Abstract
The incidence of type 2 diabetes mellitus (T2DM) is increasing worldwide, and certain population subgroups are especially vulnerable to the disease. To reduce T2DM risk and progression at the population level, preventative strategies are needed that can be implemented on a population-wide scale with minimal cost and effort. Chronic low-grade inflammation resulting from oxidative stress and imbalances in the innate immune system has been associated with obesity, metabolic syndrome, and insulin resistance – critical stages in the development and progression of T2DM. Therefore, inflammation may play a causal role in the pathogenesis of T2DM, and reducing it via modulation of oxidative stress and the innate immune response could lead to a status of improved insulin sensitivity and delayed disease onset. Dietary supplementation with anti-inflammatory and antioxidant nutritional factors, such as micronutrients, might present a novel strategy toward the prevention and control of T2DM at the population level. This review examines current knowledge linking oxidation, inflammatory signaling pathways, and vitamin supplementation or intake to the risk of T2DM. The concept that micronutrients, via attenuation of inflammation, could be employed as a novel preventive measure for T2DM is evaluated in the context of its relevance to public health.
Introduction
Type 2 diabetes mellitus (T2DM) has reached global epidemic proportions. The disease affects over 150 million people worldwide, a number that is expected to double by 2025.Citation1 It is estimated that six people die every minute from T2DM globally, a figure that will soon make the disease one of the world’s most prevalent causes of preventable mortality.Citation2 Rates of disease incidence increase with age, obesity, and a sedentary lifestyle, are elevated in certain ethnicities (Hispanics, Africans, and Aboriginals), and have been increasingly noted in children.Citation3,Citation4
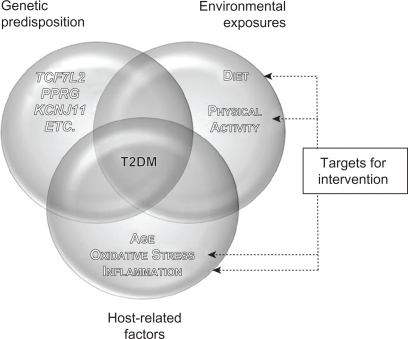
T2DM is a multifactorial disease characterized by chronic hyperglycemia, altered insulin secretion, and insulin resistance – a state of diminished responsiveness to normal concentrations of circulating insulin.Citation5,Citation6 T2DM is also defined by impaired glucose tolerance (IGT) that results from islet β-cell dysfunction, followed by insulin deficiency in skeletal muscle, liver, and adipose tissues.Citation7,Citation8 In individuals with IGT, the development of T2DM is governed by genetic predisposition and environmental variables (a hypercaloric diet and the consequent visceral obesity or increased adiposity in liver and muscle tissues) and host-related factors (age, imbalances in oxidative stress, and inflammatory responses)Citation1,Citation5,Citation9–Citation16 (). Clinical complications of T2DM include both microvascular diseases (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, myocardial infarction, peripheral vascular disease, and stroke).Citation5 The macrovascular diseases are considered to be the leading cause of mortality among diabetics.Citation17
Figure 1 Interaction between genetic predisposition, environment, and host-related factors affecting T2DM and potential areas of focus for intervention. The development of T2DM results from the interaction between genetic predisposition, environmental exposures, and various host-related factors. single-nucleotide polymorphisms within several genes, such as TCF7L2, PPARG, and KCNJ11, have been associated with T2DM risk via candidate gene studies and genome-wide associations. Lack of physical activity and a hypercaloric diet, with the resulting visceral obesity and increased adiposity in liver and muscle tissue, are associated with T2DM risk as well. Finally, host-related factors such as age, oxidative stress, and chronic inflammation also play a role in the development of the disease. Therapeutic interventions aimed at modifying lifestyle and/or levels of oxidative stress and chronic inflammation may aid in T2DM prevention and control.
A significant body of evidence highlights the key role of abnormal innate immune responses and chronic low-grade inflammation in the pathogenesis of insulin resistance and the development of T2DM.Citation9,Citation18–Citation20 Inflammation results from the activation of the innate immune response – the body’s immediate, nonspecific reaction against environmental insults such as pathogens and chemical or physical injury.Citation9 Inflammation plays a role in prevention of tissue damage, restoration of tissue homeostasis, and destruction of infectious agents.Citation9,Citation21 It is the result of the acute phase response, a systemwide process during which several proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, are released, primarily by macrophages.Citation22 These cytokines can enhance insulin resistance directly in adipocytes, muscle, and hepatic cells,Citation23,Citation24 leading to systemic disruption of insulin sensitivity and impaired glucose homeostasis.Citation25 Increased levels of proinflammatory cytokines lead to hepatic production and secretion of acute-phase proteins such as C-reactive protein (CRP), plasminogen activator inhibitor-1 (PAI-1), amyloid-A, α1-acid glycoprotein, and haptoglobin. These proteins, collectively known as inflammatory markers, appear in the early stages of T2D, and their circulating concentrations increase as the disease progresses.Citation20,Citation26
Chronic inflammation is closely associated with oxidative stress, an exaggerated presence of highly reactive molecular species, which leads to potential tissue damage.Citation10,Citation27 Oxidative stress results either from an increase in free radical production, a decrease in endogenous antioxidant defenses, or both.Citation5,Citation28,Citation29 Obesity and the metabolic syndrome, conditions which are considered key steps in the progression of insulin resistance to T2DM, are associated with both oxidative stress and inflammation.Citation8,Citation30,Citation31 Hyperglycemia, increases in plasma levels of free fatty acids (FFAs), and hyperinsulinemia have all been linked to increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS).Citation10,Citation32–Citation36 ROS and RNS activate nuclear factor κB (NFκB), a proinflammatory transcription factor, that triggers a signalling cascade leading to a continued synthesis of oxidative species and the low-grade chronic inflammation.Citation37–Citation39
Given that obesity and the metabolic syndrome are steps toward the development of T2DM, their link with oxidative stress and inflammation points to a potential causative role for these factors in the progression of the disease.Citation8 Thus, aiming to reduce oxidative imbalances and inflammation could lead to improved insulin sensitivity and delayed disease onset. Measures that prevent the development of oxidative stress and inflammation may present a feasible strategy for T2DM prevention and control. Dietary intake of micronutrients has been associated with reduced levels of oxidative stress, proinflammatory cytokines, and risk of T2DM in various cross-sectional and interventional studies,Citation8,Citation40–Citation45 an approach that may facilitate the development of novel strategies for the prevention of T2DM.
This review was undertaken in an attempt to examine current knowledge linking micronutrients intake to oxidation and inflammatory signaling pathways in the pathogenesis of T2DM. The possibility that attenuating oxidative stress and inflammation by micronutrients can be employed as a novel approach for the prevention of T2DM is evaluated from the public health perspective.
Oxidative stress, inflammation, and T2DM
Oxidation is a chemical process whereby electrons are removed from molecules and highly reactive free radicals are generated.Citation40 Free radicals include ROS such as superoxide and hydroperoxyl and RNS such as nitric oxide and nitrogen dioxide.Citation5,Citation10,Citation17 Reactive species arise as natural by-products of aerobic metabolism, and they play a role in numerous signaling cascades and physiological processes, such as phagocytosis, vasorelaxation, and neutrophil function.Citation5,Citation17,Citation46,Citation47 However, excessive oxidation can trigger cytotoxic chain reactions that are damaging to membrane lipids, proteins, nucleic acids, and carbohydrates.Citation17,Citation27,Citation46,Citation48 Therefore, the capacity of serum to control production of free radicals is defined as the ‘total antioxidant status’.
