ABSTRACT
Seeking new bioactive compounds in drug research relies on the invention of new synthetic molecules and the discovery of natural phytochemicals from medicinal plants. Thus, natural products are one of the pillars of drug discovery. Leptadenia pyrotechnica is a promising edible herbal plant, which has common traditional uses in Asia and Africa to cure various serious diseases. Numerous research studies reported that this plant has many valuable bioactive constituents with several pharmacological activities. Therefore, this plant could be a source for the development of novel lead pharmaceutical compounds. This review discusses the significance of this plant to human health and thus drug discovery by providing a comprehensive report on the recorded data of folk uses, phytochemicals along with their analysis techniques, toxicity, and pharmacological effects of this plant. This will help researchers find out the recent drug discovery research progress that was accomplished on this plant as well as the available gaps to fulfill. A systemic search was conducted according to PRISMA at which 15 primary databases were explored. The results showed that 38 pharmacological and 44 chemical studies were conducted on this plant leading to the determination of more than 200 phytochemicals and 17 biological activities of this plant.
GRAPHICAL ABSTRACT
Introduction
Drug discovery is a lengthy process that involves several steps each of which requires a great deal of effort, time, and financial resources. The first step of this process is the identification of new “lead compounds”, in which we usually rely on medicinal plants or synthesis as a source for these compounds.[Citation1,Citation2] In fact, herbal preparations have been used in the treatment of lots of a large variety of disorders in ancient civilizations. The so-called natural products comprise a wide variety of biologically active compounds derived from natural sources such as plants, fungi and marine organisms.[Citation3] Consequently, about 35% of the available drugs in the market are directly derived and isolated from natural sources.[Citation1,Citation4] For example, vincamine alkaloid and its derivatives, which are produced naturally by Vinca species that belongs to the family Apocynaceae, are used to treat various brain disorders and they show neuroprotective potentiality in mice models.[Citation5] Also, three vinca alkaloids, including vinblastine, vinorelbine, and vincristine, are approved for clinical use as cancer drugs in the United States of America.[Citation6]
The family Asclepiadaceae (subfamily of Apocynaceae) is a valuable family for the identification of new bioactive compounds. It comprises around 250 genera and about 2000 species, which are of pharmaceutical interest as they possess a wide array of biologically active constituents.[Citation7,Citation8] One of these genera is Leptadenia which encloses about 6 species; pyrotechnica (Forssk.), hastata (Pers.) Decne., arborea (Forssk.) Schweinf (syn. heterophylla (Del.) Decne.), reticulata (Retz.) Wight & Arn. (syn. madagascariensis Decne.), and jazanicaY, ephedriformis.[Citation9–12]
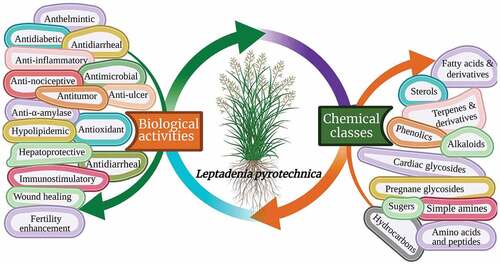
Among these species, L. pyrotechnica Forssk. Decne is a worthful and auspicious herbal medicine that belongs to genus Leptadenia and Family Asclepiadaceae. It is a highly branched, often erect leafless shrub, which grows in the hot arid sandy zones where the sand is non-saline and fairly deep. It is distributed from Northern Sahel Region throughout northern Africa, Arabian Peninsula, and western India. L. pyrotechnica can be found in Mauritania, Eretria, Somalia, Egypt, United Arab of Emirates, Qatar, Bahrain, Saudi Arabia reaching Pakistan and India. It is commonly known as kheep, Khip, Khimp, burning bush, desert broom, and markh.[Citation10,Citation11,Citation13–15] Other common synonyms of L. pyrotechnica are L. spartium, Cynanchum pyrotechnicum, and Periploca pyrotechnica.[Citation11,Citation15,Citation16] This species exhibits vast economic and medicinal values; especially that it is used as a traditional medicine in most of the countries where it grows and is utilized in the treatment of several serious diseases ranging from simple (e.g. stomach) to complex (e.g., hepatitis) types and is pharmacologically widely tested. Relevant studies showed that this plant exhibits many substantial pharmacological activities such as antimicrobial, wound healing, antitumor, antidiabetic, antioxidant, anti-lipoxygenase, anthelmintic, anti-atherosclerotic, hypolipidemic and cytotoxic activities. L. pyrotechnica is known to be rich with precious phytochemicals such as flavonoids, phenolic acids, cardiac glycosides, pregnane glycosides, alkaloids, fatty acids, terpenes, sterols, hydrocarbons, amino acids, and sugars.[Citation11,Citation17–21]
Based on previous studies, this plant is considered as a promising natural remedy which may be significant in the identification of potential new drugs. Thus, the aim of this review is to emphasize the progresses and advances which were carried out on L. pyrotechnica, highlighting its possible applications in pharmaceutical industry, and summarizing its ethnomedicinal use, phytoconstituents, their separation and analysis techniques, pharmacological activities, and toxicity. Hence, this will help highlight the scientific gaps in the current literature and provide further new insights for future research work on this plant.
Methodology
Search strategy
Relevant literature was addressed through conducting a systemic search in September 2021 following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[Citation22] Fifteen primary databases were investigated from outset until 2022, i.e., ACS, BMC, Cambridge core, FSTA, Nature, Oxford journals, PubMed, SAGE, Science direct, Sci-Finder, Scopus, Springer, Taylor and Francis, Web of science, and Wiley. In addition, a manual search was carried out using forward and backward techniques to detect more data. The search terms included the plant name “ Leptadenia pyrotechnica” and its synonyms ;Citation16 Cynanchum pyrotechnicum, Gymnema spartium, Leptadenia jacquemontiana, Leptadenia spartum, Microloma angustifolium, Microloma pyrotechnicum, Periploca pyrotechnica, and Sarcostemma pyrotechnicum. The authors (Rana Ahmed El-Fitiany (RE) and Mohammad A. Khasawneh (MK)) determined and evaluated the search terms through searching for synonyms of the plant understudy in the previously published records.
Inclusion and exclusion criteria
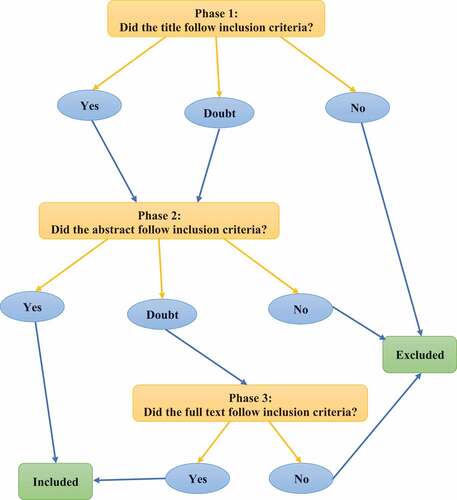
Inclusion criteria selected in the current review were as follow: (1) The study was published in English language; (2) The study discussed the traditional/folk/ethnomedicinal uses of L. pyrotechnica; (3) The study reported identification of chemical constituents of L. pyrotechnica; (4) The study stated isolation of pure chemical compounds from L. pyrotechnica; (5) The study described separation and analysis techniques of the plant chemical constituents; (6) The study testified the toxicity of L. pyrotechnica and/or its isolated and/or identified compounds; and (7) The study reported and/or evaluated and/or assessed pharmacological activity of L. pyrotechnica and/or its isolated and/or identified compounds. Studies which did not match the above stated inclusion criteria were excluded from the review. Thus, screening was conducted by RA to filter the obtained references and MK double-checked the sorted reference list to avoid any inconsistency. The screening process was conducted through three phases as shown in . The first phase was performed on the titles of the articles as the titles which did not follow the aforementioned inclusion criteria were excluded. Afterwards, the second phase was conducted on the abstracts of the studies which were accepted in the first phase in order to confirm their inclusion. The third phase was executed on the full texts of the studies in which their titles and abstracts were suspicious to follow the inclusion criteria.
Data extraction
Extraction of data was accomplished by both authors and collected in an Excel sheet according to a standardized form. In other words, wherever applicable, the selected information from the included manuscripts contained the year of publication, title of manuscript, tested organs of the plant, the country where the plant was collected, the tested extracts and/or fractions, its biological activity, toxicity, isolated and identified compounds, methods, and techniques of analysis and/or separation, tested concentrations, folk uses and methods of preparation.
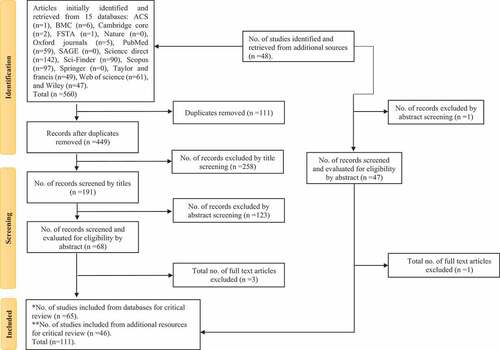
As shown in , a total number of 560 articles were perceived from the initial search of the 15 databases mentioned above, in addition to 48 studies from other resources. Regarding the databases’ extracted articles, there were 111 duplicate studies were detected and removed, then the rest of studies (n = 449) went through three phases of screening according to the inclusion criteria. The first phase “Title screening” led to the exclusion of 258 articles, while 127 studies were left out in the second phase “abstract screening”. The remaining number (n = 68) were evaluated for eligibility through screening their full texts at which three manuscripts were omitted. In contrast, only two articles were dismissed from the additional resources’ retrieved articles, resulting in a total of 111 studies included for review in this study.
Among these studies, 81 articles were conducted in countries belonging to Asia and 18 manuscripts belonging to those in Africa. These contributed countries are India, Pakistan, United Arab Emirates, Saudi Arabia, Iran, Qatar, Palestine, Yemen, Egypt, Sudan, Mali, Nigeria, and Algeria ().
Table 1. Study areas of the included articles.
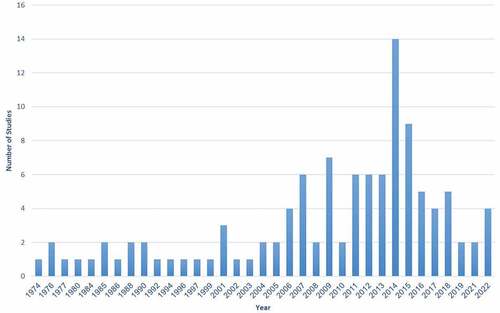
The included studies were published in the period between 1974 and 2022 as illustrated in . Also, it can be noticed that the number of studies of the chemistry, biology and ethnomedicine of L. pyrotechnica gradually increased from 1 article in 1974 reaching 7 articles in 2009, then decreased again on the span of 2010 and 2013. Afterward, they rocketed in 2014 reaching 14 studies, followed by 9 studies in 2015, and gradually ran short to 4 publications in 2022.
Results and discussion
Ethnomedicinal uses
The different organs of L. pyrotechnica are used traditionally in the treatment of about 53 human and livestock diseases in its native countries in Africa and Asia. The treated diseases along with the plant’s used parts, its traditional preparation methods, doses, and routes of administration are shown comprehensively in .
Table 2. Ethnomedicinal uses of Leptadenia pyrotechnica (Forsk.) Decne in different countries in Africa and Asia.:
Phytochemistry
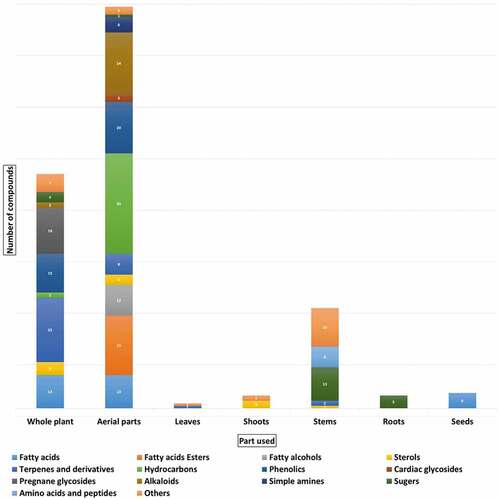
Although there are few reports regarding the chemistry of L. pyrotechnica. A total of 273 compounds were detected in the various organs of the plant; 50 of them were isolated. These compounds belong to a wide array of chemical classes (), including fatty acids, fatty acid esters, sterols, terpenes, phenolics, pregnane glycosides, cardiac glycosides, alkaloids, simple amines, hydrocarbon, sugars, amino acids, and peptides.[Citation11,Citation17,Citation19,Citation20,Citation63] Most of the reported constituents are hydrocarbons constituting 41 compounds of the total recorded compounds. summarizes the reported compounds in terms of their chemical names, classes, plant organ source, area at which they were detected, and method of investigation.
