Abstract
Aim
To investigate the efficacy and safety of dendritic cell (DC) vaccine combined with cytokine-induced killer (CIK) cell therapy in colorectal carcinoma (CRC).
Patients and methods
PubMed, Embase, and Cochrane Library databases were searched systematically for clinical trials of DC vaccine and CIK cell therapy combined with chemotherapy for CRC. The primary and secondary endpoints were overall survival (OS) and disease-free survival (DFS), respectively. Pooled risk ratios were used to assess the treatment efficacy. Both random and fixed effects models were used for statistical analysis. The study population consisted of 871 CRC patients enrolled in four trials.
Results
OS and DFS were significantly improved in patients who received chemotherapy combined with DC vaccine and CIK cells, and no severe adverse events were shown.
Conclusions
The study demonstrated that the addition of DC vaccine and CIK cell therapy to chemotherapy is feasible and effective in patients with CRC.
Introduction
Colorectal carcinoma (CRC) is now the third most common cancer, accounting for 9% of the new cancer cases in men and 8% of the new cancer cases in women in 2017, as estimated by the American Cancer Society.Citation36 More importantly, about 25% of patients with CRC have distant metastasis upon diagnosis, with liver metastasis in most cases. Only 20% of liver metastasis cases are eligible for radical surgery.Citation34 Furthermore, traditional therapies, such as chemotherapy and radiotherapy, usually fail to eradicate colorectal tumors completely, and their clinical applications are limited by accompanying adverse events.Citation11,Citation35 Thus, novel treatment and early diagnosis of CRC are pressing needs, and immunotherapy may serve as an alternative due to the encouraging results.Citation2,Citation14,Citation30
Recently, how to better fight cancer by restoring and enhancing immune function has attracted great interest. Clinical evidence demonstrates that immunotherapy may be an effective and safe supplementary approach to cancer treatment.Citation7,Citation9,Citation15,Citation22 Some studies reported that immunotherapy could improve the efficacy of chemotherapy and radiotherapy for CRC patients.Citation6,Citation10,Citation23 Many immunotherapy investigations are ongoing, including those of cancer vaccines, adoptive cellular therapy, and immunomodulatory antibodies such as ipilimumab and pembrolizumab, and obtaining sufficient numbers of immune effectors to improve the efficiency of recognizing tumor targets is one of the most notable problems.
Dendritic cells (DCs) are critical for the robust presentation of immunogenic peptides and activation of T cells.Citation38 DCs can be generated from human peripheral blood mononuclear cells (PBMCs) ex vivo by stimulating with interleukin (IL)–4, granulocyte macrophage colony-stimulating factor (GM-CSF), and tumor-associated antigen. Then, mature DCs can be transferred back into patients as a cancer vaccine to elicit an antigen-specific immune reaction.Citation27,Citation29,Citation33 Clinical studies demonstrated that DC vaccines could elicit different clinical benefits in metastatic melanoma, lymphoma, non-small-cell lung cancer, and CRC.Citation6,Citation41,Citation43 Sipuleucel-T (APC8015) is one of the DC vaccines, proliferated from PBMCs by culturing with a prostatic acid phosphatase (PAP), a fusion protein of prostate cancer antigen and GM-CSF, and it could prolong overall survival in prostate cancer patients;Citation1 thus, it was approved by the FDA for the treatment of metastatic prostate cancers.
Cytokine-induced killer (CIK) cells are immune effector cells that are easy to proliferate from PBMCs through stimulating with interferon (IFN)-γ, CD3 monoclonal antibody, and IL-2. These cells show a high proliferation rate of the CD3+ CD56+ phenotype, take advantage of the body’s natural ability to eliminate tumor cells by stimulating and restoring the immune system to recognize and kill tumor cells, exhibit robust antitumor activity, and have no major histocompatibility complex restrictions;Citation11,Citation18,Citation21 thus, they are frequently used in cellular immunotherapy for a variety of malignancies.Citation46,Citation51 As reported by Hontscha,Citation11 CIK treatment could significantly improve disease-free survival (DFS) rates and prevent recurrence for cancer patients, compared with the control group.
