Abstract
Berberine (BBR) has been extensively studied in vivo and vitro experiments. BBR inhibits cell proliferation by regulating cell cycle and cell autophagy, and promoting cell apoptosis. BBR also inhibits cell invasion and metastasis by suppressing EMT and down-regulating the expression of metastasis-related proteins and signaling pathways. In addition, BBR inhibits cell proliferation by interacting with microRNAs and suppressing telomerase activity. BBR exerts its anti-inflammation and antioxidant properties, and also regulates tumor microenvironment. This review emphasized that BBR as a potential anti-inflammation and antioxidant agent, also as an effective immunomodulator, is expected to be widely used in clinic for cancer therapy.
Introduction
Cancer is a major cluster of diseases that seriously affects human health. Therefore, development of strategies to prevent and treat cancer is critical.Citation1 Berberine (BBR), a small molecule isoquinoline alkaloid extracted from the rhizomes of coptis chinensis and hydrastis canadensis, is traditionally used to treat bacterial diarrhea.Citation2 Recent studies showed that BBR reduced lipid levels and glycemic index, and exerted anti-tumor effects.Citation3–Citation7 BBR lowered lipid levels via competitive inhibition of HMG-CoA reductase, and by interacting with the 3ʹ-UTR of the LDL receptor (LDLR) to improve the stability of LDLR mRNA.Citation8 In vivo experiment showed that BBR alleviated nonalcoholic fatty liver by activating SIRT3.Citation9 In foam cells, BBR promoted cholesterol efflux by increasing ROS production, and induced autophagy by inhibiting mTOR and Akt phosphorylation.Citation10 The mechanisms of the hypoglycemic effects of BBR have also been studied extensively. Studies showed that BBR improved insulin action through inhibition of mitochondrial and activation of AMPK.Citation11,Citation12 In liver and muscle cells, BBR restored insulin sensitivity by up-regulating InsR expression.Citation13 In vitro experiments showed that BBR affected glucose uptake by down-regulating miR29-b and increasing Akt expression.Citation14 Recent studies have shown that BBR exerted anti-tumor effects against lung cancer, cervical cancer, liver cancer, leukemia, and other malignancies.Citation15–Citation18
BBR inhibits cancer cell proliferation through various mechanisms. Here, in this review, we discussed the effects of BBR on cell cycle, cell apoptosis, cell autophagy, ability of inhibiting cell invasion and proliferation, expression of microRNA, telomerase activity, and tumor microenvironment. Currently, BBR is widely used in basic researches and clinical trials. This review clarified the potential of BBR as an anti-cancer drug, which may speed up its clinical application and eventually benefit cancer patients.
BBR Inhibits Cell Proliferation
BBR Regulates Cell Cycle
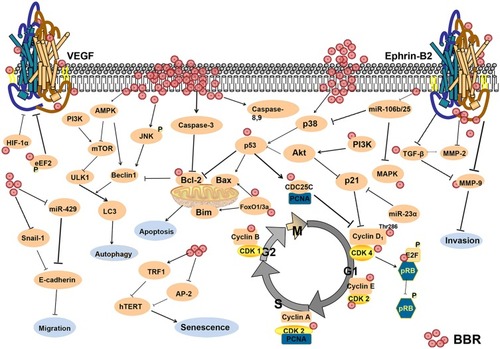
Alterations in the cell cycle promote the development of cancer.Citation19,Citation20 Studies showed that BBR regulated cell cycle and inhibited cell proliferation in multiple cancers.Citation6,Citation21,Citation22 BBR induced G1 phase cycle arrest in A549 lung cancer cells through inhibition of the expression of Cyclin D1 and Cyclin E1.Citation21 In addition, a combination of an Hsp90 inhibitor and BBR inhibited cell growth via inhibition of CDK4 expression and modulation of cyclin D1 in colorectal cancer cells.Citation22 In HepG2 human hepatoma cells, BBR suppressed cyclin D1 expression in vitro and in vivo.Citation6 Furthermore, BBR arrested the cell cycle at G1 via reduced expression of cyclin B1 and indirect inhibition of CDC2 kinase in several cancer cells.Citation23 In HBT-94 chondrosarcoma cells, BBR up-regulated the expression of p53 and p21 by modulating activation of the PI3K/Akt and p38 signaling pathways, which resulted in G2/M phase arrest.Citation24 In MDA-MB-231 breast cancer cells, BBR arrested cells in S phase, which contributed to high sensitivity of cancer cells to chemotherapy.Citation25 BBR has also been shown to influence cell cycle through regulation of Rb. Specifically, BBR acted on the 3ʹ-UTR of Rb, which resulted in inhibition of Rb mRNA degradation, stabilization of Rb translation, and inhibition of cell cycle progression.Citation26 BBR also inhibited phosphorylation of Rb protein, which prevented dissociation of the transcriptional activator E2F from Rb, and resulted in inhibition of the transition from G1 to S phase.Citation27 Together, BBR inhibits cancer cell proliferation by affecting cell cycle progression.
