Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent joint inflammation, systemic inflammation, and immunological abnormalities. Because cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 play a major role in the development of RA, their targeting could constitute a reasonable novel therapeutic strategy for treating RA. Indeed, worldwide clinical trials of TNF inhibiting biologic disease modifying antirheumatic drugs (bDMARDs) including infliximab, adalimumab, golimumab, certolizumab pegol, and etanercept as well as the humanized anti-human IL-6 receptor antibody, tocilizumab, have demonstrated outstanding clinical efficacy and tolerable safety profiles, resulting in worldwide approval for using these bDMARDs to treat moderate to severe active RA in patients with an inadequate response to synthetic disease modifying antirheumatic drugs (sDMARDs). Although bDMARDs have elicited to a paradigm shift in the treatment of RA due to the prominent efficacy that had not been previously achieved by sDMARDs, a substantial percentage of patients failed primary or secondary responses to bDMARD therapy. Because RA is a heterogeneous disease in which TNF-α and IL-6 play overlapping but distinct pathological roles, further studies are required to determine the best use of TNF inhibitors and tocilizumab in individual RA patients.
Introduction to rheumatoid arthritis (RA) and the development of targeted therapies
RA, a chronic disease affecting 0.5%–1% of adults, is characterized by persistent synovitis, systemic inflammation, and immunological abnormalities.Citation1,Citation2 Uncontrolled active RA causes joint damage, disability, diminished quality of life, and cardiovascular and other comorbidities. Although its exact pathogenesis is not fully understood, a multistep progression has been proposed for the development of RA.Citation1 Environment–gene interactions promote a loss of tolerance to self-antigens that contain a citrulline residue generated by posttranslational modification, leading to an anticitrulline response by both T-cells and B-cells. Thereafter, the inflammatory response becomes localized in the joints and synovitis is initiated and perpetuated by positive feedback loops, promoting systemic disorders. Lymphocytes, other inflammatory cells, and their products contribute to the development of RA. For instance, many cytokines have been implicated in the pathogenesis of RA, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-7, IL-15, IL-17A, IL-17F, IL-18, IL-21, IL-23, IL-32, IL-33, and granulocyte-macrophage colony stimulating factor.Citation1
Because TNF-α is an important mediator responsible for joint inflammation and destruction, it was the first cytokine to be targeted in the treatment of RA.Citation2,Citation3 TNF-α is overexpressed in the synovial fluid of patients with RA. Moreover, TNF-α transgenic mice spontaneously develop arthritis. The first biologic disease modifying antirheumatic drugs (bDMARD) generated was infliximab (IFX), a chimeric monoclonal antibody (mAb) to TNF-α. Clinical trials of IFX proved that TNF blockade is highly efficacious in the treatment of RA and led to the development of other TNF inhibitors.
Another cytokine that has been targeted in the treatment of RA is IL-6, a typical cytokine featuring redundancy and pleiotropic activity that plays a key role in the development of RA.Citation4–Citation6 IL-6 promotes the development of an imbalance between Th17 and regulatory T (Treg) cells and the production of autoantibodies, such as rheumatoid factor and anticitrullinated peptide antibody. IL-6 also promotes synovial inflammation and cartilage and bone destruction and has systemic effects in cardiovascular, psychological, and skeletal disorders. The first generated bDMARD targeting IL-6 was tocilizumab (TCZ), a humanized anti-IL-6 receptor antibody. Now, other IL-6 inhibitors are also being developed and clinical trials for these agents are in progress.Citation6 These include fully human anti-IL-6 receptor mAb (REGN88/SAR153191 [sarilumab]), anti-IL-6 receptor nanobody (ALX-0061), anti-IL-6 Abs (CNTO136 [sirukumab], ALD518 [BMS-945429], CDP6038 [olokizumab], and MEDI5117). In this review, we highlight current data regarding the comparative efficacy and safety of TCZ and TNF inhibitors. We also discuss the positions of these agents in the treatment of RA.
Differential pharmacology of TCZ, adalimumab (ADA), and other TNF inhibitors
Several bDMARDs are currently available for the treatment of moderate to severe active RA, including five TNF inhibitors (IFX, ADA, golimumab [GOL], certolizumab pegol [CEP], and etanercept [ETA]), an IL-6 blocker (TCZ), a T-cell stimulator blocker (abatacept), a B-cell depletory (rituximab), and an IL-1 receptor antagonist (anakinra).Citation4,Citation6 The characteristic features of TCZ and five TNF inhibitors are shown in .
Table 1 Characteristics of tocilizumab and tumor necrosis factor inhibitors
TCZ is a humanized IgG1 class anti-IL-6 receptor mAb that was generated by grafting the complementarity determining regions of a mouse antihuman IL-6 receptor antibody (Ab) into human IgG1.Citation7 TCZ blocks IL-6 mediated signal transduction by inhibiting the binding of IL-6 to both transmembrane and soluble IL-6 receptors. TCZ can be administered intravenously or subcutaneously.
IFX was the first TNF inhibitor developed and it is a chimeric immunoglobulin (Ig) composed of a murine variable region and a human constant region against TNF-α. Due to immunogenicity and response failure issues, IFX is licensed to be used with methotrexate (MTX) by intravenous injection. ADA and GOL are fully human mAbs to TNF-α and can be used subcutaneously every 2 weeks and every 4 weeks, respectively. CEP is a humanized Fab fragment conjugated to polyethylene glycol (PEG). The attachment of PEG prolongs the drug’s half-life, whereas the absence of an Fc fragment prevents effector functions such as Ab-dependent cellular cytotoxicity and complement-dependent cellular cytotoxicity, as well as active transfer of CEP across the placenta during pregnancy. CEP is used subcutaneously every 2 weeks.
In contrast to these TNF inhibitors, ETA is a fusion protein consisting of two TNF receptor 2 (also known as p75TNF receptor) extracellular domains and a human Fc fragment of the IgG1 class. As TNF-α and lymphotoxin binds to TNF receptor 2, ETA neutralizes the biological activity of both cytokines. ETA is administered subcutaneously once or twice weekly.
Comparative efficacy studies of TCZ, ADA, and other TNF inhibitors
TCZ
The efficacy of TCZ administered alone or in combination with MTX or other synthetic disease modifying antirheumatic drugs (sDMARDs) was verified for active RA in seven Phase III trials. The three Phase III trials AMBITION, SAMURAI, and SATORI were designed to examine the efficacy of TCZ monotherapy.Citation8–Citation10 The AMBITION trialCitation8 involved active RA patients for whom previous treatment with MTX and TNF inhibitors had not failed. The SAMURAI trialCitation9 involved patients with an inadequate response to sDMARDs, and the SATORI trialCitation10 involved patients with an inadequate response to MTX. In all three studies, patients treated with TCZ had superior American College of Rheumatology (ACR) 20 responses and lower disease activity score (DAS) 28 at 24 weeks than controls treated with MTX or other sDMARDs.
