Abstract
Thymosin α1 (Tα1), an epithelial cell (EC)-derived cytokine, has the strong ability to modulate signals delivered through innate immune receptors on dendritic cells (DCs), thus instructing the initiation of appropriate immune responses to T cells. In its ability to activate indoleamine 2,3-dioxygenase 1-dependent tolerogenic programs in DCs, Tα1 pivotally contributes to the maintenance of self-tolerance by regulating the function of regulatory T (Treg) cells. How Tα1 may contribute to the Treg cell ontogeny is not known. The transcriptional regulator autoimmune regulator (AIRE) is known to control central and peripheral tolerance. AIRE is highly expressed in thymic medullary ECs where it controls the ectopic expression of tissue restricted antigens for negative selection. The absence of AIRE-induced tissue-specific antigens in the thymus can lead to autoimmunity in the antigen-expressing target organ. Recently, AIRE protein has been detected in peripheral lymphoid organs, suggesting that peripheral AIRE may play a complementary role. We have addressed the possible relationship between AIRE and Tα1 and discovered an intricate crosstalk, whereby AIRE may promote prothymosin cleavage to Tα1, and Tα1 in turn transcriptionally regulates AIRE expression. Thus, similar to other members of thymic stromal poietins, Tα1 expressed within the thymus and peripheral tissues regulates the EC/DC crosstalk required for salutary immune homeostasis.
1. Introduction
Thymosin α1 (Tα1, commercial name ZADAXIN®) is a naturally occurring thymic peptide first described and characterized by Goldstein and Goldstein and Garaci Citation[1,2]. Tα1 is found in highest concentrations in the thymus. However, it is produced in small amounts in several peripheral lymphoid and non-lymphoid, tissues, such that circulating levels measured in healthy adults by immunoassays are in the 252 – 1158 pg/ml range Citation[3]. Prepared as a 28mer synthetic amino-terminal acylated peptide, Tα1 is in clinical trials worldwide for the treatment of some viral infections, either as a monotherapy or in combination with IFN-α Citation[4]. Additional indications are some immunodeficiencies, malignancies and HIV/AIDS Citation[5]. Tα1 has shown an excellent safety profile and does not induce the side effects and toxicities commonly associated with immunomodulatory agents. The mechanism of action of the synthetic polypeptide is thought to be related to its immunomodulating activities, centered primarily on the augmentation of T-cell function. To do this, Tα1 primarily acts on cells of the innate immune system, including macrophages, neutrophils and dendritic cells (DCs) Citation[6-8]. As such, Tα1 acts as an endogenous regulator of both the inflammatory and adaptive immune responses Citation[6,9].
2. From pleiotropy to molecular mechanisms of a system-oriented molecule
The complex pathophysiology of inflammatory-driven immune and autoimmune diseases, from infections to cancer, is self-amplifying and redundant at multiple levels. Thus, the intrinsic robustness of living systems against various perturbations is a key factor that prevents most anti-inflammatory drug from being fully successful. This implicates that pharmacological therapy for inflammatory diseases would only be successful if addressing these complex network systems. Any successful drug would have to be pleiotropic, and work on the many components of the inflammatory/autoimmune cascade at a time. Tα1 exceptionally fulfils this requirement, qualifying itself as a context-dependent molecule capable of multi-target interactions in physiological and non-physiological conditions. Despite living up to its promises, Tα1 continues to amaze us with its pleiotropy, multi-targeting activity, and ability to utilize different molecular mechanisms depending on context. Recent insights into the molecular mechanisms of Tα1’s pleiotropy have revealed its central role in modulating DC function, which is consistent with the ability of Tα1 to induce different forms of immunity and tolerance Citation[10]. Results have shown that Tα1: i) primed DCs for antifungal T-helper (Th)1 resistance through Toll-like receptor (TLR)/MyD88-dependent signaling and this translated in vivo to protection against fungal infections Citation[6]; ii) activated plasmacytoid DCs via the TLR9/MyD88-dependent viral recognition, thus leading to the activation of IFN regulatory factor 7 and the promotion of the IFN-α/IFN-γ-dependent effector pathway, and this resulted in protection against primary murine cytomegalovirus infection Citation[11]; and iii) induced indoleamine 2,3-dioxygenase (IDO)1 activity in DCs, thus affecting tolerization toward self as well as microbial non-self antigens, and this resulted in vivo in transplantation tolerance Citation[8] and protection from inflammation and allergy Citation[12]. Evidence suggests that the ability of Tα1 to signal through disparate TLRs on DC subsets may occur through the interaction with multiple, yet distinct, TLR ligands, either endogenous or exogenous Citation[10]. Heterocomplexes and collaborative interactions between endogenous and/or exogenous TLR agonists and Tα1 may lead to novel reciprocal trends of immune surveillance and effector mechanisms aiding safe disposal of pathogens and/or pathogenic insults whereby excessive inflammatory responses and tissue injury may effectively be prevented. NF-κB, a family of seven structurally related transcription factors that play a central role in the stress response and inflammation Citation[13], are exploited by Tα1 for its pleiotropic activity Citation[10]. This finding not only qualifies Tα1 as a context-oriented molecule but may also offer molecular explanations for a successful drug working on the many components of the inflammatory/immune response, that is, for successful pleiotropy.