BBR Regulates Cell Apoptosis
Apoptosis is a gene-controlled form of cell death that plays an important role in health and disease.Citation28 BBR has been shown to promote apoptosis by activating caspases. In leukemia, BBR contributed to cell apoptosis by increasing the expression of caspase-8 and caspase-9, and inhibiting the expression of bcl-2 through activation of caspase-3.Citation29 BBR activated caspases through increased levels of cytochrome C,Citation30 activation of AMPK, and increased ROS production.Citation31,Citation32 Mitochondria are central to regulation of apoptosis.Citation33 A study showed that external stimulation increased the permeability of the mitochondrial membrane, which activated the caspase cascade, and resulted in apoptosis.Citation33 This signaling cascade was also involved in BBR-induced apoptosis in hepatoma cells.Citation34 BBR increased phosphorylation of p53 through activation of JNK/p38, which promoted the entry of the apoptotic proteins Bax and Bim into mitochondria.Citation35 In addition, studies showed that BBR promoted apoptosis through increased acetylation of foxo1/3a and increased expression of Bim and Bax.Citation36,Citation37 In colon cancer cells, BBR promoted cell apoptosis by inducing the expression of ATF3 protein through increased p53 transcription activity.Citation38 In MDM2-overexpressing tumor cells, BBR treatment led to the degradation of MDM2, which induced cell apoptosis in acute lymphoblastic leukemia.Citation39 In conclusion, BBR contributes to cell death by inducing cell apoptosis through different mechanisms.
BBR Regulates Cell Autophagy
Autophagy is a form of programmed cell death that plays an important role in maintaining cellular homeostasis.Citation40 In glioblastoma, BBR targeted the AMPK/mTOR/ULK1 pathway and resulted in activation of autophagy.Citation41 In breast cancer, BBR induced autophagic death by modulating phosphorylation of JNK and contributing to dissociation of the bcl-2/beclin-1 complex.Citation42 In hepatoma cells, BBR induced autophagy by promoting the release of beclin-1 from the bcl-2/beclin-1 complex.Citation43 In addition, BBR has been shown to induce autophagy by increasing the binding capacity of GRP78 and VPS34 in cancer cells.Citation44 BBR also indirectly inhibited the expression of SREBP-1 through activation of AMPK, which activated the ULK1/mTOR1 signaling pathway to promote autophagy.Citation45 Studies have shown that autophagy-mediated drug resistance plays an important role in cancer development and that tumor cells evade apoptosis through regulation of autophagy.Citation46 BBR may be able to reverse drug resistance by regulating autophagy through activation of AMPK. A previous study showed that BBR promoted binding of the miR30 family with the beclin1 3ʹ-UTR region, which resulted in the inhibition of autophagy in adipocytes.Citation47 From the above findings, we found that the effects of BBR on autophagy are complicated, BBR either inhibited autophagy or induced autophagy to exert its anti-tumor effects. Regulation of BBR on autophagy has been widely studied; however, additional studies are needed to further characterize the mechanisms by which BBR may regulate autophagy.
BBR Inhibits Cell Invasion and Metastasis
Patients with cancer die due to destruction of tissues and organs resulting from uncontrolled cell proliferation, and tumor cell invasion and metastasis. In breast cancer cells, BBR inhibited cell proliferation and metastasis by targeting ephrin-B2 and inhibiting the expression of MMP-2 and MMP-9.Citation48 In addition, BBR inhibited the expression of MMP-2 and MMP-9 via downregulation of TGF-β1.Citation49 In melanoma, BBR inhibited the EMT through downregulation of RARα and upregulation of RARβ, which resulted in inhibition of the PI3K/Akt signaling pathway.Citation50 In triple-negative breast cancer cells, BBR inhibited cell proliferation by down-regulating IL8 expression through inhibiting the EGFR/MEK/ERK signaling pathway.Citation51 In addition, BBR has been shown to inhibit the COX-2/PGE2-JAK2/STAT3 signaling pathway, which resulted in reduced expression of MMP2 and MMP9.Citation52 Angiogenesis plays a key role in cancer progression and metastasis.Citation53 In vitro and in vivo studies showed that BBR inhibited the expression of VEGF mRNA in tumor cells,Citation54 induced phosphorylation of eEF2,Citation55 down-regulated the activity of HIF-1α, which resulted in reduced expression of VEGF,Citation56 and inhibited the PI3K/AKT signaling pathway.Citation57 In SW480 colorectal cancer cells, BBR inhibited proliferation and migration by down-regulating expression of GRP78.Citation58 In addition, BBR inhibited tumor metastasis by reducing the expression of the transcription factor snail-1.Citation16 In all, BBR inhibits cell invasion and metastasis by affecting the expression of tumor-related signaling pathways and proteins.
