Abstract
Epoetin zeta was granted marketing authorization in October 2007 by the European Medicines Agency as a recombinant human erythropoietin erythropoiesis-stimulating agent to treat symptomatic anemia of renal origin in adult and pediatric patients on hemodialysis and adults on peritoneal dialysis, as well as for symptomatic renal anemia in adult patients with renal insufficiency not yet on dialysis. Currently, epoetin zeta can be administered either subcutaneously or intravenously to correct for hemoglobin concentrations ≤10 g/dL (6.2 mmol/L) or with dose adjustment to maintain hemoglobin levels at desired levels not in excess of 12 g/dL (7.5 mmol/L). This review article focuses on epoetin zeta indications in chronic kidney disease, its use in managing anemia of renal origin, and discusses its pharmacology and clinical utility.
Introduction
Renal anemia occurs as a common complication of chronic kidney disease (CKD). CKD is a complex disease characterized by impaired renal function. The Kidney Disease Improving Global Outcomes (KDIGO) initiative defines CKD as the presence of structural or functional abnormalities of the kidneys resulting in kidney damage, for instance, pathologic abnormalities or markers of kidney damage to include proteinuria, renal tubular syndromes or imaging abnormalities, or level of kidney function measured by glomerular filtration rate (GFR) <60 mL/minute/1.73 m2 lasting ≥3 months.Citation1 There are five stages to disease progression based on estimated GFR levels calculated from serum creatinine levels and levels of proteinuria. These stages range from kidney damage with normal or increased GFR (stage I) to kidney failure (stage 5).Citation2 shows the KDIGO classifications for the five stages of CKD. The term CKD refers to the presence of any stage of CKD (stage 1 through 5) with or without kidney transplant, and includes both nondialysis and dialysis dependent disease.Citation3
Table 1 Stages of CKD, from the Kidney Disease Improving Global Outcomes (KDIGO) initiative
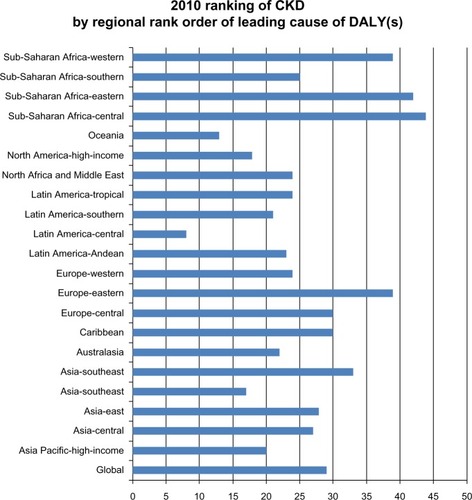
Prevalence, disease burden, and treatment costs for CKD are increasing in the US and globally. Overall, in the US, an estimated one in ten adults, or about 20 million individuals, have CKD, and, during the years 1999–2004, data show a higher prevalence among females than males (females 15.0% versus males 11.1%).Citation4–Citation6 Although, in 2011, new cases of end stage renal disease (ESRD) declined for the first time in 30 years in the US, there was an overall increase in patients receiving treatment for ESRD.Citation6 In the UK, CKD affects an estimated 6% of the population.Citation7 Little data exist for European prevalence.Citation8 The Global Burden of Disease project’s ranking of leading causes of disability-adjusted life years (DALYs) for 291 specific diseases ranks CKD 29th overall globally with regional geographical rankings ranging from 8–44 (see ).Citation9 Between 1990 and 2010, DALYs (per 100,000) for CKD show an overall increase of 16.7%, with an even greater percent increase for CKD due to diabetes mellitus (36.1%) or hypertension (42.2%).Citation9
Figure 1 2010 ranking of CKD by regional rank order of leading cause of DALY(s).
© Elsevier 2012. Adapted with permission from Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010; a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223.Citation9
Abbreviations: CKD, chronic kidney disease; DALYs, disability adjusted life years.
Risk factors for developing CKD include age, gender, race, diabetes, and genetic makeup, as well as modifiable factors such as hypertension, proteinuria, anemia, metabolic disturbances, and dyslipidemia.Citation10,Citation11 Disease progression to CKD increases the risk for cardiovascular disease, hospitalization, and death,Citation12,Citation13 with some suggesting an increased risk for a cardiorenal syndrome.Citation14
Hemoglobin (Hb) levels often gradually decline with the decline in renal function.Citation15 The prevalence of anemia increases as kidney function declines.Citation16 Renal anemia is associated with adverse patient outcomes, including decreased exercise capacityCitation17 and quality of life,Citation18 and increased hospitalization, cardiovascular events, and chance of death.Citation19–Citation21 Renal anemia arises from CKD-induced oxidative stress, inflammation, and a relative deficiency in the renal production of erythropoietin (EPO)Citation22–Citation25 due to loss of EPO synthesis or inhibitors of EPO.Citation2,Citation26 EPO is an endogenous protein produced in the kidneys to stimulate red blood cell production under hypoxic conditions.Citation24,Citation27–Citation29 Similar to the anemia of chronic disease, renal anemia is normochromic, normocytic, and characteristically hypoproliferative, however different from anemia of chronic disease, renal anemia also shows low EPO and some iron deficiency.Citation1,Citation15
The definition of renal anemia has evolved over the years. Currently, the KDIGO, the European Renal Best Practice group (ERBP), and the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) give Hb levels to serve as a guide when diagnosing anemia. The current KDIGO guidelines use the World Health Organization definition of anemia while the ERBP position statement takes into consideration Hb set point differences for European populations.Citation30 In the US, the NKF KDOQI continues to use the 2006 KDOQI definition.Citation31 shows the differences between guidelines for diagnosing renal anemia among NKF KDOQI 2006 US guidelines, KDIGO updated 2012 guidelines, and the ERBP 2013 position statement.Citation30,Citation31
Table 2 Guidelines for diagnosing renal anemia and making further evaluation based on Hb levels
After ruling out other causes of symptomatic anemia in the presence of CKD, and once renal anemia has been diagnosed, renal anemia treatment guidelines suggest a trial of iron therapy before beginning erythropoiesis-stimulating agent (ESA) therapy and based on transferrin saturation (TSAT) and ferritin levels. Iron supplementation improves Hb levels and cellular response to ESA stimulation, which may in turn help reduce the ESA dosage. This ESA response to iron supplementation may arise from how the hepcidin-ferroportin axis helps regulate iron homeostasis by controlling the entry of iron from dietary sources into the bloodstream. The iron mediated hepcidin pathway is suppressed in the presence of hypoxia and anemia, while ESA administration causes hepcidin excess. Hepcidin excess impedes the absorption of dietary iron and the release of iron stores. Therefore, administering iron supplementation, either intravenously for hemodialysis patients, or through an oral agent for nondialyzed CKD patients, for TSAT <30% and ferritin level <300 ng/mL is recommended. Iron supplementation should be used with caution for TSAT >30% and ferritin levels exceeding 500 ng/mL.Citation30,Citation32
In 1988 in Europe, and subsequently for the year 1989 in the US, recombinant human erythropoietin (rHuEPO) was introduced to treat renal anemia. Its ability to normalize Hb led to rHuEPO eventually replacing blood transfusions and the use of androgenic steroids as the mainstay treatment for renal anemia. Moreover, renal anemia was independently associated with the development of left ventricular hypertrophy (LVH) and heart failure. Normalizing Hb with epoetin alfa arrested the progression of LVH in CKD with associated improvements in cardiovascular-related morbidity and mortality.Citation33 Additionally, early rHuEPO research reported improved quality of life scores in CKD contributing to the eventual widespread use of rHuEPO in treating renal anemia.Citation34–Citation37 Over time, various epoetin analogues (epoetin alfa, epoetin beta, darbepoetin alfa) were developed that effectively increased Hb levels. Patent expirations for rHuEPO biopharmaceuticals led to the development of biosimilars, including epoetin zeta.Citation38
Currently, epoetin zeta carries indications for treating symptomatic renal anemia, for intravenous (IV) and subcutaneous administration in adults and pediatric patients undergoing hemodialysis and in adult patients on peritoneal dialysis, or for those not yet undergoing dialysis.Citation39,Citation40 Contraindications include hypersensitivity, patients who develop epoetin-induced antibody-mediated pure red cell aplasia (PRCA) following treatment, uncontrolled hypertension, and patients who for any reason cannot receive adequate antithrombotic prophylaxis.Citation39 The purpose of this article is to review the differential pharmacology and clinical utility of epoetin zeta.
