Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with multiple comorbidities, which impact negatively on patients and are often underdiagnosed, thus lacking a proper management due to the absence of clear guidelines.
Purpose
To elaborate expert recommendations aimed to help healthcare professionals to provide the right care for treating COPD patients with comorbidities.
Methods
A modified RAND-UCLA appropriateness method consisting of nominal groups to draw up consensus recommendations (6 Spanish experts) and 2-Delphi rounds to validate them (23 Spanish experts) was performed.
Results
A panel of Spanish internal medicine experts reached consensus on 73 recommendations and 81 conclusions on the clinical consequences of the presence of comorbidities. In general, the experts reached consensus on the issues raised with regard to cardiovascular comorbidity and metabolic disorders. Consensus was reached on the use of selective serotonin reuptake inhibitors in cases of depression and the usefulness of referring patients with anxiety to respiratory rehabilitation programmes. The results also showed consensus on the usefulness of investigating the quality of sleep, the treatment of pain with opioids and the evaluation of osteoporosis by lateral chest radiography.
Conclusion
This study provides conclusions and recommendations that are intended to improve the management of the complexity of patients with COPD and important comorbidities, usually excluded from clinical trials.
Introduction
Chronic obstructive pulmonary disease (COPD) is a serious health problem that constitutes the fourth cause of mortality worldwide.Citation1 In Spain, its prevalence among the population between 40 and 80 years of age is 10.2%.Citation2 COPD is related with multiple associated conditions and comorbidities which often share characteristics and risk factors.Citation3 The comorbidities most commonly associated with COPD include cardiovascular disease, endocrine and metabolic disorders, neuropsychiatric diseases, anemia, neoplasms (especially lung cancer), and gastrointestinal diseases,Citation4,Citation5 conditions often linked with smoking, systemic inflammation, airflow limitation, and aging.Citation3,Citation4,Citation6
The presence of comorbidities impacts negatively on COPD patients, reducing quality of life and increasing the probability of hospital admission and mortality.Citation3,Citation4,Citation6 Indeed, the presence of more than 1 comorbidity more than doubles the risk of mortality, and many patients with COPD die because of their comorbidities rather than their COPD, particularly in the mild and moderate phases.Citation4
The proper management of multimorbidity in patients with COPD involves the early detection and treatment of comorbidities. In this regard, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend evaluating separately the presence of comorbiditiesCitation5 when assessing these patients.
Often, however, comorbidities in COPD are underdiagnosed, because of a lack of a standardized protocol for their determination and difficulties in differentiating comorbidities from severe COPD.Citation4,Citation7 Moreover, the influence of the different treatments indicated for comorbidities in COPD and vice versa remains unclear. The outcome is failure to treat patients according to their needs.Citation3,Citation7
In the absence of clear guidelines on the treatment of patients with COPD and comorbidities, these expert recommendations aim to help healthcare professionals treating these patients to provide the right care.
Methods
This project has been conducted using a qualitative synthesis of the scientific evidence and a modification of the RAND/UCLA appropriateness method,Citation8 which combines Delphi consensus and nominal group techniques, collecting the agreement of a group of experts taking into account their clinical experience, the scientific evidence, and the collective judgment of the group.
Process Phases
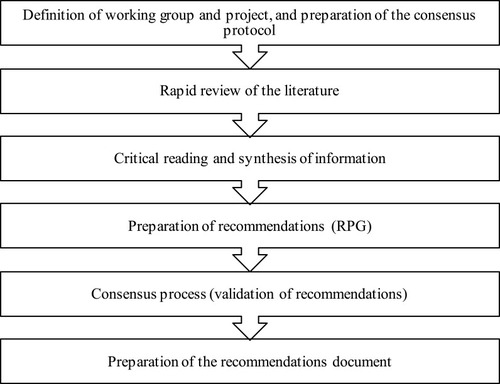
Definition of working group and project and preparation of the consensus protocol. Preparation of the document began with the constitution of the team of 2 experts that made up the group coordinator (GC), and the recommendations preparation group (RPG), composed of 4 Spanish experts in internal medicine. All members of the working group (group coordinator and recommendations preparation group) belong to the COPD working group of the Spanish internal medicine society. The GC met in person and then held a kick-off meeting with the RPG to define the objectives and fundamental criteria of the consensus document (justification, objectives, scope, target population, topics, clinical questions to be answered, formulated according the PICO (Problem/Patient/Population, Intervention/Indicator, Comparison, Outcome) structure whenever possible, participants, and methodology) and the working protocol.
