Abstract
Objective: Extravasation of circulating cancer cells is an important step of the metastatic cascade and a potential target for anti-cancer strategies based on vasoprotective drugs. Reports on anti-cancer effects of fenofibrate (FF) prompted us to analyze its influence on the endothelial barrier function during prostate cancer cell diapedesis.
Research design and methods: In vitro co-cultures of endothelial cells with cancer cells imitate the ‘metastatic niche’ in vivo. We qualitatively and quantitatively estimated the effect of 25 μM FF on the events which accompany prostate carcinoma cell diapedesis, with the special emphasis on endothelial cell mobilization.
Results: Fenofibrate attenuated cancer cell diapedesis via augmenting endothelial cell adhesion to the substratum rather than through the effect on intercellular communication networks within the metastatic niche. The inhibition of endothelial cell motility was accompanied by the activation of PPARα-dependent and PPARα-independent reactive oxygen species signaling, Akt and focal adhesion kinase (FAK) phosphorylation, in the absence of cytotoxic effects in endothelial cells.
Conclusions: Fenofibrate reduces endothelial cell susceptibility to the paracrine signals received from prostate carcinoma cells, thus inhibiting endothelial cell mobilization and reducing paracellular permeability of endothelium in the metastatic niche. Our data provide a mechanistic rationale for extending the clinical use of FF and for the combination of this well tolerated vasoactive drug with the existing multidrug regimens used in prostate cancer therapy.
1. Introduction
Agonists of PPARs are known to control the expression of genes involved in glucose and lipid metabolism. They have been used in the treatment of diabetes, hyperlipidemia and cardiovascular diseases Citation[1,2]. Fenofibrate (propan-2-yl 2-{4-[(4-chlorophenyl) carbonyl] phenoxy}-2-methylpropanoate) is an FDA-approved, PPARα agonist characterized by excellent safety and tolerability profile after chronic and prolonged treatments Citation[3,4]. It is widely used in clinical practice to lower serum levels of triglycerides and cholesterol, improve low-density lipoproteins; high-density lipoproteins ratio and prevent atherosclerosis Citation[5-8]. In parallel, the vascular protecting activity of fenofibrate (FF), independent of its lipid-lowering activity, has been reported, including its activity in improving physiological neovascularization Citation[9-11]. Conversely, several reports indicate that FF may suppress endothelial cell proliferation and migration, leading to the attenuation of tumor vasculature Citation[12-15]. Vascularization of primary tumors and the interactions of cancer cells with endothelial continua are crucial for cancer development Citation[16,17]; therefore, these facts prompted questions about possible application of FF in cancer therapy Citation[18].
According to the simplified model of cancer promotion and progression, the formation of extensively proliferating cell subpopulations characterizes early steps of carcinogenesis, while cancer progression is triggered by the clonal appearance of cells, capable of invading distant organs Citation[19]. Potential interference of FF with cancer promotion was illustrated by the attenuation of growth and survival of glioblastoma Citation[20], hepatoma Citation[21,22], medulloblastoma Citation[23] and melanoma cells Citation[24]. At the level of cancer progression, FF was demonstrated to reduce invasive potential of cancer cells Citation[24-27]. Both these activities have been ascribed to PPARα activation Citation[3,24,26,28-30] but also to the PPARα-independent effect of FF on the accumulation of reactive oxygen species (ROS) Citation[21,31]. Importantly, ROS have been implicated in the PPARα-dependent Citation[26] and PPARα-independent Citation[27] inhibition of cancer cell motility. Converging effects of FF on endothelial and cancer cell properties, its excellent safety and tolerability profile along with anti-inflammatory activity Citation[32], warrant the intensive investigations into the possible interference of FF with the progression of cancers characterized by long latency periods, such as prostate cancer.
Penetration of the endothelial layer lining up vessel walls by circulating cancer cells (cancer cell diapedesis) is a multistep process, which determines the effectiveness of the metastatic cascade and malignant dissemination Citation[33]. It is initiated by the stabilization of cancer cell adhesion to the endothelial layer inside microcapillaries Citation[34-36] and followed by local remodeling of endothelial continuum in the ‘metastatic niche’ of cancer cells. Prompted by previous reports on the interference of FF with tumor vasculature Citation[15], we undertook an in-depth examination of its effects on endothelial reactivity to the signals generated by cancer cells. Monitoring of the events accompanying the penetration of endothelial continuum by DU-145 cells gave us the opportunity to recapitulate the complexity of systems that regulate endothelial susceptibility to challenge by a single cancer cell. This approach enabled us to comprehensively address FF effects on the efficiency of prostate carcinoma cell diapedesis.
2. Methods
2.1 Cell culture
Human umbilical vein endothelial cells (HUVECs, Life Technologies Corp.) were routinely cultured in endothelial basal medium (EBM) supplemented with 10% fetal bovine serum (FBS) and supplement cocktail (recombinant human epidermal growth factor [rhEGF], bovine brain extract, hydrocortisone, gentamicin, amphotericin-B; all from Lonza). For end point experiments, the cells were used within 2–6 passages in serum-free EBM medium with supplements. Human prostate carcinoma DU-145 and PC-3 cells were routinely cultivated in DMEM-F12 HAM (Sigma) medium supplemented with 10% FBS and antibiotics Citation[37]. Co-culture experiments were performed on HUVEC monolayers between 70 and 98% confluence (indicated in the text). DU-145 or PC-3 cells were seeded into HUVEC cultures at the density of 1300 cells/cm2 in serum-free conditions. Fenofibrate, GW9662 and N-acetyl-L-cysteine (NAC; all from Sigma) were administered at the time points and concentration(s) indicated in the text.