Methods
We began by identifying general information about epoetin zeta; that this biosimilar was a rHuEPO preparation of epoetin alfa with a Chemical Abstracts Service registry number 604802-70-2 and a chemical name 1-165-Erythropoetin (human clone B03XA01) marketed under various brand names in different regions of the world (Retacrit® [HOSPIRA Inc, Lake Forest Illinois, USA, for distribution in Europe], Silapro® [STADA Arzneimittel AG, Bad Vilbel, Germany], Epobel® [STADA Arzneimittel AG, Bad Vilbel, Germany, licensed to NOBEL, IIac Pazarlama ve Sanayii Lt STI, for distribution inTurkey]), see . Based on this information, we developed a search incorporating concepts related to “epoetin zeta” and “chronic renal failure.” Our final PubMed search strategy used the following terms: (epoetin zeta OR EPO zeta OR erythropoietin zeta OR Retacrit OR Silapro) AND (kidney* OR renal OR uremia OR uremic OR ESRD OR ESRF OR CRD OR CRF OR CKD) AND (chronic OR insufficien* OR sufficien* OR disease OR fail* OR damage* OR irreversible OR end stage* OR anemia OR anemic). Searches were conducted in PubMed (publication n=10), Embase (publication n=61), Cochrane CENTRAL (publication n=4), Web of Science (publication n=24), ClinicalTrials.gov (publication n=3), and Google Scholar (publication n=278). Additionally, we gathered information from regulatory authorities including the European Medicines Agency (EMA), US Food and Drug Administration (FDA), and US Centers for Medicare and Medicaid Services (CMS), as well as from the professional association The National Kidney Foundation, Inc. Additional pertinent references were identified from the bibliographies of retrieved publications. Finally, we excluded non-English language publications and duplicates.
Table 3 Epoetin zeta, substance, cell line, brand name and manufacturer
Background
Patent expirations in 2004 for epoetin alfa and 2005 for epoetin beta in the European Union led to the development of biosimilar epoetin. The term “biosimilar”, as used in Europe (termed “follow-on-biologics” in the US and Japan, and called “subsequent-entry biologic” in Canada),Citation40 refers to officially-approved subsequent versions of innovator biotechnological products with the same primary amino acid sequence as the referent product used at the same dose to treat the same disease.Citation24,Citation28,Citation41,Citation42 Biosimilars differ from low molecular weight pharmaceutical generics in respect to the size and complexity of the active substance, the heterogeneity of the originator materials, and variability in manufacturing processes.Citation38 Pharmaceutical generics are low molecular weight formulations with similar pharmacokinetic and pharmacodynamic profiles as the original that are synthesized from commercially available reagents using standardized procedures.Citation43
Biotechnologically derived proteins have highly complex large molecular structures and a high degree of heterogeneity. This creates challenging formulation and manufacturing production processes that prevent the formulation and manufacture of exact copies of the original.Citation44,Citation45 Unlike generics with commercially available reagents, the biotechnologically derived biosimilars engineer their cellular clones in-house using living organisms or extracted tissues.Citation44–Citation47 Further, both originator biotechnologicals and biosimilars can undergo changes such as glycosylation or amino acid sequence variation (contamination), among others, that can induce immune effects.Citation43,Citation48–Citation50 PRCA, an anti-EPO antibody-associated severe anemia that results in transfusion dependence, is one such immune effect.Citation50,Citation51 Complex manufacturing processes can influence the chemical and clinical characteristics of a biosimilar or affect biosimilar quality. The complex molecular structure, as well as other issues including formulation and manufacture, results in biosimilars being similar to the referent product but not identical. However, biosimilars demonstrate comparable pharmacokinetics and therapeutic equivalence to the reference product.Citation33,Citation45,Citation52–Citation54 Minor differences in the microheterogeneity pattern of the molecule between the reference product and biosimilar are acceptable only when the difference does not impact safety and efficacy.Citation55
Regulatory approval designed to ensure therapeutic equivalence for biosimilars differs from that for generic drug formulations and addresses the complex formulation and manufacture processes. Since the formulation and manufacturing process can affect molecular similarity, regulators face challenges in establishing efficient and appropriate regulatory review and approval pathways to ensure equivalent therapeutic efficacy and safety.Citation47 The EMA provides regulatory oversight for biosimilars for the EU, with Australia also adopting the EMA comparability and quality guidelines.Citation56 The EMA guidelines require comparability studies between an authorized originator (termed reference product) biotechnologically derived product and the biosimilar in regards to clinical and nonclinical quality, safety, efficacy,Citation44,Citation45,Citation53,Citation57–Citation60 and delineate standards for immunogenicity assessment.Citation58,Citation61,Citation62 Further, the EMA provides specific epoetin guidelines for recombinant EPO biosimilars.Citation62 In the US, the Biosimilar Price Competition and Innovation Act, passed as part of the 2010 Patient Protection and Affordable Care Act, establishes the framework for the FDA regulatory pathway for biosimilars. To date, the FDA guidance is formative, with the fourth guidance draft released in the spring of 2013.Citation63 The EMA and FDA have a biosimilar cluster to enhance global development.Citation63 Also in the US, the CMS issues rules and regulations for physician fee schedule and other Medicare Part B payment systems for biosimilar biological products,Citation64 as well as billing procedures and other policies affecting the use of EPO stimulating agents in the US Medicare and Medicaid population.Citation65,Citation66
Pharmacology
Epoetin zeta is a rHuEPO biosimilar preparation to epoetin alfa. In adults, endogenous EPO production occurs primarily in interstitial kidney cells, as well as in smaller amounts in the liver and central nervous system.Citation67 Although anemia associated with renal impairment is multifactorial, in the presence of hypoxia, endogenous EPO production declines. Several theories give rationale explaining why hypoxic conditions in renal disease result in reduced EPO production instead of the normal increase in EPO production. Citation24,Citation30,Citation68,Citation69 Some theories suggest dysfunctional hypoxic cell signaling,Citation30,Citation69 whereas others suggest shortened life span of circulating red blood cells, nutritional deficiencies, or inflammation.Citation24 Dysfunctional hypoxic cell signaling in the kidneys appears to play an important role in the lack of upregulation of EPO in CKD patients. Normally, tissue hypoxia increases the expression of hypoxia inducible transcription factors that bind to a hypoxia-responsive portion of the EPO gene that, in turn, results in increased EPO production.Citation67,Citation69 In CKD, the normal oxygen dependent hydroxylation of hypoxia-inducible transcription factors are impaired.Citation70 Instead of increasing EPO expression, there is an inhibition of EPO production or loss of synthesis of EPOCitation67,Citation70 with no corresponding increase in red blood cell production.Citation70