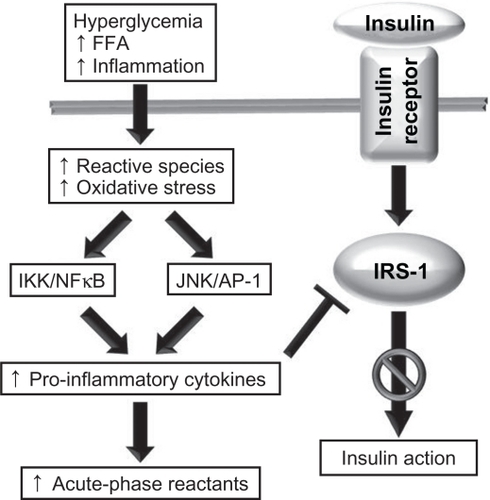
The signaling transduction role of ROS stems from their ability to activate a number of stress-sensitive kinases whose downstream effects mediate insulin resistance.Citation10 Activation of these kinases upregulates and activates NFκB and activator protein-1 (AP-1),Citation49 which subsequently (a) activates c-Jun N-terminal kinase (JNK) and inhibitor of NFκB kinase-β (IKK), (b) transcriptionally upregulates proinflammatory cytokine genes,Citation22 and (c) increases the synthesis of acute-phase reactants.Citation50–Citation52 This molecular cascade reduces the downstream signaling elicited by insulin through dysregulation of the insulin receptor (IR) substrate-1 (IRS-1), the primary molecular target of IRCitation53,Citation54 (). Concurrently, the ensuing inflammation leads to an enhanced production of reactive oxidant species, further tipping the balance in favor of elevated oxidative stress and NFκB-mediated proinflammatory pathways.Citation5,Citation37,Citation47 Because the JNK–AP-1 and IKK–NFκB axes are the major inflammatory pathways that disrupt insulin signaling, modulating their action with anti-oxidant or anti-inflammatory factors is believed to improve insulin sensitivity and glucose homeostasis.Citation24
Figure 2 The role of oxidative stress and inflammation in insulin resistance. A number of stimuli, such as hyperglycemia, high levels of circulating free fatty acid (FFA), and chronic inflammation, lead to increases in the production of reactive molecular species, and this in turn may lead to oxidative stress. Oxidants activate the JNK/AP-1 and IKK–NFκB axes, leading to an upregulation in the transcription of proinflammatory cytokine genes and increased production of cytokines and acute-phase reactants. Cytokines impair the action of the insulin receptor substrate, resulting in impaired insulin action.
A number of studies have highlighted a direct link between oxidative stress and diabetes through the measurement of markers of oxidative stress (eg, plasma and urinary F2-isoprostanes and plasma and tissue levels of nitrotyrosine and superoxide).Citation17,Citation55–Citation59 Oxidative stress in diabetes arises from various pathways, including nonenzymatic, enzymatic, and mitochondrial processes. Hyperglycemia modifies the redox balance through the polyol pathway (where glucose is reduced to sorbitol, with subsequent decreases in levels of NADPH and reduced glutathione), activates oxidases, and interferes with the mitochondrial electron transport chain.Citation35,Citation60–Citation63 These processes generate by-products that can trigger various signaling cascades, for example activation of protein kinase C to further increase the synthesis of reactive oxidative species.Citation35,Citation62,Citation64 Nonenzymatically, glucose autoxidation generates hydroxyl radicalsCitation65 and leads to the formation of advanced glycation end products that influence the transcription of proinflammatory genes to promote further oxidative stress.Citation63,Citation66 In healthy subjects, hyperglycemia has been also associated with oxidative stress, as measured through plasma levels of nitrotyrosine.Citation67
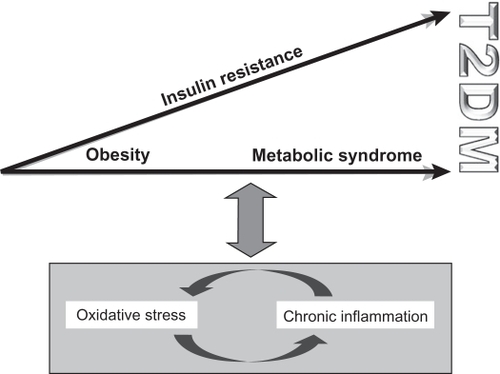
Oxidative stress and chronic inflammation are closely linked via positive feedback mechanisms. Both factors are associated with obesity and a range of metabolic syndromes (). High circulating FFA levels, which are characteristic of obesity and T2DM, influence oxidative stress via β-oxidative phosphorylation in mitochondria.Citation5,Citation6,Citation35,Citation38,Citation62,Citation68 In fact, plasma levels of the antioxidant glutathione have been shown to decrease by FFA infusion.Citation62,Citation69 Furthermore, levels of malondialdehyde (MDA), a marker of oxidative stress, and expression of NFκB are elevated in insulin-resistant states when hyperglycemia is absent in vascular, adipose, and muscular tissues.Citation62,Citation69,Citation70
Figure 3 The interaction between oxidative stress, chronic inflammation, and the progression toward T2DM. Oxidative stress and chronic inflammation are closely linked via positive feedback mechanisms and are both associated with obesity and the metabolic syndrome.
Reactive species can play a role directly in insulin sensitivity, secretion, and action in both animal and human models.Citation33,Citation71–Citation73 For example, nondiabetic rats that were infused with high levels of glucose and administered either of two antioxidants, that is, N-acetylcysteine or taurine, did not develop insulin resistance despite being hyperglycemic, suggesting that oxidative stress may play a role in glucose-induced insulin resistance and that this effect can be prevented by antioxidative factors.Citation74 Oxidative stress has also been noted to coexist with insulin resistance in patients with T2DM,Citation75,Citation76 in obese subjects, and at various stages of the metabolic syndrome.Citation10 For example, insulin resistance has been noted in obese women with reduced total antioxidant statusCitation30 and in men with plasma levels of 8-epi-prostaglandin F2α (PGF2α), a marker for lipid peroxidation.Citation31 Furthermore, suboptimal concentrations of circulating antioxidantsCitation77 and elevated levels of several markers of oxidative stress (eg, MDA, homocysteine, and ceruloplasmin) were found in subjects with metabolic syndrome.Citation78
The effects of reactive oxidative species can be modified by enzymatic action (eg, superoxide dismutase, thioredoxin, catalase, and glutathione peroxidase)Citation5 and/or by nonenzymatic antioxidants, for example, vitamins (A, C, E, and B), folate, glutathione, coenzyme Q10, α-lipoic acid (LA), carotenoids, flavonoids, and trace elements (Cu, Zn, Mg, and Se).Citation17,Citation79 Antioxidants often work in synergy with target specific reactive species,Citation79 and through their ameliorating effects on oxidative stress, they also attenuate inflammation at the molecular level.
Micronutrients in T2DM: attenuation of oxidative stress and inflammation
Factors that attenuate oxidative stress and proinflammatory cascades could provide an important public health tool to reduce the burden of chronic diseases linked to these conditions, such as obesity, T2DM, and cardiovascular diseases. A number of reports have evaluated the efficacy of anti-inflammatory and antioxidant pharmaceutical agents on manifestation and outcome of T2DM and cardiovascular disease (see below). For example, studies in animal models and humans have demonstrated that disrupting the IKKβ–NFκB pathway improves obesity-related insulin sensitivity.Citation80 Blocking JNK activity in diabetic animals improved systemic glucose homeostasis and insulin sensitivity,Citation81 whereas inhibition of IKK by salicylates led to enhanced insulin action.Citation82
The major current therapeutic agents to treat T2DM, sulfonylureas, metformin, and insulin-sensitizing glitazones, all have been associated with normalization of circulating inflammation and oxidative markers.Citation12–Citation14,Citation83–Citation85 This effect may be mediated, at least partly, by the action of the therapeutic agents on the innate immune system to retain the homeostasis of the oxidative and inflammatory status. Accordingly, glitazones (peroxisome proliferator-activated receptor-γ [PPAR-γ] agonists) improved the activities of superoxide dismutase and catalase and glutathione peroxidase in the lung tissue of hyperglycemic rabbits.Citation12,Citation86 Treatment of these animals with repaglinide, an antioxidant often used in T2DM treatment, resulted in higher glutathione levels and normalization of nitrotyrosine.Citation83 Similarly, several therapeutic agents prescribed in T2DM, such as metformin and sulfonylureas, exhibit antioxidant and anti-inflammatory activity, an effect that may be partially related to their therapeutic efficacy.Citation13,Citation14,Citation84,Citation85 These observations provide an additional line of evidence to emphasize that targeting the inflammation-related and oxidation-related pathways can provide a feasible approach in the prevention of T2DM.
