ABSTRACT
Inflammation plays a major role in etiology of multiple diseases and it has become an imperative therapeutic target in pharmacological interventions. Over the years, natural products originating from plants have made great contributions in the drug discovery process. Belonging to the Asteraceae family, Stevia rebaudiana (S. rebaudiana) is exploited at a large scale for its purpose as a natural sweetener. Even so, researchers have begun to notice other bioactive potential use of stevia such as anti-inflammatory and anti-cancer activities, which are conferred by compounds present in the leaves including stevioside, rebaudioside and isosteviol. In this review, we provide a brief overview of S. rebaudiana plant and its bioactive compounds and highlight their anti-inflammatory potential for therapeutic applications.
Introduction
Inflammation is a natural response that protects host organisms against external injuries and pathogens (Li et al. Citation2019). Upon activation, the innate immune system of body defense recruits immune cells to target the inflammation site by the production of pro-inflammatory mediators (Al-Kharashi Citation2018). These mediators like interleukins (ILs) recruit immune cells to assist in the fight against pathogens, foreign bodies or even cancer cells. However, when the inflammatory process gets out of control, the excess production of pro-inflammatory mediators can induce various acute and chronic human diseases (Medzhitov Citation2010; Kunnumakkara et al. Citation2018). Nowadays, the commonly prescribed therapeutic drugs for inflammatory diseases include corticosteroids, immunosuppressive agents and non-steroidal anti-inflammatory drugs. Having said that, the usage of these drugs is frequently associated with some unwanted side effects which significantly limited their usage, including the development of drug tolerance, addiction and gastrointestinal toxicity (Xin et al. Citation2017). Thus, many researchers have turned to natural resources to hunt for anti-inflammatory agents with higher potency and ideally without toxicity.
Native to South America (Paraguay and Brazil), Stevia rebaudiana (S. rebaudiana) is a perennial shrub of the Asteraceae family (Hossain et al. Citation2017). The practice of using S. rebaudiana leaves as natural sweetener begins among the Paraguayan Indians population; only a few leaves are required to increase the sweetness of drinks like herbal teas (Lewis Citation1992). Ever since, its usage as a natural sweetener has become popular in various regions of the world, particularly within the Asia region (Figure ; Ohtani and Yamasaki Citation2004). Besides being used as natural food additives, steviol glycosides in S. rebaudiana have shown to have high pharmaceutical potential. In fact, evidence is piling up on the potential use of stevia for biological activities, other than its use as a natural sweetener such as anti-cancer, antitumor, immunomodulatory and anti-inflammation without causing any detrimental side effects (Kinghorn Citation2002; Yasukawa et al. Citation2002; Gregersen et al. Citation2004; Chatsudthipong and Muanprasat Citation2009; Boonkaewwan and Burodom Citation2013; Wang et al. Citation2014a; Latha et al. Citation2017; López et al. Citation2017; Ruiz-Ruiz et al. Citation2017; Liu et al. Citation2018; Casas-Grajales et al. Citation2019a). Thus, the current review aims to provide an overview on the anti-inflammatory activity of S. rebaudiana and highlight its potential as a therapeutic agent against various inflammation-related diseases.
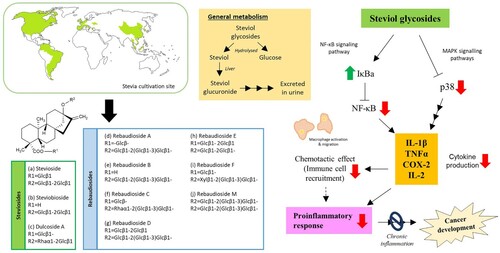
Figure 1. General information about native South American plant, S. rebaudiana, and its anti-inflammatory activity (adapted from Ferrazzano et al. Citation2015). The colored regions on the map show cultivation sites of S. rebaudiana.
Botanical description and distribution
Even though the genus Stevia contains 230 species, only S. rebaudiana gives the sweet essence (Ruiz-Ruiz et al. Citation2017). Known as ‘the sweet herb of Paraguay’, S. rebaudiana was firstly coinedd by an Italian botanist, Moises S. Bertoni (Soejarto et al. Citation1983). The plant of S. rebaudiana is described as a perennial low shrub with extensive roots, brittle stems and small, elliptical leaves (Table ) (Ferrazzano et al. Citation2015; Gutiérrez et al. Citation2016). Given its economic value as natural sweetener, the cultivation of this plant has exceeded its native habitat (i.e. Paraguay) and it is now widely grown in numerous countries, particularly in India, Japan, Taiwan, Korea, Thailand and Indonesia (Kinghorn Citation2002; Chatsudthipong and Muanprasat Citation2009). In fact, the induction load of stevia in India has reached more than 2000 kg/hectare in 2005 with the total annual output is close to 600 tonnes. In terms of growth and flowering period, it varies depending on its cultivation site, mainly affected by photoperiod, temperature and water availability of the soil (Ramesh et al. Citation2006). For example, when cultivated under long-day conditions, S. rebaudiana plant produce bigger leaves with a higher content of glycosides (Singh and Rao Citation2005). These information about growth preference of S. rebaudiana is useful to optimize cultivation condition to ensure healthy growth of the plant, and subsequently increase its bioactive compound(s) yield.
Table 1. Botanical classification and morphological features of Stevia rebaudiana (modified from Gutiérrez et al. (Citation2016)).
Chemical constituent and extraction of the bioactive compounds from S. rebaudiana leaves
S. rebaudiana is rich in carbohydrates, protein, crude fiber, minerals and glycosides (Supplementary Table 1; Ijaz et al. Citation2015). As a matter of fact, the economic value of S. rebaudiana heavily relies on the steviol glycosides content in its leaves (Table ; Figure ) (Wölwer-Rieck Citation2012; Aranda-González et al. Citation2015; Wald and Morlock Citation2017; Debnath et al. Citation2018; Gardana and Simonetti Citation2018; Gomes et al. Citation2018). The chemical structure of steviol glycoside present as an aglycone (steviol) and three molecules of glucose (Chatsudthipong and Muanprasat Citation2009). To date, at least 12 steviol glycosides have been identified from stevia leaves, which one of the best-known being stevioside (Rojas et al. Citation2018). Other steviol glycosides are rebaudioside (A–F), steviolbioside and dulcoside A (Shibata et al. Citation1995; Rojas et al. Citation2018). As cultivation conditions may affect the content of glycosides in stevia leaves, the yield of steviosides and rebaudioside A can from 16.3–95 mg/g and 6.86–44.1 mg/g of dry leaves weight, respectively (Wood et al. Citation1955). Nonetheless, it is worthy to take note that these compounds are at least 50 times sweeter than sucrose, fitting its role as a natural sweetener (Prakash et al. Citation2008; Ozdemir et al. Citation2015). At the same time, many research groups have indicated bioactive potentials of these compounds (Geuns Citation2003; Yadav and Guleria Citation2012). Therefore, researchers around the world have put in a reasonable amount of effort to develop multiple preservation, extraction and purification strategies to obtain bioactive compounds from the plant (Li et al. Citation2012; Gallo et al. Citation2017).
Table 2. Steviol glycosides present in S. rebaudiana leaves (LOD, limit of detection).
Drying is the first processing stage for stevia leaves, where solar drying is the common practice for preservation purpose. However, solar drying is lacking systemic process control that ultimately affect dried product quality (Lemus-Mondaca et al. Citation2016). In order to extend stevia leaves shelf life and preserving their stability and quality, it is crucial to deliver a quality product with a proper application of drying method and temperature. Numerous drying methods have been developed to obtain the dried stevia leaves with desirable active compounds, namely sun drying, oven drying, microwave drying, shade drying and freeze-drying method. Conventional sun drying method that exposes fresh leaves under direct sun light for 5 days produces sun-dried stevia leaves with the highest moisture, ash, protein and fat content (Gasmalla et al. Citation2014). Under a range of relatively stable temperature (38.5–58.5 °C) and air humidity (11.5–53.5%), sun-dried stevia leaves extract shows strong anti-inflammatory response against phorbol 12-myristate 13-acetate (TPA)-induced inflammation in ear edema model (Lemus-Mondaca et al. Citation2018). The microwave drying method is fast and effective against inflammation. It retains the highest amount of carbohydrate, reducing sugar and higher pH values in dried leaves when compared with sun-dried and oven-dried methods (Gasmalla et al. Citation2014). This method dries leaves between 6 and 8 min at 700–800 W, and it yields a potent anti-inflammatory extract in both arachidonic acid (AA)-induced and TPA-induced ear edema inflammation models (Gasmalla et al. Citation2014; Lemus-Mondaca et al. Citation2018). On the other hand, shade drying, hot air drying and freeze drying have no effect on rebaudioside A and C concentration (Periche et al. Citation2015). These three drying processes have been shown to reduce stevioside content while increase dulcoside A content; nonetheless both compounds achieved the highest value in shade drying method (Periche et al. Citation2015). A 3-min of hot air-drying process at 180°C also gives the highest content of total phenol, flavonoids and antioxidants in dried stevia leaves (Periche et al. Citation2015; Lemus-Mondaca et al. Citation2016). Lemus-Mondaca et al. (Citation2016)’s work on the effect of hot air drying at 30, 40, 50, 60, 70 and 80°C under a constant air velocity of 2.0 ± 0.2 m/s on fresh stevia leaves showed that 40°C is the optimal drying temperature to yield high steviol glycosides content (stevioside, steviolbioside, rebaudioside A, B and C, rubusoside and dulcoside) with best antioxidant activity compared to other temperatures. They also showed that hot air-drying temperatures at 60°C and above has a negative impact on the total phenolic and flavonoid. Later, they tested seven drying methods (freeze-drying, vacuum drying, infrared drying, shade drying, sun drying, microwave drying and hot air drying) on fresh stevia leaves and found that freeze-drying method produced the highest antioxidant capacity, higher total polyphenol, total flavonoids content and phenolic compounds (chlorogenic acid, caffeic acid and trans-ferulic acid) when compared with other drying methods and fresh stevia leaves extract (Lemus-Mondaca et al. Citation2018). Other than ther infrared drying method, the rest of the drying methods reduced inflammation in ear edema models; vacuum drying and shade drying methods showed the strong anti-inflammatory effect on AA-induced and TPA-induced inflammation, respectively (Lemus-Mondaca et al. Citation2018). Taken together, drying method and temperature on fresh stevia leaves are important to generate the leaves extract for desirable bioactivity or compound isolation.