2.2 Immunocytochemistry and fluorescence microscopy
For the immunofluorescence analysis, cells on coverslips were fixed with 3.7% formaldehyde (20 min), permeabilized (0.1% Triton X-100 in PBS, 5 min), blocked with 3% BSA and incubated with a primary antibody (rabbit polyclonal anti-VE-cadherin IgG, mouse monoclonal anti-vinculin IgG, mouse monoclonal anti-ZO-1 IgG; all from Sigma; Citation[38]). Subsequently, the cells were stained with a secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 488-conjugated goat anti-mouse IgG; Life Technologies), and counterstained with TRITC-conjugated phalloidin and/or Hoechst 33258 (both from Sigma). Where indicated, cancer cells were stained with CellTracker Orange CMRA (10 μM, Invitrogen). For gap junctional intercellular coupling (GJIC) analyses, calcein-loaded (Invitrogen) donor (DU-145) cells were plated on monolayers of acceptor (HUVEC) cells grown on coverslips in Petri dishes at the ratio of 1:50 Citation[39] to evaluate intercellular calcein transfer and coupling index defined as the percentage of donor cells coupled with at least one acceptor cell. To determine the cytotoxic effect of FF, HUVECs incubated with various concentrations of FF for 6, 24 and 48 h were harvested, and the number of viable cells was determined by the fluorescence diacetate/ethidium bromide test. Image acquisition was performed with a Leica DMI6000B microscope (DMI7000 version; Leica Microsystems, Wetzlar, Germany) equipped with the Total Internal Reflection Fluorescence, Nomarski Differential Interference Contrast (DIC) and Interference Modulation Contrast (IMC) modules. LAS-AF deconvolution software was used for image processing as described previously Citation[39]. Three dimensional (3D) images were registered using a Leica TCS SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) using 63× HCX PL APO CS oil immersion (NA 1.4) objective lens. The following instrumental parameters were used: excitation 405 nm (pulsed), 488 nm (Ar) and 543 nm (HeNe); emission detection bands: 430 nm – 480 nm for Hoechst (DNA counterstaining), and 500 nm – 575 nm for AlexaFluor488 or 560 nm – 630 nm (for TRITC); voxel size: 70 × 70 × 210 nm, registration in sequential mode, scanning 700 Hz, 4× line-averaged. 3D stacks of images were deconvolved and visualized as 3D projections using SVI 3D Huygens Deconvolution & Analysis Software (Scientific Volume Imaging B.V., Hilversum, Netherlands).
2.3 Transmigration and transendothelial permeability assays
HUVECs were seeded on coverslips at 2 × 104 cells/well and grown to confluence for 72 h. Thereafter, 1300 DU-145 or PC-3 cells/cm2 were seeded on a HUVEC monolayer on coverslips and incubated for 6 and 24 h, before F-actin/DNA staining and microscopic estimation of the percentage of cancer cells capable of disrupting the endothelial continuum (endothelial penetration index-EPI). For permeability studies, HUVECs were plated at 1 × 105 cells into polycarbonate Transwell inserts (3 μm pore size, 6.5 mm diameter; Corning) and nonadherent cells were removed after 6 h. Inserts were used for experiments 3 days after plating and transendothelial permeability was measured as previously described Citation[40]. Briefly, fluorescein isothiocyanate (FITC)-dextran (MW 42000; 1 mg/ml) was added to the upper compartment of Transwell after 6 h of FF and/or DU-145 cell addition in serum-free media. Samples were taken after 15 min, 30 min and 1 h from the lower compartment and equal volume of media was re-added to the lower chamber. The amount of FITC-dextran was determined with a microplate reader Infinite M200PRO (Tecan Group Ltd.), using excitation wavelength of 492 nm and emission detection at 521 nm. All inserts were fixed and then stained with eozine and phenol red after the experiment to verify the confluence and general appearance of the HUVEC monolayer.
2.4 Cell motility
The movement of HUVECs in mono- and co-cultures was time-lapse recorded using Leica DMI6000B time-lapse system equipped with a temperature chamber (37 ± 0.2°C/5% CO2), IMC contrast optics and a cooled, digital DFC360FX CCD camera. HUVECs were seeded at density of 500 cells/cm2 and cultured for 4 days to form islets (70% confluence). Then cultures were treated with FF and/or GW9662/NAC and recorded for 7 h with 5 min intervals. In the case of co-culture, 1300 DU-145 or PC-3 cells/cm2 were added to endothelial cell culture, recorded for 7 h and only HUVECs that had direct contact with cancer cells were analyzed. The tracks of individual cells were determined from a series of changes in the cell centroid positions. The data were pooled and analyzed to estimate basic cell motility parameters, including: i) total length of the cell trajectory (μm); ii) the total length of cell displacement, that is, the distance from the starting point directly to the cell’s final position (μm); iii) the average speed of cell movement, that is, total length of cell trajectory/time of recording (um/h); and iv) the average rate of cell displacement, that is, the distance from the starting point directly to the cell’s final position/time of recording (μm/h). Cell trajectories from no less than three independent experiments (number of cells > 50) were obtained for analysis by the non-parametric Mann–Whitney test Citation[41].
2.5 FACS analyses of ROS
HUVECs in mono- and co-cultures with DU-145 cells were treated with the EGM medium containing 25 μM FF and/or 10 μM GW9662/5 mM NAC, in the presence of DHR 123 (dihydorhodamine 123; 2 μM, Life Technologies) for 4 h. Subsequently, the cells were harvested, washed and suspended in PBS. Measurements of DHR 123 fluorescence intensity were carried out with a FACSCalibur flow cytometer (Becton Dickinson; excitation – 488 nm).
2.6 Immunoblotting and angioactive protein arrays
Cells for immunoblotting were dissolved in a lysis buffer, cellular proteins (20 μg/lane) were applied to 10% SDS-polyacrylamide gels, followed by transfer to a nitrocellulose membrane. Membranes were exposed to primary antibody (rabbit monoclonal anti-pSer473Akt IgG, rabbit monoclonal anti-Akt IgG, rabbit polyclonal anti-pThr202/pTyr204 Erk1/2 IgG, rabbit anti-Erk1/2 IgG, rabbit monoclonal anti-pTyrFAK IgG, rabbit monoclonal anti-Focal Adhesion Kinase [FAK] IgG (all from Cell Signalling); mouse monoclonal anti-vinculin IgG, mouse anti-α-tubulin IgG and mouse anti-actin IgG (all from Sigma)) followed by counterstaining with relevant HRP-conjugated secondary antibody (Invitrogen), their detection and semiquantification with SuperSignal West Pico Substrate (Pierce, Rockford, IL) and the MicroChemii imaging system (SNR Bio-Imaging Systems, Jerusalem, Israel) Citation[42]. Angiogenesis-related protein expression in HUVECs was estimated by semiquantitative technique based on antibody array kit (Proteome Profiler™ Human Angiogenesis Array Kit, R&D Systems) according to the manufacturer’s protocol. Samples were mixed with a cocktail of biotinylated detection antibodies and then incubated with nitrocellulose membranes to enable their binding to cognate immobilized capture antibodies, detection with streptavidin-HRP and semiquantification with the MicroChemii imaging system and Quantity One software. The signal was produced at each spot in proportion to the amount of the analyte bound. The results were expressed as fold changes above or below the relevant control.
2.7 Quantitative reverse transcription polymerase chain reaction
Total mRNA from the cells was prepared using the RNeasy Mini Kit Plus (Qiagen, Inc.) and reverse-transcribed with high capacity reverse transcription kit (Applied Biosystems). Detection of vinculin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels was performed by real-time reverse transcription polymerase chain reaction (RT-PCR) assay using 7500Fast System (Applied Biosystems). For detection of specific cDNAs, TaqMan gene expression assay was used including 6-carboxyfluorescein-labeled probes: Hs00419715 m1 (vinculin) and Hs99999905_m1 (GAPDH) (Applied Biosystems). GAPDH was used as a reference gene. Results are presented as a ΔΔCt value.