The recommendations validation group (RVG) was formed at a later date.
Rapid review of the literature. A non-exhaustive systematic review of the literature was conducted to identify the publications that could answer the consensus questions, using a search strategy that included studies published in the Medline database (PubMed) in the previous 5 years. The search was completed with publications relevant to the topic that the experts felt were of interest. Items for review were then selected and prioritized using two strategies, the first of which took into account title and abstract first, and then the complete document, type, and year of publication; the second strategy took into account title and abstract.
Critical reading and synthesis of information. The complete text of the selected articles was read critically, and the information was synthesized using templates which included a brief description of the study, design, patients, interventions used, main results, and conclusions. The studies were classified according to the quality of the work, using an adaptation of the Scottish Intercollegiate Guidelines Network developers’ handbookCitation9 and sent to the RPG for reading, analysis, and preparation of recommendations.
Formulation of recommendations. After reading and analyzing the information, the RPG formulated the recommendations and conclusions that corresponded to each of the clinical questions, including the level of evidence and grade of recommendation according to the Oxford Centre for Evidence Based Medicine system.Citation10
Consensus process. The process of debate and drawing up consensus recommendations consisted of an initial in-person consensus session, using nominal group methodology, in which participants defended or modified the recommendations as appropriate, and then voted on their inclusion in or exclusion from the document. The recommendations document subsequently underwent a process of external validation using a 2-round Delphi technique, in which 23 Spanish specialists participated in the first round, and 21 in the second. The panel of experts consisted of specialists in internal medicine from various hospitals and geographical areas of Spain belonging to the COPD working group of the Spanish society of internal medicine, selected according to the different hospital sizes. (see acknowledgements). Their experience in the clinical management of COPD in their daily practice was taken into account for inclusion in the panel. In each round, the members of the RVG expressed their degree of agreement or disagreement with the recommendations in an online questionnaire on a Likert scale of 1 to 5 (1 being “strongly disagree” and 5 “strongly agree”) and provided reasons for their response. Grades 1 and 2 on this scale were considered disagreement, grade 3 was neither agreement nor disagreement, and grades 4 and 5 were taken as agreement. According to this grouping, the recommendation was considered to be unanimously accepted when the entire panel of experts were in 100% agreement; consensus was accepted when at least 80% of the panelists agreed with the recommendation; divergence was defined as less than 80% of the panelists agreeing with the recommendation.
Preparation of the recommendations document. A document was prepared that contained the recommendations and conclusions of the RPG, together with the percentage of final agreement reached after the external validation rounds, which was validated by the GC and the RPG. Three recommendations/conclusions were deleted after inconsistencies were detected.
The different phases of the process are shown in .
Evidence Summary and Recommendations
This document contains a total of 73 recommendations and 81 conclusions (Supplementary Tables 1–5) on the management of patients with COPD and comorbidities, which were agreed among the expert members of the RVG. These conclusions and recommendations are grouped for each comorbidity by a) clinical consequences of the different comorbidities in the COPD patient and b) the appropriate treatment of patients with COPD and each of the comorbidities assessed.
COPD as a Disease with Extrapulmonary Manifestations and Comorbidities
The RVG achieved unanimity for conclusion C1 (Supplementary Table 1), based on the evidence that the systemic inflammation present in COPD, probably caused by the inflammatory process that develops in the airway, the parenchyma, and pulmonary circulation of COPD patients,Citation11 has been flagged as a possible etiopathogenic mechanismCitation12 common to other clinical manifestations, such as weight loss, muscle dysfunction, osteoporosis, and depression.Citation3
Cardiovascular Risk Factors
Obesity
The prevalence of obesity in COPD patients is between 29.1% and 43%,Citation13 depending on the region, field of study, and disease stage.Citation14 Obesity causes respiratory function changesCitation15 that lead to a reduction in patients’ quality of life and exercise toleranceCitation13,Citation16 and an increase in the use of health resources.Citation17 Patients with COPD and obesity should receive recommendations on nutrition and physical activity (Supplementary Table 2A).
