keeping their genes intact. Kostka et al.. (2000) found the toxin gene of B. thuringiensis was constructed in the endogenous bacterium for biological control of corn borers, which reduced the insect damage by 26%−72%, which was the earliest record of endophytic bacteria as the carrier of the insecticidal gene [Citation170]. In addition, Metarhizium robertsii, an endophytic fungus, obtained a sterol vector gene, Mr-npc2a, which is a promoter that helps drive the expression of insecticidal proteins from an insect host by horizontal gene transfer [Citation171]. Transmission of antibiotic resistance genes in plant tissues was made possible by using genetic engineering techniques to transfer the conjugative RP4 plasmid, which carries three antibiotic resistance genes, into endophytic bacteria after introduction into Escherichia coli [Citation159]. Endophytes can act as vectors for horizontal gene transfer, introducing genes coding for some metabolic pathways into host cells and thus acquiring new metabolic pathways [Citation172]. The above studies suggest that endophytes may play a role in exogenous gene carriers.
Conclusion and prospect
The effect of endophytes on host plants is directly achieved through the production of IAA, ACC, phosphates, and other probiotics, and indirectly through the induction of certain substances in plants or the expression of plant genetic genes. In addition, endophytes can produce secondary metabolites to increase crop productivity, and the growth-promoting effect may be more pronounced under abiotic stress conditions. Many studies have shown that endophytes can mitigate damage caused by abiotic stresses and increase plant tolerance to abiotic stresses by participating in osmoregulation, photosynthesis, improved plant nutrient uptake and enhanced antioxidant systems.
The positive response of endophytes to plants under abiotic stress is the main focus of this review, but we cannot ignore that endophytes may also have some negative effects on plants under abiotic stress. Complex stress factors may even exacerbate the negative effects of endophytes and worsen plant growth. In fact, the effect of endophytes on host plants under abiotic stress is limited, and this pro-biotic effect may be weakened or even counteracted when the stress level reaches a certain degree, and the interaction between endophytes and plants is in a dynamic process, which may change with the external environment or with the plants themselves. Most of the current studies on endophytes are usually conducted by isolating endophytes from host plants into culture media and studying the structure of endophytes and the active substances they produce; however, the interaction between endophytes and host plants may also affect endophytes to a certain extent. Therefore, in field trials, endophyte growth promotion may be greatly reduced. Moreover, studies on the process of endophyte colonization of plants and other aspects still need to be added and completed, so future work can be carried out in the following aspects.
To study in depth the interactions between endophytes and plants and the factors affecting these interactions, as well as what beneficial effects such interactions may bring to plants.
Identify more effective methods for isolating endophytes and replicating the results of endophyte-controlled laboratories in large-scale field trials, which are needed to ensure the activity of the endophytes and their efficacy as bioproducts.
Finding genes that respond to both endophytes and abiotic stresses, perfecting the gene network of endophytes responding to abiotic stresses, and analyzing the mechanism of endophytes responding to abiotic stresses, in order to solve the problem of growth limitation of plants under abiotic stresses at the genetic level.
In summary, endophytes have great potential to respond to abiotic stress. Utilizing endophytes to promote plant growth under abiotic stresses can improve crop plant resilience, reduce the need for chemical treatment of land, and contribute to environmental protection. Future research is expected to reveal more details about the role of endophytes in plant adaptation and survival strategies, providing a more comprehensive perspective for developing sustainable agriculture and ecosystem management.
Author contributions
Conceptualization, L.L., L.X., and J.C.; methodology, J.C., Y.Z., and L.L.; figure and table analysis, J.C., and J.W.; investigation, L.X., J.C., D.Z., and F.N.; writing – original draft preparation, J.C., L.L., and L.Q.; writing – review and editing, L.L., and D.Z., and L.X. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Oulhen N, Schulz BJ, Carrier TJ. English translation of Heinrich Anton de Bary’s 1878 speech, ‘Die Erscheinung der Symbiose’ (‘De la symbiose’). Symbiosis. 2016;69(3):131–183. doi: 10.1007/s13199-016-0409-8
- Aamir M, Rai KK, Zehra A, et al. Fungal endophytes: Classification, diversity, ecological role, and their relevance in sustainable agriculture. 2020. doi: 10.1016/B978-0-12-818734-0.00012-7
- Kandasamy GD, Kathirvel P. A review: insights into bacterial endophytic diversity and isolation with a focus on their potential applications. Microbiol Res. 2023;266:127256. doi: 10.1016/j.micres.2022.127256
- Afzal I, Shinwari ZK, Sikandar S, et al. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001
- Ali M, Ali Q, Sohail MA, et al. Diversity and taxonomic distribution of endophytic bacterial community in the rice plant and its prospective. Int J Mol Sci. 2021;22(18):10165. doi: 10.3390/ijms221810165
- Chaudhary P, Agri U, Chaudhary A, et al. Endophytes and their potential in biotic stress management and crop production. Front Microbiol. 2022;13:933017. doi: 10.3389/fmicb.2022.933017
- Collinge DB, Jensen B, Jørgensen HJ. Fungal endophytes in plants and their relationship to plant disease. Curr Opin Microbiol. 2022;69:102177. doi: 10.1016/j.mib.2022.102177
- VanWallendael A, Soltani A, Emery, NC, et al. A molecular view of plant local adaptation: incorporating stress-response networks. Annu Rev Plant Biol. 2019;70(1):559–583. doi:10.1146/annurev-arplant-050718-100114
- Gong Z, Xiong L, Shi H. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–674. doi: 10.1007/s11427-020-1683-x
- Shekhawat K, Fröhlich K, García-Ramírez GX, et al. Ethylene: a master regulator of plant-microbe interactions under abiotic stresses. Cells. 2022;12(1):31.