Micronutrient and/or trace element supplementation can modify oxidative stress and innate immune-related responses and, subsequently, reduce the burden of a range of chronic conditions. A recent study showed an interdependent and inverse association between total antioxidant capacity of the diet and CRP serum levels in nondiabetic subjects.Citation87 This observation suggests the value of using nutritional supplementation to modulate inflammation and oxidative stress in a population-based setting. With respect to T2DM, the consensus of available information suggests that micronutrient intake modulates oxidative stress and the innate immune systemCitation88,Citation89 to subsequently influence the predisposition to (and prevention of) disease.Citation88,Citation90,Citation91 Therefore, it is possible to monitor the outcome of nutritional supplementation simply by evaluating its modifying action on the levels of inflammatory biomarkers.
Many micronutrients exhibit well-characterized anti-inflammatory or immunomodulatory functions (see below). Vitamins (eg, D, E, and C) and trace elements (eg, Se, Zn, Cu, and Fe) are known to improve the overall function of the immune system, prevent excessive expression of inflammatory cytokines, and increase the ‘oxidative burst’ potential of macrophages.Citation88 Natural health products (NHPs) that contain pertinent micronutrients (eg, so-called adaptogenic medicinal plants) or modulate the innate immune response (eg, omega-3 fatty acids and probiotics) can also be explored for their preventive efficacy in chronic diseases despite their controversial benefits.Citation92 Dietary intake of omega-3 fatty acids is known to inhibit the production of proinflammatory cytokines, including IL-1β and TNF-α.Citation93 Furthermore, supplementation with Allium sativum (garlic), Curcuma longa (long turmeric), Panax quinquefolius (American ginseng), and Panax ginseng (Asian ginseng), selected candidates of a large group of antioxidant, anti-inflammatory, and adaptogenic plants, was reported to downregulate the oxidative stress and the synthesis of proinflammatory cytokines, effects related to their overall action on enhancing the innate immune response.Citation94–Citation98 Similarly, some trace elements (eg, Zn) could play a role in preventing T2DM by regulating dysglycemia and decreasing insulin insensitivity. For example, it is well known that T2DM can be accompanied by a slow loss of intracellular Zn and hyperzincuria.Citation99 Supplementation with Zn, therefore, has been shown to lower oxidative stress-related byproducts and to attenuate the synthesis of TNF-α and IL-1β.Citation100–Citation103 This observation may substantiate an antidiabetes action for Zn, and perhaps other trace elements, via its antioxidative and anti-inflammatory characteristics.
Exploring the possibility that supplementation with selected micronutrients, trace elements, and/or NHPs can attenuate inflammation and, subsequently, delay the onset of T2DM should be considered alongside existing public health practices to reduce the rising incidence of the disease. Here, we review the evidence for immunomodulatory, antioxidant, and anti-inflammatory effects of specific micronutrients, namely vitamins D, C, and E, and their overall effect on the prevention of T2DM and the related metabolic syndromes.
Vitamin D
The role of vitamin D in calcium and phosphorous homeostasis and bone metabolism is well understood. However, more recently, vitamin D and calcium homeostasis have also been linked to a number of conditions, such as neuromuscular function, cancer, and a wide range of chronic diseases, including autoimmune diseases, atherosclerosis, obesity, cardiovascular diseases, diabetes, and associated conditions such as the metabolic syndrome and insulin resistance.Citation8,Citation91,Citation104,Citation105 In T2DM, the role of vitamin D was suggested from the presence of vitamin D receptors (VDR) in the pancreatic β-islet cells.Citation106 In these cells, the biologically active metabolite of vitamin D (ie, 1,25-dihydroxy-vitamin D; 1,25(OH)2D)Citation88 enhances insulin production and secretion via its action on the VDR.Citation106 Indeed, the presence of vitamin D binding protein (DBP), a major predictor of serum levels of 25(OH) D and response to vitamin D supplementation,Citation107,Citation108 and VDR initiated several studies demonstrating a relationship between single-nucleotide polymorphisms (SNPs) in the genes regulating VDR and DBP and glucose intolerance and insulin secretion.Citation109–Citation111 This further supports a role for vitamin D in T2DM and may explain the reduced overall risk of the disease in subjects who ingest >800 IU/day of vitamin D.Citation91,Citation112 However, an alternative, perhaps related, explanation was recently proposed for the role of vitamin D in the prevention of T2DM based on its potent immunomodulatory functions.Citation113–Citation115 1,25(OH)2D modulates the production of the immunostimulatory IL-12 and the immunosuppressive IL-10,Citation116 and VDRs are present in most types of immune cells.Citation117 In this respect, supplementation with vitamin DCitation118 or its bioactive form, 1,25(OH)2D,Citation88 improved insulin sensitivity by preventing the excessive synthesis of inflammatory cytokines. This effect of vitamin D on cytokine synthesis is due to its interaction with vitamin D response elements present in the promoter region of cytokine-encoding genes. This interaction downregulates the transcriptional activities of cytokine genes and attenuates the synthesis of the corresponding proteins.Citation118 Vitamin D also deactivates NFκB, which transcriptionally regulates the proinflammatory cytokine-encoding genes.Citation119 Downregulating the expression of NFκB and downstream cytokine genes inhibits β-cell apoptosis and promotes their survival.Citation118
As reviewed by Pittas et al,Citation91,Citation120 a number of cross-sectional studies in both healthy and diabetic cohorts have shown an inverse association between serum 25(OH)D and glycemic status measures such as fasting plasma glucose, oral glucose tolerance tests, hemoglobin A1c (HbA1c), and insulin resistance as measured by the homeostatic model assessment (HOMA-R), as well as the metabolic syndrome.Citation3,Citation121–Citation125 For example, data from the National Health and Nutrition Examination Survey showed an inverse, dose-dependent association between serum 25(OH)D and diabetes prevalence in non-Hispanic whites and Mexican Americans, but not in non-Hispanic blacks.Citation122,Citation125 The same inverse trend was observed between serum 25(OH)D and insulin resistance as measured by HOMA-R, but there was no correlation between serum levels of vitamin D and β-cell function, as measured by HOMA-β.Citation122,Citation125 Data from the same cohort also showed an inverse association between serum 25(OH)D and prevalence of the metabolic syndrome.Citation122
In prospective studies, dietary vitamin D intake has been associated with incidence of T2DM. For example, data from the Women’s Health Study showed that among middle-aged and older women, taking >511 IU/day of vitamin D reduced the risk of developing T2DM compared to ingesting 159 IU/day.Citation126 Furthermore, data from the Nurses Health Study also found a significant inverse correlation between total vitamin D intake and T2DM risk, even after adjusting for BMI, age, and nondietary covariates.Citation112 Intervention studies have shown conflicting results about the effect of vitamin D on T2DM incidence. One study reported that supplementation with 1,25(OH)2D for 1 week did not affect fasting glucose or insulin sensitivity in 18 young, healthy men.Citation127 Another study found that among 14 T2DM patients, supplementing with 80 IU/day of 1,25(OH)2D ameliorated insulin secretion but did not improve glucose tolerance after a 75-g oral load.Citation128 Yet another study showed that among 65 middle-aged men who had IGT or mild T2DM and adequate serum vitamin D levels at baseline, supplementation with 30 IU/day of 1,25(OH)2D for 3 months affected neither fasting nor stimulated glucose tolerance.Citation129 However, in a crossover design, 20 diabetics with inadequate vitamin D serum levels who were given 40 IU/day of 1,25(OH)2D for 4 days had improved insulin secretion, but showed no changes in fasting or stimulated glucose or insulin concentrations.Citation130 Although the short duration of this crossover trial may account for the lack of a significant overall effect, the results suggest that improving vitamin D status can modulate factors associated with the development and progression of T2DM.
The data from a 2-year-long trial designed to assess the effects of vitamin D or 1,25(OH)2D supplementation on bone health in nondiabetic postmenopausal women were analyzed a posteriori and found no significant effect on fasting glucose levels.Citation131 A post hoc analysis of data from a 3-year-long trial for bone health showed that daily supplementation with 700 IU of vitamin D3 and 500 mg of calcium citrate malate did not change blood glucose levels or insulin resistance in elderly adults with normal glucose tolerance. These measures, however, were significantly improved in subjects with IGT at baseline.Citation90 In this trial, the effect of fasting glucose levels in the high-risk group (ie, IGT) was similar to that observed in the Diabetes Prevention Program after either an intensive lifestyle intervention or metformin treatment.Citation132 Another recent randomized, controlled trial found that daily supplementation with 400 IU of vitamin D3 for 6 months in insulin-resistant, nondiabetic, vitamin D-deficient South Asian women living in New Zealand resulted in improved insulin resistance and sensitivity.Citation133 Taken together, the available information warrants exploring the possibility that vitamin D, either alone or in combination with calcium supplementation, can be employed in developing population-based strategies for T2DM prevention and control.