Four Phase III trials were performed to evaluate the efficacy of TCZ combination therapy with MTX or another sDMARD. The OPTION trial was designed to evaluate the efficacy of TCZ in combination with MTX, and the results showed that combination therapy is effective for moderate to severe active RA.Citation11 The TOWARD trial demonstrated that TCZ combined with a sDMARD such as MTX, chloroquine, gold, sulphasalazine, azathioprine, or leflunomide is effective for reducing RA disease activity in patients with an inadequate response to monotherapy with any one of the sDMARDs.Citation12 The RADIATE trial proved that TCZ plus MTX is effective for achieving rapid and sustained improvements in signs and symptoms in patients whose RA is refractory to TNF inhibitors.Citation13 Moreover, the LITHE trial, which was designed to evaluate not only disease activity but also structural joint damage, demonstrated that TCZ plus MTX is efficacious at suppressing disease activity.Citation14 Radiographic evidence from the LITHE trial showed that progression of joint destruction is significantly inhibited after 52 weeks of combination treatment.Citation14 All of these studies enrolled patients with an inadequate response to all previous treatments, including MTX, TNF inhibitors, or other sDMARDs, and all of the studies showed that TCZ combination therapy is effective for these patient populations.
ADA
The efficacy and safety of ADA was examined in the ARMADA trial.Citation15 A total of 271 patients with active RA who had an inadequate response to MTX were randomized to continue MTX in combination with either placebo or ADA (20, 40, or 80 mg subcutaneously every other week). ACR20 responses at week 24 were 47.8, 67.2, and 65.8% in the 20, 40, and 80 mg groups, respectively, whereas the response rate was 14.5% for the placebo group. Subsequently, the PREMIER study, which involved 799 patients with early and aggressive RA who had no previous MTX use, confirmed that ADA plus MTX combination therapy is vastly superior to either MTX alone or ADA alone in improving clinical signs and symptoms, inhibiting radiographic progression of joint destruction, and effecting clinical remission.Citation16
IFX
In the Phase III trial ATTRACT, 428 RA patients with active disease activity and an inadequate response to MTX were randomized to receive MTX with either placebo or IFX (3 mg/kg every 4 weeks, 3 mg/kg every 8 weeks, 10 mg/kg every 4 weeks, or 10 mg/kg every 8 weeks).Citation17 At week 30, patients in the IFX treated groups achieved an ACR20 response rate of 50%–58%, versus an ACR20 response rate of only 20% in the placebo group. Structural damage was also assessed with the modified van der Heijde-Sharp score at week 102.Citation18 Compared with the MTX only regimen, erosion and joint space narrowing scores from baseline to week 102 with early RA patients decreased significantly with each of the IFX dose regimens.
GOL
In the Phase III trial GO-FORWARD, 444 active RA patients who had an inadequate response to MTX were randomly assigned to receive placebo subcutaneous injections plus MTX, GOL 100 mg plus placebo capsules, GOL 50 mg plus MTX, or GOL 100 mg plus MTX.Citation19 The proportion of patients who achieved an ACR20 response at week 14 was 33.1% in the placebo plus MTX group, 44.4% (P=0.059) in the GOL 100 mg plus placebo group, 55.1% (P=0.001) in the GOL 50 mg plus MTX group, and 56.2% (P<0.001) in the GOL 100 mg plus MTX group. At week 24, median Health Assessment Questionnaire Disease Index (HAQ-DI) score improvements from baseline for the placebo plus MTX, GOL 100 mg plus placebo, GOL 50 mg plus MTX, and GOL 100 mg plus MTX groups were 0.13, 0.13 (P=0.240), 0.38 (P<0.001), and 0.50 (P<0.001), respectively.
CEP
In the Phase III trial Rapid-1, 982 active RA patients were randomized to receive subcutaneous CEP at an initial dose of 400 mg given at weeks 0, 2, and 4, with a subsequent dosage of 200 or 400 mg every 2 weeks plus MTX, or placebo plus MTX.Citation20 At week 24, the ACR20 response rates were 13.6%, 58.8%, and 60.8% for the placebo, CEP 200 mg, and CEP 400 mg groups, respectively. At week 52, mean radiographic progression from baseline was reduced in patients treated with CEP 200 mg (0.4 Sharp units) or 400 mg (0.2 Sharp units), compared with placebo treated patients (2.8 Sharp units, P<0.001).
ETA
In a Phase II study, 234 active RA patients who had an inadequate response to previous treatment regimens including MTX were randomly assigned to receive twice weekly subcutaneous injections of ETA (10 or 25 mg) or placebo for 24 weeks. At week 24, the ACR20 response rates were 51%, 59%, and 11% in the ETA 10 mg, ETA 20 mg, and placebo groups, respectively.Citation21 In the subsequent Phase III TEMPO trial, 682 patients with active RA were randomly allocated to treatment with ETA 25 mg (subcutaneously twice weekly), oral MTX, or the combination.Citation22 The numeric index of the ACR response area under the curve over the first 24 weeks was significantly greater in the combination group than the ETA alone or MTX alone groups (P<0.0001). Moreover, at week 52, the combination was more efficacious than ETA alone or MTX alone in protecting against joint damage (mean total Sharp score: −0.54 versus 0.52, P=0.0006; −0.54 versus 2.80, P<0.0001, respectively).