- Flores-Torres G, Solis-Hernández AP, Vela-Correa G, et al. Pioneer plant species and fungal root endophytes in metal-polluted tailings deposited near human populations and agricultural areas in Northern Mexico. Environ Sci Pollut Res Int. 2021;28(39):55072–55088. doi: 10.1007/s11356-021-14716-6
- Parmar S, Sharma VK, Li T, et al. Fungal seed endophyte FZT214 improves dysphania ambrosioides Cd tolerance throughout different developmental stages. Front Microbiol. 2022;12:783475. doi: 10.3389/fmicb.2021.783475
- Murphy BR, Doohan FM, Hodkinson TR. From concept to commerce: developing a successful fungal endophyte inoculant for agricultural crops. J Fungi. 2018;4(1):24. doi: 10.3390/jof4010024
- Verma A, Shameem N, Jatav HS, et al. Fungal endophytes to combat biotic and abiotic stresses for climate-smart and sustainable agriculture. Front Plant Sci. 2022;13:953836. doi: 10.3389/fpls.2022.953836
- Ling L, Tu Y, Ma W, et al. A potentially important resource: endophytic yeasts. World J Microbiol Biotechnol. 2020;36(8):110. doi: 10.1007/s11274-020-02889-0
- Goodwin PH. The endosphere microbiome of ginseng. Plants. 2022;11(3):415. doi: 10.3390/plants11030415
- Alam A, Adhikary SK, Ahmed M. Morphological characterization of Colletotrichum gloeosporioiedes identified from anthracnose of Mangifera indica L. Asian J Plant Pathol. 2017;11(3):102–117. doi: 10.3923/ajppaj.2017.102.117
- Cräutlein M, Helander M, Korpelainen H, et al. Genetic diversity of the symbiotic fungus epichloë festucae in naturally occurring host grass populations. Front Microbiol. 2021;12:756991. doi: 10.3389/fmicb.2021.756991
- Harrison JG, Griffin EA. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here? Environ Microbiol. 2020;22(6):2107–2123. doi: 10.1111/1462-2920.14968
- Materatski P, Varanda C, Carvalho T, et al. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019;123(1):66–76. doi: 10.1016/j.funbio.2018.11.004
- Pastorino GN, Alcántara VM, Malbrán L, et al. Ensifer (sinorhizobium) fredii interacted more efficiently than bradyrhizobium japonicum with soybean. J Agric Ecol Res Int. 2015;2(1):10–19. doi:10.9734/JAERI/2015/13163
- Nalini MS, Prakash HS. Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Lett Appl Microbiol. 2017;64(4):261–270. doi: 10.1111/lam.12718
- Liu YH, Wei YY, Mohamad OAA, et al. Diversity, community distribution and growth promotion activities of endophytes associated with halophyte Lycium ruthenicum Murr. 3 Biotech. 2019;9(4):144. doi:10.1007/s13205-019-1678-8
- Yang ZH, Xing Y, Ma JG, et al. Epichloë fungal endophytes have more host-dependent effects on the soil microenvironment than on the initial litter quality. J Fungi (Basel). 2022;8(3):237. doi: 10.3390/jof8030237
- Mutungi PM, Wekesa VW, Onguso J, et al. Culturable bacterial endophytes associated with shrubs growing along the Draw-Down Zone of Lake Bogoria, Kenya: assessment of Antifungal Potential Against Fusarium solani and Induction of Bean Root Rot Protection. Front Plant Sci. 2022;12:796847. doi: 10.3389/fpls.2021.796847
- Mathew SA, Helander M, Saikkonen K, et al. Endophytes shape the foliar endophytic fungal microbiome and alter the auxin and salicylic acid phytohormone levels in two meadow fescue cultivars. J Fungi. 2023;9(1):90 doi:10.3390/jof9010090
- Hyde KD, Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33:163–173.