3. Promoting tolerance by Tα1 via tryptophan catabolism
The induction of tolerance is critical for the maintenance of immune homeostasis. Within the new paradigm of resistance and tolerance mechanisms as two alternative but complementary host defense strategies Citation[14], boosting tissue tolerance is likely to be a useful strategy in infectious and non-infectious diseases. The mammalian intestinal and respiratory mucosal surfaces are continuously exposed to a complex and dynamic community of microorganisms. These microbes establish symbiotic relationships with their hosts, making important contributions to immune fitness and metabolism efficiency Citation[15,16]. The immune system is pivotal in mediating the interactions between host and microbiota that shape the mucosal environment. At the mucosal surfaces, a system of tolerance and controlled inflammation to limit the response to dietary or pathogen-derived antigens is present. This regulation is achieved by a number of cell populations acting through a set of shared regulatory pathways Citation[17,18]. Regulatory T (Treg) cells constitute a pivotal mechanism of immunological tolerance. The potential role of malfunctioning Treg cells in chronic inflammatory immune and autoimmune diseases is well-documented Citation[19]. Recent studies have demonstrated a crucial role for tryptophan catabolism and kynurenine production in the induction of peripheral tolerance Citation[20,21]. IDO1 is an intracellular heme-containing enzyme that catalyzes the initial and rate-limiting step in tryptophan degradation along the kynurenine pathway. IDO1 is widely expressed in a variety of human tissues as well as in macrophages and DCs and is induced in inflammatory states by IFN-γ and other proinflammatory cytokines. Studies have demonstrated a complex and crucial role for IDO1 in immunoregulation during infection, pregnancy, autoimmunity, transplantation and neoplasia Citation[22]. By inducing IDO1 and promoting Treg cells, Tα1 successfully ameliorated respiratory allergy and intestinal inflammation in different experimental models Citation[12]. Thus, maintaining diplomatic relations between mammals and microbial communities could be added to the therapeutic and immunomodulatory properties of Tα1.