2.8 Statistical analysis
The statistical significance was determined by the Student’s t-test with p < 0.01 (transmigration, fluorimetric and viability tests) or p < 0.05 (qRT-PCR) considered to indicate significant differences; by the non-parametric Mann–Whitney test (p < 0.01; time-lapse analyses) and Wilcoxon signed-rank test (p < 0.05; permeability tests). Each parameter was calculated as the mean and standard error of the mean.
3. Results
3.1 Fenofibrate enhances endothelial barrier function to DU-145 cells
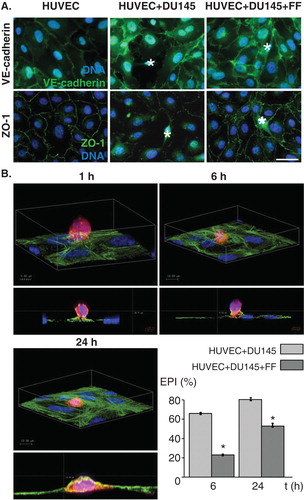
We used co-cultures of HUVECs with human prostate carcinoma DU-145 cells to estimate the effect of FF on the efficiency of DU-145 cell diapedesis. Confluent monolayers of adherent HUVECs imitate the endothelial layer at the interface between the blood and the interstitial tissues. VE-cadherin-mediated intercellular adhesion, together with ZO-1-dependent tight junctions, stabilized endothelial integrity in vitro (). Disturbance of adherents and tight junctions was observed in the proximity of DU-145 cells shortly after their seeding onto confluent endothelial cells in control conditions. Concomitantly, DU-145 cells efficiently penetrated endothelial layer as visualized and quantified by confocal microscopy (). The value of EPI estimated in control conditions increased from 66 to 79% between 6th and 24th hour of co-incubation. 25 μM FF considerably delayed transendothelial penetration of DU-145 cells (EPI = 24 and 55% after 6 and 24 h of co-incubation, respectively). This effect was accompanied by less prominent disturbances of endothelial continuum in the presence of FF ().
Figure 1. Fenofibrate inhibits penetration of endothelial layers by DU-145 cells. A. DU-145 cells (marked with *) were seeded onto the monolayer of HUVECs (98% confluence, serum-free; left) at the density of 1300 cells/cm2 in the absence (middle) or presence of 25 μM FF (right). After 6 h, specimens were fixed with 3.7% FA, permeabilized, stained for VE-cadherin (upper panel) or ZO-1 (lower panel) and counterstained with Hoechst33258. B. Representative XY and XZ reconstructions of transmigrating DU-145 cells registered 1, 6 and 24 h. after seeding in the absence of FF. DU-145 cells were stained with CellTracker, seeded and fixed as in A, stained against F-actin, counterstained with Hoechst33258 and visualized with confocal laser scanning microscopy. Transendothelial penetration indices were estimated for DU-145 cells seeded onto the HUVEC monolayer in the absence and presence of FF at the indicated time points. Statistical significance versus the relevant control at p < 0.01 (t-Student test, n = 3). Scale bar - 40 μm. Note that attenuating effect of FF on a disruption of intercellular contacts within the endothelium in the proximity of DU-145 correlates with delayed penetration of DU-145 through endothelial layers in the presence of 25 μM FF. All results are representative of three independent experiments.
3.2 Fenofibrate targets DU-145-induced HUVEC mobilization
Actin cytoskeleton determines endothelial barrier function through the effect on endothelial cell adhesion Citation[43]. Therefore, we further estimated the effect of DU-145 cells on actin cytoskeleton organisation in HUVEC. Short and scattered stress fibers attached to relatively small focal adhesions (FAs), were accompanied by cortical belts of F-actin in control HUVECs in serum-free conditions (). The seeding of DU-145 cells evoked reorganization of F-actin in proximal HUVECs, which was illustrated by the formation of prominent stress fibers and FAs. Concomitant time-lapse analyses of proximal HUVECs revealed an induction of their motility in subconfluent co-cultures with DU-145 cells (). These data demonstrate that cancer cells affect endothelial barrier function through the mobilization of endothelial cells. Noteworthy, the enhancement of endothelial barrier function by FF () was correlated with its inhibitory effect on DU-145-induced HUVEC motility (). In contrast, a synergy of FF and DU-145 effects on HUVEC cytoskeleton was illustrated by increased numbers of mature FAs and more prominent stress fibers in proximal HUVECs cultivated in the presence of FF (). Corresponding effects of FF on HUVEC mobilization by human prostate cancer PC-3 were observed (Figure S1 in supplementary data). Thus, augmentation of HUVEC adhesion to underlying extracellular matrix may reduce their susceptibility to the signals from cancer cells and improve endothelial barrier function.
Figure 2. Fenofibrate evokes cytoskeletal rearrangements and reduces DU-145-induced HUVEC motility. A. DU-145 cells (marked with *) were seeded onto the monolayer of HUVECs at the density of 1300 cells/cm2 in the absence (upper right), in the presence of 25 μM FF (lower left), or in the presence of 25 μM FF and 10 μM GW9662 (lower right). After 24 h, specimens were fixed with 3.7% FA, permeabilized, and stained for F-actin and vinculin. A number of FAs per single cell was calculated and plotted from TIRF photomicrographs. Statistical significance versus the relevant control at p < 0.01 (Student’s t-test, n = 3). B. HUVEC motility was visualized by time-lapse videomicroscopy in the conditions ascertaining their basal motility (70% confluence). Cells were analyzed in the control (serum-free) conditions (upper left), in the presence of DU-145 cells (upper right), in the presence of DU-145 cells and 25 μM FF (lower left), or in the presence of DU-145 cells, 25 μM FF and 10 μM GW9662 (lower right). Cell trajectories are depicted as circular diagrams (axis scale in µm) drawn with the initial point of each trajectory placed at the origin of the plot (registered for 7 h; n > 50). Inserts depict cell morphology visualized by IMC. Dot-plots and column chart show movement parameters of proximal cells at the single cell and population level, respectively. Statistical significance was estimated with the non-parametric Mann-Whitney test. Error bars represent SEM. Scale bar: 25 µm. Note that increased sizes and numbers of vinculin(+) FAs in FF-treated HUVECs correlate with their attenuated motility. GW9662 counteracts the effects of FF on HUVEC cytoskeleton but not on their motility. All results are representative of three independent experiments.