Iron deficiency also contributes to renal anemia due to insufficient iron and chronic inflammation.Citation71,Citation72 Iron facilitates new red blood cell production whereas iron insufficiency limits the quantity of new red blood cells.Citation71,Citation72 Chronic inflammation reduces the amount of iron released from storage when it is needed.Citation72 Supplementing EPO treatments with iron reduces the risk for iron deficiency. Epoetin zeta is a rHuEPO therapy that can be supplemented with iron.Citation68
Epoetin zeta is a derivative of the endogenous protein erythropoietinCitation68 and is a biosimilar of the rHuEPO epoetin alfa, the first commercialized rHuEPO (Epogen®, AMGEN Inc., Thousand Oaks, CA, USA; Procrit®, JANSSEN Biotech, Inc., Horsham, PA, USA; Eprex®, JANSEEN-CILAG Ltd., Buckinghamshire, UK; Epoetin®, AMGEN Inc., Thousand Oaks, CA, USA). As a biosimilar to epoetin alfa, epoetin zeta has the same 165-amino-acid structure and comparable carbohydrate composition with only minor differences in the glycosylation pattern.Citation24,Citation41,Citation48,Citation74 Glycosylation patterns largely influence immunogenicity and half-life.Citation48 More complex glycosylation patterns have a higher number of sialic acid residues with longer half-lives.Citation68,Citation74 Sialic acid carbohydrate content affects serum half-life, with increased sialic acid content resulting in longer half-life. Epoetin alfa and zeta both have 14 sialic acid residues,Citation68 and are short acting drugs with a half-life of 6–8 hours when administered intravenously or 19–24 hours if administered subcutaneously.Citation75 The short half-life allows epoetin zeta to be administered up to three times per week to treat anemia due to CKD.Citation24,Citation76
Epoetin alfa and epoetin zeta are pharmacodynamically comparable both in vitro and in in vivo tests. Pharmacokinetic studies also report bioequivalence. Krivoshiev et alCitation28,Citation41 report the pharmacokinetics of epoetin zeta and epoetin alfa (the reference product) in healthy volunteers after intravenous and subcutaneous administration, respectively. The pharmacokinetic study assessing intravenous administration was a monocentric, open, randomized, single dose, and two-period crossover trial in 24 volunteersCitation28 with 21 volunteers included for statistical analysis. Compared to epoetin alfa, the biosimilar epoetin zeta showed nearly identical pharmacokinetic parameters including area under the concentration/time curve, maximal concentration after administration, volume of distribution, total plasma clearance of drug after administration, mean residence time, and terminal half-life.Citation28 A second study assessing subcutaneous administration of epoetin zeta reported results for a monocentric, double-blind, randomized, single dose, three-period crossover trial in 48 volunteers. This study evaluated the subcutaneous bioavailability and pharmacokinetic properties of epoetin zeta compared with epoetin alfa (reference product).Citation41 The subcutaneous bioavailability of epoetin zeta is 24%, which is equivalent to epoetin alpha 20%.Citation41 Additionally, no significant differences in the aforementioned pharmacokinetic parameters were observed between subcutaneous epoetin zeta and epoetin alfa.Citation41 The nearly identical pharmacokinetic characteristics suggest bioequivalence of epoetin zeta to epoetin alfa.
The dosage for epoetin zeta is 93–97 IU/kg/week to treat anemia due to loss of endogenous production of EPO in patients with chronic renal failure.Citation41 Despite its differences in the glycosylation pattern from epoetin alfa, epoetin zeta stimulates erythropoiesis by binding to the EPO receptor on cells.Citation68 Once epoetin zeta is bound to the receptor, the receptor dimerizes and activates the Janus kinase 2 signal pathway, which goes on to activate the transcription 5 pathway.Citation68 This pathway continues to activate the EPO gene needed for proliferation and maturation of red blood cells and to treat anemia.Citation77 As a biosimilar using the endogenous pathway, there is concern that epoetin zeta could lead to neutralizing antibodies.Citation48,Citation57 Neutralizing antibodies react against endogenous EPO and result in PRCA. PRCA occurs when there is no endogenous production of EPO due to antibodies clearing endogenous EPO and rHuEPO. The clearing of all EPO results in severe anemia requiring blood transfusion intervention.Citation77 However, epoetin zeta shows a different immunogenicity profile,Citation48 mitigating the likelihood of PRCA.Citation41,Citation52
Efficacy
Efficacy studies test the comparability of biosimilars to the referent product for specific endpoints as well as product interchangeability. Epoetin zeta demonstrates comparable therapeutic efficacy to the reference product (epoetin alfa) for IV and subcutaneous routes of administration in the correction and maintenance phases of treating renal anemia.Citation24,Citation27,Citation28,Citation78
Hemoglobin endpoints were measured in two premarket authorization studies for the correction and maintenance phases of treating renal anemia in patients receiving hemodialysis. These premarket studies were designed to assess the clinical efficacy of SB309 (epoetin zeta) to the referent product, epoetin alfa (Erypo® formulation, Johnson & Johnson, New Brunswick, NJ, USA). One randomized, double-blind, verum controlled, multiple dose, parallel group, multicenter study (study 411-54-04-05-0000) assessed the therapeutic equivalence of SB309 to the referent product in the correction phase of renal anemia when administered IV. Another randomized, double-blind, crossover, verum controlled, multiple dose, multination Phase III trial (study 411-54-04-04-0000) tested the therapeutic equivalence of SB309 to the referent product in maintaining Hb levels, also when administered IV. Both studies met designated therapeutic Hb endpoints in correcting and maintaining target Hb levels when administered IV compared to the referent product.Citation61 Additional supportive open-label uncontrolled safety trials were conducted, reporting similar safety profiles between epoetin zeta and the referent product.Citation61 These premarket studies, demonstrating a high degree of similarity between epoetin zeta and the referent product, led to the approval of epoetin zeta as a biosimilar to epoetin alfa. After receiving authorization for IV administration, a later premarket authorization randomized trial assessed the therapeutic equivalence of subcutaneously administered epoetin zeta versus epoetin alfa. This study showed therapeutic equivalence in maintaining target Hb levels (epoetin zeta mean Hb 10.94 ± 0.84 g/dL, epoetin alfa mean Hb 11.02±0.94 g/dL [95% CI −0.28 g/dL to 0.12 g/dL]) and safety profiles.Citation41
Postmarket authorization research reports epoetin zeta as meeting or maintaining target Hb levels when administered IV, over the long term, or when patients switch from other ESAs to epoetin zeta. A study assessing the long-term safety and tolerability of IV epoetin zeta administration reports maintaining target Hb levels of 10.5–12.5 g/dL (after 56 weeks in n=745 patients, mean Hb=11.3–11.5 g/dL; after 108 weeks in n=164 patients, mean Hb=11.1–11.6 g/dL) with stable dosing and no neutralizing antibodies against erythropoietin.Citation79 One German postmarketing surveillance study of IV administered epoetin zeta in 322 eligible elderly (age ≥75 years) patients from 40 centers showed epoetin zeta as effective at maintaining Hb levels with no significant difference in mean dose requirements or safety concerns.Citation80 A study by Bajraktar et alCitation81 assessing interchangeability switched 33 patients in two dialysis centers from various ESAs to epoetin zeta. The Bajraktar et al study reported that epoetin zeta maintained target Hb with equivalent mean dose, no PRCA, and a safety profile in line for the study population.