4. En route to tolerance: Tα1 meets AIRE
The transcription factor autoimmune regulator (AIRE) is encoded by the homonymous gene, AIRE, situated in the region q22.3 of chromosome 21 Citation[23,24]. The homologous murine gene, AIRE is located in the chromosome 10 and shows 77% nucleotide correspondence with human AIRE Citation[25]. AIRE expression displays the highest levels in the thymus, especially in medullary epithelial cells (mTECs) and, less markedly, in DCs. Peripheral lymph nodes, fetal liver and spleen also express AIRE, albeit at low levels Citation[26]. Consistent with its high rate of expression in mTECs, AIRE is considered a major player towards the acquisition of central tolerance. The mTECs express a large number of genes, including tissue-specific antigens (TSAs) that are normally present only in specialized peripheral organs and are apparently not required for the direct functioning of mTECs Citation[27]. During negative selection, these encoded TSAs are presented by mTECs or DCs as self-antigens to differentiating thymocytes, leading to the induction of tolerance either by clonal deletion, functional inactivation of self-reactive T cells or clonal deviation Citation[28,29]. The thymic expression of many of these TSAs is dependent on the AIRE gene Citation[30] and mutations in AIRE lead to severe, multiorgan, tissue-specific autoimmunity in both mice and humans Citation[28]. Although these results reveal a role of thymic AIRE, self-tolerance must continue to be enforced after T cells leave the thymus. In this regard, human peripheral lymphoid tissues contain AIRE-expressing DCs Citation[31] and a population of extrathymic AIRE-expressing cells (eTACs) that may play an important and previously uncharacterized role in self-tolerance have been described Citation[32]. The eTACs share certain characteristics with mTECs, including being equipped to act as professional antigen-presenting cells and the AIRE-regulated expression of TSAs. The set of AIRE-regulated TSAs expressed in eTACs appears to have little overlap with thymic AIRE-regulated antigens. The lack of overlap suggests that there may be a higher order of AIRE-dependent transcriptional regulation of TSA expression, whether direct or indirect, that differs between the thymus and the periphery. The eTACs may represent a safety net within the entire immunological periphery, which functions to screen out naïve autoreactive T-cell clones that escape thymic negative selection and, finally, may play an increasingly important role with advancing age, as the thymus involutes and the burden of maintaining self-tolerance shifts to the periphery Citation[32].
With AIRE influencing intrathymic and extrathymic expression of numerous TSAs Citation[28], it becomes apparent that the regulation of AIRE will be an important determinant in self-tolerance control. Tα1 is highly expressed in human thymus (at the levels ranging from 45.0 to 52.1 ng/mg protein in normal thymuses) Citation[33] and is localized on ECs, lying singly, grouped, in Hassall’s corpuscles Citation[34]. Human Hassall’s corpuscles also express thymic stromal lymphopoietin (TSLP) that is known to condition thymic CD11c-positive DCs to induce the proliferation and differentiation of CD4(+)CD8(−)CD25(−) thymic T cells into CD4(+)CD25(+)forkhead box P3 (FoxP3[+]) Treg cells Citation[35]. These findings suggested that Hassall’s corpuscles have a critical role in DC-mediated secondary positive selection of medium-to-high affinity self-reactive T cells, leading to the generation of CD4(+)CD25(+) Treg cells within the thymus. It is of interest that human TSLP appears to induce expression of AIRE in human DCs Citation[36], although the functional significance and in vivo relevance of this finding is not known.
5. Tα1 promotes AIRE expression
Given the many functional similarities between TSLP Citation[37] and Tα1 Citation[12], we hypothesized that Tα1 could also be able to activate AIRE expression on ECs. To this purpose, we have assessed the ability of Tα1 to activate AIRE expression on murine ECs isolated from the gastrointestinal tract of naïve mice and exposed to the fungus Candida albicans in vitro. We found that Tα1 significantly increased AIRE expression on ECs (). On the same cells, Tα1 also induced the expression of Indo1 gene coding for IDO1 () and Il10 gene coding for IL-10 () which were both dependent on AIRE, being negated, at least partially, in AIRE0/0 ECs (). To determine whether endogenously produced Tα1 also regulates the expression of these genes, we assessed AIRE, Indo1 and Il10 gene expression on ECs on inhibition of prothymosin alpha (Ptma) by specific small interfering RNA (siRNA). It is known that Tα1 is produced in vivo by cleavage of Ptma in diverse mammalian tissues Citation[38]. We found that AIRE, and even more, the Indo1 and Il10 genes failed to upregulate in response to the fungus in the presence of siRNA targeting the Ptma gene (). Preliminary as they are, these data suggest that Tα1 may contribute to AIRE expression and function that includes IDO1 activation and IL-10 production.
Figure 1. Tα1 promotes Aire and Indo1 gene expression on murine ECs. ECs were exposed to Candida albicans and assessed for Aire (A and D), Indo1 (B and E) and Il10 (C and F) gene expression in the presence of Tα1 (A – C) or siPtma (prothymosin alpha) (D – F). ECs were purified from murine intestine as described Citation[40] and stimulated (106) with inactivated Candida yeasts (1:1 ratio), with and without 100 μg/ml of Tα1 (SciClone Pharmaceuticals, Inc) for 12 h at 37°C. ECs were exposed to 1 µM siPtma (Dharmacon RNAi Technologies) for 72 h, as described Citation[42] before the addition of Candida. Gene expression was evaluated by real-time RT-PCR (Stratagene) with specific primers Citation[42]. The Aire primers were as follows: Forward, 5′-CCTGGATTTCTGGAGGATTCTCT-3′; reverse, 5′-CCGTCCAGGATGCTATGCA-3′. *p < 0.05, ‡p < 0.01, §p < 0.001, Tα1-treated versus untreated cells and Aire0/0 versus Aire+/+ cells. The data are pooled from two experiments.