Vitamin C
Vitamin C (ascorbic acid or ascorbate) is the primary hydrophilic antioxidant found in human plasma.Citation134 Absorption of vitamin C occurs in the small intestine via active transport through the sodium-dependent vitamin C transporter type 1 (SVCT1). SVCT1 also appears in the renal proximal tubules, where it reabsorbs filtered ascorbate and is expressed throughout the body.Citation135 Circulating concentrations of ascorbate in blood are considered adequate if it is at least 28 μM, but they are considerably higher in most cells due to active transport by SVCT2. The daily recommended dietary allowance for vitamin C is 75 mg for women and 90 mg for men, with an additional 35 mg for smokers due to the higher metabolic turnover of the vitamin in this group compared to nonsmokers.Citation37 Ascorbate appears in the urine at intakes of roughly 60 mg/day. However, in a depletion/repletion study in healthy young women, ascorbate plasma and white blood cell concentrations saturated at intakes of 200 mg/day or higher.Citation136 These results suggest that for some individuals, the current dietary recommendations may not provide tissue-saturating ascorbate concentrations.Citation135 Recent epidemiologic findings suggest that serum ascorbic acid deficiency may be relatively common. For example, a recent cross-sectional survey of healthy young adults of the Toronto Nutrigenomics and Health (TNH) Study reported that one out of seven individuals is deficient in serum ascorbic acid.Citation137
Vitamin C has an important role in immune function and various oxidative/inflammatory processes, such as scavenging ROS and RNS, preventing the initiation of chain reactions that lead to protein glycation,Citation37,Citation40 and protecting against lipid peroxidation.Citation37,Citation138 The oxidized products of vitamin C, ascorbyl radical and dehydroascorbic acid, are easily regenerated to ascorbic acid by glutathione, NADH, or NADPH.Citation37 In addition, ascorbate can recycle vitamin E and glutathione back from their oxidized forms.Citation37,Citation135 For this reason, there has been interest in determining whether vitamin C might be used as a therapeutic agent against the oxidative stress and subsequent inflammation associated with T2DM.
A variety of epidemiologic studies have assessed the effect of vitamin C on biomarkers of oxidation, inflammation, and/or T2DM risk.Citation134,Citation139–Citation144 A large cross-sectional evaluation of healthy elderly men from the British Regional Heart Study reported that plasma vitamin C, dietary vitamin C, and fruit intake were inversely correlated with serum CRP and tissue plasminogen activator (tPA), a biomarker of endothelial dysfunction.Citation134 However, only plasma vitamin C was inversely associated with fibrinogen levels.Citation134 Another cross-sectional study of adolescents aged 13–17 found inverse associations between intake of fruit, vegetables, legumes, and vitamin C and urinary F2-isoprostane, CRP, and IL-6.Citation145 A recent cross-sectional evaluation of healthy young adults from the TNH Study demonstrated that serum ascorbic acid deficiency is associated with elevated CRP and other factors related to the metabolic syndrome such as waist circumference, BMI, and blood pressure.Citation137 Finally, the European Prospective Investigation of Cancer-Norfolk Prospective Study examined the association between fruit and vegetable intake and plasma levels of vitamin C and risk of T2DM. During a 12-year follow-up, 735 incident cases of diabetes were identified among nearly 21,000 participants. A significant inverse association was found between plasma levels of vitamin C and risk of diabetes (odds ratio [OR] = 0.38, 95% confidence interval [CI]: 0.28–0.52). In the same study, a similar association was observed between fruit and vegetable intake and T2DM risk (OR = 0.78, 95% CI: 0.60–1.00).Citation41
Despite epidemiologic findings generally pointing toward an association between increased vitamin C and reduced oxidation and inflammation, intervention trials assessing the effect of vitamin C supplementation on various markers of T2DM have yielded inconsistent results. One randomized, crossover, double-blind intervention trial reported no improvement in fasting plasma glucose and no significant differences in levels of CRP, IL-6, IL-1 receptor agonist, or oxidized low-density lipoprotein (LDL) after supplementation with 3000 mg/day of vitamin C for 2 weeks in a group of 20 T2DM patients, compared to baseline levels.Citation146 Chen and colleagues performed a randomized, controlled, double-blind intervention on a group of 32 diabetic subjects with inadequate levels of vitamin C and found no significant changes in either fasting glucose or fasting insulin after intake of 800 mg/day of vitamin C for 4 weeks.Citation147 Furthermore, Tousoulis et al reported that treatment with 2000 mg/day for 4 weeks had no effect on levels of CRP, IL-6, TNF-α, or soluble vascular cell adhesion molecule-1 in 13 T2DM patients.Citation148
On the other hand, Wang and colleagues showed that the red blood cell sorbitol/plasma glucose ratio was reduced after supplementation with 1000 mg/day vitamin C for 2 weeks in a group of eight diabetics, although no differences were found in fasting plasma glucose.Citation42 Because sorbitol is a product of the pro-oxidative polyol pathway, this observation may suggest an inhibition of the polyol pathway by vitamin C among subjects with diabetes. Another study has shown that daily intake of ascorbic acid at 2000 mg/day improved fasting plasma glucose, HbA1c, cholesterol levels, and triglycerides in 56 diabetics.Citation149 In agreement, Paolisso et al found that 1000 mg/day of vitamin C for 4 months improved LDL and total cholesterol, fasting plasma insulin, and free radicals, although it did not affect triglycerides or HDL levels in a group of 40 diabetics.Citation43
Overall, it remains unclear whether vitamin C intake has an effect on factors related to T2DM. Although the epidemiologic evidence suggests that vitamin C, whether as a supplement or as part of a diet rich in fruits and vegetables, beneficially affects inflammatory markers and disease risk, the results of intervention trials in T2DM are conflicting. Small sample sizes, genetic variation, short intervention duration, insufficient dosage, and disease status of the assessed cohorts may account for the lack of effect and the inconsistent outcomes observed in intervention studies. However, it is also possible that the status of vitamin C deficiency is a result of the oxidative and proinflammatory challenges associated with T2DM, rather than a determinant of disease pathogenesis. Therefore, further research and long-term prospective studies are needed to elucidate the role of vitamin C as a modulator of inflammation and T2DM risk and to evaluate its potential role as a preventive agent at a population level.