In terms of extraction, one of the conventional methods involves the use of organic solvents such as chloroform–methane, glycerol and propylene glycol (Kinghorn and Soejarto Citation1985; Pasquel et al. Citation2000). Taking into considerations that some organic solvents may not be environmentally friendly and/or poses some health hazards to operators, researchers have suggested the use of water to be used instead as stevioside is water-soluble too (Phillips Citation1987; Bovanová et al. Citation1998; Vaněk et al. Citation2001; Pól et al. Citation2007). As extraction methods evolved, more sophisticated techniques have been incorporated into the extraction process of stevioside, including pressurized liquid extraction (PLE), pressurized hot water extraction (PHWE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE) and supercritical fluid extraction (SFE) (Tan et al. Citation1988; Kienle Citation1990; Kovylyaeva et al. Citation2007; Liu et al. Citation2010). By the same token, these techniques offer greater advantages over conventional solvent extraction systems. For instance, the typical solvent volume required for MAE is about 10 ml/g of plant sample, which is much lesser than that required in conventional extraction methods (Soxhlet extraction: 30 ml/g of plant sample) (Javad et al. Citation2014). Jaitak et al. (Citation2009) compared three different extraction methods – conventional solvent extraction, MAE and UAE. Based on their results, MAE yielded highest stevioside (8.64%) and rebaudioside A (2.34%), followed by conventional extraction (stevioside 6.54%; rebaudioside A 1.20%) and UAE (stevioside 4.20%; rebaudioside A 1.98%). A strong evidence presented by the same team emphasized the advantage of MAE and UAE over conventional solvent extraction is the time required for the extraction. The whole extraction process with MAE is much quicker, dropping extraction time from 16 h (using Soxhlet extraction) to just a few minutes – ∼30 min for UAE and only 1 min for MAE (Jaitak et al. Citation2009; Ferrazzano et al. Citation2015). Similarly, a research team from Singapore compared the extraction efficiency of MAE to another modern green approach known as PHWE, which use superheated or sub-critical water to extract steviol glycosides present in stevia leaves (Teo et al. Citation2009). Even though the team had successfully extracted steviosides using PHWE, MAE appeared to be a better choice with higher extraction efficiency as PHWE requires temperature optimization to ensure good yield (Teo et al. Citation2009; Kovačević et al. Citation2018). Similar results were reported by Periche et al. (Citation2015) which compared three different methods – conventional (heating) method, MAE and UAE. Compared to conventional method, MAE and UAE extracted higher yield of different steviol glycosides – (a) Dulcoside A: 2.03 and 2 mg/g, (b) Rebaudioside A: 17.03 and 14.12 mg/g, and (c) Rebaudioside C: 6.6 and 6.25 mg/g (d) Stevioside: 46.48 and 39.06 mg/g, respectively.
Aside from these techniques, SFE is another extraction method deployed to obtain steviol glycosides from stevia plant, yet studies have expressed ‘mixed feelings’ over the extraction efficacy of this technique (Pasquel et al. Citation2000; Choi et al. Citation2002; Yoda et al. Citation2003; Erkucuk et al. Citation2009; Ameer et al. Citation2017). For instance, the total yield of glycosides obtained by Erkucuk and team using SFE (45.13–54.26 mg/g) was similar to those obtained using conventional water extraction (64.49 mg/g) and slightly higher than ethanol extraction (48.60 mg/g), thus implying the cost-effectiveness of SFE for industrial-scale application. On the other side, Ameer et al. (Citation2017) demonstrated that the optimization of SFE via response surface methodology and artificial neural network modeling could generate higher targeted extraction (i.e. steviol glycosides at) than conventional extraction, offering a faster, lower energy, and eco-friendly extraction method with lesser CO2 emissions and reduced solvent use.
Another important factor for practical and industrial applications is the purity of acquired bioactive compound, particularly eliminating the signature bitter-off taste of stevia. For this reason, Zhang et al. (Citation2000) developed a membrane chromatography technique to obtain high purity steviol glycoside while removing most of the bitter-tasting components. Lately, a team from Italy has described a newer solid–liquid extraction known as rapid solid–liquid dynamic extraction (RSLDE) (Gallo et al. Citation2017; Gallo et al. Citation2018). Compared to the Soxhlet method, this technique is a much ‘greener’ option, operating at low (room) temperatures while maintaining extraction reproducibility (Gallo et al. Citation2017; Naviglio et al. Citation2019). For the extraction of stevia, Gallo et al. (Citation2017, Citation2018) discovered that ‘complete’ extractions of rebaudioside A and stevioside can be achieved in 40 min. Compared to traditional heating method or maceration, the leaves were more intact and remained in a filter bag, bypassing the need of additional filtering steps to remove solid materials and more suitable for further use in agronomy and animal nutrition (Gallo et al. Citation2017, Citation2018). In truth, Phillips (Citation1987) has suggested a pre-treatment with solvents like chloroform or hexane prior to actual extraction processes to remove other essential oils, lipids, chlorophyll and other non-polar substances during the late 1980s. Besides reducing impurities, Formigoni et al. (Citation2018) have shown that the pre-treatment (with ethanol) increased the yield of steviol glycosides from stevia leaves, reduced its bitter aftertaste while achieving sensory profile similar to sucralose. Along with this, some researchers have also pointed out that using solvent alone may not be able to penetrate into the sample core to ensure complete release target compounds, which in turn result in lower extraction efficiency (Teo et al. Citation2009). Pulsed electric fields (PEF) technology and high-voltage electrical discharges (HVED) are examples of highly innovative extraction methods that involve electrical treatments of short time (from several nanoseconds to several milliseconds) to electrically ‘pierced’ biological membrane, enabling higher extraction and recovery of compound of interest (Barba et al. Citation2015a, Citation2015b; Carbonell-Capella et al. Citation2017; Li et al. Citation2019). Both PEF and HVED improved the extraction of rebaudioside A and stevioside compared to traditional grinding, shortening ‘buffer time’ before subsequent purification and concentration steps (Barba et al. Citation2017). Coupled with analytical methods like ultraviolet and visible spectrophotometer (UV–Vis) and Fourier transform infrared spectroscopy (FTIR), the extraction and identification technologies of the chemical constituent present in S. rebaudiana have progressed much since the discovery of the plant, enabling higher efficiency and faster access for future drug development work.
Metabolism of stevioside in the body system
In general, S. rebaudiana plants have long been used by the Paraguayan populations with no toxicity reported previously (Curry and Roberts Citation2008; Momtazi-Borojeni et al. Citation2017). Stevioside was found to bear a very low acute oral toxicity (LD50 between 8.2 and 17 g/kg) in the mouse, rat and hamster (Medon et al. Citation1982; Toskulkac et al. Citation1997; Sharma et al. Citation2009; Brahmachari et al. Citation2011). Furthermore, no carcinogenicity was observed in the standard AMES test and rodent models (Hagiwara et al. Citation1984; Suttajit et al. Citation1993; Toyoda et al. Citation1997; Eriko et al. Citation2003). The European Food Safety Authority (EFSA) has published a scientific report in 2015, highlighting that stevioside is neither carcinogenic nor genotoxic with no risks associated with reproductive or developmental toxicity (EFSA ANS Panel Citation2015). When ingested, steviol glycoside is not digested by human but rather hydrolyzed by the gut microbes into steviol, by cutting off their glucose units (Koyama et al. Citation2003; Geuns et al. Citation2007; JECFA Citation2008; Wheeler et al. Citation2008; Ashwell Citation2015). Consequently, steviol is absorbed via the portal vein before undergoing glucuronidation process to yield the final metabolite steviol glucuronide, which is then excreted in the urine (Wingard et al. Citation1980; Koyama et al. Citation2003; Roberts and Renwick Citation2008). Looking at pharmacokinetics data, Wheeler et al. (Citation2008) have reported that steviol glucuronide was detected in the plasma of all human subjects after administration of rebaudioside A (5 mg/kg) or stevioside (4.2 mg/kg), with the median time taken to reach the maximum concentration (Tmax) values of 12 and 8 h post-dose, respectively. In the same study, no safety concerns or adverse events were reported. With the growing evidence supporting the safety of stevia use, World Health Organization (WHO) approved stevia as a food additive in 2004 with a recommended acceptable daily intake (ADI) to be up to 4 mg/kg body weight (JECFA Citation2008). Likewise, the use of stevioside as food additive is also approved under the General Standard for Food Additives (GSFA), in countries like the USA and Canada (Martyn et al. Citation2018; Perrier et al. Citation2018). Nevertheless, a study by Robert et al. in 2016 claimed that the current ADI is assumed as particularly conservative; using rat and human as study subjects, the group proposed that the higher ADI should be implemented, falling between 6 and 16 mg/kg body weight per day. Altogether, these studies supported the safe use of stevia and its metabolites, which subsequently promote its potential to be exploited for pharmaceutical use.
