ABSTRACT
Redox medicine improvement could come from integrating insights from both the mitochondria and redox fields. One-step further is finding new clues with a comprehensive discussion of the two fields, and their relationship, including through the reactive species interactome (RSI). Here, the review provides a platform for future studies focusing on both mitochondria and the RSI. Mitochondria are discussed at length with focus on the mitochondrial permeability transition pore, the regulation of cell death types, the protein quality control system, calcium homoeostasis, ionome, metabolome, mitochondrial carriers, and organelle interaction. Then, the RSI is discussed focusing on its relationship with mitochondria. Finally, the mitochondria-RSI axis is proposed as a brand-new perspective, including with the involvement of the circadian clock and bacterial microbiota, as the first stone of the concept of MitoRedox Medicine.
Introduction
For a long time, probably for historical reasons, the mitochondria and redox fields have developed separately. This separation has led to too much lack of consideration of the two fields together. The redox field has accelerated its mutation recently with the concept of the reactive species interactome (RSI), which goes beyond the sole framework of the much studied reactive oxygen species (ROS) (Cortese-Krott et al. Citation2017; Sies and Jones Citation2020; Sies Citation2020). The RSI stands for the interactome of ROS, reactive nitrogen species (RNS), reactive sulphur species (RSS), reactive carbonyl species (RCS), notable redox enzymes and their downstream biological targets (Cortese-Krott et al. Citation2017; Bourgonje et al. Citation2020; Malard et al. Citation2021). In addition to reactive species identification and interaction, their concentration is one of the most important parameters. Oxidative eustress is the physiological exposure to reactive species, while oxidative distress is the supra-physiological exposure to reactive species (Sies Citation2017; Malard et al. Citation2021). There are so many papers proposing to target the redox system in medicine, both redox enzymes and the reactive species, mainly ROS, that it becomes difficult to cite them all. Precision redox targeting is of major importance and one of the latest steps in the right direction (Meng et al. Citation2021).
As much as the mitochondria field has largely focused on mitochondrial ROS, with primarily the bioenergetic oxidative phosphorylation (OXPHOS) system as major source. There are more and more papers which also propose targeting mitochondria in medicine, including in 2013 by Edeas and Weissig in different pathologies (Edeas and Weissig Citation2013). Mitochondria and the redox interactome, which integrates redox catalysis, homoeostasis, flux, landscape, homeorhesis and evolution, are two deeply connected fields (Santolini et al. Citation2019). Among different examples, three are highlighted. A concrete example is the finding in 2023 that the deficiency in mitochondrial thiosulphate sulfurtransferase (TST) or rhodanese, which is involved in RSS H2S metabolism by detoxifying cyanide, alters both the RSI through ROS, RSS polysulphides and the glutathione system, and the mitochondria through OXPHOS remodelling in brain cortex (Luo et al. Citation2023). Another example is the proposed targeting of the mitochondrial redox imbalance, specially ROS, in cancer through both antioxidant and pro-oxidant approaches (Mani et al. Citation2022). Exactly the same is proposed in neurodegenerative diseases, including Alzheimer’s disease, again mainly with ROS modulation (Carvalho and Cardoso Citation2021).
Therefore, the time has come to propose unifying both the mitochondria and redox fields with this detailed review focusing on the mitochondria-RSI axis as first step. First, selected elements of mitochondrial characteristics and roles are discussed at length. Special focus is done on the mitochondrial permeability transition pore, apoptosis-necroptosis-ferroptosis death triangle, the protein quality control system, ionome homoeostasis, metabolome, and mitochondrial carriers. Intracellular interactions of mitochondria with other organelles that affect mitochondrial homoeostasis are discussed. Then, mitochondrial reactive species sources are presented, including with enzymes involved in both mitochondria and the RSI. Finally, the mitochondria-RSI axis is proposed as a new concept to investigate, including considering chronobiology and bacterial microbiota as key players in this axis.
Major mitochondrial characteristics
Key mitochondrial characteristics are summarised in . An outer membrane (OM), an intermembrane space (IMS), an inner membrane (IM), and a matrix constitute the semi-autonomous organelles mitochondria, which structural dynamics oscillate between fragmented and tubular networks, depending on metabolic status (Harbauer et al. Citation2014; Pfanner et al. Citation2019; Giacomello et al. Citation2020). Mitochondria are ancient bacterial symbionts, whose matrix is organised with a characteristic shape of IM cristae maintained by MICOS, the mitochondrial contact site and cristae organising system (Wallace Citation2005; Pfanner et al. Citation2019; Giacomello et al. Citation2020). Mitochondrial matrix pH ranges from 7.8 to 8.1, so more basic than the cytosol with pH 7.2 (Casey et al. Citation2010). In addition, mitochondria are fully functional at close to 50°C, meaning more than 10°C from cytosolic temperature (Chrétien et al. Citation2018).
Figure 1. (a) key mitochondrial characteristics. OM, outer membrane; IMS intermembrane space; IM, inner membrane; MIA, IMS import and assembly machinery; MICOS, mitochondrial contact site and cristae organizing system; MIM, mitochondrial inner membrane complex; mtDNA, mitochondrial DNA; OXA, oxidase assembly machinery; OXPHOS, oxidative phosphorylation; PAM, presequence translocase-associated motor; SAM, sorting and assembly machinery; TIM, IM translocase complex; TOM, OM translocase complex. (b) Major inter-connected roles of mitochondria. Major roles of mitochondria are summarized, as well focus improving the understanding of mitochondria functions, namely apoptosis-uncontrolled necrosis-necroptosis-ferroptosis death triangle, amino acid metabolism, fatty acid metabolism, iron-sulfur clusters, ionome, mitochondrial metabolome, and the redox system. AGC, aspartate/glutamate carrier; ANT, adenine nucleotide transporter; CAC, carnitine/acylcarnitine carrier; CIC, citrate carrier; GC, glutamate carrier; MCU, mitochondrial calcium uniporter; MPC, mitochondrial pyruvate carrier; SLC25, solute carrier family 25.
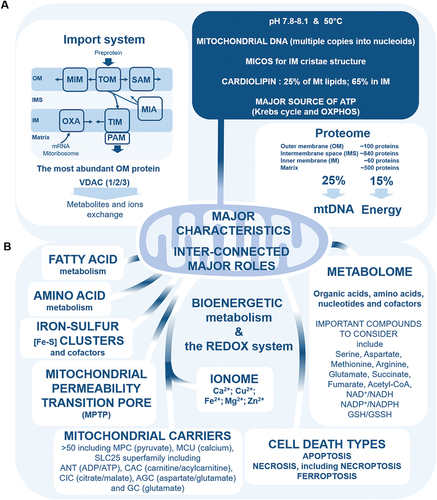
Importantly, mitochondria contain cardiolipin, which is mitochondria-specific phospholipid (Gonzalvez et al. Citation2008; Li et al. Citation2015). Cardiolipin constitutes 25% of mitochondrial membrane lipids, and 65% are found in IM (Li et al. Citation2015). Cardiolipin is highly sensitive to peroxidation by ROS, and is involved in programmed cell death apoptosis, through formation of mitochondrial membrane pores (Gonzalvez et al. Citation2008; Li et al. Citation2015). Cardiolipin is also a biomarker of damaged mitochondria when translocated to OM, and when interacting with microtubule-associated protein 1A/1B-light chain 3 (LC3) autophagic marker, which is essential for autophagosome formation in mitophagy, a quality control mechanism to degrade damaged, and dysfunctional mitochondria (Gonzalvez et al. Citation2008; Li et al. Citation2015). In addition, cardiolipin is involved (1) in the stabilisation of OXPHOS (oxidative phosphorylation) system complexes I, III, IV, and ATP synthase for mitochondrial ATP generation, (2) in mitochondrial fission, through interaction with dynamin-related protein 1 (DRP1), and (3) in the stabilisation of mitochondrial carriers such as ADP/ATP carrier which plays role in mitochondrial pore transition, respiratory supercomplexes and cell death (Gonzalvez et al. Citation2008; Klingenberg Citation2009; Li et al. Citation2015).
Importantly too, mitochondria contain their own genome, namely mitochondrial DNA (mtDNA), in multiple copies packaged into nucleoids, which are essential for mitochondrial functions, and all dedicated machineries for mtDNA replication, mtDNA transcription, and mtRNAs translation (Taylor and Turnbull Citation2005; Harbauer et al. Citation2014; Ricchetti Citation2018; Giacomello et al. Citation2020). Mitochondrial DNA alterations and disorders related to mutations, replication, and transcription affect from cellular energy metabolism to cell death (Taylor and Turnbull Citation2005; Ricchetti Citation2018).
Proteomic studies showed that mitochondria contain ~1,500 different proteins in human, 99% being encoded by the nuclear genome, and 1% by the mitochondrion genome (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019). About 100 proteins are present in OM, ~60 proteins in IMS, more than 840 proteins in IM, and ~500 proteins in mitochondrial matrix (Bohovych et al. Citation2015). Between 20% and 25% of the mitochondrial proteome regulates mtDNA, and ~15% of the mitochondrial proteome is involved in energy metabolism, including in OXPHOS (Pfanner et al. Citation2019). Adaptation of the mitochondrial proteome to bioenergetics needs is the mitochondrial proteome plasticity (Pfanner et al. Citation2019).
One of the most important mitochondrial pathway involved in mitochondrial proteome plasticity is the mitochondrial protein import system (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). This system is part of the functional mitochondrial network, including mitochondrial dynamics and architecture, OXPHOS-related bioenergetic metabolism, mtDNA regulation, protein quality control system, and Endoplasmic Reticulum–Mitochondria interaction (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). At the OM, mitochondrial import complexes include OM translocases (TOM), mitochondrial import complex (MIM), and sorting and assembly machinery (SAM) (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). Intermembrane space contains IMS import and assembly machinery (MIA) (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). Inner membrane contains IM translocases (TIM), presequence translocase-associated motor (PAM), and oxidase assembly machinery (OXA), which is involved in the synthesis of several OXPHOS subunits from matrix mitoribosomes (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). The entry of proteins into mitochondria is managed through four import pathways (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). The presequence pathway is dedicated for preproteins through TOM complex, including TOM22 and TOM40, to TIM23 complex, to ultimately integrate the IM or the matrix thanks to PAM (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). The carrier pathway is for hydrophobic proteins dedicated to the IM through TOM complex, including TOM22 and TOM40, and TIM22 complex (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). The oxidative folding pathway is dedicated for IMS proteins, through TOM complex, and MIA, including MIA40 (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). The transport pathway is finally dedicated for OM proteins, through TOM complex, chaperones, and SAM complex, including SAM50 and MDM10 (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021).
Finally, voltage-dependent anion channel (VDAC), which is the most abundant OM protein, is the major regulator for metabolites, and ions exchange between mitochondria and cytosol (Caterino et al. Citation2017; Camara et al. Citation2017; Zinghirino et al. Citation2021; Najbauer et al. Citation2021). This mitochondrial porin is proposed as cellular hub linking bioenergetic metabolism to many intracellular pathways (Pittalà et al. Citation2021). Gene expression of the three mammalian isoforms, VDAC1, VDAC2, and VDAC3, depends on transcription factors involved in regulating bioenergetic metabolism, apoptosis, and cell proliferation (Zinghirino et al. Citation2021). In addition to the well described role of VDAC in regulating mitochondrial Ca2+ flux, porin also being regulated by Ca2+, VDAC is involved in apoptosis, bioenergetic metabolism, and the interaction between mitochondria and the cytoskeleton (Caterino et al. Citation2017; Najbauer et al. Citation2021; Sander et al. Citation2021). Interestingly, VDAC isoforms display common and distinct protein partners allowing specific isoform functions (Caterino et al. Citation2017). Protein partners of VDAC1 suggest pro-apoptotic and cell homoeostasis functions, for VDAC2 anti-apoptotic function, and for VDAC3 marker of oxidative status, and mitochondrial protein quality control function (Caterino et al. Citation2017). Mitochondrial-associated hexokinase (HK) interaction with VDAC1 contributes to inhibit the pro-apoptotic function of VDAC1, by delaying its mitochondrial translocation (Dubey et al. Citation2016; Camara et al. Citation2017). Tubulin and α-synuclein block VDAC pores by direct interaction, thus increasing VDAC’s sensitivity to mitochondrial membrane potential, and regulating mitochondrial ATP generation (Rostovtseva et al. Citation2021). In addition, post-translational modifications of VDACs, including acetylation, cysteine oxidation, methionine oxidation, succination, and phosphorylation, affect their interaction with protein partners, and therefore their functions (Pittalà et al. Citation2021). For example, post-translational modifications of VDAC1 affect its interaction with the antioxidant defence enzyme superoxide dismutase SOD1 (Pittalà et al. Citation2021). Moreover, the abundance of thiols in VDAC2 suggests that this isoform can neutralise ROS through cysteine oxidation (Maurya and Mahalakshmi Citation2016). Finally, interaction of VDAC2 with steroidogenic acute respiratory protein (StAR) also suggests that VDAC2 is involved in cholesterol import into mitochondria for steroid hormone biosynthesis, and in mitochondria-associated Endoplasmic Reticulum interaction (Maurya and Mahalakshmi Citation2016).
Studies are needed for extracellular mitochondria and mitovesicles
Extracellular intact mitochondria can be found circulating in the blood, “free” or inside extracellular vesicles (EVs) (Al Amir Dache et al. Citation2020; Stier Citation2021). Interestingly, lysosomal dysfunction leads to increased secretion of mitochondria-containing EVs during heart pathophysiology (Liang et al. Citation2023). In addition, mitochondrial ROS generated by the electron transport chain contribute to regulate EVs secretion rate (Nørgård et al. Citation2023), and mitochondrial dysfunction contributes to generate a specific subpopulation of EVs, the mitovesicles, which are mitochondria-derived EVs that contain multiple mitochondrial proteins (D’Acunzo et al. Citation2021). However, the functional role of “free” extracellular mitochondria, mitochondria in EVs and mitovesicles is still debated, including as possible extracellular signalling organelle, and as new potential source of ATP to be recruited during inflammation and in chronic diseases (Al Amir Dache et al. Citation2020; Esch et al. Citation2020; Stier Citation2021; D’Acunzo et al. Citation2021). Therefore, due to a lack of data and studies that are still needed, this review will not focus on extracellular mitochondria and mitovesicles, although one day soon when the data will be provided, this review will be of very great interest with greater reasoning in physiology and pathology. Intracellular mitochondria are debated in this review.
Major mitochondria roles
Major mitochondrial roles are summarised in . The most well-known roles are the bioenergetic metabolism through the Krebs cycle or TriCarboxylic Acid (TCA) cycle and the OXPHOS system for the ATP generation, and the programmed cell death caspase-dependent and -independent apoptosis (Bock and Tait Citation2020). Mitochondria also play roles in the biosynthesis of iron-sulphur [Fe-S] clusters and cofactors, the metabolism of amino acids, the metabolism of lipids, including β-oxidation of long chain fatty acids (LCFAs) and medium chain fatty acids (MCFAs), namely the mitochondrial fatty-acid oxidation (mitoFAO) to generate acetyl-CoA for the ATP synthesis (Rouault Citation2016; Shan and Cortopassi Citation2016; Lee et al. Citation2017; Li and Hoppe Citation2023). Importantly, mitochondria play roles in the calcium buffering to handle intracellular calcium levels, the copper homoeostasis, the iron homoeostasis, the neglected magnesium and zinc metabolisms, mitochondrial permeability transition pore, and the redox signalling (Hacker and Medler Citation2008; Ghosh et al. Citation2014; Baker et al. Citation2017; Borchard et al. Citation2018; Pfanner et al. Citation2019; Jung et al. Citation2020; Bernardi et al. Citation2023). Finally, it is important to note that mitochondria play a major role in the dynamics of intracellular metabolites through mitochondrial carriers (Palmieri Citation2013; Palmieri and Monné Citation2016; Cunningham and Rutter Citation2020). The next eight sections focus on notable mitochondrial roles that should be strengthened.
The mitochondrial permeability transition pore (mPTP)
Among all the mitochondrial channels, the mPTP, which is a non-selective and Ca2+-dependent mega-channel, regulates the permeability of the mitochondrial IM (Halestrap et al. Citation2002; Bernardi et al. Citation2015, Citation2023; Carraro and Bernardi Citation2023). To make a short story on more than 70 years of discoveries and several thousand publications, while retaining a flavour of the mPTP, several non-exhaustive aspects are addressed. First, mitochondrial matrix pH, membrane potential, quinones and lipids contribute to modulate the mPTP opening (Nicolli et al. Citation1993; Bernardi et al. Citation2015). Inducers include thiol oxidants and matrix Ca2+, and inhibitors include cyclosporine, which binds matrix cyclophilin D, Mg2+, Mn2+ and adenine nucleotides (Nicolli et al. Citation1993; Bernardi et al. Citation2015). In addition, mPTP is sensitive to oxidation-reduction events, and regulates the electron flux within OXPHOS complex I, therefore linking mPTP with both mitochondrial bioenergetics and the redox system, including at minimum ROS (Bernardi et al. Citation2015, Citation2023). Always open to debate with different interpretations, including related to the estimation of the currents crossing the induced mPTP in different conditions, the precise molecular identity of the mPTP crystallises the attentions. One interpretation is a model for the mPTP in which adenine nucleotide translocator (ANT), VDAC and cyclophilin D are the key components (Halestrap et al. Citation2002). Another model also includes F-ATP synthase, ADP/ATP carriers (AACs) and mitochondrial phosphate carrier (PiC) as key components too (Chevrollier et al. Citation2011; Trisolini et al. Citation2021). Finally, it is important to emphasise the enigmatic dual role of F-ATP synthase and ANT in the formation of mPTP, in which F-ATP synthase monomers and dimers could be a key element in resolving this long debate (Nicolli et al. Citation1993; Giorgio et al. Citation2013; Bernardi et al. Citation2015; Carraro and Bernardi Citation2023). Interestingly, still with open questions, MPTP is formed from and the F-ATP synthase (Giorgio et al. Citation2013; Bernardi et al. Citation2015, Citation2023; Carraro and Bernardi Citation2023). Then, both the number of open pores and the open time of individual pores influence the consequences of MPTP opening, from mitochondrial depolarisation to ATP hydrolysis by the F-ATP synthase, the release of cytochrome C, the generation of at minimum ROS, mitochondrial damage and different cell death types (Bernardi et al. Citation2015, Citation2023; Bauer and Murphy Citation2020; Carraro and Bernardi Citation2023). There are still questions regarding the link between MPTP, apoptosis (Kinnally et al. Citation2011; Rodriguez et al. Citation2021; Bernardi et al. Citation2023) necrosis including necroptosis that is a regulated form of necrosis (Karch et al. Citation2015), and ferroptosis (Bai et al. Citation2021). This link between MPTP and cell death types ultimately positions MPTP as a major gatekeeper (Kinnally et al. Citation2011), including with ROS and possibly other reactive species yet to discover, for different cell death types that will be discussed in the next section.
The apoptosis-necroptosis-ferroptosis death triangle
Mitochondria are major regulators of the programmed cell death apoptosis, including with cardiolipin, and both B-cell lymphoma 2 (BCL-2) effector proteins Bax (Bcl-2 associated X), and Bcl-2 antagonist/killer (BAK) activation and oligomerization (Susin et al. Citation1998, Citation1999; Gonzalvez et al. Citation2008; Li et al. Citation2015; Lopez and Tait Citation2015; Bano and Prehn Citation2018; Bock and Tait Citation2020). Apoptosis is characterised by several events, including the release of cytochrome c, serine protease high temperature requirement protein A2 (OMI/HTRA2), apoptosis-inducing factor (AIF), and endonuclease G (ENDOG), apoptosome formation, and the nuclear genome destruction (Susin et al. Citation1998, Citation1999; Gonzalvez et al. Citation2008; Li et al. Citation2015; Lopez and Tait Citation2015; Bano and Prehn Citation2018; Bock and Tait Citation2020). Classical apoptosis is caspase-dependent with caspase activation, including caspase 3 and 7 (Barbier et al. Citation2009; Brentnall et al. Citation2013). Importantly, apoptosis can also be caspase-independent (Candé et al. Citation2002). In both forms of apoptosis, the major factor is AIF (Lorenzo et al. Citation1999; Candé et al. Citation2002) which is also involved in other cell death types such as necrosis (Boujrad et al. Citation2007), including necroptosis (Baritaud et al. Citation2012), and ferroptosis (Wu et al. Citation2023). The ROS hydrogen peroxide (H2O2) is a key inducer of apoptosis by inducing mitochondrial membrane disruption, and by activating key mitochondrial apoptotic proteins, including Bcl2/adenovirus E1B 19 kDa-interacting protein 3 (BNIP3), Bax, and Bak (Alexandre et al. Citation2006; Li et al. Citation2012; Galluzzi et al. Citation2018). Although apoptosis is the most investigated cell death type regarding mitochondria, more than ten cell death types are described, including uncontrolled necrosis, controlled necrosis form necroptosis, and ferroptosis (Galluzzi et al. Citation2018; Hadian and Stockwell Citation2023). Interestingly, H2O2 is a key reactive species linking apoptosis, uncontrolled necrosis, necroptosis and ferroptosis (Teramoto et al. Citation1999; Saito et al. Citation2006; Dixon et al. Citation2012; Stockwell et al. Citation2017; Galluzzi et al. Citation2018; Hadian and Stockwell Citation2023).
Necroptosis, which is induced by H2O2, initiates by tumour necrosis factor-α (TNFα), kinase receptor-interacting proteins (RIPK), including RIPK1 and RIPK3, and is characterised by necrosome formation (Nakao et al. Citation2008; Marshall and Baines Citation2014; Lu et al. Citation2017; Choi et al. Citation2019; Deragon et al. Citation2020; Yin et al. Citation2020). For example, in human lung fibroblasts, low concentration of H2O2 less than 100 microM induced apoptosis, while high concentration from 1 mM induced necroptosis (Teramoto et al. Citation1999). Another example in human T-lymphoma Jurkat cells showed that 50 microM H2O2 induced apoptosis through caspase-3/-9 activation, while 500 microM H2O2 did not activate caspase, and induced necroptosis by decreasing intracellular ATP (Saito et al. Citation2006). Regulation of intracellular ATP level coupled with H2O2 concentration contributes to determine which cell death type will be activated (Saito et al. Citation2006). Finally, importantly, mitochondrial calcium overload and accumulation of mitochondrial ROS superoxide anion (O2−) induce necroptosis (Marshall and Baines Citation2014; Lu et al. Citation2017; Choi et al. Citation2019; Deragon et al. Citation2020).
Ferroptosis could also be regulated by mitochondria. Ferroptosis is characterised by the accumulation of active iron Fe2+ and H2O2, and cytosolic and mitochondrial glutathione peroxidase 4 (GPX4) dysfunction (Dixon et al. Citation2012; Stockwell et al. Citation2017; Gan Citation2021). Glutathione peroxidase 4 is an antioxidant defence enzyme which regulates GSH/GSSG system to reduce polyunsaturated fatty acids (PUFAs) peroxidation (Dixon et al. Citation2012; Stockwell et al. Citation2017; Gan Citation2021). Ultimately, PUFAs peroxidation and RCS accumulation, including malondialdehyde (MDA), lead to ferroptosis (Dixon et al. Citation2012; Stockwell et al. Citation2017; Gan Citation2021). It is important to note that H2O2 accumulation can be caused by dysfunction of enzymes that neutralise H2O2, including GPX and catalase, and by overactivation of both NADPH-cytochrome P450 reductase (POR) and NADH-cytochrome b5 reductase (CYB5R1), two enzymes attached to both mitochondria and the Endoplamisc Reticulum (ER) membranes (Dixon et al. Citation2012; Stockwell et al. Citation2017; Šrejber et al. Citation2018; Yan et al. Citation2021). Mitochondrial O2− and H2O2, and mitochondrial dihydroorotate dehydrogenase (DHODH) and GPX4, two enzymes which detoxify lipid peroxides, are involved in ferroptosis regulation (Stockwell et al. Citation2017; Gan Citation2021).
Altogether, the most important investigation would be the role of mitochondria as a nexus linking apoptosis, necrosis including necroptosis and ferroptosis, through (1) different mitochondrial reactive species, and (2) related redox enzymes that will be discussed.
The mitochondrial protein quality control system
Protein quality control system in mitochondria contributes to maintain healthy mitochondria with both a mitochondrial network of chaperones and proteases, and cytosolic proteolytic mechanisms, including ubiquitin-proteasome system (UPS), and heat shock proteins (Hsp), such as HSP70 and HSP90 (Bohovych et al. Citation2015).
Outer membrane proteins include the E3 ubiquitin ligase autosomal recessive Parkinson’s disease 2 (PARKIN), ubiquitin ligase activator of NFKB1 (MULAN), membrane-associated ring finger C3HC4 5 (MARCH-V), mitochondrial distribution and morphology 30 (MDM30), PTEN-induced putative kinase 1 (PINK1), mitofusin (MFN), and AAA+ ATPases associated with various cellular activities mitochondrial sorting of proteins 1 (MSP1) (Bohovych et al. Citation2015). Intermembrane space proteins include HtrA2 serine protease, and ATP synthase 23 (ATP23) metalloprotease (Bohovych et al. Citation2015). Inner membrane proteins include GTPase (OPA1), i-AAA, inner membrane-embedded AAA protease, overlapping with mitochondrial AAA m-AAA protease (OMA1) ATP-independent metalloprotease, presenilin-associated rhomboid-like serine protease (PARL) protease, and inner membrane mitochondrial protease (IMP protease) (Bohovych et al. Citation2015). Matrix proteins include chaperones such as HSP10, HSP60, HSP78, mitochondrial HSP70 (MTHsp70), presequence metallopeptidase (PreP) metalloprotease, mitochondrial intermediate peptidase (MIP), mitochondrial processing peptidase (MPP) protease, several ATP-dependent serine proteases such as AAA+ serine protease LON, caseinolytic mitochondrial matrix peptidase chaperone subunit P (CLPP), and Clp subunit X (CLPX) (Bohovych et al. Citation2015). Matrix Lon and CLPX/P proteases, which are protein quality safeguards, exert critical role in regulating proteolysis for mitochondrial proteins involved in mtDNA maintenance and transcription, such as mitochondrial Transcription Factor A (TFAM), and in OXPHOS maintenance (Szczepanowska and Trifunovic Citation2021).