Safety
Several studies report adverse events associated with using erythropoiesis stimulating agents (ESAs), including epoetin alfa, when trying to achieve supraphysiologic target Hb levels or when using high doses to achieve normalization of Hb levels in poorly responsive renal anemia.Citation30,Citation60,Citation62,Citation82 When treating anemia in CKD with ESAs, using high or supraphysiologic Hb thresholds, and/or high target Hb levels, and/or high ESA dosing levels to guide therapy a significant association is shown, with an increased risk for mortality, morbidity, cardiovascular events and stoke, and tumor progression. Also, supraphysiologic Hb thresholds significantly increase the risk for an immunogenic response termed ESA-induced PRACA.Citation30,Citation60,Citation62,Citation82
Three important studies – the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR),Citation82 Cardiovascular Reduction Early Anemia Treatment Epoetin beta (CREATE),Citation83 and Trial to Reduce cardiovascular Events with Aranesp Therapy (TREAT)Citation84 – reported pivotal findings influencing current thinking about safety and practice guidelines related to target Hb ranges and dosing for all ESAs, including biosimilars such as epoetin zeta when treating renal anemia. CHOIR and CREATE reported findings from an open-label research design, while the TREAT study reported results from a placebo controlled randomized double-blind controlled trial. The CHOIR study (EPO alfa) included 1,432 CKD patients, approximately 50% of whom had coexistent diabetes, and had an intention-to-treat Hb range of 13.5 g/dL versus 11.3 g/dL. The study was stopped due to the number and severity of adverse safety events in the high Hb group.Citation82 Another study, CREATE, assessed EPO beta with Hb intention-to-treat (13–15 g/dL versus 10.5–11.5 g/dL) in 603 patients with CKD and anemia (n=301 and n=302, respectively) with results showing significant progression to ESRD in the higher target Hb group.Citation83 The study also showed no significant difference between groups for cardiovascular outcomes and LVH.Citation83 A third study, TREAT, included patients with CKD, type 2 diabetes, and anemia (n=2,012 treated with darbepoetin alfa targeting Hb 13 g/dL versus the placebo control group n=2,026 treated with rescue doses of darbepoetin alfa for Hb <9 g/dL) and reported no significant difference in hazard rates for cardiovascular events, death, myocardial ischemia, or ESRD, and a significant nearly two-fold increased risk of stroke.Citation84
Current clinical practice recommendations suggest lower than previously accepted parameters for target Hb ranges in CKD for those not yet on dialysis and for patients on dialysis, as well as for low risk and high risk patients with other comorbid conditions. Guidelines continue to evolve in light of the CHOIR, CREATE, and TREAT findings and subsequent secondary analysis of their data, among other research using secondary analysis of the data, with revisions being made to previous standards for appropriate Hb levels when initiating treatment as well as target Hb ranges for maintenance therapy. To give context, the 2008 ERBP guidelines for the management of anemia in CKD revised earlier guidelines and emphasize safety concerns when using ESAs.Citation85 More recently, in April 2013, the KDIGO position paper on anemia management guidelines in CKD summarizes, in chapter 3, the recent evidence about earlier EPO safety concerns and recommends adapting the guidelines for the European population.Citation29 When considering the risks and benefits of initiating ESA therapy, the KDIGO recommends caution in high risk groups (stroke, vascular access loss, or hypertension) and great caution for those with CKD and active malignancy, and suggests Hb levels for low-risk patients (no higher than 12 g/DL) and high risk patients (Hb between 9 and 10 g/dL) when initiating therapy.Citation29 The report discusses Hb normalization and suggests individualized therapy, but cautions against using ESAs to maintain Hb levels above 11.5 g/dL.Citation30 The KDIGO group goes on to say ESAs should not be used to intentionally increase Hb concentrations above 13 g/dL (130 g/L).Citation30 In the US, in March 2007, the FDA issued a public health advisory with an updated black box warning for approved ESAs in the US (epoetin alfa and darbepoetin alfa) with the recommendation to monitor Hb levels and adjust ESA dose to the lowest possible to avoid blood transfusion.Citation62 Collaterally, the CMS put forth a position statement for ESA use in the treatment of anemia in adults with CKD, including patients on dialysis and patients not on dialysis, after reviewing the evidence related to safety.Citation65,Citation66
The safety profile for epoetin zeta is similar to epoetin alfa, the reference product. Reports of ESA-induced PRACA in all commercially available ESAs are a key safety concern. Although the mechanism behind ESA-induced PRACA remains unknown, the highest incidence arose from epoetin alfa manufactured outside the US (Eprex®/Erypro®; Ortho-biologics, LLC, Manati, Puerto Rico).Citation86 Our review found no reports for ESA-induced PRACA related to manufacture site. The Baldamus et al study assessing the long-term safety of IV administered epoetin zeta showed no incidence of PRACA.Citation79 Three other postmarket clinical trials reported no cases of anti-epoetin antibodies or PRCA.Citation24,Citation28,Citation41
A few studies have compared epoetin alfa’s safety profile to that of epoetin zeta. Del Vecchio and Locatelli’s review article examined cardiovascular safety (in particular stroke and hypertension), cancer progression, and immunogenicity associated with using ESA to treat anemia in patients with chronic disease.Citation57 To avoid high ESA dosing, the authors suggest using a conservative and individualized approach based on a risk/benefit evaluation, and targeting intermediate Hb levels.Citation75,Citation86
Eight studies reported the safety of epoetin zeta in patients with renal anemia. Of those, there are four clinical trials,Citation24,Citation28,Citation41,Citation79 three observational studies,Citation76,Citation81,Citation87 and one post hoc analysis based on two clinical trials.Citation88 summarizes the common adverse events observed in more than 5% of the patients in the four clinical trials. Infections and infestations were the most common adverse events reported in the clinical trials (12.5%–34.1%) followed by gastrointestinal disorders (5.2%–21.9%) and injury, poisoning, and procedural complications (7.2%–25.8%). Baldamus et alCitation79 reported 715 serious adverse events in 278 patients with cardiac disorders (8.6% of patients), vascular disorders (6.6% of patients), and injury, poisoning, and procedural complications (7.0% of patients) as the most common groups of serious adverse events occurring in more than 5% of patients. Krivoshiev et alCitation41 assessed epoetin zeta administered subcutaneously for maintenance treatment of renal anemia. The study reported 38 patients as having 91 serious adverse events, with the three most common serious adverse events being medical surgical and medical procedures (4.7% of patients), cardiac disorders (3.4% of patients), and nervous system disorders (3.4%). Krivoshiev et alCitation28 also compared therapeutic effects of epoetin zeta and epoetin alfa in the correction of renal anemia, where 54 out of 305 patients treated with epoetin zeta reported serious events. Wizemann et al’sCitation24 crossover design clinical trial showed 43 patients experiencing 71 serious adverse events, with injuries, poisoning, and procedural complications as the most reported serious adverse events (23.3% of patients).