Use of stevia as ‘top-pick’ for sugar substitute in metabolic diseases
Coming with a zero calories content, stevia represents a good and healthy substitute for sugars, particularly for diabetic patients. Diabetes mellitus (DM) is a multifaceted metabolic disease characterized by glucose dysregulation which results in hyperglycemia (Care Citation2006). Type 1 (T1D) and type 2 (T2D) diabetes are categorized by their pathogenesis, where T1D involves immune-modulated pancreatic beta cells destruction while T2D primarily demonstrates insulin resistance and deficiency (Tsalamandris et al. Citation2019). Piling evidence have associated inflammation with the progression of DM via detection of circulatory inflammatory markers such as IL-6 in epidemiological studies and animal models (Table ) (Duncan et al. Citation2003; Marques-Vidal et al. Citation2012; Tsalamandris et al. Citation2019). Insulin resistance and deficiency in pancreatic β cells insulin secretion are considered as pro-inflammatory states as they have close links with inflammatory responses (Hotamisligil et al. Citation1993; Lohner et al. Citation2017; Zhong et al. Citation2017). Stevia and its extracts are known to possess insulinotropic, glucagonostatic, antihyperglycemic and blood-pressure-suppressing effects in multiple DM models (Suanarunsawata et al. Citation2004; Holvoet et al. Citation2015; Latha et al. Citation2017). Stevioside treatment at 25, 30 and 300 mg/kg on Goto-Kakizaki rats (a non-obese genetic T2D experimental model) have consistently displayed higher first-phase insulin responses and lower glucagon level during insulin-assisted glucose tolerance tests (IAGT) (Jeppesen et al. Citation2002, Citation2003, Citation2006). It significantly reduces hyperglycemia condition, both systolic and diastolic blood pressure, as well as plasma cholesterol level when administered for 5 weeks (Jeppesen et al. Citation2002, Citation2003, Citation2006). Administration of stevia leaves extracts, leaves powder and also the extracted polyphenols improves and alleviates DM symptoms such as water intake and body weight gain while protecting the kidney, pancreas and liver organs in both streptozotocin (STZ)-induced (Suanarunsawata et al. Citation2004; Chang et al. Citation2005; Sumon et al. Citation2008; Ozbayer et al. Citation2011; Saravanan et al. Citation2012; Shivanna et al. Citation2013; Akbarzadeh et al. Citation2015; Assaei et al. Citation2016; Perumal et al. Citation2016; Ahmad and Ahmad Citation2018; Elhassaneen Citation2019) and alloxan-induced DM rat/mouse models (Raskovic et al. Citation2004; Kujur et al. Citation2010; Hossain et al. Citation2011; Misra et al. Citation2011; Sharma et al. Citation2012; Singh et al. Citation2013; Ilić et al. Citation2017), as well as T2D Zucker fatty rats (Dyrskog et al. Citation2005), KKAy mice (Nordentoft et al. Citation2008) and immobilization stress-induced hyperglycemic rabbits (Aghajanyan et al. Citation2017). Besides lowering blood (random and fasting) and urine glucose levels, reducing blood pressure, as well as elevating plasma insulin level and improves biochemical profiles of triglycerides (TG), stevia extracts increases kidney nitric oxide synthase (NOS), liver catalase (CAT), glutathione (GSH) and superoxide dismutase (SOD) activities while normalizing elevated pancreatic lipid peroxidation (Ozbayer et al. Citation2011; Sharma et al. Citation2012; Shivanna et al. Citation2013; Akbarzadeh et al. Citation2015; Assaei et al. Citation2016; Perumal et al. Citation2016). These beneficial effects may result from the regulatory role of stevia and its extracts on key insulin regulatory genes (GLUT2, Ins1, Ins2, Pdx1/Ipf1, Beta2/Neurod1, Pax6 and11-beta-HSD-1) and pancreatic beta-cell transcription factors (Nkx2-2, Nkx6-1, C/EBPalpha and FoxA2) observed in isolated islets of the KKAy mice (Nordentoft et al. Citation2008). On its insulinotropic effect, stevioside modulates insulin release by potentiating the calcium ion-dependent activity of TRPM5 ion channel and augmenting glucose-induced calcium ion oscillations in pancreatic islets (Philippaert et al. Citation2017). Like stevia and its extracts, Rebaudioside A also shows blood glucose-lowering, glucagonostatic and insulinotropic effects on STZ-induced diabetic rats (Saravanan et al. Citation2012). On the contrary, both acute intraperitoneal and chronic oral administration of rebaudiosides B, C, D, dulcoside A and steviolbioside do not exert antihyperglycemic acitivity in intraperitoneal glucose tolerance test (IPGTT) examined on STZ-induced diabetic rats (Aranda-González et al. Citation2016). In the context of the anti-inflammation activity, a higher PPARγ gene expression is reported in aqueous stevia extract-treated STZ-induced diabetic rats (Assaei et al. Citation2016). PPARγ is a glucose metabolism regulator and an established anti-inflammatory player that negatively regulates macrophages and inhibits pro-inflammatory cytokines production (Jiang et al. Citation1998; Reddy et al. Citation2008; Ahmadian et al. Citation2013). One study on non-obese diabetic mice with Sjogren’s syndrome showed that PPARγ treatment significantly ameliorated lymphocytes infiltration and inflammatory responses in salivary glands (Li et al. Citation2014). Stevia too alleviates STZ-induced lymphocyte infiltration in the liver (Perumal et al. Citation2016) which may indicate the involvement of PPARγ pathway in its anti-inflammatory effect. Besides PPARγ activation, PI3K/Akt pathway is also a potential anti-inflammatory mechanism for stevia and its extracts as steviol glycosides induces Glut4 translocation to the plasma membrane by activating the PI3K/Akt pathway then further increase glucose uptake into rat fibroblasts (Prata et al. Citation2017). Along with these findings, researchers have discovered that stevia offers much more benefits than just antihyperglycemic in diabetic patients, particularly in improving wound healing and prevent scar formation (Gans Citation2003; Das Citation2013; Babakhanyan et al. Citation2017; Siraj et al. Citation2019). Individuals suffering from DM often present delayed healing, or even formation of necrotic tissues. Firstly, bacteriostatic properties of stevioside would prevent wound colonization and infection by harmful pathogens, thereby highlighting its potential in regulating immunoresponse and improving vascularity promote wound closure and healing (Babakhanyan et al. Citation2017).
Table 3. Use of stevia leaves and its bioactive compounds in diabetes animal model (N.S., not stated; N.R., not relevant; IVGT, intravenous glucose tolerance; IAGT, intra-arterial glucose tolerance; IPGT, intraperitoneal glucose tolerance; IAGT, intra-arterial glucose tolerance; OGTT, oral glucose tolerance test; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; GSH, reduced glutathione; SOD, superoxide dismutase; TG, triglycerides).
Besides that, there are few reports highlighting the protective and preventive properties of stevia against atherosclerosis, which was formerly considered as a bland lipid storage disease accompanied with the apparent inflammatory response (Ross Citation1999; Libby et al. Citation2002). As stevia can improve serum lipid profiles such as higher high-density lipoprotein (HDL) level and lower total lipids, total cholesterol, triglycerides and low-density lipoprotein (LDL) levels in overweight rats and immobilization stress-induced hyperglycemic rabbits (Elnaga et al. Citation2016; Aghajanyan et al. Citation2017), studies have been carried out to examine its anti-atherogenic effects. Aqueous stevia leaves extract shows its anti-atherogenic effect by inhibiting CuSO4-induced LDL oxidation, which is a part of the pathogenesis of atherosclerosis (Elhassaneen Citation2019). Stevioside (10 mg/kg) administration also reduces plaque volume in the aortic arch of obese insulin-resistant mice by lowering the macrophage, lipid and oxidized LDL content in the plaque (Geeraert et al. Citation2010). Its antioxidant effect on both the adipose tissue and the vascular wall induces plaque stabilization while inhibiting atherosclerotic plaque development in the same study. Doing more good than harm, the stevia plant and its steviol glycosides appear to be an attractive source for anti-inflammatory agent, so as to expand its applications beyond metabolic disorders such as DM and cardiovascular diseases.
More than just sweetener: anti-inflammatory activity of S. rebaudiana
Over the years, inflammation has been recorded as the key player in the development of various human diseases including arthritis, inflammatory bowel disease and atherosclerosis (Shoelson et al. Citation2006; Audial and Bonnotte Citation2015). The development of the natural product, like steviol glycosides, for the prevention of pro-inflammation process, may essentially be developed as a safe, efficient and cost-effective pharmaceutical intervention (Roberts and Munro Citation2009; Auyeung et al. Citation2016). Several studies have indicated the anti-inflammatory properties of stevia and its components using both in vitro and in vivo models (Table ). For instance, numerous groups began their work by studying anti-inflammatory activity of stevia extracts. Few reports showed that hydroalcoholic extract of stevia leaves (500 mg/kg) can prevent inflammation and also reduce oxidative damage in the liver, predominantly via altering the level of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (Holvoet et al. Citation2015; Latha et al. Citation2017). Generally, cytokines are produced as important signaling molecules during physiological and pathological processes by immune cells like macrophages, lymphocytes and various stromal cells. One of the studies showed that stevioside can suppress the secretion of pro-inflammatory cytokines in macrophages after being challenged with lipopolysaccharides in a dose-dependent manner (Fengyang et al. Citation2012). Similar observations were reported by Meng et al. (Citation2018), whereby the group stated that stevioside at 200 µM suppressed titanium particle-induced inflammatory response in bone marrow-derived macrophages and prevented osteolysis in mice treated with titanium particles (with a dose of 10 or 30 mg/kg). Based on their findings, stevioside conferred anti-inflammatory activity via downregulation of two major pathways, mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathways. Similar findings were also reported by another team in Thailand, which explained the stevioside suppressed pro-inflammatory cytokines release of immune cells (i.e. TNF-α, IL-1β and IL-6), and eventually inhibited NF-κB pathways and activated of its inhibitor, IκBα protein (Boonkaewwan et al. Citation2006; Boonkaewwan and Burodom Citation2013). Comparably, stevioside was able to reduce inflammation in a Staphylococcus aureus-infected mouse model via actions on MAPK and NF-κB pathways (Wang et al. Citation2014a, Citation2014b). The research group initially conducted a study on primary mouse mammary epithelial cells (MMECs) infected with S. aureus and discovered that stevioside was able to inhibit the secretion of inflammatory cytokines like TNF-α, IL-1β and IL-6, probably via actions on MAPK and NF-κB pathways. Besides effectively assisting in the treatment of S. aureus-induced mastitis, stevioside was able to prevent cell death of the S. aureus-infected MMECs by downregulating the expression of type 2 Toll-like receptors (TLRs), which is a key player in regulating inflammation and apoptosis (Wang et al. Citation2014a). Taking this information further, Wang and his team have investigated the anti-inflammatory potential of stevioside in the S. aureus-infected mouse mammary gland by administering the bacteria intraperitoneally (Wang et al. Citation2014b). Through this study, it was unveiled that stevioside reduced inflammatory cell infiltration and preserved the histological structure of the mammary glands (e.g. complete lobules, acinus). These findings were consistent with those from primary culture, stevioside was shown to possess protective and anti-inflammatory effects against S. aureus-infected mouse mammary gland, via regulatory actions on TLR2 expression, cytokines and proteins of the NF-κB and MAPK pathways. Collectively, these results suggest that stevia and its important constituent, stevioside prevents inflammation to occur by inhibiting the production of cytokines in immune, stromal and/or epithelial cells via downregulation of MAPK and NF-κB signaling pathways.