One interesting example is the HtrA serine protease family, which includes OMI/HTRA2 and HTRA3 (Chatre et al. Citation2015; Hu et al. Citation2019; Crochemore et al. Citation2019). The HTRA2 serine protease is involved in mitochondria homoeostasis and apoptosis (Hu et al. Citation2019). In addition, the HTRA3 serine protease, a cytosolic and mitochondrial protease that is upregulated by the nitroso-redox balance, regulates mitochondrial proteins, including (POLG1), and OXPHOS activity, as it has been demonstrated in the progeroid Cockayne syndrome (Chatre et al. Citation2015). Replicative P21-dependent senescence associated with mitochondrial impairment is also regulated by HTRA3 (Crochemore et al. Citation2019).
Finally, the mitochondrial protein quality control system contributes to mitochondrial unfolded protein response (MT UPR), a response to mitochondrial proteotoxic stress (Jovaisaite et al. Citation2014). In mammals, activating transcription factor 5 (ATF5) regulates MT UPR function to maintain mitochondrial homoeostasis and bioenergetic metabolism during mitochondrial stress (Fiorese et al. Citation2016). Activation of the MT UPR with mitochondrial chaperones, including HSP70, and proteases, including LON protease, is involved in longevity, in cardioprotection, and in ameliorating neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease (Zhu et al. Citation2021; Gu et al. Citation2021; Wang et al. Citation2022). Dysfunction of MT UPR aggravates heart diseases by deregulating proteostasis, and contributes to inflammation, and cancer (Zhu et al. Citation2021; Gu et al. Citation2021; Wang et al. Citation2022). Finally, MT UPR and mitochondrial protein import system are suggested to be synchronised to avoid proteotoxicity, and to enhance oxidative capacity (Richards et al. Citation2022). Together, regulation of mitochondrial protein import and folding, proteolysis of mitochondrial proteome, proteotoxic stress response, and OXPHOS maintenance make this system as a major one to further investigate (Song et al. Citation2021).
The mitochondrial calcium homeostasis
Mitochondria are important regulators of many calcium-dependent intracellular functions by buffering, sequestering, and releasing calcium ions (Giorgi et al. Citation2018). In resting conditions, mitochondrial Ca2+ concentration is 100–200 nM as cytoplasm, while mitochondria can accumulate 10- to 20-fold more Ca2+ than cytoplasm during calcium stimulation (Giorgi et al. Citation2018). Through mitochondrial Ca2+ uniporter (MCU) complex, mitochondria capture calcium ions from cytosolic and the ER sources (De Stefani et al. Citation2011; Baughman et al. Citation2011). In addition, the role of VDACs in mitochondrial Ca2+ uptake is much more complex than expected (Sander et al. Citation2021). In addition, VDAC posttranslational modification, lipidic environment, including with phosphatidylethanolamine (PE) and cardiolipin, protein partners, and subcellular localisation in Ca2+ microdomains are now being discussed as a complex physiological regulator of mitochondrial Ca2+ (Sander et al. Citation2021).
Mitochondrial activity, which is reflected by mitochondrial membrane potential, is an active force for Ca2+ uptake, while mitochondrial Na+/Ca2+ exchanger (MT NCX) and mitochondrial H+/Ca2+ exchanger (MT HCX) mainly contribute to mitochondrial calcium ions efflux (Giorgi et al. Citation2018; Garbincius and Elrod Citation2022). During oxidative distress, hypoxia, and inflammation, MCU complex are targeted by ROS, and pro-apoptotic BCL2-family proteins interact with VDAC1 and VDAC3, thus linking mitochondrial calcium homoeostasis with oxidative distress and apoptosis (Baughman et al. Citation2011; Giorgi et al. Citation2018). In addition, mitochondrial Ca2+ influx and efflux contribute to remodel intracellular Ca2+ dynamics and signalling, including in the ER, the major calcium ions storage site (Giorgi et al. Citation2018; Garbincius and Elrod Citation2022). Moreover, mitochondrial Ca2+ buffering contributes to maintain basal intracellular Ca2+ level, and to limit glutamate excitotoxicity (Rueda et al. Citation2016; Angelova et al. Citation2019; Verma et al. Citation2022). Finally, what makes the story more attractive is that calcium ions are involved in apoptosis, necroptosis, autophagy, insulin secretion, heart activity, inflammation, cancer, neuronal activity, stroke, neuromuscular disorders, and neurodegeneration (Giorgi et al. Citation2018; Garbincius and Elrod Citation2022; Rodríguez et al. Citation2022).
The mitochondrial copper homeostasis
Mitochondria also contain key cuproenzymes involved in bioenergetics such as cytochrome c oxidase (COX) from the OXPHOS system, and in the redox system such as the superoxide dismutase 1 (SOD1) (Hilton et al. Citation2018; Zischka and Einer Citation2018). Cuproenzymes are metalloenzymes whose essential cofactor is copper (Cu2+) (Hilton et al. Citation2018). Copper dysregulation within mitochondria can cause mitochondrial disorders, given that mitochondria are a major copper storage site (Baker et al. Citation2017; Garza et al. Citation2023). These mitochondrial disorders include encephalopathy, cardiomyopathy, hepatopathy, the Menkes disease with neurodevelopment defects, and the Wilson disease with psychiatric symptoms, hepatomegaly and kidney dysfunction (Baker et al. Citation2017; Zischka and Einer Citation2018; Garza et al. Citation2023). Making this so neglected long story short, copper imbalance is also associated with neurodegeneration, including Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis, and cancer, including the triple negative breast cancer (Giampietro et al. Citation2018; Ruiz et al. Citation2021; Cui et al. Citation2021). Interestingly, the so-called metformin, which inhibits OXPHOS complex I and that is used as first-line antidiabetic agent and explored as potential agent against neurodegenerative diseases, induces ROS-mediated oxidative distress by targeting mitochondrial copper through direct binding in cancer cells (Müller et al. Citation2018). On one hand, intervening in copper homoeostasis with Cu2+ overload triggers apoptosis, necroptosis, ferroptosis and cuprotosis, a copper-dependent form of cell death characterised by copper binding to lipoylated proteins involved in the TCA cycle, and iron-sulphur cluster protein loss (Tsvetkov et al. Citation2022; Tang et al. Citation2023). On the other hand, Cu2+ overload drives inflammation through ROS H2O2 accumulation, NAD+ maintenance and epigenetic changes including in macrophages, which ultimately suggests that Cu2+ overload is harmful for the immunity during infections and cancer (Solier et al. Citation2023). Altogether, the tight mitochondrial copper balance requires more investigation in physiology and pathology.
The mitochondrial other divalent ions homeostasis: iron, magnesium and zinc
Iron, magnesium and zinc are the three divalent ions that require more studies including mitochondria. First, in its forms Fe2+, in haem and in [Fe-S] cluster, iron is a major divalent ion involved in OXPHOS function through cytochrome oxidase and the TCA cycle, and ferroptosis activation (Dixon et al. Citation2012; Zhang et al. Citation2022). Complexes I, II and III from OXPHOS use more than ten [Fe-S] clusters (Rouault Citation2016). Iron deficiency is associated with altered glucose metabolism through hyperglycaemia, and altered lipid metabolism through hyperlipidaemia. Iron overload is associated with reduced glucose uptake, ROS-mediated oxidative distress, dysregulated autophagy and insulin resistance (Zhang et al. Citation2022). Notable proteins involved in iron metabolism are iron regulatory protein (IRP), ferritin, mitoferrins, and frataxin (Rouault Citation2016). Interestingly, many focus are on frataxin, a mitochondrial matrix protein that is essential for [Fe-S] cluster formation (Anzovino et al. Citation2014). Frataxin deficiency causes the inherited neurodegenerative disorder Friedreich’s ataxia (FRDA) characterised by iron overload, ROS-mediated oxidative distress, accumulation of lipid droplets, mitochondrial dysfunction, and dysregulation of ferroptosis (Li et al. Citation2020; Lynch and Farmer Citation2021). Ultimately, FRDA is characterised by both hypertrophic cardiomyopathy and neurodegeneration, which cause early death, and promising therapies focus on both ferroptosis inhibitor and iron chelator (Anzovino et al. Citation2014; Lynch and Farmer Citation2021). Finally, accumulation of mitochondrial labile iron in FRDA increases the sensitivity of skin fibroblast to UVA, which triggers oxidative distress damage, mitochondrial dysfunction with OXPHOS downregulation, and skin alteration (Reelfs et al. Citation2019). Then, the other so neglected divalent ion is magnesium (Mg2+), the main intracellular antagonist of calcium by regulating mitochondrial VDAC (Pilchova et al. Citation2017). Mitochondria are an intracellular Mg2+ storage site that is also stored in the ER (Pilchova et al. Citation2017). Many bioenergetic proteins are sensitive to magnesium, including glycolytic hexokinase and pyruvate kinase, and mitochondrial enzymes, including Krebs cycle enzymes isocitrate dehydrogenase (IDH) and 2-oxoglutarate dehydrogenase complex (OGDH) and OXPHOS complex V F0/F1-ATPase (Pilchova et al. Citation2017). Finally, it is important to note that magnesium also contributes to regulate apoptosis (Pilchova et al. Citation2017). The last so neglected divalent ion is zinc (Zn2+) that is required for many intracellular functions, including DNA synthesis, mitosis, apoptosis, and the antioxidant activity of SOD1 (Lu et al. Citation2016). Free chelatable zinc is found in the ER, the Golgi apparatus and mitochondria (Lu et al. Citation2016). Altered zinc homoeostasis is associated with neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, type 2 diabetes, and Crohn’s disease (Lu et al. Citation2016). Zinc supplementation improves SOD activity, intracellular pyruvate transport, Krebs cycle [Fe-S] enzyme succinate dehydrogenase subunit B (SDHB) and remodels the OXPHOS complex I to restore mitochondrial function in immortalised human embryonic kidney cells HEK293 (Yang et al. Citation2017). Finally, zinc supplementation can also restore cardiac function by improving mitochondrial respiration and by reducing mitochondrial ROS generation in a model of reperfusion post-ischaemia in isolated rat heart (Zhang et al. Citation2018). Altogether, this ionome platform composed of calcium, copper, iron, magnesium and zinc would be of great interest in mitochondria physiology and pathology, which requires many studies including all the five notable divalent ions together with their interactions with bioenergetics and the redox system.
The mitochondrial metabolome
Another level to take care is the mitochondrial metabolome. Finding metabolomic signatures in physiology, including during ageing (Adav and Wang Citation2021), and pathology, including in cancer (Pandey et al. Citation2017) and in neurodegenerative diseases (Novotny et al. Citation2023), contributes to reveal new molecular pathways and networks (Gucek and Sack Citation2021). Mitochondrial metabolomics allow extraction, detection, and data analysis for many mitochondrial metabolites such as amino acids, organic acids, nucleotides and cofactors, and their involvement in metabolisms, including energy metabolism, lipid metabolism, amino acid metabolism, carbohydrate metabolism, and cofactor metabolism (Go et al. Citation2014). Non-exhaustively, this section discusses several examples of how mitochondrial metabolome is so important to study. For example, metabolomic analysis of different mitochondrial diseases in patients, including with deficiency in OXPHOS complex I and III, Kearns-Sayre syndrome with mtDNA deletion, Pearson syndrome with mtDNA deletion, and Leigh syndrome showed that urine lactate as well as most of the Krebs/TCA cycle intermediates were not useful to discriminate between different mitochondrial disorders (Barshop Citation2004). Only fumarate and malate allowed some discriminations still with no clear metabolomic signature (Barshop Citation2004).
Another example is for the role of amino acid in mitochondria, whose excess induces the formation of mitochondrial-derived compartments, including with major metabolite exchangers through mitochondrial IM (Schuler et al. Citation2021; Li and Hoppe Citation2023). The activation of mammalian target of rapamycin (mTOR) by amino acid prevents oxidative distress-mediated mitochondrial damage and inhibits autophagy (Meijer et al. Citation2015; Tedesco et al. Citation2020; Li and Hoppe Citation2023). The TCA cycle is involved in anabolism and catabolism of amino acid, which links bioenergetics and amino acid metabolism in mitochondria (Ryan et al. Citation2021; Li and Hoppe Citation2023). Moreover, inhibition or disruption of the TCA cycle contributes to alter amino acid metabolism and to activate a response to amino acid deprivation (Ryan et al. Citation2021; Li and Hoppe Citation2023). Genetic ablation of TCA cycle enzyme fumarase and succinate dehydrogenase induces an increase in several amino acid, including serine and asparagine (Ryan et al. Citation2021; Li and Hoppe Citation2023). In addition, mitochondrial glutamate metabolite related to glutamine metabolism and glutaminase activity, is involved in OXPHOS activity, mitochondrial nucleotide pool maintenance with NAD+/NADH and NADP+/NADPH (NAD phosphate), and antioxidant glutathione GSH/GSSG system regulation (Yoo et al. Citation2020). Reactive oxygen species are regulated by glutamine metabolism through GSH/GSSG system (Yoo et al. Citation2020). Glutamate affects other metabolites such as pyruvate, lactate, 2-ketoglutarate, and Krebs cycle intermediates, an exacerbated effect during hypoxia (Yoo et al. Citation2020). Moreover, from glutamine metabolism, several oncometabolites are of interest, including α-KG, R-2-hydroxyglutarate (R-2-HG), succinate, and fumarate (Yoo et al. Citation2020). Metabolic reprogramming is induced by glutamine and derived metabolites, which makes this metabolism of interest to target in mitochondria, including in cancer (Yoo et al. Citation2020). It is important to note that glutamate is a powerful neurotransmitter as gamma aminobutyric acid (GABA) neurotransmitter (Hillen and Heine Citation2020). Moreover, without mentioning all the amino acids, biosynthesis of mitochondrial aspartate, whose deficiency alters the regeneration of NAD+ and induces inflammation (Wu et al. Citation2021), is supported by the TCA cycle (Sullivan et al. Citation2015; Alkan and Bogner-Strauss Citation2019) and many amino acids, including aspartate, serine, phenylalanine, isoleucine, valine, methionine, histidine, glutamate and arginine are converted to TCA cycle metabolites (Hara et al. Citation2021).
Last example is for nucleotide. In HEK293 cells, during a study looking for an association between mtDNA replication activation and mitochondrial metabolites dysregulation in an induced metabolic stress condition with glycolytic inhibitor 2-deoxyglucose, nucleotides and nicotinamide adenine dinucleotide (NAD+) were identified as important metabolites (Nomiyama et al. Citation2022). Supplementation with a precursor of NAD+, namely β-nicotinamide mononucleotide (β-NMN), induced an increase in nucleotides and improved the rate of mtDNA replication (Nomiyama et al. Citation2022). Metabolomic analysis in the context of diabetic kidney disease also reveals that many metabolites, including uric acid, stearic acid, palmitic acid, aconitic acid, isocitric acid, 4-hydroxybutyrate, and glycolic acid, are involved in mitochondrial and fatty acid metabolism (Li et al. Citation2017). Metabolomic studies contribute to improve the understanding of mitochondrial dysfunction, including in mitochondrial diseases (Esterhuizen et al. Citation2017). Specific metabolites, such as NAD+/NADH, acetyl-CoA, succinate, citrate, lactate, pyruvate, fumarate, malate, and 2-ketoglutarate are identified as key metabolites to track, and to regulate in mitochondrial diseases, and related pathways, including energy metabolism and cell death (Esterhuizen et al. Citation2017). It is important to remember that mitochondrial Krebs cycle metabolites, including acetyl-CoA, fumarate, succinate, α-ketoglutarate (α-KG), methionine, S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) are critical regulators of the epigenome, including through chromatin modifications, and DNA methylation (Martínez-Reyes and Chandel Citation2020; Santos Citation2021; Morin et al. Citation2022). Acetyl-CoA, whose major source is mitochondria, and NAD+ also affect histone acetyltransferase activity, and histone deacetylase complex, thus histone acetylation and gene expression (Lozoya et al. Citation2019; Zhu et al. Citation2020; Morin et al. Citation2022). Altogether, linking metabolomics studies with mitochondrial function, and focusing on key metabolites, including Krebs cycle intermediates, should reveal new mitochondrial roles.
The mitochondrial carriers
Finally yet importantly, there is a world within the world of mitochondrial metabolites and ions which already has its reviews and which in this paragraph is discussed with some examples. It is the world of the mitochondrial carriers (MCs), which has all its importance in this review with a future scope not to be neglected. More than 50 MCS are characterised for this superfamily of intracellular membrane transporters with implications in physiology and diseases (Palmieri Citation2013; Palmieri and Monné Citation2016; Cunningham and Rutter Citation2020; Ruprecht and Kunji Citation2020). Multiple pathways regulate MCs, from carrier gene expression with transcriptional regulation to changes in driving forces, kinetic parameters, concentrations of substrates and ions dynamics, and interactions with activators and inhibitors (Palmieri and Monné Citation2016). In mammals, the vast majority of MCs are members of the solute carrer family 25 (SLC25) (Palmieri Citation2013; Palmieri and Monné Citation2016). Non-exhaustively, the SLC25 family includes dicarboxylate carriers (SLC25A10; DIC) that transport malate, succinate, malonate, Pi, sulphite, sulphate and thiosulphate, ornithine carriers (SLC25A15/2; ORC1-2) that transport ornithine, citrulline, arginine and lysine for ORC1 and ORC2, and in addition histidine and homoarginine for ORC2, and thiamine pyrophosphate carriers (SLC25A19; TPC) that transport desoxynucleoside diphosphates (dNDPs), ADP, GDP, CDP and UDP (Palmieri Citation2013; Palmieri and Monné Citation2016). The SLC25 family also includes S-adenosylmethionine carriers (SLC25A26; SAMC) that transport S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAHC), and oxodicarboxylate carriers (SLC25A21; ODC) that transport 2-oxodipate, 2-oxoglutarate, 2-aminoadipate and citrate (Palmieri Citation2013; Palmieri and Monné Citation2016). Adenine nucleotide transporters (SLC25A4/5/6; ANT1-3) transport ADP and ATP, ATP-Mg/phosphate carriers (SLC25A24/23/25/41; APC1-4) exchange ATP-Mg for Pi, and also transport ADP and AMP, ADP/ATP carrier isoform 4 (SLC25A31; AAC4) transports ADP, ATP, dADP and dATP, mitoferrin 2 (SLC25A28; MFRN2) transports Fe2+, and four-carbon metabolite/phosphate carrier (SLC25A7/8/9; UCP1-3) transports aspartate, malate, malonate, oxaloacetate, Pi+H+ and sulphate+H+ (Palmieri Citation2013; Palmieri and Monné Citation2016). Interestingly, carnitine/acylcarnitine carrier (SLC25A20; CAC) that transports carnitine and acylcarnitine, is considered as redox sensor through its interactions with thiol-reactive compounds glutathione GSH and GSSG, GSH being activator and GSSG inhibitor of CAC activity, and is inhibited by ROS H2O2, RNS NO and RSS H2S (Palmieri and Monné Citation2016; Tonazzi et al. Citation2021). Another example is the citrate carrier (SLC25A1; CIC), which transports citrate, isocitrate, malate and phosphoenolpyruvate, that is critical for the export of citrate from the Krebs cycle to the cytosol that will be further used by pathways involved in oxaloacetate and acetyl-CoA synthesis, as well as in malate import into mitochondria, which is ultimately the citrate-malate shuttle (Palmieri Citation2013; Palmieri and Monné Citation2016; Guo et al. Citation2023). The mitochondrial import of malate is also coupled to the export of aspartate, which is the malate-aspartate shuttle, a so important shuttle involved in the NAD+/NADH system, the Krebs cycle, glycolysis, and de novo serine biosynthesis (Guo et al. Citation2023; Mh et al. Citation2023). Back to aspartate, aspartate-glutamate carriers (SLC25A12/13; AGC1-2) exchange aspartate for glutamate and cysteine sulphonate, and glutamate carriers (SLC25A22/18; GC1-2) transport glutamate (Palmieri Citation2013; Palmieri and Monné Citation2016). Here again, interestingly, glutamate transport into and out mitochondria is increasingly investigated, as glutamate is a powerful neurotransmitter as gamma aminobutyric acid (GABA) neurotransmitter (Hillen and Heine Citation2020). Mitochondrial dysfunction, neuronal activity disturbance, global cerebral hypomyelination, neonatal intrahepatic cholestasis and early infantile epileptic encephalopathy are induced by AGC and GC dysregulation (Wibom et al. Citation2009; Fiermonte et al. Citation2011; Lemattre et al. Citation2019; Hillen and Heine Citation2020). In GABAergic neurons, mitochondrial OXPHOS hyperactivity induces GABA neurotransmitter accumulation into mitochondria due to Aralar activity, which contributes to social behavioural deficits (Pardo et al. Citation2013; Kanellopoulos et al. Citation2020; Zhang et al. Citation2020). More largely, from the superfamily SLC25, MCs dysfunction and deficiency affect the Krebs cycle, the urea cycle, fatty acid synthesis, OXPHOS, mtDNA transcription and replication, acetylation of proteins, gluconeogenesis, necrosis and the RSS metabolism (Palmieri Citation2013; Palmieri and Monné Citation2016; Cunningham and Rutter Citation2020). Finally, it is just as important to mention four MCs who are not members of the SLC25 family. The ATP-binding cassette subfamily B (ABCB) transporters transport iron, nutrient and other unknown substrates, and sideroflexins transport serine and possibly other substrates important for OXPHOS complex I (Cunningham and Rutter Citation2020). Then, the transport of cytosolic pyruvate into the mitochondria is provided by the mitochondrial pyruvate carriers (MPC1-2), which is a crucial step for the whole mitochondrial bioenergetics function (McCommis and Finck Citation2015; Cunningham and Rutter Citation2020). This MC is a gatekeeper for pyruvate entry to take care given that alteration of this carrier impacts on mitochondrial function, gluconeogenesis, the regulation of blood glucose levels, and is associated with diabetes and cancer (Bender and Martinou Citation2016). Last but not least, the mitochondrial calcium uniporter channel (MCU) is of great importance given that mitochondrial Ca2+ uptake is provided by MCU (Kamer and Mootha Citation2015; Alevriadou et al. Citation2021). Interestingly MCU, which is a redox sensor with at miminum ROS H2O2, interacts with the MC APC2 (SLC25A23), cardiolipins, is involved in mitochondrial respiration, and cell death including apoptosis, necrosis and ferroptosis through Ca2+ dynamics (Kamer and Mootha Citation2015; Alevriadou et al. Citation2021). To close this short loop, once again, MCs are regulated by many factors including ions Ca2+ and Mg2+, ATP, and reactive species with at minimum ROS whose oxidation can impair their carrier function (Palmieri and Monné Citation2016; Palmieri Citation2013; Cunningham and Rutter Citation2020; McCommis and Finck Citation2015; Kamer and Mootha Citation2015).
Interactions between mitochondria and other organelles
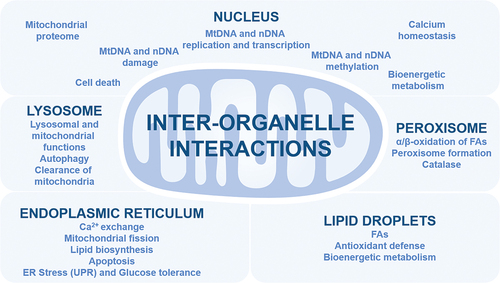
Mitochondria interact with other organelles using membrane contact sites, vesicle transport, and molecules transduction for many functions, including bioenergetic metabolism, immune response, cell death, organelle biogenesis, lipid biosynthesis and exchange including PS (phosphatidylserine), cholesterol metabolism, and nuclear DNA function (Xia et al. Citation2019). Several notable interactions are discussed in the next four sections ().
Mitochondria and the Endoplasmic Reticulum
Mitochondria interact with the ER using stable close membrane contact sites called mitochondria-associated ER membranes (MAMs), a maintained interaction during ER movement along the cytoskeleton (Xia et al. Citation2019; Gordaliza‐Alaguero et al. Citation2019; Gao et al. Citation2020). The MAMs contain many proteins, including VDACs, MFN, inositol 1,4,5 triphosphate receptor (IP3R), BCL-2 proteins, glucose-regulated protein 75 (GRP-75/MORTALIN/MTHSP70), ER BiP (HSP70 member 5, GRP78), and ER Sarcoplasmic/ER calcium ATPase (SERCA) (Xia et al. Citation2019; Gordaliza‐Alaguero et al. Citation2019; Gao et al. Citation2020; Cheema et al. Citation2021). Mitochondria-ER contact sites are involved in Ca2+ exchange, enzymes involved in lipid biosynthesis, including PS and phosphatidylethanolamine (PE), cholesterol transport, apoptosis control, including ER stress-mediated apoptosis, the ER stress response called unfolded protein response (UPR), insulin signalling, and glucose tolerance (Xia et al. Citation2019; Gordaliza‐Alaguero et al. Citation2019; Gao et al. Citation2020; Cheema et al. Citation2021). In addition, mitochondria-ER contact sites where mitochondrial division occurs, coordinate mtDNA replication with mitochondrial division, and promote mtDNA translocation to new mitochondria (Friedman et al. Citation2011; Lewis et al. Citation2016; Qin et al. Citation2020). Finally, mitochondria also communicate with the Golgi apparatus, in close contact with the ER, the precise communication being unclear, and possibly dependent on Ca2+ homoeostasis (Gordaliza‐Alaguero et al. Citation2019).