BBR Regulates Tumor Microenvironment
Tumor cells secrete cytokines to alter the surrounding tumor microenvironment, which promotes tumor cell proliferation and metastasis.Citation59–Citation61 In osteosarcoma, BBR altered the inflammatory microenvironment by down-regulating the caspase-1/IL-1β signaling pathway, which resulted in cell apoptosis.Citation62 In autoimmune diseases, BBR suppressed the Th17 response through direct interaction with T cells and DC cells.Citation63 BBR has been shown to improve osteoarthritis through inhibition of IL-1β signaling, and inhibition of cartilage damage.Citation64 In all, BBR regulates tumor microenvironment by affecting inflammatory response and immune molecules.
BBR’s Antiinflammatory Properties
In inflammatory macrophages, BBR induced Nrf2 activation in an AMPK-dependent manner and inhibited inflammation.Citation65 BBR reduced oxidized low-density lipoprotein (ox-LDL)-induced inflammation by regulating the AMPK/mTOR signaling pathway.Citation66 In hepatic fibrosis, BBR inhibited the inflammation induced by thioacetamide treatment.Citation67 In skeletal progenitor cells, BBR exerted its anti-inflammatory activities by activating the AMPKα-SIRT-1-PGC-1α signaling pathway and inhibiting the mitogen-activated protein kinase 4 (MKK4)-SAPK/JNK-C-JUN.Citation68 A study showed that BBR treatment down-regulated TNFα, IL-1β and IL-6, which suppressed the seizure-like behavior in Zebrafish.Citation69 In adjuvant arthritis in mice, BBR significantly alleviated joint destruction and inflammatory cell infiltration by regulating the AMPK/NF-кB pathway.Citation70 Together, BBR inhibits inflammation by regulating different signaling pathways and cytokines.
BBR’s Antioxidant Activities
In thioacetamide-induced liver fibrosis, BBR suppressed hepatic fibrosis by elevating hepatic antioxidant enzymes.Citation67 In spiral ganglion cells, BBR exerted antioxidant effects via mediating the generation of reactive oxygen species.Citation71 In experimental varicocele, BBR as an antioxidant agent, promoted testicular antioxidant potential, promoted spermatogenesis, and upregulated the sperm quality.Citation72 A study showed that BBR protected PC-12 cells from oxidative injury by inhibiting ROS via PI3K/AKT/mTOR signaling pathways.Citation73 In the pentylenetetrazole-induced kindling model of epilepsy in rats, BBR exerted antioxidant properties and anti-epileptogenic effects by up-regulating superoxide dismutase levels.Citation74 In the intestinal tissue of mice, BBR treatment enhanced the antioxidant status by increasing the activities of catalase and glutathione peroxidase enzymes.Citation75 These findings demonstrated that BBR exerts antioxidant properties through various mechanisms, and BBR might be the potential antioxidant agent.
BBR Acts as an Effective Candidate for Tumor Immunotherapy
Immunotherapy for the treatment of tumors has received increased attention. BBR has been shown to exert positive effects on tumor immunotherapy. A study showed that BBR acted as a dopamine D1- and D2-like receptor antagonist to inhibit secretion of IFN-γ, TNF-α, IL-6, and IL-1β from LPS-stimulated lymphocytes.Citation76 BBR also improved autoimmune neuropathy by down-regulating TNF-α and IL-1 levels, and by inhibiting proliferation of CD4+ T cells.Citation77 Moreover, BBR inhibited the phosphorylation of STAT1, which resulted in inhibition of IFN-γ-induced IDO1 expression.Citation78 These results indicated that BBR is a potential therapeutic candidate for tumor immunotherapy.