Table 3. Previously isolated and identified compounds of Leptadenia pyrotechnica (Forsk.) Decne.:
Phytochemical screening, proximate analysis, and nutritional values
Ethanolic extract of the Indian whole plant and its fractions (petroleum ether, chloroform, ethyl acetate, and methanol) went through preliminary phytochemical screening, and they revealed the presence of alkaloids, amino acids, carbohydrates, proteins, terpenes, flavonoids, glycosides, saponins, tannins, and steroids.[Citation93] Also, the methanol extract of the whole plant revealed positive results for alkaloids test (Wagner and Hager), while negative for Mayer in a study conducted on the Saudi Arabian plant.[Citation94] Interestingly, the callus and different plant parts were subjected to primary metabolite quantification. The maximum soluble sugars were found in callus, while the maximum amount of starch, protein and phenolic contents were found in the stems and the maximum lipids were found in the leaves.[Citation95]
The phytochemical screening of the methanolic extract of the aerial parts of L. pyrotechnica showed the presence of cardiac glycosides, flavonoids, alkaloids, sterols and/or triterpenes, carbohydrates and/or glycosides, coumarins, saponins, catechol, pyrogallol tannins. Whereas the aerial parts are free from crystalline sublimate, volatile oils, volatile amines, cyanogenic glycosides, anthraquinones and iridoids.[Citation72] Another study conducted in UAE demonstrated the presence of terpenes and/or sterols, saponins, flavonoids, and tannins in the 95% ethanolic extract of the arial parts.[Citation96]
In an Indian study, the phytochemical screening of the aerial parts’ ethanolic extract and its fractions, including petroleum ether, chloroform, ethyl acetate, and methanol, showed that they contain carbohydrates, alkaloids, flavonoids, tannins, steroids, proteins, saponins, glycosides and terpenoids.[Citation97] Additional Egyptian study conducted a preliminary phytochemical analysis on the ethanolic extract of the aerial parts. The results referred to the presence of flavonoids and anthraquinone in it.[Citation98] The aqueous methanolic extract (80:20% v/v) of the aerial parts of the Nigerian plant was subjected to preliminary phytochemical screening, which showed the presence of cardiac glycoside, tannins, terpenoids, alkaloids and flavonoids.[Citation99] Also, the proximate mass composition of the aerial parts was found to contain 4.39% for lipids, 4.89% for ash, 5.60% for protein, 15.61% for carbohydrates, 46.70% for crude fiber, and 6.77% for moisture in Qatar.[Citation100]
Hexane, chloroform, acetone, ethyl acetate, butanol, ethanol, methanol and water extracts of the roots and aerial parts were subjected to qualitative and quantitative screening for four major groups of phytochemicals, viz. alkaloids, flavonoids, saponins and tannins. The qualitative screening displayed the presence of all major groups of phytochemicals in the extracts of both parts, while it was noted that the methanolic extract was the most efficient one. Concerning the quantitative screening, it was clear from the data that the aerial parts contain more saponins, flavonoids, and tannins, representing 0.46%, 139.448 mg of QE/100 g of extract, and 154.961 mg of TAE/100 g of extract, respectively. In contrast, the roots presented more alkaloids (3.267%).[Citation101] The primary metabolites (carbohydrates, lipids, proteins, and phenols) of the dry powders of the roots and shoots were quantified. The results showed that the shoots contain more lipids, total soluble sugars, and phenols than the roots, representing 140, 5.5, and 0.26 mg/g dry weight, respectively. However, the roots are more rich in proteins and starch representing 15 and 4 mg/g dry weight, respectively.[Citation102] Mineral content analysis reported that the mineral content in the fruits are 156 mg for calcium, 317 mg for phosphorus, and 3.18 mg for iron, and the energy content is 68 Cal./100 g of the pulp.[Citation103]
Fatty acids
A total of 29 fatty acids were determined in the whole plant, aerial parts and seeds of L. pyrotechnica. Most of them were identified in aerial parts.[Citation65,Citation68,Citation70] On the one hand, lauric, myristic, palmitic, palmitoleic, oleic, linoleic, alpha-linolenic, arachidic and behenic acids were detected in the petroleum ether extract of markh grown in Qatar.[Citation70] On the other hand, n-hexadecanoic, heptadecanoic, linoleic, and α- linolenic acids were characterized in the hexane fraction of the aerial parts of the UAE grown Markh.[Citation68] Interestingly, only six compounds, named stearic, oleic, palmitic, linoleic, linolenic, and vernolic acids, were recognized in the seeds oil.[Citation69,Citation71] Vernolic acid is an oxygenated fatty acid, which is called an epoxy fatty acid. It was isolated chromatographic techniques and identified using spectroscopic analysis.[Citation69]
Fatty acid esters
The methyl and ethyl ester derivative of the fatty acids of the hexane and petroleum ether extracts of the aerial parts of the plant were studied by GC/MS analysis in UAE and Egypt. The investigations resulted in the identification of nine fatty acid ethyl esters from the hexane extract in the study conducted in UAE, while, in the study conducted in Egypt, 14 methyl esters were detected in the saponifiable matter derived from acetone soluble fraction of petroleum ether extract.[Citation68,Citation72]
Fatty alcohols
Fatty alcohols content of the aerial parts of Egyptian and Indian L. pyrotechnica were also studied. Cetyl alcohol was isolated from the benzene and the ethanol extracts using spectral techniques in India.[Citation73] Whereas eleven fatty alcohols, named nonacosanol, triacontanol, hentriacontanol, dotriacontanol, tritriacontanol, tetratriacontanol, pentatriacontanol, hexatriacontanol, heptatriacontanol, octatriacontanol, and nonatriacontanol, were isolated and identified from the acetone insoluble fraction of the petroleum ether extract of aerial parts grown in Egypt.[Citation72]
Sterols
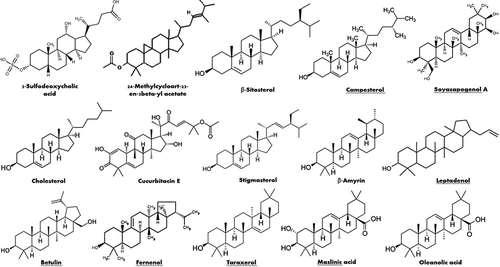
Seven sterols were found in the literature to be recognized in different parts of the plant (). β-sitosterol was detected in different organs of the plants in several studies. It was identified and quantified in the methanolic extracts of the whole plants of three populations in India, viz. P-I Bikaner, P-II Churu, and P-III Ajmer, and the compound concentrations were found to be 19.79 ± 0.05, 16.69 ± 0.02, and 15.75 ± 0.07 mg/g dry weight of plant, respectively.[Citation82] Moreover, it was isolated from the whole plant in another study in India.[Citation76] β-Sitosterol was also identified and quantified in a previous study in the plant shoots using chromatographic and spectral techniques.[Citation83] β-Sitosterol was also identified in the hexane, and the unsaponifiable matter derived from acetone soluble fraction of petroleum ether extract aerial parts in UAE and Egypt.[Citation68,Citation72] However, it was isolated from benzene fraction of the ethanolic extract in two studies conducted in India,[Citation73,Citation84] in addition to another study in Egypt using chromatographic and spectroscopic methods.[Citation79] Stems of the plants growing in Southwest Asia and north Africa were found to contain β-sitosterol as well.[Citation40]
Campesterol was identified and quantified in the Indian shoots of the plant,[Citation83] and detected in the hexane fraction and unsaponifiable matter derived from acetone soluble fraction of petroleum ether extract of the aerial parts in UAE and Egypt, respectively.[Citation68,Citation72] Stigmasterol was detected in the shoots and isolated from the whole plant in India. Furthermore, it was determined in the hexane and petroleum ether extracts of the aerial parts in UAE and Egypt, respectively.[Citation68,Citation72,Citation76,Citation83] Another study revealed the presence of cholesterol in the unsaponifiable matter derived from acetone soluble fraction of petroleum ether extract of the aerial parts.[Citation72] Also, cucurbitacin E, 24-methylcycloart-23-en-3β-yl acetate, and 3-sulfodeoxycholic acid were detected in the methanolic crude extract of the whole plant in Pakistan.[Citation65]
Terpenes and their derivatives
Thirty-four terpene derivatives were reported in the whole plant, aerial parts, leaves and stems. presents the structures of some important terpenes. Regarding the detected triterpenes, lupeol was detected and quantified in India in the methanolic extract of the whole plant. The compound concentrations were found to be 9.44 ± 0.40, 10.39 ± 0.35, and 12.29 ± 0.15 mg/g dry weight of plant, respectively.[Citation82] In addition, it was identified in the hexane fraction and unsaponifiable matter derived from the petroleum ether fraction of the aerial parts in UAE and Egypt, respectively.[Citation68,Citation72] Lupeol, β-Amyrin and botulin were isolated in a previous study conducted in Egypt using various chromatographic and spectroscopic methods.[Citation79] The triterpene oleanolic acid was quantified in the methanolic extracts of the whole plant to be 10.61 ± 0.05, 12.45 ± 0.08, and 18.88 ± 0.01 mg/g dry weight, respectively, in three Indian populations, viz. P-I Bikaner, P-II Churu, and P-III Ajmer.[Citation76,Citation82] A glycol-oleanolic acid conjugate, pyrotechnoic acid, was isolated from the butanol fraction of the leaves’ ethanolic extract in Pakistan.[Citation86] Furthermore, squalene was found in the hexane fraction of the aerial parts of the Emirati khimp, in addition to the unsaponifiable matter yielded from acetone soluble fraction of petroleum ether extract of the Egyptian grown markh.[Citation68,Citation72] Taraxerol was identified in the aerial parts’ unsaponifiable matter that was derived from acetone soluble fraction of petroleum ether extract. Additionally, it was isolated from the benzene fraction, which was derived from acid insoluble residue of ethanolic extract of the aerial parts.[Citation40,Citation72,Citation84] Moreover, fernenol was reported in the stems, and isolated from benzene fraction which was yielded from acid insoluble residue of the aerial parts’ ethanolic extract.[Citation40,Citation84] β-Amyrin acetate was isolated, in an Indian study, from benzene and ethanol extracts of the aerial parts.[Citation73] Leptadenol, a pentacyclic triterpenoid, was isolated from the hexane extract of the plant grown in Pakistan.[Citation85]
Lupanol 3-O-diglucoside was isolated from benzene and ethanolic extracts of the aerial parts.[Citation73] The triterpene alcohol, hopenol B, was recognized in the hexane fraction of the aerial parts of the plant grown in UAE.[Citation68] Finally, phytol was detected in hexane and petroleum ether extracts of the aerial parts in UAE and Egypt.[Citation68,Citation72] Other monoterpenes, diterpenes, triterpenes, and sesquiterpenes were identified through metabolic profiling of the methanolic crude extract of the Pakistani whole plant using RP-UHPLC/MS.[Citation65]
Hydrocarbons
Forty-one hydrocarbons were detected in the whole plant and hexane, petroleum ether, benzene and ethanol extracts of the aerial parts. These compounds belong to alkanes, alkenes, aromatic hydrocarbons, ketones and alcohols.[Citation68,Citation72,Citation73,Citation76]
Phenolics
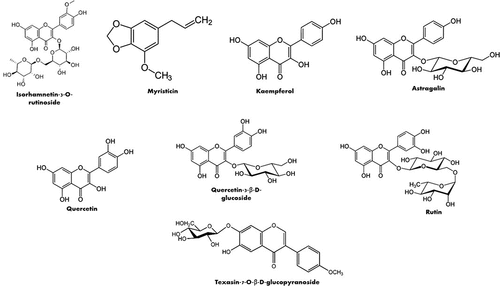
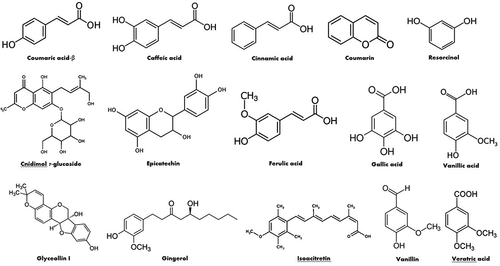
Phenolic acids, phenolic aldehydes, flavonoids, phenylpropene, coumarins, and benzenediol were also identified in the aerial parts of L. pyrotechnica in several studies conducted in India, Pakistan, UAE, and Egypt (). Caffeic acid, vanillic acid, p-coumaric acid, ferulic acid, veratric acid, and cinnamic acid were identified and quantified in the methanolic extract of the aerial parts. Caffeic acid showed the highest concentration (3.3%) in the tested extract followed by vanillin (0.018%).[Citation77] In another study, caffeic, vanillic, gallic and cinnamic acids were also found in the ethyl acetate fraction of the aerial parts. In contrast, ethanol and butanol extracts contain caffeic, vanillic, and gallic acids, whereas the water fraction contains only vanillic and gallic acids. The study revealed that vanillic acid presents with the highest concentration in the tested extracts among the detected phenolic acids representing 7.03, 8.10, 3.42, and 1.20 mg/g extract in the ethanolic, ethyl acetate, butanol, and water fractions, respectively.[Citation78]
In the flavonoid family, epicatechin and quercetin-3-β-D-glucoside were identified in the ethanol, ethyl acetate, and butanol fractions of the aerial parts showing the highest concentration of epicatechin in the ethanol extract (8.76 mg/g extract) and quercetin-3-β-D-glucoside in the ethyl acetate fraction (16.72 mg/g extract). Both compounds were not detected in the water fraction.[Citation78] Another study led to the isolation of six flavonoids from the ethyl acetate extract of the aerial parts, named kaempferide-3-O-α-l-rhamnopyranosyl (1′″→6″)-O-β-d-glucopyranoside, kaempferol-3-O-β-d-rhamnopyranosyl (1′″→6′′)-O-β-d-glucopyranoside, kaempferide3-O-α-l-rhamnopyranosyl (1′″→6′′)-O-β-d-glucopyranoside, kaempferol-3-o-β-d-glucopyranoside (astragalin), texasin-7-o-β-d-glucopyranoside, and kaempferol.[Citation80] Astragalin, quercetin, isorhamnetin-3-O-rutinoside, quercetin-3’- O-β-D-glucoside, and rutin were also isolated from khip in another study conducted in Egypt.[Citation79]
Other benzenediols, phenolic aldehydes, phenylpropenes, and coumarins were also detected and quantified in the methanolic extract of the aerial parts. These constituents are resorcinol (0.008%), vanillin (0.181%), myristicin (0.013%), coumarin (0.004%).[Citation77] Furthermore, 15 phenolic compounds were characterized and identified in the crude methanolic extract of the whole plant of Pakistan using RP-UHPLC/MS through matching the resulted data with database. At the same study, the methanolic extract in addition to its hexane, ethyl acetate and water fractions were studied for their total phenolic and flavonoid contents through conducting Folin-Ciocalteu and AlCl3 assays, respectively. The highest contents were found in the hexane fraction since they were 25.79 ± 0.11 mg GAE/g and 20.64 ± 0.33 mg RE/g, respectively.[Citation65] Purohit et al. determined the total flavonoid and phenolic contents of the ethanolic extract of the whole plant to be 34.85 mg QE/g DW and 49.47 mg GAE/g DW, respectively, in India.[Citation93] Alqasoumi et al. determined the total phenolic, and flavonoid contents of the ethanolic extract of the aerial parts in Egypt. Folin-Ciocalteu method which involves gallic acid as a standard was used for the estimation of total phenolics, while colorimetric method using a quercetin calibration curve was applied to determine total flavonoid content. The tested extract showed a high phenolic content (158.3 ± 6.25 mg GAE per g extract) and flavonoid content (89.0 ± 3.40 mg QE per g extract).[Citation104] Furthermore, the polyphenolic content of the ethanolic crude extract, ethyl acetate, n-butanol and water fractions of the aerial parts were investigated using Folin-Ciocalteu method. The results revealed that ethyl acetate fraction displayed the highest phenolic content (252.27 ± 2.84 mg gallic acid/g of dry extract).[Citation78] Moreover, the total phenolic content of the hexane fraction of the aerial parts was studied and it was 10.53 mg gallic acid/g.Citation68 Additional study showed that the total phenolic content was found to be 2.11 ± 0.86 mg GAE/g in the 80% methanolic extract of the leaves of Pakistan.[Citation105] Interestingly, the 70% ethanolic extract of the Egyptian green fruits were examined for their total phenolic contents, and it was found to be 59.1 mg GAE/g.[Citation106]
Pregnane glycosides