DCs have been proved to play an important role in CIK activation, proliferation, phenotype expression, and cytokine secretion by direct contact and secreted IL-12, IFN-γ, and TNF-a, which enhance antitumor effects.Citation13,Citation22,Citation32,Citation47 After co-culture with DCs, CD3+ CD56+ cells, which are the main effector cells enhancing CIK cytotoxicity, were increased, whereas CD4+ CD25+ regulatory T cells, which inhibit CIK antitumor activity, were decreased.Citation32,Citation47 In addition, CIK cells promote DC maturation and expression of co-stimulatory molecules, such as CD40, CD80, and CD86,Citation24,Citation47 and the combination of DC and CIK provides a remarkably increased cytotoxic activity.Citation46 It has also been found that the combination of DC and CIK is more effective than CIK treatment.Citation53
Combination therapy with CIK cells and DC vaccine has been successfully applied to treat cancers, such as glioblastoma, non-small-cell lung cancer, and advanced renal cell carcinoma, and it has been demonstrated that this therapy can enhance host cellular immune responses, prolong survival time, and improve quality of life.Citation19,Citation40,Citation45,Citation49 Mao et alCitation20 reported that DC vaccine and CIK cell combined with chemotherapy are able to improve the disease control rate (DCR) and living quality of patients, with no severe side effects in patients with metastatic breast cancer. However, recent clinical trials on the DC vaccine and CIK cells in CRC patients are still based on small sample size and a lack of high-quality data from multicenter studies. So, we conducted this meta-analysis with the aim of investigating the efficacy and safety of combination therapy with DC vaccine and CIK cell for CRC, which will help future clinical trials.
Methods
Literature search strategy
PubMed, Embase, and Cochrane Library databases were searched with no date restrictions for “colorectal cancer,” “cytokine-induced killer cells,” and “dendritic cells.” All of the articles were subjected to strict reviews, including full text, authors, and research institutions, to eliminate duplicate versions.
Selection criteria
The articles we identified in this search were required to meet the following inclusion criteria for the present meta-analysis: (1) the report was of a human trial published in English; (2) the patients had histologically or cytologically proven CRC diagnosis; (3) the combination therapy group received both chemotherapy and more than one cycle of DC vaccine and CIK cell therapy, whereas the control group received chemotherapy alone; and (4) the primary outcome measure in the study was overall survival (OS). Case studies were excluded.
Data extraction
Data extraction was conducted by two investigators using a standardized approach. Divergences in what to collect were adjudicated by a third investigator after reading the original publications. We collected information from the publication, including the authors’ name, sample size per arm, tumor stage, and the percentages of OS and DFS. Randomized controlled trials were assessed using the Jadad et alCitation12 format, and the quality of the retrospective studies was assessed using the Newcastle-Ottawa Scale.Citation37
Efficacy assessments
We evaluated both OS and DFS of the four studies. OS was defined as the time from the start of a clinical trial to death due to any cause, and DFS was defined as the time from surgery to tumor recurrence or death by authors of included studies. We used Engauge Digitizer to extract data from the survival curves of the included studies,Citation55 and then used the Excel table provided by Tierney et alCitation42 for data reconstruction.
Statistical analysis
Statistical analysis was performed using the Review Manager software program (version 5.3; Cochrane, London, UK). P-values less than 0.05 were considered as significant. Risk ratios (RRs) and their 95% confidence intervals (CIs) were used for calculations. Heterogeneity analysis of the included trials was performed before combining data using Cochran’s chi-squared test (Q test), with significance set at P-values less than 0.10. Because heterogeneity in the four studies may be difficult to detect, we evaluated both random effects and fixed effects.