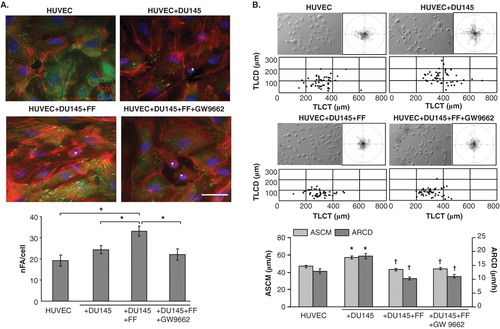
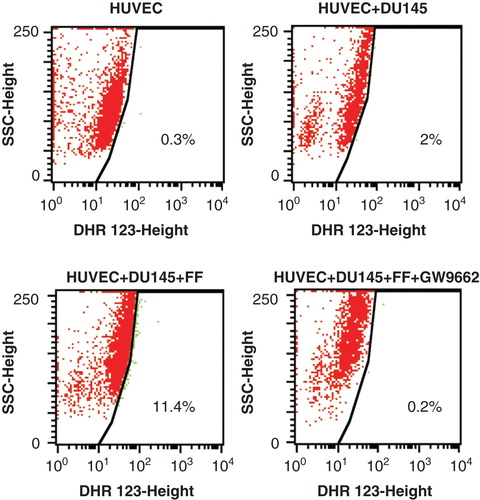
Because FF was found to exert its biological effects through the activation of PPARα/ROS-dependent signaling Citation[3], we further estimated how PPAR antagonist GW9662 influenced the effect of FF on HUVEC mobilization by DU-145 cells. GW9662 attenuated FF-induced cytoskeleton reorganization () but failed to restore FF-inhibited HUVEC motility in the co-cultures with DU-145 cells (). Similar correlation was observed in HUVEC/PC-3 co-cultures (Figure S1 in supplementary data). These effects were accompanied by ROS generation in FF-treated HUVEC/DU-145 co-cultures and downregulation of ROS levels by GW9662 (). Because 10 μM GW9662 inhibits the activity of PPARα Citation[44], our data indicate that FF attenuates DU-145-induced HUVEC mobilization at least partly through PPARα/ROS-dependent effect on the adhesive status of HUVECs. However, PPARα-independent pathways may also be involved in HUVEC reactions to FF.
Figure 3. Fenofibrate induces ROS production in HUVECs co-cultured with DU-145 cells in a PPARα – dependent manner. DU-145 cells were seeded onto the monolayer (98% of confluence; serum-free) of HUVECs at the density of 1300 cells/cm2 in the absence (upper right), in the presence of 25 μM FF (lower left), or in the presence of 25 μM FF and 10 μM GW9662 (lower right). Co-cultures were incubated in the presence of DHR123 (2 μM) for 4 h and analyzed with FACSCalibur flow cytometer. DHR123-specific signal was collected in green channel (505 nm – 560 nm). Dot-plots comprise 20,000 events gated according to SSD properties Inserts show data quantification according to the gates indicated in the plots. All results are representative of three independent experiments.
3.3 Fenofibrate improves endothelial barrier function independently of intercellular communication in the ‘metastatic niche’
FF may directly target endothelial cells or enhance their barrier function through indirect effect on the efficiency of intercellular communication loops in the metastatic niche. Paracrine loops and GJIC between cancer and endothelial cells has long been suggested to facilitate cancer cell diapedesis Citation[33,45]. Actually, FF significantly reduced GJIC intensity between DU-145 cells and HUVECs. However, this effect was not counteracted by GW9662 (). Furthermore, transient silencing of Cx43 expression in DU-145 cells attenuated their mobilizing effect on HUVEC motility but chemical block of GJIC by 18-α-glicyrrhetinic acid exerted only minute effects on HUVEC motility and transendothelial penetration of DU-145 cells (). Thus, interference of FF with GJIC is hardly responsible for its effect on HUVEC mobilization in co-cultures, nor could the augmentation of endothelial barrier function by FF be ascribed to its interference with paracrine loops in the metastatic niche. The shifts in the expression profiles of 22 out of 55 analyzed angioactive factors were seen after DU-145 cell seeding on HUVEC monolayers. Only CXCL16 expression was considerably upregulated in HUVEC/DU-145 co-cultures, whereas downregulation of anti-angiogenic endostatin and of pro-angiogenic IGFBP-2, angiopoietin-2, uPA and TGF-β1 was seen. FF attenuated the expression of pro-angiogenic IGFBP-1 and IL-8, and of anti-angiogenic TIMP-4, TIMP-1, but had no effect on the CXCL16 expression levels in co-cultures (, Figure S2 in supplementary data).
Figure 4. Communication networks within the metastatic niche do not participate in augmentation of HUVEC barrier function by fenofibrate. A. Calcein-loaded DU-145 cells were seeded onto HUVEC monolayers (98% of confluence) in the absence (upper left), in the presence of 25 μM FF (upper right) or in the presence of FF and GW9662 (lower left). The inhibitory effect of FF on GJIC in HUVEC/DU-145 co-cultures is illustrated by quantification of calcein transfer assay (coupling index, Ci, lower right; 2 h). Statistical significance versus the relevant control at p < 0.01 (t-Student test, n = 3). Scale bar: 25 µm. B. The effect of siRNA Cx43 silencing in DU-145 cells and AGA (100 μM) on EPI and HUVEC motility was compared (see legend to and ). Statistical significance was estimated with the non-parametric Mann-Whitney test (* vs HUVEC control; † vs HUVEC+DU-145; p ≤ 0.01). Error bars represent SEM. C. HUVECs were cultured in control conditions (98% of confluence), in the presence of DU-145 cells, and in the presence of DU-145 cells and 25 μM FF for 24 h. Then, expression of angioactive proteins was estimated by semiquantitive technique based on antibody array kit (see Methods). Plots show the densitometrically estimated dot intensities illustrating the protein amounts in HUVEC/DU-145 co-cultures in the absence and in the presence of 25 μM FF relative to HUVEC control. Note that FF had minute effect on the expression pattern of angiogenic factors in the co-cultures. D. HUVEC/DU-145 co-cultures were established as in C, and pSer473Akt and pThr202/pTyr204 ERK1/2 levels were visualized by immunoblotting at the indicated time points in the absence and presence of 25 μM FF. Numerical values represent results of densitometric analyses, normalized against housekeeping gene expression (α-tubulin) and FBS control and compared to control (HUVEC = 1). Note the increase of ERK1/2 and Akt phosphorylation in HUVECs in co-cultures with DU-145, and of Akt phosphorylation in the presence of 25 μM FF, respectively. Results are representative of three independent experiments.