A study conducted in Mali revealed the isolation of 18 pregnane glycosides from the chloroform-methanol 9:1 (v/v) extract of the whole plant. The aglycone moieties include deacetylmetaplexigenin, sarcostin, and 11-hydroxysarcostin. C-12 and/or C-20 of the identified aglycones were linked with ester moieties, viz. acetyl, benzoyl, cinnamoyl, p-coumaroyl, and nicotinoyl. The determined sugar molecules were found to be linked to C-3 of the aglycons; they include hexopyranose, 6-deoxy-3-Omethylhexopyranose, and 2,6-dideoxy-3-O-methylhexopyranose sugars.[Citation81]
Cardiac glycosides
Three cardiac glycosides were isolated from the areal parts’ latex and their defatted methanol extract in Egypt, named 14,19-Dihydroxycard-20 (22)-enolide-3-O-β-d-digitoxoside,14,19-Dihydroxycard-20 (22)-enolide-3-O-[β-d-glucopyranosyl-β-d-digitoxoside], and 14,19-Dihydroxycard-20 (22)-enolide-3-O-[β-d-glucopyranosyl-β-d-glucopyranoside].[Citation67]
Alkaloids and simple amines
A sum of 24 alkaloids, including pyridine, pyrrole, pyrazine, and indole derivatives, were identified through GC-MS analysis of the alkaloid fraction of the defatted methanol extract of the aerial parts. Additionally, six simple amines, named N-ethyl-N-hydroxyethanamine, 4-aminobenzene-1,3-diol, 2-methylazetidine, N, N’- diphenylcarbodiimide, N-(prop-1-yn-1yl) acetamide, 1,2-dimethylazetidine, were detected.[Citation64] Another study indicated the identification of two alkaloids, including 4-formyl Indole and ibogaine, in the methanolic extract of the whole plant using RP-UHPLC/MS analysis.[Citation65]
Sugars and glycosides
Leptidin was isolated from the benzene and ethanol extracts of the aerial parts of the Indian L. pyrotechnica.[Citation73] Additional ten sugars, including glucose, sucrose, fructose, ionositol, fucose, raffinose, maltose, xylose, arabinose, and rhamnose were detected in the whole plant, stem, its exudate, fibers, and root.[Citation66,Citation74–76]
Amino acids and peptides
The stems of L. pyrotechnica were found to be rich in amino acids and peptides. In an Indian study, two dipeptides, viz. DL-alanyl-L-alanine, and glycyl-L-alanine, and six amino acids, namely L-lysine, L-arginine, L-alanine, L-threonine, L-methionine, and L-isoleucine, were determined in the stem.[Citation66]
Miscellaneous
Other compounds, belonging to additional scattered chemical classes, were reported in various parts of the plant in different studies. α-Cellulose was detected in the whole plant, stem, and its fibers. It was found that its content in the stem of Sudan and India was 44.3% and 30.51%, respectively.[Citation88,Citation89] In contrast, the α-cellulose content of stem fibers was the highest as it was found to be 75.26%.[Citation74,Citation90,Citation91] Moreover, hemicellulose and holocellulose were found in the stems of Sudan and India with a percentages of 22.1, and 64.3, respectively.[Citation88,Citation89]
The lignin content of the stems has the highest content of lignin compared to other parts with percentage of 21.7% in Sudan. In the Indian plant, the stems, fibers, and whole plant contain 16.12%, 4.93%, and 16.12%, respectively.[Citation74,Citation88–91]
The ash content was investigated in the Indian whole plant, stem, and its fibers, representing 3.12%, 3.12%, and 2.77%, respectively. The Sudan plants’ stems contain 2.4% only.[Citation74,Citation88–90] High amounts of 3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetrasiloxane and penicillamine were found in the plant shoots in India.[Citation83] Also, lanostan-3-β-ol, α -tocopherol (Vitamin E), and ismiarenol were characterized in hexane extract of the aerial parts in UAE.[Citation68] Pentosan and pectin were quantified in the whole plant (18.97% and 2.14%, respectively), stem (18.97% and 2.14%, respectively), and its fibers (5.15% and 3.84%, respectively) in India.[Citation74,Citation89–91] Stem fibers, also, demonstrated the presence of uronic anhydride and nitrogenous matter with amounts of 6.2% and 0.44%, respectively.[Citation74] Additionally, proline amounts were quantified in the leaves during different seasons (stressed, winter, and rainy) in an Indian study, and their concentrations were ranging from 0.77% to 2.93%.[Citation92] Enzymes were also detected in the plant as acid phosphatase in the Indian plant stems.[Citation87]
Recently, a metabolic profiling study in Pakistan was performed at which 1-Octen-3-yl glucoside and 6’-Hydroxymethylsimvastatin were detected in the methanolic extract of the whole plant using RP-UHPLC/MS.[Citation65]
Separation and analysis techniques
Chromatographic techniques
Chromatographic techniques are the most common techniques that are used in the fractionation, isolation, and purification of bioactive compounds. These techniques include Paper Chromatography (PC), Column Chromatography (CC), Thin Layer Chromatography (TLC), Vacuum Liquid Chromatography (VLC), High-Performance Thin-Layer Chromatographic (HPTLC), Low Pressure Liquid Chromatography (LPLC), Medium Pressure Liquid Chromatography (MPLC), High Performance Liquid Chromatography (HPLC), Reversed Phase-Ultra High Performance Liquid Chromatography (RP-UHPLC/MS), Preparative Paper Chromatography (PPC), Two-Dimensional Paper Chromatography (TDPC), Gas Liquid Chromatography (GLC), Gas Chromatography/Mass Spectrometry (GC/MS), Gas Chromatography-Flame Ionization Detection (GC-FID), and Rotation Locular Counter Current Chromatography (RLCCC).
Metabolic profiling was performed on the Pakistani whole plant using RP-UHPLC/MS. A total number of 57 metabolites, belonging to phenolics, flavonoids, terpenoids, steroids, fatty acids, and alkaloids, were determined by matching the resulted data with databases.[Citation65] For the detection of lipoidal matter, CC and TLC were used for the isolation of fatty alcohols, hydrocarbons, terpenes, and sterols from the unsaponifiable matter of the petroleum ether extract of the aerial parts of the plant under review grown in Egypt. The utilized stationary phase in CC was silica gel, while the mobile phase was petroleum ether, followed by an increasing ratio of a mixture of petroleum ether and benzene, then a mixture of benzene and methanol with a ratio of 75:25.[Citation72] Both techniques were used in another study for the isolation of two phytosterols and one triterpene compound as the ethanolic extract of the aerial part was acidified with HCl. The acid insoluble residue was applied to CC packed with silica gel as stationary phase, and the mobile phases were petroleum ether and benzene. The benzene fraction yielded the three previously mentioned compounds.[Citation84] Furthermore, VLC was used for the fractionation of the butanol fraction of the leaves. The eluted fraction, using the mobile phase mixture solvent of chloroform and methanol (30:70), was then subjected to CC using CHCl3 and methanol as mobile phases, yielding a triterpene which is called pyrotechnic acid.[Citation86] Also, HPTLC was used to develop a sensitive analytical method to estimate the concentrations of β-sitosterol, lupeol, and oleanolic acid, which were used as analytical markers, in the methanolic extracts of whole plants from three populations to ensure the therapeutic dose in herbal formulations, standardization, and quality control of bulk drugs.[Citation82] GLC and GC/MS were used in several studies to carry out quantitative and qualitative analysis of the lipid content of L. pyrotechnica organs. Five fatty acids were identified and quantified from the seed oils of the plant in Pakistan using GLC.[Citation71] An additional study in Pakistan isolated, characterized and identified an epoxy fatty acid in the seed oil using standard Gunstone’s method of direct acetolysis beside GC analysis.[Citation69] Moreover, fatty acids of the aerial parts of the plant grown in Qatar were liberated by acidification of saponifiable matter with H2SO4 and extracted by ether; afterward, their methyl esters were analyzed using GC.[Citation70] Another study in UAE applied GC/MS technique to investigate the hexane fraction of Khimp aerial parts, resulting in the detection of sterols, hydrocarbons, terpenes, fatty acids and their derivatives.[Citation68] Likewise, in the investigation of the petroleum ether extract of the aerial parts of the Egyptian grown plant as GC, GC-MS, GC-FID as well as comparison with published data were applied to identify the isolated contents of the saponifiable and unsaponifiable matters, including fatty acid esters, fatty alcohols, terpenes, hydrocarbons, and sterols. The study reported that GC/MS gave better results than GC-FID as they are more reliable and easier in interpretation.[Citation72] GC-MS was operated in another study for the qualitative and quantitative determination of phytosterols in shoots of the plant in India.[Citation83]
For phenolics, HPLC was used in numerous studies to investigate the phenolic content of plant extracts qualitatively and quantitatively. It was used in a previous study to develop and optimize a simple, rapid, analytical HPLC method for the simultaneous separation and quantification of ten phenolic constituents from the crude methanolic extract of the aerial parts of the plant. The column which was used for the separation process was Atlantis T3 column, using isocratic elution of acetonitrile and water mixture as mobile phase with a flow rate of 0.8 ml/min. The apparatus was coupled with a diode-array detector held at 25°C, in which spectra were obtained at 200–600 nm. The determination of the compounds was carried out through peak areas and Rf values.[Citation77] Additional study operated HPLC for the quantitative and qualitative determination of the phenolic content of the aqueous ethanolic crude extract of the aerial parts, in addition to its ethyl acetate, butanol and water partitioning fractions, using authentic standards of six polyphenolic compounds, viz. gallic acid, vanillic acid, caffeic acid, epicatechin, trans-cinnamic acid, and quercetin-3-β-D-glucoside. The utilized column was C18 reversed phase, using a gradient elution of acetic acid-water and methanol as mobile phase with a flow rate of 0.8 mL/min. Diode array detector (DAD) was coupled with the equipment as the spectra for detection was at 254, 280, 320 and 370 nm. The determination of the compounds was done using retention times and calibration curves, respectively.[Citation78] Also, LPLC, PPC, TLC, TDPC and HPLC were used for the isolation of six flavonoids from the ethyl acetate fraction of the aerial parts. The ethyl acetate fraction was fractionated into four subfractions through its application to Sephadex LH-20 as adsorbent on LPLC, using an eluant of 95% methanol/water. TLC spotting of column chromatography fractions and subfractions was conducted, and the spots were detected via ultra-violet light and by spraying with AlCl3 reagent. PPC was used to complete the isolation process. Further purification of fractions, subfractions and isolated compounds was done on Sephadex LH-20 LPLC, CC and TDPC.[Citation80]
Regarding glycosides, RLCCC, HPLC-diode array, and TLC were applied to isolate cardiac glycosides from the arial parts’ latex and their defatted methanol extract.[Citation57] In Mali, 18 pregnane glycosides were isolated from the chloroform-methanol (9:1) extract via CC using sephadex LH20 as stationary phase and MeOH as mobile phase, beside MPLC using silica gel as stationary phase and gradient elution of CHCl3-MeOH as mobile phase. RP-HPLC on a C18 was employed to purify the obtained pregnane glycosides’ fractions. Furthermore, GC analysis was used for the determination of the absolute configuration of glucose moieties in the isolated pregnane glycosides from the chloroform-methanol extract of the whole plant in Mali using Chiral-Val column. The resulted retention times were compared with those of D- and L-glucose authentic samples.[Citation81]
For detection of alkaloids and simple amines, TLC was utilized to detect the alkaloids and amines constituents of the aerial parts in an Egyptian study, as the study utilized various adsorbents and elution systems. The adsorbents included silica gel and alumina, while the elution systems consisted of (S1) chloroform-acetone-diethylamine (85:15:1), silica gel, (S2) benzene-ethyl acetate-diethylamine (70:20:10), silica gel, (S3) toluene-dichloromethane (9:1), silica gel, (S4) ethyl acetate-n-hexane (80:20) +3 drops of ammonia, alumina, (S5) chloroform-acetone (85:15), alumina, (S6) benzene-methanol (90:10) +3 drops of ammonia, alumina and (S7) n-butanol-acetic acid-water (80:3:17), cellulose. The best resolution was achieved with the plates coated with silica gel G and eluted with S1, S2, S3 and S4. Visualization of the resulted spots were done by using ultraviolet light with the wavelength of 254 and 366 nm, in addition to dragendorff’s and iodoplatinic acid as spraying reagents with 10% sulfuric acid to increase the detection sensitivity if needed. VLC was also used for fractionation of the alkaloid extract and further isolation of alkaloid fractions, which were derived from the defatted methanolic extract. It was packed with silica gel and/or aluminum oxide as stationary phases. The mobile phases consisted of different ratios of toluene, dichloromethane, methanol, cyclohexane, and acetone. GC-MS was operated to analyze and determine the alkaloids that constitutes the alkaloid fractions, as the identification process relied on comparing the relative retention times and mass spectra of the tested samples with authentic ones and/or the National Bureau of Standards (NBS) and the spectra of Wiley Library, in addition to comparing their retention indices with literature data.[Citation64] Also, sugar content of khimp stem fibers was identified and quantified using PC and GLC coupled with FID.[Citation74]
Spectral Techniques
Nuclear magnetic resonance (NMR), mass spectrometry (MS), and Infrared (IR)
In lipid content, a study applied a combination of spectral techniques to determine the structure of a glycol-oleanolic acid conjugate triterpene which is called pyrotechnoic acid in L. pyrotechnica leaves. This study used 1D NMR techniques, viz. 1H-NMR, 13C-NMR, and DEPT 13C-NMR to determine numbers, types and coupling patterns of carbons and hydrogens. 2D NMR techniques were employed as well, including H-COSY and HMBC, in order to get more information regarding the correlation between the carbon and hydrogen atoms. Additionally, IR was carried out to know the functional groups in the structure, in addition to Electron ionization-Mass spectroscopy (EI-MS), Field desorption mass spectroscopy (FDMS), High resolution mass spectrometry (HR-MS), which were operated to get more details about the compound’s molecular weight and fragment ions.[Citation86] In other studies, in India, IR, 1 H-NMR, and MS techniques were conducted, leading to the identification of isolated fatty acid alcohol, sterols, glycoside, hydrocarbon, terpene and its derivative in the benzene and ethanol extracts of the aerial parts of khip. Comparison with authentic samples were also carried out to asses in the identification process.[Citation73,Citation84] Also, IR was used in the identification of the lipid content in the aerial parts’ petroleum ether extract in Egypt, and determination of phytosterols of the shoots in India.[Citation72,Citation83]
In case of phenolic compounds, the chemical structures of isolated six flavonoids from the ethyl acetate fraction of the aerial parts were elucidated with the aid of mass spectrometry (FAB-, ESI- and EI-techniques), 1D, and 2D NMR (1H-, 13C- and COSY).[Citation80]
The structures of three isolated cardiac glycosides were elucidated using integrated spectroscopic equipment, including fast-atom bombardment (FAB) and electrospray ionization (ESI) mass spectrometry and NMR.[Citation67] Additionally, determination of isolated pregnane glycosides’ structures, which were isolated from the whole plant chloroform-methanol extract, was done with the aid of mass spectroscopy (ESIMS, ESIMSn (multistage MSn analysis)), 1D NMR (1H NMR, 13C NMR and DEPT), and 2D NMR (HSQC, HMBC, TOCSY, DQF-COSY, ROESY) techniques.[Citation81]
Ultraviolet spectroscopy (UV)