Results
Selection of the trials
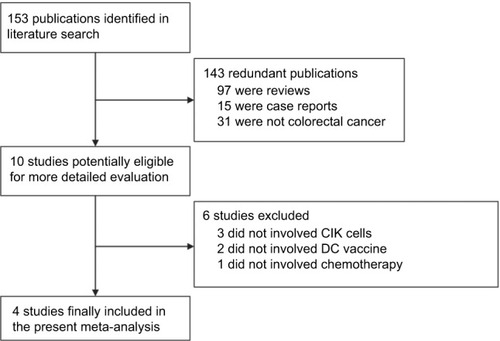
The initial literature search found 153 related articles, but 143 publications were excluded because 97 were reviews, 15 were case reports, and 31 were not CRC. After referring to full texts, six publications that failed to meet the inclusion criteria were excluded because three did not involve CIKs, two did not involve DCs, and one did not involve chemotherapy combined with DCs and CIK cells. Ultimately, there were only four clinical trials, which involved a total of 871 patients, that matched our inclusion criteria.Citation8,Citation17,Citation25,Citation54 The selection procedure is shown in .
Characteristics of the eligible trials
All four trials were fully published and consisted of two randomized studies and two retrospective analyses. The researchers in the studies prepared DC vaccines by culturing PBMCs of CRC patients in the presence of tumor lysates, GM-CSF, tumor necrosis factor, and IL-4. In addition, investigators used CD3 monoclonal antibody, IFN-γ, and IL-2 in the CIK cell cultures in all of these trials. The mean number of DCs transfused into the patients in these studies was 1×107 per course, whereas that of CIK cells was more than 1×109 per course. The information about the publication, including the authors’ name, sample size per arm, tumor stage, and the percentages of OS and DFS, were also collected ().
Table 1 Clinical information on the patients in the four eligible trials
Six-month OS
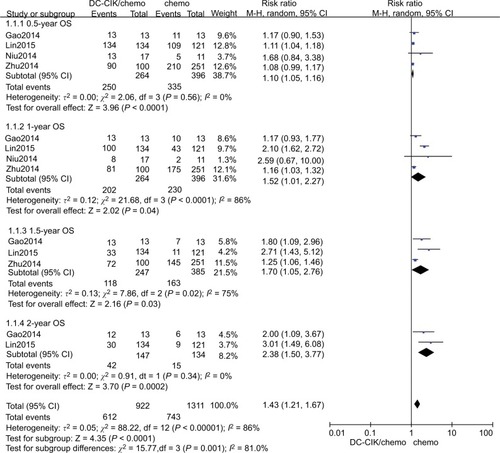
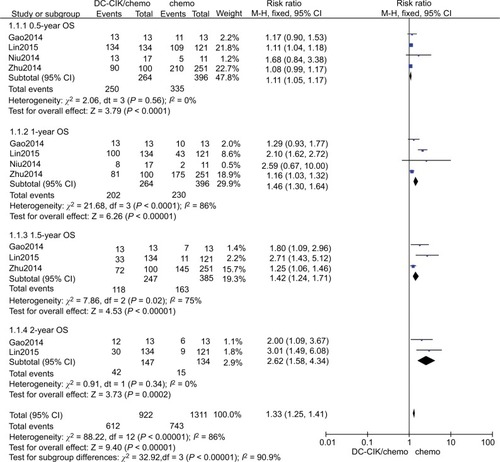
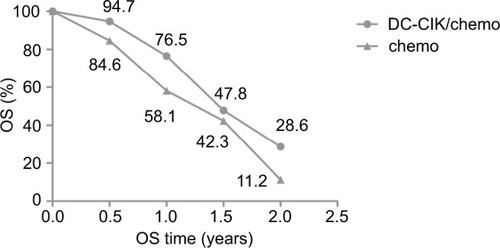
We found that DC-CIK cell therapy significantly improved six-month OS, whether using fixed or random effects models for statistical analysis (fixed effects model: RR=1.11 [95% CI=1.05–1.17], P=0.0001; random effects model: RR=1.10 [95% CI=1.05–1.16], P<0.001). The 6-month OS rate was 94.7% (250/264) in the patients who received the combination therapy and 84.6% (335/396) in those who received chemotherapy only. We did not detect statistically significant heterogeneity in the four studies (χ2=2.06, df=3, P=0.56, ICitation2=0%) ( and ).