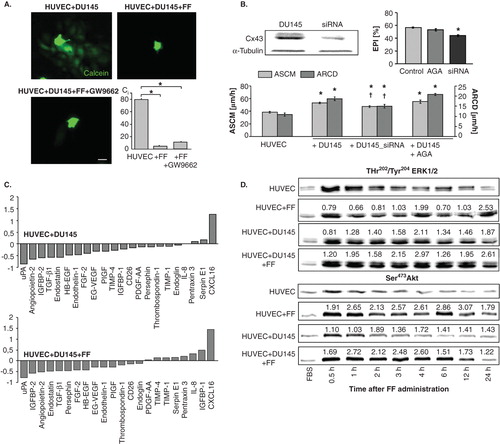
Mobilization of HUVECs and the shifts in the expression of angioactive factors were accompanied by increased extracellular signal-regulated kinase(ERK)1/2-dependent (ERK)1/2-dependent signaling activity in HUVECs co-cultured with DU-145 cells (, see also ). A pronounced and prolonged ERK1/2 and Akt phosphorylation was seen when FBS depletion was followed by DU145 cell seeding. FF slightly increased ERK1/2 phosphorylation in co-cultures but did not considerably affect ERK1/2 phosphorylation in HUVEC monolayers. In contrast, we observed increased levels of phosphorylated Akt both in FF-treated HUVEC monolayers and HUVEC/DU-145 cultures. Interestingly, the dynamics of Akt phosphorylation in HUVECs upon DU-145 seeding was more similar to control. These data indirectly indicate the involvement of Akt -dependent pathway in the augmentation of endothelial barrier function by FF.
3.4 Fenofibrate enhances endothelial barrier function through the direct effect on endothelial cell adhesion
Further analyses of HUVEC reactions to FF administered in the absence of DU-145 cells unequivocally confirmed its direct effect on endothelial cells. Akt phosphorylation () was accompanied by oscillations of FAK phosphorylation levels and correlated with up-regulation of vinculin levels in HUVECs at the protein but not mRNA level (). In conjunction with cytoskeletal rearrangements, in particular with the increased numbers of FAs in FF-treated HUVECs (), this observation is indicative of the recruitment and sequestration of vinculin to FAs. In similar to co-cultures, FF induced cytoskeletal rearrangements and accumulation of ROS in HUVECs () in a PPARα-dependent manner because both reactions were attenuated by GW9662 ( cf. B). Notably, GW9662 did not counteract inhibitory effect of FF on HUVEC motility (). These findings confirm that FF directly enhances endothelial barrier function partly via direct PPARα-dependent effects on endothelial cell adhesion.
Figure 5. Fenofibrate directly targets endothelial cell cytoskeleton and motility. A. HUVECs were cultivated in the absence or in the presence of 25 μM FF. Tyr397FAK levels and vinculin expression at protein and mRNA level were analyzed at the indicated time points by immunoblotting and qRT-PCR (quantified as described in Figure 4D). B. Cytoskeletal architecture of HUVECs cultured in control conditions (upper left), in the presence of 25 μM FF (upper right), or in the presence of 25 μM FF + 10 μM GW9662 (lower left) was visualized and the numbers of FAs per single cell quantified as in Figure 2 (lower right). C. HUVECs (98% of confluence) were cultivated in the in the presence of 25 μM FF (upper plot), or in the presence of 25 μM FF and 10 μM GW9662 (lower plot). Cells were incubated in the presence of DHR123 (2 μM) for 4 h and analyzed with FACSCalibur flow cytometer as in . D. HUVEC motility in the control conditions (70% of confluence; left), in the presence of 25 μM FF (middle left) 25 μM FF + 10 μM GW9662 (middle right) was visualized by time-lapse videomicroscopy and analyzed as in Figure 2B (right). Statistical significance was estimated with the non-parametric Mann-Whitney test (* vs HUVEC control; p ≤ 0.01). Error bars represent SEM. Results are representative of three independent experiments.
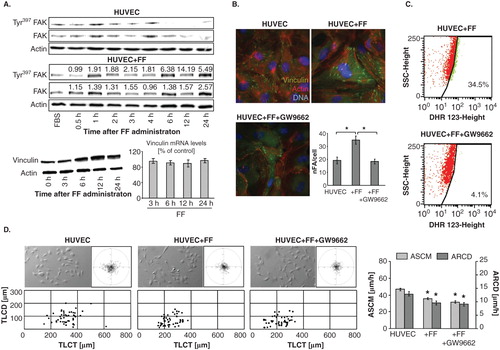
On the other hand, the lack of GW9662 effects on FF-treated HUVEC motility suggested the involvement of PPAR-independent pathways in HUVEC reactions to FF. Because we observed intracellular ROS accumulation in HUVECs after FF treatment, PPARα-independent/ROS-dependent pathways may be involved in the observed phenomena. To verify this notion, we analyzed the effect of NAC on HUVEC reactions to FF. NAC attenuated FF-induced cytoskeletal rearrangements () and significantly restored FF-inhibited HUVEC motility (). NAC strengthens cellular antioxidant defense systems Citation[46], therefore these findings would confirm an involvement of PPARα-independent/ROS-dependent signaling in the determination of HUVEC responses to FF. On the other hand, eradication of HUVEC responses to FF by NAC was accompanied by the increase of ROS levels in FF-treated cells (). Notably, FF did not influence the structure of tight junctions in HUVEC monolayers and its effect on their permeability to solutes was similar in the presence and absence of DU-145 cells (). No pronounced changes in the expression pattern of angioactive factors were seen either (). When administered at concentrations of up to 100 μM, FF prompted the maturation of focal contacts and reduced their motility in a dose-dependent fashion, but exerted no effects on their viability (; ). NAC-induced normalization of HUVEC phenotype in the presence of high ROS levels indicates the involvement of a discrete ROS pool in HUVEC-reactions to FF. Thus, ROS generation is a physiological response of endothelial cells to FF, unrelated to FF cytotoxicity.
Figure 6. PPARα-independent signaling is involved in the augmentation of endothelial barrier function by fenofibrate. A. Cytoskeletal architecture of HUVECs cultured in control conditions (left), in the presence of 25 μM FF (middle left), 25 μM FF + 5 mM NAC (middle right) is visualized and the numbers of FAs per single cell quantified as in Figure 2 (right). Statistical significance versus the relevant control at p < 0.01 (t-Student test, n = 3). B. HUVEC motility in the control conditions (70% of confluence), in the presence of 25 μM FF and 25 μM FF + 5 mM NAC was visualized by time-lapse videomicroscopy and analyzed as in Figure 2B. Statistical significance versus the relevant control at p < 0.01 (t-Student test, n = 3). C. HUVEC monolayers (98% of confluence) treated with 25 μM FF (left), or with 25 μM FF and 5 mM NAC (right) were incubated in the presence of DHR123 and analyzed with FACSCalibur flow-cytometer as in Figure 3. Note that NAC abrogates HUVEC reactions to FF but does not down-regulate intracellular ROS levels.