![Figure 1. Tα1 promotes Aire and Indo1 gene expression on murine ECs. ECs were exposed to Candida albicans and assessed for Aire (A and D), Indo1 (B and E) and Il10 (C and F) gene expression in the presence of Tα1 (A – C) or siPtma (prothymosin alpha) (D – F). ECs were purified from murine intestine as described Citation[40] and stimulated (106) with inactivated Candida yeasts (1:1 ratio), with and without 100 μg/ml of Tα1 (SciClone Pharmaceuticals, Inc) for 12 h at 37°C. ECs were exposed to 1 µM siPtma (Dharmacon RNAi Technologies) for 72 h, as described Citation[42] before the addition of Candida. Gene expression was evaluated by real-time RT-PCR (Stratagene) with specific primers Citation[42]. The Aire primers were as follows: Forward, 5′-CCTGGATTTCTGGAGGATTCTCT-3′; reverse, 5′-CCGTCCAGGATGCTATGCA-3′. *p < 0.05, ‡p < 0.01, §p < 0.001, Tα1-treated versus untreated cells and Aire0/0 versus Aire+/+ cells. The data are pooled from two experiments.](/cms/asset/0d12cc31-69b1-4110-a570-8822fd10bb7c/iebt_a_1044895_f0001_oc.jpg)
6. AIRE drives IDO1 production
We have already shown that AIRE0/0 mice on BALB/c background are much more susceptible than AIRE+/+ mice to mucosal candidiasis, a hallmark of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy disease caused by AIRE mutations Citation[23,24]. IL-10+FoxP3+ T cells were significantly decreased in these mice, a finding pointing to a tolerance defect correlated with AIRE deficiency Citation[39]. To assess whether, similar to the in vitro findings, AIRE regulates IDO1 expression in vivo, we assessed IDO1 protein expression in the mesenteric lymph nodes of AIRE0/0 and AIRE+/+ mice intragastrically infected with C. albicans. Consistent with previous data Citation[39], AIRE0/0 mice were clearly more susceptible than AIRE+/+ to candidiasis, as revealed by the extensive inflammatory cell recruitment and signs of immunopathology in the stomach of AIRE0/0 mice (), likely accounting for the ability of these mice to better control the growth of the fungus as compared to AIRE+/+ mice (). In terms of IDO1 protein expression, we found that this expression was defective in AIRE0/0 mice on infection (), a finding further pointing to the contribution of AIRE to the generation of self-tolerance Citation[28]. These findings clearly suggest that Tα1 and AIRE cooperate in the induction of IDO1-dependent immune tolerance. As a matter of fact, lack of tolerance occurred in Indo−/− mice infected intragastrically with C. albicans, as judged by signs of extensive immunopathology in the stomach () and imbalanced Il17a/Il10 gene expression (), as already demonstrated Citation[40]. Of interest, treatment with Tα1 failed to restore immune tolerance in Indo–/– mice (), a finding further pointing to IDO1 as an important mediator of Tα1-induced tolerance.