UV spectrophotometer was applied to measure the total phenolic content of ethanolic crude extract of the aerial parts of L. pyrotechnica, beside its ethyl acetate, butanol and water fractions, using Folin-Ciocalteu method. The absorbance of the resulted color was measured at 765 nm, and results were calculated as averages of three measurements and expressed as milligrams of gallic acid equivalent per gm dry weight of plant material.[Citation78] Moreover, it is used for the determination of sugar parts of flavonoids, as it was used in the structure elucidation process of six isolated flavonoids from the ethyl acetate fraction of the aerial parts.[Citation80]
Others
Densitometer scanner was employed to integrate cardiac glycoside fractions of the latex from aerial parts of L. pyrotechnica.[Citation67]
Toxicity
Acute toxicity of the extracts of the aerial parts of the Egyptian plant were examined on brine shrimp. The results revealed that the lethal concentration 50 (LC50) for the petroleum ether extract (lipid fraction) was 35.48 ppm, which indicates that the extract is highly toxic.[Citation72] In another study, the ethyl acetate, methanolic and defatted methanolic extracts of the aerial parts, in addition to the flavonoid subfractions were evaluated by the same authors via brine shrimp lethality test applying the Reed-Muench method. The results showed that flavonoids exhibited higher LC50 values and less toxicity than the other tested extracts and fractions. In addition, the mortality rates of the isolated flavonoids; fraction E-I, kaempferol-3-O-α-l-rhamnopyranosyl (1′″→6″)-O-β-d-glucopyranoside, and kaempferol-3-O-β-d-rhamnopyranosyl (1′″→6″)-O-β-d-glucopyranoside, were higher than the other tested fractions of flavonoids at the concentration of 100 ppm, and the study suggested that this may be related to their long glycosidic moiety.[Citation80] Correspondingly, additional study conducted by the same authors, using the same method at which the acute toxicities of the methanol and defatted methanol extracts of the arial parts, beside its latex were investigated. The resulted LC50 were found to be 11.89, 28.19, and 18.84 ppm, respectively. Regarding the mortality rates, on the one hand, the results showed 100% mortality with concentration of 1000 ppm in latex (cardiac glycosides) and methanol extract, whereas 98.1% were shown in the defatted methanol extract. On the other hand, all the investigated extracts demonstrated high mortality with 100 ppm concentration, ranging from 79.41% to 98.83%. Lower mortality rates were found with the concentration of 10 ppm, as they were fluctuating between 25% and 45.1%.[Citation67] Likewise, the same authors determined the mortality rate and LC50 of the alkaloid extract and compared it with those of the methanol and defatted methanol extracts of the aerial parts using the same previous method. The results showed that the mortality rate of the alkaloids was lower, representing 58.21% and 14.10% at 100 and 10 ppm concentrations, respectively. The estimated LC50 of the total alkaloids was 63.09 ppm.[Citation64] In another study, the same authors performed a comparative study between the toxicity of the previously studied extracts of the aerial parts (latex (cardiac glycosides), methanol, defatted methanol, petroleum ether (lipids), total alkaloids, and ethyl acetate (total flavonoids)), in addition to other sequential extracts including dichloromethane, methanol/dichloromethane (1:1), defatted methanol/dichloromethane (1:1), butanol, and aqueous. They determined their LC50 through conducting brine shrimp lethality test. The results showed that the extracts which exhibited higher toxicities were latex, methanol, methanol/dichloromethane (1:1), defatted methanol/dichloromethane (1:1), defatted methanol and dichloromethane extracts, while the others demonstrated lower toxicity.[Citation107] Additional study in India recorded the signs of toxicity and mortality within 24–72 h on Wistar Albino rats with weights ranges of 150–180 gm. The tested rats received 100, 200,500, 1000, 2000, and 4000 mg/kg BW of the crude extract of the aerial parts of the plant and its corresponding fractions. The results revealed that the ethanolic extract of the aerial parts and its petroleum ether, chloroform, ethyl acetate, and methanol fractions did not show signs of toxicity or mortality up to a dose of 4000 mg/kg upon oral administration.[Citation97] Furthermore, the 70% methanolic extract of the aerial parts of Pakistan was assessed for its safety through oral administration of different doses of the tested extract (0.3, 1.0, 3.0 and 10.0 g/kg) by Swiss albino mice (18–30 g). Behavioral abnormalities and toxicity signs as tremors, salivation, sweating, convulsions, lacrimation, somatomotor activity, and writhing reflex were observed. It was found that the crude extract is safe up to 10 g/kg upon oral administration, as no indications of toxicity, or behavioral changes were observed; moreover, no mortality was recorded after 2 days.[Citation108]
The lethal dose 50 (LD50) of the ethanolic extract of the aerial parts was determined at concentrations of 1000, 2000 and 4000 mg/kg in an Egyptian study using male Wistar rats (200–220 g). Signs of toxicity and recording of death numbers per dose were observed for twenty-four hours. The study suggested that the oral LD50 of the tested extracts were higher than 4000 mg/kg, with no signs of toxicity including diarrhea, restlessness, hematuria, respiratory distress, and uncoordinated muscle movements, or mortality within 24 hours of observation. Serum levels of liver enzymes; alanine transaminase (ALT) and aspartate transaminase (AST); and kidney biomarkers (urea and creatinine) were measured to study their effect on liver and kidney after 65 days of administration. The results showed that the tested doses did not cause significant change in the serum levels of these biomarkers and enzymes, which indicates that the tested doses are not nephrotoxic or hepatotoxic.[Citation98]
In another study, the LD50 of the ethanol extracts of the aerial parts of Egyptian L. pyrotechnica was determined on male Wistar albino rats (180–200 g) using the doses of 1000, 2000, or 4000 mg/kg of the tested extract. The oral administration of the tested extract showed no toxicity in doses up to 4000 mg/kg, as no symptoms of toxicity or mortality were reported within 24 hours of observation. Moreover, the sub-chronic toxicity of the same extract was studied through giving the Male Wistar albino rats doses of 200 and 400 mg/kg of the tested extract orally for 35 consecutive days. Biochemical analysis of liver and kidney biomarkers was done, by measuring the serum levels of ALT, AST, total bilirubin, total proteins, albumin, urea, and creatinine. The resulted data did not show any significant change on the serum levels of ALT, AST, total bilirubin, total proteins, urea, creatinine, and albumin when compared to control.[Citation104] In additional study, the subacute liver toxicity of intraperitoneal administration of the ethanolic extract of the aerial parts of L. pyrotechnica was examined on Wister albino rats (120–200 g) for 21 days at three doses, viz. 50 mg/kg, 100 mg/kg and 150 mg/kg. The serum levels of liver enzymes, ALT, AST, and alkaline phosphatase (ALP), in addition to total protein and albumin, were assayed. The resulted data refer to a mild hepatotoxic effect of the tested doses with no significant increase in the assessed levels of AST, ALT, albumin, and total protein in the liver serum was observed. However, a significant increase in ALP was reported as compared with the control group. This indicates that there is no major damage in the liver cells.[Citation109]
Interestingly, the toxicity of phytol isolated from L. pyrotechnica was assessed by MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2 H-tetrazolium bromide) in a study performed in Iran. A series of concentrations of phytol (62.5, 125, 250, 500, 1000 µg/mL) were incubated at 37°C with mouse skin cells for periods of 12, 24, and 36 hours, then the cell viability percentage was calculated. The results unraveled that phytol toxicity is dose and time dependent as the percentage of cell viability decreases with high concentrations and long exposure times, showing that the lowest concentration (31.2 µg/mL) caused the highest cell viability, which was determined as 97%, and vice versa.[Citation110]
Pharmacology
As shown in , the various parts of L. pyrotechnica along with its isolated fractions and compounds showed significant and promising pharmacological activities in various studies. These bioactivities will be discussed in the following sections.
Table 4. Reported pharmacological activities of L. pyrotechnica (Forsk.) Decne.
Anthelmintic activity
Two concentrations of the whole plant’s methanolic extract (50 and 100 mg/mL) were examined for their anthelmintic activity against Pheretima posthuman, using Albendazole (20 mg/mL) as a standard and CMC (0.5%) as control. Time of paralysis and time of death of the worm were determined. The results indicated that the tested extract demonstrated a remarkable anthelmintic capacity at high concentration (100 mg/mL) in comparison with the reference drug.[Citation111]
Antidiabetic
The antidiabetic potential of the methanolic extract of aerial parts of L. pyrotechnica was determined at concentrations of 100, 200, 300 mg/kg, using diabetic rats induced by streptozotocin, and glibenclamide (10 mg/kg) as a reference standard. The serum levels of total proteins, triglycerides, cholesterol, liver glycogen, plasma glucose and body weight of different groups were estimated. The results of the study showed that the investigated extract possesses potent antidiabetic effect as it significantly restored the elevated serum cholesterol and triglyceride levels to normal limits after treatment for three weeks. Moreover, it considerably ameliorated the depleted levels of glycogen and total protein in diabetic rats, bringing them close to their normal levels. The other positive outcomes that were observed were the improvements of weights of the diabetic rats upon treatment with the tested extract because the rats experienced loss in their weights upon diabetic induction. Dose dependent lowering effect of the extract was determined against the elevated serum level of glucose.[Citation112]
Antidiarrheal
Antidiarrheal effects of the aqueous methanolic extract (80:20% v/v) of the aerial parts and the defatted one were evaluated in a study conducted in Nigeria through using castor-oil induced diarrhea in albino rats. Both extracts were administered orally at doses of 300, 600 and 1200 mg/kg and diphenoxylate HCl was used as a standard reference drug (5 mg/kg). The results revealed that the oral administration of the tested extracts significantly inhibited the number of defecations in the treated rats compared to the control group; however, the defatted extract demonstrated the highest activity at the dose of 1200 mg/kg.[Citation99]
Anti-Inflammatory
The anti-inflammatory activity of the oral administration of the ethanolic extract of the Egyptian aerial parts was examined in male Wistar albino rats through calculating the % inhibition of paw edema using a carrageenan-induced paw edema test. The examined doses of the extract understudy were 200 and 400 mg/kg, and dexamethasone (0.2 mg/kg) was used as a standard. The study showed that the extract exhibited a good anti-inflammatory and anti-edematogenic effect at which 50.60% reduction of paw swelling after 3 h of carrageenan injection with a dose of 400 mg/kg was recorded. The authors presumed that this strong effect may be attributed to the phenolic and/or flavonoid constituents of the tested extract.[Citation104] The lipoxygenase (LOX) inhibitory activity of the aerial parts’ ethanolic crude extract, and its ethyl acetate, n-butanol and water partitioning fractions were also evaluated. Ethyl acetate extract demonstrated the highest potentiality with IC50 of 1.41 µg/mL.Citation78 The anti-inflammatory capacity of the ethanolic extract and its fractions (petroleum ether, chloroform, ethyl acetate, and methanol) of the aerial parts were studied at doses of 200 and 400 mg/kg in-vivo against carrageenan and histamine induced paw edema, using indomethacin as a standard drug (10 mg/kg). The results demonstrated that the tested extract and fractions significantly inhibited the increased paw edema induced by carrageenan and histamine. The authors suggested that this effect was due to the plant content of bioactive phytoconstituents, such as triterpenes, alkaloids, polyphenols, and flavonoids.[Citation97]
In a recent study, Lepseq5, which is an isolated endophyte from L. pyrotechnica leaves was tested for its anti-inflammatory potential using carrageenan-induced foot edema model in rats, which is an acute inflammation model. Intraperitoneal treatment of its aqueous extract was given to the rats in doses between 50 and 100 mg/kg, and percentage of edema suppression was calculated after 1, 2, 3,4, 5, 6 and 24 hours. The results revealed that the tested extract exhibits a promising anti-inflammatory effect since it showed a dose-dependent inhibition of the foot edema in rats after 24 hours.[Citation113]
Antimicrobial
Several records showed that the plant possesses a good antimicrobial effect.[Citation114] Dichloromethane, methanol, and aqueous extracts of the Yemeni whole plant were screened in-vitro for their antibacterial and antifungal activities against three Gram +ve bacteria (Staphylococcus aureus, Bacillus subtilis, Micrococcus flavus), two Gram -ve bacteria (Escherichia coli, Pseudomonas aeruginosa), and six fungal strains (Candida maltose, Candida albicans, Candida krusei, Aspergillus fumigatus, Trichophyton mentagrophytes, Absidia corymbifera). On the one hand, the examined extracts showed very weak antibacterial activity. On the other hand, the tested extract did not show any antifungal activity against the tested fungal strains.[Citation115] Also, the antibacterial and antifungal activities of the ethanolic extract of the Indian whole plant and its petroleum ether, chloroform, ethyl acetate, and methanol fractions were evaluated by disc diffusion method, at which minimum inhibitory, bactericidal, fungicidal concentrations, and zone of inhibitions against the bacterial and fungal strains were measured. The tested bacterial strains and the used standards were Escherichia coli/Norfloxacin 26 mg/mL, Pseudomonas aeruginosa/Tobramycin 28 mg/mL, Salmonella typhi/Ciprofloxacin 25 mg/mL, and Staphylococcus aureus /Ampicillin 24 mg/mL, while the used fungal strains included Candida albicans, Candida krusei, Candida tropicalis, and Candida parapsilosis, using Fluconazole 25 mg/mL as a standard drug. The results showed the effectiveness of different extract and fractions at various concentrations against the tested bacterial and fungal strains, as the study assumed that their antimicrobial potency is most probably due to their high phenolic, terpenoid, and flavonoid contents and their antioxidant capacity.[Citation93]
The mother tinctures of L. pyrotechnica aerial parts in Pakistan was evaluated for its antifungal potential against potentially pathogenic fungal species, such as Aspergillus niger, Aspergillus flavus, Aspergillus ustus, and Candida albicans using agar disc diffusion method. Fluconazole (2 mg/ml) was used as a standard antifungal drug. The study revealed that the mother tincture of the plant exhibits a potential antifungal effect, as its ratio against Aspergillus niger, Aspergillus flavus, Aspergillus ustus, and Candida albicans was 32 mm/27 mm, 30 mm/28 mm, 17 mm/30 mm, and 24 mm/30 mm, respectively.[Citation116] In another study in India, the antibacterial activity of the methanolic extract of the aerial parts was investigated against two Gram +ve (Staphylococcus epidermidis, Staphylococcus aureus), and two Gram -ve bacteria (Salmonella typhi and Salmonella paratyphi-A). The minimum inhibitory concentration (MIC) was determined in concentrations ranged between 0.125 and 8.0 mg/ml, ciprofloxacin (20 µl/ml) was used as a standard and 100% DMSO as a negative control. The resulted zone of inhibition was 6 mm against Staphylococcus aureus and zero against the other bacteria, which reflects that the tested extract has a weak antibacterial activity against the used bacteria compared to the standard drug.[Citation117] Also, the 95% ethanolic extract of the UAE arial parts showed no activity against the following tested microorganisms; Staphylococcus aureus, Streptococcus faecalis, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Serratia marcescens, and Candida albicans, at concentrations ≤3 mg/ml.[Citation96]