Table 4. Anti-inflammation activity of stevioside and related compounds (N.S., not stated; N.R., not relevant; TPA, 12-O-tetradecanoylphorbol-13-acetate; ID50, half maximum inhibitory dose; LPS, lipopolysaccharides; qRT-PCR, quantitative real-time PCR; MPO, Myeloperoxidase; ELISA, enzyme-linked immunosorbent assay; IHC, Immunohistochemistry; Nrf2, nuclear factor (erythroid-derived 2)-like 2; TAA, thioacetamide).
Apart from that, the accumulation of free radicals often causes detrimental effects to the host. Free radicals can modify or damage biological macromolecules such as protein, lipid and DNA. These damages consequently compromise the functioning of crucial processes including DNA repair system, which ultimately leads to mutation or even development of cancer (Pacifici and Davies Citation1991; Bartsch and Nair Citation2006; Klaunig Citation2018). Stevioside has been shown to be able to reduce oxidative stress and inflammation by several mechanism of actions. Ramos-Tovar et al. (Citation2018a) revealed that stevia extract (100 mg/kg) and stevioside (100 mg/kg) can prevent oxidative damage by activating nuclear factor erythroid 2-related factor 2 (Nrf2) response, an important transcription factor that can avert inflammation. Similar findings were reported by a recent study by Casas-Grajales in 2019, in which they demonstrated that rebaudioside A preserved expression of Nrf2 as well as downregulated/prevented the expression of pro-inflammatory pathway and genes such as NF-κB, TGF-β1, Smad7 and MMP-13 protein (Casas-Grajales et al. Citation2019a, Citation2019b).
The idea of crosstalk between Nrf2 antioxidant response and NF-κB-inflammatory response has been proposed few years back (Li et al. Citation2008; Ma Citation2013). It has been speculated that the activators of Nrf2 can hinder IKK/IκB phosphorylation and p65 NF-κB subunit nuclear translocation, and then preventing NF-κB signaling pathway. Based on the proposed theory, stevioside may possibly prevent inflammation (a) via direct actions on NF-κB signaling pathway which then inhibiting the production of pro-inflammatory cytokines, and/or (b) via activating Nrf2 antioxidant response which then inhibits NF-κB signaling pathway (Bunprajun et al. Citation2012; Yingkun et al. Citation2013; Garcia-Arroyo et al. Citation2016; Ramos-Tovar et al. Citation2018a, Citation2018b; Casas-Grajales et al. Citation2019a, Citation2019b).
Potential development of stevioside as chemopreventive agent
Over these years, there has been substantial evidence to support the notion that chronic inflammation can increase cancer risk (Shacter and Weitzman Citation2002). Given that the conventional cancer treatment regime like chemotherapy often come with unwanted side effects (e.g. alopecia, mouth ulceration, nausea and vomiting), researchers are yet to design or find a ‘perfect cure’ that has got high drug specificity, targeting only cancer cells but not normal cells (Chabner and Roberts Citation2005; Chari Citation2007). As a hallmark feature of cancer, inflammation plays an important role in carcinogenesis – from the formation and development of cancer cells (i.e. initiation, progression) to migration to other sites (i.e. metastasis) (Germano et al. Citation2008; Grivennikov et al. Citation2010; Hanahan and Weinberg Citation2011; Arvelo et al. Citation2016). The anti-cancer and antioxidant potential of stevia and its chemical constituents have been suggested by researchers for more than 10 years (Konoshima and Takasaki Citation2002; Yasukawa et al. Citation2002; Mizushina et al. Citation2005; Masuda et al. Citation2006; Bhattacharyya et al. Citation2009; Chen et al. Citation2018). As a high intake of antioxidants can prevent the accumulation of free radicals or oxidative stress generated by injury or even infections, they can essentially decrease cancer risk. Besides diterpenoids like austroinulin and 6-O-acetyl-austroinulin, stevioside as the major component of stevia plant can scavenge free radicals and eliminate cancer cells by altering pathways pivotal for their survival (Paul et al. Citation2012; Cho et al. Citation2013; Yildiz-Ozturk et al. Citation2015). Additionally, some researchers pointed out that rather than just reducing the accumulation of free radicals alone, it may be important to target both oxidative stress and inflammatory processes together to ensure a fruitful therapeutic outcome (Biswas Citation2016; Pillon Barcelos et al. Citation2017). In the case of stevia plant and its metabolites, researchers described their antioxidant activities based on both biochemical and cell-based assays, reducing the accumulation of free radicals by activating antioxidant pathways that drive expression of enzymes such as superoxide dismutase and catalase (Rao et al. Citation2014; Bender et al. Citation2015; Prata et al. Citation2017). Based on these studies, steviol glycosides have been highlighted to have a significantly beneficial role in human health maintenance. That being said, it is truly fascinating that on top of antioxidant activity, stevioside can modulate immune responses and influence recruitment of immune cells, which may be beneficial to ensure the elimination of cancer cells (Boonkaewwan et al. Citation2006; Arango Duque and Descoteaux Citation2014). Boonkaewwan et al. (Citation2006) reported that stevioside suppressed TNF-α, IL-1β and nitric oxide release in monocytes after stimulated with lipopolysaccharide (LPS), whereas those unstimulated monocytes treated with stevioside induced a higher level of TNF-α, IL-1β and nitric oxide. Therefore, the consumption of stevioside may be useful to enhance innate immunity, particularly in augmenting macrophage function (e.g. phagocytic activity) to ensure the eradication of cancer cells (Sehar et al. Citation2008). Taken altogether, stevioside represents an impressive drug candidate which deserves further investigations as the compound can inhibit oxidative stress while modulating immune responses, both processes which are said to be critical players in the development of human chronic diseases.
Future recommendations and direction
Reflecting on the past, it has been over a century since the discovery of S. rebaudiana. Yet, researchers are still trying to study the chemical constituents of the stevia and make use of every molecules that is isolated from the plant. Stevioside has always been in the limelight, owing to its commercial importance as a natural sweetener. Even so, growing evidence reveals that stevioside may possibly be developed as a valuable pharmaceutical agent against inflammation, given that the compound itself does not exhibit any cytotoxicity (Curry and Roberts Citation2008; Tandel Citation2011). Hence, there have been much efforts that has been poured in to ‘improvise’ its chemical structure, in the hope of exploiting it for medicinal purposes. Through acid hydrolysis, stevioside is converted into another beyerane diterpene known as isosteviol. Isosteviol has been shown to possess bioactivities such as antihyperglycemic (Ma et al. Citation2007), anti-inflammatory (Yasukawa et al. Citation2002) and anti-cancer effects (Mizushina et al. Citation2005). However, one of the major flaws of isosteviol is that it has poor water solubility, rendering the drug formulation process complicated. Thus, a research group has successfully generated isosteviol sodium (STVNa) with higher water solubility by adding a sodium group to the lead structure isosteviol, without jeopardizing its bioactive potentials (Sun et al. Citation2018). Similar action as stevioside, STVNa was shown to be able to regulate NF-κB signaling pathway and transforming growth factor (TGF)-β-signaling pathways facilitated by cylindromatosis (CYLD) protein (Lim et al. Citation2012; Tang et al. Citation2018; Zhang et al. Citation2018). For instance, STVNa (8 mg/kg) suppressed oxidative stress and inflammation in the heart of diabetic rodent model (induced by streptozotocin), via inhibitory actions on MAPK and NF-κB pathway (Tang et al. Citation2018). All in all, these findings showed that the derivatization of stevioside and its associated metabolites might be worthwhile for the search of bioactive compounds with potent anti-inflammatory actions.
Despite that, microbial biotransformation may also be another powerful manufacturing tool in the chemical and pharmaceutical industries (Xu et al. Citation2007; Parshikov et al. Citation2012; Hegazy et al. Citation2015). The idea is that, through microbial biotransformation, some naturally occurring inactive compounds may occasionally be transformed into its active form by microbes (Musharraf et al. Citation2010; Parshikov et al. Citation2012). As a green chemistry, this biotransformation method complements with the Sustainability Development Goals proposed by the United Nations (https://www.un.org/sustainabledevelopment/), reducing energy consumption and minimizing and/or eliminating the use of hazardous substances. Akihisa et al. (Citation2004) demonstrated that fungus can be used to modify isosteviol structure, yielding different or new compounds based on the fungus used (e.g. Aspergillus niger, Glomerella cingulate, Mortierella elongate). Using Raji cell models to study the activation of Epstein–Barr virus early antigen, five of the products generated from biotransformation exhibited more potent inhibitory effects (92–96% inhibition rate) than isosteviol and stevioside, while preserving high viability (60%) of Raji cells. Complementary with the use of microbes to generate next-generation sweeteners, this biotransformation process may improve the taste of stevia by diminishing the lingering bitter taste, and simultaneously increase its bioactive potentials for pharmaceutical purposes (Olsson et al. Citation2016).
Along with that, several groups have decided to take a closer look at the mechanisms of anti-inflammatory activity conferred by stevia and steviol glycosides, focusing on the changes in intestinal microbiome following the intake of this natural sugar substitute. Without displaying any obvious toxicity to humans, some studies hinted that the intake of non-caloric sweeteners can affect the microbial population in the gut (Suez et al. Citation2014, Citation2015; Bian et al. Citation2017; Wang et al. Citation2018; Nettleton et al. Citation2019; Vamanu et al. Citation2019). Instead of being absorbed into the bloodstream, these sweeteners may accumulate in the lumen of the gut, causing dysbiosis or imbalance in the gut microbiome. Stevia extract containing steviol glycosides is able to kill pathogens like Escherichia coli O157:H7, but not probiotics Bifidobacterium and Lactobacillus (Tomita et al. Citation1997). In gastrointestinal diseases like inflammatory bowel disease, stevioside has been shown to be capable of ameliorating inflammatory symptoms, while preserving histo-architecture of the mice colon (Alavala et al. Citation2019), which could be attributed to the restoration of the gut microbiome or via regulation of their metabolites (Atteh et al. Citation2008). In spite of that, a study in Romania by Vamanu et al. (Citation2019) investigated the changes in microbiome and metabolomics after exposed several sweeteners using an in vitro static system (GIS1 model), including three forms of commercially purchased steviol (i.e. powder, tablet or combination with brown sugar). The team noted mixed findings; even though the total number of bacterial cells did not change, steviol capsules significantly reduced Gram-negative strains and Bifidobacteria, while steviol powder and steviol containing brown sugar increased the number of Bifidobacteria. Undeniably, the authors highlighted the probable interference by carrier ingredients such as sodium bicarbonate. These results then urge further research to unravel potential anti-inflammatory action of stevia acting via regulation of gut microbiota.