Vitamin E
Vitamin E occurs as four common types: α tocopherol, β tocopherol, γ tocopherol, and δ tocopherol, and these differences in chemical structure have different molecular functions and impacts.Citation40 α Tocopherol is the major form recognized by the α tocopherol transfer protein in the liver and is, therefore, incorporated into very low-density lipoprotein, whereas γ tocopherol is metabolized and thus not retained. This results in much higher concentrations of α than γ tocopherol in the body.Citation37 Although vitamin E supplements consist primarily of α tocopherol, γ tocopherol is the main dietary source of the vitamin, as it is found in various vegetable oils, seeds, and nuts.Citation37 Vitamin E intake increases the rate of lymphocyte proliferation by enhancing the ability of T cells to undergo cell division cycles.Citation150 Its anti-inflammatory action, however, has been substantiated from observations demonstrating its ability to modulate the expression of the IL-2 and IL-4 genes and the IL-1 receptor antagonist following supplementation in animal models.Citation151 In T2DM patients, vitamin E reduced the serum levels of IL-1β, IL-6, TNF-α, PAI-1, and CRP.Citation15
The anti-inflammatory actions of vitamin E are believed to be related to its post-transcriptional inhibitory action on 5-lipoxygenase,Citation152 a member of the lipoxygenase family of enzymes involved in the synthesis of the inflammatory prostaglandins. Additionally, vitamin E downregulates NFκB and exerts potent lipophilic antioxidant effect on internal and external cell membranes as well as plasma lipoproteins, notably LDL.Citation15 Indeed, studies in both animal models and humans have demonstrated that vitamin E intake blocks LDL lipid peroxidation, prevents the oxidative stress linked to T2DM-associated abnormal metabolic patterns (hyperglycemia, dyslipidemia, and elevated levels of FFAs), and, subsequently, attenuates cytokine gene expression.Citation15,Citation62,Citation151,Citation153
A number of epidemiological studies demonstrated an inverse association between vitamin E and markers of oxidation, inflammation, and T2DM incidence.Citation154–Citation156 For example, a 4-year prospective assessment of 944 nondiabetic Finnish men found that below-median plasma vitamin E levels were associated with a 3.9-fold higher relative risk of diabetes (95% CI: 1.8–8.6).Citation154 Another case-control study (n = 106 cases, 201 controls) reported an inverse association between vitamin E serum levels and T2DM status, although the association became statistically insignificant after adjusting for cardiovascular disease risk factors (ie, serum cholesterol, obesity, smoking, and hypertension).Citation155 The Insulin Resistance Atherosclerosis Study, a 5-year prospective study of nearly 900 nondiabetic adults, found that plasma concentrations of α tocopherol offered a significant protective effect against T2DM (OR = 0.12, 95% CI: 0.02–0.68).Citation156 In contrast, various epidemiologic studies and intervention trials (see below) reported inconsistent findings. For example, supplementation with 750 IU/day of mixed tocopherols for 6 weeks reduced plasma but not urinary F2-isoprostanes, a marker of oxidative stress in vivo, in two different studies on subjects with T2DM.Citation157,Citation158 This level of supplementation did not alter the serum concentration of CRP, IL-6, TNF-α, or MCP-1 in one studyCitation158 and was associated with increased blood pressure and heart rate in another trial.Citation157
Data from the Women’s Health Study, assessing women health at baseline, demonstrated that supplementation with 600 IU of α tocopherol for 10 years on alternate days had no significant benefit for T2DM.Citation159 However, in another trial of 15 diabetics and 10 control individuals, 4 months of daily supplementation with 1350 IU of α tocopherol significantly improved plasma glucose levels, triglycerides, total cholesterol levels, LDL, and HbA1c, but did not improve the response of β cells to glucose or FFAs.Citation44 In a trial involving 34 Mexican diabetic women, daily supplementation with 800 IU of α tocopherol for 6 weeks improved total antioxidant status and reduced MDA levels, but had no effect on serum glucose, lipids, or HbA1c levels.Citation160 Daily supplementation with 1600 IU of α tocopherol for 10 weeks in a group of 21 diabetic men had no effect on HbA1c, fasting blood glucose, or glycated plasma protein concentrations, but had a beneficial effect on LDL oxidation.Citation161
Despite these conflicting findings, a recent study evaluated the effects of daily supplementation of a combination of vitamin C (20,000 IU) and vitamin E (400 IU) for 4 weeks on insulin sensitivity in untrained (n = 19) and trained (n = 20) healthy young men.Citation162 The study concluded that such a regimen may preclude the exercise-induced amelioration of insulin resistance in humans.Citation162 This may relate to the source of vitamin E used, that is, α, β, γ, or δ tocopherols.Citation163 Indeed, one study assessed the differences between α and γ tocopherols on factors related to the metabolic syndrome.Citation164 Subjects with the metabolic syndrome were administered either form of vitamin E or an equal mixture of the two at doses of 1200 IU/day for 6 weeks. Plasma levels of IL-1β, TNF-α, IL-6, CRP, and markers of oxidative stress were measured. Serum levels of CRP were decreased after supplementation with both α and γ tocopherols, and only the combined treatment yielded a significant lowering effect on the inflammatory markers. TNF-α decreased with α and mixed tocopherols, but neither IL-1β nor IL-6 were affected by any treatment regimen. Markers of oxidative stress, such as MDA and lipid peroxides, were significantly reduced with all treatments.Citation164 These results suggest that supplementation with combined α and γ tocopherols may be more beneficial in reducing oxidative stress and inflammation than either isoform alone. Furthermore, given the role of vitamin C in regeneration of oxidized vitamin E (see above), combined vitamin C and vitamin E may be more effective than administering either micronutrient on its own. For example, 13 elderly men with impaired fasting glucose were given a daily combination of 1000 mg α tocopherol and 1000 IU vitamin C for 4 weeks. The combined supplement yielded significant reductions in levels of fasting plasma insulin, glucose, TNF-α, and 8-isoprostane.Citation45
In general, inconsistent results from studies evaluating the effect of vitamin E on inflammation, oxidation, and T2DM risk may result partly from genetic differences between individuals that could lead to variations in response to micronutrient exposure. For example, SNPs in the TNF-α gene (eg, −308 G>A) affected the inflammatory response of elderly individuals to supplementation with 273 IU/day of α tocopherol for 1 year.Citation165 When carriers of the A allele received vitamin E, they had lower TNF-α synthesis upon exposure to lipopolysaccharides compared to their nonsupplemented counterparts. Because the A allele has been previously associated with higher TNF-α levels, it was concluded that the anti-inflammatory effects of vitamin E are specific to those who are genetically predisposed to develop inflammatory responses upon exposure to stimuli.Citation165 This observation is critical in identifying subjects of the general population who will benefit more from vitamin E supplementation based on their genetic predisposition to disease-related factors.
Conclusion and future directions
Introducing novel and effective prevention strategies in a public health setting necessitates considering approaches with the least (if any) side effects and the maximal preventive efficacy and outcome. In this context, applying nutritional intervention to attenuate inflammation and oxidative stress would be a feasible public health strategy for prevention of T2DM. This approach should be explored in prediabetic subjects and the outcome should be compared to the effect(s) of modifying current practices, such as lifestyle change, dietary intervention, and exercise. The effectiveness of lifestyle-change intervention programs for prediabetes also shows a promising effect on the reduction of overall incidence of T2DM or its complications, and it can be implemented in general clinical practice.Citation166 A lifestyle-change program including increased exercise and change in diet (either by reduction in glycemic load or reduced-fat diet) demonstrated a significant difference between control and intervention groups in markers for risk of progression to T2DM including weight, BMI, and waist circumference.Citation166 In general, current approaches for the prevention of T2DM have been shown to be effective in delaying or preventing the progression from prediabetes to diabetes.Citation167 In patients with insulin resistance, these practices are known to improve insulin sensitivity and the overall predisposition to T2DM.Citation168 On the other hand, increasing intake of vitamin D to >800 IU daily along with 1200 mg of calcium was reported to reduce the risk of developing T2DM by 33%.Citation91 In agreement, healthy older adults with impaired fasting glucose showed significant improvement in attenuating the glycemic response and insulin resistance when they increased their intake of vitamin D by 700 IU/day and calcium by 500 mg/day for 3 years.Citation112
It seems reasonable, therefore, to suggest that the two preventive approaches for T2DM, that is micronutrient supplementation and lifestyle change, may be combined into a single successful intervention program. This strategy may be more efficient in reducing the burden of the disease in the general population and in vulnerable subpopulations than when a single approach is proposed. Moreover, such a combined approach may be introduced into the general practice setting and to the general population with low expenditure and minimal side effects.Citation8
Overall, the current state of knowledge warrants further study into the extent to which micronutrients can modify the association between markers of inflammation and oxidative stress and early stages of T2DM. There is evidence supporting the idea that vitamin supplementation can modify the genotype–phenotype association within the innate immune response (ie, the proinflammatory and inflammatory markers) and that it has an ameliorating effect on oxidative stress and the subsequent proinflammatory signaling. This proposition may provide the mechanism by which nutritional factors prevent or delay disease development and can be introduced into the general population, as well as susceptible subpopulations. In relation to the current preventive approaches for T2DM, for example, lifestyle changes, exercise, and dietary intervention, exploring the efficacy of micronutrient supplementation on attenuating oxidative stress, the innate immune response, and the ensuing inflammation and evaluating the outcome of this strategy on T2DM incidence may be assessed through a series of prospective population-based studies, first, to determine the feasibility and effectiveness of this protocol; second, to validate and evaluate this strategy and ensure replication of results; and, third, to monitor the outcome to quantify the overall preventive response in comparison with the current approaches. As reviewed by Willcox et al,Citation169 study design is an issue to consider when assessing the efficacy of antioxidants in reducing the risk of developing cardiometabolic diseases. Intervention trials should be designed with sufficient power to measure T2DM as a primary, rather than a secondary, outcome. Furthermore, these studies should investigate the effects of antioxidant vitamins and trace elements in representative populations so that the results are applicable to high-risk groups, and they should take into account genetic variation accounting for interindividual differences in response to supplementation. Consideration should be also given to the antioxidants given in specific studies, as different antioxidants exert their effects through different mechanisms.Citation169
Disclosure
The authors report no conflicts of interest in this work.
