Abstract
Objective
To evaluate the comparative effectiveness of available tumor necrosis factor-α inhibitors (anti-TNFs) for the management of psoriatic arthritis (PsA) in patients with an inadequate response to disease-modifying antirheumatic drugs (DMARDs).
Methods
We used an exhaustive search strategy covering randomized clinical trials, systematic reviews and health technology assessments (HTA) published on anti-TNFs for PsA. We performed indirect comparisons of the available anti-TNFs (adalimumab, etanercept, golimumab, and infliximab) measuring relative risks (RR) for the psoriatic arthritis response criteria (PsARC), mean differences (MDs) for improvements from baseline for the Health Assessment Questionnaire (HAQ) by PsARC responders and non-responders, and MD for the improvements from baseline for the psoriasis area and severity index (PASI). When the reporting of data on intervention group response rates and improvements were incomplete, we used straightforward conversions based on the available data.
Results
We retrieved data from 20 publications representing seven trials, as well as two HTAs. All anti-TNFs were significantly better than control, but the indirect comparison did not reveal any statistically significant difference between the anti-TNFs. For PsARC response, golimumab yielded the highest RR and etanercept the second highest; adalimumab and infliximab both yielded notably smaller RRs. For HAQ improvement, etanercept and infliximab yielded the largest MD among PsARC responders. For PsARC nonresponders, etanercept, infliximab, and golimumab yielded similar MDs, and adalimumab a notably lower MD. For PASI improvement, infliximab yielded the largest MD and golimumab the second largest, while etanercept yielded the smallest MD. In some instances, the estimated magnitudes of effect were notably different from the estimates of previous HTA indirect comparisons.
Conclusion
There is insufficient statistical evidence to demonstrate differences in effectiveness between available anti-TNFs for PsA. Effect estimates seem sensitive to the analytic approach, and this uncertainty should be taken into account in future economic evaluations.
Introduction
Psoriatic arthritis (PsA) is an inflammatory disease affecting joints and connective tissues.Citation1 PsA affects up to 30% of individuals with psoriasis, a chronic skin condition affecting 1%–2% of the general population.Citation1 It can be a destructive disabling joint disease, with the severity increasing over time.Citation1 There are no cures for PsA and so the focus of treatment has been on controlling symptoms and preventing damage to joints. Patients are typically treated first with nonsteroidal anti-inflammatory drugs (NSAIDS), that help to reduce pain and inflammation of the joints.Citation2 In patients with more severe disease, disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, are often a first course of treatment.Citation2 More recently, therapies that inhibit the pro-inflammatory protein – tumor necrosis factor (TNF) – are increasingly being used in patients who have failed traditional DMARD therapy.Citation2
Currently, four anti-TNFs are indicated for the treatment of PsA in combination with methotrexate (MTX). So far two comparative effectiveness assessments of available anti-TNFs for PsA have been conducted, both in connection with a health technology assessment (HTA).Citation3,Citation4 However, because of methodological shortcomings and limitations, the inferences from these analyses are weakened. The first HTA included adjusted indirect comparisons of only three of the four indicated anti-TNFs (adalimumab, infliximab, and etanercept).Citation3 In addition, although this HTA provided summary tables of the trial outcomes at different time points (eg, 14 weeks and 24 weeks), it was not clear which time points were used for producing the pooled comparative effectiveness estimates. The second HTA attempted to model only a few outcomes that lend themselves well to an economic model (ie, PsA response criteria [PsARC], Health Assessment Questionnaire [HAQ] by PsARC responders and nonresponders, and Psoriasis Area and Severity Index [PASI] mean change as a continuous variable).Citation4 However, the shortage in available data on these outcomes led the authors to conduct what was effectively a Bayesian imputation analysis. Given the scarcity of the data used, it is evident that the effectiveness estimates and any accompanying cost-effectiveness estimates will be sensitive to the imputation assumptions, and that the “noninformative” priors elicited in the model may carry a relatively high degree of information, and thus bias the estimates of effect.Citation5,Citation6
To address the shortcomings of previous indirect comparisons, and in particular the most recent HTA report, we performed an exhaustive literature search and data extraction of all trial publications, data available in published meta-analyses, and data available from HTAs. We used all available data on outcomes to calculate previously missing trial results, and thereby obviated the shortcomings of the Bayesian approach. We then re-ran the indirect comparison to obtain “improved” estimates of effect on the outcomes used to derive quality-adjusted life year (QALY) estimates in a recent National Institute for Health and Clinical Excellence (NICE) HTA.Citation4
Methods
Eligibility criteria
We included randomized controlled trials (RCTs) examining the efficacy of anti-TNF biological agents (adalimumab, etanercept, golimumab, and infliximab) for the treatment of PsA. RCTs studying adult populations with active and progressive PsA with an inadequate response to previous DMARD therapy were eligible. We included RCTs of any treatment dose and duration of the above-specified anti-TNF biologics. We excluded trials conducted among PsA populations that had an adequate response to DMARD therapy, or were naïve to DMARD therapy. We also excluded trials conducted among PsA populations with prior experience with anti-TNF agents, including an inadequate response. Furthermore, trials that did not have a placebo control and that examined non-anti-TNF biological agents were excluded.