Figure 2. Aire controls IDO1 protein expression in vivo. Aire0/0 and Aire+/+ mice on the BALB/c background were injected intragastrically with 108 Candida albicans and assessed a week later for (A) stomach histology by periodic acid-Schiff (PAS) staining (note the presence of abundant inflammatory cells and signs of acanthosis and parakeratosis in the stomachs of Aire0/0) and fungal growth (B), expressed as CFU per organ (mean ± SE). (C) IDO1 expression in mesenteric lymph nodes (immunoblotting was performed with rabbit polyclonal IDO1-specific antibody, as described in Ref. Citation[43]. The positive control consisted of IDO1-expressing MC24 transfectants and the negative control of mock-transfected MC22 cells). Corresponding pixel density ratio was normalized against β-tubulin. ***p = 0.001, Aire0/0 versus Aire+/+ mice. The data reported are from one experiment representative of three with similar results. (D) Stomach histology (PAS staining) is shown and (E) Il17a and Il10 expression shown on stomach total cells from C57BL/6 or Indo–/– mice intragastrically infected with 108 C. albicans, treated with 200 μg/kg/i.p. of Tα1 daily for a week and immediately sacrificed. Images were acquired with a 40× objective. Bars indicate magnification. Data are representative (histology) or pooled from two experiments. *p < 0.01, ‡p < 0.001, Tα1-treated versus untreated (none) mice (naïve, uninfected and untreated mice).
![Figure 2. Aire controls IDO1 protein expression in vivo. Aire0/0 and Aire+/+ mice on the BALB/c background were injected intragastrically with 108 Candida albicans and assessed a week later for (A) stomach histology by periodic acid-Schiff (PAS) staining (note the presence of abundant inflammatory cells and signs of acanthosis and parakeratosis in the stomachs of Aire0/0) and fungal growth (B), expressed as CFU per organ (mean ± SE). (C) IDO1 expression in mesenteric lymph nodes (immunoblotting was performed with rabbit polyclonal IDO1-specific antibody, as described in Ref. Citation[43]. The positive control consisted of IDO1-expressing MC24 transfectants and the negative control of mock-transfected MC22 cells). Corresponding pixel density ratio was normalized against β-tubulin. ***p = 0.001, Aire0/0 versus Aire+/+ mice. The data reported are from one experiment representative of three with similar results. (D) Stomach histology (PAS staining) is shown and (E) Il17a and Il10 expression shown on stomach total cells from C57BL/6 or Indo–/– mice intragastrically infected with 108 C. albicans, treated with 200 μg/kg/i.p. of Tα1 daily for a week and immediately sacrificed. Images were acquired with a 40× objective. Bars indicate magnification. Data are representative (histology) or pooled from two experiments. *p < 0.01, ‡p < 0.001, Tα1-treated versus untreated (none) mice (naïve, uninfected and untreated mice).](/cms/asset/47bbf202-158a-4251-871b-6cd58bb04fac/iebt_a_1044895_f0002_oc.jpg)
7. AIRE promotes Tα1 production
Tα1 is produced in vivo by cleavage of Ptma in diverse mammalian tissues Citation[38]. Evidence indicates that a lysosomal asparaginyl endopeptidase, called legumain, is able to process Ptma to generate Tα1 Citation[38]. AIRE expression in human monocyte-derived DCs was found to correlate with a slight downregulation of legumain-specific transcript in unstimulated cells Citation[41]. We assessed legumain production in purified intestinal DCs exposed to C. albicans yeasts or hyphae. Surprisingly, we found a significant increase in the production of legumain in DCs exposed to the hyphae, an effect that was not observed in AIRE0/0 DCs (). Therefore, AIRE expression correlates with upregulation of legumain protein production. To assess whether a similar regulation occurs in vivo, we evaluated legumain expression in the stomach of AIRE0/0 and AIRE+/+ mice intragastrically infected with C. albicans. We found a profound downregulation of legumain mRNA expression in conditions of AIRE deficiency (), associated with a lower Tα1 production (). Lower Tα1 production was similarly observed in purified AIRE0/0 ECs on in vitro stimulation with C. albicans (), a finding suggesting that Aire may promote cleavage of Ptma in ECs via legumain.
Figure 3. Aire promotes Tα1 production. (A) Legumain production in DCs purified from the mesentheric lymph nodes and pulsed for 6 h with live Candida albicans yeasts and hyphae (at the cell:fungi ratio of 1:1) as described Citation[43]. Immunoblotting was done on cell lysates with the anti-legumain specific antibody (Santa Cruz). Corresponding pixel density ratio was normalized against β-actin. (B) Lgmn (Legumain) gene expression by RT-PCR and (C) Tα1 production (by specific ELISA from Immundiagnostik) in Aire0/0 and Aire+/+ mice a week after intragastric infection with 108 C. albicans are shown. Assays were done on stomach total cells or homogenates (pooled from 6 mice). (D) Tα1 production by intestinal ECs is isolated from naïve mice and stimulated with Candida as in legend to ). *p < 0.01, Aire0/0 versus Aire+/+ mice and cells. The data are pooled from two experiments.