References
- ZimmetPAlbertiKGShawJGlobal and societal implications of the diabetes epidemicNature2001414686578278711742409
- WildSRoglicGGreenASicreeRKingHGlobal prevalence of diabetes: estimates for the year 2000 and projection for 2030Diabetes Care20042751047105315111519
- MokdadAHBowmanBAFordESVinicorFMarksJSKoplanJPThe continuing epidemics of obesity and diabetes in the United StatesJAMA2001286101195120011559264
- VenkataramanRNandaNCBawejaGParikhNBhatiaVPrevalence of diabetes mellitus and related conditions in Asian Indians living in the United StatesAm J Cardiol200494797798015464696
- LambREGoldsteinBJModulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular functionInt J Clin Pract20086271087109518489578
- SavageDBPetersenKFShulmanGIMechanisms of insulin resistance in humans and possible links with inflammationHypertension200545582883315824195
- StumvollMGoldsteinBJvan HaeftenTWType 2 diabetes: principles of pathogenesis and therapyLancet200536594671333134615823385
- BadawiAKlipAHaddadPType 2 diabetes mellitus and inflammation: prospects for biomarkers of risk and nutritional interventionDiabetes Metab Syndr Obes20103173186
- PickupJCInflammation and activate innate immunity in the pathogenesis of type 2 diabetesDiabetes Care200427381382314988310
- EvansJLMadduxBAGoldfineIDThe molecular basis for oxidative stress-induced insulin resistanceAntioxid Redox Signal200577–81040105215998259
- AlbertiKGTreating type 2 diabetes–today’s targets, tomorrow’s goalsDiabetes Obes Metab20013Suppl 1S3S1011685827
- DandonaPAljadaABandyopadhyayAInflammation: the link between insulin resistance, obesity and diabetesTrends Immunol20042514714698276
- DandonaPAljadaAA rational approach to pathogenesis and treatment of type 2 diabetes mellitus, insulin resistance, inflammation, and atherosclerosisAm J Cardiol2002905A27G33G
- DandonaPAljadaAChaudhuriABandyopadhyayAThe potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetesJ Clin Endocrinol Metab20038862422242912788837
- SinghUJialalIAnti-inflammatory effects of alpha-tocopherolAnn N Y Acad Sci2004103119520315753145
- IsharwalSMisraAWasirJSNigamPDiet and insulin resistance: a review and Asian Indian perspectiveIndian J Med Res2009129548549919675375
- JohansenJSHarrisAKRychlyDJErgulAOxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practiceCardiovasc Diabetol200541515862133
- HoustisNRosenEDLanderESReactive oxygen species have a causal role in multiple forms of insulin resistanceNature2006440708694494816612386
- PickupJCMattockMBChusneyGDBurtDNIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome XDiabetologia19974011128612929389420
- PickupJCCrookMAIs type II diabetes mellitus a disease of the innate immune system?Diabetologia19984110124112489794114
- ConeJBInflammationAm J Surg2001182655856211839317
- MedzhitovRJanewayCJrInnate immunityN Engl J Med2000343533834410922424
- BilanPJSamokhvalovVKoshkinaASchertzerJDSamaanMCKlipADirect and macrophage-mediated actions of fatty acids causing insulin resistance in muscle cellsArch Physiol Biochem2009115417619019671019
- HotamisligilGSInflammation and metabolic disordersNature2006444712186086717167474
- HotamisligilGSArnerPCaroJFAtkinsonRLSpiegelmanBMIncreased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistanceJ Clin Invest1995955240924157738205
- Fernandez-RealJMPickupJCInnate immunity, insulin resistance and type 2 diabetesTrends Endocrinol Metab2008191101618082417
- FridlyandLEPhilipsonLHReactive species and early manifestation of insulin resistance in type 2 diabetesDiabetes Obes Metab20068213614516448517
- RosenPNawrothPPKingGMollerWTritschlerHJPackerLThe role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes SocietyDiabetes Metab Res Rev200117318921211424232
- WestICRadicals and oxidative stress in diabetesDiabet Med200017317118010784220
- FenkciVFenkciSYilmazerMSerteserMDecreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular diseaseFertil Steril200380112312712849813
- UrakawaHKatsukiASumidaYOxidative stress is associated with adiposity and insulin resistance in menJ Clin Endocrinol Metab200388104673467614557439
- BrownleeMThe pathobiology of diabetic complications: a unifying mechanismDiabetes20055461615162515919781
- EvansJLGoldfineIDMadduxBAGrodskyGMAre oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction?Diabetes20035211812502486
- FacchiniFSHuaNWReavenGMStoohsRAHyperinsulinemia: the missing link among oxidative stress and age-related diseases?Free Radic Biol Med200029121302130611118820
- InoguchiTLiPUmedaFHigh glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cellsDiabetes200049111939194511078463
- TripathyDMohantyPDhindsaSElevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjectsDiabetes200352122882288714633847
- CalderPCAlbersRAntoineJMInflammatory disease processes and interactions with nutritionBr J Nutr2009101Suppl 1S1S4519586558
- EvansJLGoldfineIDMadduxBAGrodskyGMOxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetesEndocr Rev200223559962212372842
- EspositoKNappoFMarfellaRInflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stressCirculation2002106162067207212379575
- BartlettHEEperjesiFNutritional supplementation for type 2 diabetes: a systematic reviewOphthalmic Physiol Opt200828650352319076553
- HardingAHWarehamNJBinghamSAPlasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer–Norfolk prospective studyArch Intern Med2008168141493149918663161
- WangHZhangZBWenRRChenJWExperimental and clinical studies on the reduction of erythrocyte sorbitol-glucose ratios by ascorbic acid in diabetes mellitusDiabetes Res Clin Pract1995281187587907
- PaolissoGBalbiVVolpeCMetabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabeticsJ Am Coll Nutr19951443873928568117
- PaolissoGD’AmoreAGiuglianoDCerielloAVarricchioMD’OnofrioFPharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patientsAm J Clin Nutr19935756506568480681
- RizzoMRAbbatecolaAMBarbieriMEvidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin actionJ Am Coll Nutr200827450551118978171
- CartaSCastellaniPDelfinoLTassiSVeneRRubartelliADAMPs and inflammatory processes: the role of redox in the different outcomesJ Leukoc Biol200986354955519564570
- NavabMGharaviNWatsonADInflammation and metabolic disordersCurr Opin Clin Nutr Metab Care200811445946418542007
- BeattySKohHPhilMHensonDBoultonMThe role of oxidative stress in the pathogenesis of age-related macular degenerationSurv Ophthalmol200045211513411033038
- TakedaKAkiraSTLR signaling pathwaysSemin Immunol20041613914751757
- BaumannHGauldieJThe acute phase responseImmunol Today199415274807512342
- SteelDMWhiteheadASThe major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A proteinImmunol Today199415281888155266
- GabayCKushnerIAcute-phase proteins and other systemic responses to inflammationN Engl J Med199934064484549971870
- MontecuccoFSteffensSMachFInsulin resistance: a proinflammatory state mediated by lipid-induced signaling dysfunction and involved in atherosclerotic plaque instabilityMediators Inflamm2008200876762318604303
- AguirreVUchidaTYenushLDavisRWhiteMFThe c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307)J Biol Chem2000275129047905410722755
- GuzikTJWestNEBlackEVascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factorsCirc Res2000869E85E9010807876
- GuzikTJMussaSGastaldiDMechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P) H oxidase and endothelial nitric oxide synthaseCirculation2002105141656166211940543
- Vega-LopezSDevarajSJialalIOxidative stress and antioxidant supplementation in the management of diabetic cardiovascular diseaseJ Investig Med20045212432
- ObergBPMcMenaminELucasFLIncreased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney diseaseKidney Int20046531009101614871421
- CerielloAMercuriFQuagliaroLDetection of nitrotyrosine in the diabetic plasma: evidence of oxidative stressDiabetologia200144783483811508267