Mitochondria, peroxisome and lipid droplets
Mitochondria interact with the single membrane-bound peroxisomes, which are involved in many pathways, including α- and β-oxidation of very long chain fatty acids (VLCFAs) to generate MCFAs, ROS H2O2 and acetyl-CoA, glyoxylate metabolism, amino acid regulation, and antioxidant defence through catalase (Shai et al. Citation2016; Xia et al. Citation2019; Tanaka et al. Citation2019; Guillén-Samander et al. Citation2021). Peroxisomal membrane contains “mitochondrial-like” solute carrier family transporters, including SLC16 and SLC27, for metabolites and essential cofactors including CoA, NAD+, NADP+, FAD, FMN and ATP used for the β-oxidation of fatty acids (Chornyi et al. Citation2021). Peroxisomal integrity maintenance through mitochondria close interaction is essential (Shai et al. Citation2016; Xia et al. Citation2019). Mitochondria contribute to the formation of peroxisomes, including through peroxisome proliferator-activated receptors (PPARs), and the transcription factor peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), which modulates antioxidant enzymes, peroxisomal and mitochondrial biogenesis and functions (Peeters et al. Citation2015; Xia et al. Citation2019). Peroxisomes can fuse and divide, and are degraded in a lysosomal manner, called pexophagy, a process that is regulated by mitochondrial OM E3-ubiquitin ligase Membrane-associated RING-CH protein V (MARCH5) (Xia et al. Citation2019; Zheng et al. Citation2021). Mitochondria-peroxisomes interaction occurs with peroxisomal proteins, including peroxin-11 (PEX11) that binds to mitochondrial MDM34 protein (Xia et al. Citation2019). Mitochondria-peroxisomes interaction also occurs using vesicular transport, and diffusion of reactive species, including ROS, and metabolites, including fatty acids and acetyl-coA (Xia et al. Citation2019; Wang et al. Citation2022). Alteration of peroxisome functions impact on mitochondrial functions (Peeters et al. Citation2015; Tanaka et al. Citation2019; Xia et al. Citation2019). In addition, both peroxisomes and mitochondria interact with lipid droplet for fatty acids and antioxidant enzyme dynamics (Shai et al. Citation2016; Ravi et al. Citation2021). Mitochondria actively interact with lipid droplet for fatty acid biosynthesis, including triacylglycerol (TAG), and ATP generation (Gordaliza‐Alaguero et al. Citation2019). This interaction, which is dependent on nutrient availability, impacts on fatty acid metabolism, mitochondrial dynamics, and bioenergetic metabolism (Gordaliza‐Alaguero et al. Citation2019).
Mitochondria and lysosome
Lysosomes actively degrade mitochondrial proteins and damaged mitochondria (Todkar et al. Citation2017; Gordaliza‐Alaguero et al. Citation2019; Deus et al. Citation2020). The mitochondria-lysosomes crosstalk affects both mitochondrial and lysosomal functions, including through lysosomal small GTPase RAB5 and RAB7 (ras-related protein), mitochondrial fission 1 (FIS1) and MFN2, and calcium exchange (Todkar et al. Citation2017; Gordaliza‐Alaguero et al. Citation2019; Deus et al. Citation2020). This interaction, which could play a role in autophagy, is dependent on glucose, nutrient availability, and mammalian target of rapamycin (mTOR response) (Todkar et al. Citation2017; Gordaliza‐Alaguero et al. Citation2019; Deus et al. Citation2020). Interestingly, peroxisomes also communicate with lysosomes for cholesterol metabolism (Shai et al. Citation2016).
Mitochondria and the nucleus
The mitochondrial genome is only coding for 13 proteins of OXPHOS, 2 ribosomal RNAs, and 22 transfer RNAs (Ricchetti Citation2018; Xia et al. Citation2019). All the other mitochondrial proteins are encoded by the nuclear genome, including RNA polymerase mitochondrial (POLRMT), POLG1/2 mtDNA polymerase subunits, mitochondrial transcription factor A (TFAM), PGC-1α, and the key antioxidant defence and OXPHOS modulator nuclear factor, erythroid 2-related factor 2 transcription factor (NRF2) (Xia et al. Citation2019). Linked to a transitory giant mitochondrial tubular network for high ATP generation, mitochondrial DNA transcription and initiation of replication are prevalently coordinated with nuclear DNA synthesis phases, which includes a mtDNA replication and transcription pause during S-phase of the cell cycle (Chatre and Ricchetti Citation2013). Dysfunctional crosstalk between the nucleus and mitochondria induces bioenergetic metabolism alterations, oxidative distress with DNA damage in both organelles, calcium overload, and mTOR pathway disturbance (Guaragnella et al. Citation2018; Xia et al. Citation2019). Noncoding RNAs, including long noncoding RNAs (lncRNAs), produce both by the nuclear genome and mtDNA are part of the communication between the two organelles, for mitochondrial stability, apoptosis, and nuclear genome reprogramming (Xia et al. Citation2019). In addition, it is important to note that nuclear-encoded microRNAs modulate mitochondrial respiration (Xia et al. Citation2019). Moreover, mitochondrial DNA fragments named nuclear DNA sequences of mitochondrial origin (NUMTs) colonise the nuclear genome (Ricchetti et al. Citation2004; Chatre and Ricchetti Citation2011). Nuclear DNA methylation by DNA methyltransferases (DNMTs), including DNMT1 and DNMT3, affects mitochondria through methylation of genes involved in mitochondria homoeostasis, including PGC-1α, in mtDNA, including POLG1, and in OXPHOS, including NADH:ubiquinone oxidoreductase subunit (NDUF), ubiquinol-cytochrome c reductase core protein 2 (UQCR2) for complex III, and cytochrome c oxidase subunit 7B (COX7B) for complex IV (Lopes Citation2020). Mitochondrial DNA is also methylated, including by the unique mitochondrial DNMT1, but the functional role remains unclear (Lopes Citation2020). Interestingly, several vitamins play roles in both mitochondria and the nucleus (Janssen et al. Citation2019). For example, B-vitamins, including thiamine (B1), riboflavin (B2), niacin (B3), pantothenate (B5) and pyridoxal phosphate (B6) are involved in the TCA cycle than OXPHOS, by contributing to the biosynthesis of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), and by regulating many redox reactions such as antioxidant GSH/GSSG system, NAD+/NADH system, NADP+/NADPH system and the biosynthesis of [Fe-S] biosynthesis (Janssen et al. Citation2019). In addition, B-vitamin B9/B11 is involved in folate metabolism (Janssen et al. Citation2019). The hypoxia response Hypoxia-inducible factor 1 (HIF-1) transcription factor, as well methylation and demethylation of DNA and histones are regulated by B-vitamins, including B2, B6 and cobalamin (B12), by affecting HIF-1 stability, ten-eleven translocation family of DNA demethylase (TET demethylase) regulation, and histone lysine demethylase (KDM demethylase) regulation respectively (Janssen et al. Citation2019). Histone acylation is also modulated by histone acetyltransferase (HAT) and lysine acetyltransferase (KAT) fuelled by mitochondria-derived acetyl-coA, and by sirtuin deacetylases, which are dependent on B3 vitamin (Janssen et al. Citation2019). Modulation of mitochondrial respiration by B3 for OXPHOS complex I, and B2 for OXPHOS complex II, affects mitochondrial ROS generation, and oxidative distress that will further affect nuclear DNA stability and function (Janssen et al. Citation2019). Moreover, during oxidative distress, accumulation of mouse double minute 2 (MDM2) into mitochondria, which is an oncoprotein that regulates nuclear transcription factors including tumour protein P53, reduces the generation of mtRNA NADH-dehydrogenase 6 (ND6), alters OXPHOS complex I function, and induces mitochondrial ROS generation (Arena et al. Citation2018). In addition, storkhead box 1 (STOX1) winged-helix transcription factor, whose accumulation causes human gestational disease pre-eclampsia characterised by hypertension, proteinuria, and cardiac hypertrophy, induces a switch of the nitroso-redox balance from ROS H2O2 to RNS ONOO−, and promotes mitochondrial hyperactivity at 20% O2 and in hypoxia in trophoblast cells (Doridot et al. Citation2014). The STOX1 effects in vitro and in vivo are successfully counteracted by supplementation in nitric oxide synthase cofactor tetrahydrobiopterin (BH4), which also restores gestational tension and cardiac function (Chatre et al. Citation2022). Finally, mitochondrial dysfunctions alter telomere stability in the nuclear genome, and TERT (telomerase reverse transcriptase), which cycles between mitochondria and the nucleus, with a possible mitochondrial telomere-independent function of telomerase, positively regulates mitophagy through PINK1 modulation (Zheng et al. Citation2019; Shin and Chung Citation2020). Upon oxidative distress in cancer cells, including ROS accumulation, over translocation of TERT into mitochondria could contribute to reduce mtROS generation, and prevent nuclear DNA damage and apoptosis (Singhapol et al. Citation2013). Another example is the nuclear-encoded ENDOG, which degrades nuclear DNA during apoptosis, that removes damage mtDNA, and that stimulates mtDNA replication initiation (Wiehe et al. Citation2018). Altogether, many different ways for mitochondria-nucleus communication.
What makes this story more interesting is the involvement of ROS, and more largely, of the reactive species, including mitochondrial reactive species, at all discussed levels.
The reactive species interactome
The concept of the Reactive Species Interactome (RSI) is the interaction between ROS, RNS, RSS, RCS, notable redox enzymes and their downstream biological targets (Sies Citation2015; Cortese-Krott et al. Citation2017, Citation2020; Bourgonje et al. Citation2020; Malard et al. Citation2021) . Originally defined as a redox system, the RSI could be at the heart of the redox interactome, and requires investigation to be fully demonstrated in physiology and pathology (Cortese-Krott et al. Citation2017, Citation2020; Santolini et al. Citation2019; Bourgonje et al. Citation2020; Malard et al. Citation2021). To overview, the ROS metabolism initiates from the precursor O2 to form O2−-derived reactive species (Chen et al. Citation2009; Hayyan et al. Citation2016; Sies and Jones Citation2020) including H2O2 (Sies Citation2020), and notable ROS enzymes are cytosolic and mitochondrial SODs that metabolise O2− to generate H2O2 (Fukai and Ushio-Fukai Citation2011), peroxisomal catalase that metabolises H2O2 to generate water and O2 (Glorieux and Calderon Citation2017), peroxisomal xanthine oxidoreductase (XOR) that generates both O2− and H2O2 from O2 (Bortolotti et al. Citation2021), and myeloperoxidase (MPO) that metabolises H2O2 with Cl− to hypochlorous acid (HOCl) (Ray and Katyal Citation2016). The RNS metabolism generates nitric oxide (NO)-derived reactive species (Farah et al. Citation2018), including toxic peroxynitrite (ONOO−) (Bartesaghi and Radi Citation2018), nitrite (NO2−) (Castiglione et al. Citation2012) and nitrate (NO3−) (Feelisch Citation2012), and the notable RNS enzyme is nitric oxide synthase (NOS) with neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) (Roe and Ren Citation2012; Forstermann and Sessa Citation2012; Hong et al. Citation2021). The RSS metabolism, which depends on cysteine metabolism, generates hydrogen sulphide (H2S)-derived reactive species (Cao et al. Citation2019; Olson Citation2020) including polysulphides H2Sn (Olson Citation2018), through many RSS notable enzymes that include cystathionine-γ-lyase (CSE) that metabolises L-homocysteine and L-cysteine to L-homolanthionine and H2S (Kolluru et al. Citation2013; Olson et al. Citation2018; Cao et al. Citation2019) and cystathionine b-synthase (CBS) that metabolises L-cysteine to generate L-serine, L-lanthionine and H2S (Kolluru et al. Citation2013; Olson et al. Citation2018; Cao et al. Citation2019). The RCS metabolism, which connects ROS, RNS, and RSS, includes malondialdehyde (MDA) (Cighetti et al. Citation2001), methylglyoxal (MGO) (Schalkwijk and Stehouwer Citation2020) and 4-hydroxy-trans-2-nonenal (4-HNE) (Di Domenico et al. Citation2017), and mainly originates from polyunsaturated fatty acids (PUFAs) peroxidation (Gaschler and Stockwell Citation2017; Altomare et al. Citation2021). The notable RCS enzymes that block this peroxidation are the selenoenzymes glutathione peroxidase (GPX) that metabolise H2O2 and reduced glutathione GSH to generate oxidised GSSG and water (Espinoza et al. Citation2008; Dixon et al. Citation2012) and peroxiredoxin (PRDX) that metabolise H2O2 and other peroxides to generate water (Garcia-Bonilla and Iadecola Citation2012; Park et al. Citation2016).
Figure 3. (a) key reactive species from the reactive species interactome. Major reactive species are presented for each family. Reactive species target mostly any intracellular and extracellular compounds, including DNA, proteins, lipids and themselves. Non-exhaustively, several notable modifications induced by different reactive species are presented. (b) Only ROS in mitochondria? Presence of ROS, RNS, RSS, RCS in mitochondria is highlighted, which includes notable enzymes involved in both RSI dynamics, and mitochondria homeostasis. CAT/GOT, cysteine aminotransferase/glutamate oxaloacetate transaminase; CytC, cytochrome c; COX, cytochrome c oxidase; DHODH, dihydroorotate dehydrogenase; DLD, dihydrolipoamide dehydrogenase; ER, Endoplasmic Reticulum; ETF-QOR, electron transfer flavoprotein-ubiquinone oxidoreductase; G3PDH, glycerol 3-phosphate dehydrogenase; GPX4, glutathione peroxidase 4; hydroxy-aa, hydroxyl-amino acid; MnSOD, manganese superoxide dismutase; MPO, myeloperoxidase; mtNOS, mitochondrial nitric oxide synthase; NO, nitric oxide; NOX4, NADPH oxidase 4; ODH, 2-oxoglutarate dehydrogenase; OGDH, 2-oxoglutarate dehydrogenase; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; RCS, reactive carbonyl species; SQOR, sulfide quinone oxidoreductase; XOR, xanthine oxidoreductase.
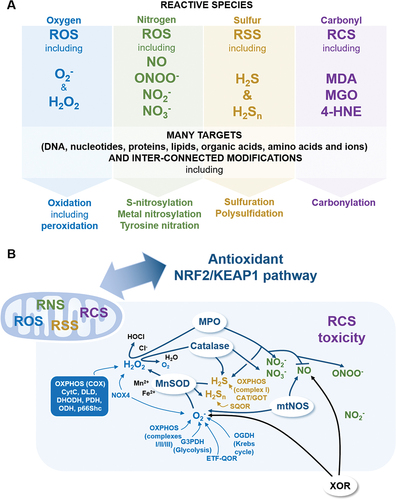
Importantly, notable redox enzymes link ROS, RNS, RSS and RCS together. Superoxide dismutase regulates RSS by oxidising H2S into polysulphides H2Sn (Olson et al. Citation2018). Then, catalase is also able to produce nitrite NO2− and nitrate NO3− (Ogino et al. Citation2001). Catalase could also be a sulphide-sulphur oxidoreductase to metabolise H2S as sulphide oxidase, to generate H2S as sulphide reductase, and to eliminate H2Sn (Olson et al. Citation2017). Xanthine oxidoreductase (XOR) is involved in purine catabolism to form uric acid (Vergeade et al. Citation2012; Snezhkina et al. Citation2019; Bortolotti et al. Citation2021). Importantly, XOR also contributes to produce NO through nitrite and nitrate reduction (Vergeade et al. Citation2012; Snezhkina et al. Citation2019; Bortolotti et al. Citation2021). Interestingly, MPO is also a major NO scavenger, and oxidises nitrite to generate NO2 or peroxynitrite (ONOO−), which is the MPO-H2O2-nitrite system (Burner et al. Citation2000; Galijasevic et al. Citation2003). Furthermore, MPO also oxidises H2S, and generates different RCS, including acetone and acrolein, from hydroxy-amino acids (Sena and Chandel Citation2012; Pálinkás et al. Citation2015; Garai et al. Citation2017). Regarding NOS, coupled NOS generates NO, while uncoupled NOS generates O2−, and NO reacts with O2− to form ONOO−, and with H2S to form nitroxyl (Olson Citation2018; Wu et al. Citation2018; Snezhkina et al. Citation2019). Finally, RCS, including malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), regulate XOR and GPX activities (Cighetti et al. Citation2001; Park et al. Citation2003).
Reactive species are diffused in the entire cytoplasm, including in mitochondria. Therefore, next four sections focus on mitochondrial reactive species sources, notable redox enzymes linking the RSI and mitochondria, namely SOD, catalase, XOR, MPO, NOS, and GPX, and the role of the antioxidant NRF2/KEAP1 (nuclear factor E2-related factor 2/Kelch-like ECH-associated protein 1) signalling pathway in mitochondria.
Only ROS as mitochondrial reactive species?
A big mistake is to claim that mitochondria are the main contributor in ROS generation, which is nowadays not true (Tirichen et al. Citation2021; Zhang and Wong Citation2021). Mitochondria are significant sources of ROS but not necessarily the main contributor (Zhang and Wong Citation2021). For example, the ER is another significant contributor of intracellular ROS (Cao and Kaufman Citation2014; Tirichen et al. Citation2021). This debate still requires investigation improving the understanding of organelle contribution. In addition, in most studies and reviews, mitochondrial oxidative distress, which is the imbalance between ROS generation and antioxidant defence activity, is oversimplified to mitochondrial ROS (mtROS), mainly O2− and H2O2 (Murphy Citation2009; Sabharwal and Schumacker Citation2014; Tirichen et al. Citation2021). Only mtROS in mitochondria?
Mitochondrial ROS
Mitochondrial ROS O2− is generated by different mitochondrial proteins, including glycerol 3-phosphate dehydrogenase (G3PDH) during glycolysis, 2-oxoglutarate dehydrogenase (OGDH) in the tricarboxylic acid TCA/Krebs cycle, OXPHOS complexes I, and III (Murphy Citation2009; Sabharwal and Schumacker 2Citation014; Sies Citation2015, Citation2017; Snezhkina et al. Citation2019; Sies and Jones Citation2020; Tirichen et al. Citation2021). Mitochondrial OXPHOS complex II, and electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-QOR) also produce O2− at low level (Kao et al. Citation2010; Mailloux et al. Citation2013; Tirichen et al. Citation2021). Mitochondrial NADPH oxidase 4 (NOX4) generates O2−, and preferentially H2O2 (Shanmugasundaram et al. Citation2017; Tirichen et al. Citation2021). Reduction of O2− to H2O2 is catalysed by SOD, including in mitochondria with antioxidant isoform MnSOD SOD2 (Ganini et al. Citation2018; Palma et al. Citation2020; Kitada et al. Citation2020; Tirichen et al. Citation2021). Interestingly, related to iron overload and manganese deficiency, a prooxidant alternative isoform also exists, namely peroxidase FeSOD2, which uses H2O2 to overoxidize many cellular components (Ganini et al. Citation2018; Palma et al. Citation2020; Kitada et al. Citation2020; Tirichen et al. Citation2021). Although Cu/Zn SOD1 is not the dedicated mitochondrial SOD, accumulation of mutant SOD1 in amyotrophic lateral sclerosis (ALS) decreases mitochondrial protein import, and induces mitochondrial dysfunction (Li et al. Citation2010; Tafuri et al. Citation2015). In addition, accumulation of misfolded SOD1 has a possible pathological role in ALS (Paré et al. Citation2018; Benkler et al. Citation2018). Moreover, aggregation of SOD1 can spread from neuron to neuron in ALS, and a treatment based on anti-SOD1 antibodies is considered as a promising ALS therapy (Paré et al. Citation2018; Benkler et al. Citation2018). Furthermore, microtubule-associated protein tau affects mitochondria-lysosomes communication, and mitochondrial function by increasing nutrient-induced mitochondrial activity (NiMA), and both suppressing SOD1 activity and mtDNA replication (Norambuena et al. Citation2022). Accumulation of amyloid-β oligomers in Alzheimer’s disease inhibits tau effects, including in SOD1 activity, and mitochondrial function (Norambuena et al. Citation2022). In addition, OXPHOS IV cytochrome c oxidase (COX), cytochrome c and dihydrolipoamide dehydrogenase (DLDH) regulate H2O2 metabolism through direct interaction with this reactive species, adaptor protein p66Shc is a source of H2O2, and dihydroorotate dehydrogenase (DHODH), pyruvate dehydrogenase (PDH) and 2-oxoglutarate dehydrogenase (ODH) are sources of O2− and H2O2 (Lambert and Brand Citation2009; Brand Citation2010; Kao et al. Citation2010; Mailloux et al. Citation2013; Cortese-Krott et al. Citation2017; Snezhkina et al. Citation2019; Sies and Jones Citation2020; Tirichen et al. Citation2021). Then, peroxisomal catalase, thanks to two peroxisomal targeting signals (Petrova et al. Citation2004), which dismutates H2O2 to O2 and H2O, is also found in mitochondria, thanks to also a non-canonical mitochondrial targeting signal (Petrova et al. Citation2004; Tirichen et al. Citation2021). The question still remains open on the competition of peroxisomal and mitochondrial targeting signals for the dual localisation of catalase (Petrova et al. Citation2004; Ast et al. Citation2013). Next, XOR also contributes to produce mitochondrial O2−, although this redox enzyme is not found in mitochondria (Vergeade et al. Citation2012; Snezhkina et al. Citation2019; Malard et al. Citation2021; Bortolotti et al. Citation2021). Finally, MPO has been recently found in mitochondria in addition to the cytosol, vesicles, and the nucleus (Sena and Chandel Citation2012; de Araujo et al. Citation2013; Pálinkás et al. Citation2015; Garai et al. Citation2017; Malard et al. Citation2021).
Mitochondrial RNS, RSS, and RCS
As previously mentioned, SOD and catalase are both present in mitochondria. Given that SOD also oxidises H2S into polysulphides H2Sn (Olson et al. Citation2018), mitochondrial SOD is involved in ROS and RSS metabolisms. In addition, given that catalase is also able to produce NO2− and NO3− (Ogino et al. Citation2001), and that catalase could also be a sulphide-sulphur oxidoreductase (Olson et al. Citation2017), mitochondrial catalase is involved in ROS, RNS and RSS metabolisms. Moreover, as MPO is also a major NO scavenger that oxidises NO2− to generate NO2 or ONOO− (Burner et al. Citation2000), that oxidises H2S (Pálinkás et al. Citation2015; Garai et al. Citation2017) and that generates several RCS , mitochondrial MPO is involved in ROS, RNS, RSS and RCS. Then, mitochondria contain their own calcium-sensitive mitochondrial NOS (MTNOS) (Ghafourifar and Cadenas Citation2005; Lacza et al. Citation2009). Importantly, GPX4, which neutralises lipid hydroperoxides, the major source of RCS, is localised to the nucleoplasm and mitochondria (Cighetti et al. Citation2001; Park et al. Citation2003; de Bari et al. Citation2020; Tadokoro et al. Citation2020).
Finally, other mitochondrial proteins are involved in the RSI dynamics, in particular through the RSS family. The accessory sulfurtransferase subunit of OXPHOS complex I is a source of H2S (Olson Citation2018). The enzyme cysteine aminotransferase (CAT), which is involved in H2S generation, is also a glutamate oxaloacetate transaminase (GOT) that metabolises oxaloacetate to produce aspartate, α-ketoglutarate, and glutamate in mitochondria (Miyamoto et al. Citation2014; Mellis et al. Citation2021). Hydrogen sulphide catabolism to form thiosulphate occurs, including in mitochondria, and mitochondrial sulphide quinone oxidoreductase (SQOR) generates persulfides H2S2 (Kolluru et al. Citation2013; Olson Citation2018; Cao et al. Citation2019). Last, rhodanese or thiosulphate sulfurtransferase (TST) is a mitochondrial enzyme that converts RSS SO32− to S2O32−, and that is connected to OXPHOS, including complex I system for ROS generation (Krueger et al. Citation2010; Paul et al. Citation2020).
The implication of the antioxidant NRF2/KEAP1 pathway in mitochondrial function
It is ultimately impossible to talk about the RSI without addressing the major transcription factor for the oxidative dis/eu-stress response, namely NRF2. The NRF2/KEAP1 signalling pathway is based on the transcriptional regulation by NRF2 at the antioxidant response element (ARE) of the promotors of many cytoprotective genes involved in the redox system, including the glutathione system, GPX, thioredoxin (TXN), NADPH regeneration, and haem and iron metabolism (Tonelli et al. Citation2018). This transcriptional regulation is mediated by the tight interaction between NRF2 and KEAP1, a major sensor of oxidative and electrophilic stress and the adaptor subunit of CULLIN3-E3 ubiquitin ligase (Tonelli et al. Citation2018). The NRF2 activity is regulated by many pathways, including inflammatory stimuli, xenobiotics, microRNAs, cofactors, coactivators and competitors, which makes altogether this signalling pathway complex and multifactorial (Tonelli et al. Citation2018). The NRF2 activity maintains redox homoeostasis, including redox enzymes and reactive species, promotes angiogenesis, and exhibits both anti-cancer and anti-inflammation effects (Wu et al. Citation2019). Interestingly, on one hand, H2S can bind to cysteine residues of NRF2 and KEAP1, which contributes to regulates this signalling pathway (Scammahorn et al. Citation2021), on the other, NRF2/KEAP1 pathway induces increase in persulfides that belong to the sulfane sulphur family from RSS, which are intermediates in the biosynthesis of [Fe-S] proteins (Alam et al. Citation2023). The targeting of NRF2/KEAP1 pathway is then a very promising strategy to limit inflammation, including in inflammatory bowel disease (Geertsema et al. Citation2023), liver injury (Hiemstra et al. Citation2022), and cancer (Wu et al. Citation2019).