Table 4 Adverse events reported in epoetin zeta clinical trials
Lonnemann and Wrenger’sCitation76 small clinical observational study assessing the use of epoetin zeta in ESRD reported 17 out of 18 patients as switching from various ESAs to epoetin zeta with no side effects after 6 months of epoetin zeta treatment. Bajraktar et alCitation81 evaluated the safety of epoetin zeta in 33 dialysis patients and reported that 3% of the patients develop hypotension, in-dialyzers clotting, or thrombosis of the arteriovenous fistula. Perani et alCitation87 examined the immunological reaction of epoetin zeta in 12 patients with CKD. This study showed no adverse events after switching from methoxy polyethylene glycol epoetin beta to epoetin zeta.
In a post hoc analysis of three large Phase III clinical trials, Wiecek et alCitation88 reported the safety of switching epoetin alfa and epoetin zeta. The post hoc analysis includes 481 patients from the maintenance trial (n=239) as well as from the induction trial (n=242). The results show no difference in the treatment-emergent adverse event profiles when patients switch from epoetin alfa to epoetin zeta. The same type of treatment-emergent events occurred as would be seen in ≥10% of the patient population.
Overall, the biosimilar epoetin zeta shows tolerable adverse effects in patients with renal anemia compared to the reference product epoetin alfa.Citation52,Citation89 However, regulations require a detailed risk management plan to monitor serious and severe safety events and unknown potential safety issues for pharmacovigilance.Citation77 For instance, assessing safety issues in the populations underrepresented in clinical trials, such as elderly patients or patients with various comorbidities, would add to risk management plan requirements. Additional postmarketing pharmacovigilant activities to evaluate unanticipated issues that may happen when patients switch between a biosimilar and their referent product could include the close monitoring of PRCA and thromboembolic events, as well as drug utilization and tracking of rare but serious adverse events.Citation57,Citation89 illustrates the adverse events reported in epoetin zeta clinical trials.
Current treatment in CKD
Patients with symptomatic renal anemia subsequent to ESRD or CKD of at least stage 3 (estimated GFR <60) whose Hb levels are less than or equal to 10 g/dL are eligible for treatment.
For all CKD indications, current EMA guidelines recommend specific target Hb ranges and dosing regimens for initiating treatment (termed the correction phase) as well as for maintenance therapy. Target Hb levels are particularly important due to the safety concerns surrounding the increased risk for mortality and morbidity associated with epoetin use to achieve relatively high target Hb levels.Citation90 The target Hb ranges are 10–12 g/dL (6.2–7.5 mmol/L) for adults and 9.5–11 g/dL (5.9–6.8 mmol/L) in pediatric patients. Clinicians should avoid maintaining Hb levels >12 g/dL (7.5 mmol/L) and should be vigilant to avoid a rise in Hb levels >2g/dL (1.25 mmol/l) over a 4 week period.Citation90
Licensed health care providers face patient care challenges related to the interchangeability of biotherapeutics and ESA drug safety concerns including those for epoetin zeta.Citation91,Citation92 Interchangeability can occur between biosimilar products and the reference product, as well as among a biopharmaceutical product class (eg, any ESAs). Salem and Harvie discuss the nurse’s role and responsibilities regarding biosimilar medicines and their clinical use.Citation93 Inadequate knowledge of biosimilar products increases the risks for medication errors and adverse events, and/or may affect desired treatment outcomes. Due to the complex nature of biopharmaceutical products, the study authors suggest providing additional professional training and educational opportunities for healthcare providers who prescribe, dispense, or administer biopharmaceutical products or biosimilars.Citation93 Health care providers should be well informed about the differences between biosimilars and their reference products, gain evidence-based knowledge about biosimilar use in clinical practice, and track adverse events when patients switch between biosimilar and reference products.Citation78,Citation92,Citation93
Pharmacoeconomic considerations
ESAs bring significant clinical benefits to patients with renal anemia. However, ESAs are very expensive and access to them is likely to be restricted. With the advent of biosimilars, drug access and treatment options improved adding potential health economic benefits over the reference biopharmaceutical ESA therapy.Citation87,Citation88,Citation93,Citation94,Citation96 In one survey in Spain, the epoetin zeta cost analysis reported a total cost saving of nearly 45% with respect to epoetin alfa.Citation97 Costs per patient per month declined from €298 for epoetin alfa therapy to €177 per patient per month for epoetin zeta.Citation96 Additionally, a UK cost minimization study showed that epoetin zeta minimizes the costs for treating renal anemia compared to epoetin alfa.Citation98 Epoetin zeta decreased the cost by £7.9 per week for a hemodialysis patient in the Hb correction phase and by £3.9–£15.6 per week in a hemodialysis patient at Hb maintenance phase, based on a 70 kg patient.Citation98 Low acquisition costs for epoetin zeta contribute to the potential cost savings. However, the risk of immunogenicity and the safety profile for serious adverse events raises uncertainties for the long-term safety and effectiveness of biosimilars. Future epoetin zeta studies could assess patient outcomes and costs for target Hb levels and further compare results between epoetin zeta and epoetin alfa. Additionally, Simoens suggests conducting further pharmacoeconomic analyses at multiple time points throughout the life cycle of biological pharmaceutical products.Citation99
Utility
Utility measures the value of health states from a specified perspective (eg, society, payer, or patient perspective) using a standard measure such as a quality adjusted life year (QALY). Our search did not find any formal cost utility analysis or direct elicitation measures, such as standard gamble or time trade-off, for epoetin zeta in CKD. We found only one study reporting quality of life (QOL) for epoetin zeta in chemotherapy-induced anemia.Citation100