Regarding the treatment of patients with COPD and obesity, the use of systemic corticosteroids can contribute to weight gain,Citation18 while roflumilast can cause weight loss.Citation19 Moreover, rehabilitation programs have been shown to produce equivalent improvements in obese and non-obese patients,Citation20,Citation21 so these programs are recommended in patients with COPD and obesity. In overweight or obese patients, a higher fat-free mass index is associated with greater exercise tolerance, so weight loss treatment for reducing weight while preserving lean body mass should be consideredCitation22 (Supplementary Table 2A).
Arterial Hypertension
Arterial hypertension (AHT) is the most common comorbidity in COPD patientsCitation23 It is associated with a greater degree of dyspnea, lower tolerance for physical activity,Citation24 and airflow obstruction,Citation25 and correlates inversely with lung function.Citation26 Although AHT per se does not influence the natural history of COPD, it is a vascular risk factor for the development of cardiovascular disease.Citation5 Thus, in patients with COPD, AHT can promote the onset of heart failure (HF), ischemic heart disease (IHD), or chronic kidney disease, which worsen the prognosis of COPD.Citation4,Citation27 A comprehensive examination must therefore be performed to detect the risk factors associated with COPD and AHT in all patients with both diseases (Supplementary Table 2B).
With regard to the appropriate treatment of patients with COPD and AHT, 2 conclusions and 4 recommendations were formulated (Supplementary Table 2B). Although no definitive conclusions can be made regarding the influence of long-acting bronchodilators (formoterol, salmeterol, indacaterol, tiotropium) and anti-inflammatory drugs (roflumilast, inhaled corticosteroids)Citation28–Citation30 on the control of AHT, it can be concluded that bronchodilators (LAMA and LABA) are safe and well tolerated in hypertensive patients with COPD.Citation3
According to evidence on the treatment of AHT in patients with COPD, calcium antagonists have no significant clinical effect on COPD,Citation31 while both renin-angiotensin-aldosterone system inhibitors and beta-blockers (BBs) may provide a benefit.Citation32,Citation33 The use of cardioselective BBs is preferable if the patient has COPD and AHT, ICD, HF, or arrhythmias.Citation34,Citation35 Retrospective and observational studies have also shown that the use of BB in patients hospitalized for COPD exacerbations was well tolerated and associated with a 40% reduction in mortality,Citation36 and a lower mortality rate and risk of exacerbation, respectively.Citation37 Patients receiving diuretics and atenolol must be closely monitored, and treatment with potassium supplements or potassium-sparing diuretics in the first case,Citation38 and reduction of doses of atenolol in the second,Citation4 may be considered.
Diabetes Mellitus
The prevalence of diabetes mellitus (DM) in COPD patients is higher than in the general population, and an estimated 29.4%–37% of COPD patients hospitalized are diabetic.Citation39,Citation40 Diabetic patients also have an increased risk of COPD (as well as asthma, cystic fibrosis, and pneumonia), due to lung function decline and increased incidence of respiratory infections caused by chronic hyperglycemia.Citation41
DM has implications for COPD patients. In the first place, it has been associated with a lower quality of life in several studies,Citation42–Citation47 which has also been shown in a recent study to be the result of a decline in lung function and the consequent decrease in exercise tolerance.Citation47 DM in COPD patients reduces time to first hospitalization, prolongs the length of stay, and increases mortality due to exacerbations,Citation48 as a result of both greater numbers of exacerbations of infectious originCitation49 and poorer outcomes with non-invasive mechanical ventilation during these events.Citation50 Furthermore, in the 24 months after hospitalization of patients with COPD and DM2 for exacerbations, the risk of mortality is twice that of those who do not have DM227, and an increase in 5-year mortality has also been reported.Citation39,Citation51,Citation52 Another study found that DM in COPD increases mortality by 3-fold, regardless of lung function,Citation53 and it is associated with restrictive changes in respiratory function that can lead to increased dyspnea and a greater predisposition to exacerbations.Citation54 Based on this evidence, we recommend to rule out DM in COPD patients, and to maintain an optimal degree of glycemic control in COPD patients with DM (Supplementary Table 2C).