![Figure 3. Aire promotes Tα1 production. (A) Legumain production in DCs purified from the mesentheric lymph nodes and pulsed for 6 h with live Candida albicans yeasts and hyphae (at the cell:fungi ratio of 1:1) as described Citation[43]. Immunoblotting was done on cell lysates with the anti-legumain specific antibody (Santa Cruz). Corresponding pixel density ratio was normalized against β-actin. (B) Lgmn (Legumain) gene expression by RT-PCR and (C) Tα1 production (by specific ELISA from Immundiagnostik) in Aire0/0 and Aire+/+ mice a week after intragastric infection with 108 C. albicans are shown. Assays were done on stomach total cells or homogenates (pooled from 6 mice). (D) Tα1 production by intestinal ECs is isolated from naïve mice and stimulated with Candida as in legend to Figure 1). *p < 0.01, Aire0/0 versus Aire+/+ mice and cells. The data are pooled from two experiments.](/cms/asset/93032aaa-6822-4e8f-9f28-dcf66e4961c1/iebt_a_1044895_f0003_oc.jpg)
8. Conclusion
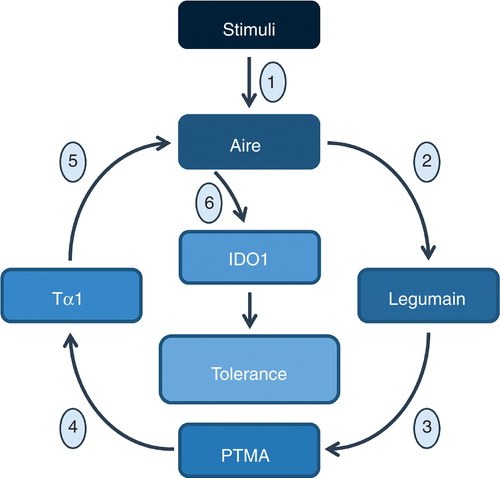
The discovery of AIRE as an unexpected partner in Tα1’s physiological role may represent an important advance in the field of therapeutic development of Tα1. Our data would indicate the existence of a crosstalk between AIRE and Tα1, whereby AIRE may promote Ptma cleavage to Tα1 through regulation of the asparaginyl endopeptidase legumain activity and Tα1, in turn, regulates AIRE expression at the transcriptional level (). Although more work is needed to provide mechanistic insights governing the reciprocal regulation between AIRE and Tα1 in ECs and/or other types of cells and the possible in vivo significance, AIRE exploitation by Tα1 qualifies Tα1 as a master regulator of central and peripheral immune tolerance.
Figure 4. Schematic representation of the possible pathways underlying the Tα1/Aire synergistic crosstalk in tolerance. Induced in infection (1), Aire may activate the asparaginyl endopeptidase legumain activity (2) to PTMA cleavage (3) leading to the production of Tα1 (4) that in turn activates Aire transcription (5) which is required for the activation of the enzyme IDO1 (6).
Acknowledgment
The authors thank Dr Cristina Massi Benedetti for digital art and editing.
Declaration of interest
This paper is part of a supplemental issue, sponsored by SciClone. The research in this paper was supported by the Specific Targeted Research Project FunMeta (ERC-2011-AdG-293714) to L Romani. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents, received or pending, or royalties.