Indirect comparisons of the efficacy of TCZ and TNF inhibitors
As indicated above, the efficacy of TCZ and TNF inhibitors in treating moderate to severe RA in patients who experienced an inadequate response to MTX has been demonstrated in separate studies. Although several systematic reviews have indirectly compared the efficacy of TCZ and TNF inhibitors in treating RA, only one trial, the ADACTA, has directly compared the efficacy of these agents.Citation23
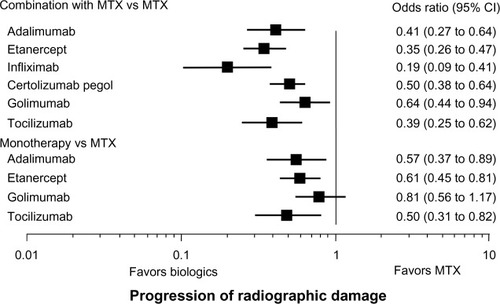
Bergman et al conducted a systematic literature review of double blind, randomized, placebo-controlled trials that spanned an 18-year period and investigated the effectiveness of TCZ (three trials; OPTION, LITHE, and TOWARD) and TNF inhibitors ADA, IFX, and ETA (total 11 trials) in treating RA in patients who experienced an inadequate response to sDMARDs.Citation24 The effectiveness of TCZ is comparable to that of each of the TNF inhibitors with respect to ACR20 and ACR50 responses, but greater than that of the TNF inhibitors with respect to ACR70 response. Another systematic review of selected clinical trials involving combination therapy with MTX concluded that there was no difference in efficacy on the basis of ACR50 response criterion at 24/30 weeks between TNF inhibitors and TCZ.Citation25 Turkstra et al reported a mixed treatment comparison of the short-term efficacy of nine bDMARDs, including TNF inhibitors and TCZ in patients with established RA.Citation26 They found that the ACR50 response rate of TCZ at 6 months is comparable to that of ADA, ETA, GOL, and IFX. In an indirect comparison, Salliot et al found no significant difference in the efficacy of TCZ and GOL in treating RA patients who had an inadequate response to TNF inhibitors (ADA, ETA, and IFX).Citation27 Orme et al reported the results of a network meta-analysis of the efficacy of bDMARDs with or without sDMARDs.Citation28 Odds ratios (covariate analysis) of ACR20/50/70 responses for ADA plus sDMARDs and TCZ plus sDMARDs versus sDMARDs alone were 3.374/4.203/4.58 and 4.363/5.797/9.23, respectively. In contrast, odds ratios (fixed effect) of ACR20/50/70 responses for ADA and TCZ versus placebo were 4.95/4.82/11.42 and 26.17/46.94/55.54, respectively. Pierreisnard et al also reported that there were no significant differences between the various TNF inhibitors and TCZ in terms of clinical efficacy (ACR50) in patients who had an inadequate MTX response.Citation29 Jones et al summarized the evidence regarding radiographic damage with bDMARDs, either alone or in combination with MTX.Citation30 For biologic monotherapy, TCZ, ADA, and ETA were significantly better than MTX, with TCZ ranking first, whereas GOL had no significant effect (). For a bDMARD in combination with MTX compared with MTX alone, TCZ and all TNF inhibitors were effective at slowing X-ray progression. Taken together, the evidence from these indirect comparisons indicates that the efficacy of TCZ is comparable to that of TNF inhibitors when used in combination with MTX and that TCZ monotherapy is superior to TNF inhibitor monotherapy.
Figure 1 Indirect comparisons of the suppressive effects of tocilizumab and tumor necrosis factor inhibitors on radiographic damage.
Abbreviations: CI, confidence interval; MTX, methotrexate.
Direct comparisons of the efficacy of TCZ and ADA
The head-to-head ADACTA trial compared the efficacy of TCZ with that of ADA as monotherapy for RA.Citation23 A total of 325 patients were randomly assigned to receive either TCZ 8 mg/kg intravenously every 4 weeks plus placebo subcutaneously every 2 weeks or ADA 40 mg subcutaneously every 2 weeks plus placebo intravenously every 4 weeks for 24 weeks. At week 24, patients treated with TCZ had a greater decrease in DAS28 than patients treated with ADA (−3·3 versus −1·8; P<0.0001). The proportion of patients attaining DAS28 remission was 39.9% with TCZ and 10.5% with ADA. ACR20, ACR50, and ACR70 response rates were achieved in 65% and 49.4% (P<0.01), 47.2% and 27.8% (P<0.01), and 32.5% and 17.9% (P<0.01) of patients treated with TCZ and ADA, respectively. These results demonstrated the overall superiority of monotherapy with TCZ compared with monotherapy with ADA for the treatment of RA. Clinical evidence demonstrated that coadministration of TNF inhibitors and MTX is more efficacious than administration of TNF inhibitors alone in treating RA.Citation31 In contrast, the findings of the ACT-RAY trial comparing the efficacy of TCZ plus MTX therapy with that of TCZ monotherapy in a setting that closely resembled a real life clinical practice showed that TCZ monotherapy is not clinically inferior to TCZ combination therapy,Citation32 indicating that as monotherapy, TCZ appears to be more effective than TNF inhibitors at suppressing disease activity.
In a Japanese cohort, the Tsurumai Biologics Communication Registry, the proportion of patients who achieved low disease activity, clinical remission, and a moderate or good European League Against Rheumatism (EULAR) response at 24 weeks was determined following treatment with ADA or TCZ.Citation33 A total of 120 patients were treated with ADA (77% of patients in combination with MTX), while 99 patients were treated with TCZ (36% of patients in combination with MTX). There was no significant difference between ADA and TCZ treated patients with respect to the proportion of low disease activity and remission, but a higher proportion of patients treated with TCZ achieved a moderate or good EULAR response.
Comparative safety and tolerability studies
The integrated safety of TCZ was evaluated in clinical trials through comparisons of adverse events (AEs) between a control population (4,199) and a TCZ treated population (4,009), and the results were reported in 2011.Citation34 Total exposure to TCZ was 8,580 patient-years (PYs), and the total duration of observation was 9,414 PYs. Overall AE and serious AE rates were 278.2/100 PYs and 14.4/100 PYs, respectively. AEs included serious infection (4.7/100 PYs), opportunistic infection (0.23/100 PYs), gastrointestinal perforation (0.28/100 PYs), malignancy (1.1/100 PYs), myocardial infarction (0.25/100 PYs), and stroke (0.19/100 PYs).
In another systematic review in which the total duration of observation was 12,293 PYs, infections were also the most common AE and serious AE identified, and the rate of serious infections was 4.5/100 PYs.Citation35 The short-term (28 weeks) safety of TCZ was monitored in a postmarketing surveillance study in Japan involving 7,901 patients.Citation36 The incidence of total AEs and serious AEs was 43.9% and 9.6%, respectively. Infection and infestation were the most frequent (11.1%) and serious (0.5%) AEs. Analysis of long-term clinical trial safety data showed that rates of serious AEs, serious infections, and cardiovascular events remained stable during continued exposure to TCZ. Infection was identified as the most frequent serious AE. The most common infections reported in randomized controlled trials (RCTs) were pneumonia (0.9/100 PYs) and skin or soft tissue infections (0.9/100 PYs). These results led to the conclusion that infections are the most frequent AEs associated with TCZ. A meta-analysis comparing the safety profile of TCZ with those of TNF inhibitors (7–9/100 PYs) showed similar rates of serious infections,Citation37,Citation38 although among TNF inhibitors, an increased risk of serious infection was observed with IFX.
As TNF-α plays a crucial role in the host defense against intracellular pathogens (eg, TNF-α activates macrophages and stimulates the formation and maintenance of granulomas to protect against Mycobacterium tuberculosis infection), TNF inhibitors increase the risk of tuberculosis reactivation, as evidenced by clinical trials showing an incidence of 0.4% with IFX.Citation39 Within the anti-TNF biologic cohort, IFX and ADA are associated with a 3- to 4-fold higher risk of reactivation than ETA.Citation40 It seems likely that the incidence of reactivation of tuberculosis is lower during TCZ treatment than during anti-TNF treatment, as there are only six reported cases in the worldwide TCZ clinical trials database, which covers >10,000 PYs of exposure.Citation41 Moreover, according to QuantiFERON assay data, TNF inhibitors (but not TCZ) influence tuberculosis-antigen-induced IFN-γ production,Citation42 suggesting that TCZ may be safer than TNF inhibitors with respect to reactivation of latent tuberculosis.