One-year OS
The 1-year OS rates were 58.1% (230/396) and 76.5% (202/264) in the control and combination therapy groups, respectively. The estimated pooled RR for the four trials improved significantly in 1-year OS for patients in the combination group (fixed effects model: RR=1.46 [95% CI=1.30–1.64], P<0.0001; random effects model: RR=1.52 [95% CI=1.01–2.27], P=0.040). However, we found statistically significant heterogeneity in the four trials (χ2=21.68, df=3, P<0.001, ICitation2=86%) ( and ).
One and a half-year OS
Data on 1.5-year OS were available for only three trials.Citation8,Citation17,Citation54 The 1.5-year OS rates were 47.8% (118/247) and 42.3% (163/385) in the combination therapy and control groups, respectively. The estimated pooled RR for the three trials exhibited significant improvements (fixed effects model: RR=1.45 [95% CI=1.24–1.71], P=0.02; random effects model: RR=1.70 [95% CI=1.05–2.76], P=0.030). We found statistically significant heterogeneity in the three trials (χ2=7.86, df=2, P=0.02, ICitation2=75%) ( and ).
Two-year OS
Data on 2-year OS were available for two trials.Citation8,Citation17 The 2-year OS rate was 28.6% (42/147) in the combination therapy group and 11.2% (15/134) in the control group. The estimated pooled RR for the two trials demonstrated that DC-CIK cell therapy significantly improved the 2-year OS in CRC patients (fixed effects model: RR=2.62 [95% CI=1.58–4.34], P=0.0002; random effects model: RR, 2.38 [95% CI=1.50–3.77]; P=0.0002). We did not detect statistically significant heterogeneity in the two trials ((χ2=0.91, df=1, P=0.34, ICitation2=0%) ( and ).
To determine the differences in OS between the combination therapy and control groups, mean OS rates and the result of paired t-tests of included studies are shown in , with a P-value of 0.025, indicating that DC-CIK cell therapy significantly improved OS rates in patients with CRC.
Figure 4 Mean survival curve for the combination therapy (DC-CIK/chemo) and control (chemo) groups.
Notes: A paired t-test was used for statistical analysis (P=0.025; P-values less than 0.05 were considered significant).
Abbreviations: DC-CIK/chemo, DC-CIK immunotherapy combined with chemotherapy; chemo, chemotherapy alone; OS, overall survival.
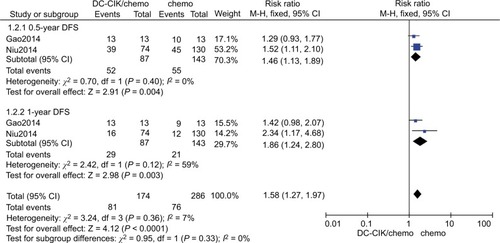
Six-month DFS
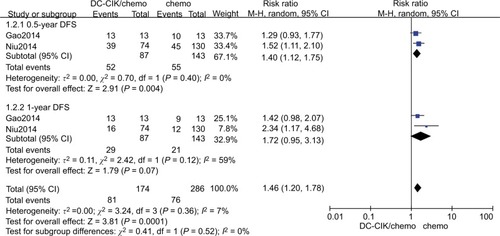
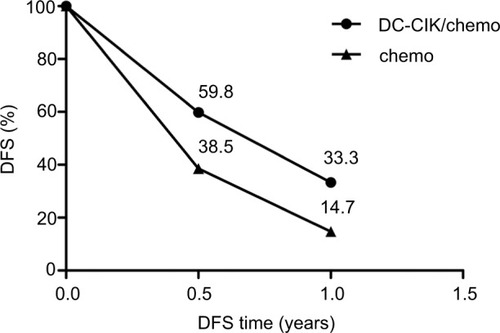
Data on 6-month DFS were available for two trials.Citation8,Citation25 The 6-month DFS rates were 59.8% (52/87) and 38.5% (55/143) in the combination therapy and control groups, respectively. The combined RR (fixed effects model: RR=1.46 [95% CI=1.13–1.89], P=0.004; random effects model: RR=1.40 [95% CI=1.12–1.75]; P=0.004) indicated that DC-CIK cell therapy combined with chemotherapy was markedly effective in terms of its effect on DFS. We found no statistical heterogeneity in the two trials (χ2=0.70, df=1, P=0.40, ICitation2=0%) ( and ).