- Galindo-Solís JM, Fernández FJ. Endophytic fungal terpenoids: Natural role andbioactivities. Microorganisms. 2022;10(2):339. doi: 10.3390/microorganisms10020339
- Rodriguez RJ, White Jr JJF, Arnold AE, et al. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182(2):314–330. doi: 10.1111/j.1469-8137.2009.02773.x
- Mundt JO, Hinkle NF. Bacteria within ovules and seeds. Appl environ microbiol. 1976;32(5):694–698. doi: 10.1128/aem.32.5.694-698.1976
- Singh M, Kumar A, Singh R, et al. Endophytic bacteria: a new source of bioactive compounds. 3 Biotech. 2017;7(5):315. doi: 10.1007/s13205-017-0942-z
- Liu H, Carvalhais LC, Crawford M, et al. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol. 2017;8:2552. doi: 10.3389/fmicb.2017.02552
- Lechevalier HA, Solotorovsky M, Mcdurmont CI. A new genus of the actinomycetales: micropolyspora gen. nov. J Gen Microbiol. 1961;26(1):11–18. doi: 10.1099/00221287-26-1-11
- Gangwar M, Dogra S, Gupta UP, et al. Diversity and biopotential of endophytic actinomycetes from three medicinal plants in India. African J Microbiol Res. 2014;8(2):184–191. doi:10.5897/AJMR2012.2452
- Singh R, Dubey AK. Diversity and applications of endophytic actinobacteria of plants in special and other ecological niches. Front Microbiol. 2018;9:1767. doi: 10.3389/fmicb.2018.01767
- Khalil AMA, Hassan SE, Alsharif SM. Isolation and Characterization of Fungal Endophytes Isolated from Medicinal Plant Ephedra pachyclada as Plant Growth-Promoting. Biomolecules. 2021;11(2):140. doi: 10.3390/biom11020140
- Hamaoka K, Aoki Y, Suzuki S, et al. Isolation and Characterization of Endophyte Bacillus velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants. 2021;10(9):1815. doi:10.3390/plants10091815
- Khan MS, Gao J, Chen X, et al. Isolation and characterization of plant growth-promoting endophytic bacteria Paenibacillus polymyxa SK1 from lilium lancifolium. Bio Med Res Int. 2020;2020:1–17. doi: 10.1155/2020/8650957
- Zhang D, Sun W, Xu W, et al. Antimicrobial and cytotoxic activity of endophytic fungi from Lagopsis supina. J Microbiol Biotechnol. 2023;33(4):543–551. doi:10.4014/jmb.2211.11055
- Dubey A, Saiyam D, Kumar A, et al. BacterialRoot endophytes: characterization of their competence and plant growth promotion in soybean (glycine max (L.) merr.) under drought stress. Int J Environ Res Public Health. 2021;18(3):931. doi: 10.3390/ijerph18030931
- Soni SK, Singh R, Ngpoore NK, et al. Isolation and characterization of endophytic fungi having plant growth promotion traits that biosynthesizes bacosides and withanolides under in vitro conditions. Braz J Microbiol. 2021;52(4):1791–1805. doi: 10.1007/s42770-021-00586-0
- Duong B, Nguyen HX, Phan HV, et al. Identification and characterization of Vietnamese coffee bacterial endophytes displaying in vitro antifungal and nematicidal activities. Microbiol Res. 2021;242:126613. doi: 10.1016/j.micres.2020.126613
- Zhang S, Xu Q, Ji C, et al. Study on secondary metabolites of endophytic fungus Diaporthe sp. AC1 induced by tryptophan analogs. Front Microbiol. 2023;14:1254609. doi: 10.3389/fmicb.2023.1254609
- Zhang H, Yang Z, Jiang Z, et al. Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China. J Fungi. 2023;9(2):247. doi:10.3390/jof9020247
- Li D, Li Y, Wang X, et al. Engineered pine endophytic Bacillus toyonensis with nematocidal and colonization abilities for pine wilt disease control. Front Microbiol. 2023;14:1240984. doi: 10.3389/fmicb.2023.1240984
- Tanaka A, Christensen MJ, Takemoto D, et al. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell. 2006;18(4):1052–1066. doi:10.1105/tpc.105.039263
- Martínez-Arias C, Sobrino-Plata J, Medel D, et al. Stem endophytes increase root development, photosynthesis, and survival of elm plantlets (Ulmus minor mill.). J Plant Physiol. 2021;261:153420. doi: 10.1016/j.jplph.2021.153420
- Zhao J, Shuai W, Zhu X, et al. Isolation and characterization of nodules endophytic bacteria pseudomonas protegens Sneb1997 and serratia plymuthica Sneb2001 for the biological control of root-knot nematode. Appl Soil Ecol. 2021;164:103924. doi: 10.1016/j.apsoil.2021.103924
- Gao JL, Khan MS, Sun YC, et al. Characterization of an endophytic antagonistic bacterial strain bacillus halotolerans LBG-1-13 with multiple plant growth-promoting traits, stress tolerance, and its effects on Lily growth. Bio Med Res Int. 2022;2022:1–12. doi: 10.1155/2022/5960004
- Gupta S, Pandey S, Sharma S. Decoding the plant growth promotion and antagonistic potential of bacterial endophytes from Ocimum sanctum Linn. Against root rot pathogen fusarium oxysporum in Pisum sativum. Front Plant Sci. 2022;13:813686. doi: 10.3389/fpls.2022.813686
- Agrios GN. Plant pathology. San Diego: Academic Press; 1997. p. 447.
- Costa Pinto LSR, Azevedo JL, Pereira JO, et al. Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytol. 2000;147(3):609–615. doi:10.1046/j.1469-8137.2000.00722.x
- Arnold AE, Engelbrecht BMJ. Fungal endophytes nearly double minimum leaf conductance in seedlings of a neotropical tree species. J Trop Ecol. 2007;23(3):369–372. doi: 10.1017/S0266467407004038
- Terna TP, Mohamed NMI, Zakaria L. Histopathology of corn plants infected by endophytic fungi. Biology. 2022;11(5):641. doi: 10.3390/biology11050641
- Fang D, Chen J, Yi C, et al. First report of Fusarium fujikuroi causing bulb rot on Lilium lancifolium in China. Plant Dis. 2021;105(8):2254. doi: 10.1094/PDIS-06-20-1197-PDN
- Lu H, Wei T, Lou H, et al. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with Biotic and abiotic stresses. J Fungi. 2021;7(9):719. doi:10.3390/jof7090719
- Samakovli D, Tichá T, Vavrdová T, et al. YODA-HSP90 module regulates phosphorylation-dependent inactivation of speechless to control stomatal development under acute heat stress in arabidopsis. Mol Plant. 2020;13(4):612–633. doi: 10.1016/j.molp.2020.01.001
- Ying M, Yue W, Xiao-Jun S, et al. Mechanism and application of plant growth-promoting bacteria in heavy metal bioremediation. Environ Sci. 2022;43(9):4911–4922.