In contrast to TNF inhibitors, gastrointestinal perforation appears to be an AE specific to TCZ, with an incidence rate of 1.9–2.8/1,000 PYs.Citation34,Citation43 This rate is between the 3.9/1,000 PYs for corticosteroids and 1.3/1,000 PYs for TNF inhibitors, as indicated in the United Health Care database.Citation43 A total of 17 of 29 (59%) reported events involved colonic diverticular perforation, suggesting that TCZ should not be used in patients with a history of diverticulitis.
Increases in mean fasting levels of plasma lipids, such as total cholesterol (TC), low-density lipoprotein (LDL), triglycerides, and high-density lipoprotein (HDL), occur in 20%–30% of patients treated with TCZ, which appeared higher in patients treated with TNF inhibitors.Citation34,Citation36 A 24-week, double blind, randomized multicenter, two part, Phase III trial followed by an 80-week open label trial (MEASURE) evaluated lipid and lipoprotein levels, HDL particle composition, markers of coagulation, and thrombosis in 132 patients with RA receiving either TCZ or placebo.Citation44 At week 12, median TC, LDL-cholesterol (LDL-C), and triglyceride levels increased in TCZ recipients versus placebo recipients (12.6% versus 1.7%, 28.1% versus 2.2%, 10.6% versus −1.9%, respectively; all P<0.01). There were no significant differences in the concentrations of mean small LDL, mean oxidized LDL, or total HDL-C, but the HDL associated serum amyloid A (SAA) content decreased in TCZ treated patients. TCZ also induced reductions (>30%) in secretory phospholipase A2-IIA, lipoprotein (a), fibrinogen, and D-dimers and an elevation in the level of paraoxonase (all P<0.0001 versus placebo). These data constitute detailed evidence that TCZ modulates lipoprotein particles and other surrogates of vascular risk.
Comparisons of drug survival with TNF inhibitors have been reported in some registries. In the Consortium of Rheumatology Researchers of North America registry, the 24-month persistence for biologically naive patients on the new anti-TNF treatments IFX, ETA, and ADA was 63%, 53%, and 53% respectively.Citation45 The Lombardy Rheumatology Network registry reported 2.5-year treatment continuation rates for IFX, ETA, and ADA of approximately 56%, 72%, and 57%, respectively.Citation46 The Swiss Clinical Quality Management for Rheumatoid Arthritis registry reported 2.5-year drug survival rates for IFX, ETA, and ADA of approximately 51%, 58%, and 61%, respectively.Citation47 An Italian study group (Gruppo Italiano di Studio sulle Early Arthritides registry) reported 2.5-year continuation rates for IFX, ETA, and ADA of approximately 52%, 65%, and 52%, respectively.Citation48
There are few reports describing TCZ drug survival. The Danish Nationwide Rheumatological Database registry reported 48-, 96-, and 144-week TCZ adherence rates of 61%, 54%, and 47%, respectively.Citation49 In contrast, the Danish Nationwide Rheumatological Database registry reported 48-month drug survival rates for IFX, ETA, and ADA of 41%, 56%, and 52%, respectively.Citation50 The Japanese Osaka University Biologics for Rheumatic Diseases registry reported 1-year drug continuation rates for TCZ, IFX, ETA, and ADA of 89%, 73%, 86%, and 78%, respectively, and 2.5-year rates of 79%, 47%, 78%, and 55%, respectively.Citation51 In this registry, the continuation rates for TCZ and ETA are significantly higher than those for IFX and ADA. The most frequent reasons given for discontinuation are AEs for TCZ and a lack of efficacy for ADA and IFX. The Registry of Japanese Rheumatoid Arthritis Patients for Long-term Safety reported significantly lower discontinuation rates due to lack of efficacy for patients taking ETA compared with patients taking IFX or TCZ.Citation52 Finally, the Cohort of Arthritis Biologic Users at Kameda Institute registry reported that the drug survival and safety profiles of TCZ are similar to those of TNF inhibitors (IFX, ETA, and ADA).Citation53 The results regarding tolerability are summarized in . These reports indicate that tolerability of TCZ is comparable to or better than that of TNF inhibitors.
Table 2 Comparative tolerability of tocilizumab with tumor necrosis factor inhibitors
Comparative patient focused perspectives, such as quality of life, patient satisfaction/acceptability, adherence, and uptake
In all Phase III trials modified HAQ-DI scores significantly improved with TCZ treatment. Moreover, based on functional assessment of chronic illness therapy (FACIT), the OPTION and TOWARD studies reported that TCZ had an ameliorative effect, and the Short-Form (SF)36 Health Survey indicated both mental and physical (SF36-mental and SF-physical) effects.Citation11,Citation12 In addition, the RADIATE study found that at week 24 versus placebo, TCZ treatment at 8 mg/kg was associated with significantly greater improvements in HAQ-DI, FACIT, and SF36-physical, and that TCZ treatment at 4 mg/kg was associated with greater improvements in HAQ-DI and SF36-physical.Citation54 Components of the Arthritis Impact Measurement Scale 2 (AIMS-2) (eg, physical score, symptom, and affect score) and those of SF36 (eg, bodily pain, general health, vitality, and mental health) improved in 39 patients in a clinical practice after 4 weeks of TCZ therapy, but there was no improvement in the social interaction component of AIMS-2 after 24 weeks of treatment.Citation55
The Tocilizumab and DMARDs: Achievements in Rheumatoid Arthritis study reported improvements in diary documented fatigue, pain, and morning stiffness with TCZ treatment.Citation56 The mean FACIT-Fatigue score increased from 28.8±11.2 at baseline to 35.3±11.5 at week 4 and to 37.4±12.2 at week 24, and the mean HAQ-DI score decreased from 1.48±0.65 to 1.15±0.68 at week 4 and to 1.00±0.75 at week 24 or the last visit. Favorable mean changes from baseline to week 24 or the last visit were also observed in each of the domains of the SF36, especially in the physical domains. The Treatment Satisfaction Questionnaire for Medication, which was completed at the end of the study, showed a high level of patient agreement/satisfaction for each of the derived domains: “effectiveness” (69.4%), “side effects” (88.7%), “convenience” (72.4%), and “global satisfaction” (74.7%).
Fatigue represents an important symptom for patients with RA. Chauffier et al assessed the effect of biotherapies on fatigue based on data from ten RCTs involving patients with established RA.Citation57 Unfortunately, with respect to fatigue, they found that the overall effect size of all bDMARDs versus placebo at week 24 of treatment is small in established RA. In inadequate responders to sDMARDs, the effect size is similar for TNF inhibitors and nonanti-TNF bDMARDs including TCZ. Strand et al reported that ADA plus MTX significantly improved physical function and health-related quality of life in patients with early RA after 2 years of treatment.Citation58 However, no clinically meaningful differences between patients on ADA monotherapy or MTX were observed. In a recent meta-analysis, Callhoff et al studied the impact of bDMARDs including five TNF inhibitors but not TCZ on the physical function of patients with RA, as evaluated by Health Assessment Questionnaire.Citation59 Overall, bDMARDs produced greater improvement in physical function than sDMARDs, with an Health Assessment Questionnaire standardized mean difference of 0.44 (95% confidence interval [CI]: 0.38, 0.50). No significant differences between TNF inhibitors were observed.