One-year DFS
Data on 1-year DFS were available for two trials.Citation8,Citation25 The 1-year DFS rates were 33.3% (29/87) and 14.7% (21/143) in the combination therapy and control groups, respectively. When we used a fixed effects model for analysis, we found that the combination therapy could prolong 1-year DFS (fixed effects model: RR=1.86 [95% CI=1.24–2.80], P=0.003). However, when a random effects model was used for analysis, there was no significant difference between the two groups in 1-year DFS (random effects model: RR=1.72 [95% CI=0.95–3.31]; P=0.07). We did not find statistical heterogeneity in the two trials ((χ2=2.42, df=1, P=0.12, ICitation2=59%) ( and ).
To determine the differences in DFS between the combination therapy and control groups, mean DFS rates and the results of paired t-tests of included studies are shown in , with a P-value of 0.043, indicating that DC-CIK cell therapy significantly improved DFS in patients with CRC.
Figure 7 Mean DFS rates in the combination therapy (DC-CIK/chemo) and control (chemo) groups.
Notes: A paired t-test was used for statistical analysis (P=0.043; P-values less than 0.05 were considered significant).
Abbreviations: DC-CIK/chemo, DC-CIK immunotherapy combined with chemotherapy; Chemo, chemotherapy alone; DFS, disease-free survival.
Adverse effects
None of the study patients had any severe adverse effects due to their treatment. The proportion of fever in the combination therapy group was higher than that in the control group, and the transfusion of DC vaccine and CIK cells has been thought to be the main reason. Most of the patients with fever in our study had temperatures ranging from 37.5°C to 40.0°C, and the fever was resolved spontaneously without any treatment in most patients, except some who received antipyretic treatment. There were no significant differences between the two groups in the incidence of other adverse effects, such as insomnia, anorexia, joint soreness, skin rash, or leukopenia ().
Table 2 Adverse effects after DC vaccine and CIK cell therapy combined with chemotherapy
Discussion
In recent years, immunotherapy has become the most promising approach for cancer therapy.Citation3 CIK cell therapy is a subset of immunotherapy that has attracted more and more attention due to its strong antitumor activity.Citation39 The combination of DC vaccine and CIK cells can enhance the antitumor response, and this combination therapy made satisfying achievements in clinical applications of various tumors. A randomized controlled study reported that 1-year OS and DFS rates in gastric cancer and CRC patients who received DC vaccine and CIK cell therapy combined with chemotherapy were significantly higher than those in patients who received chemotherapy alone.Citation8 Furthermore, a recent meta-analysis included randomized controlled trials (RCTs) which applied chemotherapy with and without DC-CIK (DCs co-cultured with CIK cells before infusion or infusion of DC vaccine and CIK cells, respectively), and demonstrated that the combination therapy can markedly improve 2- and 3-year OS as well as 1- and 3-year DFS rates over chemotherapy alone for solid tumors, such as non-small-cell lung cancer, breast cancer, and gastrointestinal cancer. In addition, the combination therapy has been more effective for gastrointestinal cancers than for non-small-cell lung cancer in terms of OS.Citation14
This meta-analysis focused on chemotherapy combined with DC vaccine and CIK in CRC treatment. Considering that the quality of published literature may lead to bias of the meta-analysis, we enrolled four relatively high-quality clinical trials that were published in English and met the inclusion criteria. We used both random and fixed effects models for statistical analysis so that the results would be more objective and reliable. Our analysis found that the combination treatment had favorable results for 6-month, 1-year, 1.5 year, and 2-year OS, and 6-month DFS in both the fixed and random effects models. However, no significant difference was found in the random effects model analysis of 1-year DFS. Data on 1-year DFS were available for only two trials, and these trials had small samples, which may affect the results. Therefore, more trials about DC vaccine and CIK cell treatment in CRC are needed to evaluate DFS in the future.