No specific recommendations are currently available for the treatment of DM in COPD patients,Citation4 who can be managed according to standard guidelines,Citation3–Citation5 as reflected in our recommendation (Supplementary Table 2C). According to the available evidence, the use of systemic corticosteroids for acute COPD exacerbations is associated with an increased risk of hyperglycemia,Citation55 an effect that does not occur with inhaled corticosteroids (ICS).Citation56,Citation57 Insufficient evidence is available on the effect of roflumilast on blood glucose.Citation58 As in diabetic patients without COPD, metformin is the first choice, and this drug must be suspended in the same situations as in diabetic patients without COPD. The adjustment of antidiabetic treatment and closer monitoring of blood glucose should be evaluated in diabetic patients with COPDCitation4, (Supplementary Table 2C).
Dyslipidemia
The prevalence of dyslipidemia in patients with chronic obstructive pulmonary disease (COPD) varies widely, depending on the type of study and the criteria selected to establish the dyslipidemia diagnosis. The prevalence in Spain is around 35%Citation59,Citation60 although studies such as CONSISTE reported rates of up to 48%.Citation61 According to several studies, COPD patients characteristically present a lipid pattern with similar total cholesterol and low density lipoprotein cholesterol (LDL-C), lower triglycerides (TG), and greater high-density lipoprotein cholesterol (HDL-C) values than the general population.Citation4,Citation62
Although the role of dyslipidemia in the clinical course of COPD is still unclear, it must be monitored, as it represents a risk factor for the development of cardiovascular disease in these patients.Citation3 However, the available evidence is still insufficient to establish specific conclusions (Supplementary Table 2D).
Very few studies have evaluated the effect of the different COPD treatments on dyslipidemia. No significant clinical effects have been described with inhaled anticholinergics, and studies with methylxanthines and inhaled corticosteroids (ICS) point to an increase in total cholesterol and HDL-C with high doses of ICS in healthy patientsCitation4 and an increase in HDL-C in rheumatology patients receiving prednisoneCitation63 and inhaled beta-2 adrenergic agonists, such as terbutaline.Citation64 The scientific evidence on lipid-lowering treatments in COPD is insufficient to establish solid recommendations on their use, as studies published to date vary widely in terms of statins and doses, and the follow-up period is generally limited.Citation65,Citation66 Among the effects observed, low-dose pravastatin was found to improve exercise tolerance in non-dyslipidemic patients,Citation67 and in several studies, treatment with statins reduced the number of exacerbationsCitation68 in COPD patients with pulmonary hypertensionCitation69 and concomitant cardiovascular disease,Citation70 as well as the number of hospitalizations.Citation71,Citation72 In 1 study, however, the number of exacerbations and time to first exacerbation did not decrease in patients who received simvastatin.Citation68 The reduction in respiratory mortality and all-cause mortality does not appear to be due to the lipid-lowering effect of the statin, but rather to its antiinflammatory action.Citation73 Reductions in respiratory mortality and all-cause mortality of 55% and 21%, respectively, have been reported in patients with COPD who received statins.Citation74 Another study found no association between statin use, reduction of all-cause mortality, time to first hospitalization for exacerbation, or time to first pneumonia.Citation75 A meta-analysis of 10 randomized clinical trials studying the effect of statins in COPD patients showed an improvement in exercise tolerance, lung function, and quality of life in COPD patients with a history of cardiovascular disease treated with statins.Citation76 Given this evidence, our conclusions and recommendations about dyslipidemia in COPD patients are shown in Supplementary Table 2D.
Concomitant Cardiovascular Disease
Heart Failure
The prevalence of heart failure (HF) ranges from 5% to 60% in the general COPD population,Citation77 and increases in line with COPD severity, being approximately 30% in patients hospitalized for COPD exacerbations and rising to 75% in patients undergoing mechanical ventilation.Citation78–Citation82 HF and COPD exacerbations share precipitating factors, such as respiratory infections, and the main symptoms of both diseases are similar (increased dyspnea), which can complicate diagnosis. The determination of natriuretic peptides is useful for ruling out the presence of HF in patients with COPD exacerbationsCitation83 and a positive finding is associated with a worse prognosis in both patients hospitalized for COPD exacerbations and in patients with stable phase COPD,Citation84 (Supplementary Table 3A).