Huynh et al examined patient treatment preference.Citation60 The most frequent reason given for choosing intravenous treatment was “safety” (62%), followed by “easy to manage” (39%). The two most frequent reasons given for choosing self-injection at home were “time constraints” and “easy to manage” (both 57%). The majority of RA patients already treated with bDMARDs in that study preferred the route of administration they were used to. The majority of the patients not currently treated with a bDMARD preferred subcutaneous treatment at home. A feeling of safety was important to patients who preferred intravenous treatment. Health professionals as a group may be biased toward the use of subcutaneous treatment. It is now possible to administer TCZ subcutaneously as well as intravenously.Citation61,Citation62 Although subcutaneous injection of TCZ is disadvantageous in heavy patients, the fact that patients can now choose the administration route is a positive development.
Comparison of the cost-effectiveness of TCZ and TNF inhibitors
Although demonstrations of the outstanding efficacy of TNF inhibitors and TCZ have led to a paradigm shift with respect to the management of RA, the relatively high cost of these drugs imposes a large burden on both patients and society.Citation63 The Swedish Early Interventions In Rheumatoid Arthritis project demonstrated that drug costs increased primarily due to the introduction of biologics.Citation64 Sick leave decreases during the first year, but disability pensions increase, resulting in no change in indirect costs. Over the following years, disability pensions increase further and indirect costs also increase. In the 6 years after diagnosis of early RA, drug costs are partially offset by decreasing outpatient visits, but indirect costs remain unchanged and total costs increase. Therefore, the cost of bDMARDs is a significant problem. bDMARDs significantly increase the quality-adjusted life years (QALYs) gained when compared to MTX alone. QALY is a measure of disease burden affecting the quality and quantity of the life lived. In Finland, TCZ plus MTX was found to be more cost-effective than ADA plus MTX or ETA plus MTX in comparison with MTX alone.Citation65 A QALY gained with retail priced (wholesale priced) TCZ plus MTX costs Euro (€)18,957 (€17,057) more than MTX alone. Diamantopoulos et al reported the cost utility of TCZ in RA patients with an inadequate response to sDMARDs from a payer’s perspective in Italy.Citation66 Replacement of TNF inhibitors (ADA, ETA, and IFX) with TCZ reduces total costs over a patient’s lifetime (base-case analysis, TCZ: €141,100 versus TNF inhibitors: €143,500). Patients receiving TCZ realize more QALYs than patients receiving standard of care (9.8881 QALYs versus 9.3502 QALYs). When TCZ is added to standard of care without replacing TNF inhibitors, the incremental cost-effectiveness ratio becomes €17,100 per QALY.
In the ADACTA study, economic evaluation of the cost per response or remission of TCZ versus ADA was reported for Spain.Citation67 The cost per ACR20/50/70 response is lower with TCZ than with ADA (€8,105/11,162/16,211 versus €11,553/20,529/31,882). The cost of attaining DAS28 remission with TCZ and ADA is €13,204 and €54,352, respectively. Treatment with TCZ was dominant in all scenarios analyzed. Similar economic evaluation of TCZ versus ADA from the ADACTA trial was conducted in Australia.Citation68 TCZ monotherapy was found to result in lower total treatment costs (in Australian dollars [$]) per patient over 24 weeks compared with ADA monotherapy ($9,739 versus $10,722).
In the UK, the addition of TCZ in combination with MTX to treat severe active RA in patients with an inadequate response to sDMARDs was found to produce a gain of 1.17 QALYs per patient, at an incremental cost of UK pound (£)23,253.Citation69 This equates to an incremental cost-effectiveness ratio (ICER) of £19,870. The addition of TCZ in combination with MTX to the current Scottish standard of care in adult TNF inhibitor-inadequate responders with moderate to severe active RA produces a gain of 1.234 QALYs per patient, at an incremental cost of £27,465.Citation70 This equates to an ICER of £22,254. Tanaka et al reported the cost-effectiveness of TCZ in Japan.Citation71 The lifetime cumulative costs and QALYs were 35.4 million Japanese yen (¥) and 11.7, respectively, in the TCZ group and ¥23.3 million and 9.3, respectively, in the MTX group. The ICER for TCZ was ¥4.94 million, with a 66.2% probability of falling below the allowable threshold based upon probabilistic sensitivity analysis. These findings suggest that TCZ is more cost-effective than TNF inhibitors, including ADA, ETA, and IFX.
Conclusion and place in therapy
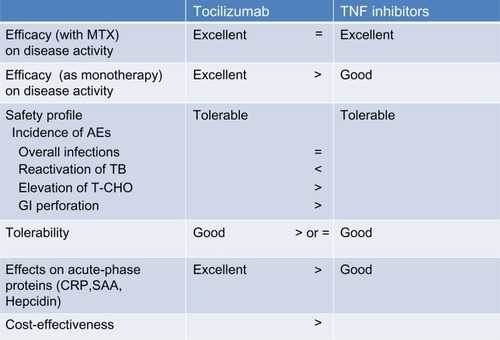
The property of TCZ and TNF inhibitors is summarized in . Based upon recent findings, the EULAR recommendations for the management of RA were updated in 2013.Citation72 In patients responding insufficiently to MTX and/or other sDMARDs, with or without glucocorticoids, use of bDMARDs should commence with MTX. First line bDMARDs include TNF inhibitors, abatacept, and TCZ, and under certain circumstances, rituximab. If biologic monotherapy must be initiated, only TCZ has supportive evidence. However, TCZ, TNF inhibitors, and other bDMARDs do not produce beneficial effects in all active RA patients. Therefore, to determine the optimal strategy for using particular bDMARDs in individual RA patients, the characteristic features of these drugs should be clarified.Citation73
Figure 2 Properties of tocilizumab and tumor necrosis factor inhibitors in the management of rheumatoid arthritis.
RA animal models have provided some clarification. The most well-known animal model of RA is collagen-induced arthritis, which involves injection of mice with type II collagen to produce an immune response directed at connective tissues. Both IL-6 and TNF-α have been shown to play a major role in the development and progression of joint destruction in the collagen-induced arthritis model. Immunization with type II collagen in this model primarily increases the frequency of Th17 cells. Treatment of immunized mice with anti-IL-6 receptor Ab during priming leads to marked suppression of both the induction of Th17 cells and arthritis development, whereas administration of soluble TNF receptor-Fc fusion protein from day 0 to 14 fails to suppress Th17 differentiation and arthritis development.Citation74 Anti-type II collagen Ab-induced arthritis (CAIA) is a model in which the priming phase of T-cell dependent Ab generation is skipped. Although TNF-α and IL-6 are also elevated in this model, arthritis is suppressed in TNF-α- but not in IL-6-deficient mice, indicating that TNF-α plays a more significant role than IL-6 in joint inflammation in CAIA.Citation75 These findings suggest that IL-6 is essential for the induction of immunological abnormalities and the development of arthritis and that the pathological role of IL-6 is different from that of TNF-α, which is primarily involved in the development of joint inflammation.