Furthermore, methanol, diethyl ether, hexane, aqueous extracts of the leaves were evaluated for its antifungal activity against Aspergillus niger, Aspergillus flavus, Fusarium oxysporium, and Fusarium moniliformis using disc diffusion method. A standard antibiotic (Fluconazole) was used as a reference drug. The resulted zone of inhibition diameters indicated that the tested extracts exhibit remarkable antifungal effect against the tested fungal species since they ranged from 4.5 to 18.2 mm, suggesting that the examined plant extracts might be promising sources of antifungal drugs.[Citation118]
Interestingly, phytol was evaluated for its antimicrobial activity, toxicity, and surface stability to find out its efficiency as a natural disinfectant. Serial concentrations of the compound were tested against Escherichia coli, Staphylococcus aureus, Candida albicans, and Aspergillus niger, then the MIC was measured for each microorganism. The results revealed that phytol can be used as an effective disinfectant for surfaces, as it showed a high antimicrobial activity, as its MIC50 was 62.5 μg/mL against E. coli, C. albicans, and A. niger, while >1000 μg/mL against S. aureus. Other reasons include being nontoxic and stable on stone, MDF, and steel surfaces for thirty six hours.[Citation110] Also, the antibacterial activities of the hexane, chloroform, acetone, ethyl acetate, butanol, methanol, ethanol and water extracts of roots and fruits were examined against Staphylococcus epidermidis and S. aureus by applying agar-well diffusion assay. Gentamycin and DMSO were used as positive and negative controls, respectively. The resulted zones of inhibition, and MICs for all the tested extracts, indicated that the extracts of both parts have a good antibacterial activity against the tested strains. However, the methanolic extracts of both parts showed the best results against the tested pathogens. In general, potency of the activity was found to be affected by the solvent type and extract concentration.[Citation119]
Another interesting recent study has shown that five isolated endophytes from the leaves of L. pyrotechnica demonstrated considerable antibacterial effect against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Candida albicans and Aspergillus fumigatus. However, the aqueous extract of one of the isolated endophytes; named Lepseq5 (Aspergillus foetidus); displayed the highest antibacterial activity against all the tested pathogens. In this study, gentamicin and ketoconazole antibiotics were used as reference drugs against the tested bacterial and fungal strains, respectively.[Citation113]
Anti-Nociceptive
The anti-nociceptive effect of the aerial parts’ ethanolic extract and its fractions (petroleum ether, chloroform, ethyl acetate, and methanol) were investigated against acetic acid induced writhing, formalin induced paw licking and tail flick test. The used standard drugs were diclofenac (10 mg/kg), aspirin (50 mg/kg), and diclofenac sodium (50 mg/kg) in acetic acid induced writhing method, formalin-induced hind paw-licking, and tail-flick test, respectively. The tested extract and fractions displayed a significant analgesic capacity, as they reduce writhing, paw licking, pain rate and tail withdrawal response significantly. The study assumed that this potentiality is due to phytocompounds like triterpenes, alkaloids, polyphenols, and flavonoids found in the extract.[Citation97]
Antioxidant
The antioxidant capacity of the ethanolic extract of the Indian whole plant and its fractions, including petroleum ether, chloroform, ethyl acetate, and methanol fractions were evaluated through carrying out DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical scavenging assay. Quercetin and gallic acid were used as standards. The ethanolic extract and its fractions displayed the best antioxidant effect at the concentration of 100 µg/mL.[Citation93] The methanolic extract of the whole plant was also examined for its free radical scavenging activity in-vitro through performing the hydrogen peroxide (H2O2) radical scavenging activity and DPPH assay. The results showed that the extract exhibited a significant inhibition percentage of free radicals in a dose-dependent manner, with IC50 of 152.46 µg/mL in DPPH assay and 65.48 µg/mL in hydrogen peroxide radical scavenging assay. This activity was attributed to the polyphenolic content and other phytochemical contents of the plant.[Citation120] Recently, DPPH, ABTS, FRAP, CUPRAC and total antioxidant (phosphomolybdenum assay) assays were done to study the antioxidant capacity of the whole plant methanolic extract and its ethyl acetate, hexane, and water fractions. Its metal chelating effects were also studied using ethylenediaminetetraacetic acid (EDTA) as a standard. The utilized doses of the extract were 0.5–5 mg/mL. Ethyl acetate showed the highest free radical scavenging effect in DPPH and ABTS assays, giving the values of 21.05 ± 0.45 mg TE/g and 68.12 ± 0.53 mg TE/g, respectively. Also, it showed the highest activity in CUPRAC and FRAP assays (44.93 ± 1.66 and 117.42 ± 1.28 mg TE/g extract, respectively). In contrast, the sharpest metal chelation effect and total antioxidant capacity were found in the hexane fraction (11.57 ± 0.29 mg EDTAE/g, 1.52 mmol TE/g extract, respectively). Accordingly, Zubair et al. assume that non-phenolic constituents of the plant are the most chelating agents with metal ions. Furthermore, according to conducted multivariate analysis, they suggest that the high antioxidant property of the plant is due to its phenolic and terpenoid contents [38].
The antioxidant effect of the ethanolic extract of the Egyptian aerial parts was evaluated through DPPH assay using Trolox as a reference, and the results were expressed as Trolox equivalent antioxidant capacity (TEAC). The extract displayed high antioxidant and DPPH radical scavenging activities (1.84 TEAC).[Citation104] Moreover, the ethanolic extract of L. pyrotechnica aerial parts, and its ethyl acetate, butanol and water fractions were examined for their antioxidant effect using the FRAP (Ferric Reducing Ability of Plasma), DPPH, ABTS (2,2’ -azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and β-carotene bleaching assays. IC50 of ethyl acetate fraction exhibited the best antioxidant activity (1.2, 0.57, 0.45 mmol ascorbic acid equivalent/g in the FRAP, ABTS and DPPH assays, respectively).[Citation78] Additionally, the antioxidant activity of hexane fraction of the aerial parts was investigated using FRAP and ABTS methods, comparing the results with those of the ethanolic extract and its ethyl acetate, butanol, and water fractions, which were previously reported. The results demonstrated that the hexane fraction exhibits the lowest antioxidant capacity (0.049 and 0.049 µmol/mg in FRAP and ABTS assays, respectively).[Citation68] Also, the aerial parts and roots’ methanolic extracts showed a significant free radical scavenging activity, which increases by the increase of concentration, compared to a standard synthetic antioxidant, butylated hydroxy anisole (BHA). The study evaluated this activity through multiple assays conducted in-vitro, viz. DPPH, hydrogen peroxide scavenging and reducing power assays. The crude extract of the roots demonstrated higher activity than that of the aerial parts in the DPPH and hydrogen peroxide scavenging assays, in addition to the reducing power assay till the concentration of 40 µg/mL. However, in concentrations more than 40 µg/mL, the aerial parts displayed higher effect than roots in reducing power assay.[Citation121] Another study investigated the antioxidant activity of the powders of roots and shoots via FRAP, peroxidase (POXA), and lipid peroxidation (LPO) assays. The shoots were found to have better antioxidant capacity than the roots.[Citation102]
Regarding the leaves, 80% aqueous methanolic extract of the leaves were investigated for antioxidant effect by applying DPPH and FRAP assays. The results showed IC50 of 991.62 μg/mL for DPPH assay and 1.69 mMol Fe+2/g for FRAP assay.[Citation105]
Interestingly, 70% aqueous ethanolic extract of the Egyptian green fruits demonstrated a promising dose-dependent scavenging activity with an IC50 value of 89.3 μg/mL in DPPH assay, when subjected to different assays to detect its antioxidative effect. These assays were DPPH radical scavenging at a concentration of 0.1 mg/mL, reducing power at a concentration of 0.2 mg/mL, Fe+ chelating at a concentration of 2.0 mg/mL, and H2O2 scavenging at a concentration of 40 μg/mL.[Citation106]
Antitumor, antiproliferative, and cytotoxic activities
Methanol extract of the Yemeni whole plant was evaluated in-vitro for its cytotoxicity It was measured by the neutral red uptake assay using human amniotic epithelial cell line FL-cells. The resulted IC50 % (µg/mL) of the methanol extract was 550 against FL-cells.[Citation115] The aqueous ethanolic crude extract of the aerial parts was also investigated for its cytotoxicity on MCF-7 human breast cancer cell line model, along with its ethyl acetate, n-butanol and water fractions using MTT assay. Ethyl acetate fraction displayed the best cytotoxic effect (IC50 = 43.16 µg/mL) against human breast cancer cell line.[Citation78] Additional study assessed the cytotoxicity effect of the 80% ethanolic extract and its fractions (n-hexane, ethyl acetate, n-butanol and water) against colon cancer cell lines (HCT 116 wild type and HCT 116 p53−/−). The results revealed that the hexane fraction exhibits the strongest cytotoxic effect against colon cancer cell lines as it reduces the cell viability of colon cancer cells in a dose- and time-dependent manner with IC50 of 100 μg/mL after 24 h, inducing a P53-dependent apoptosis, which is possibly mediated by ROS generation and involved multiple intrinsic and extrinsic pathways.[Citation68]
Potato disc bioassay was done to perform antitumor screening on the methanolic, defatted methanolic, dichloromethane, and ethyl acetate extracts of the aerial parts, in addition to the flavonoid subfractions and isolated flavonoids. The output data showed that the methanol and ethyl acetate extracts have the strongest antitumor activity (−49.30 and −43.20%, respectively). The authors suggested that the strong effect of L. Pyrotechnica extract may be due to its high flavonoid content.[Citation80] Moreover, the same method was carried out to determine the antitumor activity of the latex (total cardiac glycosides) and compare it with the activity of total methanol and defatted methanol extracts of the aerial parts. The crude extract and cardiac glycosides demonstrated a significant antitumor effect, −49.3% and −30.8%, respectively.[Citation67] Similarly, another study conducted by the same authors evaluated the antitumor activity of total alkaloid extract of the aerial parts and compared it with the methanol and defatted methanol extracts. The resulted data showed that the most active fractions were the methanol and alkaloid extracts, representing −49.30% and −33.60%, respectively.[Citation64] The same authors carried out a comparative study between the antitumor activities of the previously studied extracts and other extracts derived from the aerial parts using the same test. The results revealed that methanol, ethyl acetate and alkaloids extracts exhibit better activity than the other tested extracts as their percentages of inhibitions were −49.30, −43.20, and −33.60%, respectively.[Citation107]
Pregnane glycosides isolated from the chloroform-methanol (9:1) extract of the whole plant were evaluated for their antiproliferative activity using three continuous murine and human culture cell lines, J774.A1, HEK293, and WEHI-164. The activity was found to depend on the type of the aglycone, its linked moieties and number of sugar moieties. In other words, compounds which possess deacethylmetaplexigenin aglycon and linked cinnamoyl ester moiety to the aglycone’s C-12 had the highest activity. In contrast, compounds containing sarcostin as aglycon were the least active. The study suggested that the aromatic group linked to the C-12 of deacetylmetaplexigenin enhances the cytotoxic effect whereas the acetyl group inhibits the activity in other compounds as it restores cell proliferation. It was also presumed that the number of sugar moieties is inversely proportional to potency of the activity as the compounds with fewer sugar molecules are more active.[Citation81]
Anti-Ulcer
Methanolic extracts of the whole plant of L. pyrotechnica was investigated for its anti-ulcer effect by applying experimentally induced gastric ulcer by pyloric ligation model, using Wistar albino rats and ranitidine at a dose of 50 mg/kg as a standard drug. The output of the experiment indicates that the crude extract possesses gastroprotective against pyloric ligation induced ulcer model in rats. In particular, the study suggested that it can be potentially used for treating ulcer, as it significantly decreased the gastric acid secretion, pH and ulcer index compared to the control group.[Citation122] The anti-ulcer effect of the ethanolic extract of the Egyptian aerial parts as the oral pretreatment with a dose of 400 mg/kg was also investigated against ulcerative colitis (UC) induced by intracolonic administration of acetic acid in male Wistar albino rats. Dexamethasone (0.2 mg/kg) was used as a reference drug as it is widely used for the treatment of colitis. The results showed a significant reduction of carrageenan-induced paw edema since it protected against diarrhea, colonic ulceration, mucosal damage, and myeloperoxidase (MPO) activity elevation in colonic mucosa of rats with colitis valuable effect of L. pyrotechnica aerial parts’ ethanolic extract against acetic acid-induced ulcerative colitis. The authors suggested that this potent effect was most likely due to their significant antioxidant, anti-inflammatory activities, phenolic and flavonoid contents.[Citation104]
Antiviral
The aqueous-alcoholic extracts of aerial parts of the Egyptian L. pyrotechnica showed inactivity against herpes simplex-1 virus (HSV), poliomyelitis-1 virus (POLIO) and vesicular stomatitis virus (VSV) when subjected to antiviral screening bioassay. The activity was estimated through the end point titration technique (EPTT), which measures the capability of the dilutions of the tested extract to decrease the resulted effect of cytopathogenic; this is expressed as reduction factor (Rf) of the viral titer.[Citation123]
Enzyme inhibition activity
Enzyme inhibition performance of the methanolic extract and its ethyl acetate, hexane, and water fractions of the whole plant was studied in-vitro against acetylcholinesterase (AChE), butyryl-cholinesterase (BChE), tyrosinase, α-amylase and α-glucosidase enzymes. The tested extracts demonstrated efficient inhibition of cholinesterase in AChE assay at which the ethyl acetate and water fractions exhibited the best performance (2.43 and 2.40 mg GALAE/g extract, respectively). For BChE assay, α-glucosidase and α-amylase inhibitory activities, the hexane fraction possessed the highest capacity, showing values of 5.98 ± 0.44 mg GALAE/g, 7.72 ± 0.14 mmol ACAE/g and 0.55 ± 0.01 mmol ACAE/g extract, respectively. Water fraction was the best to inhibit tyrosinase (59.35 ± 0.29 mg KAE/g extract).