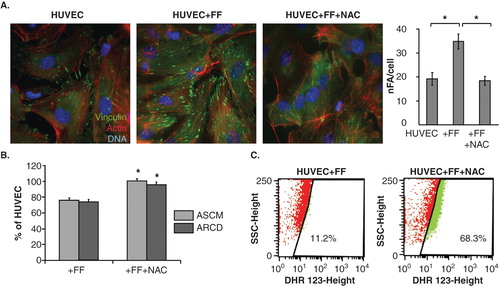
Figure 7. FF does not exert sub-lethal effects in HUVECs. A. Functional status of ZO-1-mediated intercellular contacts in control conditions (upper left), in the presence of 25 μM FF (upper right), or in the presence of 25 μM FF + 10 μM GW9662 (lower left); and the effect of DU-145 and 25 μM FF on solute permeability of HUVEC monolayers was analyzed as described (see Figure 1 and Methods, respectively). B. HUVECs were cultured in control conditions (98% of confluence) and in the presence of 25 μM FF for 24 h. Then, angioactive proteins were analyzed as in . C. HUVECs were cultivated in the presence of FF (25-100 μM) and their cytoskeleton architecture (see Figure 6A for control; upper panel) motility (middle), and viability (lower plot) was quantified. Statistical significance: * versus the relevant control at p < 0.01, using the Wilcoxon signed-rank test (A), Mann–Whitney (B) and Student t-test (C). Note the dose-dependent effects of FF on HUVEC cytoskeleton and motility but not on HUVEC viability.
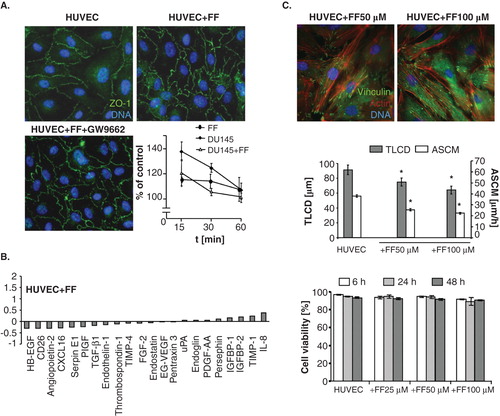
Table 1. Effect of fenofibrate on the motile activity of HUVECs.
4. Discussion
Penetration of endothelial layer by circulating cancer cells is a prerequisite for their homing in interstitial compartments underlying the endothelium. This process is crucial for the metastatic cascade Citation[47] and its efficiency depends on a number of intrinsic cancer cell traits: their motile activity and nanomechanical elasticity, competence for GJIC, expression of cell adhesion receptors and secretion of metalloproteinases Citation[34,48-50]. The interference of FF with tumor progression has predominantly been considered in terms of its effect on these properties Citation[23,26]. The susceptibility of the endothelial layer to a challenge by a single cancer cell is an equally important, yet underestimated determinant of diapedesis Citation[48,51]. FF effect on the properties of endothelial continuum in the metastatic niche has not yet been assessed. This study fills this gap because it demonstrates for the first time that FF enhances endothelial barrier function through the direct effect on endothelial cell susceptibility to the signals generated by prostate cancer cells. The influence of FF on endothelial cell behavior in the metastatic niche illustrates a new mechanism of its anti-cancer activity. It confirms previous suggestions about the interference of FF with the metastatic cascade which were based on in vivo approaches Citation[18,25]. It is noteworthy that the enhancement of endothelial barrier function to prostate cancer cells was observed in the presence of 25 μM FF. This concentration of FF in the culture medium remains within the limits defined by the content of fenofibric acid, an active FF metabolite, in the blood of patients who take FF on regular bases (up to 200 mg/day). Pharmacodynamic studies have revealed that maximal concentrations of fenofibric acid in the sera of FF-treated patients considerably exceed 25 μM Citation[52-55], confirming the biological significance of our findings.
Monitoring of endothelial cell behavior in the proximity of cancer cells gives the opportunity to reconstruct the events in the ‘metastatic niche’ of circulating cancer cells during cancer cell diapedesis. It provides a tool to evaluate the role of endothelial cell mobilization in the progress of the transendothelial penetration of cancer cells. DU-145 cell line, which was used in this study to imitate circulating prostate carcinoma cells, had been propagated from prostate cancer metastases to brain. Thus, DU-145 cells represent the progeny of cells capable of penetrating tissue barriers. The interference of FF with their intrinsic properties might thus have participated in the observed attenuation of transendothelial penetration in HUVEC/DU-145 co-cultures. Actually, we have previously shown that FF inhibits the motility and GJIC in DU-145 populations Citation[27]. Here, we observed the retraction of endothelium in the proximity of DU-145 cells, which was accompanied by induction of HUVEC motility and counteracted by FF. Interference of FF with endothelial cell mobilization by PC-3 cells (derived from prostate cancer metastasis to bone) additionally illustrates the biological significance of our data. Corresponding HUVEC responses to FF seen in the absence and presence of cancer cells show that FF effectors are situated down-stream of intercellular communication loops within the metastatic niche. FF inhibited GJIC between cancer and endothelial cells Citation[56], but this communication route plays a secondary role in HUVEC mobilization by DU-145 cells. Concomitantly, the expression of angioactive factors, which might mobilize HUVECs in the ERK1/2-dependent manner, was not affected by FF. These data indicate that FF enhances endothelial barrier function in the ‘metastatic niche’ of prostate cancer cells through a direct effect on endothelial cell properties.
PPARα/ROS-dependent pathway has previously been implicated in the inhibition of cancer cell invasive potential Citation[26]. Our observations indicate that PPARα-dependent, ROS-dependent signaling is also involved in the regulation of endothelial barrier function in the ‘metastatic niche’ by FF. This was illustrated by a partial abrogation of endothelial cell responses to FF seen in the presence of PPARα antagonist. It was accompanied by attenuation of ROS accumulation in FF-treated HUVECs. Akt and FAK phosphorylation observed in FF-treated HUVECs suggest that both effectors participate in the endothelial cell reactions to FF. Notably, it has previously been shown that endothelial cell reactions (incl. inhibition of cell motility and angiogenesis) to FF and other PPARα agonists are mediated by Akt Citation[57]. Whether Akt provides a mechanistic link between PPARα pathway and cytoskeletal rearrangements in endothelial cells requires a more elaborate study based on PPARα knock-out approach. Such an approach should also consider the interrelations between FF-induced ROS signaling in HUVEC and activation of Akt-dependent pathway.