Search strategy
In consultation with a medical librarian, two investigators (ED, KT) independently conducted a systematic literature search for RCTs. The search terms included “psoriatic arthritis,” “ biologic,” “anti-TNF,” and the generic and brand names of each of the agents (eg, “adalimumab,” “etanercept,” “golimumab,” “infliximab”). The following electronic databases (from inception to week 15 [April 9–15], 2012) were searched: MEDLINE, EMBASE, and Cochrane CENTRAL. Searches were limited to RCTs in humans, but not limited by language. Additionally, we searched for published HTAs and systematic reviews to further identify completed RCTs and/or obtain additional data on the published clinical trials. Lastly, some additional data were provided by Merck-Shire-Dome, UK. The exact search strategy is available from the authors upon request.
Study selection
Following the systematic literature searches, the same two investigators (ED, KT) obtained the full manuscripts of relevant trials, and independently assessed the relevance of each to determine whether or not it fit the eligibility criteria listed above. Any discrepancies between the two investigators were resolved by consulting a third investigator (EM) if necessary. Trials that did not meet the eligibility criteria were excluded and their reference listed with reasons for exclusion. Eligible trials underwent a quality assessment by one investigator (ED), using a modified Jadad scale.Citation7
Data abstraction
Data were extracted by one investigator (ED) and independently checked by a second investigator (KT). Disagreements between the data extracted were resolved by consulting a third investigator (EM) if necessary. We abstracted data on anti-inflammatory response as derived from the PsARC. Response of psoriatic skin lesions, as determined by the PASI, was also abstracted. Finally, functional status, as determined by the HAQ score, was abstracted overall, and by PsARC response, where possible. Definitions of each for the outcomes are presented in the Supplementary materials (Table S1). The following trial characteristics were also abstracted: study design, number of subjects, trial duration, outcome measures used, treatment dose and duration, concomitant therapies, and participant characteristics.
Data synthesis
Outcomes
We considered the same three outcomes as a previous HTA: the PsARC response, the HAQ mean change from baseline for PsARC responders and nonresponders, and the PASI mean change from baseline. Our primary endpoint was the last observed time point in the trial, before allowed dose escalation or treatment cross-over. We chose this because patients with escalated dose and patients that have crossed over are no longer comparable to patients on a fixed dose treatment in terms of estimating efficacy.
Dealing with incomplete data
The PsARC response was reported completely across all trial publications, and thus did not require any transformations or imputations. The HAQ mean change by PsARC responders and non-responders were made available to us through the full version of a recently published HTA.Citation4 However, the HAQ scores from the Mease 2000Citation8 and Mease 2004Citation9 studies had been combined in this HTA, and the available placebo HAQ response had been compiled across Mease 2000, Mease 2004, and the IMPACT trials.Citation16–Citation18 For this reason we made use of the overall HAQ baseline and mean change scores extracted from the trial publications to calculate the summary statistics which were not reported (note all missing data points were fully derived and no imputations were needed). Table S2 provides a detailed overview of necessary data conversions for the HAQ outcome.
For the PASI mean change only IMPACT and IMPACT 2 had complete data. For the remaining trials except for Mease 2004, baseline PASI and associated standard deviations (SDs) as well as PASI50, PASI70, and PASI90 were available. We assumed that the absolute percentage mean change approximately followed a normal distribution and approximated the mean and standard deviation from the PASI50, PASI70, and PASI90 data. We then used the approximated distribution with the available baseline distribution to produce PASI mean changes, using simulations. For Mease 2004, where no baseline data was available, we imputed data by random sampling from the other trials. Appendix 2 provides a detailed overview of necessary data conversions and imputations for the PASI outcome.