Focusing on mitochondria, the NRF2/KEAP1 signalling pathway is central to the RSI and mitochondria by regulating cytoprotective agents in mitochondria, including the mitochondrial GSH pool and redox enzymes such as SOD, catalase and GPX, mitochondrial FAO, the Krebs cycle, the OXPHOS system, mitochondrial calcium homoeostasis, mitochondrial integrity and mitophagy (Holmström and Finkel Citation2014; Esteras and Abramov Citation2022). The NRF2 activity mediates mitochondrial activation through mitochondrial SQOR and RSS metabolism by increasing cysteine pool availability (Alam et al. Citation2023). The activation of NRF2 also contributes to remove damage mitochondria by PARKIN/PINK1-dependent mitophagy (Gumeni et al. Citation2021). In addition, disruption of the mitochondrial GSH pool activates the NRF2/KEAP1 signalling pathway, which links NRF2 to mitochondrial redox system (Cvetko et al. Citation2021). This relationship is strengthened in colon cancer where NRF2 silencing upregulates microRNAs through the central inflammation transcription factor nuclear factor-κB (NF-KB), which ultimately alteres cytochrome c oxidase subunit-1 (MT-CO1) of the OXPHOS complex IV system (Jung et al. Citation2017). Another example is in vascular smooth muscle cells where the silencing of NRF2 reduces the expression of mitochondrial OXPHOS complex I NAD(P)H:quinone oxidoreductase (NQO1), which contributes to alter H2S dynamics and to inhibit H2S calcification-suppressing activity (Aghagolzadeh et al. Citation2017). Altogether, the NRF2/KEAP1 signalling pathway is highly active in mitochondria, and mitochondrial dysfunction alters this central pathway.
The mitochondria-RSI axis in mitochondrial diseases and dysfunction
Here, the aim is not to compile all the diseases in which, together, mitochondria and reactive species are involved. Several key mitochondrial diseases and diseases with mitochondrial dysfunction are discussed.
First, a mitochondrial disease is described as a mitochondrial impairment caused by mutations at mitochondrial genes or nuclear genes coding for mitochondrial proteins (Gorman et al. Citation2016; Suomalainen and Battersby Citation2018; Bruni Citation2021). Mitochondrial diseases can affect any organ, including brain with neurodegeneration, strokes, ataxia, Parkinson’s Disease and psychiatrics symptoms, liver, muscle, kidney, and the heart (Suomalainen and Battersby Citation2018). The LHON disease (Leber’s hereditary optic neuropathy), caused by LHON-associated mtDNA mutations in a subunit of OXPHOS complex I NADH:ubiquinone oxidoreductase, and discovered by Douglas C. Wallace and colleagues in 1988, is the first example of mitochondrial disorder with bioenergetic failure (Wallace et al. Citation1988; Wallace Citation2005, Citation2013; Kirches Citation2011). The MELAS disease (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes) is another well-known mitochondrial disease, which is a myopathy caused by mutations in the mitochondrial tRNALeu(UUR) gene in more than 80% of MELAS patients (Wallace Citation2013; Ikeda et al. Citation2018; Bruni Citation2021). Mitochondrial DNA mutations are also associated with carotid intima-media thickness, atherosclerosis, and cardiovascular diseases (Kirichenko et al. Citation2020; Bruni Citation2021). For examples, mtDNA rearrangement mutations, mutations in mitochondrial DNA polymerase POLG, mtDNA maintenance protein twinkle, mitochondrial thymidine kinase 2 (TK2), mitochondrial deoxyguanosine kinase (DGUOK), mitochondrial malate dehydrogenase 2 (MDH2), and mitochondrial GTPase optic atrophy protein 1 (OPA1) lead to mtDNA deletion and depletion that affect muscle, pancreas with type II diabetes, the heart, and brain, including with neurodegeneration (Wallace Citation2005, Citation2013; Sánchez-Caballero et al. Citation2016; Ait-El-Mkadem et al. Citation2017; Suomalainen and Battersby Citation2018). Moreover, mitochondrial protein synthesis defects associated with mitochondrial tRNA mutations, and disruption of mitochondrial protein quality control, including with mAAA protease and chaperone complex, are other examples of mitochondrial diseases (Suomalainen and Battersby Citation2018). Finally, mitochondrial diseases are also associated with obesity, dyslipidemia, and arterial hypertension (Wallace Citation2005).
Then, diseases with mitochondrial dysfunction are diseases that involve one or several factors or events or alterations of nuclear gene(s) whose impairment can affect mitochondrial function, non-exhaustively including bioenergetic failure, oxidative distress through ROS, and cell death types modulation, including apoptosis and ferroptosis (Wallace Citation2005; Cao and Kaufman Citation2014; Gladyshev Citation2014; Sies Citation2015; Cortese-Krott et al. Citation2017, Citation2020; Stockwell et al. Citation2017; Santolini et al. Citation2019; Bourgonje et al. Citation2020; Malard et al. Citation2021; Tirichen et al. Citation2021). While most of studies focused on ROS, here, the scientific community from both redox and mitochondria fields is invited at improving the roles of other reactive species linking mitochondrial dysfunction and many diseases. Mitochondrial dysfunction contribute to inflammation, and many pathologies, including neuromuscular diseases, cancer, neurodegenerative disorders, and premature ageing (Chatre et al. Citation2015; Bajzikova et al. Citation2019; Bruni Citation2021). Low mtDNA replication, and mitochondrial 7S DNA accumulation are associated with Parkinson’s Disease (Podlesniy et al. Citation2019). Furthermore, alteration and aberrant function of mitochondrial kinase PINK1, which is involved in mitochondrial protein quality control and mitophagy, contribute to the pathophysiology of Parkinson’s Disease (Podlesniy et al. Citation2019; Martín-Jiménez et al. Citation2020; Bruni Citation2021). Moreover, regarding mitochondrial protein import system, in heart mitochondria, increase in TOM complex stability is cardioprotective, including through TOM20 and TOM22, and favours the integration of VDAC in OM, a key protein involved in mitochondrial permeability transition pore (mPTP), and calcium dynamics (Zhao and Zou Citation2021; Sander et al. Citation2021). Heart dysfunction, failure, and cardiomyopathies are associated with mitochondrial import system impairment (Zhao and Zou Citation2021). In addition, impairment of TOM or TIM import activity induces mitochondrial stress response, through mitochondrial protein quality control system activation (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). This activation includes mitochondrial PINK1 and E3 ubiquitin ligase PARKIN, two key proteins involved in Alzheimer’s disease, Parkinson’s disease, and possibly in cancer development (Harbauer et al. Citation2014; Pfanner et al. Citation2019). Mitochondrial protein import system is therefore an essential pathway for mitochondrial stability and functions (Schmidt et al. Citation2010; Harbauer et al. Citation2014; Pfanner et al. Citation2019; Zhao and Zou Citation2021). Another good example is the progeroid disorder Cockayne Syndrome, which is caused by DNA repair proteins CSA or CSB mutations, and that is characterised by accelerated ageing, neurological and developmental abnormalities, and hypersensitivity to oxidative distress (Nardo et al. Citation2009; Chatre et al. Citation2015; Alupei et al. Citation2018). In the Cockayne Syndrome, caused by nitroso-redox imbalance, meaning reactive species dysregulation, the accumulation of HtrA3 serine protease impairs mitochondrial bioenergetic metabolism, including by deregulating mitochondrial POLG1, and leads to excessive replicative senescence (Chatre et al. Citation2015; Crochemore et al. Citation2019). This example is one example amongst more and more examples, with reactive species associated with mitochondrial diseases and mitochondrial dysfunction in diseases, including through OXPHOS alteration (Suomalainen and Battersby Citation2018). Another concern is ageing, which is a physiological process associated with mitochondrial dysfunction, and many diseases. The link between mitochondria and ageing is a long story, including through mitochondrial ROS (Wallace Citation2005; Gladyshev Citation2014). Originally, the free radical theory of aging was the concept of ageing caused by ROS, and accumulation of oxidative damage (Wallace et al. Citation1988; Gladyshev Citation2014). Nowadays, improving knowledge of ageing, many questions are still opened regarding ROS toxicity and roles, oxidative and other types of damage, mtDNA mutations, and nutrition related to mitochondrial bioenergetic metabolism (Wallace Citation2005; Gladyshev Citation2014). Oxidative distress mediated by several reactive species exerts a pro-ageing effect, namely (1) ROS, including O2− and H2O2, by increasing senescence, (2) RNS, including NO and ONOO−, by increasing nitrative damage, and (3) RCS, including 4-HNE and MDA (Malard et al. Citation2021). Interestingly, oxidative distress mediated by RSS, including H2S and H2Sn, exerts an anti-ageing effect by inhibiting ROS and RNS generation (Malard et al. Citation2021). In addition, antioxidant defense decline, including SOD and catalase activity, and key enzymes overactivation, including XOR and MPO, are pro-ageing (Malard et al. Citation2021).
Finally, given that NRF2 is deficient or dysregulated in mitochondrial-related disorders, in cancer, in neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease, the mitochondria-RSI axis will have to be investigated including the NRF2/KEAP1 signalling pathway (Holmström et al. Citation2016; Jung et al. Citation2017; Wu et al. Citation2019; Song and Long Citation2020; Cvetko et al. Citation2021; Hiemstra et al. Citation2022; Geertsema et al. Citation2023).
In this RSI-mitochondria axis, in physiology and pathology, two players may potentiate the effects, which is the bacteria microbiota and the circadian clock.
The bacterial microbiota in the mitochondria-RSI axis
One of the most studied bacterial microbiota is the gut microbiota, which includes the most abundant phyla Firmicutes and Bacteroidetes (Liu et al. Citation2020).
The microbiota-gut-brain axis, which is defined as a bidirectional communication system between the gut and the brain, is related to metabolic pathways, through multiple bacterial metabolites, such as SCFAs, namely acetate, propionate, and butyrate (Dumitrescu et al. Citation2018; Liu et al. Citation2020). In the gut, Bacteroides, Bifidobacterium, Parabacteroides and Escherichia spp. generate neurotransmitter GABA (Shandilya et al. Citation2022). The gut bacterial microbiota in the microbiota-gut-brain axis could be a susceptibility factor for neurological diseases, including epilepsy, Huntington’s disease, Parkinson’s disease, autism spectrum disorder, and Alzheimer’s disease (Dumitrescu et al. Citation2018; Cryan et al. Citation2020). The gut bacterial microbiota is also involved in neurodevelopment, including neurogenesis, myelination, and microglial activation, through SCFAs and other metabolites (Dumitrescu et al. Citation2018; Cryan et al. Citation2020). In Alzheimer’s disease, gut dysbiosis leads to overgeneration of bacterial proinflammatory neurotoxins, and may promote neuroinflammation, amyloid-beta accumulation, increase in blood-brain-barrier permeability, insulin resistance, and oxidative distress (Dumitrescu et al. Citation2018; Liu et al. Citation2020; Shandilya et al. Citation2022). The gut bacterial microbiota is suggested either to contribute to reactive species generation and/or to interfere with antioxidant defence (Dumitrescu et al. Citation2018; Liu et al. Citation2020). In Parkinson’s disease, gut bacterial microbiota dysbiosis, including with low abundance of bacterial SCFAs, and reduced plasma levels of NAD (nicotinamide adenine dinucleotide) and TUDCA (Tauroursodeoxycholic acid), could contribute to mitochondrial dysfunction (Dumitrescu et al. Citation2018; Liang et al. Citation2021; Shandilya et al. Citation2022). Bacterial produced SCFA butyrate prevents mitochondrial bioenergetic failure by providing acetyl-CoA, bacterial produced TUDCA is an anti-apoptotic metabolites that upregulates mitophagy, and regulates OXPHOS complex I, and bacterial produced NAM (nicotinamide), the amide form of niacin, contributes to regulate the plasma level of NAD, which improves mitochondrial function, including energy generation (Liang et al. Citation2021).
The bacterial microbiota–mitochondria interaction could also be a crucial player in inflammatory diseases (Mottawea et al. Citation2016; Kirichenko et al. Citation2020). In Crohn’s disease, which is a type of inflammatory bowel disease, alteration of the gut bacterial microbiota, including H2S producer Atopobium parvulum, associated with decrease in butyrate content, impairs mitochondrial function, including mitochondrial OXPHOS, and mitochondrial proteins involved in H2S regulation, such as sulphur dioxygenase (ETHE1), and rhodanese/TST (Mottawea et al. Citation2016; Kirichenko et al. Citation2020). Bacterial butyrate producer may cause mitochondrial dysfunction, and may contribute to overgenerate mtROS (Liu et al. Citation2020). Mitochondrial ROS accumulation also affects the composition of the gut bacterial microbiota (Mottawea et al. Citation2016; Kirichenko et al. Citation2020). Then, in atherosclerosis, which is characterised by (1) fats and cholesterol deposition in the wall of the artery, (2) inflammation, and (3) increase in risk of myocardial dysfunction and stroke, bacterial metabolite trimethylamine N-oxide (TMAO) may deregulate lipid metabolism (Kirichenko et al. Citation2020). Increase in plasma TMAO is associated with increase in fats and cholesterol deposition in the wall of the artery (Kirichenko et al. Citation2020). In addition, mitochondrial dysfunction, through mtROS accumulation and associated with inflammation, is involved in the progression of atherosclerosis (Kirichenko et al. Citation2020).
Then, the gut bacterial microbiota-mitochondria axis is also involved in obesity and type 2 diabetes (T2D), through bacterial metabolites dysregulation, including SCFAs, and mitochondrial dysfunction at OXPHOS level due to mitochondrial ROS, NO, and ONOO− accumulation (Vezza et al. Citation2020). Thus, gut bacterial microbiota dysbiosis is also a serious concern, including with low abundance of Bacteriodetes and Akkermansia muciniphila in obese and T2D patients, and a correlation between Clostridium and Lactobacillus species with insulin resistance (Vezza et al. Citation2020). In this line, genetic variants of the mtDNA affect mitochondrial bioenergetic function, and both the composition and the activity of the gut bacterial microbiota, including with different diet, and during exercise (Clark and Mach Citation2017). High protein consumption during resting days and training could negatively affect the gut bacterial microbiota composition and activity, including through overgeneration of H2S, and so consequently possibly with mitochondrial dysfunction (Clark and Mach Citation2017).
Another recent good example is the viral infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – COVID19. During viral infection in COVID-19 pathogenesis, COVID-19 viral proteins can bind to host mitochondrial proteins, including the mitochondrial import receptor subunit TOM70 (Gordon et al. Citation2020, Citation2020) nonstructural protein 4 and 8 (NSP4 and NSP8), which are involved in the regulation of the mitochondrial membrane potential and permeability through interaction with ANT and VDAC (Gordon et al. Citation2020, Citation2020) citrate synthase (Stukalov et al. Citation2021) and fumarate hydratase (Stukalov et al. Citation2021) from the Krebs cycle, OXPHOS subunits including ATP synthase subunits (Stukalov et al. Citation2021), and ORF9B (Stukalov et al. Citation2021) and ORF9C (Gordon et al. Citation2020, Citation2020) that interacts with OXPHOS subunit. And viral microRNA 2392 from COVID-19 blocks the transcription of nuclear genes coding for OXPHOS subunits (Guarnieri et al. Citation2023). In this complex viral context, mitochondrial dysfunction linked to oxidative distress mediated by mitochondrial ROS, may contribute to bacterial microbiota dysbiosis, which will further contribute to coagulopathy, iron and ferroptosis dysregulation, and the vicious cycle of inflammation and oxidative distress in many organs (Saleh et al. Citation2020).
Regarding ageing, the diversity of the gut bacterial microbiota could be a hallmark of healthy ageing (Cryan et al. Citation2020). Ageing is associated with a reduction in diversity of the gut bacterial microbiota, a decrease in SCFA generation, including butyrate, and a colonisation by different species, including streptococci, staphylococci, and enterococci (Cӑtoi et al. Citation2020). In long living people, including centenarians, Firmicutes and Bacteroidetes are still dominants, and an enrichment has been reported in both facultative anaerobes from Proteobacteria phylum, and Akkermansia, Lactobacillus, and Bifidobacterium (Cӑtoi et al. Citation2020). Furthermore, dysregulation of the gut bacterial microbiota affects oxidative distress and mitochondrial function in microglia from aged mice, through N (6)-carboxymethyllysine metabolite, which is a member of RCS that contributes to modulate ROS generation (Mossad et al. Citation2022).
In brain, in inflammation, in metabolic disorders, and in ageing, the bacterial microbiota is linked to both mitochondria and reactive species, not only ROS (Dumitrescu et al. Citation2018; Ballard and Towarnicki Citation2020). The nexus between bacterial microbiota and mitochondria could be the RSI and related notable enzymes. As already discussed, ROS are regulating and are regulated by both bacterial microbiota and mitochondria (Ballard and Towarnicki Citation2020). For examples, Lactobacillus stimulate ROS production in gut stem cells, and gut bacterial dysbiosis is associated with decrease in colanic acid, SCFA acetate, propionate, butyrate, indole, phenolic acid, which together induce mitochondrial dysfunction, and mitochondrial ROS generation (Dumitrescu et al. Citation2018; Ballard and Towarnicki Citation2020). Then, looking at RNS and RSS, in the gut, bacteria Lactobacilli and Bifidobacterium convert nitrite and nitrate into NO, Streptococcus generate NO, while Salmonella and Escherichia coli produce H2S (Carlström et al. Citation2020; Shandilya et al. Citation2022). Commensal oral bacteria, including Firmicutes Staphylococcus, Streptococcus, Veillonella, and Actinobacteria play a crucial role in shaping the development of pulmonary hypertension, obesity, and cardiovascular diseases, through regulation of nitrate, nitrite, and NO synthesis and signalling, including with nitrite and nitrate reductase activities (Koch et al. Citation2017). In Parkinson’s disease, H2S generated by gut bacterial microbiota, including by Akkermansia muciniphila, Bilophila wadsworthia, Prevotella, and Porphyromonas, may induce the disease, with an excess of H2S that promotes mitochondrial dysfunction, increases iron content and ROS, and leads to alpha-synuclein aggregation (Murros Citation2022). In addition, during inflammation in the intestinal epithelium, H2S is overgenerated by multiple bacteria, including Fusobacterium (Blachier et al. Citation2019). Moreover, colonic inflammation and colorectal cancer are associated with increase in RCS, including 4-HNE and MDA, which alter the gut bacterial microbiota by reducing the growth of beneficial anaerobic bacteria, and by increasing the growth of redox-resistant deleterious bacteria (Lei et al. Citation2021). Moreover, the gut bacterial microbiota contributes to produce the aminated metabolite of epigallocatechin-3-gallate polyphenol, which scavenges RCS, including 4-HNE and MDA (Zhang et al. Citation2019).
Focusing on notable enzymes involved in the RSI and mitochondria, during exercise, inflammation, and gut dysbiosis, the gut bacterial microbiota regulates SOD activity, although it requires further investigation improving the relationship between the gut bacterial microbiota, SOD, and the intestinal redox balance (Clark and Mach Citation2017). The colonic bacterial microbiota contributes to infectious colitis with Citrobacter rodentium that leads to increase in Bacteroides, and reduction in both antioxidant glutathione GSH, and mitochondrial MnSOD/SOD2 (Ghosh et al. Citation2011; Clark and Mach Citation2017). To limit the immune response linked to mitochondrial dysfunction, several pathogenic bacteria tend to reduce mitochondrial ROS generation through overactivating SOD activity, such as with Mycobacterium tuberculosis, and Ehrlichia chaffeensis (Liu et al. Citation2012; Saint-Georges-Chaumet and Edeas Citation2016; Clark and Mach Citation2017). Healthy gut bacterial microbiota, including with Bifidobacterium adolescentis, contributes to improve healthspan and lifespan, and to limit osteoporosis and neurodegeneration by regulating catalase activity (Chen et al. Citation2021). Bacterial microbiota-derived tryptophan indoles protect tissues during intestinal inflammation, by selectively inhibiting MPO (Alexeev et al. Citation2021). Reduction in generation of hypochlorous acid by MPO through indoles, contributes to reduce intestinal damage, and inflammation (Alexeev et al. Citation2021). The catecholate siderophore enterobactin produced by Escherichia coli is also an inhibitor of MPO, which contributes to gain a survival advantage for E.coli during inflammatory gut diseases (Singh et al. Citation2015; Dumitrescu et al. Citation2018). Together, the MPO inhibition links the bacterial microbiota, MPO within the RSI, and innate immune system (Singh et al. Citation2015; Alexeev et al. Citation2021). Interestingly, brain damage due to extensive stroke lead to gut bacterial dysbiosis, which also affects immune system, thus contributing to pro-inflammation, and systemic oxidative distress (Khoshnam et al. Citation2017; Dumitrescu et al. Citation2018). In addition, regarding NOS enzyme, the gut bacterial microbiota is regulated by NO produced by NOS, while bacterial infection can lead to both upregulation of NOS and increase in NO content, which in turn activate the immune response (Hong et al. Citation2021). Furthermore, in mice, lactic acid bacteria, including Lactobacillus rhamnosus and Lactobacillus reuteri, contribute to reduce uric acid in serum and urine, through reduction in XOR activity, by increasing SCFAs generation (Ni et al. Citation2021). Finally, intestinal ischemia/reperfusion (I/R) alters gut microbiota, and induces ferroptosis (Deng et al. Citation2021). Interestingly, in mice, gut bacterial microbiota capsiate metabolite reduces I/R-induced ferroptosis by enhancing GPX4 expression (Deng et al. Citation2021).
Finally, growing evidences on the relationship between NRF2 and gut microbiota strongly suggest including the NRF2/KEAP1 pathway in the mitochondria-RSI axis considering at minimum bacterial microbiota studies in physiology and pathology. For example, the gut-resident Lactobacillus rhamnosus activates NRF2 to protect against oxidative distress induced by liver injury mediated by acute ethanol toxicity, and acetaminophen overdose (Saeedi et al. Citation2020). The microbial metabolite urolithin A enhances the gut barrier integrity by activating the NRF2 pathway (Singh et al. Citation2019). In addition, dysregulated gut-microbial-derived tryptophan metabolites alter the NRF2 signalling pathway, which induces liver oxidative distress and injury (Shang et al. Citation2023). Last example is the activation of NRF2 that regulates the gut-microbiota-testis axis through steroidogenesis, which includes testosterone with both pro- and anti-oxidant effects (Zhao et al. Citation2020). Altogether, several points were discussed to encourage investigating the mitochondria-RSI (including NRF2 pathway) axis by including bacterial microbiota as a new key player ().
Figure 4. The gut bacterial microbiota and the mitochondria-RSI axis. Key links between the gut bacterial microbiota and the mitochondria-RSI axis are summarized. GPX4, glutathione peroxidase 4; MPO, myeloperoxidase; mtRS, mitochondrial reactive species; NAD, nicotinamide adenine dinucleotide; NOS, nitric oxide synthase; OXPHOS, oxidative phosphorylation; RCS, reactive carbonyl species; RNS, reactive nitrogen species; ROS, reactive oxygen species; RSS, reactive sulfur species; SCFAs, short-chain fatty acids; SOD, superoxide dismutase; TMAO, trimethylamine N-oxide; TUDCA, tauroursodeoxycholic acid; XOR, xanthine oxidoreductase.
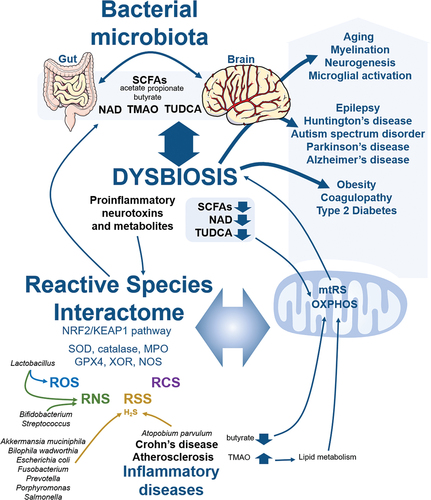
The circadian clock in the mitochondria-RSI axis
The circadian rhythm is a 24-hour cycle that oscillates in light/dark solar day, infradian rhythm being for a period greater than 24 h and ultradian rhythm for a period less than 24 h (Tartour et al. Citation2022). This oscillation has at its heart the molecular circadian clock, which orchestrates physiology and behaviour in living organisms to follow the solar day and to adapt to environmental changes (Tartour et al. Citation2022). The mammalian circadian clock is based on a transcriptional-translational feedback loop with the oscillation of activators that peak in expression during the light phase, namely circadian locomoter output cycles kaput (CLOCK) (Gekakis et al. Citation1998) and brain and muscle ARNT-like 1 (BMAL1) (Lande-Diner et al. Citation2013; Doruk et al. Citation2020) and repressors peak in expression during the dark phase, namely period (PER1, PER2 and PER3) (Ye et al. Citation2014; Tartour et al. Citation2022) and cryptochrome (CRY1 and CRY2) (Ye et al. Citation2014; Tartour et al. Citation2022). Mass-spectrometry based proteomics on isolated mitochondria from mice liver showed that rate-limiting mitochondrial enzymes, including carnitine palmitoyl-transferase 1 (CPT1), which is involved in the conversion of palmitoyl-CoA to palmitoylcarnitine in the presence of carnitine and for the entry of LCFAs into mitochondria, and short chain 3-hydroxyacyl-CoA dehydrogenase (HADH) for the FAO pathway, several members of the OXPHOS system and of the TIM/TOM import machinery were cycling with a peak during the light phase and dependent on PER1/2 (Neufeld-Cohen et al. Citation2016). Again in mice liver, the bioenergetic and redox metabolite NAD+ has a clock-driven cycle with a rhythmic biosynthesis (Peek et al. Citation2013). This metabolite also controls the circadian clock through the regulation of the nuclear translocation of PER2 (Levine et al. Citation2020). In addition, mitochondrial dynamin-related protein 1 (DRP1) involved in mitochondrial fission, ROS generation and bioenergetic is controlled by the circadian clock through the modulation of its phosphorylation, including with a peak of phosphorylated DRP1 during the light phase (Schmitt et al. Citation2018). Interestingly, the oscillation of mitochondrial respiratory activity is synchronised with BMAL1 expression, and the expression of BMAL1 is altered by potassium cyanide (KCN) that inhibits OXPHOS complex IV, and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) that is an uncoupler of OXPHOS (Scrima et al. Citation2016). This rhythmic mitochondrial respiration is also regulated by mitochondrial Ca2+, whose influx disturbance by inhibiting MCU dysregulates the expression of both BMAL1 and CLOCK (Scrima et al. Citation2020). In pacemaker neurons from Drosophila, the mitochondrial cation antiporter leucine zipper-EF-handcontaining transmembrane protein 1 (LETM1) contributes to drive ionic rhythms, which connect ionome, the circadian clock and bioenergetics together (Morioka et al. Citation2022).