BBR’s Other Functions
Effects of BBR on microRNA
MicroRNA is a class of single-stranded RNA involved in post-transcriptional regulation.Citation79 More than 2500 microRNAs have been identified, and over-expression of many microRNAs has been shown to be closely related to onset and development of tumors.Citation80–Citation82 In endometrial cancer cells and multiple myeloma cells, BBR inhibited cancer invasion and metastasis by interacting with microRNAs.Citation83–Citation85 In hepatoma cells, BBR mediated the transcriptional activation of p21 and GADD45α by up-regulating the expression of miR-23a.Citation86 In colon cancer cells, BBR down-regulated miR-429 and inhibited E-cadherin expression.Citation87 Together, BBR inhibits cancer invasion and metastasis by regulating the expression of microRNAs or interacting with them. Although studies have shown that BBR inhibited cancer development by interacting with microRNAs, the interaction sites and mechanisms associated with these inhibitory effects need to be further explored.
BBR Regulates Telomerase Activity
Telomerase activity is inhibited in normal cells, and abnormal activation of telomerase results in immortalization in tumor cells.Citation88 BBR inhibited binding of AP-2 to the hTERT promoter, which resulted in reduced expression of hTERT and reversal of tumor cell immortalization.Citation89 Another study showed that BBR stabilized the structure of the endogenous telomere G-quadruplex,Citation90 which resulted in inhibition of binding of hTR to this complex and inhibition of telomerase activity.Citation91 Together, BBR affects cell proliferation by inhibiting telomerase activity.
Discussion and Conclusion
The traditional Chinese medicine BBR has been shown to affect cell cycle, cell apoptosis, cell autophagy, and the tumor microenvironment. BBR has also been shown to exert anti-inflammatory and antioxidant effects. Tumor immunotherapy is a hotspot for tumor therapy in recent years, immune-suppressants such as PD-1/PD-L1 suppressants have emerged one after another. However, it is difficult to be widely used in clinic due to their high prices. BBR as an effective immunomodulator and a kind of cheap Chinese traditional drug, is expected to be widely used in clinical practice as an ideal drug for immunotherapy.
As studies showed, BBR exerted its role on autophagy through different mechanisms. In several cancer cells, BBR inhibited cell proliferation by inducing autophagy and also reversed drug resistance by regulating cell autophagy.Citation45,Citation46 However, in mature adipocytes, BBR maintained the cellular homeostasis by inhibiting autophagy.Citation47 Studies showed that autophagy plays an important role in maintaining a stable intracellular environment.Citation92,Citation93 We inferred that autophagy plays different roles in cells. On the one hand, tumor cells evaded apoptosis through decreasing autophagy level; therefore, BBR treatment up-regulated autophagy and led to cancer cell death. On the other hand, BBR treatment lowered the original high level of autophagy in mature adipocytes to contribute to maintenance of a stable intracellular environment. Regulation of BBR on autophagy is complicated; therefore, studies are needed to make further progress on regulation of BBR on autophagy.
Although BBR exerts beneficial effects that may aid in the treatment of tumors, the efficacy of BBR is limited by poor solubility in water, rapid metabolism, and low absorption rate in intestines. Therefore, development of formulations that improve absorption of BBR in the intestines may have great potential for treatment of cancer. Xiao et al successfully designed nanoparticles that significantly increased the bioavailability of BBR, which demonstrated that nanotechnology may be a promising strategy for improving the pharmacokinetics of BBR.Citation94 Clinical trials mainly focused on the function of BBR on lowering lipid levels and regulating blood sugar. However, nowadays, anti-tumor effects of BBR are being investigated in a number of clinical trials.
In this review, we discussed the anti-tumor mechanisms of BBR (), and its potential as an anti-cancer drug. BBR has been shown to inhibit cell proliferation and angiogenesis through modulating cell cycle, cell apoptosis, cell autophagy, and the tumor microenvironment. This review emphasized that BBR as a potential anti-inflammation and antioxidant agent, also as an effective immunomodulator, is expected to achieve clinical application for cancer therapy.
Acknowledgments
This review is supported by The Provincial Natural Science Foundation of Hubei (No. 2015BCA270) and Joint Fund of Wuhan Union Hospital (No.02.03.2019-95).