Collectively, there have been much research work revolving identification of chemical constituents in stevia plant, as well as exploring their potential as a natural sweetener. Lest we ignore that as a natural source of anti-inflammatory products with low toxicity, the continuous efforts on stevia studies prove that it is more than just a sweetener and warrants it for further investigation to be developed as valuable pharmaceutical or therapeutic agents against important human diseases like cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Aghajanyan A, Movsisyan Z, Trchounian A. 2017. Antihyperglycemic and antihyperlipidemic activity of hydroponic Stevia rebaudiana aqueous extract in hyperglycemia induced by immobilization stress in rabbits. Biomed Res Int. 2017:9251358.
- Ahmad U, Ahmad RS. 2018. Antidiabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complement Altern Med. 18(1):179.
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. 2013. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 19(5):557–566.
- Akbarzadeh S, Eskandari F, Tangestani H, Bagherinejad ST, Bargahi A, Bazzi P, Daneshi A, Sahrapoor A, O'Connor WJ, Rahbar AR. 2015. The effect of Stevia rebaudiana on serum omentin and visfatin level in STZ-induced diabetic rats. J Diet Suppl. 12(1):11–22.
- Akihisa T, Hamasaki Y, Tokuda HM, Kimura Y, Nishino H. 2004. Microbial transformation of isosteviol and inhibitory effects on Epstein-Barr virus activation of the transformation products. J Nat Prod. 67(3):407–410.
- Al-Kharashi AS. 2018. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol. 32(4):318–323.
- Alavala S, Sangaraju R, Nalban N, Sahu BD, Jerald MK, Kilari EK, Sistla R. 2019. Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against dextran sulphate sodium-induced ulcerative colitis in mice. Eur J Pharmacol. 855:192–201.
- Ameer K, Chun BS, Kwon JH. 2017. Optimization of supercritical fluid extraction of steviol glycosides and total phenolic content from Stevia rebaudiana (Bertoni) leaves using response surface methodology and artificial neural network modeling. Ind Crops Prod. 109:672–685.
- Aranda-González I, Moguel-Ordoñez Y, Betancur-Ancona D. 2015. Validation of HPLC-UV method for determination of minor glycosides contained in Stevia rebaudiana Bertoni leaves. Biomed Chromatogr. 29(5):733–738.
- Aranda-González I, Moguel-Ordóñez Y, Chel-Guerrero L, Segura-Campos M, Betancur-Ancona D. 2016. Evaluation of the antihyperglycemic effect of minor steviol glycosides in normoglycemic and induced-diabetic Wistar rats. J Med Food. 19(9):844–852.
- Arango Duque G, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 5:491.
- Arvelo F, Sojo F, Cotte C. 2016. Tumour progression and metastasis. Ecancermedicalscience. 10:617.
- Ashwell M. 2015. Stevia, nature's zero-calorie sustainable sweetener: a new player in the fight against obesity. Nutr Today. 50(3):129–134.
- Assaei R, Mokarram P, Dastghaib S, Darbandi S, Darbandi M, Zal F, Akmali M, Ranjbar Omrani GH. 2016. Hypoglycemic effect of aquatic extract of stevia in pancreas of diabetic rats: PPARγ-dependent regulation or antioxidant potential. Avicenna J Med Biotechnol. 8(2):65–74.
- Atteh J, Onagbesan O, Tona K, Decuypere E, Geuns J, Buyse J. 2008. Evaluation of supplementary Stevia (Stevia rebaudiana Bertoni) leaves and stevioside in broiler diets: effects on feed intake, nutrient metabolism, blood parameters and growth performance. J Anim Physiol Anim Nutr. 92:640–649.
- Audial S, Bonnotte B. 2015. Inflammation. Rev Prat. 65(3):403–408.
- Auyeung KK, Han QB, Ko JK. 2016. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 44(1):1–22.
- Babakhanyan MA, Nahapetyan K, Hovhannisyan LE, Simonyan KV, Avetisyan LG, Avetisyan RA, Chavushyan VA. 2017. Wound healing potential of hydroponic Stevia rebaudiana in rats. Phys Med Rehab Res. 2(3):1–4.
- Barba FJ, Grimi N, Vorobiev E. 2015a. Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. J Food Eng. 149:222–228.
- Barba FJ, Koubaa M, Grimi N, Vorobiev E. 2017. Selective extraction of biocompounds from Stevia rebaudiana Bertoni leaves using electrotechnologies. In: Miklavcic Damijan, editor. Handbook of electroporation. Cham, Switzerland: Springer; p. 2751–2761. doi:https://doi.org/10.1007/978-3-319-26779-1_123-1.
- Barba FJ, Parniakov O, Pereira SA, Wiktor A, Grimi N, Boussetta N, Lebovka N. 2015b. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res Int. 77:773–798.
- Bartsch H, Nair J. 2006. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 391(5):499–510.
- Bender C, Graziano S, Zimmermann BF. 2015. Study of Stevia rebaudiana Bertoni antioxidant activities and cellular properties. Int J Food Sci Nut. 66(5):553–558.
- Bhattacharyya D, Ghanta S, Banerjee A, Chattopadhyay S. 2009. Stevia rebaudiana, a novel source of phytoceuticals with anticancer potential. Planta Med. 75(09):SL46.
- Bian X, Tu P, Chi L, Gao B, Ru H, Lu K. 2017. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem Toxicol. 107:530–539.
- Biswas SK. 2016. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Ox Med Cell Long. 2016:5698931.
- Boonkaewwan C, Burodom A. 2013. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J Sci Food Agric. 93(15):3820–3825.
- Boonkaewwan C, Toskulkao C, Vongsakul M. 2006. Anti-inflammatory and immunomodulatory activities of stevioside and its metabolite steviol on THP-1 cells. J Agric Food Chem. 54(3):785–789.
- Bovanová L, Brandšteterov E, Baxa S. 1998. HPLC determination of stevioside in plant material and food samples. Z Lebensm Unters Forsch. A. 207(5):352–355.
- Brahmachari G, Mandal LC, Roy R, Mondal S, Brahmachari AK. 2011. Stevioside and related compounds–molecules of pharmaceutical promise: a critical overview. Archiv Der Pharmazie. 344(1):5–19.
- Bunprajun T, Yimlamai T, Soodvilai S, Muanprasat C, Chatsudthipong V. 2012. Stevioside enhances satellite cell activation by inhibiting of NF-κB signaling pathway in regenerating muscle after cardiotoxin-induced injury. J Agric Food Chem. 60(11):2844–2851.
- Carbonell-Capella JM, Šic Žlabur J, Rimac Brnčić S, Barba FJ, Grimi N, Koubaa M, Vorobiev E. 2017. Electrotechnologies, microwaves, and ultrasounds combined with binary mixtures of ethanol and water to extract steviol glycosides and antioxidant compounds from Stevia rebaudiana leaves. J Food Process Preserv. 41(5):e13179.
- Care D. 2006. Diagnosis and classification of diabetes mellitus. Diabetes Care. 37(Suppl 1):S81–S90.
- Casas-Grajales S, Ramos-Tovar E, Chávez-Estrada E, Alvarez-Suarez D, Hernández-Aquino E, Reyes-Gordillo K, Cerda-García-Rojas CM, Camacho J, Tsutsumi V, Lakshman MR, et al. 2019a. Antioxidant and immunomodulatory activity induced by stevioside in liver damage: in vivo, in vitro and in silico assays. Life Sci. 224:187–196.
- Casas-Grajales S, Reyes-Gordillo K, Cerda-García-Rojas CM, Tsutsumi V, Lakshman MR, Muriel P. 2019b. Rebaudioside A administration prevents experimental liver fibrosis: an in vivo and in vitro study of the mechanisms of action involved. J Appl Toxicol. 39(8):1118–1131.
- Chabner BA, Roberts TG. 2005. Chemotherapy and the war on cancer. Nat Rev Cancer. 5(1):65–72.
- Chang JC, Wu MC, Liu IM, Cheng JT. 2005. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Horm Metab Res. 37(10):610–616.
- Chari RV. 2007. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 41:98–107.
- Chatsudthipong V, Muanprasat C. 2009. Stevioside and related compounds: therapeutic benefits beyond sweetness. Pharmacol Ther. 121(1):41–54.
- Chen J, Xia Y, Sui X, Peng Q, Zhang T, Li J, Zhang J. 2018. Steviol, a natural product inhibits proliferation of the gastrointestinal cancer cells intensively. Oncotarget. 9(41):26299–26308.
- Cho BO, Ryu HW, So Y, Cho JK, Woo HS, Jin CH, Seo KI, Park JC, Jeong IY. 2013. Anti-inflammatory effect of austroinulin and 6-O-acetyl-austroinulin from Stevia rebaudiana in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem Toxicol. 62:638–644.
- Choi YH, Kim I, Yoon KD, Lee SJ, Kim CY, Yoo KP, Choi YH, Kim J. 2002. Supercritical fluid extraction and liquid chromatographic-electrospray mass spectrometric analysis of stevioside from Stevia rebaudiana leaves. Chromatographia. 55(9–10):617–620.
- Curry LL, Roberts A. 2008. Subchronic toxicity of rebaudioside A. Food Chem Toxicol. 46(7):S11–S20.
- Das K. 2013. Wound healing potential of aqueous crude extract of Stevia rebaudiana in mice. Rev Saude Publica. 23(2):351–357.
- Debnath M, Ashwath N, Hill CB, Callahan DL, Dias DA, Jayasinghe NS, Midmore DJ, Roessner U. 2018. Comparative metabolic and ionomic profiling of two cultivars of Stevia rebaudiana Bert. (Bertoni) grown under salinity stress. Plant Physiol Biochem. 129:56–70.
- Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G. 2003. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities’ study. Diabetes. 52:1799–1805.
- Dyrskog SE, Jeppesen PB, Colombo M, Abudula R, Hermansen K. 2005. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism. 54(9):1181–1188.
- [EFSA ANS Panel] EFSA Panel on Food Additives and Nutrient Sources Added to Food. 2015. Scientific opinion on the safety of the proposed amendment of the specifications for steviol glycosides (E 960) as a food additive. EFSA J. 13(12):4316.
- Elhassaneen YA. 2019. Stevia (Stevia rebaudiana) leaves: chemical composition, bioactive compounds, antioxidant activities, antihyperglycemic and antiatherogenic effects. J Stud Search Spec Edu. 5(1):157–180.