Analyses of various markers during biologic treatment are also helpful to clarify the characteristics of bDMARDs. Both TNF inhibitors and TCZ lead to improvements in serological and urinary markers related to bone and cartilage metabolism. Several immunological studies have sought to clarify the mechanisms underlying the effects of TCZ. Of particular importance is to determine whether TCZ can correct the Th17/Treg imbalance, which is thought to be a fundamental immunological abnormality in RA.Citation76 The results of preliminary studies suggest that inhibition of IL-6 function by TCZ corrects the imbalance between Th17 and Treg cells in the peripheral CD4-positive T-cell population.Citation77,Citation78 In contrast, TNF-α suppresses Treg function by dephosphorylating serine 418 in the C-terminal DNA-binding domain of the forkhead box P3, whereas anti-TNF therapy can restore Treg cell function.Citation79 Moreover, a study involving eight patients with RA demonstrated that 6 months of treatment with TCZ causes a selective decrease in IL-21 production by memory/activated T-cells.Citation80 IL-21 is known to promote plasma cell differentiation and induce IgG4 production, and TCZ treatment leads to a reduction in the serum levels of IgG4-specific anticitrullinated peptide antibody, indicating the presence of a pathway involving IL-6, IL-21, and IgG4 autoantibodies in RA. In another study, Roll et al examined the in vivo effect of TCZ on the B-cell compartment in 16 RA patients and found that TCZ induces a significant reduction in peripheral preswitch and postswitch memory B-cells.Citation81 In addition, TCZ (but not ETA) significantly reduces somatic hypermutation in immunoglobulin gene rearrangements in preswitch memory B-cells,Citation82 suggesting modulation of memory B-cells as a possible mechanism for TCZ. Further evaluation is required to clarify the effects of bDMARDs in treating the immunological abnormalities associated with RA.
IL-6, which was found to be identical to hepatocyte-stimulating factor, induces the expression of various acute phase proteins, such as C-reactive protein, hepcidin, SAA, and fibrinogen, indicating that IL-6 plays a role in the development of systemic inflammatory symptoms, signs, and complications. TCZ treatment is expected to ameliorate the inflammatory effects and inhibit the development of complications. Increased production of hepcidin predominantly induced by IL-6 leads to anemia associated with chronic disorders.Citation83 A comparative evaluation of the effects of TCZ and TNF inhibitors on serum hepcidin and anemia found that significant improvement in anemia and reduction in serum hepcidin levels are more pronounced in the TCZ treated patients than in TNF inhibitor treated patients.Citation84 Amyloid A amyloidosis is a serious complication of RA, as amyloid fibril deposition causes progressive deterioration in various organs,Citation85 although due to a marked progression of antirheumatic treatment, the incidence of amyloid A amyloidosis has recently decreased.Citation86,Citation87 SAA is an amyloid fibril precursor protein. Because the synthesis of SAA depends primarily on IL-6, TCZ injection promptly reduces the serum concentration of SAA, just as in the case of C-reactive protein, and the suppressive activity of TCZ on the serum SAA level is more powerful than that of TNF inhibitors.Citation88,Citation89 Case reports and series studies published to date have demonstrated the marked ameliorative effect of TCZ on gastrointestinal symptoms and renal dysfunction caused by amyloid A amyloidosis.Citation90–Citation92
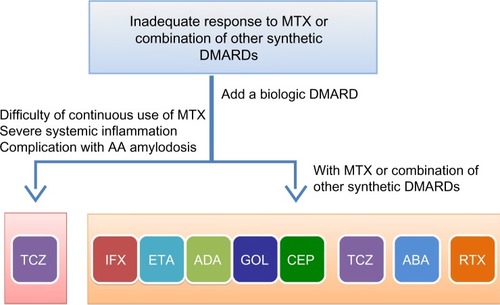
On the basis of these findings, we suggest that TCZ can be selected as the first line biologic for patients who 1) cannot continue treatment with MTX or other sDMARDs, 2) present with severe inflammatory findings, and 3) have or who are at high risk of developing amyloid A amyloidosis (). Moreover, medication adherence and cost-effectiveness appears to favor TCZ in comparison with TNF inhibitors. However, further evaluation and clarification of the characteristic features of bDMARDs are essential to determine the optimal treatment for individual RA patients.
Figure 3 Selection of biologic disease modifying antirheumatic drugs.
Abbreviations: ABA, abatacept; ADA, adalimumab; CEP, certolizumab pegol; DMARDs, disease modifying antirheumatic drugs; ETA, etanercept; GOL, golimumab; IFX, infliximab; MTX, methotrexate; RTX, rituximab; TCZ, tocilizumab.
Disclosure
T Tanaka has received a grant and payment for lectures including service on speakers’ bureaus from Chugai Pharmaceutical Co, Ltd. Y Hishitani has received a payment for lectures including service on speakers’ bureaus from Chugai Pharmaceutical Co, Ltd. A Ogata has received a consulting fee as a medical adviser, a grant, and payment for lectures including service on speakers’ bureaus from Chugai Pharmaceutical Co, Ltd.