- DunlopMAldose reductase and the role of the polyol pathway in diabetic nephropathyKidney Int Suppl200077S3S1210997684
- KashiwagiAAsahinaTIkebuchiMAbnormal glutathione metabolism and increased cytotoxicity caused by H2O2 in human umbilical vein endothelial cells cultured in high glucose mediumDiabetologia19943732642698174840
- ScottJAKingGLOxidative stress and antioxidant treatment in diabetesAnn N Y Acad Sci2004103120421315753146
- CerielloATestaRAntioxidant anti-inflammatory treatment in type 2 diabetesDiabetes Care200932Suppl 2S232S23619875557
- Rask-MadsenCKingGLProatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistanceArterioscler Thromb Vasc Biol200525348749615637306
- TurkoIVMarcondesSMuradFDiabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferaseAm J Physiol Heart Circ Physiol20012816H2289H229411709394
- BrowningLMJebbSANutritional influences on inflammation and type 2 diabetes riskDiabetes Technol Ther200681455416472050
- MarfellaRQuagliaroLNappoFCerielloAGiuglianoDAcute hyperglycemia induces an oxidative stress in healthy subjectsJ Clin Invest2001108463563611518739
- DouilletCBostMAccominottiMBorson-ChazotFCiavattiMEffect of selenium and vitamin E supplementation on lipid abnormalities in plasma, aorta, and adipose tissue of Zucker ratsBiol Trace Elem Res19986532212369892495
- PaolissoGGambardellaATagliamonteMRDoes free fatty acid infusion impair insulin action also through an increase in oxidative stress?J Clin Endocrinol Metab19968112424442488954022
- ItaniSIRudermanNBSchmiederFBodenGLipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alphaDiabetes20025172005201112086926
- RudichATiroshAPotashnikRKhamaisiMBashanNLipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein kinase B and glucose transport in 3T3-L1 adipocytesDiabetologia199942894995710491755
- RudichAKozlovskyNPotashnikRBashanNOxidant stress reduces insulin responsiveness in 3T3-L1 adipocytesAm J Physiol19972725 Pt 1E935E9409176196
- TiroshARudichAPotashnikRBashanNOxidative stress impairs insulin but not platelet-derived growth factor signalling in 3T3-L1 adipocytesBiochem J2001355Pt 375776311311139
- HaberCALamTKYuZN-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stressAm J Physiol Endocrinol Metab20032854E744E75312799318
- BruceCRCareyALHawleyJAFebbraioMAIntramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanismDiabetes20035292338234512941774
- PaolissoGD’AmoreAVolpeCEvidence for a relationship between oxidative stress and insulin action in non-insulin-dependent (type II) diabetic patientsMetabolism19944311142614297968598
- FordESMokdadAHGilesWHBrownDWThe metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination SurveyDiabetes20035292346235212941775
- LeeKUOxidative stress markers in Korean subjects with insulin resistance syndromeDiabetes Res Clin Pract200154Suppl 2S29S3311733106
- MaritimACSandersRAWatkinsJBIIIDiabetes, oxidative stress, and antioxidants: a reviewJ Biochem Mol Toxicol2003171243812616644
- KingGLThe role of inflammatory cytokines in diabetes and its complicationsJ Periodontol2008798 Suppl1527153418673007
- LiuGRondinoneCMJNK: bridging the insulin signaling and inflammatory pathwayCurr Opin Investig Drugs2005610979987
- HundalRSPetersenKFMayersonABMechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetesJ Clin Invest2002109101321132612021247
- GumieniczekAKrzywdzinskaMNowakMModulation of nitrosative/oxidative stress in the lung of hyperglycemic rabbits by two antidiabetics, pioglitazone and repaglinideExp Lung Res200935537137919842839
- AdameovaAXuYJDuhamelTATappiaPSShanLDhallaNSAnti-atherosclerotic molecules targeting oxidative stress and inflammationCurr Pharm Des200915273094310719754384
- FukuzawaMSatohJQiangXInhibition of tumor necrosis factor-alpha with anti-diabetic agentsDiabetes Res Clin Pract199943314715410369423
- DelerivePFruchartJCStaelsBPeroxisome proliferator-activated receptors in inflammation controlJ Endocrinol2001169345345911375115
- BrighentiFValtuenaSPellegriniNTotal antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjectsBr J Nutr200593561962515975160
- MagginiSWintergerstESBeveridgeSHornigDHSelected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responsesBr J Nutr200798Suppl 1S29S3517922955
- SooryMRelevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targetingInfect Disord Drug Targets20099440041419689382
- PittasAGHarrisSSStarkPCDawson-HughesBThe effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adultsDiabetes Care200730498098617277040
- PittasAGLauJHuFBDawson-HughesBReview: the role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysisJ Clin Endocriol Metabol200792620172029
- HaddadPSAzarGAGroomSBoivinMNatural health products, modulation of immune function and prevention of chronic diseasesEvid Based Complement Alternat Med20052451352016322809
- JamesMJGibsonRAClelandLGDietary polyunsaturated fatty acids and inflammatory mediator productionAm J Clin Nutr2000711 Suppl343S348S10617994
- LiuCTSheenLYLiiCKDoes garlic have a role as an antidiabetic agent?Mol Nutr Food Res200751111353136417918164
- WeisbergSPLeibelRTortorielloDVDietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesityEndocrinology200814973549355818403477
- SrinivasLShaliniVKDNA damage by smoke: protection by turmeric and other inhibitors of ROSFree Radic Biol Med19911132772831937145
- SrivastavaRSrimalRCModification of certain inflammation-induced biochemical changes by curcuminIndian J Med Res1985812152233159662
- VuksanVStavroMPSievenpiperJLSimilar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetesDiabetes Care20002391221122610977009
- SprietsmaJESchuitemakerGEDiabetes can be prevented by reducing insulin productionMed Hypotheses199442115238196555
- HashemipourMKelishadiRShapouriJEffect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese childrenHormones (Athens)20098427928520045801
- SongYWangJLiXKCaiLZinc and the diabetic heartBiometals200518432533216158224
- PrasadASZinc in human health: effect of zinc on immune cellsMol Med2008145–635335718385818
- PrasadASBaoBBeckFWKucukOSarkarFHAntioxidant effect of zinc in humansFree Radic Biol Med20043781182119015451058
- Botella-CarreteroJIAlvarez-BlascoFVillafruelaJJBalsaJAVazquezCEscobar-MorrealeHFVitamin D deficiency is associated with the metabolic syndrome in morbid obesityClin Nutr200726557358017624643
- TeegardenDDonkinSSVitamin D: emerging new roles in insulin sensitivityNutr Res Rev2009221829219555519
- HolickMFDiabetes and the vitamin d connectionCurr Diab Rep20088539339818778589
- AnanFTakahashiNNakagawaMOoieTSaikawaTYoshimatsuHHigh-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patientsMetabolism200554455255815798966
- FuLYunFOczakMWongBYViethRColeDECommon genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementationClin Biochem20094210–111174117719302999
- SzathmaryEJThe effect of Gc genotype on fasting insulin level in Dogrib IndiansHum Genet19877543683723552957
- HiraiMSuzukiSHinokioYVariations in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose toleranceJ Clin Endocrinol Metab20008551951195310843180
- BaierLJDobberfuhlAMPratleyREHansonRLBogardusCVariations in the vitamin D-binding protein (Gc locus) are associated with oral glucose tolerance in nondiabetic Pima IndiansJ Clin Endocrinol Metab1998838299329969709981
- PittasAGDawson-HughesBLiTVitamin D and calcium intake in relation to type 2 diabetes in womenDiabetes Care200629365065616505521
- HayesCENasholdFESpachKMPedersenLBThe immunological functions of the vitamin D endocrine systemCell Mol Biol (Noisy-le-grand)200349227730012887108
- GriffinMDXingNKumarRVitamin D and its analogs as regulators of immune activation and antigen presentationAnnu Rev Nutr20032311714512651965
- CantornaMTZhuYFroicuMWittkeAVitamin D status, 1,25-dihydroxyvitamin D3, and the immune systemAm J Clin Nutr2004806 Suppl1717S1720S15585793
- DeLucaHFCantornaMTVitamin D: its role and uses in immunologyFASEB J200115142579258511726533