- Citao L, Bigang M, Dingyang Y, et al. Salt tolerance in rice: physiological responses and molecular mechanisms. Crop J. 2022;10(1):13–25.
- Alengebawy A, Abdelkhalek ST, Qureshi SR, et al. Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 2021;9(3):42. doi: 10.3390/toxics9030042
- Genchi G, Sinicropi MS, Lauria G, et al. The effects of cadmium toxicity. Int J Environ Res Public Health. 2020;17(11):3782. doi: 10.3390/ijerph17113782
- Ministry of Environmental Protection of the People’s Republic of China. The investigation communique on national soil pollution condition from ministry of environmental protection of the People’s Republic of China and Ministry of Land and Resources of the People’s Republic of China. Land and rural environment. 2014. [cited 2014 April 17].
- Sperdouli I, Adamakis IS, Dobrikova A, et al. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in salvia sclarea plants. Toxics. 2022;10(1):36.
- Domka A, Rozpądek P, Ważny R, et al. Mucor sp.–an endophyte of Brassicaceae capable of surviving in toxic metal-rich sites. J Basic Microbiol. 2019;59(1):24–37.
- Deng Z, Cao L, Huang H, et al. Characterization of Cd- and Pb-resistant fungal endophyte Mucor sp. CBRF59 isolated from rapes (Brassica chinensis) in a metal-contaminated soil. J Hazard Mater. 2011;185(2–3):717–724. doi: 10.1016/j.jhazmat.2010.09.078
- Xu R, Li T, Cui H, et al. Diversity and characterization of Cd-tolerant dark septate endophytes (DSEs) associated with the roots of Nepal alder (alnus nepalensis) in a metal mine tailing of southwest China. Appl Soil Ecol. 2015;93:11–18. doi: 10.1016/j.apsoil.2015.03.013
- Khan AR, Ullah I, Waqas M, et al. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf. 2017;136:180–188. doi: 10.1016/j.ecoenv.2016.03.014
- DalCorso G, Manara A, Furini A. An overview of heavy metal challenge in plants: from roots to shoots. Metallomics Integr Biometal Sci. 2013;5(9):1117–1132. doi: 10.1039/c3mt00038a
- Yan X, Huang Y, Song H, et al. A MYB4-MAN3-Mannose-MNB1 signaling cascade regulates cadmium tolerance in Arabidopsis. PloS Genet. 2021;17(6):e1009636. doi: 10.1371/journal.pgen.1009636
- Mwamba TM, Li L, Gill RA, et al. Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotoxicol Environ Saf. 2016;134(1):239–249.
- Xiao Y, Dai MX, Zhang GQ, et al. Effects of the dark septate endophyte (DSE) exophiala pisciphila on the growth of root cell wall polysaccharides and the cadmium content of Zea mays L. under cadmium stress. J Fungi. 2021;7(12):1035.
- Gao MY, Chen XW, Huang WX, et al. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J Hazard Mater. 2021;416:125894. doi: 10.1016/j.jhazmat.2021.125894
- Sobrino-Plata J, Meyssen D, Cuypers A, et al. Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil. 2014;377(1–2):369–381. doi: 10.1007/s11104-013-2006-4
- Su Z, Zeng Y, Li X, et al. The endophytic fungus piriformospora indica-assisted alleviation of cadmium in tobacco. J Fungi (Basel). 2021, Aug 20;7(8):675. doi: 10.3390/jof7080675
- DeGroote KV, McCartha GL, Pollard AJ. Interactions of the manganese hyperaccumulator Phytolacca americana L. with soil pH and phosphate. Ecol Res. 2017;33(4):749–755. doi: 10.1007/s11284-017-1547-z
- Zhu S, Ho S-H, Jin C, et al. Nanostructured manganese oxides: Natural/artificial formation and their induced catalysis for wastewater remediation. Environ Sci Nano. 2020;7(2):368–396. doi: 10.1039/C9EN01250H
- Li Y, Liu K, Zhu J, et al. Manganese accumulation and plant physiology behavior of camellia oleifera in response to different levels of nitrogen fertilization. Ecotoxicol Environ Saf. 2019 Nov 30;184:109603.
- Alejandro S, Höller S, Meier B, et al. Manganese in Plants: From Acquisition to Subcellular Allocation. Front Plant Sci. 2020;11:300. doi: 10.3389/fpls.2020.00300
- Wu Q, Lin X, Li S, et al. Endophytic Bacillus sp. AP10 harboured in Arabis paniculata mediates plant growth promotion and manganese detoxification. Ecotoxicology and environmental safety. 2023;262:115170. doi: 10.1016/j.ecoenv.2023.115170
- Tang Y, Kang H, Qin Z, et al. Significance of manganese resistant bacillus cereus strain WSE01 as a bioinoculant for promotion of plant growth and manganese accumulation in Myriophyllum verticillatum. The Science of the total environment. 2020;707:135867. doi: 10.1016/j.scitotenv.2019.135867
- Zhang WH, He LY, Wang Q. Inoculation with endophytic Bacillus megaterium 1Y31 increases Mn accumulation and induces the growth and energy metabolism-related differentially-expressed proteome in Mn hyperaccumulator hybrid pennisetum. Journal of hazardous materials. 2015;300:513–521. doi:10.1016/j.jhazmat.2015.07.049
- Fatemi H, Zaghdoud C, Nortes PA, et al. Differential aquaporin response to distinct effects of two Zn concentrations after foliar application in Pak Choi (brassica rapa L.) plants. Agronomy. 2020;10(3):450. doi: 10.3390/agronomy10030450
- Michael PI, Krishnaswamy M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ Exp Bot. 2021;74:171–177. doi: 10.1016/j.envexpbot.2011.05.016
- Yan J, Pan Y, He J, et al. Toxic vascular effects of polystyrene microplastic exposure. Sci Total Environ. 2023;905:167215. doi: 10.1016/j.scitotenv.2023.167215
- Cao GH, Li XG, Zhang CR, et al. Physiological response mechanism of heavy metal-resistant endophytic fungi isolated from the roots of polygonatum kingianum. Environ Microbiol Rep. 2023;15(6):568–581.