In addition to mitochondria, the circadian clock is also tightly linked to the redox system through the GSH system, redox enzymes, including SOD (Colepicolo et al. Citation1992; Singh et al. Citation2003) catalase (Singh et al. Citation2003; Navigatore-Fonzo et al. Citation2014) GPX (Singh et al. Citation2003; Navigatore-Fonzo et al. Citation2014) XOR (Kanemitsu et al. Citation2017), NADPH oxidase (NOX) (Anea et al. Citation2013), PRDX (Edgar et al. Citation2012), MPO (Deng et al. Citation2018), and reactive species (Stangherlin and Reddy Citation2013; Mezhnina et al. Citation2022). The circadian clock influences the ROS generation through redox enzymes, and ROS disturbance alters the circadian clock (Tamaru et al. Citation2013; Mezhnina et al. Citation2022). In addition, alteration of NO metabolism impairs both the circadian rhythm during ageing (Kunieda et al. Citation2008), and the rhythmicity of autophagy (Mitter et al. Citation2010). Moreover, deficiencies in H2S and GSH in muscle impair circadian clock genes, including CLOCK, BMAL1, PER2 and CRY2 (Parsanathan and Jain Citation2019). In skeletal muscle, the circadian clock system also contributes to the RSS-related trans-sulphuration pathway linked to mitochondrial PGC1A and mitofusin-2 (MFN-2) (Tyagi et al. Citation2023). Last but not least, the loss of the antioxidant transcription factor NRF2 function in mice fibroblasts and liver alters the circadian clock genes CLOCK and BMAL1 through its interaction with CRY2 (Wible et al. Citation2018). The NRF2/KEAP1 signalling pathway is also regulated by the circadian clock through the binding of BMAL1 to the promoter of NRF2 (Sun et al. Citation2021). Finally, circadian clock disruption is associated with many diseases, which are shared with mitochondrial dysfunction and redox dysregulation, including chronic inflammation, cancer and neurodegenerative diseases (Altman et al. Citation2015; Padmanabhan and Billaud Citation2017; Deng et al. Citation2018; Douma and Gumz Citation2018; Lananna and Musiek Citation2020; Ahmed et al. Citation2021; Li et al. Citation2022). Altogether, the circadian clock is closely linked to both mitochondria and the RSI, which makes this player a crucial pathway to study within the mitochondria-RSI axis for future investigations.
It is all the more important that the circadian clock also shares with mitochondria and the redox system another player, the bacterial microbiota. In mice, in a gender-dependent manner, deletion of BMAL1 that induces a circadian clock disruption alters the faecal bacterial composition, thus showing that the gut microbiota is controlled by the circadian clock (Liang et al. Citation2015). In mice, CLOCK mutation also induces gut bacterial dysbiosis (Voigt et al. Citation2016). In a Drosophila model, both the gut bacterial microbiota and the rhythmicity of feeding regulate the circadian transcriptome, including through epigenetic with histone acetylation (Zhang et al. Citation2023). Moreover, the circadian clock and the gut bacterial microbiota together synchronise the host’s response to diet, including with bioenergetics and redox metabolisms (Gutierrez Lopez et al. Citation2021). Therefore, this interplay between the circadian clock and the gut bacterial microbiota, including with mitochondria and the redox system, is of major importance in daily life, with different diets and environmental changes. Finally, this interplay is also of major importance in space biology, in which regulating the circadian clock combined with a controlled diet and sleep to keep a healthy gut microbiota is the object of all attention and new studies (Guo et al. Citation2014; Voorhies et al. Citation2019; Siddiqui et al. Citation2021). Making major discoveries will require integrating the mitochondria-RSI axis, including the circadian clock and gut bacterial microbiota ().
Figure 5. The interplay between the circadian clock, the gut microbiota and mitochondria-RSI axis. BMAL1, brain and muscle ARNT-like 1; CLOCK, circadian locomoter output cycles kaput; CRY1/2, cryptochrome1/2; FA, fatty acid; GPX, glutathione peroxidase; KEAP1, kelch-like ECH-associated protein 1; MPO, myeloperoxidase; NAD, nicotinamide adenine dinucleotide; NRF2, nuclear factor E2-related factor 2; OXPHOS, oxidative phosphorylation; PER1/2/3, period 1/2/3; RNS, reactive nitrogen species; ROS, reactive oxygen species; RSS, reactive sulfur species; SOD, superoxide dismutase; TIM, IM translocase complexe; TOM, OM translocase complex; XOR, xanthine oxidoreductase.
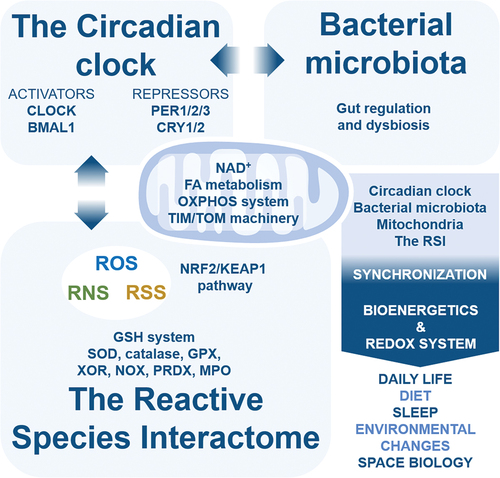
Gender effects, genetic and epigenetic, and environmental factors
Other factors and influences, which will strengthen the mitochondria-RSI axis, will have to be better considered in the near future. Even if this is not the main subject of this already well-developed review, a few lines are necessary to raise awareness of what will need to be integrated into redox medicine. Integrating insights from both the mitochondria and redox fields for the mitochondria-RSI axis is one step, including the circadian clock and gut bacterial microbiota is a second one, and the third one is reminding that genetic, epigenetic, gender and environmental factors will play a significant role in shaping the redox medicine field. (1) The gender effect. For example, greater resistance of mitochondrial OXPHOS function is associated with reduced lipid peroxidation and greater antioxidant enzyme activities in sedentary females compared with males rats (Farhat et al. Citation2017). In addition, exercise training better improves mitochondrial function oxidative capacities in males (Farhat et al. Citation2017). Another example is for sexual hormones, which decline during aging, that affect the NRF2/KEAP1 signaling pathway, the oxidative distress management, mitochondrial function and the inflammation status in the brain (Torrens-Mas et al. Citation2020). In female, estrogens affect epigenetic through the histone deacetylases sirtuins, which regulate the expression of multiple genes involved in oxidative stress response including NRF2, in mitochondrial function and biogenesis including PGC1A, in DNA damage response including tumor suppressor P53, and in inflammation including NF-KB (Torrens-Mas et al. Citation2020). Gender effect is also crucial in cancer incidence, cancer therapy and survival through multiple facets with at minimum higher ROS levels and mitochondrial DNA alteration in males, DNA damage and repair, sexual hormones, chromatin organization, anticancer immunity, and longevity influences (Rubin Citation2022; Özdemir et al. Citation2022). (2) The environmental exposure. Environmental exposures, including altered respiration with different contaminants, are linked to oxidative distress, mitochondrial dysfunction and multiple organ dysfunctions (Caito and Aschner Citation2015; Reddam et al. Citation2022). Contaminants can be carcinogen, teratogen, toxicant, xenobiotic, developmental/reproductive toxicant, endocrine disrupter, and different toxins (Caito and Aschner Citation2015; Reddam et al. Citation2022). This includes cigarette smoke, air pollutants, pesticides, nanomaterials and heavy metals (Caito and Aschner Citation2015; Reddam et al. Citation2022). Mitochondrial DNA alterations, heteroplasmy and mitochondrial respiration are biomarkers for environmental health (Reddam et al. Citation2022). At minimum accumulation of ROS and accumulation of DNA damage due to overoxidation, and NRF2 response alterations are also biomarkers of environmental health (Zheng et al. Citation2020; Reddam et al. Citation2022). Moreover, environmental exposure also comprises infections. For example, the coronavirus 2 (SARS-CoV-2) pandemic teached us that COVID-19 pathogenesis with hyper-inflammatory status, hyper-ferritinemia and hyper-coagulability, is in fact a redox disease with an “oxidative” and “metabolic” storm (Edeas et al. Citation2020; Cumpstey et al. Citation2021). (3) Another crucial environmental factor is lifestyle diet. This factor is of great interest for the mitochondria function, the redox status, the gut bacterial microbiota and even the circadian clock. Many studies are looking for the best diet to reduce at minimum ROS, to control the redox balance and to improve bioenergetics, which includes the type of diet such as Mediterranean diet (Pollicino et al. Citation2023), high-fat diet (Kyriazis et al. Citation2022), caloric restriction (Kyriazis et al. Citation2022), ketogenic diet (Kyriazis et al. Citation2022), fasting and Nordic diet (Kyriazis et al. Citation2022). (4) Combining the type of diet and exercise training is also another lifestyle influence for the redox and mitochondrial systems (Pileggi et al. Citation2022), for the gut microbiota function and ultimately to delay aging (Strasser et al. Citation2021). (5) Combining lifestyle diet and the circadian rhythm is also an emerging approach to regulate oxidative stress and mitochondrial metabolism through a possible personalized chrononutrition (Galimberti and Mazzola Citation2021). (6) The genetic etiology. Complex genetic etiology, which includes nuclear and mitochondrial genetic variants in human population, complicates the situation of metabolic dysfunction and diseases with both mitochondria and redox alterations (Brown et al. Citation2020). (7) Finally, the redox system with ROS and RNS regulates the epigenetic machinery with histone acetylation/deacetylation, histone methylation/demethylation, and DNA methylation/demethylation, which will affect global gene expression (Wu et al. Citation2022). Altogether, different but interconnected factors that should be deeper investigated considering the mitochondria-RSI axis.
Conclusion
An improved understanding of the multifaceted role of the reactive species interactome in mitochondria will open perspectives in key mechanisms involved in physiology and pathology. Mitochondrial permeability transition pore, regulation of cell death type switch, the protein quality control system, ionome homoeostasis, metabolome and the mitochondrial carriers are key mechanisms to further investigate. Importantly, the circadian clock and the gut bacterial microbiota have to be more investigated as the two major players in the mitochondria-RSI axis. Also to consider in the near future for the mitochondria-RSI axis will be the gender effect, the environmental exposure, the lifestyle diet, the combination of diet with exercise and chronobiology, the contribution of genetic variation and epigenetics. This review provides the basis for building and developing new studies combining both the mitochondria and redox fields, chronobiology and bacterial microbiota for the new proposed concept of “MitoRedox Medicine” of which this review is the first stone.
Acknowledgments
The author acknowledges the use of Servier Medical Art image bank. The figures were partly generated using Servier Medical Art, licensed under a Creative Commons Attribution 3.0 unported license.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Adav SS, Wang Y. 2021. Metabolomics signatures of aging: recent advances. Aging Dis. 12:646–38. doi: 10.14336/AD.2020.0909.
- Aghagolzadeh P, Radpour R, Bachtler M, van Goor H, Smith ER, Lister A, Odermatt A, Feelisch M, Pasch A. 2017. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis. 265:78–86. doi: 10.1016/j.atherosclerosis.2017.08.012.
- Ahmed R, Nakahata Y, Shinohara K, Bessho Y. 2021. Cellular senescence triggers altered circadian clocks with a prolonged period and delayed phases. Front Neurosci. 15:638122. doi: 10.3389/fnins.2021.638122.
- Ait-El-Mkadem S, Dayem-Quere M, Gusic M, Chaussenot A, Bannwarth S, François B, Genin EC, Fragaki K, Volker-Touw CLM, Vasnier C, et al. 2017. Mutations in MDH2, encoding a Krebs cycle enzyme, cause early-onset severe encephalopathy. Am J Hum Genet. 100:151–159. doi: 10.1016/j.ajhg.2016.11.014.
- Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, et al. 2020. Blood contains circulating cell‐free respiratory competent mitochondria. FASEB J. 34:3616–3630. doi: 10.1096/fj.201901917RR.
- Alam MM, Kishino A, Sung E, Sekine H, Abe T, Murakami S, Akaike T, Motohashi H. 2023. Contribution of NRF2 to sulfur metabolism and mitochondrial activity. Redox Biol. 60:102624. doi: 10.1016/j.redox.2023.102624.
- Alevriadou BR, Patel A, Noble M, Ghosh S, Gohil VM, Stathopulos PB, Madesh M. 2021. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol. 320:C465–C482. doi: 10.1152/ajpcell.00502.2020.
- Alexandre J, Batteux F, Nicco C, Chéreau C, Laurent A, Guillevin L, Weill B, Goldwasser F. 2006. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 119:41–48. doi: 10.1002/ijc.21685.
- Alexeev EE, Dowdell AS, Henen MA, Lanis JM, Lee JS, Cartwright IM, Schaefer REM, Ornelas A, Onyiah JC, Vögeli B, et al. 2021. Microbial-derived indoles inhibit neutrophil myeloperoxidase to diminish bystander tissue damage. FASEB J Publ Fed Am Soc Exp Biol. 35:e21552. doi: 10.1096/fj.202100027R.
- Alkan HF, Bogner-Strauss JG. 2019. Maintaining cytosolic aspartate levels is a major function of the TCA cycle in proliferating cells. Mol Cell Oncol. 6:e1536843. doi: 10.1080/23723556.2018.1536843.
- Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah SY, Stine ZE, Walton ZE, Gouw AM, Venkataraman A, Li B, Goraksha-Hicks P, et al. 2015. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 22:1009–1019. doi: 10.1016/j.cmet.2015.09.003.
- Altomare A, Baron G, Gianazza E, Banfi C, Carini M, Aldini G. 2021. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: limits and perspectives. Redox Biol. 101899. doi: 10.1016/j.redox.2021.101899.
- Alupei MC, Maity P, Esser PR, Krikki I, Tuorto F, Parlato R, Penzo M, Schelling A, Laugel V, Montanaro L, et al. 2018. Loss of proteostasis is a pathomechanism in Cockayne syndrome. Cell Rep. 23:1612–1619. doi: 10.1016/j.celrep.2018.04.041.
- Anea CB, Zhang M, Chen F, Ali MI, Hart CMM, Stepp DW, Kovalenkov YO, Merloiu A-M, Pati P, Fulton D, et al. 2013. Circadian clock control of Nox4 and reactive oxygen species in the vasculature. PLoS One. 8:e78626. doi: 10.1371/journal.pone.0078626.
- Angelova PR, Vinogradova D, Neganova ME, Serkova TP, Sokolov VV, Bachurin SO, Shevtsova EF, Abramov AY. 2019. Pharmacological sequestration of mitochondrial calcium uptake protects neurons against glutamate excitotoxicity. Mol Neurobiol. 56:2244–2255. doi: 10.1007/s12035-018-1204-8.
- Anzovino A, Lane DJR, Huang ML-H, Richardson DR. 2014. Fixing frataxin: ‘ironing out’ the metabolic defect in Friedreich’s ataxia. Br J Pharmacol. 171:2174–2190. doi: 10.1111/bph.12470.
- Arena G, Cissé MY, Pyrdziak S, Chatre L, Riscal R, Fuentes M, Arnold JJ, Kastner M, Gayte L, Bertrand-Gaday C, et al. 2018. mitochondrial MDM2 regulates respiratory complex I activity independently of p53. Mol Cell. 69:594–609.e8. doi: 10.1016/j.molcel.2018.01.023.
- Ast J, Stiebler AC, Freitag J, Bölker M. 2013. Dual targeting of peroxisomal proteins. Front Physiol. 4:297. doi: 10.3389/fphys.2013.00297.
- Bai L, Yan F, Deng R, Gu R, Zhang X, Bai J. 2021. Thioredoxin-1 rescues MPP+/MPTP-induced ferroptosis by increasing glutathione peroxidase 4. Mol Neurobiol. 58:3187–3197. doi: 10.1007/s12035-021-02320-1.
- Bajzikova M, Kovarova J, Coelho AR, Boukalova S, Oh S, Rohlenova K, Svec D, Hubackova S, Endaya B, Judasova K, et al. 2019. Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 29:399–416.e10. doi: 10.1016/j.cmet.2018.10.014.
- Baker ZN, Cobine PA, Leary SC. 2017. The mitochondrion: a central architect of copper homeostasis. Met Integr Biometal Sci. 9:1501–1512. doi: 10.1039/c7mt00221a.
- Ballard JWO, Towarnicki SG. 2020. mitochondria, the gut microbiome and ROS. Cell Signal. 75:109737. doi: 10.1016/j.cellsig.2020.109737.
- Bano D, Prehn JHM. 2018. Apoptosis-inducing factor (AIF) in physiology and disease: The tale of a repented natural born killer. EBioMedicine. 30:29–37. doi: 10.1016/j.ebiom.2018.03.016.
- Barbier S, Chatre L, Bras M, Sancho P, Roué G, Virely C, Yuste VJ, Baudet S, Rubio M, Esquerda JE, et al. 2009. Caspase-independent type III programmed cell death in chronic lymphocytic leukemia: the key role of the F-actin cytoskeleton. Haematologica. 94:507–517. doi: 10.3324/haematol.13690.
- Baritaud M, Cabon L, Delavallée L, Galán-Malo P, Gilles M-E, Brunelle-Navas M-N, Susin SA. 2012. AIF-mediated caspase-independent necroptosis requires ATM and DNA-PK-induced histone H2AX Ser139 phosphorylation. Cell Death Disease. 3:e390–e390. doi: 10.1038/cddis.2012.120.
- Barshop BA. 2004. Metabolomic approaches to mitochondrial disease: correlation of urine organic acids. Mitochondrion. 4:521–527. doi: 10.1016/j.mito.2004.07.010.
- Bartesaghi S, Radi R. 2018. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 14:618–625. doi: 10.1016/j.redox.2017.09.009.
- Bauer TM, Murphy E. 2020. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res. 126:280–293. doi: 10.1161/CIRCRESAHA.119.316306.
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 476:341–345. doi: 10.1038/nature10234.
- Bender T, Martinou J-C. 2016. The mitochondrial pyruvate carrier in health and disease: To carry or not to carry? Biochim Biophys Acta BBA - Mol Cell Res. 1863:2436–2442. doi: 10.1016/j.bbamcr.2016.01.017.
- Benkler C, O’Neil AL, Slepian S, Qian F, Weinreb PH, Rubin LL. 2018. Aggregated SOD1 causes selective death of cultured human motor neurons. Sci Rep. 8:16393. doi: 10.1038/s41598-018-34759-z.
- Bernardi P, Gerle C, Halestrap AP, Jonas EA, Karch J, Mnatsakanyan N, Pavlov E, Sheu S-S, Soukas AA. 2023. Identity, structure, and function of the mitochondrial permeability transition pore: controversies, consensus, recent advances, and future directions. Cell Death Differ. 30:1869–1885. doi: 10.1038/s41418-023-01187-0.
- Bernardi P, Rasola A, Forte M, Lippe G. 2015. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 95:1111–1155. doi: 10.1152/physrev.00001.2015.
- Blachier F, Beaumont M, Kim E. 2019. Cysteine-derived hydrogen sulfide and gut health: a matter of endogenous or bacterial origin. Curr Opin Clin Nutr Metab Care. 22:68–75. doi: 10.1097/MCO.0000000000000526.
- Bock FJ, Tait SWG. 2020. mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 21:85–100. doi: 10.1038/s41580-019-0173-8.
- Bohovych I, Chan SSL, Khalimonchuk O. 2015. Mitochondrial protein quality control: The mechanisms guarding mitochondrial health. Antioxid Redox Signal. 22:977–994. doi: 10.1089/ars.2014.6199.
- Borchard S, Bork F, Rieder T, Eberhagen C, Popper B, Lichtmannegger J, Schmitt S, Adamski J, Klingenspor M, Weiss K, et al. 2018. The exceptional sensitivity of brain mitochondria to copper. Toxicol In Vitro. 51:11–22. doi: 10.1016/j.tiv.2018.04.012.
- Bortolotti M, Polito L, Battelli MG, Bolognesi A. 2021. Xanthine oxidoreductase: one enzyme for multiple physiological tasks. Redox Biol. 41:101882. doi: 10.1016/j.redox.2021.101882.
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. 2007. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle Georget Tex. 6:2612–2619. doi: 10.4161/cc.6.21.4842.
- Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H. 2020. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med. 26:1034–1046. S147149142030157X. doi: 10.1016/j.molmed.2020.06.006.
- Brand MD. 2010. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 45:466–472. doi: 10.1016/j.exger.2010.01.003.
- Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. 2013. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 14:32. doi: 10.1186/1471-2121-14-32.
- Broeks MH, Meijer NW, Westland D, Bosma M, Gerrits J, German HM, Ciapaite J, van Karnebeek CD, Wanders RJ, Zwartkruis FJ, et al. 2023. The malate-aspartate shuttle is important for de novo serine biosynthesis. Cell Rep. 42. doi: 10.1016/j.celrep.2023.113043.
- Brown JA, Sammy MJ, Ballinger SW. 2020. An evolutionary, or “Mitocentric” perspective on cellular function and disease. Redox Biol. 36:101568. doi: 10.1016/j.redox.2020.101568.
- Bruni F. 2021. Mitochondria: from physiology to pathology. Life. 11:991. doi: 10.3390/life11090991.
- Burner U, Furtmüller PG, Kettle AJ, Koppenol WH, Obinger C. 2000. Mechanism of reaction of myeloperoxidase with nitrite. J Biol Chem. 275:20597–20601. doi: 10.1074/jbc.M000181200.
- Caito SW, Aschner M. 2015. Mitochondrial redox dysfunction and environmental exposures. Antioxid Redox Signal. 23:578–595. doi: 10.1089/ars.2015.6289.
- Camara AKS, Zhou Y, Wen P-C, Tajkhorshid E, Kwok W-M. 2017. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol. 8. doi: 10.3389/fphys.2017.00460.
- Candé C, Cecconi F, Dessen P, Kroemer G. 2002. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 115:4727–4734. doi: 10.1242/jcs.00210.
- Cao X, Ding L, Xie Z, Yang Y, Whiteman M, Moore PK, Bian J-S. 2019. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal. 31:1–38. doi: 10.1089/ars.2017.7058.
- Cao SS, Kaufman RJ. 2014. Endoplasmic reticulum stress and oxidative stress in cell Fate decision and human disease. Antioxid Redox Signal. 21:396–413. doi: 10.1089/ars.2014.5851.
- Carlström M, Moretti CH, Weitzberg E, Lundberg JO. 2020. Microbiota, diet and the generation of reactive nitrogen compounds. Free Radic Biol Med. 161:321–325. doi: 10.1016/j.freeradbiomed.2020.10.025.
- Carraro M, Bernardi P. 2023. The mitochondrial permeability transition pore in Ca2+ homeostasis. Cell Calcium. 111:102719. doi: 10.1016/j.ceca.2023.102719.
- Carvalho C, Cardoso S. 2021. Diabetes–Alzheimer’s disease link: targeting mitochondrial dysfunction and redox imbalance. Antioxid Redox Signal. 34:631–649. doi: 10.1089/ars.2020.8056.
- Casey JR, Grinstein S, Orlowski J. 2010. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 11:50–61. doi: 10.1038/nrm2820.
- Castiglione N, Rinaldo S, Giardina G, Stelitano V, Cutruzzolà F. 2012. Nitrite and nitrite reductases: from molecular mechanisms to dignificance in human health and disease. Antioxid Redox Signal. 17:684–716. doi: 10.1089/ars.2011.4196.
- Caterino M, Ruoppolo M, Mandola A, Costanzo M, Orrù S, Imperlini E. 2017. Protein–protein interaction networks as a new perspective to evaluate distinct functional roles of voltage-dependent anion channel isoforms. Mol Biosyst. 13:2466–2476. doi: 10.1039/C7MB00434F.
- Chatre L, Biard DSF, Sarasin A, Ricchetti M. 2015. Reversal of mitochondrial defects with CSB-dependent serine protease inhibitors in patient cells of the progeroid Cockayne Syndrome. Proc Natl Acad Sci. 112:E2910–E2919. doi: 10.1073/pnas.1422264112.