CIK cell-based immunotherapy is effective for metastatic CRC, as demonstrated by improved OS and progression-free survival rates.Citation16,Citation52 Moreover, researchers have demonstrated that the infusion of DC vaccine and CIK cells can induce a strong immune response with a positive response of delayed-type hypersensitivity (DTH) in cancer patients, such as those with advanced renal cell carcinoma, hepatocellular carcinoma, and pancreatic cancer.Citation45,Citation50
The superior therapeutic effect of DC vaccine and CIK cell therapy over that of chemotherapy alone may be explained by three factors. First, cancer stem cells, which are resistant to chemotherapy and radiotherapy, and target proliferating cells, have the abilities of self-renewal, cancer initiation, and further maintenance of tumors. Since proliferation is not a prerequisite for the identification and destruction of tumor cells by immune mechanisms, DC vaccine and CIK cell therapy may be an effective way to eliminate cancer stem cells.Citation26 Second, CIK cells, which have the benefits of T lymphocytes and natural killer cells, exhibit strong antitumor activity, but not major histocompatibility complex restriction.Citation28,Citation31 This was demonstrated by higher serum IL-12 and IFN-γ levels in the combination group than in the chemotherapy-alone group, as reported by Gao et al,Citation8 indicating that CIK cell therapy may specifically promote a Th1 immune response to kill tumor cells. Third, combining with DC vaccine increases the antitumor activities of CIK cell therapy, both in theory and in practice.Citation4,Citation5,Citation44,Citation48
A higher incidence of fever was observed in the combination therapy group than that in the control group, and the transfusion of DC vaccine and CIK cells has been thought to be the main reason and, at the same time, may indicate a strong immune response. The frequencies of other treatment-related side effects, such as leukopenia, anemia, nausea, vomiting, and abnormal liver function, seem to be lower in the combination group compared with the control group, indicating that the infusion of DC vaccine and CIK cells is safe for patients with CRC.
Our meta-analysis has some limitations that may affect interpretation of the results. First, no statistical analysis about side effects was performed due to the limited data. Second, data on DFS were only available for two trials, which may have led to bias. Third, we reconstructed data from published articles rather than using the original records of the respective experiments, which may have led to deviation from the real data. Fourth, limited to research data, we just compared DC vaccine and CIK cells plus chemotherapy with chemotherapy alone, without the comparisons of DC vaccine plus chemotherapy, or CIK cells plus chemotherapy. Thus, we expect more related research to verify the safety and efficacy of DC vaccine and CIK cell therapy for CRC.
In conclusion, our analysis demonstrated that DC vaccine and CIK cell combination therapy was applicable for patients with CRC, which is a feasible choice to prolong survival and prevent tumor recurrence. This study may contribute to the widespread use of DC vaccine and CIK cells in CRC and provide valid evidence for the clinical application of other malignancies.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81500030) and the Natural Science Foundation of Guangdong Province (2016A030313272, 2016A030313277, 2017A030313573).