Health-related quality of life is worse in patients with COPD and concomitant HF (Supplementary Table 3A), These patients present a greater risk of mortality and hospital admissionCitation85 according to a study of 1264 patients with HF and mild or moderate COPDCitation86 and another study of 7800 patients.Citation87 On the basis of 2 studies in which it was noted that readmission after hospitalization or COPD exacerbation was more frequent in patients with HF,Citation88 the latter being the most frequent reason for readmission,Citation89 and the fact that the presence of HF worsens the short and long-term prognosis of patients hospitalized for COPD exacerbation,Citation79,Citation90 it was concluded that HF worsens the prognosis of COPD patients in terms of both new exacerbations and hospitalizations and mortality (Supplementary Table 3A). It has also been observed that COPD worsens the prognosis of patients hospitalized with HF,Citation91,Citation92 and patients with both comorbidities have a higher risk of death and hospital admissions.
Long-acting bronchodilators, ICS, and LABA-LAMA fixed-dose combinations are safe in patients with HFCitation3,Citation93,Citation94 and do not increase the risk of heart disease.Citation95,Citation96 Some treatments for HF (cardioselective BBs, such as carvedilol) were also found to be safe in COPD patients,Citation33,Citation97,Citation98 and the use of BB was associated with a reduced risk of mortalityCitation99 and COPD exacerbations.Citation100,Citation101 Therefore, the presence of HF does not require a change in the treatment in patients with COPD.Citation93,Citation97,Citation102 (Supplementary Table 3A).
Ischemic Heart Disease
According to a systematic review, the prevalence of ischemic heart disease (IHD) in patients with COPD ranges between 7.1% and 31.3%, depending on the study population,Citation103 while in studies based on administrative databases, this prevalence has been estimated to be between 22% and 33.6%.Citation104–Citation106 Cohort studies conducted in patients hospitalized for COPD exacerbations have shown a prevalence of IHD of between 17% and 22%.Citation78,Citation79 The presence of both conditions in conjunction worsens patient prognosis, with COPD patients showing a significantly increased risk of having IHD,Citation107 a well-recognized cause of mortality in COPD patients.Citation97 In fact, IHD is one of the most frequent causes of mortality in COPD patients.Citation108 Several studies have found that quality of life is significantly worse in patients with COPD and concomitant IHD.Citation109–Citation111 Moreover, the presence of IHD doubles the risk of hospitalization in patients with COPD,Citation112 and is associated with an increase in 3-month mortality,Citation79 and after an exacerbation, the risk of a coronary event in the following weeks is more than doubled.Citation113,Citation114 In this regard, we recommend to rule out the possibility of IHD in all patients with COPD (Supplementary Table 3B).
Concerning the need for prescribing appropriate treatments in patients with COPD and IHD, the conclusions and recommendation are in Supplementary Table 3. In the treatment of COPD, no significant differences were found in mortality for combinations of drugs for COPD and IHD, such as salmeterol/fluticasoneCitation115 or vilanterol/fluticasone.Citation94 The prognosis after an acute coronary event in COPD patients treated with ICS was better than in those who did not receive ICS.Citation116 The combination of ICS with LABA or LABA + LAMA also gave rise to lower mortality,Citation117,Citation118 and LABA and LAMA are safe in patients with COPD and IHD.Citation29,Citation114,Citation119,Citation120 In the treatment of IHD, statins may have a beneficial effect in COPD, although this was not demonstrated in a clinical trial evaluating the usefulness of statins in reducing COPD exacerbations.Citation114 The use of statins and angiotensin converting enzyme inhibitors was significantly associated with a decline in 90-day mortality in patients hospitalized for acute exacerbations;Citation121 BBs decreased mortality in COPD patients after myocardial infarctionCitation99 and the risk of exacerbations in COPD patients with cardiovascular disease.Citation100 In another retrospective study, continuing treatment with BBs during hospitalization for COPD exacerbations was not associated with an increase in mortality or readmissions at 30 days,Citation122 while the use of antiplatelet drugs was associated with reduced mortality.Citation123
Peripheral Artery Disease
A number of cohort studies have shown a prevalence of peripheral artery disease (PAD) in COPD patients of 8.8%,Citation124 and between 13% and 16%,Citation78,Citation79 increasing according to the severity of obstruction.Citation124 Patients with COPD and PAD have less exercise tolerance and poorer quality of life,Citation124 and the presence of COPD is associated with a higher readmission rate after PAD is treated with bypass or percutaneous interventions.Citation125 In patients with stable COPD, a direct relationship was found between an ankle-brachial index of < 1 and an independent increase in 4-year mortality.Citation126 In hospitalized COPD patients, the presence of PAD was associated with an increase of mortality at 3 months.Citation79 In view of this evidence, the presence of PAD should be ruled out in COPD patients (Supplementary Table 3C).