- Elnaga NA, Massoud MI, Yousef M I, Mohamed HH. 2016. Effect of stevia sweetener consumption as non-caloric sweetening on body weight gain and biochemical’s parameters in overweight female rats. Ann Agric Sci. 61(1):155–163.
- Eriko K, Norifumi S, Yuji O, Ken K, Osamu I, Kunio K, Fujino A, Ui M. 2003. Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem Toxicol. 41(6):875–883.
- Erkucuk A, Akgun IH, Yesil-Celiktas O. 2009. Supercritical CO2 extraction of glycosides from Stevia rebaudiana leaves: identification and optimization. J Supercrit Fluids. 51(1):29–35.
- Fengyang L, Yunhe F, Bo L, Zhicheng L, Depeng L, Dejie L, Wen Z, Yongguo C, Naisheng Z, Xichen Z, et al. 2012. Stevioside suppressed inflammatory cytokine secretion by downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation. 35(5):1669–1675.
- Ferrazzano GF, Cantile T, Alcidi B, Coda M, Ingenito A, Zarrelli A, Di Fabio G, Pollio A. 2015. Is Stevia rebaudiana Bertoni a noncariogenic sweetener? A review. Molecules. 21(1):E38.
- Formigoni M, Milani PG, da Silva Avíncola A, Dos Santos VJ, Benossi L, Dacome AS, Pilau EJ, da Costa SC. 2018. Pretreatment with ethanol as an alternative to improve steviol glycosides extraction and purification from a new variety of stevia. Food Chem. 241:452–459.
- Gallo M, Formato A, Formato G, Naviglio D. 2018. Comparison between two solid-liquid extraction methods for the recovery of steviol glycosides from dried stevia leaves applying a numerical approach. Processes. 6(8):105.
- Gallo M, Vitulano M, Andolfi A, DellaGreca M, Conte E, Ciaravolo M, Naviglio D. 2017. Rapid solid-liquid dynamic extraction (RSLDE): a new rapid and greener method for extracting two steviol glycosides (stevioside and rebaudioside A) from stevia leaves. Plant Foods Hum Nutr. 72(2):141–148.
- Gans A. 2003 Oct 20. Method for treating wounds to promote healing. United States patent US 7,318,934.
- Garcia-Arroyo FE, Cristobal M, Arellano-Buendia AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa MA, Roncal-Jiménez C, Bankir L, et al. 2016. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol. 311(1):R57–R65.
- Gardana C, Simonetti P. 2018. Determination of steviol glycosides in commercial extracts of Stevia rebaudiana and sweeteners by ultra-high-performance liquid chromatography Orbitrap mass spectrometry. J Chromatogr A. 1578:8–14.
- Gasmalla M, Yang R, Amadou I, Hua X. 2014. Nutritional composition of Stevia rebaudiana Bertoni leaf: effect of drying method. Trop J Pharm Res. 13(1):61–65.
- Geeraert B, Crombé F, Hulsmans M, Benhabilès N, Geuns JM, Holvoet P. 2010. Stevioside inhibits atherosclerosis by improving insulin signaling and antioxidant defense in obese insulin-resistant mice. Int J Obes. 34(3):569–577.
- Germano G, Allavena P, Mantovani A. 2008. Cytokines as a key component of cancer-related inflammation. Cytokine. 43(3):374–379.
- Geuns JM. 2003. Stevioside. Phytochem. 64(5):913–921.
- Geuns JM, Buyse J, Vankeirsbilck A, Temme EH. 2007. Metabolism of stevioside by healthy subjects. Eur J Clin Invest. 232(1):164–173.
- Gomes EN, Moterle D, Biasi LA, Koehler HS, Kanis LA, Deschamps C. 2018. Plant densities and harvesting times on productive and physiological aspects of Stevia rebaudiana Bertoni grown in southern Brazil. An Acad Bras Cienc. 90(4):3249–3264.
- Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. 2004. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 53(1):73–76.
- Grivennikov SI, Greten FR, Karin M. 2010. Immunity, inflammation, and cancer. Cell. 140(6):883–899.
- Gutiérrez DG, Muñoz-Schick M, Grossi MA, Rodríguezcravero JF, Morales V, Moreira-Muñoz A. 2016. The genus Stevia (Eupatorieae, Asteraceae) in Chile: a taxonomical and morphological analysis. Phytotaxa. 282(1):001–018.
- Hagiwara A, Fukushima S, Kitaori M, Shibata M, Ito N. 1984. Effects of three sweeteners on rat urinary bladder carcinogenesis initiated by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Gan. 75(9):763–768.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–674.
- Hegazy ME, Mohamed TA, ElShamy AI, Mohamed AE, Mahalel UA, Reda EH, Shaheen AM, Tawfik WA, Shahat AA, Shams KA, et al. 2015. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway: a review. J Adv Res. 6(1):17–33.
- Holvoet P, Rull A, Garcia-Heredia A, Lopez-Sanroma S, Geeraert B, Joven J, Camps J. 2015. Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: a transcriptomic and metabolomic study. Food Chem Toxicol. 77:22–33.
- Hossain MS, Alam MB, Asadujjaman M, Islam MM, Rahman MA, Islam MA, Islam A. 2011. Antihyperglycemic and antihyperlipidemic effects of different fractions of Stevia rebaudiana leaves in alloxan induced diabetic rats. Int J Pharm Sci Res. 2(7):1722.
- Hossain F, Islam MT, Islam MA, Akhta S. 2017. Cultivation and uses of Stevia (Stevia rebaudiana Bertoni): a review. Afr J Food Agri Nut Dev. 17(04):12745–12757.
- Hotamisligil GS, Shargill NS, Spiegelman BM. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259(5091):87–91.
- Ijaz M, Pirzada AM, Saqib M, Latif M. 2015. Stevia rebaudiana: an alternative sugar crop in Pakistan–a review. J Med Spice Plants. 20(2):88–96.
- Ilić V, Vukmirović S, Stilinović N, Čapo I, Arsenović M, Milijašević B. 2017. Insight into anti-diabetic effect of low dose of stevioside. Biomed Pharmacother. 90:216–221.
- Jaitak V, Bikram Singh B, Kaul VK. 2009. An efficient microwave-assisted extraction process of stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni). Phytochem Anal. 20(3):240–245.
- Javad S, Naz S, Ilyas S, Tariq A, Aslam F. 2014. Optimization of the microwave assisted extraction and its comparison with different conventional extraction methods for isolation of stevioside from Stevia rebaudiana. Asian J Chem. 26(23):8043–8048.
- [JECFA] Joint FAO/WHO Expert Committee on Food Additives, Compendium of Food Additive Specifications: Joint FAO/WHO Expert Committee on Food Additives, 69th Meeting. 2008. Compendium of food additive specifications. FAO Food Nutr Pap. 52(2):783–1589.
- Jeppesen PB, Dyrskog SE, Agger A, Gregersen S, Colombo M, Xiao J, Hermansen K. 2006. Can stevioside in combination with a soy-based dietary supplement be a new useful treatment of type 2 diabetes? An in vivo study in the diabetic Goto-Kakizaki rat. Rev Diabet Stud. 3(4):189–199.
- Jeppesen PB, Gregersen S, Alstrup KK, Hermansen K. 2002. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effects in vivo: studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine. 9(1):9–14.
- Jeppesen P. B., Gregersen S., Rolfsen S. E., Jepsen M., Colombo M., Agger A., Xiao J., Kruhøffer M., Ørntoft T., Hermansen K. 2003. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism. 52(3):372–378.
- Jiang C, Ting AT, Seed B. 1998. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 391(6662):82–86.
- Kienle U. 1990. Method of making a natural sweetener based on Stevia rebaudiana, and use thereof. United States patent US 5,112,610.
- Kinghorn AD. 2002. Stevia: the genus Stevia. London, UK: Taylor & Francis.
- Kinghorn AD, Soejarto DD. 1985. Current status of stevioside as a sweetening agent for human use. In: Wagner H, Hikino H, Farnsworth NR, editors. Progress in economic and medicinal plant research. Vol. 1. London: Academic Press; p. 1–52.
- Klaunig JE. 2018. Oxidative stress and cancer. Curr Pharm Des. 24(40):4771–4778.
- Konoshima T, Takasaki M. 2002. Cancer-chemopreventive effects of natural sweeteners and related compounds. Pure Appl Chem. 74(7):1309–1316.
- Kovačević BD, Barba FJ, Granato D, Galanakis CM, Herceg Z, Dragović-Uzelac V, Putnik P. 2018. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 254:150–157.
- Kovylyaeva GI, Bakaleinik GA, Strokina IY, Gubskaya VI, Sharipova RR, Alfonsov VA, Kataev VE, Tolstikov AG. 2007. Glycosides from Stevia rebaudiana. Chem Nat Compd. 43(1):81–85.
- Koyama E, Kitazawa K, Ohori Y, Izawa O, Kakegawa K, Fujino A, Ui M. 2003. In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food Chem Toxicol. 41(3):359–374.
- Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, Roy BK. 2010. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacognosy Res. 2(4):258–263.
- Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, Gupta SC, Bharti AC, Aggarwal BB. 2018. Chronic diseases, inflammation, and spices: how are they linked? J Transl Med. 16(1):14.
- Latha S, Chaudhary S, Ray SR. 2017. Hydroalcoholic extract of Stevia rebaudiana bert. leaves and stevioside ameliorates lipopolysaccharide induced acute liver injury in rats. Biomed Pharmacother. 95:1040–1050.
- Lemus-Mondaca R, Ah-Hen K, Vega-Gálvez A, Honores C, Moraga NO. 2016. Stevia rebaudiana leaves: effect of drying process temperature on bioactive components, antioxidant capacity and natural sweeteners. Plant Foods Hum Nutr. 71(1):49–56.
- Lemus-Mondaca R, Vega-Gálvez A, Rojas P, Stucken K, Delporte C, Valenzuela-Barra G, Pasten A. 2018. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: effect of different drying methods. J Appl Res Med Aroma Plants. 11:37–46.
- Lewis WH. 1992. Early uses of Stevia rebaudiana (Asteraceae) leaves as a sweetener in Paraguay. Eco Bot. 46(3):336–337.