- Lou X, Zhang X, Zhang Y. The synergy of Arbuscular Mycorrhizal Fungi and exogenous abscisic acid benefits Robinia pseudoacacia L. Growth through altering the distribution of Zn and endogenous abscisic acid. J Fungi. 2021;7(8):671. doi: 10.3390/jof7080671
- Li J, Wu H, Pu Q, et al. Complete genome of Sphingomonas paucimobilis ZJSH1, an endophytic bacterium from dendrobium officinale with stress resistance and growth promotion potential. Arch Microbiol. 2023;205(4):132. doi: 10.1007/s00203-023-03459-2
- Iqbal S, Ali U, Fadlalla T, et al. Genome wide characterization of phospholipase a & C families and pattern of lysolipids and diacylglycerol changes under abiotic stresses in brassica napus L. Plant Physiol Biochem. 2020;147:101–112. doi: 10.1016/j.plaphy.2019.12.017
- Abdel-Mawgoud M, Bouqellah NA, Korany SM, et al. Arbuscular mycorrhizal fungi as an effective approach to enhance the growth and metabolism of soybean plants under thallium (TI) toxicity. Plant Physiol Biochem. 2023;203:108077. doi: 10.1016/j.plaphy.2023.108077
- Wang W, Xing L, Xu K, et al. Salt stress-induced H2O2 and Ca2+ mediate K+/Na+ homeostasis in Pyropiahaitanensis. J Appl Phycol. 2020;32(6):4199–4210. doi: 10.1007/s10811-020-02284-0
- Murphy MP, Bayir H, Belousov V, et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab. 2022;4(6):651–662. doi: 10.1038/s42255-022-00591-z
- Sabeem M, Abdul Aziz M, Mullath SK, et al. Enhancing growth and salinity stress tolerance of date palm using piriformospora indica. Front Plant Sci. 2022 Nov 25;13:1037273.
- Bokhari A, Essack M, Lafi FF. Bioprospecting desert plant bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci Rep. 2019;9(1):18154. doi: 10.1038/s41598-019-54685-y
- Liu Z, Xu N, Pang Q, et al. A salt-tolerant strain of trichoderma longibrachiatum HL167 is effective in alleviating salt stress, promoting plant growth, and managing fusarium wilt disease in cowpea. J Fungi. 2023;9(3):304.
- An C, Ma S, Shi X, et al. Diversity and ginsenoside biotransformation potential of cultivable endophytic fungi associated with panax bipinnatifidus var. bipinnatifidus in Qinling mountains, China. Front Pharmacol. 2022;13:762862. doi: 10.3389/fphar.2022.762862
- Xu F, Liang Y, Wang X, et al. Synergic mitigation of saline-alkaline stress in wheat plant by silicon and Enterobacter sp. FN0603. Front Microbiol. 2022;13:1100232. doi: 10.3389/fmicb.2022.1100232
- Navarro-Torre S, Barcia-Piedras JM, Caviedes MA, et al. Bioaugmentation with bacteria selected from the microbiome enhances arthrocnemum macrostachyum metal accumulation and tolerance. Mar Pollut Bull. 2017;117(1–2):340–347. doi: 10.1016/j.marpolbul.2017.02.008
- Gupta A, Singh AN, Tiwari RK, et al. Salinity alleviation and reduction in oxidative stress by Endophytic and rhizospheric microbes in two rice cultivars. Plants. 2023, Feb 21;12(5):976. doi: 10.3390/plants12050976
- Brotman Y, Landau U, Cuadros-Inostroza Á, et al. Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLOS Pathogens. 2013;9(3):e1003221.
- Chen L, Liu Y, Wu G, et al. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol Plant. 2016;158(1):34–44. doi: 10.1111/ppl.12441
- Zhang H, Kim MS, Sun Y, et al. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant Microbe Interact. 2008;21(6):737–744. doi: 10.1094/MPMI-21-6-0737
- Zawoznik MS, Ameneiros M, Benavides MP, et al. Response to saline stress and aquaporin expression in Azospirillum-inoculated barley seedlings. Appl Microbiol Biotechnol. 2011;90(4):1389–1397. doi: 10.1007/s00253-011-3162-1
- Liu S, Hao H, Zhao X, et al. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Scientific reports. 2017;7(1):10795. doi:10.1038/s41598-017-11308-8
- Vaishnav A, Kumari S, Jain S, et al. Putative bacterial volatile-mediated growth in soybean (glycine max L. Merrill) and expression of induced proteins under salt stress. Journal of Applied Mmicrobiology. 2015;119(2):539–551.