Notes
Bibliography
- Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin alpha 1. Expert Opin Biol Ther 2009;9:593-608
- Garaci E. Thymosin alpha1: a historical overview. Ann N Y Acad Sci 2007;1112:14-20
- Weller FE, Shah U, Cummings GD, et al. Serum levels of immunoreactive thymosin alpha 1 and thymosin beta 4 in large cohorts of healthy adults. Thymus 1992;19:45-52
- Billich A. Thymosin alpha1. SciClone Pharmaceuticals. Curr Opin Investig Drugs 2002;3:698-707
- Tuthill CW, King RS. Thymosin Apha 1–A Peptide Immune Modulator with a Broad Range of Clinical Applications. Clin Exp Pharmacol 2013;3:133
- Romani L, Bistoni F, Gaziano R, et al. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood 2004;103:4232-9
- Zhang P, Chan J, Dragoi AM, et al. Activation of IKK by thymosin alpha1 requires the TRAF6 signalling pathway. EMBO Rep 2005;6:531-7
- Romani L, Bistoni F, Perruccio K, et al. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood 2006;108:2265-74
- Pierluigi BD, Angelo C, Fallarino F, et al. Thymosin alpha1: the regulator of regulators? Ann N Y Acad Sci 2010;1194:1-5
- Romani L, Moretti S, Fallarino F, et al. Jack of all trades: thymosin alpha1 and its pleiotropy. Ann N Y Acad Sci 2012;1269:1-6
- Bozza S, Gaziano R, Bonifazi P, et al. Thymosin alpha1 activates the TLR9/MyD88/IRF7-dependent murine cytomegalovirus sensing for induction of anti-viral responses in vivo. Int Immunol 2007;19:1261-70
- Romani L, Bistoni F, Montagnoli C, et al. Thymosin alpha1: an endogenous regulator of inflammation immunity and tolerance. Ann N Y Acad Sci 2007;1112:326-38
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004;25:280-8
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012;335:936-41
- Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010;10:159-69
- Thorburn AN, Macia L, Mackay CR. Diet metabolites and “western-lifestyle” inflammatory diseases. Immunity 2014;40:833-42
- Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol 2013;25:352-7
- Roncarolo MG, Gregori S, Bacchetta R, et al. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol 2014;380:39-68
- Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 2014;259:88-102
- Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol 2007;7:817-23
- McGaha TL, Huang L, Lemos H, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 2012;249:135-57
- Munn DH, Mellor AL. Indoleamine 23 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34:137-43
- Finnish-German AC. An autoimmune disease APECED caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 1997;17:399-403
- Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet 1997;17:393-8
- Mittaz L, Rossier C, Heino M, et al. Isolation and characterization of the mouse AIRE gene. Biochem Biophys Res Commun 1999;255:483-90
- Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol 2008;8:948-57
- Derbinski J, Gabler J, Brors B, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 2005;202:33-45
- Mathis D, Benoist C. Aire. Annu Rev Immunol 2009;27:287-312
- Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev 2011;241:89-103
- Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002;298:1395-401
- Poliani PL, Kisand K, Marrella V, et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am J Pathol 2010;176:1104-12
- Gardner JM, Devoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 2008;321:843-7
- Naruse H, Hashimoto T, Yamakawa Y, et al. Immunoreactive thymosin alpha 1 in human thymus and thymoma. J Thorac Cardiovasc Surg 1993;106:1065-71
- Dalakas MC, Engel WK, McClure JE, et al. Immunocytochemical localization of thymosin-alpha 1 in thymic epithelial cells of normal and myasthenia gravis patients and in thymic cultures. J Neurol Sci 1981;50:239-47
- Watanabe N, Wang YH, Lee HK, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005;436:1181-5
- Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol 2004;5:426-34
- Roan F, Bell BD, Stoklasek TA, et al. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol 2012;91:877-86
- Sarandeses CS, Covelo G, Diaz-Jullien C, et al. Prothymosin alpha is processed to thymosin alpha 1 and thymosin alpha 11 by a lysosomal asparaginyl endopeptidase. J Biol Chem 2003;278:13286-93
- Ahlgren KM, Moretti S, Lundgren BA, et al. Increased IL-17A secretion in response to Candida albicans in autoimmune polyendocrine syndrome type 1 and its animal model. Eur J Immunol 2011;41:235-45
- Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013;39:372-85
- Sillanpaa N, Magureanu CG, Murumagi A, et al. Autoimmune regulator induced changes in the gene expression profile of human monocyte-dendritic cell-lineage. Mol Immunol 2004;41:1185-98
- de Luca A, Bozza S, Zelante T, et al. Non-hematopoietic cells contribute to protective tolerance to Aspergillus fumigatus via a TRIF pathway converging on IDO. Cell Mol Immunol 2010;7:459-70
- De Luca A, Montagnoli C, Zelante T, et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol 2007;179:5999-6008