- Li J, Chen Z, Di D. 2012. Preparative separation and purification of rebaudioside A from Stevia rebaudiana Bertoni crude extracts by mixed bed of macroporous adsorption resins. Food Chem. 132(1):268–276.
- Li Z, Fan Y, Xi J. 2019. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 277:246–260.
- Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. 2008. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 76(11):1485–1489.
- Li CL, Tan LH, Wang YF, Luo CD, Chen HB, Lu Q, Li YC, Yang XB, Chen JN, Liu YH, et al. 2019. Comparison of anti-inflammatory effects of berberine, and its natural oxidative and reduced derivatives from Rhizoma Coptidis in vitro and in vivo. Phytomedicine. 52:272–283.
- Li W, Zhang S, Liu H, Wang L, Zhang C, Leng J, Yu Z, Yang X, Tian H, Hu G. 2014. Different associations of diabetes with beta-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care. 37(9):2533–2539.
- Libby P, Ridker PM, Maseri A. 2002. Inflammation and atherosclerosis. Circulation. 105(9):1135–1143.
- Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, Matsuno T, Xu X, Huang Y, Zhang W, et al. 2012. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat Commun. 3(1):771.
- Liu Q, Hu H, Hu T, Han T, Wang A, Huang L, Tan Q, Tan W. 2018. STVNa attenuates right ventricle hypertrophy and pulmonary artery remodeling in rats induced by transverse aortic constriction. Biomed Pharmacother. 101:371–378.
- Liu J, Li JW, Tang J. 2010. Ultrasonically assisted extraction of total carbohydrates from Stevia rebaudiana Bertoni and identification of extracts. Food Bioprod Process. 88(2–3):215–221.
- Lohner V, Brookes RL, Hollocks MJ, Morris RG, Markus HS. 2017. Apathy, but not depression, is associated with executive dysfunction in cerebral small vessel disease. PLoS ONE. 12:0176943.
- López V, Pérez S, Vinuesa A, Zorzetto C, Abian O. 2017. Stevia rebaudiana ethanolic extract exerts better antioxidant properties and antiproliferative effects in tumour cells than its diterpene glycoside stevioside. Food Funct. 7(4):2107–2113.
- Ma Q. 2013. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 53:401–426.
- Ma J, Ma Z, Wang J, Milne RW, Xu D, Davey AK, Evans AM. 2007. Isosteviol reduces plasma glucose levels in the intravenous glucose tolerance test in Zucker diabetic fatty rats. Diabetes Obes Metab. 9(4):597–599.
- Marques-Vidal P, Schmid R, Bochud M, Bastardot F, Von Känel R, Paccaud F, Glaus J, Preisig M, Waeber G, Vollenweider P. 2012. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PLoS ONE. 7(12):e51768.
- Martyn D, Darch M, Roberts A, Lee HY, Yaqiong Tian T, Kaburagi N, Belmar P. 2018. Low-/No-Calorie sweeteners: a review of global intakes. Nutrients. 10(3):357.
- Masuda T, Yamashita D, Maekawa T, Sone Y, Yamaguchi H, Takeda Y, Yamana T. 2006. Identification of antioxidative compounds from Stevia (Stevia rebaudiana). Nippon Shokuhin Kagaku kaogaku kaishi. 53(12):597–602.
- Medon PJ, Pezzuto JM, Hovanecbrown J, Nanayakkara NPD, Soejarto DD, Kamath SK, Kinghoen AD. 1982. Safety assessment of some Stevia rebaudiana sweet principles. Fed Proc. 41(5):1568–1568.
- Medzhitov R. 2010. Inflammation 2010: new adventures of an old flame. Cell. 140(6):771–776.
- Meng J, Zhou CH, Hu B, Luo M, Wu H, Yang Y, Wang W, Jiang G, Hong J, Li S, et al. 2018. Stevioside prevents wear particle-induced osteolysis by inhibiting osteoclastogenesis and inflammatory response via the suppression of TAK1 activation. Front Pharmacol. 9:1053.
- Misra H, Soni M, Silawat N, Mehta D, Mehta BK, Jain DC. 2011. Antidiabetic activity of medium-polar extract from the leaves of Stevia rebaudiana Bert (Bertoni) on alloxan-induced diabetic rats. J Pharm Bioallied Sci. 3(2):242.
- Mizushina Y, Akihisa T, Ukiya M, Hamasaki Y, Murakami-Nakai C, Kuriyama I, Takeuchi T, Sugawara F, Yoshida H. 2005. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life Sci. 77(17):2127–2140.
- Momtazi-Borojeni AA, Esmaeili SA, Abdollahi E, Sahebkar A. 2017. A review on the pharmacology and toxicology of steviol glycosides extracted from Stevia rebaudiana. Curr Pharm Des. 23(11):1616–1622.
- Musharraf SG, Najeeb A, Khan S, Pervez M, Ali RA, Choudhary MI. 2010. Microbial transformation of 5α-hydroxycaryophylla-4 (12), 8(13)-diene with Macrophomina phaseolina. J Mol Ca B: Enzymatic. 66(1–2):156–160.
- Naviglio D, Scarano P, Ciaravolo M, Gallo M. 2019. Rapid solid-liquid dynamic extraction (RSLDE): a powerful and greener alternative to the latest solid-liquid extraction techniques. Foods. 8(7):245.
- Nettleton JE, Klancic T, Schick A, Choo AC, Shearer J, Borgland SL, Chleilat F, Mayengbam S, Reimer RA. 2019. Low-dose stevia (Rebaudioside A) consumption perturbs gut microbiota and the mesolimbic dopamine reward system. Nutrients. 11(6):1248.
- Nordentoft I, Jeppesen PB, Hong J, Abudula R, Hermansen K. 2008. Isosteviol increases insulin sensitivity and changes gene expression of key insulin regulatory genes and transcription factors in islets of the diabetic KKAy mouse. Diabetes Obes Metab. 10(10):939–949.
- Ohtani K, Yamasaki K. 2004. Methods to improve the taste of the sweet principles of Stevia rebaudiana. Cheminform. 35(17):1–10.
- Olsson K, Carlsen S, Semmler A, Simón E, Mikkelsen MD, Møller BL. 2016. Microbial production of next-generation stevia sweeteners. Micro Cell Fact. 15(1):207.
- Ozbayer C, Kurt H, Kalender S, Ozden H, Gunes HV, Basaran A, Cakmak EA, Civi K, Kalender Y, Degirmenci I. 2011. Effects of Stevia rebaudiana (Bertoni) extract and N-nitro-L-arginine on renal function and ultrastructure of kidney cells in experimental type 2 diabetes. J Med Food. 14(10):1215–1222.
- Ozdemir C, Arslaner A, Ozdemir S, Allahyari M. 2015. The production of ice cream using stevia as a sweetener. J Food Sci Technol. 52(11):1–4.
- Pacifici RE, Davies KJA. 1991. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 37(1–3):166–180.
- Parshikov IA, Netrusov AI, Sutherland JB. 2012. Microbial transformation of antimalarial terpenoids. Biotechnol Adv. 30(6):1516–1523.
- Pasquel A, Meireles MAA, Marques MOM, Petenate AJ. 2000. Extraction of stevia glycosides with CO2 + water, CO2 + ethanol, and CO2 + water + ethanol. Braz J Chem Eng. 17(3):271–282.
- Paul S, Sengupta S, Bandyopadhyay TK, Bhattacharyya A. 2012. Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutr Cancer. 64(7):1087–1094.
- Periche A, Castelló ML, Heredia A, Escriche I. 2015. Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves. Food Chem. 172:1–6.
- Perrier JD, Mihalov JJ, Carlson SJ. 2018. FDA regulatory approach to steviol glycosides. Food Chem Toxicol. 122:132–142.
- Perumal V, Manickam T, Bang KS, Velmurugan P, Oh BT. 2016. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int J Biol Macromol. 92:63–69.
- Philippaert K, Pironet A, Mesuere M, Sones W, Vermeiren L, Kerselaers S, Pinto S, Segal A, Antoine N, Gysemans C, et al. 2017. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 8:14733.
- Phillips KC. 1987. Stevia: steps in developing a new sweetener. In: Grenby TH, editor. Developments in sweeteners -3. London: Elsevier Applied Science; p. 1-43.
- Pillon Barcelos R, Freire Royes LF, Gonzalez-Gallego J, Bresciani G. 2017. Oxidative stress and inflammation: liver responses and adaptations to acute and regular exercise. Free Rad Res. 51(2):222–236.
- Pól J, Varadová Ostrá E, Karásek P, Roth M, Benesová K, Kotlaríková P, et al. 2007. Comparison of two different solvents employed for pressurised fluid extraction of stevioside from Stevia rebaudiana: methanol versus water. Anal Bioanal Chem. 388(8):1847–1857.
- Prakash I, Dubois GE, Clos JF, Wilkens KL, Fosdick LE. 2008. Development of rebiana, a natural, non-caloric sweetener. Food Chem Toxicol. 46(7):S75–S82.
- Prata C, Zambonin L, Rizzo B, Maraldi T, Angeloni C, Vieceli Dalla Sega F, Fiorentini D, Hrelia S. 2017. Glycosides from Stevia rebaudiana Bertoni possess insulin-mimetic and antioxidant activities in rat cardiac fibroblasts. Ox Med Cell Long. 2017:3724545.
- Ramesh K, Singh V, Megeji NW. 2006. Cultivation of Stevia [Stevia rebaudiana (Bert.) Bertoni]: a comprehensive review. Adv Agro. 89(05):137–177.
- Ramos-Tovar E, Flores-Beltrán RE, Galindo-Gómez S, Vera-Aguilar E, Diaz-Ruiz A, Montes S, Camacho J, Tsutsumi V, Muriel P. 2018a. Stevia rebaudiana tea prevents experimental cirrhosis via regulation of NF-kappaB, Nrf2, transforming growth factor beta, Smad7, and hepatic stellate cell activation. Phytother Res. 32(12):2568–2576.
- Ramos-Tovar E, Hernández-Aquino E, Casas-Grajales S, Buendia-Montano LD, Galindo-Gomez S, Camacho J, Tsutsumi V, Muriel P. 2018b. Stevia prevents acute and chronic liver injury induced by carbon tetrachloride by blocking oxidative stress through nrf2 upregulation. Oxid Med Cell Longev. 2018:3823426.