Disclosure
The authors report no conflicts of interest in this work.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.2010721296855
- Habtemariam S. Berberine and inflammatory bowel disease: a concise review. Pharmacol Res. 2016;113(Pt A):592–599. doi:10.1016/j.phrs.2016.09.04127697643
- Gu L, Li N, Gong J, et al. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. 2011;203(11):1602–1612. doi:10.1093/infdis/jir14721592990
- Shan YQ, Ren G, Wang YX, et al. Berberine analogue IMB-Y53 improves glucose-lowering efficacy by averting cellular efflux especially P-glycoprotein efflux. Metabolism. 2013;62(3):446–456. doi:10.1016/j.metabol.2012.09.00923079743
- Zhang R, Qiao H, Chen S, et al. Berberine reverses lapatinib resistance of HER2-positive breast cancer cells by increasing the level of ROS. Cancer Biol Ther. 2016;17(9):925–934. doi:10.1080/15384047.2016.121072827416292
- Wang N, Wang X, Tan HY, et al. Berberine suppresses cyclin D1 expression through proteasomal degradation in human hepatoma cells. Int J Mol Sci. 2016;17:11. doi:10.3390/ijms17111899
- Yao J, Kong W, Jiang J. Learning from berberine: treating chronic diseases through multiple targets. Sci China Life Sci. 2015;58(9):854–859. doi:doi:10.1007/s11427-013-4568-z.24174332
- Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–1351. doi:10.1038/nm113515531889
- Xu X, Zhu XP, Bai JY, et al. Berberine alleviates nonalcoholic fatty liver induced by a high-fat diet in mice by activating SIRT3. FASEB j. 2019;33(6):7289–7300. doi:10.1096/fj.201802316R30848932
- Kou JY, Li Y, Zhong ZY, et al. Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis. 2017;8(1):e2558. doi:10.1038/cddis.2016.35428102849
- Turner N, Li JY, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57(5):1414–1418. doi:10.2337/db07-155218285556
- Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi:10.2337/db06-000616873688
- Chen C, Zhang Y, Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397(3):543–547. doi:10.1016/j.bbrc.2010.05.15320515652
- Zhao HL, Sui Y, Qiao CF, et al. Sustained antidiabetic effects of a berberine-containing Chinese herbal medicine through regulation of hepatic gene expression. Diabetes. 2012;61(4):933–943. doi:10.2337/db11-116422396199
- Qi HW, Xin LY, Xu X, et al. Epithelial-to-mesenchymal transition markers to predict response of berberine in suppressing lung cancer invasion and metastasis. J Transl Med. 2014;12:22. doi:10.1186/1479-5876-12-2224456611
- Chu SC, Yu CC, Hsu LS, et al. Berberine reverses epithelial-to-mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol Pharmacol. 2014;86(6):609–623. doi:10.1124/mol.114.09403725217495
- Jie S, Li H, Tian Y, et al. Berberine inhibits angiogenic potential of Hep G2 cell line through VEGF down-regulation in vitro. J Gastroenterol Hepatol. 2011;26(1):179–185. doi:10.1111/j.1440-1746.2010.06389.x21175812
- Li H, Guo L, Jie S, et al. Berberine inhibits SDF-1-induced AML cells and leukemic stem cells migration via regulation of SDF-1 level in bone marrow stromal cells. Biomed Pharmacother. 2008;62(9):573–578. doi:10.1016/j.biopha.2008.08.00318805669
- Matera R, Saif MW. New therapeutic directions for advanced pancreatic cancer: cell cycle inhibitors, stromal modifiers and conjugated therapies. Expert Opin Emerg Drugs. 2017;22(3):223–233. doi:10.1080/14728214.2017.136238828783977
- Jahagirdar D, Gore CR, Patel H, et al. Induction of apoptotic death and cell cycle arrest in HeLa cells by extracellular factors of breast cancer cells. Asian Pac J Cancer Prev. 2018;19(12):3307–3316. doi:10.31557/apjcp.2018.19.12.330730583335
- Xiao Y, Tian C, Huang T, et al. 8-cetylberberine inhibits growth of lung cancer in vitro and in vivo. Life Sci. 2018;192:259–269. doi:10.1016/j.lfs.2017.11.01229138118
- Su YH, Tang WC, Cheng YW, et al. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with berberine for treatment of colorectal cancer. Biochim Biophys Acta. 2015;1853(10Pt A):2261–2272. doi:10.1016/j.bbamcr.2015.05.01225982393
- Li X-K, Motwani M, Tong W, et al. Huanglian, A chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharmacol. 2000;58(6):1287–1293. doi:10.1124/mol.58.6.128711093765