Author contributions
Haiqing Ma and Zhong Lin conceived and designed the experiments. Xiuling Zhou and Xiangqiong Mo performed the experiments. Shuncong Wang, Cuiling Zhou and Yonghui Su analyzed the data. Junlan Qiu and Jingjing Zhao contributed to the reagents, materials and analysis tools. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BotrelTEClarkOPompeoACImmunotherapy with Sipuleucel-T (APC8015) in patients with metastatic castration-refractory prostate cancer (mCRPC): a systematic review and meta-analysisInt Braz J Urol201238671772723302410
- ChenLZhangXPrimary analysis for clinical efficacy of immunotherapy in patients with pancreatic cancerImmunotherapy20168222323426565954
- Couzin-FrankelJBreakthrough of the year 2013. Cancer immunotherapyScience201334261651432143324357284
- CuiYYangXZhuWLiJWuXPangYImmune response, clinical outcome and safety of dendritic cell vaccine in combination with cytokine-induced killer cell therapy in cancer patientsOncol Lett20136253754124137363
- EllebaekEAndersenMHSvaneIMStratenPTImmunotherapy for metastatic colorectal cancer: present status and new optionsScand J Gastroenterol201247331532422214467
- FongLHouYRivasAAltered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapyProc Natl Acad Sci U S A200198158809881411427731
- GalluzziLVacchelliEEggermontATrial Watch: Adoptive cell transfer immunotherapyOncoimmunology20121330631522737606
- GaoDLiCXieXAutologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patientsPLoS One201494e9388624699863
- GaoJBernatchezCSharmaPRadvanyiLGHwuPAdvances in the development of cancer immunotherapiesTrends Immunol2013342909823031830
- HarrisJERyanLHooverHCAdjuvant Active Specific Immunotherapy for Stage II and III Colon Cancer with an Autologous Tumor Cell Vaccine: Eastern Cooperative Oncology Group Study E5283J Clin Oncol20001814815710623705
- HontschaCBorckYZhouHMessmerDSchmidt-WolfIGClinical trials on CIK cells: first report of the international registry on CIK cells (IRCC)J Cancer Res Clin Oncol2011137230531020407789
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials19961711128721797
- KirkwoodJMButterfieldLHTarhiniAAZarourHKalinskiPFerroneSImmunotherapy of cancer in 2012CA Cancer J Clin201262530933522576456
- LanXPChenYGWangZImmunotherapy of DC-CIK cells enhances the efficacy of chemotherapy for solid cancer: a meta-analysis of randomized controlled trials in Chinese patientsJ Zhejiang Univ Sci B201516974375626365116
- LesterhuisWJHaanenJBPuntCJCancer immunotherapy – revisitedNat Rev Drug Discov201110859160021804596
- LiXDaiXShiLPhase II/III Study of Radiofrequency Ablation Combined with Cytokine-Induced Killer Cells Treating Colorectal Liver MetastasesCell Physiol Biochem2016401–213714527855365
- LinTSongCChuoDYZhangHZhaoJClinical Effects of Autologous Dendritic Cells Combined with Cytokine-Induced Killer Cells Followed by Chemotherapy in Treating Patients with Advanced Colorectal Cancer: A Prospective StudyTumour Biol20163744367437226499782
- LuPHNegrinRSA novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiencyJ Immunol19941534168716967519209
- MaggiET-cell responses induced by allergen-specific immunotherapyClin Exp Immunol20101611101820408857
- MaoQLiLZhangCSunYLiuSCuiSClinical effects of immunotherapy of DC-CIK combined with chemotherapy in treating patients with metastatic breast cancerPak J Pharm Sci2015283 Suppl1055105826051718
- MargolinKANegrinRSWongKKChatterjeeSWrightCFormanSJCellular immunotherapy and autologous transplantation for hematologic malignancyImmunol Rev19971572312409255634
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- MosolitsSNilssonBMellstedtHTowards therapeutic vaccines for colorectal carcinoma: a review of clinical trialsExpert Rev Vaccines20054332935016026248
- MuYZhouCHChenSFEffectiveness and safety of chemotherapy combined with cytokine-induced killer cell /dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysisCytotherapy20161891162117727421742
- NiuJRenYZhangTRetrospective comparative study of the effects of dendritic cell vaccine and cytokine-induced killer cell immunotherapy with that of chemotherapy alone and in combination for colorectal cancerBiomed Res Int20142014214727725210706