References
- MclnnesIBSchettGThe pathogenesis of rheumatoid arthritisN Engl J Med2011365232205221922150039
- SmolenJSAletahaDKoellerMWeismanMHEmeryPNew therapies for treatment of rheumatoid arthritisLancet200737096021861187417570481
- ThalayasingamNIsaacsJDAnti-TNF therapyBest Pract Res Clin Rheumatol201125454956722137924
- TanakaTOgataANarazakiMTocilizumab for the treatment of rheumatoid arthritisExpert Rev Clin Immunol20106684385420979549
- TanakaTNarazakiMKishimotoTTherapeutic targeting of the interleukin-6 receptorAnnu Rev Pharmacol Toxicol20125219921921910626
- TanakaTOgataAKishimotoTTargeting of interleukin-6 for the treatment of rheumatoid arthritis: a review and updateRheumatol Curr Res2013S4002
- SatoKTsuchiyaMSaldanhaJReshaping a human antibody to inhibit the interleukin-6 dependent tumor cell growthCancer Res19935348518568428365
- JonesGSebbaAGuJComparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION studyAnn Rheum Dis2010691889619297346
- NishimotoNHashimotoJMiyasakaNStudy of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x-ray reader-blinded randomized controlled trial of tocilizumabAnn Rheum Dis20076691162116717485422
- NishimotoNMiyasakaNYamamotoKStudy of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapyMod Rheumatol2009191121918979150
- SmolenJSBeaulieuARubbert-RothAOPTION InvestigatorsEffect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trialLancet2008371961798799718358926
- GenoveseMCMcKayJDNasonovELInterleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy studyArthritis Rheum200858102968298018821691
- EmeryPKeystoneETonyHPIL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumor necrosis factor biologicals: results from a 24-week multicentre randomized placebo controlled trialAnn Rheum Dis200867111516152318625622
- KremerJMBlancoRBrzoskoMTocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one yearArthritis Rheum201163360962121360490
- WeinblattMEKeystoneECFurstDEAdalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trialArthritis Rheum2003481354512528101
- BreedveldFCWeismanMHKavanaughAFThe PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatmentArthritis Rheum2006541263716385520
- MainiRSt ClariEWBreedveldFInfliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study GroupLancet199935491941932193910622295
- BreedveldFCEmeryPKeystoneEInfliximab in active early rheumatoid arthritisAnn Rheum Dis200463214915514722203
- KeystoneECGenoveseMCKlareskogLGolimumab, a human antibody to tumour necrosis factor alpha given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD StudyAnn Rheum Dis200968678979619066176
- KeystoneEvan der HeijdeDMasonDJrCertolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group studyArthritis Rheum200858113319332918975346
- MorelandLWSchiffMHBaumgartnerSWEtanercept therapy in rheumatoid arthritis. A randomized, controlled trialAnn Intern Med1999130647848610075615
- KlareskogLvan der HeijdeDde JagerJPTEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigatorsTherapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trialLancet2004363941067568115001324
- GabayCEmeryPvan VollenhovenRADACTA Study InvestigatorsTocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trialLancet201338198771541155023515142
- BergmanGJHochbergMCBoersMWintfeldNKielhornAJansenJPIndirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugsSemin Arthritis Rheum201039642544120223500
- Gallego-GalisteoMVilla-RubioAAlegre-del ReyEMarquez-FernandezERamos-BaezJJIndirect comparison of biological treatments in refractory rheumatoid arthritisJ Clin Pharm Ther201237330130721831256
- TurkstraENgSKScuffhamPAA mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritisCurr Med Res Opin201127101885189721848493
- SalliotCFinckhAKatchamartWIndirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysisAnn Rheum Dis201170226627121097801
- OrmeMEMacgilchristKSMitchellSSpurdenDBirdASystematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70Biologics2012642946423269860
- PierreisnardAIssaNBarnetcheTRichezCSchaeverbekeTMeta-analysis of clinical and radiological efficacy of biologics in rheumatoid arthritis patients naive or inadequately responsive to methotrexateJoint Bone Spine201380438639223141718
- JonesGDarian-SmithEKwokMWinzenbergTEffect of biologic therapy on radiological progression in rheumatoid arthritis: what does it add to methotrexate?Biologics2012615516122848148
- EmeryPSebbaAHuizingaTWBiologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritisAnn Rheum Dis201372121897190423918035
- DougadosMKisselKSheeranTAdding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY)Ann Rheum Dis2013721435022562983
- TakahashiNKojimaTKanekoAClinical efficacy of abatacept compared to adalimumab and tocilizumab in rheumatoid arthritis patients with high disease activityClin Rheumatol2014331394724057092
- SchiffMHKremerJMJahreisAVernonEIsaacsJDvan VollenhovenRFIntegrated safety in tocilizumab clinical trialsArthritis Res Ther2011135R14121884601
- GenoveseMCRubbert-RothASmolenJSLongterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposureJ Rheumatol201340676878023457383
- KoikeTHarigaiMInokumaSEffectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in JapanJ Rheumatol2014411152324187110
- SinghJAWellsGAChristensenRAdverse effects of biologics: a network meta-analysis and Cochrane overviewCochrane Database Syst Rev2011162CD00879421328309
- JainASinghJAHarms of tumor necrosis factor inhibitions in rheumatic diseases: a focused systematic review of the literatureImmunotherapy20135326529923444956
- Gómez-ReinoJJCarmonaLValverdeVRMolaEMMonteroMDBIOBADASER GroupTreatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance reportArthritis Rheum20034882122212712905464
- TubachFSalmonDRavaudPResearch Axed on Tolerance of Biotherapies GroupRisk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registryArthritis Rheum20096071884189419565495
- van VollenhovenRFNishimotoNYamanakaHExperience with Mycobacterium tuberculosis infection reported in the tocilizumab worldwide RA safety databaseAnn Rheum Dis200968Suppl 3567
- OgataAMoriMHashimotoSMinimal influence of tocilizumab on IFN-gamma synthesis by tuberculosis antigensMod Rheumatol201020213013319898919
- GoutTOstörAJNisarMKLower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature reviewClin Rheumatol201130111471147421833686
- MclnnesIBThompsonLGilesJTEffect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled studyAnn Rheum Dis Epub12242013
- GreenbergJDReedGDecktorDCORRONA InvestigatorsA comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registryAnn Rheum Dis20127171134114222294625
- MarchesoniAZaccaraEGorlaRTNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practiceAnn N Y Acad Sci2009117383784619758236
- Du PanSMDehlerSCiureaASwiss Clinical Quality Management PhysiciansComparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritisArthritis Rheum200961556056819405000
- IannoneFFerraccioliGGremeseELapadulaGGroup Italiano di Studio sulle Early Arthritis (GISEA)Drug survival on TNF inhibitors: 2003–2004 data from Italian National Register (GISEA Registry)Ann Rheum Dis Epub12242013
- LeffersHCBØstergaardMGlintborgBThree-year drug survival and effectiveness of Abatacept and Tocilizumab in patients with rheumatoid arthritis treated in routine care. Results from the Nationwide Danish Danbio RegistryArthritis Rheum201365Suppl 10S612
- HetlandMLChristensenIJTarpUAll Departments of Rheumatology in DenmarkDirect comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registryArthritis Rheum2010621223220039405