- Eo SH, Kim JH, Kim SJ. Induction of G(2)/M arrest by berberine via activation of PI3K/Akt and p38 in human chondrosarcoma cell line. Oncol Res. 2014;22(3):147–157. doi:10.3727/096504015x1429812291558326168133
- Gao X, Wang J, Li M, et al. Berberine attenuates XRCC1-mediated base excision repair and sensitizes breast cancer cells to the chemotherapeutic drugs. J Cell Mol Med. 2019;23(10):6797–6804. doi:10.1111/jcmm.1456031338966
- Chai YS, Yuan ZY, Lei F, et al. Inhibition of retinoblastoma mRNA degradation through poly (A) involved in the neuroprotective effect of berberine against cerebral ischemia. PLoS One. 2014;9(6):e90850. doi:10.1371/journal.pone.009085024603897
- Wu HL, Chuang TY, Al-Hendy A, et al. Berberine inhibits the proliferation of human uterine leiomyoma cells. Fertil Steril. 2015;103(4):1098–1106. doi:10.1016/j.fertnstert.2015.01.01025682924
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/0192623070132033717562483
- Okubo S, Uto T, Goto A, et al. Berberine induces apoptotic cell death via activation of caspase-3 and −8 in HL-60 human leukemia cells: nuclear localization and structure-activity relationships. Am J Chin Med (Gard City N Y). 2017;45(7):1497–1511. doi:10.1142/s0192415x17500811
- Li DX, Zhang J, Zhang Y, et al. Inhibitory effect of berberine on human skin squamous cell carcinoma A431 cells. Genet Mol Res. 2015;14(3):10553–10568. doi:10.4238/2015.September.8.1726400287
- Yang X, Huang N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol Med Rep. 2013;8(2):505–510. doi:10.3892/mmr.2013.150623732865
- Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol. 2008;229(1):33–43. doi:10.1016/j.taap.2007.12.02718275980
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41(1):11–22. doi:10.5483/bmbrep.2008.41.1.01118304445
- Hwang JM, Kuo HC, Tseng TH, et al. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80(2):62–73. doi:10.1007/s00204-005-0014-816189662
- Park GB, Park SH, Kim D, et al. Berberine induces mitochondrial apoptosis of EBV-transformed B cells through p53-mediated regulation of XAF1 and GADD45alpha. Int J Oncol. 2016;49(1):411–421. doi:10.3892/ijo.2016.350227121748
- Shukla S, Rizvi F, Raisuddin S, et al. FoxO proteins’ nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic Biol Med. 2014;76:185–199. doi:10.1016/j.freeradbiomed.2014.07.03925128467
- Shukla S, Sharma A, Pandey VK, et al. Concurrent acetylation of FoxO1/3a and p53 due to sirtuins inhibition elicit Bim/PUMA mediated mitochondrial dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol Appl Pharmacol. 2016;291:70–83. doi:10.1016/j.taap.2015.12.00626712469
- Piyanuch R, Sukhthankar M, Wandee G, et al. Berberine, a natural isoquinoline alkaloid, induces NAG-1 and ATF3 expression in human colorectal cancer cells. Cancer Lett. 2007;258(2):230–240. doi:10.1016/j.canlet.2007.09.00717964072
- Zhang X, Gu L, Li J, et al. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010;70(23):9895–9904. doi:10.1158/0008-5472.can-10-154620935220
- Maes H, Rubio N, Garg AD, et al. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19(7):428–446. doi:10.1016/j.molmed.2013.04.00523714574
- Wang J, Qi Q, Feng Z, et al. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget. 2016;7(41):66944–66958. doi:10.18632/oncotarget.1139627557493
- Wang K, Zhang C, Bao J, et al. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci Rep. 2016;6:26064. doi:10.1038/srep2606427263652
- Wang N, Feng Y, Zhu M, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010;111(6):1426–1436. doi:10.1002/jcb.2286920830746
- La X, Zhang L, Li Z, et al. Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells. Oncotarget. 2017;8(13):20909–20924. doi:10.18632/oncotarget.1495928157699
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi:10.1038/ncb232921892142
- Piya S, Andreeff M, Borthakur G. Targeting autophagy to overcome chemoresistance in acute myleogenous leukemia. Autophagy. 2017;13(1):214–215. doi:10.1080/15548627.2016.124526327797294
- Deng Y, Xu J, Zhang X, et al. Berberine attenuates autophagy in adipocytes by targeting BECN1. Autophagy. 2014;10(10):1776–1786. doi:10.4161/auto.2974625126729
- Ma W, Zhu M, Zhang D, et al. Berberine inhibits the proliferation and migration of breast cancer ZR-75-30 cells by targeting Ephrin-B2. Phytomed. 2017;25:45–51. doi:10.1016/j.phymed.2016.12.013