Early research suggests improved health outcomes and symptom management for rHuEPO (EPO alfa), such as exercise capacity,Citation36 and health related QOL in patients with renal anemia.Citation37,Citation101,Citation102 A recent post hoc analysis of the Canadian Erythropoietin Study Group trial ([EPO alfa] included patients on dialysis >3 months with anemia (Hb <9 g/dL), randomized to (n=38, epoetin zeta intravenous treatment group versus 40, placebo), intention-to-treat Hb range 9.5–11 g/dL EPO alfa treatment group versus Hb 11.5–13 g/dL placebo control) showed significantly that fatigue, shortness of breath, and weakness were lessened, and energy was improved in the epoetin alfa treatment group compared to controls.Citation103 Another post hoc, multicenter, open-label prospective study using epoetin alfa to treat anemia in patients with nondialysis CKD showed that, across every 2 g/dL change in Hb, the greatest incremental improvement in QOL occurred when the Hb level reached 11–12 g/dL.Citation102 A meta-analysis of epoetin alfa clinical trials by Jones et al reported improvements in Hb levels and QOL, and reductions in hospitalizations, overall transfusion rate, and number of units of blood transfused.Citation104 Another study showed improvements in QOL when treating anemia with epoetin alfa in patients with human immunodeficiency virus infection/acquired immunodeficiency syndrome and coexistent CKD.Citation105
However, the CHOIR trial reported serious increased risk of death, myocardial infarction, congestive heart failure, and stroke with no incremental improvement in QOL when correcting renal anemia with epoetin alfa with target Hb levels of 13.5 g/dL.Citation83 Target Hb levels remain an important consideration in the risk benefit profile for epoetin alfa and in the relationship to utility measures and health outcomes. A review by Leaf and Goldfarb to assess the magnitude and nature of ESA-associated improvements in health related QOL found the maximal increase in health related QOL occurs for Hb ranging between 10 and 12 g/dL, with health-related quality of life (HRQL). improvements blunted beyond that range.Citation106 Additional utility research is needed to assess QALYs, symptom management using the current standard of care for appropriate Hb levels when initiating therapy, and Hb target ranges to correct renal anemia with epoetin zeta compared to other EPAs or biosimilars.
Although we did not find any studies reporting QOL outcomes generally or in terms of QALY, or by symptom management for epoetin zeta in managing anemia in CKD, one study reported improvements in QOL when treating patients with chemotherapy-induced anemia with epoetin zeta.Citation100 Future research could further our knowledge about utility outcomes for epoetin zeta in CKD, by the different stages of CKD, or among patients with different risk profiles as compared to the reference product (EPO alfa), or as compared to other ESAs or other biosimilars.
Conclusion
Our review of the biosimilar epoetin zeta found that epoetin zeta has therapeutic equivalence and a comparable safety profile to the reference product (EPO alfa). Epoetin zeta shows comparable efficacy to epoetin alfa in the correction and maintenance phases of CKD anemia treatment for both IV and subcutaneous administration, as well as when interchanged with other ESAs. Although two studies report cost savings, the small number of rigorous studies devoted to cost effectiveness limits the generalizability of suggesting epoetin zeta as a cost effective alternative to current standard treatment or other ESAs.Citation107 Although one study assessed health related QOL in cancer, to date, we found no utility studies assessing measures of health related QOL, when treating renal anemia with epoetin zeta. Evidence based practice guidelines for ESAs for both originator products and biosimilars, including epoetin zeta, continue to evolve in light of safety concerns, and include recent recommendations about contraindications, when to initiate therapy, dosing, and parameters for Hb levels and target maintenance Hb ranges. Further research is needed to expand our knowledge of the cost effectiveness and health related QOL outcomes for epoetin zeta.
Disclosure
The authors report no conflicts of interest in this work.
References
- KDOQI Clinical Practice Guidelines: KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease [webpage on the Internet]New YorkNational Kidney Foundation2006 Available from: http://www.kidney.org/professionals/kdoqi/guidelines_anemia/cpr12.htmAccessed September 09, 2013
- KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and StratificationNew YorkNational Kidney Foundation2002 Available from: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/bibliography.htm#267Accessed September 09, 2013
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work GroupKDIGO Clinical Practice Guideline for Anemia in Chronic Kidney DiseaseKidney Int20122279335
- CoreshJSelvinEStevensLAPrevalence of chronic kidney disease in the United StatesJAMA2007298172038204717986697
- El NahasAMBelloAKChronic kidney disease: the global challengeLancet2005365945633134015664230
- US GovernmentRenal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of HealthNational Institute of Diabetes and Digestive and Kidney DiseasesBethesda, MD2013
- RoderickPRothMMindellJPrevalence of chronic kidney disease in England: findings from the 2009 health survey for EnglandJ Epidemiol Community Health201165Suppl IA12
- ZoccaliCKramerAJagerKChronic kidney disease and end-stage renal disease a review produced to contribute to the report ‘status of health in the European union: towards a healthier Europe’NDT Plus201033213224
- MurrayCJVosTLozanoRDisability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010; a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592197222323245608
- LevinAIdentification of patients and risk factors in chronic kidney disease: evaluating risk factors and therapeutic strategiesNephrol Dial Transplant200116Suppl 7576011590259
- ChadbanSHowellMTwiggSCARIThe CARI guidelines. Cost-effectiveness and socioeconomic implications of prevention and management of chronic kidney disease in type 2 diabetesNephrology (Carlton)201015Suppl 1S19520320591031
- GoASChertowGMFanDMcCullochCEHsuCYChronic kidney disease and the risks of death, cardiovascular events, and hospitalizationsN Engl J Med2004351131296130515385656
- WeinerDETighiouartHAminMGChronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studiesJ Am Soc Nephrol20041551307131515100371
- RoncoCHaapioMHouseAAAnavekarNBellomoRCardiorenal syndromeJ Am Coll Cardol2008521915271539
- AstorBCMuntnerPLevinAEustaceJACoreshJAssociation of kidney function with aneamia; The Third national Health and Nutrition Examination Survey (1988–1994)Arch Intern Med2002162121401140812076240
- McClellanWAronoffSLBoltonWKThe prevalence of anemia in patients with chronic kidney diseaseCurr Med Res Opin20042091501151015383200
- PainterPMooreGCarlsonLEffects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of lifeAm J Kidney Dis200239225726511840365
- PerlmanRLFinkelsteinFOLiuLQuality of life in chronic kidney disease (CKD); a cross-sectional analysis in the Renal Research Institute-CKD studyAm J Kidney Dis200545465866615806468
- BaigentCBurburyKWheelerDPremature cardiovascular disease in chronic renal failureLancet2000356922414715210963260
- PortolésJGorrizJLRubioEfor NADIR-3 Study GroupThe development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney diseaseBMC Nephrol201314223295149