Statistical models
We performed frequentist indirect comparison meta-analyses using random-effects models.Citation13 We obtained comparative relative risks (RR) with 95% confidence intervals for PsARC, and mean difference (MD) estimates with 95% confidence intervals for HAQ (PsARC responders and nonresponders) and PASI. All analyses were performed using StatsDirect (StatsDirect Ltd, Altrincham, UK) and R v. 2.14 (The R Project for Statistical Computing; http://www.R-project.org/).
For PsARC we pooled the response rate in the placebo group from all trials, and used simulation to produce the expected response rate with each of the treatments using the indirect RR estimates and associated (log) standard error estimates. For HAQ and PASI we pooled the control group mean responses from baseline across trials, and used simulation to produce the expected mean response with each of the treatments using the indirect MD estimates and associated standard error estimates. Our primary analysis was of the outcomes observed at last time point (before allowed dose escalation or cross-over). However, since the last observed time points across trials were not consistent, we performed sensitivity analysis where possible. For PsARC we performed sensitivity analysis using similar “short-term” (ie, 12–16 weeks) outcomes, and, separately, “long-term” (ie, 24 weeks) outcomes where available. These analyses were not possible for the HAQ and PASI outcomes as we only had data on one time point.
Results
Identified studies
Nineteen studies, representing seven RCTs, met our inclusion criteria.Citation14–Citation27 Two of these RCTs used adalimumab,Citation14–Citation19 two used etanercept,Citation8–Citation27 two used infliximab,Citation10–Citation24 and one used golimumab.Citation20 presents the characteristics of each RCT, and Table S3 presents the demographic characteristics of the patients included in each RCT. Twenty-nine studies examined in detail were excluded; reasons for exclusion are presented in Table S4. A schematic of the study selection process is presented in .
Table 1 Characteristics of the included trials
Indirect comparisons
For all treatments for all outcomes (except for adalimumab for HAQ nonresponders), there was a statistically significant difference in favor of the treatment (allowing for 5% type I error).
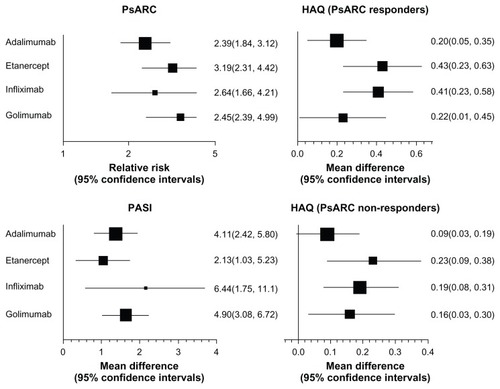
presents the direct estimates for each of the anti-TNF treatments compared with placebo. For PsARC response, golimumab yielded the highest relative risk (RR 3.45, 95% CI: 2.39, 4.99) and etanercept the second highest (RR 3.19, 95% CI: 2.31, 4.42). Adalimumab and infliximab both yielded notably smaller RRs. Sensitivity analysis using different time points did not reveal any difference in PsARC response RRs (results not shown, but available from the authors upon request). For HAQ improvement, etanercept and infliximab yielded the largest MD among PsARC responders (0.43 and 0.41, respectively). For PsARC nonresponders, etanercept, infliximab, and golimumab yielded similar MDs, and adalimumab yielded a notably lower MD. For PASI improvement, infliximab yielded the largest MD and golimumab the second largest (6.44 and 4.90, respectively), while etanercept yielded the smallest MD (3.13).
Figure 2 Forest plots of direct estimates for anti-TNFs versus placebo comparisons.
presents the indirect estimates between anti-TNF treatments. None of the four treatments were statistically significantly different for any of the outcomes.
Table 2 Head-to-head indirect estimates of the anti-TNF drugs
Lastly, presents the pooled control group responses and the expected intervention group responses using the indirect RR and MD estimates from the placebo comparison.