- Kim S, Lee J, You D, et al. Berberine suppresses cell motility through downregulation of TGF-beta1 in triple negative breast cancer cells. Cell Physiol Biochem. 2018;45(2):795–807. doi:10.1159/00048717129414799
- Naveen CR, Gaikwad S, Agrawal-Rajput R. Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomed. 2016;23(7):736–744. doi:10.1016/j.phymed.2016.03.013
- Kim S, You D, Jeong Y, et al. Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomed. 2018;50:43–49. doi:10.1016/j.phymed.2018.08.004
- Liu X, Ji Q, Ye N, et al. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10(5):e0123478. doi:10.1371/journal.pone.012347825954974
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204. doi:10.1007/s10456-017-9552-y28361267
- Tsang CM, Cheung KC, Cheung YC, et al. Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim Biophys Acta. 2015;1852(3):541–551. doi:10.1016/j.bbadis.2014.12.00425496992
- Tan HY, Wang N, Tsao SW, et al. Suppression of vascular endothelial growth factor via inactivation of eukaryotic elongation factor 2 by alkaloids in Coptidis rhizome in hepatocellular carcinoma. Integr Cancer Ther. 2014;13(5):425–434. doi:10.1177/153473541351363524363282
- Hamsa TP, Kuttan G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem Toxicol. 2012;35(1):57–70. doi:10.3109/01480545.2011.58943722145808
- Kim S, Oh SJ, Lee J, et al. Berberine suppresses TPA-induced fibronectin expression through the inhibition of VEGF secretion in breast cancer cells. Cell Physiol Biochem. 2013;32(5):1541–1550. doi:10.1159/00035659124335179
- Gong C, Hu X, Xu Y, et al. Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anticancer Drugs. 2020;31(2):141–149. doi:10.1097/cad.000000000000083531743135
- Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518(7537):107–110. doi:10.1038/nature1390525409146
- Delloye-Bourgeois C, Bertin L, Thoinet K, et al. Microenvironment-driven shift of cohesion/detachment balance within tumors induces a switch toward metastasis in neuroblastoma. Cancer Cell. 2017;32(4):427–43.e8. doi:10.1016/j.ccell.2017.09.00629017055
- De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nature Rev Cancer. 2017;17(8):457–474. doi:10.1038/nrc.2017.5128706266
- Jin H, Jin X, Cao B, et al. Berberine affects osteosarcoma via downregulating the caspase-1/IL-1beta signaling axis. Oncol Rep. 2017;37(2):729–736. doi:10.3892/or.2016.532728000894
- Yang Y, Qi J, Wang Q, et al. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci. 2013;54(4):2516–2522. doi:10.1167/iovs.12-1121723482469
- Liu SC, Lee HP, Hung CY, et al. Berberine attenuates CCN2-induced IL-1beta expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol Appl Pharmacol. 2015;289(1):20–29. doi:10.1016/j.taap.2015.08.02026344001
- Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20(4):574–588. doi:10.1089/ars.2012.511623875776
- Fan X, Wang J, Hou J, et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13:92. doi:10.1186/s12967-015-0450-z25884210
- Eissa LA, Kenawy HI, El-Karef A, et al. Antioxidant and anti-inflammatory activities of berberine attenuate hepatic fibrosis induced by thioacetamide injection in rats. Chem Biol Interact. 2018;294:91–100. doi:10.1016/j.cbi.2018.08.01630138605
- Poudel A, Zhou JY, Mekala N, et al. Berberine hydrochloride protects against cytokine-induced inflammation through multiple pathways in undifferentiated C2C12 myoblast cells. Can J Physiol Pharmacol. 2019;97(8):699–707. doi:10.1139/cjpp-2018-065331026403
- Zhang B, Wang L, Ji X, et al. Anti-inflammation associated protective mechanism of berberine and its derivatives on attenuating pentylenetetrazole-induced seizures in zebrafish. J Neuroimmune Pharmacol. 2020. doi:10.1007/s11481-019-09902-w
- Zhou J, Yu Y, Yang X, et al. Berberine attenuates arthritis in adjuvant-induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF-small ka, CyrillicB pathway. Eur J Pharmacol. 2019;852:179–188. doi:10.1016/j.ejphar.2019.02.03630796904
- Zhuang W, Li T, Wang C, et al. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic Biol Med. 2018;121:127–135. doi:10.1016/j.freeradbiomed.2018.04.57529715550