More detailed analyses are also required to identify mechanisms underlying the abrogation of FF effect on endothelial cells by NAC. Concomitantly with the attenuation of FF-induced cytoskeletal rearrangements, NAC restored the motility of FF-treated HUVECs. It demonstrates the involvement of PPARα-independent, ROS-dependent pathway in HUVEC reactions to FF. Such a pathway has been shown to mediate FF-induced inhibition of cell motility Citation[27]. As a glutathione (GSH) precursor, NAC assists intracellular antioxidant defense systems Citation[46]. However, it failed to reduce ROS levels in FF-treated endothelial cells. In the absence of FF effects on endothelial cell viability, these observations suggest the involvement of a discrete, relatively small ROS partition, generated in a PPARα-independent fashion. Preventive effect of NAC on GSH depletion in FF-treated endothelial cells, which reduces their susceptibility to ROS signaling, may provide an alternative explanation for this conundrum. Precise identification of the specific, NAC-sensitive and NAC-insensitive ROS and their subcellular origin(s) is needed to justify further speculations on the mechanisms of ROS involvement in endothelial cell reactions to FF in vitro and in vivo.
Considering the present state of knowledge, we can again only speculate about the mechanisms of the interplay between PPARα-dependent/independent pathways and adhesion of FF-treated HUVECs Citation[12,57]. Stress fibers thickening, increased vinculin expression and FA recruitment Citation[58] in FF-treated endothelial cells are characteristic for strongly adherent isometrically contracting cells Citation[59]. We can suggest that FAK-dependent adhesive status of endothelial cells cooperates in the regulation of endothelial barrier function. Increased solute permeability of FF-treated endothelia suggests that the shifts in cell adhesion strength may evoke changes in the equilibrium between cell-to-cell and cell-substratum interactions, crucial for endothelial barrier function Citation[33]. However, local loosening of intra-endothelial fascia adherens can only be sufficient for transendothelial infiltration of ‘ameboid’ cells, such as leukocytes or monocytes. These cells are elastic enough to squeeze through relatively small endothelial discontinuities Citation[60]. In contrast, cancer cells that are characterized by ‘mesenchymal’ strategy of movement display relatively low susceptibility to mechanical distortions Citation[61]. They require more prominent endothelial remodeling to penetrate transjunctional windows. Whereas DU-145 cell motility plays a secondary role in the remodeling of endothelial continuum, the inhibition of endothelial cell motility may additionally strengthen endothelial barrier function through stabilizing effect on cell-substratum adhesion. Negative feedback loops between cell migration and adhesion strength may participate in this process. Thus, converging FF effects on cancer and endothelial cells at the ‘metastatic niche’ affect the susceptibility of the endothelial continuum to the signals from cancer cells and regulate endothelial barrier function.
5. Conclusions
The in vitro model, which imitates the interface between circulating cancer cells and endothelium Citation[34,48,62], enabled us to demonstrate the attenuating effect of FF on endothelial cell responsiveness to the signals generated by cancer cells in the metastatic niche. The strengthening effect of FF on endothelial cell adhesion and impairment of motile activity by FF reduces the efficiency of prostate carcinoma cell diapedesis and potentially interferes with the prostate cancer metastatic cascade. In the context of other in vivo studies that directly and/or indirectly suggest the interference of FF with tumor progression Citation[15,18,25], this report extends the knowledge on biological effects of existing and emerging vasoactive drugs on the metastatic cascade. It fills the gap in the understanding of the links between the vasoactive, anti-atherosclerotic and anti-tumorigenic activity of FF and suggests the application of this lipid-lowering drug as a means to reducing the metastatic spread of prostate cancer cells. It also justifies further analyses of the interference of FF with the metastatic cascade of prostate cancer and other tumors with the more sophisticated genetic in vivo approaches. Comprehensive epidemiologic studies are also postulated to estimate the links between FF intake and risk of prostate cancer metastases.
IETT_A_981153_SM1860.pdf
Download PDF (991 KB)Declaration of interest
This work was financially supported by the Polish National Science Centre (grant 2011/01/B/NZ3/00004) and funds granted to the Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University to further “The Development of Young Scientists and Doctoral Students” 2011/2012 (DS/46/2011). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union (Grants No: UDA-POIG.01.03.01-14-036/09-00 - “Application of polyisoprenoid derivatives as drug carriers and metabolism regulators”; POIG.02.01.00-12-064/08 – “Molecular biotechnology for health”; POIG 01.02-00-109/99 “Innovative methods of stem cell applications in medicine”). The authors declare that they have no competing interests. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
Bibliography
- Lamers C, Schubert-Zsilavecz M, Merk D. Therapeutic modulators of peroxisome proliferator-activated receptors (PPAR): a patent review (2008-present). Expert Opin Ther Pat 2012;22:803-41
- Ahmed W, Ziouzenkova O, Brown J, et al. PPARs and their metabolic modulation: new mechanisms for transcriptional regulation? J Intern Med 2007;262:184-98
- Grabacka M, Reiss K. Anticancer properties of PPARalpha-effects on cellular metabolism and inflammation. PPAR Res 2008;2008:930705
- Grabacka M, Pierzchalska M, Reiss K. Peroxisome proliferator activated receptor alpha ligands as anticancer drugs targeting mitochondrial metabolism. Curr Pharm Biotechnol 2013;14:342-56
- Robinson JG. LDL reduction: how low should we go and is it safe? Curr Cardiol Rep 2008;10:481-7
- Adeghate E, Adem A, Hasan MY, et al. Medicinal chemistry and actions of dual and Pan PPAR modulators. Open Med Chem J 2011;5:93-8
- Balakumar P, Rohilla A, Mahadevan N. Pleiotropic actions of fenofibrate on the heart. Pharmacol Res 2011;63:8-12
- McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs 2011;71:1917-46
- Katayama A, Yamamoto Y, Tanaka K, et al. Fenofibrate enhances neovascularization in a murine ischemic hindlimb model. J Cardiovasc Pharmacol 2009;54:399-404
- Hiukka A, Maranghi M, Matikainen N, et al. PPARalpha: an emerging therapeutic target in diabetic microvascular damage. Nat Rev Endocrinol 2010;6:454-63
- Noonan JE, Jenkins AJ, Ma JX, et al. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013;62:3968-75
- Goetze S, Eilers F, Bungenstock A, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun 2002;293:1431-7
- Varet J, Vincent L, Mirshahi P, et al. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci 2003;60:810-19