Table 3 Expected response rates and 95% confidence intervals for the three considered outcomes with the four anti-TNF drugs
Discussion
Our indirect comparison of anti-TNF drugs for PsA was based on an extensive literature search and data extraction that allowed us to calculate trial results that were missing in previous indirect comparisons. No statistically significant difference was detected between the four anti-TNF drugs. When considering only the magnitude of estimated effect, the three anti-TNF drugs etanercept, infliximab, and golimumab seem to perform comparably better than adalimumab. When compared with each other, each of these three anti-TNFs performed better for one or two outcomes, but worse for one or two other outcomes (eg, golimumab yields the highest PsARC response, but the lowest average HAQ among PsARC nonresponders). In some instances, the treatment effect point estimates were also notably different from the estimates used to inform the recent NICE cost-effectiveness analysis.Citation4
Our indirect comparison comes with a number of strengths and limitations. We performed an extensive search of all trial publications (several reports have been published for each trialCitation14–Citation29), previous systematic reviews, and HTAs. This allowed us to extract enough data to calculate the results of the outcomes of interest when missing. This also removed the necessity for Bayesian imputation models driven by priors. Despite the extensive data search and extraction, one cannot avoid the fact that the trial data are relatively sparse. Thus, calculations made for missing values and inferences regarding comparative effectiveness may be considerably impacted by random error. Some data may also have been suboptimal. For HAQ improvement by PsARC responders, our etanercept data were a pooled analysis of the Mease 2000 and Mease 2004 trials. Although we were able to use trial reported HAQ scores to calculate and validate these results, some bias concerns exist with regards to Mease 2000, which we were not able to perform sensitivity analysis on. For PASI improvement, we only had continuous data available for about half of the trials. The conversion based on reported PASI50, PASI75, and PASI90 is only approximate, and may thus introduce some error. However, we do not believe this potential error is worse than the bias introduced by using falsely labeled “noninformative” priors in a Bayesian imputation model.
This incongruence between magnitudes of effect estimates in our indirect comparison and previous indirect comparisons, strongly suggests sensitivity to analytic approaches that should not be overlooked in related economic evaluations. Patient utility can be derived by already established mathematical relationships between generic quality of life instruments such as the EQ-5D and the disease outcomes of interest (PsARC, HAQ, and PASI). While previous health economic assessments did perform a wide array of sensitivity analyses, these did not cover sensitivity to different analytic approach such as the ‘imputation’ used for our indirect comparison. Given that adalimumab, etanercept, golimumab, and infliximab are approved for use in PsA in many major settings, it is unlikely that we will see additional trials assessing the efficacy of these therapies, and so, economic evaluations will need to rely on the current available evidence. As such, it seems important to undertake a revision of current cost-effectiveness models to assess whether current drug indications are based on robust results, or need reconsideration.
Conclusion
Our indirect comparison did not demonstrate any significant difference between anti-TNF drugs for the treatment of PsA. In some instances, the magnitudes of effect in our indirect comparison differed from others. Since the analyzed outcomes play an important role informing quality adjusted life years (QALYs, and thus cost per QALY) in cost-effectiveness analyses, it seems reasonable to insist that the cost-effectiveness analyses on which the current drug indications are based be revised to check the robustness of their findings.
Author contributions
KT and EM conceived the design of the study. KT drafted the first manuscript. KT and ED extracted the data. KT performed the statistical analyses. All authors contributed to the interpretation of the findings and to the writing of the final version of the manuscript.
Acknowledgments
This study was sponsored by Merck, Sharp, & Dohme, UK.
Supplementary materials
Table S1 Outcomes included in the analysis
Table S2 Imputations solutions and assumptions employed to construct PASI mean changes
Table S3 Demographic characteristics of participants in the included randomized controlled trials
Table S4 Publications excluded after detailed evaluation
Disclosure
Kristian Thorlund and Edward Mills have consulted either Merck and Co, Inc, Pfizer Ltd, Nycomed, Takeda, Novartis or GlaxoSmithkline on multiple treatment comparison and systematic review issues. Kristian Thorlund and Edward Mills have received grant funding from the Canadian Institutes of Health Research (CIHR) Drug Safety and Effectiveness Network to develop methods and educational materials on MTCs. Edward Mills receives salary support from the Canadian Institutes of Health Research through a Canada Research Chair. Kristian Thorlund receives salary support from the CIHR Drug Safety and Effectiveness Network. The above authors report no other conflicts of interest in this work. Eric Druyts and Antonio Avina-Zubieta report no conflicts of interest in this work.