- HishitaniYOgataAShimaYRetention of tocilizumab and anti-tumour necrosis factor drugs in the treatment of rheumatoid arthritisScand J Rheumatol201342425325923470089
- SakaiRTanakaMNankiTREAL Study GroupDrug retention rates and relevant risk factors for drug discontinuation due to adverse events in rheumatoid arthritis patients receiving anticytokine therapy with different target moleculesAnn Rheum Dis201271111820182622504558
- YoshidaKTokudaYOshikawaHAn observational study of tocilizumab and TNF-α inhibitor use in a Japanese community hospital: different remission rates, similar drug survival and safetyRheumatology (Oxford)201150112093209921890622
- StrandVBurmesterGROgaleSDevenportJJohnAEmeryPImprovements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE studyRheumatology (Oxford)201251101860186922753773
- FusamaMNakaharaHHamanoYImprovement of health status evaluated by Arthritis Impact Measurement Scale (AIMS-2) and Short Form-36 (SF-36) in patients with rheumatoid arthritis treated with tocilizumabMod Rheumatol201323227628322669600
- FeistERubbert-RothAKaufmannJEffectiveness after 4 and 24 weeks and safety of the novel interleukin-6 receptor inhibitors tocilizumab (TCZ) in patients with active rheumatoid arthritis (RA)–analysis of the patient-reported outcomes (PRO) in the TAMARA studyAnn Rheum Dis201069Suppl 3544
- ChauffierKSalliotCBerenbaumFEffect of biotherapies on fatigue in rheumatoid arthritis: a systematic review of the literature and meta-analysisRheumatology (Oxford)2012511606821515629
- StrandVRentzAMCifaldiMAChenNRoySRevickiDHealth-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter studyJ Rheumatol2012391637222045836
- CallhoffJWeißAZinkAListingJImpact of biologic therapy on functional status in patients with rheumatoid arthritis – a meta-analysisRheumatology (Oxford)201352122127213523946435
- HuynhTØstergaardAEgsmoseCPatients and health professional preferences to route of administration of biologics agents for RA treatmentAnn Rheum Dis201372Suppl 3431
- BurmesterGRRubbert-RothACantagrelAA randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study)Ann Rheum Dis2014731697423904473
- OgataATanimuraKSugimotoTA phase 3 study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis (MUSASHI)Arthritis Care Res (Hoboken) Epub8272013
- TsaoNWBansbackNJShojaniaKMarraCAThe issue of comparators in economic evaluations of biologic response modifiers in rheumatoid arthritisBest Pract Res Clin Rheumatol201226565967623218430
- HallertEHusbergMKalkanASkoghTBernfortLEarly rheumatoid arthritis 6 years after diagnosis is still associated with high direct costs and increasing loss of productivity: the Swedish TIRA projectScand J Rheumatol Epub12192013
- SoiniEJHallinenTAPuolakkaKVihervaaraVKauppiMJCost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritisJ Med Econ201215234035122168785
- DiamantopoulosABenucciMCapriSEconomic evaluation of tocilizumab combination in the treatment of moderate-to-severe rheumatoid arthritis in ItalyJ Med Econ201215357658522313326
- Navarro SarabiaFBlancoFJAlvaro GraciaJMEconomic evaluation of rheumatoid arthritis monotherapy with tocilizumab and adalimumabRev Esp Salud Publica2013874343350 Spanish24100773
- NgoPSorathiaRCToddCJonesGNashPAustralian economic evaluation of tocilizumab (TCZ) monotherapy vs adalimumab (ADA) monotherapy in patients with rheumatoid arthritis (RA) based on the ADACTA trialInternal Medicine Journal2013431212
- PapadakisKMcNamaraSWintfeldNA UK cost-effectiveness analysis on the use of tocilizumab (Roactemra®) with methotrexate in the treatment of adults with moderate to severe active rheumatoid arthritis who have failed to respond adequate to one or more DMARDsAnn Rheum Dis201069Suppl 3389
- McNamaraSKPapadakisKNWintfeldNAn economic evaluation on the use of tocilizumab (Roactemra®) with methotrexate in the treatment of adults with moderate to severe active rheumatoid arthritis who have failed to respond adequately to an anti-TNF drugs in ScotlandAnn Rheum Dis201069Suppl 3389
- TanakaEInoueEHoshiDCost-effectiveness of a humanized anti-intereleukin-6 (IL-6) receptor monoclonal antibody, tocilizumab, in rheumatoid arthritis using IORRA cohort databaseAnn Rheum Dis201271Suppl 366822121127
- SmolenJSLandeweRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis Epub10252013
- OgataAHiranoTHishitaniYTanakaTSafety and efficacy of tocilizumab for the treatment of rheumatoid arthritisClin Med Insights Arthritis Musculoskelet Disord20125274222438671
- FujimotoMSeradaSMiharaMInterleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responsesArthritis Rheum200858123710371919035481
- KagariTDoiHShimozatoTThe importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritisJ Immunol200216931459146612133972
- TanakaTCan IL-6 blockade rectify imbalance between Tregs and Th17 cells?Immunotherapy20135769569723829620
- SamsonMAudiaSJanikashviliNBrief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in rheumatoid arthritis patientsArthritis Rheum20126482499250322488116
- PesceBSotoLSabugoFEffect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patientsClin Exp Immunol2013171323724223379428
- NieHZhengYLiRPhosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-a in rheumatoid arthritisNat Med201319332232823396208
- CarboneGWilsonADiehlSABunnJCooperSMRinconMInterleukin-6 receptor blockade selectively reduces IL-21 production by CD4 T cells and IgG4 autoantibodies in rheumatoid arthritisInt J Biol Sci20139327928823493630
- RollPMuhammadKSchumannMIn vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartmentArthritis Rheum20116351255126421305508
- MuhammadKRollPSeiboldTImpact of IL-6 receptor inhibition on human memory B cells in vivo: impaired somatic hypermutation in preswitch memory B cells and mutational targeting in memory B cellsAnn Rheum Dis20117081507151021551509
- NemethERiveraSGabayanVIL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidinJ Clin Invest200411391271127615124018
- SongSNIwahashiMTomosugiNComparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patientsArthritis Res Ther2013155R14124286116
- ObiciLMerliniGAA amyloidosis: basic knowledge, unmet needs and future treatmentsSwiss Med Wkly2012142w1358022653707
- LaihoKTiitinenSKaarelaKHelinHIsomakiHSecondary amyloidosis has decreased in patients with inflammatory joint disease in FinlandClin Rheumatol199918212212310357116
- ImmonenKFinnePGronhagen-RiskaCA marked decline in the incidence of renal replacement therapy for amyloidosis associated with inflammatory rheumatic diseases – data from nationwide registries in FinlandAmyloid2011181252821284495
- TanakaTHagiharaKHishitaniYOgataATocilizumab for the treatment of AA amyloidosisGuvencIAAmyloidosis – An Insight to Disease of Systems and Novel TherapiesCroatiaINTECH Open Access Publisher2011155170
- OkudaYOhnishiMMatobaKComparison of the clinical utility of tocilizumab and anti-TNF therapy in AA amyloidosis complicating rheumatic diseasesMod Rheumatol201424113714324261770
- NishidaSHagiharaKShimaYRapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatmentAnn Rheum Dis20096871235123619525413
- HakalaMImmonenKKorpelaMVasalaMKauppiMJGood medium-term efficacy of tocilizumab in DMARD and anti-TNF-α therapy resistant reactive amyloidosisAnn Rheum Dis201372346446523148310
- MiyagawaINakayamadaSSaitoKStudy on the safety and efficacy of tocilizumab, an anti-IL-6 receptor antibody, in patients with rheumatoid arthritis complicated with AA amyloidosisMod Rheumatol Epub10212013