Interestingly, Zubair et al. performed docking for some compounds, which were identified in the study via RP-UHPLC/MS, against α-amylase, α-glucosidase, AChE, BChE and tyrosinase enzymes. Among the tested compounds, soyasapogenol A exhibited the highest binding affinity towards α-amylase, α-glucosidase and BChE, but lower than those of acarbose and galantamine control agents. On the other hand, glyceollin presented the highest binding affinity towards AChE and tyrosinas with lower that of the control drug in case of AChE. It is worth mentioning that all the docked compounds displayed higher affinity to tyrosinase than the control (kojic acid) [38]. Also, the anti-α-amylase activity was investigated in the 70% ethanolic extract of the Egyptian green fruits, by performing the starch-iodine method, using a concentration of 1 mg/mL of the tested extract. It showed a significant inhibition of the starch break-down in-vitro, as it showed inhibitory activity of 15.6%, 27.4% and 35.8% at the concentrations of 500, 1000 and 2000 μg/mL, respectively.[Citation106]
Fertility enhancement
The ethanolic extract of the aerial parts of L. pyrotechnica was investigated to determine its effect on the male rats’ reproductive system after prolonged treatment. Significant enhancement in the reproductive organs’ relative weight, sperm count, sperm abnormality, and sperm motility, in addition to increasing of testosterone and luteinizing hormone serum levels were determined upon oral treatment with the tested extract for 65 consecutive days (the needed period for spermatogenic cycle). So, the study suggests that the tested extract exhibits fertility enhancement effect in male rats, and it may be due to its flavonoid and anthraquinone content.[Citation98]
Hepatoprotective
The effect of oral administration of methanolic extract of the whole plant of L. pyrotechnica was determined on paracetamol induced liver damage in Wistar albino rats. The tested extract recorded a significant reduction in the elevated serum levels of hepatic enzymes, viz. Aspartate transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), ALP and total bilirubin. The histological findings showed that the treated groups with the tested extract exhibited healthy liver cells, as it generally showed mild defects in comparison with the control and intoxicated groups. Thus, the study suggested that the crude extract of the whole plant could be a promising hepatoprotective and hepato-curative natural product for hepatocellular disorders.[Citation124]
Hypolipidemic and anti-atherosclerotic efficacy
The methanolic extract of the aerial parts of L. pyrotechnica was investigated for its hypolipidemic and anti-atherosclerotic efficacy using male cholesterol fed rabbits. The tested dose of the extract was 250 mg/kg/day, which was administered orally for 60 days. The results confirmed that the simultaneous oral administration of the extract gave a considerable hypolipidemic and anti-atherosclerotic effect as it considerably prevented the rise in serum levels of total cholesterol, low-density lipoprotein (LDL)-cholesterol, very-low-density lipoprotein (VLDL)-cholesterol, triglycerides and atherogenic index. Additionally, the extract significantly reduced the total cholesterol, triglycerides, and lipid peroxidation of the liver and aorta in the extract treated rabbits. Furthermore, no atheromatic changes and plaque formation were observed in the aorta upon treatment with the extract.[Citation125]
Immunostimulatory and immunomodulatory activities
The aqueous methanolic (30:70) extract of the L. pyrotechnica aerial parts in-vivo experiments were performed in Wistar albino rats, including neutrophil adhesion test, hemagglutinating antibody (HA) titre, delayed-type hypersensitivity response, phagocytic activity, and cyclophosphamide-induced myelosuppression. The results showed that the extract possesses immunostimulatory activity and adaptive immune response. The stimulation effect was confirmed via the improvement of neutrophils to nylon pellets adhesion percentage and the increased phagocytic index, while the adaptive response is adjusted through humoral response and cell mediated immune responses. The authors suggest the folk use of these parts of the plant to alleviate the diseases that require immunity stimulation, such as infectious diseases including AIDS, tuberculosis and pneumonia.[Citation108] In another study, the immunostimulatory effect of the crude extract of the whole plant was evaluated in-vivo at which the phagocytic activity was determined through carbon-clearance rate test. The results indicated that the administration of the tested extract increases the clearance rate of carbon, as it increases the activity of phagocytes in a dose dependent manner compared to the control group.[Citation126]
Relaxant activity on intestinal smooth muscle
The effect of the aqueous extract of the aerial parts of the Emirati L. pyrotechnica on the activity of intestinal smooth muscle was examined in-vitro through determining its effect on the isolated jejunum of rabbits. The output of the study revealed that the tested extract displayed a week antispasmodic activity since the resulted percentage of maximum inhibition of spontaneous contractions of the rabbits’ isolated jejunum, upon treatment by the extract, was 42.8 ± 2.8% and the IC50 was 2.31 mg/ml. It was also found that the tested extract induced initial contraction followed by inhibition of the motility of the rabbit isolated jejunum, which was partially reversed.[Citation127]
Wound healing
A study conducted in India to evaluate the wound healing effect of the ethanolic crude extract of the roots and aerial parts of L. pyrotechnica in order to confirm its traditional use by the dwellers of Thar Desert. The activity was tested on excision wound model, using Wistar albino rats, at which rate of wound healing was indicated through wound closure rate and period of epithelialization. The tested extracts were formulated as ointments with a concentration of 4% w/w in simple ointment base, and Framycetin sulphate cream (1% w/w) was applied as a standard drug in the experiment. The resulted rate of wound closure and period of epithelialization showed that both tested extracts are effective for wound healing.[Citation128]
Conclusions and future perspectives
This detailed review provides a full inclusive report that covers the ethnomedicinal, phytochemical, analytical, toxicological, and pharmacological aspects that were previously reported on L. pyrotechnica. Moreover, the review presents the recent research progresses that were accomplished on L. pyrotechnica, giving the chance to pinpoint scientific research gaps that are related to this medicinal plant in the current knowledge.
In general, most of the conducted pharmacological studies support some of the reported traditional uses of the plant-like treatment of worms, diabetes, diarrhea, inflammation, bacteria, swelling, obesity, tumors, wounds, and fertility problems. However, their exact mechanisms of action were rarely studied and are therefore still ambiguous. Moreover, other organs, which are reported to possess valuable traditional uses, have not been investigated yet; especially that most of the performed pharmacological studies focused on the whole plant and aerial parts. Thus, there are many other organs and medicinal uses still need analysis, and high‐quality methodologies are required to be carried out to get highly accurate and precise results. Some of these medicinal uses include the capacity of the plant to treat ear ache, eye infections, bones problems like rheumatic pain, renal problems like urinary retention, lung diseases as asthma, reproductive system problems as gynecological disorders, as well as other skin and digestive system abnormalities.
Further fractionation and isolation of the major chemical constituents of the active extracts are still required accompanied by biological activity evaluation. This will help pinpoint the most active compounds and fractions since almost most of the conducted biological activities were done on the crude extracts and barely on fractions. Additionally, even though toxicological studies confirmed that the plant is generally safe, herb‐drug interactions of L. pyrotechnica is yet to be studied.
In terms of clinical research, no data are available regarding clinical trials that support the reported biological activities since all the achieved studies were mostly performed in-vitro or in-vivo but were not carried out in human beings. Subsequently, it is recommended to start clinical studies for the most promising reported pharmacological effects. It is also important to highlight that before starting such clinical trials, it is pivotal to re-evaluate the in-vitro studied activities in-vivo to take into consideration the systemic process and get more accurate conclusions.
Regarding the plant chemistry, it is clear from the collected data that the achieved chemical investigations were concentrated on vegetative parts of the plant, such as the whole plant, aerial parts, and stems, neglecting the reproductive ones as shown in . Accordingly, extensive determination of their chemical profiles is recommended to be done, as this will help understand and determine the mechanisms of their corresponding medicinal uses and biological effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Zhang, A.; Sun, H.; Wang, X. Mass Spectrometry‐driven Drug Discovery for Development of Herbal Medicine. Mass Spectrom. Rev. 2018, 37(3), 307–320. DOI: 10.1002/mas.21529.
- U.S. Food and Drug Administration. The Drug Development Process. 2018. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process.
- Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Supuran, C. T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discovery. 2021, 20(3), 200–216. DOI: 10.1038/s41573-020-00114-z.
- Calixto, J. B. The Role of Natural Products in Modern Drug Discovery. Anais da Academia Brasileira de Ciências. 2019, 91(suppl 3). DOI: 10.1590/0001-3765201920190105.
- Dey, A.; Mukherjee, A. Plant-Derived Alkaloids: A Promising Window for Neuroprotective Drug Discovery. In Discovery and Development of Neuroprotective Agents from Natural Products, Goutam Brahmachari (Ed).; Radarweg: Elsevier, 2018; pp. 237–320.
- Moudi, M.; Go, R.; Yien, C. Y. S.; Nazre, M. Vinca Alkaloids. Int. J. Preventive Med. 2013, 4(11), 1231.
- Wiart, C. Medicinal Plants of the Asia-Pacific: Drugs for the Future?; Boca Raton: World Scientific, 2006.
- Endress, M. Apocynaceae: Brown and Now. Telopea. 2004, 10(2), 525–541.
- Grubben, G. Vegetables; Wageningen, Netherlands: Prota, 2004; Vol. 2.
- Masrahi, Y. S. A New Species of Leptadenia (Apocynaceae) and Two Other New Records from Southwestern Saudi Arabia. Saudi J. Biol. Sci. 2015, 22(5), 631–636. DOI: 10.1016/j.sjbs.2015.02.003.
- Schmelzer, G. H.; Gurib-Fakim, A. Medicinal Plants 2; Prota, 2008; Vol. 11.
- Deflers, A. Les Asclépiadées de L’arabie Tropicale, 1896; Vol. 3, Egypt: Institut Égyptien.
- Norton, J.; Majid, S. A.; Allan, D.; Al Safran, M.; Böer, B.; Richer, R. An Illustrated Checklist of the Flora of Qatar; UK: Browndown Publications Gosport, 2009.
- Tewari, V. Some Important Fruit Trees and Shrubs of Hot Arid Regions of Rajasthan State in India, Their Uses and Nutritive Values. J. Plant Chem. Ecophysiol. 2016, 1(1), 1004.
- Booth, F. E.; Wickens, G. E. Non-Timber Uses of Selected Arid Zone Trees and Shrubs in Africa; Rome, Italy.: Food & Agriculture Org, 1988.
- Families, W.-W.-C.-O.-S.-P. World checklist of selected plant families. 2019. https://wcsp.science.kew.org/synonomy.do?name_id=507765.
- Javid, S., Khalil, S.; Munir, I.; Amjad, M. S.; Akbar, K.; Yasmeen, F.; Akhtar, M. S. Plant Metabolites and Pharmacological Activities of Leptadenia Pyrotechnica (Forssk.) Decne. In Natural Bio-Active Compounds, Mohd Sayeed Akhtar, Mallappa Kumara Swamy, Uma Rani Sinniah, Eds.; Singapore: Springer, 2019; pp. 551–560.
- Idrees, S.; Qureshi, R.; Bibi, Y.; Ishfaq, A.; Khalid, N.; Iftikhar, A.; Shabir, A.; Riaz, I.; Saboon, N.; Ahmad, N., et al. Ethnobotanical and Biological Activities of Leptadenia Pyrotechnica (Forssk.) Decne.: A Review. Afr. J. Traditional Complementary Altern. Med. 2016, 13(4), 88–96.
- Verma, N.; Jha, K. K.; Chaudhary, S.; Singh, O.; Kumar, A. Phytochemistry, Pharmacology and Traditional Uses of Leptadenia Pyrotechnica-An Important Medicinal Plant. Indian J. Pharm. Biol. Res. 2014, 2(1), 128. DOI: 10.30750/ijpbr.2.1.20.
- Ullah, F.; Uzair, M.; Chaudhry, B. A.; Zafar, Z. U. Phytochemical and Pharmacological Studies of Leptadenia Pyrotechnica. Pak. J. Pharm. Res. 2015, 1(2), 78–85. DOI: 10.22200/pjpr.2015278-85.
- Gajendra, S.; Arora, S.; Mathur, S. Eco-Friendly Corrosion Inhibition of Mild Steel in Hydrochloric Acid Using Leptadenia Pyrotechnica as a Green Inhibitor. Res. J. Chem. Sci. 2015, 5(8), 28–34.
- Page, M. J.; McKenzie, J. E.; Bossuyt, P. M.; Boutron, I.; Hoffmann, T. C.; Mulrow, C. D.; Shamseer, L.; Tetzlaff, J. M.; Akl, E. A.; Brennan, S. E.; Chou, R. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj . 2021, 10, 372.