- Rao GN, Rao PP, Balaswamy K, Satyanarayama A. 2014. Antioxidant activity of stevia (Stevia rebaudiana L.) leaf powder and a commercial stevioside powder. J Food Pharmaceut Sci. 2(2):32–38.
- Raskovic A, Gavrilovic M, Jakovljevic V, Sabo J. 2004. Glucose concentration in the blood of intact and alloxan-treated mice after pretreatment with commercial preparations of Stevia rebaudiana (Bertoni). Eur J Drug Metab Pharmacokinet. 29(2):87–90.
- Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, Samuel C. 2008. Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res. 14:2978–2987.
- Roberts A, Munro I. 2009. Stevioside and related compounds: therapeutic benefits beyond sweetness. Pharmacol Ther. 122(3):e1–2.
- Roberts A, Renwick AG. 2008. Comparative toxicokinetics and metabolism of rebaudioside A, stevioside, and steviol in rats. Food Chem Tox. 46(7):S31–S39.
- Rojas E, Bermudez V, Motlaghzadeh Y, Mathew J, Fidilio E, Faria J, Rojas J, de Bravo MC, Contreras J, Mantilla LP, et al. 2018. Stevia rebaudiana Bertoni and its effects in human disease: emphasizing its role in inflammation, atherosclerosis and metabolic syndrome. Curr Nutr Rep. 7(3):161–170.
- Ross R. 1999. Atherosclerosis – an inflammatory disease. N Engl J Med. 340(2):115–126.
- Ruiz-Ruiz JC, Moguel-Ordonez YB, Segura-Campos MR. 2017. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit Rev Food Sci Nutr. 57(12):2680–2690.
- Saravanan R, Vengatash babu K, Ramachandran V. 2012. Effect of Rebaudioside A, a diterpenoid on glucose homeostasis in STZ-induced diabetic rats. J Physiol Biochem. 68(3):421–431.
- Sehar I, Kaul A, Bani S, Pal HC, Saxena AK. 2008. Immune up regulatory response of a non-caloric natural sweetener, stevioside. Chem Biol Interact. 173(2):115–121.
- Shacter E, Weitzman SA. 2002. Chronic inflammation and cancer. Oncology. 16(2):217–229.
- Sharma M, Thakral NK, Thakral S. 2009. Chemistry and in vivo profile of ent-kaurene glycosides of Stevia rebaudiana Bertoni – an overview. Nat Prod Rad. 8(2):181–189.
- Sharma R, Yadav R, Manivannan E. 2012. Study of effect of Stevia rebaudiana bertoni on oxidative stress in type-2 diabetic rat models. Biomed Aging Pathol. 2(3):126–131.
- Shibata H, Sawa Y, Oka T, Sonoke S, Kim KK, Yoshioka M. 1995. Steviol and steviol-glycoside: glucosyltransferase activities in Stevia rebaudiana Bertoni-purification and partial characterization. Arch Biochem Biophys. 321(2):390–396.
- Shivanna N, Naika M, Khanum F, Kaul VK. 2013. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J Diabetes Complications. 27(2):103–113.
- Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest. 116(7):1793–1801.
- Singh S, Garg V, Yadav D. 2013. Antihyperglycemic and antioxidative ability of Stevia rebaudiana (Bertoni) leaves in diabetes induced mice. Int J Pharm Pharm Sci. 5(2):297–302.
- Singh SD, Rao GP. 2005. Stevia: the herbal sugar of 21st century. Sugar Tech. 7(1):17–24.
- Siraj ES, Pushpanjali K, Manoranjitha BS. 2019. Efficacy of stevioside sweetener on pH of plaque among young adults. Dent Res J. 16(2):104–109.
- Soejarto DD, Compadre CM, Medon PJ, Kamath SK, Kinghorn AD. 1983. Potential sweetening agents of plant origin. II. field search for sweet-tasting Stevia species. Econ Bot. 37(1):71–79.
- Suanarunsawata T, Klongpanichapaka S, Rungseesantivanona S, Chaiyabutrb N. 2004. Glycemic effect of stevioside and Stevia rebaudiana in streptozotocin-induced diabetic rats. East J Med. 9(2):51–56.
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. 2014. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 514(7521):181–186.
- Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. 2015. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes. 6(2):149–155.
- Sumon MH, Mostofa M, Jahan MS, Kayesh M, Haque MA. 2008. Comparative efficacy of powdered form of stevia (Stevia rebaudiana Bertoni) leaves and glimepiride in induced diabetic rats. Bangl J Vet Med. 6(2):211–215.
- Sun X, Yang Y, Xie Y, Shi X, Huang L, Tan W. 2018. Protective role of STVNa in myocardial ischemia reperfusion injury by inhibiting mitochondrial fission. Oncotarget. 9(2):1898–1905.
- Suttajit M, Vinitketkaumnuen U, Meevatee U, Buddhasukh D. 1993. Mutagenicity and human chromosomal effect of stevioside, a sweetener from Stevia rebaudiana Bertoni. Environ Health Perspect. 101(Suppl 3):53–56.
- Tan S, Shibuta Y, Tanaka O. 1988. Isolation of sweetener from Stevia rebaudiana. Jpn Kokai. 63(177):764.
- Tandel KR. 2011. Sugar substitutes: health controversy over perceived benefits. J Pharmacol Pharmacother. 2(4):236–243.
- Tang SG, Liu XY, Ye JM, Hu TT, Yang YY, Han T, Tan W. 2018. Isosteviol ameliorates diabetic cardiomyopathy in rats by inhibiting ERK and NF-kappaB signaling pathways. J Endocrinol. 238(1):47–60.
- Teo CC, Tan SN, Yong JW, Hew CS, Ong ES. 2009. Validation of green-solvent extraction combined with chromatographic chemical fingerprint to evaluate quality of Stevia rebaudiana Bertoni. J Sep Sci. 32(4):613–622.
- Tomita T, Sato N, Arai T, Shiraishi H, Sato M, Takeuchi M, Kamio Y. 1997. Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157: H7 and other food-borne pathogenic bacteria. Microbiol Immunol. 41(12):1005–1009.
- Toskulkac C, Chaturat L, Temcharoen P, Glinsukon T. 1997. Acute toxicity of stevioside, a natural sweetener, and its metabolite, steviol, in several animal species. Drug Chem Toxicol. 20(1–2):31–44.
- Toyoda K, Matsui H, Shoda T, Uneyama C, Takada K, Takahashi M. 1997. Assessment of the carcinogenicity of stevioside in F344 rats. Food Chem Toxicol. 35(6):597–603.
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. 2019. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 14(1):50–59.
- Vamanu E, Pelinescu D, Gatea F, Sârbu I. 2019. Altered in vitro metabolomic response of the human microbiota to sweeteners. Genes. 10(7):535.
- Vaněk T, Nepovı&´m A, Valı&´ček P. 2001. Determination of stevioside in plant material and fruit teas. J Food Comp Anal. 14(4):383–388.
- Wald JP, Morlock GE. 2017. Quantification of steviol glycosides in food products, Stevia leaves and formulations by planar chromatography, including proof of absence for steviol and isosteviol. J Chromatogr A. 1506:109–119.
- Wang QP, Browman D, Herzog H, Neely GG. 2018. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE. 13(7): e0199080.
- Wang T, Guo M, Song X, Zhang Z, Jiang H, Wang W, Fu Y, Cao Y, Zhu L, Zhang N. 2014b. Stevioside plays an anti-inflammatory role by regulating the NF-kappaB and MAPK pathways in S. aureus-infected mouse mammary glands. Inflammation. 37(5):1837–1846.
- Wang T, Song X, Zhang Z, Guo M, Jiang H, Wang W, Cao Y, Zhu L, Zhang N. 2014a. Stevioside inhibits inflammation and apoptosis by regulating TLR2 and TLR2-related proteins in S. aureus-infected mouse mammary epithelial cells. Int Immunopharm. 22(1):192–199.
- Wheeler A, Boileau AC, Winkler PC, Compton JC, Prakash I, Jiang X, Mandarino DA. 2008. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food Chem Toxicol. 46(7):S54–S60.
- Wingard RE, Brown JP, Enderlin FE, Dale JA, Hale RL, Seitz CT. 1980. Intestinal degradation and absorption of the glycosidic sweeteners stevioside and rebaudioside A. Experientia. 36(5):519–520.
- Wölwer-Rieck U. 2012. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: a review. J Agric Food Chem. 60(4):886–895.
- Wood HB, Allerton R, Diehl HW, Fletcher HG. 1955. Stevioside. I. The structure of the glucose moieties. J Org Chem. 20(7):875–883.
- Xin Q, Bai B, Liu W. 2017. The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochem Int. 103:57–64.
- Xu P, Hua D, Ma C. 2007. Microbial transformation of propenylbenzenes for natural flavour production. Trends Biotechnol. 25(12):571–576.
- Yadav SK, Guleria P. 2012. Steviol glycosides from Stevia: biosynthesis pathway review and their application in foods and medicine. Crit Rev Food Sci Nutr. 52(11):988–998.
- Yasukawa K, Kitanaka S, Seo S. 2002. Inhibitory effect of stevioside on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Biol Pharm Bull. 25(11):1488–1490.
- Yildiz-Ozturk E, Nalbantsoy A, Tag O, Yesil-Celiktas O. 2015. A comparative study on extraction processes of Stevia rebaudiana leaves with emphasis on antioxidant, cytotoxic and nitric oxide inhibition activities. Ind Crops Prod. 77:961–971.
- Yingkun N, Zhenyu W, Jing L, Xiuyun L, Huimin Y. 2013. Stevioside protects LPS-induced acute lung injury in mice. Inflammation. 36(1):242–250.
- Yoda SK, Marques MO, Petenate AJ, Meireles M. 2003. Supercritical fluid extraction from Stevia rebaudiana Bertoni using CO2 and CO2+water: extraction kinetics and identification of extracted components. J Food Eng. 57(2):125–134.
- Zhang SQ, Kumar A, Kutowy O. 2000. Membrane-based separation scheme for processing sweeteners from stevia leaves. Food Res Int. 33(7):617–620.
- Zhang H, Zhong K, Lu M, Mei Y, Tan E, Sun X. 2018. Neuroprotective effects of isosteviol sodium through increasing CYLD by the downregulation of miRNA-181b. Brain Res Bull. 140:392–401.
- Zhong J, Gong Q, Mima A. 2017. Inflammatory regulation in diabetes and metabolic dysfunction. J Diabetes Res. 2017:5165268.