- Chatre L, Ducat A, Spradley FT, Palei AC, Chéreau C, Couderc B, Thomas KC, Wilson AR, Amaral LM, Gaillard I, et al. 2022. Increased NOS coupling by the metabolite tetrahydrobiopterin (BH4) reduces preeclampsia/IUGR consequences. Redox Biol. 55:102406. doi: 10.1016/j.redox.2022.102406.
- Chatre L, Ricchetti M. 2011. Nuclear mitochondrial DNA activates replication in Saccharomyces cerevisiae. PLoS One. 6:e17235. doi: 10.1371/journal.pone.0017235.
- Chatre L, Ricchetti M. 2013. Prevalent coordination of mitochondrial DNA transcription and initiation of replication with the cell cycle. Nucleic Acids Res. 41:3068–3078. doi: 10.1093/nar/gkt015.
- Cheema JY, He J, Wei W, Fu C. 2021. The Endoplasmic Reticulum-mitochondria encounter structure and its regulatory proteins. Contact. 4:251525642110644. doi: 10.1177/25152564211064491.
- Chen Y, Azad MB, Gibson SB. 2009. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 16:1040–1052. doi: 10.1038/cdd.2009.49.
- Chen S, Chen L, Qi Y, Xu J, Ge Q, Fan Y, Chen D, Zhang Y, Wang L, Hou T, et al. 2021. Bifidobacterium adolescentis regulates catalase activity and host metabolism and improves healthspan and lifespan in multiple species. Nat Aging. 1:991–1001. doi: 10.1038/s43587-021-00129-0.
- Chevrollier A, Loiseau D, Reynier P, Stepien G. 2011. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim Biophys Acta BBA - Bioenerg. 1807:562–567. doi: 10.1016/j.bbabio.2010.10.008.
- Choi ME, Price DR, Ryter SW, Choi AMK. 2019. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 4:e128834. doi: 10.1172/jci.insight.128834.
- Chornyi S, IJlst L, van Roermund CWT, Wanders RJA, Waterham HR. 2021. Peroxisomal metabolite and cofactor transport in humans. Front Cell Dev Biol. 8. doi: 10.3389/fcell.2020.613892.
- Chrétien D, Bénit P, Ha H-H, Keipert S, El-Khoury R, Chang Y-T, Jastroch M, Jacobs HT, Rustin P, Rak M. 2018. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 16:e2003992. doi: 10.1371/journal.pbio.2003992.
- Cighetti G, Bortone L, Sala S, Allevi P. 2001. Mechanisms of action of malondialdehyde and 4-hydroxynonenal on xanthine oxidoreductase. Arch Biochem Biophys. 389:195–200. doi: 10.1006/abbi.2001.2328.
- Clark A, Mach N. 2017. The Crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 8. doi: 10.3389/fphys.2017.00319.
- Colepicolo P, Camarero VC, Hastings JW. 1992. A circadian rhythm in the activity of superoxide dismutase in the photosynthetic alga gonyaulax polyedra. Chronobiol Int. 9:266–268. doi: 10.3109/07420529209064536.
- Cortese-Krott MM, Koning A, Kuhnle GGC, Nagy P, Bianco CL, Pasch A, Wink DA, Fukuto JM, Jackson AA, van Goor H, et al. 2017. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. In: Mary AL, editor. Antioxid Redox Signal. p. 684–712. doi: 10.1089/ars.2017.7083.
- Cortese-Krott MM, Santolini J, Wootton SA, Jackson AA, Feelisch M. 2020. The reactive species interactome. In: Oxidative stress. Elsevier; p. 51–64. doi: 10.1016/B978-0-12-818606-0.00004-3.
- Crochemore C, Fernández-Molina C, Montagne B, Salles A, Ricchetti M. 2019. CSB promoter downregulation via histone H3 hypoacetylation is an early determinant of replicative senescence. Nat Commun. 10:5576. doi: 10.1038/s41467-019-13314-y.
- Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. 2020. The gut microbiome in neurological disorders. Lancet Neurol. 19:79–194. doi: 10.1016/S1474-4422(19)30356-4.
- Cui L, Gouw AM, LaGory EL, Guo S, Attarwala N, Tang Y, Qi J, Chen Y-S, Gao Z, Casey KM, et al. 2021. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat Biotechnol. 39:357–367. doi: 10.1038/s41587-020-0707-9.
- Cumpstey AF, Clark AD, Santolini J, Jackson AA, Feelisch M. 2021. COVID-19: a redox disease-what a stress pandemic can teach us about resilience and what we may learn from the reactive species interactome about its treatment. Antioxid Redox Signal. 35:1226–1268. doi: 10.1089/ars.2021.0017.
- Cunningham CN, Rutter J. 2020. Picometers under the OMM: diving into the vastness of mitochondrial metabolite transport. EMBO Rep. 21:e50071. doi: 10.15252/embr.202050071.
- Cvetko F, Caldwell ST, Higgins M, Suzuki T, Yamamoto M, Prag HA, Hartley RC, Dinkova-Kostova AT, Murphy MP. 2021. Nrf2 is activated by disruption of mitochondrial thiol homeostasis but not by enhanced mitochondrial superoxide production. J Biol Chem. 296:100169. doi: 10.1074/jbc.RA120.016551.
- Cӑtoi AF, Corina A, Katsiki N, Vodnar DC, Andreicuț AD, Stoian AP, Rizzo M, Pérez-Martínez P. 2020. Gut microbiota and aging-A focus on centenarians. Biochim Biophys Acta BBA - Mol Basis Dis. 1866:165765. doi: 10.1016/j.bbadis.2020.165765.
- D’Acunzo P, Pérez-González R, Kim Y, Hargash T, Miller C, Alldred MJ, Erdjument-Bromage H, Penikalapati SC, Pawlik M, Saito M, et al. 2021. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci Adv. 7:eabe5085. doi: 10.1126/sciadv.abe5085.
- de Araujo TH, Okada SS, Ghosn EEB, Taniwaki NN, Rodrigues MR, de Almeida SR, Mortara RA, Russo M, Campa A, Albuquerque RC. 2013. Intracellular localization of myeloperoxidase in murine peritoneal B-lymphocytes and macrophages. Cell Immunol. 281:27–30. doi: 10.1016/j.cellimm.2013.01.002.
- de Bari L, Scirè A, Minnelli C, Cianfruglia L, Kalapos MP, Armeni T. 2020. Interplay among oxidative stress, methylglyoxal pathway and S-Glutathionylation. Antioxidants. 10:19. doi: 10.3390/antiox10010019.
- Deng F, Zhao B-C, Yang X, Lin Z-B, Sun Q-S, Wang Y-F, Yan Z-Z, Liu W-F, Li C, Hu J-J, et al. 2021. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes. 13:1–21. doi: 10.1080/19490976.2021.1902719.
- Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, Cao L, Wang H, Billiar TR, Jiang J, et al. 2018. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 24:366–378. doi: 10.1016/j.celrep.2018.06.026.
- Deragon MA, McCaig WD, Patel PS, Haluska RJ, Hodges AL, Sosunov SA, Murphy MP, Ten VS, LaRocca TJ. 2020. Mitochondrial ROS prime the hyperglycemic shift from apoptosis to necroptosis. Cell Death Discov. 6:132. doi: 10.1038/s41420-020-00370-3.
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A 40 kDa protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 476:336–340. doi: 10.1038/nature10230.
- Deus CM, Yambire KF, Oliveira PJ, Raimundo N. 2020. Mitochondria–lysosome crosstalk: from physiology to neurodegeneration. Trends Mol Med. 26:71–88. doi: 10.1016/j.molmed.2019.10.009.
- Di Domenico F, Tramutola A, Butterfield DA. 2017. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med. 111:253–261. doi: 10.1016/j.freeradbiomed.2016.10.490.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. 2012. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 149:1060–1072. doi: 10.1016/j.cell.2012.03.042.
- Doridot L, Châtre L, Ducat A, Vilotte J-L, Lombès A, Méhats C, Barbaux S, Calicchio R, Ricchetti M, Vaiman D. 2014. Nitroso-redox balance and mitochondrial homeostasis are regulated by STOX1, a pre-eclampsia-associated gene. Antioxid Redox Signal. 21:819–834. doi: 10.1089/ars.2013.5661.
- Doruk YU, Yarparvar D, Akyel YK, Gul S, Taskin AC, Yilmaz F, Baris I, Ozturk N, Türkay M, Ozturk N, et al. 2020. A clock-binding small molecule disrupts the interaction between clock and bmal1 and enhances circadian rhythm amplitude. J Biol Chem. 295:3518–3531. doi: 10.1074/jbc.RA119.011332.
- Douma LG, Gumz ML. 2018. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 119:108–114. doi: 10.1016/j.freeradbiomed.2017.11.024.
- Dubey AK, Godbole A, Mathew MK. 2016. Regulation of VDAC trafficking modulates cell death. Cell Death Discov. 2:1–10. doi: 10.1038/cddiscovery.2016.85.
- Dumitrescu L, Popescu-Olaru I, Cozma L, Tulbă D, Hinescu ME, Ceafalan LC, Gherghiceanu M, Popescu BO. 2018. Oxidative Stress and the microbiota-gut-brain axis. Oxid Med Cell Longev. 2018:2406594. doi: 10.1155/2018/2406594.
- Edeas M, Saleh J, Peyssonnaux C. 2020. Iron: innocent bystander or vicious culprit in covid-19 pathogenesis? Int J Infect Dis IJID Publ Int Soc Infect Dis. 97:303–305. doi: 10.1016/j.ijid.2020.05.110.
- Edeas M, Weissig V. 2013. Targeting mitochondria: strategies, innovations and challenges: The future of medicine will come through mitochondria. Mitochondrion. 13:389–390. doi: 10.1016/j.mito.2013.03.009.
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. 2012. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 485:459–464. doi: 10.1038/nature11088.
- Esch T, Stefano GB, Ptacek R, Kream RM. 2020. Emerging Roles of blood-borne intact and respiring mitochondria as bidirectional mediators of pro- and anti-inflammatory processes. Med Sci Monit Int Med J Exp Clin Res. 26:e924337-1–e924337–3. doi: 10.12659/MSM.924337.
- Espinoza SE, Guo H, Fedarko N, DeZern A, Fried LP, Xue Q-L, Leng S, Beamer B, Walston JD. 2008. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 63:505–509. doi: 10.1093/gerona/63.5.505.
- Esteras N, Abramov AY. 2022. Nrf2 as a regulator of mitochondrial function: energy metabolism and beyond. Free Radic Biol Med. 189:136–153. doi: 10.1016/j.freeradbiomed.2022.07.013.
- Esterhuizen K, van der Westhuizen FH, Louw R. 2017. Metabolomics of mitochondrial disease. Mitochondrion. 35:97–110. doi: 10.1016/j.mito.2017.05.012.
- Farah C, Michel LYM, Balligand J-L. 2018. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 15:292–316. doi: 10.1038/nrcardio.2017.224.
- Farhat F, Amérand A, Simon B, Guegueniat N, Moisan C. 2017. Gender-dependent differences of mitochondrial function and oxidative stress in rat skeletal muscle at rest and after exercise training. Redox Rep Commun Free Radic Res. 22:508–514. doi: 10.1080/13510002.2017.1296637.
- Feelisch M. 2012. NO and hypoxia: taking nitrate to new heights. Nitric Oxide. 27:S4. doi: 10.1016/j.niox.2012.04.016.
- Fiermonte G, Parisi G, Martinelli D, De Leonardis F, Torre G, Pierri CL, Saccari A, Lasorsa FM, Vozza A, Palmieri F, et al. 2011. A new caucasian case of neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD): a clinical, molecular, and functional study. Mol Genet Metab. 104:501–506. doi: 10.1016/j.ymgme.2011.08.022.
- Fiorese CJ, Schulz AM, Lin Y-F, Rosin N, Pellegrino MW, Haynes CM. 2016. The transcription factor ATF5 Mediates a mammalian mitochondrial UPR. Curr Biol CB. 26:2037–2043. doi: 10.1016/j.cub.2016.06.002.
- Forstermann U, Sessa WC. 2012. Nitric oxide synthases: regulation and function. Eur Heart J. 33:829–837. doi: 10.1093/eurheartj/ehr304.
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. 2011. ER tubules mark sites of mitochondrial division. Sci. 334:358–362. doi: 10.1126/science.1207385.
- Fukai T, Ushio-Fukai M. 2011. Superoxide Dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 15:1583–1606. doi: 10.1089/ars.2011.3999.
- Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. 2003. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci. 100:14766–14771. doi: 10.1073/pnas.2435008100.
- Galimberti D, Mazzola G. 2021. Chapter 12 - chronobiology and chrononutrition: relevance for aging. In: Caruso C, Candore G, editors. Human aging. Cambridge: Academic Press; p. 219–254. doi: 10.1016/B978-0-12-822569-1.00006-8.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. 2018. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25:486–541. doi: 10.1038/s41418-017-0012-4.
- Gan B. 2021. Mitochondrial regulation of ferroptosis. J Cell Biol. 220:e202105043. doi: 10.1083/jcb.202105043.
- Ganini D, Santos JH, Bonini MG, Mason RP. 2018. Switch of mitochondrial superoxide dismutase into a prooxidant peroxidase in manganese-deficient cells and mice. Cell Chem Biol. 25:413–425.e6. doi: 10.1016/j.chembiol.2018.01.007.
- Gao P, Yan Z, Zhu Z. 2020. Mitochondria-Associated endoplasmic reticulum membranes in cardiovascular diseases. Front Cell Dev Biol. 8:604240. doi: 10.3389/fcell.2020.604240.
- Garai D, Ríos-González BB, Furtmüller PG, Fukuto JM, Xian M, López-Garriga J, Obinger C, Nagy P. 2017. Mechanisms of myeloperoxidase catalyzed oxidation of H2S by H2O2 or O2 to produce potent protein Cys-polysulfide-inducing species. Free Radic Biol Med. 113:551–563. doi: 10.1016/j.freeradbiomed.2017.10.384.
- Garbincius JF, Elrod JW. 2022. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 102:893–992. doi: 10.1152/physrev.00041.2020.
- Garcia-Bonilla L, Iadecola C. 2012. Peroxiredoxin sets the brain on fire after stroke. Nat Med. 18:858–859. doi: 10.1038/nm.2797.
- Garza NM, Swaminathan AB, Maremanda KP, Zulkifli M, Gohil VM. 2023. Mitochondrial copper in human genetic disorders. Trends Endocrinol Metab. 34:21–33. doi: 10.1016/j.tem.2022.11.001.
- Gaschler MM, Stockwell BR. 2017. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 482:419–425. doi: 10.1016/j.bbrc.2016.10.086.
- Geertsema S, Bourgonje AR, Fagundes RR, Gacesa R, Weersma RK, van Goor H, Mann GE, Dijkstra G, Faber KN. 2023. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol Med. 29:830–842. doi: 10.1016/j.molmed.2023.07.008.
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. 1998. Role of the CLOCK protein in the mammalian circadian mechanism. Sci. 280:1564–1569. doi: 10.1126/science.280.5369.1564.
- Ghafourifar P, Cadenas E. 2005. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 26:190–195. doi: 10.1016/j.tips.2005.02.005.
- Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, et al. 2011. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 301:G39–49. doi: 10.1152/ajpgi.00509.2010.
- Ghosh A, Trivedi PP, Timbalia SA, Griffin AT, Rahn JJ, Chan SSL, Gohil VM. 2014. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum Mol Genet. 23:3596–3606. doi: 10.1093/hmg/ddu069.
- Giacomello M, Pyakurel A, Glytsou C, Scorrano L. 2020. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 21:204–224. doi: 10.1038/s41580-020-0210-7.
- Giampietro R, Spinelli F, Contino M, Colabufo NA. 2018. The pivotal role of copper in neurodegeneration: a new strategy for the therapy of neurodegenerative disorders. Mol Pharm. 15:808–820. doi: 10.1021/acs.molpharmaceut.7b00841.
- Giorgi C, Marchi S, Pinton P. 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 19:713–730. doi: 10.1038/s41580-018-0052-8.
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, et al. 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 110:5887–5892. doi: 10.1073/pnas.1217823110.
- Gladyshev VN. 2014. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 20:727–731. doi: 10.1089/ars.2013.5228.
- Glorieux C, Calderon PB. 2017. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 398:1095–1108. doi: 10.1515/hsz-2017-0131.
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJA, Petit PX, Vaz FM, Gottlieb E. 2008. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 183:681–696. doi: 10.1083/jcb.200803129.
- Gordaliza‐Alaguero I, Cantó C, Zorzano A. 2019. Metabolic implications of organelle–mitochondria communication. EMBO Rep. 20. doi: 10.15252/embr.201947928.
- Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H, Jureka AS, Obernier K, Guo JZ, Batra J, et al. 2020. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Sci. 370:eabe9403. doi: 10.1126/science.abe9403.
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O’Meara MJ, Rezelj VV, Guo JZ, Swaney DL, et al. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 583:459–468. doi: 10.1038/s41586-020-2286-9.
- Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. 2016. Mitochondrial diseases. Nat Rev Dis Primer. 2:1–22. doi: 10.1038/nrdp.2016.80.
- Go Y-M, Uppal K, Walker DI, Tran V, Dury L, Strobel FH, Baubichon-Cortay H, Pennell KD, Roede JR, Jones DP. 2014. Mitochondrial metabolomics using high-resolution fourier-transform mass spectrometry. In: Raftery D, editor. Mass spectrometry in metabolomics. New York: Springer; p. 43–73. 10.1007/978-1-4939-1258-2_4.
- Guaragnella N, Coyne LP, Chen XJ, Giannattasio S. 2018. Mitochondria–cytosol–nucleus crosstalk: learning from saccharomyces cerevisiae. FEMS Yeast Res. 18. doi: 10.1093/femsyr/foy088.
- Guarnieri JW, Dybas JM, Fazelinia H, Kim MS, Frere J, Zhang Y, Soto Albrecht Y, Murdock DG, Angelin A, Singh LN, et al. 2023. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Sci Transl Med. 15:eabq1533. doi: 10.1126/scitranslmed.abq1533.
- Gucek M, Sack MN. 2021. Proteomic and metabolomic advances uncover biomarkers of mitochondrial disease pathophysiology and severity. J Clin Invest. 131. doi: 10.1172/JCI145158.
- Gu L-F, Chen J-Q, Lin Q-Y, Yang Y-Z. 2021. Roles of mitochondrial unfolded protein response in mammalian stem cells. World J Stem Cells. 13:737–752. doi: 10.4252/wjsc.v13.i7.737.
- Guillén-Samander A, Leonzino M, Hanna MG IV, Tang N, Shen H, De Camilli P. 2021. VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J Cell Biol. 220:e202010004. doi: 10.1083/jcb.202010004.
- Gumeni S, Papanagnou E-D, Manola MS, Trougakos IP. 2021. Nrf2 activation induces mitophagy and reverses Parkin/Pink1 knock down-mediated neuronal and muscle degeneration phenotypes. Cell Death Disease. 12:1–12. doi: 10.1038/s41419-021-03952-w.
- Guo D, He H, Meng Y, Luo S, Lu Z. 2023. Determiners of cell fates: the tricarboxylic acid cycle versus the citrate-malate shuttle. Protein Cell. 14:162–164. doi: 10.1093/procel/pwac026.
- Guo J-H, Qu W-M, Chen S-G, Chen X-P, Lv K, Huang Z-L, Wu Y-L. 2014. Keeping the right time in space: importance of circadian clock and sleep for physiology and performance of astronauts. Mil Med Res. 1:23. doi: 10.1186/2054-9369-1-23.
- Gutierrez Lopez DE, Lashinger LM, Weinstock GM, Bray MS. 2021. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 33:873–887. doi: 10.1016/j.cmet.2021.03.015.
- Hacker K, Medler KF. 2008. Mitochondrial calcium buffering contributes to the maintenance of basal calcium levels in mouse taste cells. J Neurophysiol. 100:2177–2191. doi: 10.1152/jn.90534.2008.
- Hadian K, Stockwell BR. 2023. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 22:723–742. doi: 10.1038/s41573-023-00749-8.
- Halestrap AP, McStay GP, Clarke SJ. 2002. The permeability transition pore complex: another view. Biochimie. 84:153–166. doi: 10.1016/S0300-9084(02)01375-5.
- Hara Y, Kume S, Kataoka Y, Watanabe N. 2021. Changes in TCA cycle and TCA cycle-related metabolites in plasma upon citric acid administration in rats. Heliyon. 7:e08501. doi: 10.1016/j.heliyon.2021.e08501.
- Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C. 2014. The protein import machinery of mitochondria—a regulatory hub in metabolism, stress, and disease. Cell Metab. 19:357–372. doi: 10.1016/j.cmet.2014.01.010.
- Hayyan M, Hashim MA, AlNashef IM. 2016. Superoxide Ion: Generation and chemical implications. Chem Rev. 116:3029–3085. doi: 10.1021/acs.chemrev.5b00407.
- Hiemstra S, Fehling-Kaschek M, Kuijper IA, Bischoff LJM, Wijaya LS, Rosenblatt M, Esselink J, van Egmond A, Mos J, Beltman JB, et al. 2022. Dynamic modeling of Nrf2 pathway activation in liver cells after toxicant exposure. Sci Rep. 12:7336. doi: 10.1038/s41598-022-10857-x.
- Hillen AEJ, Heine VM. 2020. Glutamate carrier involvement in mitochondrial dysfunctioning in the brain white matter. Front Mol Biosci. 7:151. doi: 10.3389/fmolb.2020.00151.
- Hilton JB, Kysenius K, White AR, Crouch PJ. 2018. The accumulation of enzymatically inactive cuproenzymes is a CNS-specific phenomenon of the SOD1G37R mouse model of ALS and can be restored by overexpressing the human copper transporter hCTR1. Exp Neurol. 307:118–128. doi: 10.1016/j.expneurol.2018.06.006.
- Holmström KM, Finkel T. 2014. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 15:411–421. doi: 10.1038/nrm3801.
- Holmström KM, Kostov RV, Dinkova-Kostova AT. 2016. The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol. 1:80–91. doi: 10.1016/j.cotox.2016.10.002.
- Hong P-P, Zhu X-X, Yuan W-J, Niu G-J, Wang J-X. 2021. Nitric Oxide synthase regulates gut microbiota homeostasis by erk-nf-κb pathway in shrimp. Front Immunol. 12:778098. doi: 10.3389/fimmu.2021.778098.
- Hu Y, Bi Y, Yao D, Wang P, Li Y. 2019. Omi/HtrA2 protease associated cell apoptosis participates in blood-brain barrier dysfunction. Front Mol Neurosci. 12:48. doi: 10.3389/fnmol.2019.00048.
- Ikeda T, Osaka H, Shimbo H, Tajika M, Yamazaki M, Ueda A, Murayama K, Yamagata T. 2018. Mitochondrial DNA 3243A>T mutation in a patient with MELAS syndrome. Hum Genome Var. 5:1–4. doi: 10.1038/s41439-018-0026-6.
- Janssen JJE, Grefte S, Keijer J, de Boer VCJ. 2019. Mito-Nuclear communication by mitochondrial metabolites and its regulation by b-vitamins. Front Physiol. 10:78. doi: 10.3389/fphys.2019.00078.
- Jovaisaite V, Mouchiroud L, Auwerx J. 2014. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 217:137–143. doi: 10.1242/jeb.090738.
- Jung H, Kim SY, Canbakis Cecen FS, Cho Y, Kwon S-K. 2020. Dysfunction of mitochondriaL CA2+ regulatory Machineries in brain aging and neurodegenerative diseases. Front Cell Dev Biol. 8. doi: 10.3389/fcell.2020.599792.
- Jung K-A, Lee S, Kwak M-K. 2017. NFE2L2/NRF2 activity is linked to MITOCHONDRIA and AMP-activated protein kinase signaling in cancers through miR-181c/mitochondria-encoded cytochrome c oxidase regulation. Antioxid Redox Signal. 27:945–961. doi: 10.1089/ars.2016.6797.
- Kamer KJ, Mootha VK. 2015. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol. 16:545–553. doi: 10.1038/nrm4039.
- Kanellopoulos AK, Mariano V, Spinazzi M, Woo YJ, McLean C, Pech U, Li KW, Armstrong JD, Giangrande A, Callaerts P, et al. 2020. Aralar Sequesters gaba into hyperactive mitochondria, causing social behavior deficits. Cell. 180:1178–1197.e20. doi: 10.1016/j.cell.2020.02.044.
- Kanemitsu T, Tsurudome Y, Kusunose N, Oda M, Matsunaga N, Koyanagi S, Ohdo S. 2017. Periodic variation in bile acids controls circadian changes in uric acid via regulation of xanthine oxidase by the orphan nuclear receptor PPARα. J Biol Chem. 292:21397–21406. doi: 10.1074/jbc.M117.791285.
- Kao MPC, Ang DSC, Pall A, Struthers AD. 2010. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens. 24:1–8. doi: 10.1038/jhh.2009.70.
- Karch J, Kanisicak O, Brody MJ, Sargent MA, Michael DM, Molkentin JD. 2015. Necroptosis interfaces with MOMP and the MPTP in mediating cell death. PLoS One. 10:e0130520. doi: 10.1371/journal.pone.0130520.
- Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. 2017. Pathogenic mechanisms following ischemic stroke. Neurol Sci J Ital Neurol Soc Ital Soc Clin Neurophysiol. 38:1167–1186. doi: 10.1007/s10072-017-2938-1.
- Kinnally KW, Peixoto PM, Ryu S-Y, Dejean LM. 2011. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013.
- Kirches E. 2011. LHON: mitochondrial mutations and more. Curr Genomics. 12:44–54. doi: 10.2174/138920211794520150.