References
- GladmanDDAntoniCMeasePCleggDONashPPsoriatic arthritis: epidemiology, clinical features, course, and outcomeAnn Rheum Dis200564Suppl 2ii14ii1715708927
- RitchlinCTKavanaughAGladmanDDTreatment recommendations for psoriatic arthritisAnn Rheum Dis20096891387139418952643
- RodgersMEpsteinDBojkeLEtanercept, infliximab, and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluationHealth Technol Assess20111510ixxi1329
- YangHCraigDEpsteinDGolimumab for the treatment of psoriatic arthritis: a NICE single technology appraisalPharmecoeconomics2012304257270
- GelmanAPrior distributions for variance parameters in hiearchical modelsBayesian Anal200613515533
- LambertPCSuttonAJBurtonPRAbramsKRJonesDRHow vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGSStat Med200524152401242816015676
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials19961711128721797
- MeasePJGoffeBSMetzJVanderStoepAFinckBBurgeDJEtanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trialLancet2000356922738539010972371
- MeasePJKivitzAJBurchFXEtanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progressionArthritis Rheum20045072264227215248226
- AntoniCEKavanaughAKirkhamBSustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT)Arthritis Rheum20055241227123615818699
- AntoniCEKavanaughAvan der HeijdeDTwo-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT)J Rheumatol200835586987618381786
- KavanaughAAntoniCEGladmanDThe Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 yearAnn Rheum Dis20066581038104316439444
- BucherHCGuyattGHGriffithLEWalterSDThe results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trialsJ Clin Epidemiol19975066836919250266
- GladmanDDMeasePJChoyEHRitchlinCTPerdokRJSassoEHRisk factors for radiographic progression in psoriatic arthritis: subanalysis of the randomized controlled trial ADEPTArthritis Res Ther2010123R11320537151
- GladmanDDMeasePJCifaldiMAPerdokRJSassoEMedichJAdalimumab improves joint-related and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the Adalimumab Effectiveness in Psoriatic Arthritis TrialAnn Rheum Dis200766216316817046964
- GladmanDDMeasePJRitchlinCTAdalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trialArthritis Rheum200756247648817265483
- MeasePJGladmanDDRitchlinCTAdalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trialArthritis Rheum200552103279328916200601
- MeasePJOryPSharpJTAdalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT)Ann Rheum Dis200968570270918684743
- GenoveseMCMeasePJThomsonGTSafety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapyJ Rheumatol20073451040105017444593
- KavanaughAMcInnesIMeasePGolimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled studyArthritis Rheum200960497698619333944
- AntoniCKruegerGGde VlamKInfliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trialAnn Rheum Dis20056481150115715677701
- KavanaughAAntoniCKruegerGGInfliximab improves health related quality of life and physical function in patients with psoriatic arthritisAnn Rheum Dis200665447147716096330
- KavanaughAAntoniCMeasePEffect of infliximab therapy on employment, time lost from work, and productivity in patients with psoriatic arthritisJ Rheumatol200633112254225916960923
- van der HeijdeDKavanaughAGladmanDDInfliximab inhibits progression of radiographic damage in patients with active psoriatic arthritis through one year of treatment: Results from the induction and maintenance psoriatic arthritis clinical trial 2Arthritis Rheum20075682698270717665424
- MeasePJKivitzAJBurchFXContinued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanerceptJ Rheumatol200633471272116463435
- MeasePJWoolleyJMBitmanBWangBCGlobeDRSinghAMinimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfactionJ Rheumatol201138112461246521885498
- MeasePJWoolleyJMSinghATsujiWDunnMChiouCFPatient-reported outcomes in a randomized trial of etanercept in psoriatic arthritisJ Rheumatol20103761221122720395648
- BaranauskaiteARaffayováHKungurovNVfor RESPOND investigatorsInfliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND studyAnn Rheum Dis201271454154821994233
- KimballABBensimonAGGuerinAEfficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: Subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trialAm J Clin Dermatol2011121516221110526
- MeasePGenoveseMCGladsteinGAbatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trialArthritis Rheum201163493994821128258
- PrinzJCFitzgeraldOBoggsRICombination of skin, joint and quality of life outcomes with etanercept in psoriasis and psoriatic arthritis in the PRESTA trialJ Eur Acad Dermatol Venereol201125555956420840349
- AsahinaANakagawaHEtohTOhtsukiMfor Adalimumab M04- 688 Study GroupAdalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled studyJ Dermatol201037429931020507398
- AttenoMPelusoRCostaLComparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugsClin Rheumatol201029439940320066450
- MeasePJSignorovitchJYuAPImpact of adalimumab on symptoms of psoriatic arthritis in patients with moderate to severe psoriasis: a pooled analysis of randomized clinical trialsDermatology201022011719940437
- SterryWOrtonneJPKirkhamBComparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trialBMJ2010340c14720124563
- ToriiHNakagawaHfor Japanese Infliximab Study investigatorsInfliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo- controlled multicenter trialJ Dermatol Sci2010591404920547039
- van KuijkAWGerlagDMVosKA prospective, randomised, placebo-controlled study to identify biomarkers associated with active treatment in psoriatic arthritis: effects of adalimumab treatment on synovial tissueAnn Rheum Dis20096881303130918647851
- BongiornoMRPistoneGDoukakiSAricòMAdalimumab for treatment of moderate to severe psoriasis and psoriatic arthritisDermatol Ther200821Suppl 2S15S20
- BrodszkyVPentekMGulacsiLEfficacy of adalimumab, etanercept, and infliximab in psoriatic arthritis based on ACR50 response after 24 weeks of treatmentScand J Rheumatol200837539940018821278
- FeldmanSRGottliebABBalaMInfliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasisBr J Dermatol2008159370471018627375
- KristensenLEGülfeASaxneTGeborekPEfficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group registerAnn Rheum Dis200867336436917644547
- RavindranVScottDLChoyEHA systematic review and metaanalysis of efficacy and toxicity of disease modifying anti-rheumatic drugs and biological agents for psoriatic arthritisAnn Rheum Dis200867685585917827183
- RevickiDWillianMKSauratJHImpact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasisBr J Dermatol2008158354955718047521
- SaadAASymmonsDPNoycePRAshcroftDMRisks and benefits of tumor necrosis factor-alpha inhibitors in the management of psoriatic arthritis: systematic review and metaanalysis of randomized controlled trialsJ Rheumatol200835588389018381787
- SpadaroACeccarelliFScrivoRValesiniGLife-table analysis of etanercept with or without methotrexate in patients with psoriatic arthritisAnn Rheum Dis200867111650165118854519
- StroberBTellerCYamauchiPEffects of etanercept on C- reactive protein levels in psoriasis and psoriatic arthritisBr J Dermatol2008159232233018503600
- FrankelEHStroberBECrowleyJJEtanercept improves psoriatic arthritis patient-reported outcomes: results from EDUCATECutis200779432232617500381
- KimballABJacksonJMSobellJMReductions in healthcare resource utilization in psoriatic arthritis patients receiving etanercept therapy: results from the educate trialJ Drugs Dermatol20076329930617373192
- Romero-MatéAGarcía-DonosoCCórdoba-GuijarroSEfficacy and safety of etanercept in psoriasis/psoriatic arthritis: an updated reviewAm J Clin Dermatol20078314315517492843
- Vander CruyssenBDe KeyserFKruithofEMielantsHVan den BoschFComparison of different outcome measures for psoriatic arthritis in patients treated with infliximab or placeboAnn Rheum Dis200766113814017178763
- FransenJAntoniCMeasePJPerformance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitorsAnn Rheum Dis200665101373137816644783
- GottliebABKircikLEisenDUse of etanercept for psoriatic arthritis in the dermatology clinic: the Experience Diagnosing, Understanding Care, and Treatment with Etanercept (EDUCATE) studyJ Dermatolog Treat200617634335217853307
- RitchlinCEfficacy and safety of infliximab for the treatment of psoriatic arthritisNat Clin Pract Rheumatol20062630030116932707
- KvienTKHeibergLieEA Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseasesClin Exp Rheumatol2005235 Suppl 39S188S19416273806
- RinaldiFProvenzanoGTerminiASpinelloMLa SetaFLong term infliximab treatment for severe psoriatic arthritis: evidence of sustained clinical and radiographic responseAnn Rheum Dis20056491375137616100346