- VeldmanCMCantornaMTDeLucaHFExpression of 1,25-dihydroxyvitamin D(3) receptor in the immune systemArch Biochem Biophys2000374233433810666315
- RiachyRVandewalleBKerr ConteJ1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20Endocrinology2002143124809481912446608
- Van EttenEMathieuCImmunoregulation by 1,25-dihydroxyvitamin D3: basic conceptsJ Steroid Biochem Mol Biol2005971–29310116046118
- PittasAGChungMTrikalinosTSystematic review: vitamin D and cardiometabolic outcomesAnn Intern Med2010152530731420194237
- ChiuKCChuAGoVLSaadMFHypovitaminosis D is associated with insulin resistance and beta cell dysfunctionAm J Clin Nutr200479582082515113720
- FordESAjaniUAMcGuireLCLiuSConcentrations of serum vitamin D and the metabolic syndrome among US adultsDiabetes Care20052851228123015855599
- HypponenEPowerCVitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesityDiabetes Care200629102244224617003300
- NeedAGO’LoughlinPDHorowitzMNordinBERelationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal womenClin Endocrinol (Oxf)200562673874115943837
- ScraggRSowersMBellCSerum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination SurveyDiabetes Care200427122813281815562190
- LiuSSongYFordESMansonJEBuringJERidkerPMDietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older US womenDiabetes Care200528122926293216306556
- FliserDStefanskiAFranekEFodePGudarziARitzENo effect of calcitriol on insulin-mediated glucose uptake in healthy subjectsEur J Clin Invest19972776296339263752
- InomataSKadowakiSYamataniTFukaseMFujitaTEffect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitusBone Miner1986131871923334207
- LjunghallSLindLLithellHTreatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance–a prospective randomized double-blind studyActa Med Scand198722243613673321925
- OrwollERiddleMPrinceMEffects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitusAm J Clin Nutr1994595108310878172095
- NilasLChristiansenCTreatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal womenInt J Obes1984854074116549176
- KnowlerWCBarrett-ConnorEFowlerSEReduction in the incidence of type 2 diabetes with lifestyle intervention or metforminN Engl J Med2002346639340311832527
- Von HurstPRStonehouseWCoadJVitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomised, placebo-controlled trialBr J Nutr2010103454955519781131
- WannametheeSGLoweGDRumleyABruckdorferKRWhincupPHAssociations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasisAm J Clin Nutr200683356757416522902
- AguirreRMayJMInflammation in the vascular bed: importance of vitamin CPharmacol Ther200811919610318582947
- LevineMWangYPadayattySJMorrowJA new recommended dietary allowance of vitamin C for healthy young womenProc Natl Acad Sci U S A200198179842984611504949
- CahillLCoreyPNEl-SohemyAVitamin C deficiency in a population of young Canadian adultsAm J Epidemiol2009170446447119596710
- YoungISTateSLightbodyJHMcMasterDTrimbleERThe effects of desferrioxamine and ascorbate on oxidative stress in the streptozotocin diabetic ratFree Radic Biol Med19951858338407797090
- FordESLiuSManninoDMGilesWHSmithSJC-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adultsEur J Clin Nutr20035791157116312947436
- HamerMChidaYIntake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysisJ Hypertens200725122361236917984654
- WoodwardMLoweGDRumleyAEpidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow MONICA Survey. II. Relationships to cardiovascular risk factors and prevalent cardiovascular diseaseBr J Haematol19979747857979217177
- WoodwardMRumleyATunstall-PedoeHLoweGDAssociations of blood rheology and interleukin-6 with cardiovascular risk factors and prevalent cardiovascular diseaseBr J Haematol1999104224625710050704
- WoodwardMRumleyALoweGDTunstall-PedoeHC-reactive protein: associations with haematological variables, cardiovascular risk factors and prevalent cardiovascular diseaseBr J Haematol2003122113514112823355
- GaoXBermudezOITuckerKLPlasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white eldersJ Nutr2004134491391815051846
- HoltEMSteffenLMMoranAFruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescentsJ Am Diet Assoc2009109341442119248856
- LuQBjorkhemIWretlindBDiczfalusyUHenrikssonPFreyschussAEffect of ascorbic acid on microcirculation in patients with type II diabetes: a randomized placebo-controlled cross-over studyClin Sci (Lond)2005108650751315675894
- ChenHKarneRJHallGHigh-dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistanceAm J Physiol Heart Circ Physiol20062901H137H14516126809
- TousoulisDAntoniadesCVasiliadouCEffects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitusHeart200793224424616914485
- ErikssonJKohvakkaAMagnesium and ascorbic acid supplementation in diabetes mellitusAnn Nutr Metab19953942172238546437
- AdolfssonOHuberBTMeydaniSNVitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacityJ Immunol200116773809381711564798
- HanSNAdolfssonOLeeCKProllaTAOrdovasJMeydaniSNVitamin E and gene expression in immune cellsAnn N Y Acad Sci200410319610115753137
- DevarajSJialalIα-Tocopherol decreases interleukin-1β release from activated human monocytes by inhibition of 5-lipoxygenaseArterioscler Thromb Vasc Biol1999191125113310195945
- ThomasSRStockerRMolecular action of vitamin E in lipoprotein oxidation: implications for atherosclerosisFree Radic Biol Med200028121795180510946221
- SalonenJTNyyssonenKTuomainenTPIncreased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in menBMJ19953117013112411277580706
- ReunanenAKnektPAaranRKAromaaASerum antioxidants and risk of non-insulin dependent diabetes mellitusEur J Clin Nutr199852289939505151
- Mayer-DavisEJCostacouTKingIZaccaroDJBellRAPlasma and dietary vitamin E in relation to incidence of type 2 diabetes: the Insulin Resistance and Atherosclerosis Study (IRAS)Diabetes Care200225122172217712453956
- WardNCWuJHClarkeMWThe effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trialJ Hypertens200725122723417143195
- WuJHWardNCIndrawanAPEffects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetesClin Chem200753351151917272491
- LiuSLeeIMSongYVitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trialDiabetes200655102856286217003353
- Ble-CastilloJLCarmona-DiazEMendezJDEffect of alpha-tocopherol on the metabolic control and oxidative stress in female type 2 diabeticsBiomed Pharmacother200559629029515932790
- ReavenPDHeroldDABarnettJEdelmanSEffects of vitamin E on susceptibility of low-density lipoprotein and low-density lipoprotein subfractions to oxidation and on protein glycation in NIDDMDiabetes Care19951868078167555507
- RistowMZarseKOberbachAAntioxidants prevent health-promoting effects of physical exercise in humansProc Natl Acad Sci U S A2009106218665867019433800
- BuijsseBFeskensEJKwapeLKokFJKromhoutDBoth alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly menJ Nutr2008138234435018203902
- DevarajSLeonardSTraberMGJialalIGamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndromeFree Radic Biol Med20084461203120818191645
- BelisleSELekaLSDelgado-ListaJJacquesPFOrdovasJMMeydaniSNPolymorphisms at cytokine genes may determine the effect of vitamin E on cytokine production in the elderlyJ Nutr2009139101855186019710156
- BarclayCProcterKLGlendenningRMarshPFreemanJMathersNCan type 2 diabetes be prevented in UK general practice? A lifestyle-change feasibility study (ISAIAH)Br J Gen Pract20085855354154718682012
- NorrisSLZhangXAvenellALong-term effectiveness of weight-loss interventions in adults with pre-diabetes: a reviewAm J Prev Med200528112613915626569
- FrostGLeedsATrewGMargaraRDornhorstAInsulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic dietMetabolism19984710124512519781629
- WillcoxBJCurbJDRodriguezBLAntioxidants in cardiovascular health and disease: key lessons from epidemiologic studiesAm J Cardiol200810110A75D86D18157969