- Asseng S, Spänkuch D, Hernandez-Ochoa IM, et al. The upper temperature thresholds of life. Lancet Planet Health. 2021;5(6):e378–e385. doi: 10.1016/S2542-5196(21)00079-6
- Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11:375. doi: 10.3389/fpls.2020.00375
- Eid AM, Fouda A, Abdel-Rahman MA, et al. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants. 2021;10(5):935. doi: 10.3390/plants10050935
- Waqas M, Khan AL, Shahzad R, et al. Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J Zhejiang Univ. 2015;16(12):1011–1018.
- Pan Y, Cheng JH, Sun DW. Metabolomic analyses on microbial primary and secondary oxidative stress responses. Compr Rev Food Sci Food Saf. 2021;20(6):5675–5697. doi: 10.1111/1541-4337.12835
- Javed J, Rauf M, Arif M, et al. Endophytic fungal consortia enhance basal drought-tolerance in moringa oleifera by upregulating the antioxidant enzyme (APX) through heat shock factors. Antioxidants. 2022;11(9):1669. doi: 10.3390/antiox11091669
- Aswini K, Suman A, Sharma P, et al. Seed endophytic bacterial profiling from wheat varieties of contrasting heat sensitivity. Front Plant Sci. 2023;14:1101818. doi: 10.3389/fpls.2023.1101818
- De Marco A, Sicard P, Feng Z, et al. Strategic roadmap to assess forest vulnerability under air pollution and climate change. Glob Chang Biol. 2022;28(17):5062–5085. doi: 10.1111/gcb.16278
- Huang B, Fan Y, Cui L, et al. Cold stress response mechanisms in another development. Int J Mol Sci. 2022;24(1):30.
- Cui X, He W, Christensen MJ, et al. Abscisic acid may play a critical role in the moderating effect of epichloë endophyte on achnatherum inebrians under drought stress. J Fungi. 2022;8(11):1140.
- Lin W, Liu L, Liang J, et al. Changes of endophytic microbial community in rhododendron simsii roots under heat stress and its correlation with leaf physiological indicators. Front Microbiol. 2022;13:1006686. doi: 10.3389/fmicb.2022.1006686
- Ismail KH, Hamayun M, Hussain A, et al. Endophytic fungus aspergillus japonicus mediates host plant growth under normal and heat stress conditions. Bio Med Res Int. 2018;2018:1–11. doi: 10.1155/2018/7696831
- Bisht N, Mishra SK, Chauhan PS. Bacillus amyloliquefaciens inoculation alters physiology of rice (Oryza sativa L. var. IR-36) through modulating carbohydrate metabolism to mitigate stress induced by nutrient starvation. Int J Biol Macromol. 2020;143:937–951. doi: 10.1016/j.ijbiomac.2019.09.154
- Waqas M, Kim YH, Khan AL, et al. Additive effects due to biochar and endophyte application enable soybean to enhance nutrient uptake and modulate nutritional parameters. J Zhejiang Univ. 2017;18(2):109–124.
- Wang T, Li F, Lu Q, et al. Diversity, novelty, antimicrobial activity, and new antibiotics of cultivable endophytic actinobacteria isolated from psammophytes collected from Taklamakan Desert. J Pharm Anal. 2021;11(2):241–250. doi: 10.1016/j.jpha.2020.06.004
- Khan A, Ali S, Khan M, et al. Parthenium hysterophorus’s endophytes: the second layer of defense against biotic and abiotic stresses. Microorganisms. 2022;10(11):2217. doi: 10.3390/microorganisms10112217
- Yung L, Sirguey C, Azou-Barré A, et al. Natural fungal endophytes from noccaea caerulescens mediate neutral to positive effects on plant biomass, mineral nutrition and Zn phytoextraction. Front Microbiol. 2021;12:689367. doi: 10.3389/fmicb.2021.689367
- Sharma VK, Parmar S, Tang W, et al. Effects of fungal seed endophyte FXZ2 on dysphania ambrosioides Zn/Cd tolerance and accumulation. Front Microbiol. 2022;13:995830. doi: 10.3389/fmicb.2022.995830
- Ismail I, Hussain A, Mehmood A, et al. Thermal stress alleviating potential of endophytic fungus rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak J Bot. 2020;52(5):52.
- Zhao Z, Kou M, Zhong R, et al. Transcriptome analysis revealed plant hormone biosynthesis and response pathway modification by epichloëgansuensis in Achnatheruminebrians under different soil moisture availability. J Fungi. 2021;7(8):640.