- ThorpMLJohnsonESYangXPetrikAFPlattRSmithDHEffect of anaemia on mortality, cardiovascular hospitalization and end-stage renal disease among patients with chronic kidney diseaseNephrology (Carlton)200914224024619207866
- LocatelliFAndrulliSMemoliBNutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patientsNephrol Dial Transplant200621499199816384825
- VaziriNDAnemia and anemia correction: surrogate markers or causes of morbidity in chronic kidney disease?Nat Clin Pract Nephrol20084843644518542121
- WizemannVRutkowskiBBaldamusCScigallaPKoytchevREpoetin Zeta Study GroupComparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatmentCurr Med Res Opin200824362563718208642
- EandiMErythropoiesis-stimulating agents in patients with chronic kidney diseaseRev Health Care201232113125
- McGonigleRJWallinJDShadduckRKFisherJWErythropoietin deficiency and inhibition of erythropoiesis in renal insufficiencyKidney Int19842524374446727139
- LonnemannGMacdougallICBiosimilar epoetins in renal anaemia â current status and insights from European practiceEur Nephrol201152101107
- KrivoshievSTodorovVVManitiusJComparison of the therapeutic effects of epoetin zeta and epoetin alfa in the correction of renal anaemiaCurr Med Res Opin20082451407141518394266
- MacdougallICRecent advances in erythropoietic agents in renal aneamiaSemin Nephrol200626431331816949470
- LocatelliFBáránayPCovicAKidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statementNephrol Dial Transplant20132861346135923585588
- KDOQIKDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney DiseaseAm J Kidney Dis2007492 Suppl 2S12S15417276798
- Zumbreanen-BulloghKBabittJLThe iron cycle in CKD: from genetics and experimental models to CKD patientsNephrol Dial Transplant Epub11132013
- JonesMSchenkelBJustJEpoetin alfa’s effect on left ventricular hypertrophy and subsequent mortalityInt J Cardiol2005100225326515823633
- BenzRLPressmanMRHovickETPetersonDDA preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (the SLEEPO study)Am J Kidney Dis19993461089109510585319
- EvansRWRecombinant human erythropoietin and the quality of life of end-stage renal disease patients: a comparative analysisAm J Kidney Dis1991184 Suppl 162701928082
- PainterPThe importance of exercise training in rehabilitation of patients with end-stage renal diseaseAm J Kidney Dis1994241 Suppl 1S2S9 discussion S31–S328023835
- RevickiDABrownREFeenyDHHealth-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patientsAm J Kidney Dis19952545485547702049
- RogerSDBiosimilars: How similar or dissimilar are they?Nephrology (Carlton)200611434134616889575
- Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant erythropoietins (Revision)LondonEuropean Medicines Agency, Committee for Medicinal Products for Human Use (CHMP)201010 [about 8 p.] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/04/WC500089474.pdfAccessed September 12, 2013
- RossertJEMEA guidelines on biosimilars and their clinical implicationsKidney Blood Press Res200730Suppl 1131717726338
- KrivoshievSWizemannVCzekalskSTherapeutic equivalence of epoetin zeta and alfa, administered subcutaneously, for maintenance treatment of renal anemiaAdv Ther201027210511720369312
- TrioloGItalian Society of NephrologyGuidelines for the treatment of aneamia in chronic renal failureG Ital Nefrol200320Suppl 24S61S82 Italian14666504
- BarosiGBosiAAbbroacchioMPKey concepts and crucial issues on epoetin and filgrastim biosimilars. A position paper from the Italian Society of Hematology, Italian Society of Experimental Hematology, and Italian Group for Bone Marrow TransplantationHaematologica201196793794221719882
- WallerCFBiosimilars and their use in hematology and oncologyCommun Oncol201296198205
- TsiftsoglouASRuizSSchneiderCKDevelopment and regulation of biosimilars: Current status and future challengesBio Drugs2013273203211
- BorgesLDifferent modalities of erythropoiesis stimulating agentsPort J Nephrol Hypert2010242137145
- SitteHHBiologicals and biosimilarsPort J Nephrol Hypert2009232135139
- TamilvananSRajaNLSaBBasuSKClinical concerns of immunogenicity produced at cellular levels by biopharmaceuticals following heir parenteral administration into human bodyJ Drug Target2010181748949820192653
- SchellekensHBiosimilar therapeutics – what do we need to consider?NDT Plus20092Suppl 1i27i3619461855
- CasadevallNEchardtKURossertJEpoetin-induced autoimmune pure red cell aplasiaJ Am Soc Nephrol200516Suppl 1S67S6915938038
- CasadevallNWhat is antibody-mediated pure red cell aplasia (PRCA)?Nephrol Dial Transplant200520Suppl 4iv3iv815827056
- AbrahamISunDBagalagelABiosimilars in 3D: Definition, development and differentiationBioengineered20134420320623714845
- AhmedIKasparBSharmaUBiosimilars: Impact of biologic product life cycle and European experience on the regulatory trajectory in the United StatesClin Ther201234240041922244050
- CombeCTredreeRLSchellekensHBiosimilar epoetins: An analysis based on recently implemented European medicines evaluation agency guidelines on comparability of biopharmaceutical proteinsPharmacother2005257954962
- WeiseMBielskyMCDe SmetKBiosimilars: what clinicians should knowBlood2012120265111511723093622
- Non-clinical guidelines: European Union guidelines adopted in Australia [webpage on the Internet]Symonston: Department of HealthTherapeutic Goods Administration2012 Available from: http://www.tga.gov.au/industry/pm-euguidelines-adopted-nonclinical.htm#nonclinicalsimilarAccessed Sept 10, 2013
- Del VecchioLLocatelliFSafety issues related to erythropoiesis-stimulating agents used to treat anemia in patients with chronic kidney diseaseExpert Opin Drug Saf201211692393122916722
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP)Guidelines on Immunogenicity Assessment of Biotechnology-Derived Therapeutic ProteinsLondonEuropean Medicines Agency2007 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003947.pdfAccessed September 09, 2013
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP)Similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issuesLondonEuropean Medicines Agency2006 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdfAccessed September 09, 2013
- JelkmannWBiosimilar epoetins and other “follow-on” biologics: update on the European experiencesAm J Hematol2010851077178020706990
- European medicines AgencyScientific discussionLondonEuropean medicines Agency2007 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000872/WC500054374.pdfAccessed September 10, 2013
- MP389 Immunogenicity of epoetin zeta in the treatment of renal anaemia [webpage on the internet]CasadevallNKrommingaAScigallaP Available from: http://www.abstracts2view.com/era_archive/view.php?nu=ERA08L_1714Accessed September 14, 2013
- Guidance for industry formal meetings between the FDA and biosimilar biological product sponsors or applicants [webpage on the Internet]Silver SpringU.S. Food and Drug Administration Available from: http://www.fda.gov/Drugs/GuidancecomplianceRegulatoryInformation/Guidances/default.htmAccessed March 2013