- Kirichenko TV, Markina YV, Sukhorukov VN, Khotina VA, Wu W-K, Orekhov AN. 2020. A Novel insight at atherogenesis: the role of microbiome. Front Cell Dev Biol. 8. doi: 10.3389/fcell.2020.586189.
- Kirichenko TV, Ryzhkova AI, Sinyov VV, Sazonova MD, Orekhova VA, Karagodin VP, Gerasimova EV, Voevoda MI, Orekhov AN, Ragino YI, et al. 2020. Impact of mitochondrial DNA mutations on carotid intima-media thickness in the novosibirsk region. Life Basel Switz. 10:E160. doi: 10.3390/life10090160.
- Kitada M, Xu J, Ogura Y, Monno I, Koya D. 2020. Manganese Superoxide dismutase dysfunction and the pathogenesis of kidney disease. Front Physiol. 11. doi: 10.3389/fphys.2020.00755.
- Klingenberg M. 2009. Cardiolipin and mitochondrial carriers. Biochim Biophys Acta. 1788:2048–2058. doi: 10.1016/j.bbamem.2009.06.007.
- Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. 2017. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015.
- Kolluru GK, Shen X, Bir SC, Kevil CG. 2013. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 35:5–20. doi: 10.1016/j.niox.2013.07.002.
- Krueger K, Koch K, Jühling A, Tepel M, Scholze A. 2010. Low expression of thiosulfate sulfurtransferase (rhodanese) predicts mortality in hemodialysis patients. Clin Biochem. 43:95–101. doi: 10.1016/j.clinbiochem.2009.08.005.
- Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. 2008. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res. 102:607–614. doi: 10.1161/CIRCRESAHA.107.162230.
- Kyriazis ID, Vassi E, Alvanou M, Angelakis C, Skaperda Z, Tekos F, Garikipati VNS, Spandidos DA, Kouretas D. 2022. The impact of diet upon mitochondrial physiology (Review). Int J Mol Med. 50:1–26. doi: 10.3892/ijmm.2022.5191.
- Lacza Z, Pankotai E, Busija DW. 2009. Mitochondrial nitric oxide synthase: current concepts and controversies. Front Biosci Landmark Ed. 14:4436–4443. doi: 10.2741/3539.
- Lambert AJ, Brand MD. 2009. Reactive oxygen species production by mitochondria. In: Stuart JA, editor. Mitochondrial DNA. Totowa, New Jersey: Humana Press; p. 165–181. doi: 10.1007/978-1-59745-521-3_11.
- Lananna BV, Musiek ES. 2020. The wrinkling of time: aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol Dis. 139:104832. doi: 10.1016/j.nbd.2020.104832.
- Lande-Diner L, Boyault C, Kim JY, Weitz CJ. 2013. A positive feedback loop links circadian clock factor CLOCK-BMAL1 to the basic transcriptional machinery. Proc Natl Acad Sci U S A. 110:16021–16026. doi: 10.1073/pnas.1305980110.
- Lee J, Choi J, Selen Alpergin ES, Zhao L, Hartung T, Scafidi S, Riddle RC, Wolfgang MJ. 2017. Loss of hepatic mitochondrial long-chain fatty acid oxidation confers resistance to diet-induced obesity and glucose intolerance. Cell Rep. 20:655–667. doi: 10.1016/j.celrep.2017.06.080.
- Lei L, Zhang J, Decker EA, Zhang G. 2021. Roles of Lipid peroxidation-derived electrophiles in pathogenesis of colonic inflammation and colon cancer. Front Cell Dev Biol. 9:665591. doi: 10.3389/fcell.2021.665591.
- Lemattre C, Imbert-Bouteille M, Gatinois V, Benit P, Sanchez E, Guignard T, Tran Mau-Them F, Haquet E, Rivier F, Carme E, et al. 2019. Report on three additional patients and genotype-phenotype correlation in SLC25A22-related disorders group. Eur J Hum Genet EJHG. 27:1692–1700. doi: 10.1038/s41431-019-0433-2.
- Levine DC, Hong H, Weidemann BJ, Ramsey KM, Affinati AH, Schmidt MS, Cedernaes J, Omura C, Braun R, Lee C, et al. 2020. NAD+ controls circadian reprogramming through PER2 Nuclear translocation to counter aging. Mol Cell. 78:835–849.e7. doi: 10.1016/j.molcel.2020.04.010.
- Lewis SC, Uchiyama LF, Nunnari J. 2016. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 353:aaf5549. doi: 10.1126/science.aaf5549.
- Liang X, Bushman FD, FitzGerald GA. 2015. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A. 112:10479–10484. doi: 10.1073/pnas.1501305112.
- Liang Y, Cui L, Gao J, Zhu M, Zhang Y, Zhang H-L. 2021. Gut microbial metabolites in Parkinson’s disease: implications of mitochondrial dysfunction in the pathogenesis and treatment. Mol Neurobiol. 58:3745–3758. doi: 10.1007/s12035-021-02375-0.
- Liang W, Sagar S, Ravindran R, Najor RH, Quiles JM, Chi L, Diao RY, Woodall BP, Leon LJ, Zumaya E, et al. 2023. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat Commun. 14:5031. doi: 10.1038/s41467-023-40680-5.
- Li J, Cao F, Yin H, Huang Z, Lin Z, Mao N, Sun B, Wang G. 2020. Ferroptosis: past, present and future. Cell Death Disease. 11:88. doi: 10.1038/s41419-020-2298-2.
- Li Q, Hoppe T. 2023. Role of amino acid metabolism in mitochondrial homeostasis. Front Cell Dev Biol. 11:1127618. doi: 10.3389/fcell.2023.1127618.
- Li X, Liu X, Meng Q, Wu X, Bing X, Guo N, Zhao X, Hou X, Wang B, Xia M, et al. 2022. Circadian clock disruptions link oxidative stress and systemic inflammation to metabolic syndrome in obstructive sleep apnea patients. Front Physiol. 13. doi:10.3389/fphys.2022.932596.
- Li X-X, Tsoi B, Li Y-F, Kurihara H, He R-R. 2015. Cardiolipin and its different properties in mitophagy and apoptosis. J Histochem Cytochem. 63:301–311. doi: 10.1369/0022155415574818.
- Liu S, Gao J, Zhu M, Liu K, Zhang H-L. 2020. Gut microbiota and dysbiosis in alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol. 57:5026–5043. doi: 10.1007/s12035-020-02073-3.
- Liu TF, Vachharajani VT, Yoza BK, McCall CE. 2012. Nad±dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 287:25758–25769. doi: 10.1074/jbc.M112.362343.
- Li Q, Vande Velde C, Israelson A, Xie J, Bailey AO, Dong M-Q, Chun S-J, Roy T, Winer L, Yates JR, et al. 2010. ALS-linked mutant superoxide dismutase 1 (SOD1) alters mitochondrial protein composition and decreases protein import. Proc Natl Acad Sci. 107:21146–21151. doi: 10.1073/pnas.1014862107.
- Li L, Wang C, Yang H, Liu S, Lu Y, Fu P, Liu J. 2017. Metabolomics reveal mitochondrial and fatty acid metabolism disorders that contribute to the development of DKD in T2DM patients. Mol Biosyst. 13:2392–2400. doi: 10.1039/C7MB00167C.
- Li R, Yan G, Li Q, Sun H, Hu Y, Sun J, Xu B. 2012. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (h2o2)-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One. 7:e44907. doi: 10.1371/journal.pone.0044907.
- Lopes AFC. 2020. Mitochondrial metabolism and DNA methylation: a review of the interaction between two genomes. Clin Epigenetics. 12:182. doi: 10.1186/s13148-020-00976-5.
- Lopez J, Tait SWG. 2015. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 112:957–962. doi: 10.1038/bjc.2015.85.
- Lorenzo HK, Susin SA, Penninger J, Kroemer G. 1999. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 6:516–524. doi: 10.1038/sj.cdd.4400527.
- Lozoya OA, Wang T, Grenet D, Wolfgang TC, Sobhany M, Ganini da Silva D, Riadi G, Chandel N, Woychik RP, Santos JH. 2019. Mitochondrial acetyl-CoA reversibly regulates locus-specific histone acetylation and gene expression. Life Sci Alli. 2:e201800228. doi: 10.26508/lsa.201800228.
- Lu B, Gong X, Wang Z, Ding Y, Wang C, Luo T, Piao M, Meng F, Chi G, Luo Y, et al. 2017. Shikonin induces glioma cell necroptosis in vitro by ROS overproduction and promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin. 38:1543–1553. doi: 10.1038/aps.2017.112.
- Lu Q, Haragopal H, Slepchenko KG, Stork C, Li YV. 2016. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int J Physiol Pathophysiol Pharmacol. 8:35–43.
- Luo Y, Chatre L, Melhem S, Al-Dahmani ZM, Homer NZM, Miedema A, Deelman LE, Groves MR, Feelisch M, Morton NM, et al. 2023. Thiosulfate sulfurtransferase deficiency promotes oxidative distress and aberrant NRF2 function in the brain. Redox Biol. 68:102965. doi: 10.1016/j.redox.2023.102965.
- Lynch DR, Farmer G. 2021. Mitochondrial and metabolic dysfunction in Friedreich ataxia: update on pathophysiological relevance and clinical interventions. Neuronal Signal. 5:NS20200093. doi: 10.1042/NS20200093.
- Mailloux RJ, McBride SL, Harper M-E. 2013. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci. 38:592–602. doi: 10.1016/j.tibs.2013.09.001.
- Malard E, Valable S, Bernaudin M, Pérès E, Chatre L. 2021. The Reactive species interactome in the brain. Antioxid Redox Signal Ars. 35:1176–1206. doi: 10.1089/ars.2020.8238.
- Mani S, Swargiary G, Ralph SJ. 2022. Targeting the redox imbalance in mitochondria: a novel mode for cancer therapy. Mitochondrion. 62:50–73. doi: 10.1016/j.mito.2021.11.002.
- Marshall KD, Baines CP. 2014. Necroptosis: is there a role for mitochondria? Front Physiol. 5. doi: 10.3389/fphys.2014.00323.
- Martínez-Reyes I, Chandel NS. 2020. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 11:102. doi: 10.1038/s41467-019-13668-3.
- Martín-Jiménez R, Lurette O, Hebert-Chatelain E. 2020. Damage in mitochondriaL DNA associated with Parkinson’s disease. DNA Cell Biol. 39:1421–1430. doi: 10.1089/dna.2020.5398.
- Maurya SR, Mahalakshmi R. 2016. VDAC-2: mitochondrial outer membrane regulator masquerading as a channel? FEBS J. 283:1831–1836. doi: 10.1111/febs.13637.
- McCommis KS, Finck BN. 2015. Mitochondrial pyruvate transport: a historical perspective and future research directions. Biochem J. 466:443–454. doi: 10.1042/BJ20141171.
- Meijer AJ, Lorin S, Blommaart EF, Codogno P. 2015. Regulation of autophagy by amino acids and MTOR-dependent signal transduction. Amino Acids. 47:2037–2063. doi: 10.1007/s00726-014-1765-4.
- Mellis A-T, Misko AL, Arjune S, Liang Y, Erdélyi K, Ditrói T, Kaczmarek AT, Nagy P, Schwarz G. 2021. The role of glutamate oxaloacetate transaminases in sulfite biosynthesis and H2S metabolism. Redox Biol. 38:101800. doi: 10.1016/j.redox.2020.101800.
- Meng J, Lv Z, Zhang Y, Wang Y, Qiao X, Sun C, Chen Y, Guo M, Han W, Ye A, et al. 2021. Precision redox: the key for antioxidant pharmacology. Antioxid Redox Signal. 34:1069–1082. doi: 10.1089/ars.2020.8212.
- Mezhnina V, Ebeigbe OP, Poe A, Kondratov RV. 2022. Circadian control of mitochondria in reactive oxygen species homeostasis. Antioxid Redox Signal. 37:647–663. doi: 10.1089/ars.2021.0274.
- Mitter SK, Rao HV, Cai J, Thampi P, Qi X, Dunn WA, Grant MB, Boulton ME. 2010. Nitric oxide affects the circadian rhythmicity of autophagy in retinal microvascular endothelial cells. Invest Ophthalmol Visual Sci. 51:5624.
- Miyamoto R, Otsuguro K, Yamaguchi S, Ito S. 2014. Contribution of cysteine aminotransferase and mercaptopyruvate sulfurtransferase to hydrogen sulfide production in peripheral neurons. J Neurochem. 130:29–40. doi: 10.1111/jnc.12698.
- Morin AL, Win PW, Lin AZ, Castellani CA. 2022. Mitochondrial genomic integrity and the nuclear epigenome in health and disease. Front Endocrinol. 13:1059085. doi: 10.3389/fendo.2022.1059085.
- Morioka E, Kasuga Y, Kanda Y, Moritama S, Koizumi H, Yoshikawa T, Miura N, Ikeda M, Higashida H, Holmes TC, et al. 2022. Mitochondrial LETM1 drives ionic and molecular clock rhythms in circadian pacemaker neurons. Cell Rep. 39:110787. doi: 10.1016/j.celrep.2022.110787.
- Mossad O, Batut B, Yilmaz B, Dokalis N, Mezö C, Nent E, Nabavi LS, Mayer M, Maron FJM, Buescher JM, et al. 2022. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat Neurosci. 25:295–305. doi: 10.1038/s41593-022-01027-3.
- Mottawea W, Chiang C-K, Mühlbauer M, Starr AE, Butcher J, Abujamel T, Deeke SA, Brandel A, Zhou H, Shokralla S, et al. 2016. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat Commun. 7:13419. doi: 10.1038/ncomms13419.
- Müller S, Versini A, Sindikubwabo F, Belthier G, Niyomchon S, Pannequin J, Grimaud L, Cañeque T, Rodriguez R. 2018. Metformin reveals a mitochondrial copper addiction of mesenchymal cancer cells. PLoS One. 13:e0206764. doi: 10.1371/journal.pone.0206764.
- Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem J. 417:1–13. doi: 10.1042/BJ20081386.
- Murros KE. 2022. hydrogen sulfide produced by gut bacteria may induce Parkinson’s disease. Cells. 11:978. doi: 10.3390/cells11060978.
- Najbauer EE, Becker S, Giller K, Zweckstetter M, Lange A, Steinem C, de Groot BL, Griesinger C, Andreas LB. 2021. Structure, gating and interactions of the voltage-dependent anion channel. Eur Biophys J. 50:159–172. doi: 10.1007/s00249-021-01515-7.
- Nakao N, Kurokawa T, Nonami T, Tumurkhuu G, Koide N, Yokochi T. 2008. Hydrogen peroxide induces the production of tumor necrosis factor-α in RAW 264.7 macrophage cells via activation of p38 and stress-activated protein kinase. Innate Immun. 14:190–196. doi: 10.1177/1753425908093932.
- Nardo T, Oneda R, Spivak G, Vaz B, Mortier L, Thomas P, Orioli D, Laugel V, Stary A, Hanawalt PC, et al. 2009. A UV-sensitive syndrome patient with a specific CSA mutation reveals separable roles for CSA in response to UV and oxidative DNA damage. Proc Natl Acad Sci. 106:6209–6214. doi: 10.1073/pnas.0902113106.
- Navigatore-Fonzo LS, Delgado SM, Gimenez MS, Anzulovich AC. 2014. Daily rhythms of catalase and glutathione peroxidase expression and activity are endogenously driven in the hippocampus and are modified by a vitamin A-free diet. Nutr Neurosci. 17:21–30. doi: 10.1179/1476830513Y.0000000062.
- Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, et al. 2016. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci. 113:E1673–E1682. doi: 10.1073/pnas.1519650113.
- Nicolli A, Petronilli V, Bernardi P. 1993. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 32:4461–4465. doi: 10.1021/bi00067a039.
- Ni C, Li X, Wang L, Li X, Zhao J, Zhang H, Wang G, Chen W. 2021. Lactic acid bacteria strains relieve hyperuricaemia by suppressing xanthine oxidase activity via a short-chain fatty acid-dependent mechanism. Food Funct. 12:7054–7067. doi: 10.1039/D1FO00198A.
- Nomiyama T, Setoyama D, Yasukawa T, Kang D. 2022. Mitochondria metabolomics reveals a role of β-nicotinamide mononucleotide metabolism in mitochondrial DNA replication. J Biochem (Tokyo). 171:325–338. doi: 10.1093/jb/mvab136.
- Norambuena A, Sun X, Wallrabe H, Cao R, Sun N, Pardo E, Shivange N, Wang DB, Post LA, Ferris HA, et al. 2022. SOD1 mediates lysosome-to-mitochondria communication and its dysregulation by amyloid-β oligomers. Neurobiol Dis. 169:105737. doi: 10.1016/j.nbd.2022.105737.
- Nørgård MØ, Lund PM, Kalisi N, Andresen TL, Larsen JB, Vogel S, Svenningsen P. 2023. Mitochondrial reactive oxygen species modify extracellular vesicles secretion rate. FASEB BioAdvances. 5:355–366. doi: 10.1096/fba.2023-00053.
- Novotny BC, Fernandez MV, Wang C, Budde JP, Bergmann K, Eteleeb AM, Bradley J, Webster C, Ebl C, Norton J, et al. 2023. Metabolomic and lipidomic signatures in autosomal dominant and late-onset Alzheimer’s disease brains. Alzheimers Dement J Alzheimers Assoc. 19:1785–1799. doi: 10.1002/alz.12800.
- Ogino K, Kodama N, Nakajima M, Yamada A, Nakamura H, Nagase H, Sadamitsu D, Maekawa T. 2001. Catalase catalyzes nitrotyrosine formation from sodium azide and hydrogen peroxide. Free Radic Res. 35:735–747. doi: 10.1080/10715760100301241.
- Olson KR. 2018. H2S and polysulfide metabolism: conventional and unconventional pathways. Biochem Pharmacol. 149:77–90. doi: 10.1016/j.bcp.2017.12.010.
- Olson KR. 2020. Are reactive sulfur species the new reactive oxygen species? antioxid. Redox Signal Ars. 33:1125–1142. 2020.8132. doi: 10.1089/ars.2020.8132.
- Olson KR, Gao Y, Arif F, Arora K, Patel S, DeLeon ER, Sutton TR, Feelisch M, Cortese-Krott MM, Straub KD. 2018. Metabolism of hydrogen sulfide (H2S) and Production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biol. 15:74–85. doi: 10.1016/j.redox.2017.11.009.
- Olson KR, Gao Y, DeLeon ER, Arif M, Arif F, Arora N, Straub KD. 2017. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 12:325–339. doi: 10.1016/j.redox.2017.02.021.
- Özdemir BC, Gerard CL, Espinosa da Silva C. 2022. Sex and gender differences in anticancer treatment toxicity: a call for revisiting drug dosing in oncology. Endocrinology. 163:bqac058. doi: 10.1210/endocr/bqac058.
- Padmanabhan K, Billaud M. 2017. Desynchronization of Circadian clocks in cancer: a metabolic and epigenetic connection. Front Endocrinol. 8. doi: 10.3389/fendo.2017.00136.
- Pálinkás Z, Furtmüller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, Nagy P. 2015. Interactions of hydrogen sulfide with myeloperoxidase: sulfide is a substrate and inhibitor of myeloperoxidase. Br J Pharmacol. 172:1516–1532. doi: 10.1111/bph.12769.
- Palma FR, He C, Danes JM, Paviani V, Coelho DR, Gantner BN, Bonini MG. 2020. Mitochondrial superoxide dismutase: what the established, the intriguing, and the novel reveal about a key cellular redox switch. Antioxid Redox Signal. 32:701–714. doi: 10.1089/ars.2019.7962.
- Palmieri F. 2013. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 34:465–484. doi: 10.1016/j.mam.2012.05.005.
- Palmieri F, Monné M. 2016. Discoveries, metabolic roles and diseases of mitochondrial carriers: a review. Biochim Biophys Acta BBA - Mol Cell Res. 1863:2362–2378. doi: 10.1016/j.bbamcr.2016.03.007.
- Pandey R, Caflisch L, Lodi A, Brenner AJ, Tiziani S. 2017. Metabolomic signature of brain cancer. Mol Carcinog. 56:2355–2371. doi: 10.1002/mc.22694.
- Pardo B, Contreras L, Satrústegui J. 2013. De novo synthesis of glial glutamate and glutamine in young mice requires aspartate provided by the neuronal mitochondrial aspartate-glutamate carrier aralar/AGC1. Front Endocrinol. 4. doi: 10.3389/fendo.2013.00149.
- Paré B, Lehmann M, Beaudin M, Nordström U, Saikali S, Julien J-P, Gilthorpe JD, Marklund SL, Cashman NR, Andersen PM, et al. 2018. Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis. Sci Rep. 8:14223. doi: 10.1038/s41598-018-31773-z.
- Park MH, Jo M, Kim YR, Lee C-K, Hong JT. 2016. Roles of peroxiredoxins in cancer, neurodegenerative diseases and inflammatory diseases. Pharmacol Ther. 163:1–23. doi: 10.1016/j.pharmthera.2016.03.018.
- Park YS, Koh YH, Takahashi M, Miyamoto Y, Suzuki K, Dohmae N, Takio K, Honke K, Taniguchi N. 2003. Identification of the Binding site of methylglyoxal on glutathione peroxidase: methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites arg 184 and 185. Free Radic Res. 37:205–211. doi: 10.1080/1071576021000041005.
- Parsanathan R, Jain SK. 2019. Hydrogen sulfide regulates circadian-clock genes in C2C12 myotubes and the muscle of high-fat-diet-fed mice. Arch Biochem Biophys. 672:108054. doi: 10.1016/j.abb.2019.07.019.
- Paul BD, Snyder SH, Kashfi K. 2020. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 38:101772. doi: 10.1016/j.redox.2020.101772.
- Peek CB, Affinati AH, Ramsey KM, Kuo H-Y, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. 2013. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Sci. 342:1243417. doi: 10.1126/science.1243417.
- Peeters A, Shinde AB, Dirkx R, Smet J, De Bock K, Espeel M, Vanhorebeek I, Vanlander A, Van Coster R, Carmeliet P, et al. 2015. Mitochondria in peroxisome-deficient hepatocytes exhibit impaired respiration, depleted DNA, and PGC-1α independent proliferation. Biochim Biophys Acta BBA - Mol Cell Res. 1853:285–298. doi: 10.1016/j.bbamcr.2014.11.017.
- Petrova VY, Drescher D, Kujumdzieva AV, Schmitt MJ. 2004. Dual targeting of yeast catalase a to peroxisomes and mitochondria. Biochem J. 380:393–400. doi: 10.1042/BJ20040042.
- Pfanner N, Warscheid B, Wiedemann N. 2019. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 20:267–284. doi: 10.1038/s41580-018-0092-0.
- Pilchova I, Klacanova K, Tatarkova Z, Kaplan P, Racay P. 2017. The Involvement of Mg2+ in Regulation of cellular and mitochondrial functions. Oxid Med Cell Longev. 2017:6797460. doi: 10.1155/2017/6797460.
- Pileggi CA, Blondin DP, Hooks BG, Parmar G, Alecu I, Patten DA, Cuillerier A, O’Dwyer C, Thrush AB, Fullerton MD, et al. 2022. Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity. EBioMedicine. 83:104192. doi: 10.1016/j.ebiom.2022.104192.
- Pittalà MGG, Conti Nibali S, Reina S, Cunsolo V, Di Francesco A, De Pinto V, Messina A, Foti S, Saletti R. 2021. Vdacs post-translational modifications discovery by mass spectrometry: impact on their hub function. Int J Mol Sci. 22:12833. doi: 10.3390/ijms222312833.
- Podlesniy P, Puigròs M, Serra N, Fernández-Santiago R, Ezquerra M, Tolosa E, Trullas R. 2019. Accumulation of mitochondrial 7S DNA in idiopathic and LRRK2 associated Parkinson’s disease. EBioMedicine. 48:554–567. doi: 10.1016/j.ebiom.2019.09.015.
- Pollicino F, Veronese N, Dominguez LJ, Barbagallo M. 2023. Mediterranean diet and mitochondria: new findings. Exp Gerontol. 176:112165. doi: 10.1016/j.exger.2023.112165.
- Qin J, Guo Y, Xue B, Shi P, Chen Y, Su QP, Hao H, Zhao S, Wu C, Yu L, et al. 2020. ER-mitochondria contacts promote mtDNA nucleoids active transportation via mitochondrial dynamic tubulation. Nat Commun. 11:4471. doi: 10.1038/s41467-020-18202-4.
- Ravi A, Palamiuc L, Loughran RM, Triscott J, Arora GK, Kumar A, Tieu V, Pauli C, Reist M, Lew RJ, et al. 2021. PI5P4Ks drive metabolic homeostasis through peroxisome-mitochondria interplay. Dev Cell. 56:1661–1676.e10. doi: 10.1016/j.devcel.2021.04.019.
- Ray RS, Katyal A. 2016. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci Biobehav Rev. 68:611–620. doi: 10.1016/j.neubiorev.2016.06.031.
- Reddam A, McLarnan S, Kupsco A. 2022. Environmental chemical exposures and mitochondrial dysfunction: a review of recent literature. Curr Environ Health Rep. 9:631–649. doi: 10.1007/s40572-022-00371-7.
- Reelfs O, Abbate V, Cilibrizzi A, Pook MA, Hider RC, Pourzand C. 2019. The role of mitochondrial labile iron in Friedreich’s ataxia skin fibroblasts sensitivity to ultraviolet A. Metallomics. 11:656–665. doi: 10.1039/C8MT00257F.