- Ahmed, N.; Mahmood, A.; Mahmood, A.; Tahir, S. S.; Bano, A.; Malik, R. N.; Hassan, S.; Ishtiaq, M. Relative Importance of Indigenous Medicinal Plants from Layyah District, Punjab Province, Pakistan. J. Ethnopharmacol. 2014, 155(1), 509–523. DOI: 10.1016/j.jep.2014.05.052.
- Ahmed, N.; Mahmood, A.; Ashraf, A.; Bano, A.; Tahir, S. S.; Mahmood, A. Ethnopharmacological Relevance of Indigenous Medicinal Plants from District Bahawalnagar, Punjab, Pakistan. J. Ethnopharmacol. Journal of Ethnopharmacology. 2015, 175, 109–123. DOI: 10.1016/j.jep.2015.08.011.
- von Maydell, H.-J. Trees and Shrubs of the Sahel, Their Characteristics and Uses. Germany: TZ Verlagsgesellschaft, 1986.
- Sarfaraz Khan, M., Mir Ajab, K.; Mushtaq, A.; Muhammad Z.; Khalid, U. Floristic Account of the Asclepiadaceous Species from the Flora of Dera Ismail Khan District, KPK, Pakistan. Am. J. Plant Sci. 2011, 3, 2012.
- Yaseen, G.; Ahmad, M.; Sultana, S.; Suleiman Alharrasi, A.; Hussain, J.; Zafar, M.; Rehman, Shafiq-Ur. Ethnobotany of Medicinal Plants in the Thar Desert (Sindh) of Pakistan. J. Ethnopharmacol. 2015, 163, 43–59. DOI: 10.1016/j.jep.2014.12.053.
- Aziz, N.; Gilani, A.; Rindh, M. Kushta (S): Unique Herbo-Mineral Preparations Used in South Asian Traditional Medicine. Med. Hypotheses. 2002, 59(4), 468–472. DOI: 10.1016/S0306-9877(02)00260-8.
- Gulshan, A. B., Dasti, A. A.; Sabir, H.; Atta, M. I.; Muhammad, Amin-ud-din. Indigenous Uses of Medicinal Plants in Rural Areas of Dera Ghazi Khan, Punjab, Pakistan. J. Agric. Biol. Sci. 2012, 7(9), 750–762.
- Wariss, H. M., et al. Ethnobotanical Studies of Dicotyledonous Plants of Lal Suhanra National Park, Bahawalpur, Pakistan. Int. J. Sci. Res. 2014, 3(6), 2452–2460.
- Ahmad, M.; Khan, M. P. Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological Survey on Medicinal Plants Used in Herbal Drinks Among the Traditional Communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186. DOI: 10.1016/j.jep.2016.02.039.
- Upadhyay, B.; Dhaker, A. K.; Kumar, A. Ethnomedicinal and Ethnopharmaco-Statistical Studies of Eastern Rajasthan, India. J. Ethnopharmacol. 2010, 129(1), 64–86. DOI: 10.1016/j.jep.2010.02.026.
- Issa, T. O.; Mohamed, Y. S.; Yagi, S.; Ahmed, R. H.; Najeeb, T. M.; Makhawi, A. M.; Khider, T. O. Ethnobotanical Investigation on Medicinal Plants in Algoz Area (South Kordofan), Sudan. J. Ethnobiol. Ethnomed. 2018, 14(1), 1–22. DOI: 10.1186/s13002-018-0230-y.
- Ghazali, E.; Abdalla, W.; Elegami, A.; Baballa, K.; Sulieman, Y.; Mubarak, F.; Hamed, H. Medicinal Plants of the Sudan Part VII: Medicinal Plants of River Nile State. In Medicinal and Aromatic Plants and Traditional Medicine Research Institute; National Centre for Research: Khartoum, Sudan, 2021; p. 77.
- Sharma, J.; Gairola, S.; Gaur, R. D.; Painuli, R. M. The Treatment of Jaundice with Medicinal Plants in Indigenous Communities of the Sub-Himalayan Region of Uttarakhand, India. J. Ethnopharmacol. 2012, 143(1), 262–291. DOI: 10.1016/j.jep.2012.06.034.
- Tounekti, T.; Mahdhi, M.; Khemira, H. Ethnobotanical Study of Indigenous Medicinal Plants of Jazan Region, Saudi Arabia. Evid. Based Complement. Altern. Med. 2019, 2019. DOI:10.1155/2019/3190670.
- Niaz, S.; Bokhari, T. Z.; Sherwani, S. K.; Younis, U.; Dasti, A. A. Ethnobotanical Study of Some Medicinal Plants of Thal Desert Punjab, Pakistan. Int. J. Pharm. Res. Biosci. 2013, 2, 31–41.
- Yadav, S. S.; Rao, A. S.; Sheoran, A.; Singh, N.; Nandal, A.; Bhandoria, M. S.; Ganaie, S. A.; Bansal, P. An Ethnomedicinal Survey of Traditionally Used Medicinal Plants from Charkhi Dadri District, Haryana: An Attempt Towards Documentation and Preservation of Ethnic Knowledge. Indian J. Traditional Knowl. (IJTK). 2021, 20(2), 436–450.
- Hammiche, V.; Maiza, K. Traditional Medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105(3), 358–367. DOI: 10.1016/j.jep.2005.11.028.
- Ghazanfar, S. A. Handbook of Arabian Medicinal Plants; Boca Raton, Florida: CRC press, 1994.
- Abu-Rabia, A. Ethnobotany Among Bedouin Tribes in the Middle East. In Medicinal and Aromatic Plants of the Middle-East, Zohara Yaniv Ed.; Heidelberg New York London: Springer, 2014; pp. 27–36.
- Abu-Rabia, A. Palestinian Plant Medicines for Treating Renal Disorders: An Inventory and Brief History. Altern. Complementary Ther. 2005, 11(6), 295–300. DOI: 10.1089/act.2005.11.295.
- Phondani, P. C.; Bhatt, A.; Elsarrag, E.; Horr, Y. A. Ethnobotanical Magnitude Towards Sustainable Utilization of Wild Foliage in Arabian Desert. J. Traditional Complementary Med. 2016, 6(3), 209–218. DOI: 10.1016/j.jtcme.2015.03.003.
- Malik, S.; Ahmad, S.; Sadiq, A.; Alam, K.; Wariss, H. M.; Ahmad, I.; Hayat, M. Q.; Anjum, S.; Mukhtar, M. A Comparative Ethno-Botanical Study of Cholistan (An Arid Area) and Pothwar (A Semi-Arid Area) of Pakistan for Traditional Medicines. J. Ethnobiol. Ethnomed. 2015, 11(1), 1–20. DOI: 10.1186/s13002-015-0018-2.
- Ahmad, S.; Alam, K.; Wariss, H. M.; Anjum, S.; Mukhtar, M. Ethnobotanical Studies of Plant Resources of Cholistan Desert, Pakistan. Int. J. Sci. Res. 2014, 3(6), 1782–1788.
- Katewa, S.; Galav, P. Additions to the Traditional Folk Herbal Medicines from Shekhawati Region of Rajasthan. Indian J. Tradit. Knowl. 2006, 5(4), 494–500.
- Safa, O.; Soltanipoor, M. A.; Rastegar, S.; Kazemi, M.; Nourbakhsh Dehkordi, K.; Ghannadi, A. An Ethnobotanical Survey on Hormozgan Province, Iran. Avicenna J. Phytomed. 2013, 3(1), 64.
- Upadhyay, B.; Singh, K.; Kumar, A. Ethno-Veterinary Uses and Informants Consensus Factor of Medicinal Plants of Sariska Region, Rajasthan, India. J. Ethnopharmacol. 2011, 133(1), 14–25. DOI: 10.1016/j.jep.2010.08.054.
- Bhatti, G. R.; Qureshi, R.; Shah, M. Ethnobotany of Qadanwari of Nara Desert. Pak. J. Bot. 2001, 33, 801–812.
- Qureshi, R. Ethnobotany of Rohri Hills, Sindh, Pakistan. Hamdard Medicus. 2002, 45(3), 86–94.
- Qasim, M.; Abideen, Z.; Adnan, M. Y.;Ansari, R.; Gul, B.; Khan, M. A. Traditional Ethnobotanical Uses of Medicinal Plants from Coastal Areas. J. Coast Life Med. 2014, 2(1), 22–30.
- Goyal, M.; Sharma, S., Traditional Wisdom and Value Addition Prospects of Arid Foods of Desert Region of North West India. 2009. Indian Journal of Traditional Knowledge. 8(4), 581–585
- Patel, S.; Desai, P.; Pandey, V. Ethnomedicinal Plants Used by the Tribals in Bhiloda Taluka of Sabarkantha District, Gujarat. Indian J. Adv. Plant Res. 2014, 1, 33–36.
- Patel, S.; Kanjaria, K.; Patel, K. Investigations on Climber Resources Used by Tribal Inhabitants of Ambaji Forest of Banaskantha District (North Gujarat) by Rs Patel_ Kv Kanjaria and Kc Patel; Gujarat, India: Life Sciences leaflets, 2010: pp. 251–257; Vol. 10.
- Raza, M. A., Prevalence of intestinal parasites in small ruminants and their senstivity to treatments with ethnobotanical remedies in Cholistan, Pakistan. ( Doctoral dissertation) 2013.
- Raza, M. A.; Younas, M.; Buerkert, A.; Schlecht, E. Ethno-Botanical Remedies Used by Pastoralists for the Treatment of Livestock Diseases in Cholistan Desert, Pakistan. J. Ethnopharmacol. 2014, 151(1), 333–342. DOI: 10.1016/j.jep.2013.10.049.
- Youssef, S. Medicinal and Non-Medicinal Uses of Some Plants Found in the Middle Region of Saudi Arabia. J. Med. Plants Res. 2013, 7(34), 2501–2517.
- Upadhyay, B.; Roy, S.; Kumar, A. Traditional Uses of Medicinal Plants Among the Rural Communities of Churu District in the Thar Desert, India. J. Ethnopharmacol. 2007, 113(3), 387–399. DOI: 10.1016/j.jep.2007.06.010.
- Almoshari, Y. Medicinal Plants Used for Dermatological Disorders Among the People of the Kingdom of Saudi Arabia: A Narrative Review. Saudi J. Biol. Sci. 2022, 29(6), 103303. DOI: 10.1016/j.sjbs.2022.103303.
- Khare, C. P. Indian Medicinal Plants: An Illustrated Dictionary; Berlin/Heidelberg: Springer Science & Business Media, 2008.
- Singh, A. Endangered Economic Species of Indian Desert. Genet. Resour. Crop Evol. 2004, 51(4), 371–380. DOI: 10.1023/B:GRES.0000023452.91250.52.
- Diallo, D.; Hveem, B.; Mahmoud, M. A.; Berge, G.; Paulsen, B. S.; Maiga, A. An Ethnobotanical Survey of Herbal Drugs of Gourma District, Mali. Pharm. Biol. 1999, 37(1), 80–91. DOI: 10.1076/phbi.37.1.80.6313.
- Rastogi, R. P.; Mehrotra, B. N. Compendium of Indian Medicinal Plants: 1985-1989; India: Central Drug Research Institute and Publications & Information Directorate, 1990; Vol. 4.
- Youssef Moustafa, A. M.; Khodair, A. I.; Saleh, M. A. GC-MS Investigation and Toxicological Evaluation of Alkaloids from Leptadenia Pyrotechnica. Pharm. Biol. 2009, 47(10), 994–1003. DOI: 10.1080/13880200902973761.
- Zubair, M.; Nazir, M.; Saleem, M.; Raiz, N.; Touseef, S.; Khan, S.; Zengin, G.; Ehsan Mazhar, M.; Imran Tousif, M. Chemodiversity, Biological Activities and Molecular Docking Studies of Leptadenia Pyrotechnica (Forssk.) Decne: A Comprehensive Approach to Validate Its Medicinal Use. Chem. Biodivers. 2022, 19(5), e202100884.
- Dhawan, A.; Singh, H. Free Pools of Amino Acids and Sugars in Leptadaenia Pyrotechnica F; India: Current science, 1976.
- Moustafa, A.M.Y.; Khodair, A. I.; Saleh, M. A. Structural Elucidation and Evaluation of Toxicity and Antitumor Activity of Cardiac Glycosides Isolated from Leptadenia Pyrotechnica. Pharm. Biol. 2009, 47(9), 826–834. DOI: 10.1080/13880200902902505.
- Khasawneh, M. A., Koch, A., Elwy, H. M., Hamza, A. A., Schneider-Stock, R. Leptadenia Pyrotechnica Induces P53-Dependent Apoptosis in Colon Cancer Cells. Nat. Prod. Chem. Res. 2015, 3, 1–8.
- Sherwani, M.; Mathur, A.; Afreena, S. Leptadenia Pyrotechnica Seed Oil: A Good Source of Epoxy Acid. J. Lipid Sci. Technol. 2009, 41, 95–97.
- Al-Easa, H. S., Fatty Acids Composition of Fifty-One Plant Species Growing in Qatar. 2004.
- Khan, M.; Majid, A. Characterization of Leptadenia Pyrotechnica (Khip) Seed Oil. J. Pure Appl. Sci. (Pakistan). 1985, 4, 37–39.
- Moustafa, A. M. Y.; Khodair, A.; Saleh, M. Phytochemical Investigation and Toxicological Studies of Lipid Constituents Isolated from Leptadenia Pyrotechnica. J. Pharmacol. Toxicol. 2007, 2, 681–697. DOI: 10.3923/jpt.2007.681.697.
- Joshi, B.; Agrawal, V.; Kishore, D. Chemical Investigation of Leptadenia Spartium Part II. Herba Polonica. 1988, 34(1–2), 1–86.
- Mojumder, P.; Mondal, S. B.; Mukhopadhya, S.; Sen, K. K. Chemical Characterization of Khimp Fibre (Leptadenia Pyrotechnica). J. Sci. Ind. Res. 2001, 60, 675–677.
- Basalah, M.O.; Al-Whaibi, M. H.; Ahmad, A. M. Some Biochemical Analysis of Stem Exudate, and Stem and Root Tissues of Leptadenia Pyrotechnica (Forssk) Decne. Saudi Biol. Soc. 1984, 7, 133–141.
- Iyer, A.; Bhasin, V.; Joshi, B. C. Chemical Investigation of Leptadenia Spartium. I. Herba Polonica. 1974, 20, 321–334.