- Rashid U, Yasmin H, Hassan MN, et al. Drought-tolerant bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2022;41(3):549–569. doi: 10.1007/s00299-020-02640-x
- Saikia K, Bora LC. Exploring actinomycetes and endophytes of rice ecosystem for induction of disease resistance against bacterial blight of rice. Eur J Plant Pathol. 2020;159(1):67–79. doi: 10.1007/s10658-020-02141-3
- Yan L, Zhu J, Zhao X, et al. Beneficial effects of endophytic fungi colonization on plants. Appl Microbiol Biotechnol. 2019;103(8):3327–3340. doi: 10.1007/s00253-019-09713-2
- Anyasi RO, Atagana HI. Endophyte: understanding the microbes and its applications. Pak J Biol Sci. 2019;22(4):154–167. doi: 10.3923/pjbs.2019.154.167
- Singh BK, Delgado-Baquerizo M, Egidi E. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol. 2023;21(10):640–656. doi: 10.1038/s41579-023-00900-7
- Rai N, Kumari Keshri P, Verma A, et al. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology. 2021;12(3):139–159. doi: 10.1080/21501203.2020.1870579
- Xu W, Li M, Lin W, et al. Effects of epichloë sinensis endophyte and host ecotype on physiology of Festuca sinensis under different soil moisture conditions. Plants. 2021;10(8):1649. doi: 10.3390/plants10081649
- Doty SL, Joubert PM, Firrincieli A, et al. Potential biocontrol activities of populus endophytes against several plant pathogens using different inhibitory mechanisms. Pathogens. 2022;12(1):13. doi: 10.3390/pathogens12010013
- Elias LM, Fortkamp D, Sartori SB, et al. The potential of compounds isolated from Xylaria spp. As antifungal agents against anthracnose. Braz J Microbiol. 2018;49(4):840–847. doi: 10.1016/j.bjm.2018.03.003
- Bengyella L, Iftikhar S, Nawaz K, et al. Biotechnological application of endophytic filamentous bipolaris and curvularia: a review on bioeconomy impact. World J Microbiol Biotechnol. 2019;35(5):69. doi: 10.1007/s11274-019-2644-7
- Miotto-Vilanova L, Jacquard C, Courteaux B, et al. Burkholderia phytofirmans PsJN confers grapevine resistance against botrytis cinerea via a direct antimicrobial effect combined with a better resource mobilization. Front Plant Sci. 2016;7:1236. doi: 10.3389/fpls.2016.01236
- Terhonen E, Sipari N, Asiegbu FO. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol Control. 2016;99:53–63. doi: 10.1016/j.biocontrol.2016.04.006
- Zhao H, Ding X, Chu X, et al. Plant immune inducer ZNC promotes rutin accumulation and enhances resistance to botrytis cinerea in tomato. Stress Biol. 2023;3(1):36.
- Kumar V, Nautiyal CS. Endophytes modulate plant genes: present status and future perspectives. Curr Microbiol. 2023;80(11):353. doi: 10.1007/s00284-023-03466-y
- Gupta S, Pandey S, Nandi SP, et al. Modulation of ethylene and ROS-scavenging enzymes by multifarious plant growth-promoting endophytes in tomato (Solanum lycopersicum) plants to combat Xanthomonas-induced stress. Plant Physiol Biochem. 2023;202:107982. doi: 10.1016/j.plaphy.2023.107982
- Fardella PA, Clarke BB, Belanger FC. The Epichloë festucae Antifungal Protein Efe-AfpA Has Activity against Numerous Plant Pathogens. Microorganisms. 2023;11(4):828. doi: 10.3390/microorganisms11040828
- Zhao Y, Mao W, Tang W, et al. Wild Rosa endophyte M7SB41-mediated Host plant’s powdery mildew resistance. J Fungi (Basel). 2023;9(6):620. doi: 10.3390/jof9060620
- Han L, Zheng W, He Z, et al. Endophytic fungus Biscogniauxia petrensis produces antibacterial substances. PeerJ. 2023;11:e15461. doi: 10.7717/peerj.15461
- Zhang J, Huang X, Yang S, et al. Endophytic Bacillus subtilis H17-16 effectively inhibits phytophthora infestans, the pathogen of potato late blight, and its potential application. Pest Manag Sci. 2023;79(12):5073–5086. doi: 10.1002/ps.7717
- Huang J, He Z, Wang J, et al. A novel effector FlSp1 inhibits the colonization of endophytic Fusarium lateritium and increases the resistance to Ralstonia solanacearum in tobacco. J Fungi (Basel). 2023;9(5):519. doi: 10.3390/jof9050519
- Pal KK, Dey R, Sherathia DN, et al. Alleviation of salinity stress in peanut by application of endophytic bacteria. Front Microbiol. 2021;12:650771. doi: 10.3389/fmicb.2021.650771
- Kachalkin A, Glushakova A, Streletskii R. Diversity of endophytic yeasts from agricultural fruits positive for phytohormone IAA production. Biotech. 2022;11(3):38. doi: 10.3390/biotech11030038
- Debnath S, Chakraborty S, Langthasa M, et al. Non-rhizobial nodule endophytes improve nodulation, change root exudation pattern and promote the growth of lentil, for prospective application in fallow soil. Front Plant Sci. 2023;14:1152875. doi: 10.3389/fpls.2023.1152875
- Barrera M, Jakobs-Schoenwandt D, Persicke M, et al. Anhydrobiotic engineering for the endophyte bacterium kosakonia radicincitans by osmoadaptation and providing exogenously hydroxyectoine. World J Microbiol Biotechnol. 2020;36(1):6. doi: 10.1007/s11274-019-2780-0
- Li Y, Bi M, Sun S, et al. Comparative metabolomic profiling reveals molecular mechanisms underlying growth promotion and disease resistance in wheat conferred by piriformospora indica in the field. Plant Signal Behav. 2023;18(1):2213934. doi: 10.1080/15592324.2023.2213934
- Heeter KJ, Harley GL, Abatzoglou JT. Unprecedented 21st century heat across the Pacific Northwest of North America. Npj Clim Atmos Sci. 2023;6(1):5. doi: 10.1038/s41612-023-00340-3
- He W, Megharaj M, Wu CY, et al. Endophyte-assisted phytoremediation: mechanisms and current application strategies for soil mixed pollutants. Crit Rev Biotechnol. 2020;40(1):31–45. doi: 10.1080/07388551.2019.1675582
- Khan NA, Asaf S, Ahmad W, et al. Diversity, lifestyle, genomics, and their functional role of cochliobolus, bipolaris, and curvularia species in environmental remediation and plant growth promotion under biotic and abiotic stressors. J Fungi. 2023;9(2):254.