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER)Draft guidance for industry formal meetings between FDA and biosimilars biological product sponsors or applicants Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM345649.pdfAccessed January 8, 2014
- Decision memo for erythropoiesis stimulating agents (ESAs) for treatment of anemia in adults with CKD including patients on dialysis and patients not on dialysis (CAG-00413N) [webpage on the Internet]BaltimoreCenters for Medicare and Medicaid Services (CMS)2011 Available from: http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=245&bc=AAAAAAAACAAAAA%3d%3d&Accessed September 20, 2013
- Erythropoietin stimulating agents policies [webpage on the Internet]BaltimoreCenters for Medicare and Medicaid Services (CMS)2013 Available from: http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/esapolicies.htmlAccessed September 20, 2013
- KapitsinouPPLiuQUngerTLHepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemiaBlood2010116163039304820628150
- MacdougallICNovel agents for anaemia management in chronic kidney diseaseEuropiean Nephrol2010413741
- HaaseVHRegulation of erythropoiesis by hypoxia-inducible factorsBlood Rev2013271415323291219
- BernhardtWMWiesenerMSScigallaPInhibition of prolyl hydroxylases increases erythropoietin production in ESRDJ Am Soc Nephrol201021122151215621115615
- EschbachJWEgrieJCDowningMRBrowneJKAdamsonJWCorrection of the anemia of end-stage renal disease with recombinant human erythropoietinN Engl J Med1987316273783537801
- AdamsonJWEschbachJWManagement of the anaemia of chronic renal failure with recombinant erythropoietinQ J Med198973272109311012694210
- HörlWHDifferentiating factors between erythropoiesis-stimulating agents: An update to selection for anaemia of Chronic Kidney DiseaseDrugs201373211713023338536
- WalshGPost-translational modifications in the context of therapeutic proteins: An introductory overviewPost-translational Modification of Protein BiopharmaceuticalsWest Sussex, UKWiley-Blackwell2009114
- LocatelliFDel VecchioLErythropoiesis-stimulating agents in renal medicineOncologist201116Suppl 3192421930831
- LonnemannGWrengerEBiosimilar epoetin zeta in nephrology: Effect of injection frequency on weekly doseInt J Clin Med20123598602
- EbbersHCMuenzbergMSchellekensHThe safety of switching between therapeutic proteinsExpert Opin Biol Ther201212111473148522849511
- CasadevallNEdwardsIRFelixTPharmacovigilance and biosimilars: Considerations, needs and challengesExpert Opinion Biol Ther201313710391047
- BaldamusCKrivoshievSWolf-PflugmannMSiebert-WeigelMKoytchevRBronnALong-term safety and tolerability of epoetin zeta, administered intravenously, for maintenance treatment of renal anemiaAdv Ther200825111215122818931828
- BachmakovIMeiBnerRBenkensteinCSa423 Comparison of long-term efficacy and safety of epoetin zeta in elderly ‘real-word’ patients with chronic kidney disease (CKD) and renal anaemia on dialysis aged ≥ and <75 yearsPoster Session: Anaemia in CKD 5
- BajraktarJLazarovaBNajdovskaEMihajlovaLDSL-011 efficacy and safety of epoetin zeta in dialysis patientsEur J Hosp Pharm Sci Pract201320A91
- SinghASaczechLTangKLCorrection of anemia with epoetin alfa in chronic kidney diseaseNew Engl J Med2006355202085209817108343
- DrüekeTBLocatelliFClyneNfor the CREATE investigatorsNormalization of hemoglobin level in patients with chronic kidney disease and anemiaN Engl J Med2006355202071208417108342
- PfefferMABurdmannEAChenCA trial of darbepoetin alfa in type 2 diabetes and chronic kidney diseaseN Engl J Med2009361212019203219880844
- ZockaliCAbramowiczDCannata-AndiaJBEuropean best practice quo vadis? From European best practice guidelines (EBPG) to European renal best practice (ERBP)Nephrol Dial Transplant2008232162216618469309
- PollockCJohnsonDWHörlWHPure red cell aplasia induced by erythropoiesis-stimulating agentsClin J Am Soc Nephrol20083119319918178785
- PeraniLScolariCBrausAGalliEGRP-179 switch from CERA to EPO zeta in patients with anaemia and chronic kidney diseaseEur J Hos Pharm Sci Pract201320A64
- WiecekAAhmedIScigallaPKoytchevRSwitching epoetin alfa and epoetin zeta in patients with renal anemia on dialysis: Posthoc analysisAdv Ther2010271294195220972656
- AbrahamIMacDonaldKClinical safety of biosimilar recombinant human erythropoietinsExpert Opin Drug Saf201211581984022880621
- Retacrit: epetin zeta. Product information: Annex I Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000872/human_med_001031.jsp&mid=WC0b01ac058001d124Accessed March 4, 2014
- LeeJSHaTKLeeSJLeeGMCurrent state and perspectives on erythropoietin productionAppl Microbiol Biotechnol20129561405141622820523
- LeeJFLittenJBGramppGComparability and biosimilarity: Considerations for the healthcare providerCurr Med Res Opin20122861053105822519391
- SalemLHarvieBBiosimilar medicines and their use: The nurse’s role and responsibilityRen Soc Aust J2010627680
- GoldsmithDGesualdoLBiosimilar epoetins in nephrology – where are we nowEur Nephrol201262124
- SimoensSVerbekenGHuysIBiosimilars and market access: A question of comparability and costs?Targeted Oncol201274227231
- GoldsmithD2009: A requiem for rHuEPOs – but should we nail down the coffin in 2010?Clin J Am Soc Nephrol20105592993520413441
- LopezMJAntoninoGVicenteIMejiaLGarciaPSanchezAErythropoietic factors in renal chronic disease: Economic managementInt J Clin Pharm2012341221222
- PearsonIVJohnsonKIA cost minimization analysis of epoetin zeta for the treatment of anemia associated with chronic kidney diseaseValue Health2008113A304
- SimoensSBiosimilar medicines and cost-effectivenessClinicoecon Outcomes Res20113293621935330
- TzekovaVMihaylovGElezovicIKoytchevREpoetin Zeta Oncology Study GroupTherapeutic effects of epoetin zeta in the treatment of chemotherapy-induced anaemia. Curr Med Res OpinJul200925716891697
- SoniRKWeisbordSDUnruhMLHealth-related quality of life outcomes in chronic kidney diseaseCurr Opin Nephrol Hypertens201019215315920051850
- LefebvrePVekemanFSarokhanBEnnyCProvenzanoRCremieuxPYRelationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfaCurr Med Res Opin200622101929193717022852
- KeownPAChurchillDNPoulin-CostelloMDialysis patients treated with epoetin alfa show improved anemia symptoms; A new analysis of the Canadian Erythropoietin Study Group trialHemodial Int201014216817320345390
- JonesMIbelsLSchenkelBZagariMImpact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysisKidney Int200465375776714871396
- KimelMLeidyNKMannixSDixonJDoes epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney diseaseValue Health2008111577518237361
- LeafDEGoldfarbDSInterpretation and review of health-related quality of life data in CKD patients receiving treatment for anemiaKidney Int20089751152418813284
- All Wales Medicines Strategy Group [homepage on the Internet]Epoetin zeta (Retacrit®): Reference 130 final appraisal recommendation Available from: http://www.awmsg.org/awmsgonline/app/appraisalinfo/130Accessed March 6, 2014