- O’BrienCAPollettAGallingerSDickJEA human colon cancer cell capable of initiating tumour growth in immunodeficient miceNature2007445712310611017122772
- PaluckaKBanchereauJDendritic-cell-based therapeutic cancer vaccinesImmunity2013391384823890062
- PievaniABorleriGPendeDDual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicityBlood2011118123301331021821703
- PyzerARAviganDERosenblattJClinical trials of dendritic cell-based cancer vaccines in hematologic malignanciesHum Vaccin Immunother201410113125313125625926
- RaberPLSierraRAThevenotPTT cells conditioned with MDSC show an increased anti-tumor activity after adoptive T cell based immunotherapyOncotarget2016714175651757827007050
- Schmidt-WolfIGLefterovaPMehtaBAPhenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cellsExp Hematol19932113167316797694868
- SchmidtJEisoldSBüchlerMWMärtenADendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinomaCancer Immunol Immunother200453111018102615185013
- SchulerPJHarasymczukMVisusCPhase I dendritic cell p53 peptide vaccine for head and neck cancerClin Cancer Res20142092433244424583792
- SevčíkováKUšákováVBartošováZSurgical treatment of metastases and its impact on prognosis in patients with metastatic colorectal carcinomaKlin Onkol2014271384424635436
- SharifSO’ConnellMJYothersGLopaSWolmarkNFOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancerCancer Invest200826995696318798075
- SiegelRLMillerKDJemalACancer Statistics, 2017CA Cancer J Clin201767173028055103
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- SteinmanRMBanchereauJTaking dendritic cells into medicineNature2007449716141942617898760
- ThanendrarajanSKimYSchmidt-WolfINew adoptive immunotherapy strategies for solid tumours with CIK cellsExpert Opin Biol Ther201212556557222444075
- ThomasAAErnstoffMSFadulCEImmunotherapy for the treatment of glioblastomaCancer J2012181596822290259
- ThurnerBHaendleIRöderCVaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanomaJ Exp Med1999190111669167810587357
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- TimmermanJMCzerwinskiDKDavisTAIdiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patientsBlood20029951517152611861263
- ToomeyPGVohraNAGhansahTSarnaikAAPilon-ThomasSAImmunotherapy for gastrointestinal malignanciesCancer Control2013201324223302905
- WangHZhouFJWangQJEfficacy of autologous renal tumor cell lysate-loaded dendritic cell vaccine in combination with cytokine-induced killer cells on advanced renal cell carcinoma – a report of ten casesAi Zheng200625562563016687087
- WangQJWangHPanKComparative study on anti-tumor immune response of autologous cytokine-induced killer (CIK) cells, dendritic cells-CIK (DC-CIK), and semi-allogeneic DC-CIKChin J Cancer201029764164820591215
- WeiXCYangDDHanXRBioactivity of umbilical cord blood dendritic cells and anti-leukemia effectInt J Clin Exp Med2015810197251973026770637
- XiangBSnookAEMageeMSWaldmanSAColorectal cancer immunotherapyDiscov Med2013158430130823725603
- ZhangLYangXSunZDendritic cell vaccine and cytokine-induced killer cell therapy for the treatment of advanced non-small cell lung cancerOncol Lett20161142605261027073525
- ZhangLZhuWLiJClinical outcome of immunotherapy with dendritic cell vaccine and cytokine-induced killer cell therapy in hepatobiliary and pancreatic cancerMol Clin Oncol20164112913326870371
- ZhangYWangJWangYAutologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomyClin Dev Immunol2013201319569124382970
- ZhaoHWangYYuJAutologous Cytokine-Induced Killer Cells Improves Overall Survival of Metastatic Colorectal Cancer Patients: Results From a Phase II Clinical TrialClin Colorectal Cancer201615322823527052743
- ZhouCLiuDLiJChemotherapy plus dendritic cells co-cultured with cytokine-induced killer cells versus chemotherapy alone to treat advanced non-small-cell lung cancer: a meta-analysisOncotarget2016752865008651027863436
- ZhuHYangXLiJSafety, and Survival and Quality of Life Outcomes for Advanced Colorectal Cancer Patients Treated with Dendritic Cell Vaccine and Cytokine-Induced Killer Cell TherapyBiomed Res Int2014201460387125136601
- ZhouZZhangTLiBExtracting and transforming of appropriate data of Meta-analysis in survival curveChin J Evid Based Cardiovasc Med20146243247 Chinese [with English abstract]