- Preet, R.; Chand Gupta, R. Simultaneous Determination of Phenolic Compounds in Leptadenia Pyrotechnica (Forssk.) Decne. By Using High-Performance Liquid Chromatography (HPLC-DAD-UV). Adv. Pharmacol. Sci. 2018, 2018, 1–4. DOI: 10.1155/2018/9604972.
- Khasawneh, M. A.; Elwy, H. M.; Hamza, A. A.; Fawzi, N. M.; Hassan, A. H. Antioxidant, Anti-Lipoxygenase and Cytotoxic Activity of Leptadenia Pyrotechnica (Forssk.) Decne Polyphenolic Constituents. Molecules. 2011, 16(9), 7510–7521. DOI: 10.3390/molecules16097510.
- Madiha, A. H.; Farid a, B.; Mahmood F, E. S., Phytochemical and Biological Investigation of Leptadenia Pyrotechnica Dence Growing in Egypt. 2002.
- Youssef Moustafa, A. M.; Khodair, A. I.; Saleh, M. A. Isolation, Structural Elucidation of Flavonoid Constituents from Leptadenia Pyrotechnica and Evaluation of Their Toxicity and Antitumor Activity. Pharm. Biol. 2009, 47(6), 539–552. DOI: 10.1080/13880200902875065.
- Cioffi, G.; Sanogo, R.; Vassallo, A.; Dal Piaz, F.; Autore, G.; Marzocco, S.; De Tommasi, N. Pregnane Glycosides from Leptadenia P Yrotechnica. J. Nat. Prod. 2006, 69(4), 625–635. DOI: 10.1021/np050493r.
- Preet, R.; Gupta, R. C.; Pradhan, S. K. Chromatographic Determination of β-Sitosterol, Lupeol, and Oleanolic Acid in Leptadenia Pyrotechnica (Forsk.) Decne.–a Botanical Source of the Ayurvedic Drug Jivanti. JPC J. Planar Chromatogr. Mod. TLC. 2018, 31(2), 150–154. DOI: 10.1556/1006.2018.31.2.9.
- Kumar, D. D.; Dhayal, K.; Shrama, R. A. Phytosterols from Leptadenia Pyrotechnica (Forsk) Decne. Elixir Int. J. Oct, 2017, 48714–48716.
- Manavalan, R.; Mithal, B. Constituents of the Aerial Parts of Leptadenia Pyrotechnica. Planta Med. 1980, 39(05), 95. DOI: 10.1055/s-2008-1074910.
- Noor, F.; Ahmed, A.; Imtiazuddin, S. M.; Khan, B. A Triterpenoid from Lepetadenia Pyrotechnica. Phytochemistry. 1992, 32(1), 211–212. DOI: 10.1016/0031-9422(92)80137-4.
- Ali, M. S.; Kausar, F.; Malik, A. Pyrotechnoic Acid: A Glycol-Oleanolic Acid Conjugate from Leptadenia Pyrotechnica (Asclepiadaceae). J. Chem. Soc. Pak. 2001, 23(3), 180–181.
- Dhawan, A.; Singh, H. Properties of Acid Phosphatase from Leptadaenia Pyrotechnica Forsk. Indian J. Exp. Biol. 1976, 14(3), 344–345.
- Saeed, H. A.; Liu, Y.; Lucia, L.; Chen, H. Sudanese Dicots as Alternative Fiber Sources for Pulp and Papermaking. Wood Ind./drvna Industrija. 2018, 69(2), 175–182.
- Bhaduri, S.; Mojumder, P. Medium Density Particle Board from Khimp Plant. Nat. Prod. Radiance. 2008, 7(2), 106–110.
- Bhaduri, S.; Ghosh, I. N.; Kundu, S. K.; Mojumder, P.; Mondal, S. B. Leptadenia Pyrotechnica-A Potential Raw Material for Pulp and Paper. IPPTA: Q. J. Indian Pulp Paper Tech. Assoc. 2006, 18(1), 59.
- Kundu, S.; Mojumder, P.; Bhaduri, S. K.; Das, B. K. Physical Characteristics of Khimp Fibre. Indian J. Fibre Text. Res. 2005, 30(2), 153–156.
- Sher, M.; Sen, D. N. Environmental Changes and Proline Content in Some Desert Plants. J. Arid Env. 1990, 19(2), 241–243. DOI: 10.1016/S0140-1963(18)30823-1.
- Purohit, S. Phytochemical Screening, Free Radical Scavenging, Antimicrobial Activity of Ethanolic Extract of Leptadenia Pyrotechnica. Int. J. Green Pharm. 2017, 11(03), 180–187.
- Woo, W.-S.; Chi, H.-J.; YunChoi, H.-S. Alkaloid Screening of Some Saudi Arabian Plants. Korean J. Pharmacogn. 1977, 8(3), 109–113.
- Rekha, S. S.; Mukesh, K.; Krishan, K. In vivo and in vitro Comparative Study of Primary Metabolites of Leptadenia Pyrotechnia. J. Microbiol. Biotechnol. Res. 2013, 3(2), 51–54.
- Tanira, M.; Bashir, A. K.; Dib, R.; Goodwin, C. S.; Wasfi, I. A.; Banna, N. R. Antimicrobial and Phytochemical Screening of Medicinal Plants of the United Arab Emirates. J. Ethnopharmacol. 1994, 41(3), 201–205. DOI: 10.1016/0378-8741(94)90033-7.
- Mishra, S. K.; Purohit, S.; Trigunayat, A.; Pandey, B. L. Anti-Inflammatory and Anti-Nociceptive Activity of Extract and Fractions of Leptadenia Pyrotechnica. Eur. J. Biomed. 2017, 4(9), 824–832.
- Soliman, G. A.; Donia, A. E. R. M.;Awaad, A. S.; Alqasoumi, S. I.; Yusufoglu, H. Effect of Emex Spinosa, Leptadenia Pyrotechnica, Haloxylon Salicornicum and Ochradenus Baccatus Extracts on the Reproductive Organs of Adult Male Rats. Pharm. Biol. 2012, 50(1), 105–112.
- Usman, H.; Aji, A.; Ahmed, I. A; Wiam, I. M.; Jambaima, M. A. Phytochemical and Antidiarrhoeal Screening of the Aerial Part of Leptadenia Pyrotechnica (Forsk.) Decne. Exp. Anim. 2017, 8, 9.
- Al-Easa, H. S., Constituents of Plants Growing in Qatar Xxxlll Proximate Composition of Thirty Four Food and Feed Plants Growing in Qatar. 2003.
- Munazir, M.; Qureshi, R.; Munir, M. Preliminary Phytochemical Screening of Roots and Aerial Parts of Leptadenia Pyrotechnica. Pak. J. Bot. 2015, 47(2), 659–664.
- Dileep Kumar, K. D.; Sharma, A. R. Biochemical Estimation of Primary Metabolites and Antioxidant Activity of Corchorus Olitorius L. And Leptadenia Pyrotechnica (Forsk) Decne. Int. J. Res. Pharm. Chem. 2018, 8(1), 183–187.
- Nandal, U.; Bhardwaj, R. L. The Role of Underutilized Fruits in Nutritional and Economic Security of Tribals: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54(7), 880–890. DOI: 10.1080/10408398.2011.616638.
- Alqasoumi, S. I.; Soliman, G. A. E. H.; Awaad, A. S.; Donia, A. E. R. M. Anti-Inflammatory Activity, Safety and Protective Effects of Leptadenia Pyrotechnica, Haloxylon Salicornicum and Ochradenus Baccatus in Ulcerative Colitis. Phytopharmacology. 2012, 2(1), 58–71.
- Qasim, M.; Abideen, Z.; Adnan, M. Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M. A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. South Afr. J. Bot. 2017, 110, 240–250. DOI: 10.1016/j.sajb.2016.10.005.
- Hossain, S.; El-Sayed, M.; Aoshima, H. Antioxidative and Anti-α-Amylase Activities of Four Wild Plants Consumed by Pastoral Nomads in Egypt. Orient. Pharm. Exp. Med. 2009, 9(3), 217–224. DOI: 10.3742/OPEM.2009.9.3.217.
- Moustafa, A.; Khodair, A. I.; Saleh, M. A. Potato Disc Bioassay and Cytotoxic Effect of Leptadenia Pyrotechnica: Comparative Study of Diverse Extracts. Pak. J. Biol. Sci. 2011, 14(18), 882–886. DOI: 10.3923/pjbs.2011.882.886.
- Rasheed, H. M. F.; Rasheed, F.; Qureshi, A. W.; Jabeen, Q. Immunostimulant Activities of the Aqueous Methanolic Extract of Leptadenia Pyrotechnica, a Plant from Cholistan Desert. J. Ethnopharmacol. 2016, 186, 244–250.
- Watafua, M.; Geidam, M. Effects of the Ethanolic Extract of Aerials of Leptadenia Pyrotechnica (Forsk.) Decnse. On the Liver Function of Wister Strain Albino Rats. Int. J. Sci. Res. Manag. 2014, 2, 605–610.
- Ghaneian, M. T.; Ehrampoush, M. H.; Jebali, A.; Hekmatimoghaddam, S.; Mahmoudi, M. Antimicrobial Activity, Toxicity and Stability of Phytol as a Novel Surface Disinfectant. Environ. Health Eng. Manage. J. 2015, 2(1), 13–16.
- Kumar, S.; Chaudhary, S.; Jha, K. Anthelminthic Activity on the Leptadenia Pyrotechnica (Forsk.) Decne. J. Nat. Prod. Plant Resour. 2011, 1(4), 56–59.
- Chaudhary, S.; Khosa, R. L.; Jha, K. K.; Kumar, S. Evaluation of Antidiabetic Activity of Whole Plant of Leptadenia Pyrotechnica (Forssk.) Decne Against Streptozotocin Induced Diabetes in Rats. Pharmacol. Online. 2011, 2, 1196–1204.
- Al-Maghraby, W. K.; Doleib, N. M.; Taha, H. M. Molecular Profiling and Potential Bioactive Characteristics of Endophytic Fungi Isolated from Leptadenia Pyrotechnica. Biomed. Pharmacol. J. 2022, 15(1), 531–541. DOI: 10.13005/bpj/2394.
- Mashwani, Z. U. R.; Wali, R.; Khan, M.F.; Abasi, F.; Khalid, N.; Raja, N. I. Antibacterial Activity of Some Selected Medicinal Plants of Pakistan, in Medicinal Plants as Anti-Infectives, François Chassagne Ed; United Kingdom: Elsevier, 2022; pp. 209–234.
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, Antimicrobial and Cytotoxic Activities of Selected Medicinal Plants from Yemen. J. Ethnopharmacol. 2007, 111(3), 657–666. DOI: 10.1016/j.jep.2007.01.018.
- Hadi, F.; Maqbool, T.; Khurshid, S.; Nawaz, A.; Aftab, S.; Tahir, S.; Awan, S. J.; Malik, A. Anti-Fungal Activity of Cressa Cretica, Leptadenia Pyrotechnica and Pulicaria Crispa, Indigenous Plants of Cholistan Desert, Pakistan. Anti-Infect. Agents. 2021, 19(3), 325–332. DOI: 10.2174/2211352518999201117144613.
- Mahida, Y.; Mohan, J., Screening of Plants for Their Potential Antibacterial Activity Against Staphylococcus and Salmonella Spp. 2007. Natural Product Radiance, 6, 301–305
- Rekha, K. K.; Krishan, K.; Monika, S. Antifungal Potential of Leptadenia Pyrotechnica Against Some Pathogenic Fungi. Int. J. Eng. Sci. Invent. 2013, 2(5), 27–29.
- Munazir, M., Qureshi, R.; Arshad, M.; Gulfraz, M. Antibacterial Activity of Root and Fruit Extracts of Leptadenia Pyrotechnica (Asclepiadaceae) from Pakistan. Pak. J. Bot. 2012, 44(4), 1209–1213.
- Partap, S.; Tewari, U.; Sharma, K.; Jha, K. K. In vitro Antioxidant Activity of Whole Plant of Leptadenia Pyrotechnica. J. Drug Delivery Ther. 2014, 4(1), 40–44. DOI: 10.22270/jddt.v4i1.742.
- Munazir, M.; Qureshi, R.; Munir, M. In vitro Antioxidant Activity of Methanolic Extracts of Various Parts of Leptadenia Pyrotechnica (Forssk.) Decne. Pak. J. Pharm. Sci. 2015, 28(2), 535–539.
- Partap, S., Ujjwal T.; Jyoti Sati, K. S.; Keshari K. J. Anti-Ulcer Activity of Methanolic Extracts of the Whole Plant of Leptadenia Pyrotechnica Against Gastric Ulcer in Rats. Res. J. Pharm. Biol. Chem. Sci. 2014, 5(1), 872–879.
- Soltan, M. M.; Zaki, A. K. Antiviral Screening of Forty-Two Egyptian Medicinal Plants. J. Ethnopharmacol. 2009, 126(1), 102–107. DOI: 10.1016/j.jep.2009.08.001.
- Partap, S.; Tewari, U.; Sharma, K.; Jha, K. K. Hepatoprotective Activity of Whole Plant Extract of Leptadenia Pyrotechnica Against Paracetamol Induced Damage in Rats. J. Drug Delivery Ther. 2014, 4(1), 36–39. DOI: 10.22270/jddt.v4i1.743.
- Jain, G., Jhalani, S.; Agarwal, S.; Jain, K. Hypolipidemic and Antiatherosclerotic Effect of Leptadenia Pyrotechnica Extract in Cholesterol Fed Rabbits. Asian J. Exp. Sci. 2007, 21(1), 115–122.
- Tewari, U. P.; Sangh, J.; Kishore, K. Immunomodulatory Activity of Whole Plant of Leptadenia Pyrotechnica. Int. Res. J. Humanities Eng. Pharm. Sci. 2014, 1(7), 8–11.
- Tanira, M.; Ali, B. H.; Bashir, A. K.; Wasfi, I. A.; Chandranath, I. Evaluation of the Relaxant Activity of Some United Arab Emirates Plants on Intestinal Smooth Muscle. J. Pharm. Pharmacol. 2011, 48(5), 545–550. DOI: 10.1111/j.2042-7158.1996.tb05971.x.
- Shaw, N.; Singh, G. Wound Healing Activity of Ethanolic Extract of Aerial and Root Part of Leptadenia Pyrotechnica (Fork.) Decne. Int. J. Pharm. Biol. Sci. 2014, 5, 671–675.