- Hassani-Bafrani H, Najaran H, Razi M, et al. Berberine ameliorates experimental varicocele-induced damages at testis and sperm levels; evidences for oxidative stress and inflammation. Andrologia. 2019;51(2):e13179. doi:10.1111/and.1317930334274
- Li Z, Jiang T, Lu Q, et al. Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cell Mol Neurobiol. 2019. doi:10.1007/s10571-019-00756-7
- Guna V, Saha L, Bhatia A, et al. Anti-oxidant and anti-apoptotic effects of berberine in pentylenetetrazole-induced kindling model in rat. J Epilepsy Res. 2018;8(2):66–73. doi:10.14581/jer.1801130809499
- Dkhil MA, Metwaly MS, Al-Quraishy S. Berberine improves the intestinal antioxidant status of laboratory mice, Mus musculus. Saudi j Biol Sci. 2017;24(7):1567–1573. doi:10.1016/j.sjbs.2015.10.01230294226
- Kawano M, Takagi R, Kaneko A, et al. Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J Neuroimmunol. 2015;289:43–55. doi:10.1016/j.jneuroim.2015.10.00126616870
- Li H, Li XL, Zhang M, et al. Berberine ameliorates experimental autoimmune neuritis by suppressing both cellular and humoral immunity. Scand J Immunol. 2014;79(1):12–19. doi:10.1111/sji.1212324354407
- Wang YX, Pang WQ, Zeng QX, et al. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur J Med Chem. 2018;143:1858–1868. doi:10.1016/j.ejmech.2017.10.07829133053
- Bhaskaran M, Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol. 2014;51(4):759–774. doi:10.1177/030098581350282024045890
- Rachagani S, Macha MA, Heimann N, et al. Clinical implications of miRNAs in the pathogenesis, diagnosis and therapy of pancreatic cancer. Adv Drug Deliv Rev. 2015;81:16–33. doi:10.1016/j.addr.2014.10.02025453266
- Yang N, Ekanem NR, Sakyi CA, et al. Hepatocellular carcinoma and microRNA: new perspectives on therapeutics and diagnostics. Adv Drug Deliv Rev. 2015;81:62–74. doi:10.1016/j.addr.2014.10.02925450260
- Banwait JK, Bastola DR. Contribution of bioinformatics prediction in microRNA-based cancer therapeutics. Adv Drug Deliv Rev. 2015;81:94–103. doi:10.1016/j.addr.2014.10.03025450261
- Ayati SH, Fazeli B, Momtazi-Borojeni AA, et al. Regulatory effects of berberine on microRNome in Cancer and other conditions. Crit Rev Oncol Hematol. 2017;116:147–158. doi:10.1016/j.critrevonc.2017.05.00828693796
- Wang Y, Zhang S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed Pharmacother. 2018;103:1287–1293. doi:10.1016/j.biopha.2018.04.16129864910
- Yin Z, Yang J, Ning R, et al. Signal pathways, diseases, and functions associated with the miR-19a/92a cluster and the use of berberine to modulate the expression of this cluster in multiple myeloma cells. J Biochem Mol Toxicol. 2018;32(6):e22057. doi:10.1002/jbt.2205729687521
- Wang N, Zhu M, Wang X, et al. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of MIR-23a in hepatocellular carcinoma. Biochim Biophys Acta. 2014;1839(9):849–857. doi:10.1016/j.bbagrm.2014.05.02724942805
- Liu H, Huang C, Wu L, et al. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016;9:4121–4127. doi:10.2147/ott.s10472927462166
- Maciejowski J, de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nature Rev Mol Cell Biol. 2017;18(3):175–186. doi:10.1038/nrm.2016.17128096526
- Fu L, Chen W, Guo W, et al. Berberine targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One. 2013;8(7):e69240. doi:10.1371/journal.pone.006924023869238
- Xiong YX, Su HF, Lv P, et al. A newly identified berberine derivative induces cancer cell senescence by stabilizing endogenous G-quadruplexes and sparking a DNA damage response at the telomere region. Oncotarget. 2015;6(34):35625–35635. doi:10.18632/oncotarget.552126462146
- Ivancich M, Schrank Z, Wojdyla L, et al. Treating cancer by targeting telomeres and telomerase. Antioxidants (Basel, Switzerland). 2017;6:1. doi:10.3390/antiox6010015
- Onorati AV, Dyczynski M, Ojha R, et al. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–3318. doi:10.1002/cncr.3133529671878
- Katheder NS, Khezri R, O’Farrell F, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541(7637):417–420. doi:10.1038/nature2081528077876
- Yu F, Ao M, Zheng X, et al. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017;24(1):825–833. doi:10.1080/10717544.2017.132106228509588