- Kracmarova-Farren M, Papik J, Uhlik O, et al. Compost, plants and endophytes versus metal contamination: choice of a restoration strategy steers the microbiome in polymetallic mine waste. Environ Microbiome. 2023;18(1):74. doi: 10.1186/s40793-023-00528-3
- Wen Z, Liu Q, Yu C, et al. The difference between rhizosphere and Endophytic Bacteria on the safe cultivation of lettuce in Cr-Contaminated Farmland. Toxics. 2023;11(4):371. doi: 10.3390/toxics11040371
- Li B, Wu B, Dong Y, et al. Endophyte inoculation enhanced microbial metabolic function in the rhizosphere benefiting cadmium phytoremediation by Phytolaccaacinosa. Chemosphere. 2023;338:139421. doi: 10.1016/j.chemosphere.2023.139421
- Kashyap S, Chandra R, Kumar B, et al. Biosorption efficiency of nickel by various endophytic bacterial strains for removal of nickel from electroplating industry effluents: an operational study. Ecotoxicology. 2022;31(4):565–580.
- Ancheeva E, Daletos G, Proksch P. Bioactive secondary metabolites from endophytic fungi. Curr Med Chem. 2020;27(11):1836–1854. doi: 10.2174/0929867326666190916144709
- Toghueo RMK. Bioprospecting endophytic fungi from fusarium genus as sources of bioactive metabolites. Mycology. 2019;11(1):1–21. doi: 10.1080/21501203.2019.1645053
- Luo HZ, Jiang H, Huang XS, et al. New sesquiterpenoids from plant-associated Irpex lacteus. Front Chem. 2022;10:905108. doi: 10.3389/fchem.2022.905108
- Puri SC, Nazir A, Chawla R, et al. The endophytic fungus trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J Biotechnol. 2006;122(4):494–510. doi: 10.1016/j.jbiotec.2005.10.015
- Devi P, Rodrigues C, Naik CG, et al. Isolation and characterization of antibacterial compound from a mangrove-endophytic fungus, penicillium chrysogenum MTCC 5108. Indian J Microbiol. 2012;52(4):617–623.
- Zhao J, Wang J, Pang X, et al. An anti-influenza A virus microbial metabolite acts by degrading viral endonuclease PA. Nat Commun. 2022;13(1):2079. doi: 10.1038/s41467-022-29690-x
- Verma A, Rai N, Gupta P, et al. Exploration of in vitro cytotoxic and in ovo antiangiogenic activity of ethyl acetate extract of penicillium oxalicum. Environ Toxicol. 2023;38(10):2509–2523. doi: 10.1002/tox.23889
- Chen C, Ye G, Tang J, et al. New Polyketides from mangrove endophytic fungus Penicillium sp. BJR-P2 and their anti-inflammatory activity. Mar Drug. 2022;20(9):583.
- Tang J, Wu L, Tang XF, et al. A new alkaloid from thespesia populnea endophytic fungus Penicillium sp. TM-Y1-1. J Asian Nat Prod Res. 2023;25(9):905–911. doi: 10.1080/10286020.2022.2162887
- Farooq S, Qayum A, Nalli Y, et al. Discovery of a secalonic acid derivative from aspergillus aculeatus, an endophyte of Rosa damascena Mill., triggers apoptosis in MDA-MB-231 triple negative breast cancer cells, an endophyte of Rosa damascena Mill., triggers apoptosis in MDA-MB-231 triple negative breast cancer cells. ACS Omega. 2020;5(38):24296–24310. doi: 10.1021/acsomega.0c02505
- Caruso G, Abdelhamid MT, Kalisz A, et al. Linking endophytic fungi to medicinal plants therapeutic activity. A case study on asteraceae. Agriculture. 2020;10(7):286. doi: 10.3390/agriculture10070286
- Liu SS, Jiang JX, Huang R, et al. A new antiviral 14-nordrimane sesquiterpenoid from an endophytic fungus Phoma spp. Phytochem Lett. 2019;29:75–78. doi: 10.1016/j.phytol.2018.11.005
- Mahmoud MM, Abdel-Razek AS, Soliman HSM, et al. Diverse polyketides from the marine endophytic Alternaria sp. LV52: structure determination and cytotoxic activities. Biotechnol Rep. 2021;33:e00628. doi: 10.1016/j.btre.2021.e00628
- Guo B, Dai JR, Ng S, et al. Cytonic acids a and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus cytonaema species. J Natural Prod. 2000;63(5):602–604. doi: 10.1021/np990467r
- Zhao H, Xu C, Lu HL, et al. Host-to-pathogen gene transfer facilitated infection of insects by a pathogenic fungus. PLOS Pathogens. 2014;10(4):e1004009. doi: 10.1371/journal.ppat.1004009
- Alam B, Lǐ J, Gě Q, et al. Endophytic fungi: from symbiosis to secondary metabolite communications or vice versa? Front Plant Sci. 2021;12:791033. doi: 10.3389/fpls.2021.791033