- Ricchetti M. 2018. Replication stress in mitochondria. Mutat Res Mol Mech Mutagen. 808:93–102. doi: 10.1016/j.mrfmmm.2018.01.005.
- Ricchetti M, Tekaia F, Dujon B. 2004. Continued colonization of the human genome by mitochondrial DNA. PLoS Biol. 2:e273. doi: 10.1371/journal.pbio.0020273.
- Richards BJ, Slavin M, Oliveira AN, Sanfrancesco VC, Hood DA. 2022. Mitochondrial protein import and UPRmt in skeletal muscle remodeling and adaptation. Semin Cell Dev Biol. S1084-9521(22)00004-0. doi: 10.1016/j.semcdb.2022.01.002.
- Rodríguez LR, Lapeña-Luzón T, Benetó N, Beltran-Beltran V, Pallardó FV, Gonzalez-Cabo P, Navarro JA. 2022. Therapeutic Strategies targeting mitochondrial calcium signaling: a new hope for neurological diseases? Antioxidants. 11(1):165. doi: 10.3390/antiox11010165.
- Rodriguez J, Li T, Xu Y, Sun Y, Zhu C. 2021. Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury. Neural Regen Res. 16:205–213. doi: 10.4103/1673-5374.290875.
- Roe ND, Ren J. 2012. Nitric oxide synthase uncoupling: A therapeutic target in cardiovascular diseases. Vascul Pharmacol. 57:168–172. doi: 10.1016/j.vph.2012.02.004.
- Rostovtseva TK, Bezrukov SM, Hoogerheide DP. 2021. Regulation of mitochondriaL Respiration by VDAC is enhanced by membrane-bound inhibitors with disordered polyanionic c-terminal domains. Int J Mol Sci. 22:7358. doi: 10.3390/ijms22147358.
- Rouault TA. 2016. Mitochondrial iron overload: causes and consequences. Curr Opin Genet Dev. 38:31–37. doi: 10.1016/j.gde.2016.02.004.
- Rubin JB. 2022. The spectrum of sex differences in cancer. Trends Cancer. 8:303–315. doi: 10.1016/j.trecan.2022.01.013.
- Rueda CB, Llorente-Folch I, Traba J, Amigo I, Gonzalez-Sanchez P, Contreras L, Juaristi I, Martinez-Valero P, Pardo B, Del Arco A, et al. 2016. Glutamate excitotoxicity and Ca 2+ -regulation of respiration: Role of the Ca 2+ activated mitochondrial transporters (CaMCs). Biochim Biophys Acta BBA - Bioenerg. 1857:1158–1166. doi: 10.1016/j.bbabio.2016.04.003.
- Ruiz LM, Libedinsky A, Elorza AA. 2021. Role of copper on mitochondrial function and metabolism. Front Mol Biosci. 8. doi: 10.3389/fmolb.2021.711227.
- Ruprecht JJ, Kunji ERS. 2020. The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem Sci. 45:244–258. doi: 10.1016/j.tibs.2019.11.001.
- Ryan DG, Yang M, Prag HA, Blanco GR, Nikitopoulou E, Segarra-Mondejar M, Powell CA, Young T, Burger N, Miljkovic JL, et al. 2021. Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism. eLife. 10:e72593.doi:10.7554/eLife.72593.
- Sabharwal SS, Schumacker PT. 2014. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 14:709–721. doi: 10.1038/nrc3803.
- Saeedi BJ, Liu KH, Owens JA, Hunter-Chang S, Camacho MC, Eboka RU, Chandrasekharan B, Baker NF, Darby TM, Robinson BS, et al. 2020. Gut-resident lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 31:956–968.e5. doi: 10.1016/j.cmet.2020.03.006.
- Saint-Georges-Chaumet Y, Edeas M. 2016. Microbiota-mitochondria inter-talk: consequence for microbiota-host interaction. Pathog Dis. 74:ftv096. doi: 10.1093/femspd/ftv096.
- Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, Noguchi N, Niki E. 2006. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 40:619–630. doi: 10.1080/10715760600632552.
- Saleh J, Peyssonnaux C, Singh KK, Edeas M. 2020. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 54:1–7. doi: 10.1016/j.mito.2020.06.008.
- Sánchez-Caballero L, Ruzzenente B, Bianchi L, Assouline Z, Barcia G, Metodiev MD, Rio M, Funalot B, van den Brand MAM, Guerrero-Castillo S, et al. 2016. Mutations in complex i assembly factor tmem126b result in muscle weakness and isolated complex i deficiency. Am J Hum Genet. 99:208–216. doi: 10.1016/j.ajhg.2016.05.022.
- Sander P, Gudermann T, Schredelseker J. 2021. A calcium guard in the outer membrane: is vdac a regulated gatekeeper of mitochondrial calcium uptake? Int J Mol Sci. 22:946. doi: 10.3390/ijms22020946.
- Santolini J, Wootton SA, Jackson AA, Feelisch M. 2019. The redox architecture of physiological function. Curr Opin Physiol. 9:34–47. doi: 10.1016/j.cophys.2019.04.009.
- Santos JH. 2021. Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic Biol Med. 170:59–69. doi: 10.1016/j.freeradbiomed.2020.11.016.
- Scammahorn JJ, Nguyen ITN, Bos EM, Van Goor H, Joles JA. 2021. Fighting oxidative stress with sulfur: hydrogen sulfide in the renal and cardiovascular systems. Antioxid Basel Switz. 10:373. doi: 10.3390/antiox10030373.
- Schalkwijk CG, Stehouwer CDA. 2020. Methylglyoxal, a Highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. 100:407–461. doi: 10.1152/physrev.00001.2019.
- Schmidt O, Pfanner N, Meisinger C. 2010. Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol. 11:655–667. doi: 10.1038/nrm2959.
- Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, Ishihara N, Mihara K, Ripperger JA, Albrecht U, et al. 2018. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 27:657–666.e5. doi: 10.1016/j.cmet.2018.01.011.
- Schuler M-H, English AM, Xiao T, Campbell TJ, Shaw JM, Hughes AL. 2021. Mitochondrial-derived compartments facilitate cellular adaptation to amino acid stress. Mol Cell. 81:3786–3802.e13. doi: 10.1016/j.molcel.2021.08.021.
- Scrima R, Cela O, Agriesti F, Piccoli C, Tataranni T, Pacelli C, Mazzoccoli G, Capitanio N. 2020. Mitochondrial calcium drives clock gene-dependent activation of pyruvate dehydrogenase and of oxidative phosphorylation. Biochim Biophys Acta BBA - Mol Cell Res. 1867:118815. doi: 10.1016/j.bbamcr.2020.118815.
- Scrima R, Cela O, Merla G, Augello B, Rubino R, Quarato G, Fugetto S, Menga M, Fuhr L, Relógio A, et al. 2016. Clock-genes and mitochondrial respiratory activity: evidence of a reciprocal interplay. Biochim Biophys Acta BBA - Bioenerg. 1857:1344–1351. doi: 10.1016/j.bbabio.2016.03.035.
- Sena LA, Chandel NS. 2012. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 48:158–167. doi: 10.1016/j.molcel.2012.09.025.
- Shai N, Schuldiner M, Zalckvar E. 2016. No peroxisome is an island — peroxisome contact sites. Biochim Biophys Acta BBA - Mol Cell Res. 1863:1061–1069. doi: 10.1016/j.bbamcr.2015.09.016.
- Shan Y, Cortopassi G. 2016. Mitochondrial Hspa9/Mortalin regulates erythroid differentiation via iron-sulfur cluster assembly. Mitochondrion. 26:94–103. doi: 10.1016/j.mito.2015.12.005.
- Shandilya S, Kumar S, Kumar Jha N, Kumar Kesari K, Ruokolainen J. 2022. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J Adv Res. 38:223–244. doi: 10.1016/j.jare.2021.09.005.
- Shang H, Huang C, Xiao Z, Yang P, Zhang S, Hou X, Zhang L. 2023. Gut microbiota-derived tryptophan metabolites alleviate liver injury via AhR/Nrf2 activation in pyrrolizidine alkaloids-induced sinusoidal obstruction syndrome. Cell Biosci. 13:127. doi: 10.1186/s13578-023-01078-4.
- Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D, Rodriguez R, Block K. 2017. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun. 8:997. doi: 10.1038/s41467-017-01106-1.
- Shin WH, Chung KC. 2020. Human telomerase reverse transcriptase positively regulates mitophagy by inhibiting the processing and cytoplasmic release of mitochondrial PINK1. Cell Death Disease. 11:425. doi: 10.1038/s41419-020-2641-7.
- Siddiqui R, Akbar N, Khan NA. 2021. Gut microbiome and human health under the space environment. J Appl Microbiol. 130:14–24. doi: 10.1111/jam.14789.
- Sies H. 2015. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4:180–183. doi: 10.1016/j.redox.2015.01.002.
- Sies H. 2017. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 11:613–619. doi: 10.1016/j.redox.2016.12.035.
- Sies H. 2020. Findings in redox biology: from H2O2 to oxidative stress. J Biol Chem. 295:13458–13473. doi: 10.1074/jbc.X120.015651.
- Sies H, Jones DP. 2020. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 21:363–383. doi: 10.1038/s41580-020-0230-3.
- Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC. 2013. Mitochondrial telomerase protects cancer cells from nuclear dna damage and apoptosis. PLoS One. 8:e52989. doi: 10.1371/journal.pone.0052989.
- Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, et al. 2019. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 10:89. doi: 10.1038/s41467-018-07859-7.
- Singh R, Singh RK, Mahdi AA, Singh RK, Kumar A, Tripathi AK, Rai R, Singh U, Cornélissen G, Schwartzkopff O, et al. 2003. Circadian periodicity of plasma lipid peroxides and other anti-oxidants as putative markers in gynecological malignancies. Vivo Athens Greece. 17:593–600.
- Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. 2015. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 6:7113. doi: 10.1038/ncomms8113.
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. 2019. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev. 2019:1–17. doi: 10.1155/2019/6175804.
- Solier S, Müller S, Cañeque T, Versini A, Mansart A, Sindikubwabo F, Baron L, Emam L, Gestraud P, Pantoș GD, et al. 2023. A druggable copper-signalling pathway that drives inflammation. Nature. 617:386–394. doi: 10.1038/s41586-023-06017-4.
- Song J, Herrmann JM, Becker T. 2021. Quality control of the mitochondrial proteome. Nat Rev Mol Cell Biol. 22:54–70. doi: 10.1038/s41580-020-00300-2.
- Song X, Long D. 2020. Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front Neurosci. 14. doi: 10.3389/fnins.2020.00267.
- Šrejber M, Navrátilová V, Paloncýová M, Bazgier V, Berka K, Anzenbacher P, Otyepka M. 2018. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J Inorg Biochem. 183:117–136. doi: 10.1016/j.jinorgbio.2018.03.002.
- Stangherlin A, Reddy AB. 2013. Regulation of Circadian clocks by redox homeostasis*. J Biol Chem. 288:26505–26511. doi: 10.1074/jbc.R113.457564.
- Stier A. 2021. Human blood contains circulating cell-free mitochondria, but are they really functional? Am J Physiol -Endocrinol Metab. 320:E859–E863. doi: 10.1152/ajpendo.00054.2021.
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. 2017. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 171:273–285. doi: 10.1016/j.cell.2017.09.021.
- Strasser B, Wolters M, Weyh C, Krüger K, Ticinesi A. 2021. The Effects of lifestyle and diet on gut microbiota composition, inflammation and muscle performance in our aging society. Nutrients. 13:2045. doi: 10.3390/nu13062045.
- Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, Haas DA, Huang Y, Oubraham L, Wang A, et al. 2021. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 594:246–252. doi: 10.1038/s41586-021-03493-4.
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. 2015. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 162:552–563. doi: 10.1016/j.cell.2015.07.017.
- Sun Q, Zeng C, Du L, Dong C. 2021. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia‑reperfusion. Exp Ther Med. 21:1. doi: 10.3892/etm.2021.9622.
- Suomalainen A, Battersby BJ. 2018. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. 19:77–92. doi: 10.1038/nrm.2017.66.
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 397:441–446. doi: 10.1038/17135.
- Susin SA, Zamzami N, Kroemer G. 1998. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta BBA - Bioenerg. 1366:151–165. doi: 10.1016/S0005-2728(98)00110-8.
- Szczepanowska K, Trifunovic A. 2021. Mitochondrial matrix proteases: quality control and beyond. FEBS J. 289:7128–7146. doi: 10.1111/febs.15964.
- Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada K, et al. 2020. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 5. doi:10.1172/jci.insight.132747.
- Tafuri F, Ronchi D, Magri F, Comi GP, Corti S. 2015. SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis. Front Cell Neurosci. 9:336. doi: 10.3389/fncel.2015.00336.
- Tamaru T, Hattori M, Ninomiya Y, Kawamura G, Varès G, Honda K, Mishra DP, Wang B, Benjamin I, Sassone-Corsi P, et al. 2013. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS One. 8:e82006. doi: 10.1371/journal.pone.0082006.
- Tanaka H, Okazaki T, Aoyama S, Yokota M, Koike M, Okada Y, Fujiki Y, Gotoh Y. 2019. Peroxisomes control mitochondrial dynamics and the mitochondrion-dependent pathway of apoptosis. J Cell Sci Jcs. 224766. doi: 10.1242/jcs.224766.
- Tang X, Yan Z, Miao Y, Ha W, Li Z, Yang L, Mi D. 2023. Copper in cancer: from limiting nutrient to therapeutic target. Front Oncol. 13. doi: 10.3389/fonc.2023.1209156.
- Tartour K, Andriani F, Folco EG, Letkova D, Schneider R, Saidi I, Sato T, Welz P-S, Benitah SA, Allier C, et al. 2022. Mammalian PERIOD2 regulates H2A.Z incorporation in chromatin to orchestrate circadian negative feedback. Nature Structural & Molecular Biology. 29:549–562. doi: 10.1038/s41594-022-00777-9.
- Taylor RW, Turnbull DM. 2005. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 6:389–402. doi: 10.1038/nrg1606.
- Tedesco L, Rossi F, Ragni M, Ruocco C, Brunetti D, Carruba MO, Torrente Y, Valerio A, Nisoli E. 2020. A Special amino-acid formula tailored to boosting cell respiration prevents mitochondrial dysfunction and oxidative stress caused by doxorubicin in mouse cardiomyocytes. Nutrients. 12:282. doi: 10.3390/nu12020282.
- Teramoto S, Tomita T, Matsui H, Ohga E, Matsuse T, Ouchi Y. 1999. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: protective roles of glutathione. Jpn J Pharmacol. 79:33–40. doi: 10.1254/jjp.79.33.
- Tirichen H, Yaigoub H, Xu W, Wu C, Li R, Li Y. 2021. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front Physiol. 12:627837. doi: 10.3389/fphys.2021.627837.
- Todkar K, Ilamathi HS, Germain M. 2017. Mitochondria and Lysosomes: discovering bonds. Front Cell Dev Biol. 5:106. doi: 10.3389/fcell.2017.00106.
- Tonazzi A, Giangregorio N, Console L, Palmieri F, Indiveri C. 2021. The mitochondrial Carnitine acyl-carnitine carrier (SLC25A20): molecular mechanisms of transport, role in redox sensing and interaction with drugs. Biomolecules. 11:521. doi: 10.3390/biom11040521.
- Tonelli C, Chio IIC, Tuveson DA. 2018. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 29:1727–1745. doi: 10.1089/ars.2017.7342.
- Torrens-Mas M, Pons D-G, Sastre-Serra J, Oliver J, Roca P. 2020. Sexual hormones regulate the redox status and mitochondrial function in the brain. Pathological Implications Redox Biol. 31:101505. doi: 10.1016/j.redox.2020.101505.
- Trisolini L, Laera L, Favia M, Muscella A, Castegna A, Pesce V, Guerra L, De Grassi A, Volpicella M, Pierri CL. 2021. Differential expression of ADP/ATP carriers as a biomarker of metabolic remodeling and survival in kidney cancers. Biomolecules. 11:38. doi: 10.3390/biom11010038.
- Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, et al. 2022. Copper induces cell death by targeting lipoylated TCA cycle proteins. Sci. 375:1254–1261. doi: 10.1126/science.abf0529.
- Tyagi S, Tyagi S, Sathnur S, Sen U, Akinterinwa O, Zheng Y, Mokshagundam S, Singh M. 2023. Role of the circadian clock system in trans-sulfuration pathway and tissue remodeling. Physiology. 38:5732949. doi: 10.1152/physiol.2023.38.S1.5732949.
- Vergeade A, Mulder P, Vendeville C, Ventura-Clapier R, Thuillez C, Monteil C. 2012. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J Cardiovasc Pharmacol. 60:538–543. doi: 10.1097/FJC.0b013e318271223c.
- Verma M, Lizama BN, Chu CT. 2022. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl Neurodegener. 11:3. doi: 10.1186/s40035-021-00278-7.
- Vezza T, Abad-Jiménez Z, Marti-Cabrera M, Rocha M, Víctor VM. 2020. Microbiota-mitochondria inter-talk: a potential therapeutic strategy in obesity and type 2 diabetes. Antioxidants. 9:848. doi: 10.3390/antiox9090848.
- Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, Vitaterna MH, Turek FW, Keshavarzian A. 2016. The Circadian Clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res. 40:335–347. doi: 10.1111/acer.12943.
- Voorhies AA, Mark Ott C, Mehta S, Pierson DL, Crucian BE, Feiveson A, Oubre CM, Torralba M, Moncera K, Zhang Y, et al. 2019. Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci Rep. 9:9911. doi: 10.1038/s41598-019-46303-8.
- Wallace DC. 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 39:359. doi: 10.1146/annurev.genet.39.110304.095751.
- Wallace DC. 2013. A mitochondrial bioenergetic etiology of disease. J Clin Invest. 123:1405–1412. doi: 10.1172/JCI61398.
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AMS, Elsas LJ, Nikoskelainen EK. 1988. Mitochondrial DNA mutation associated with leber’s hereditary optic Neuropathy. Science. 242:1427–1430. doi: 10.1126/science.3201231.
- Wang G, Fan Y, Cao P, Tan K. 2022. Insight into the mitochondrial unfolded protein response and cancer: opportunities and challenges. Cell Biosci. 12:18. doi: 10.1186/s13578-022-00747-0.
- Wang Y, Zhang X, Yao H, Chen X, Shang L, Li P, Cui X, Zeng J. 2022. Peroxisome-generated succinate induces lipid accumulation and oxidative stress in the kidneys of diabetic mice. J Biol Chem. 298:101660. doi: 10.1016/j.jbc.2022.101660.
- Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR. 2018. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. eLife. 7:e31656. doi: 10.7554/eLife.31656.
- Wibom R, Lasorsa FM, Töhönen V, Barbaro M, Sterky FH, Kucinski T, Naess K, Jonsson M, Pierri CL, Palmieri F, et al. 2009. AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med. 361:489–495. doi: 10.1056/NEJMoa0900591.
- Wiehe RS, Gole B, Chatre L, Walther P, Calzia E, Ricchetti M, Wiesmüller L. 2018. Endonuclease G promotes mitochondrial genome cleavage and replication. Oncotarget. 9:18309–18326. doi: 10.18632/oncotarget.24822.
- Wu D, Hu Q, Zhu D. 2018. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid Med Cell Longev. 2018:1–16. doi: 10.1155/2018/4579140.
- Wu R, Li S, Hudlikar R, Wang L, Shannar A, Peter R, Chou PJ, Kuo H-CD, Liu Z, Kong A-N. 2022. Redox signaling, mitochondrial metabolism, epigenetics and redox active phytochemicals. Free Radic Biol Med. 179:328–336. doi: 10.1016/j.freeradbiomed.2020.12.007.
- Wu S, Lu H, Bai Y. 2019. Nrf2 in cancers: a double-edged sword. Cancer Med. 8:2252–2267. doi: 10.1002/cam4.2101.
- Wu P, Zhang X, Duan D, Zhao L. 2023. Organelle-specific mechanisms in crosstalk between apoptosis and ferroptosis. Oxid Med Cell Longev. 2023:3400147. doi: 10.1155/2023/3400147.
- Wu B, Zhao TV, Jin K, Hu Z, Abdel MP, Warrington KJ, Goronzy JJ, Weyand CM. 2021. Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation. Nat Immunol. 22:1551–1562. doi: 10.1038/s41590-021-01065-2.
- Xia M, Zhang Y, Jin K, Lu Z, Zeng Z, Xiong W. 2019. Communication between mitochondria and other organelles: a brand-new perspective on mitochondria in cancer. Cell Biosci. 9:27. doi: 10.1186/s13578-019-0289-8.
- Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, Zhang Z, Wang X. 2021. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 81:355–369.e10. doi: 10.1016/j.molcel.2020.11.024.
- Yang X, Wang H, Huang C, He X, Xu W, Luo Y, Huang K. 2017. Zinc enhances the cellular energy supply to improve cell motility and restore impaired energetic metabolism in a toxic environment induced by OTA. Sci Rep. 7:14669. doi: 10.1038/s41598-017-14868-x.
- Ye R, Selby CP, Chiou Y-Y, Ozkan-Dagliyan I, Gaddameedhi S, Sancar A. 2014. Dual modes of CLOCK: BMAL1 inhibition mediated by cryptochrome and Period proteins in the mammalian circadian clock. Genes Dev. 28:1989–1998. doi: 10.1101/gad.249417.114.
- Yin W, Wang C, Peng Y, Yuan W, Zhang Z, Liu H, Xia Z, Ren C, Qian J. 2020. Dexmedetomidine alleviates H2O2-induced oxidative stress and cell necroptosis through activating of α2-adrenoceptor in H9C2 cells. Mol Biol Rep. 47:3629–3639. doi: 10.1007/s11033-020-05456-w.
- Yoo HC, Yu YC, Sung Y, Han JM. 2020. Glutamine reliance in cell metabolism. Exp. Mol. Med. 52:1496–1516. doi: 10.1038/s12276-020-00504-8.
- Zhang Y, Li Y, Barber AF, Noya SB, Williams JA, Li F, Daniel SG, Bittinger K, Fang J, Sehgal A. 2023. The microbiome stabilizes circadian rhythms in the gut. Proc Natl Acad Sci. 120:e2217532120. doi: 10.1073/pnas.2217532120.
- Zhang Y, Peng L, Song W. 2020. Mitochondria hyperactivity contributes to social behavioral impairments. Signal Transduct Target Ther. 5:126. doi: 10.1038/s41392-020-00239-y.
- Zhang G, Sheng M, Wang J, Teng T, Sun Y, Yang Q, Xu Z. 2018. Zinc improves mitochondrial respiratory function and prevents mitochondrial ROS generation at reperfusion by phosphorylating STAT3 at Ser727. J Mol Cell Cardiol. 118:169–182. doi: 10.1016/j.yjmcc.2018.03.019.
- Zhang Y, Wong HS. 2021. Are mitochondria the main contributor of reactive oxygen species in cells? J Exp Biol. 224:jeb221606. doi: 10.1242/jeb.221606.
- Zhang S, Xin W, Anderson GJ, Li R, Gao L, Chen S, Zhao J, Liu S. 2022. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Disease. 13:1–13. doi: 10.1038/s41419-021-04490-1.
- Zhang S, Zhao Y, Ohland C, Jobin C, Sang S. 2019. Microbiota facilitates the formation of the aminated metabolite of green tea polyphenol (-)-epigallocatechin-3-gallate which trap deleterious reactive endogenous metabolites. Free Radic Biol Med. 131:332–344. doi: 10.1016/j.freeradbiomed.2018.12.023.
- Zhao T-X, Wei Y-X, Wang J-K, Han L-D, Sun M, Wu Y-H, Shen L-J, Long C-L, Wu S-D, Wei G-H. 2020. The gut-microbiota-testis axis mediated by the activation of the Nrf2 antioxidant pathway is related to prepuberal steroidogenesis disorders induced by di-(2-ethylhexyl) phthalate. Environ Sci Pollut Res. 27:35261–35271. doi: 10.1007/s11356-020-09854-2.
- Zhao F, Zou M-H. 2021. Role of the mitochondrial protein import machinery and protein processing in heart disease. Front Cardiovasc Med. 8. doi: 10.3389/fcvm.2021.749756.
- Zheng J, Chen X, Liu Q, Zhong G, Zhuang M. 2021. Ubiquitin ligase MARCH5 localizes to peroxisomes to regulate pexophagy. J Cell Biol. 221:e202103156. doi: 10.1083/jcb.202103156.
- Zheng F, Gonçalves FM, Abiko Y, Li H, Kumagai Y, Aschner M. 2020. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 34:101475. doi: 10.1016/j.redox.2020.101475.
- Zheng Q, Huang J, Wang G. 2019. Mitochondria, telomeres and telomerase subunits. Front Cell Dev Biol. 7:274. doi: 10.3389/fcell.2019.00274.
- Zhu D, Wu X, Zhou J, Li X, Huang X, Li J, Wu J, Bian Q, Wang Y, Tian Y. 2020. NuRD mediates mitochondrial stress–induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci Adv. 6:eabb2529. doi: 10.1126/sciadv.abb2529.
- Zhu L, Zhou Q, He L, Chen L. 2021. Mitochondrial unfolded protein response: an emerging pathway in human diseases. Free Radic Biol Med. 163:125–134. doi: 10.1016/j.freeradbiomed.2020.12.013.
- Zinghirino F, Pappalardo XG, Messina A, Nicosia G, De Pinto V, Guarino F. 2021. VDAC genes expression and regulation in mammals. Front Physiol. 12:708695. doi: 10.3389/fphys.2021.708695.
- Zischka H, Einer C. 2018. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int J Biochem Cell Biol. 102:71–75. doi: 10.1016/j.biocel.2018.07.001.