- Meissner M, Stein M, Urbich C, et al. PPARalpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ Res 2004;94:324-32
- Panigrahy D, Kaipainen A, Huang S, et al. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA 2008;105:985-90
- Cao Z, Shang B, Zhang G, et al. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta 2013;1836:273-86
- Hida K, Ohga N, Akiyama K, et al. Heterogeneity of tumor endothelial cells. Cancer Sci 2013;104:1391-5
- Robison NJ, Campigotto F, Chi SN, et al. A phase II trial of a multi-agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr Blood Cancer 2014;61:636-42
- Langley RR, Fidler IJ. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 2011;128:2527-35
- Giordano A, Macaluso M. Fenofibrate triggers apoptosis of glioblastoma cells in vitro: new insights for therapy. Cell Cycle 2012;11:3154
- Jiao HL, Zhao BL. Cytotoxic effect of peroxisome proliferator fenofibrate on human HepG2 hepatoma cell line and relevant mechanisms. Toxicol Appl Pharmacol 2002;185:172-9
- Yamasaki D, Kawabe N, Nakamura H, et al. Fenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARalpha-independent mechanisms. Eur J Cell Biol 2011;90:657-64
- Urbanska K, Pannizzo P, Grabacka M, et al. Activation of PPARalpha inhibits IGF-I-mediated growth and survival responses in medulloblastoma cell lines. Int J Cancer 2008;123:1015-24
- Grabacka M, Plonka PM, Urbanska K, et al. Peroxisome proliferator-activated receptor alpha activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin Cancer Res 2006;12:3028-36
- Grabacka M, Placha W, Plonka PM, et al. Inhibition of melanoma metastases by fenofibrate. Arch Dermatol Res 2004;296:54-8
- Drukala J, Urbanska K, Wilk A, et al. ROS accumulation and IGF-IR inhibition contribute to fenofibrate/PPARalpha -mediated inhibition of Glioma cell notility in vitro. Mol Cancer 2010;9:159
- Wybieralska E, Szpak K, Gorecki A, et al. Fenofibrate attenuates contact-stimulated cell motility and gap junctional coupling in DU-145 human prostate cancer cell populations. Oncol Rep 2011;26:447-53
- Thuillier P, Anchiraico GJ, Nickel KP, et al. Activators of peroxisome proliferator-activated receptor-alpha partially inhibit mouse skin tumor promotion. Mol Carcinog 2000;29:134-42
- Saidi SA, Holland CM, Charnock-Jones DS, et al. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Mol Cancer 2006;5:13
- Panigrahy D, Kaipainen A, Kieran MW, et al. PPARs: a double-edged sword in cancer therapy? PPAR Res 2008;2008:350351
- Scatena R, Bottoni P, Giardina B. Mitochondria, PPARs, and cancer: is receptor-independent action of PPAR agonists a key? PPAR Res 2008;2008:256251
- Tomizawa A, Hattori Y, Inoue T, et al. Fenofibrate suppresses microvascular inflammation and apoptosis through adenosine monophosphate-activated protein kinase activation. Metabolism 2011;60:513-22
- Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013;13:858-70
- Wang X, Ferreira AM, Shao Q, et al. Beta3 integrins facilitate matrix interactions during transendothelial migration of PC3 prostate tumor cells. Prostate 2005;63:65-80
- Woodward J. Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell Adh Migr 2008;2:151-2
- Strell C, Niggemann B, Voss MJ, et al. Norepinephrine promotes the beta1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROalpha release. Mol Cancer Res 2012;10:197-207
- Szpak K, Wybieralska E, Niedzialkowska E, et al. DU-145 prostate carcinoma cells that selectively transmigrate narrow obstacles express elevated levels of CX43. Cell Mol Biol Lett 2011;16:625-37
- Guan K, Czyz J, Furst DO, et al. Expression and cellular distribution of alpha(v)integrins in beta(1)integrin-deficient embryonic stem cell-derived cardiac cells. J Mol Cell Cardiol 2001;33:521-32
- Ryszawy D, Sarna M, Rak M, et al. Functional links between Snail-1 and Cx43 account for the recruitment of Cx43-positive cells into the invasive front of prostate cancer. Carcinogenesis 2014;35:1920-30
- Wojciak-Stothard B, Potempa S, Eichholtz T, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001;114:1343-55
- Miekus K, Czernik M, Sroka J, et al. Contact stimulation of prostate cancer cell migration: the role of gap junctional coupling and migration stimulated by heterotypic cell-to-cell contacts in determination of the metastatic phenotype of Dunning rat prostate cancer cells. Biol Cell 2005;97:893-903
- Daniel-Wojcik A, Misztal K, Bechyne I, et al. Cell motility affects the intensity of gap junctional coupling in prostate carcinoma and melanoma cell populations. Int J Oncol 2008;33:309-15
- Quadri SK. Cross talk between focal adhesion kinase and cadherins: role in regulating endothelial barrier function. Microvasc Res 2012;83:3-11
- Leesnitzer LM, Parks DJ, Bledsoe RK, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002;41:6640-50
- Czyz J, Szpak K, Madeja Z. The role of connexins in prostate cancer promotion and progression. Nat Rev Urol 2012;9:274-82
- Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 2014;141:150-9
- van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 2011;728:23-34
- Pollmann MA, Shao Q, Laird DW, et al. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res 2005;7:R522-34
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011;147:992-1009
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol 2012;24:277-83
- Garcia-Roman J, Zentella-Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett 2013;335:259-69
- Doser K, Guserle R, Nitsche V, et al. Comparative steady state study with 2 fenofibrate 250 mg slow release capsules. An example of bioequivalence assessment with a highly variable drug. Int J Clin Pharmacol Ther 1996;34:345-8
- Kajosaari LI, Backman JT, Neuvonen M, et al. Lack of effect of bezafibrate and fenofibrate on the pharmacokinetics and pharmacodynamics of repaglinide. Br J Clin Pharmacol 2004;58:390-6
- Uetake D, Ohno I, Ichida K, et al. Effect of fenofibrate on uric acid metabolism and urate transporter 1. Intern Med 2010;49:89-94
- Hu L, Wu H, Niu F, et al. Design of fenofibrate microemulsion for improved bioavailability. Int J Pharm 2011;420:251-5
- Mroue RM, El Sabban ME, Talhouk RS. Connexins and the gap in context. Integr Biol (Camb) 2011;3:255-66
- Okayasu T, Tomizawa A, Suzuki K, et al. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci 2008;82:884-91
- Alexander JS, Zhu Y, Elrod JW, et al. Reciprocal regulation of endothelial substrate adhesion and barrier function. Microcirculation 2001;8:389-401
- Deguchi S, Sato M. Biomechanical properties of actin stress fibers of non-motile cells. Biorheology 2009;46:93-105
- Yuan SY, Shen Q, Rigor RR, et al. Neutrophil transmigration, focal adhesion kinase and endothelial barrier function. Microvasc Res 2012;83:82-8
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Exp Med 2010;207:11-19
- Nevo I, Sagi-Assif O, Meshel T, et al. The involvement of the fractalkine receptor in the transmigration of neuroblastoma cells through bone-marrow endothelial cells. Cancer Lett 2009;273:127-39
Supplementary materials are available online.
Figures S1 and S2.