Although some studies at the experimental level suggest that treatment with LABA + ICS or LAMA may decrease arterial rigidity (a marker of PAD),Citation127 in general the treatment of PAD is not affected by COPD treatment. Treatment with statins in patients with PAD and COPD is beneficial and BBs are safe and do not affect quality of life,Citation128 a finding confirmed in another study focusing on the year after hospitalization.Citation129 Smoking cessation is also recommended in the clinical practice guidelines for patients with PAD in the lower limbs.Citation130 (Supplementary Table 3C).
Atrial Fibrillation
The prevalence of arrhythmias in patients with stable COPD ranges from 5% to 15%, increasing to up to 20%-30% in patients with more severe COPD.Citation97 In 2 systematic reviews, it ranged from 5% to 29%,Citation105,Citation107 and atrial fibrillation (AF) occurred in 21% and arrhythmia in 27% of patients hospitalized for COPD exacerbation.Citation40,Citation78
The most common arrhythmia in the general population and in patients with COPD is AF, and its presence is inversely associated with FEV1 values. COPD patients are more likely to develop permanent AF and to relapse following cardioversion.Citation97 COPD exacerbations can cause AF, which in turn can be the cause of COPD exacerbations.Citation131 COPD patients with AF have a worse quality of life, and COPD increases the risk of hospitalization for AF by up to 2-fold,Citation105,Citation132 while the presence of AF increases mortality in patients hospitalized for COPD exacerbations,Citation79,Citation133–Citation135 thus worsening prognosis. Both dyspnea and hypercapnia and acidosis may predispose to AF.Citation136,Citation137
Several studies have found that treatments for COPD are associated with an increased risk of arrhythmias. The use of short-acting antimuscarinic agents (SAMA), starting treatment with LABA or SAMA in a population study in COPD patients, and oral steroids and theophylline have all been associated with an increased risk of AF and arrhythmias.Citation138–Citation141 In contrast, no increase in arrhythmias associated with the use of LABA or LAMA has been demonstrated in clinical trials.Citation29,Citation94 Conflicting results have also been reported with regard to an increase in the number of arrhythmias associated with inhaled SABA beta adrenergic receptor agonists,Citation140,Citation142 and high doses of prednisone have been associated with an increased risk of AF.Citation143 Treatments for AF may be used either to reverse AF or to control heart rhythm; propafenone and flecainide should be avoided in patients with COPD, unless the absence of structural heart disease or diastolic dysfunction can be demonstrated.Citation3
Neuropsychiatric Comorbidities
Anxiety
The prevalence of pervasive developmental disorders of anxiety in COPD patients ranges from 6% to 33%,Citation144 and up to 25% in patients with advanced COPD.Citation3,Citation4,Citation78 COPD patients often present symptoms of anxiety that influence COPD disease course and severity, and similarly, COPD symptoms can also contribute to cause anxiety.
The consequences of anxiety in COPD are shown in Supplementary Table 4A. The coexistence of COPD and anxiety has been associated with lower adherence to treatments and to rehabilitation programs or smoking cessation programs, and with a lower exercise tolerance and greater use of healthcare resources.Citation144–Citation150 Anxiety is associated with a worse quality of life, greater functional deterioration, and increased mortality, especially among young patients, women, and smokers, and patients with chronic cough, a higher St. George’s Respiratory Questionnaire score, or a history of cardiovascular disease.Citation151–Citation154 When patients were evaluated using the health-related quality of life questionnaires,Citation155 a correlation was found between the baseline level of anxiety and decline in quality of life at 1-year follow-up.Citation156 Anxiety in COPD patients also worsens prognosis, with the consequence of higher health spending, increased frequency of readmissions, and worse self-management of the disease,Citation157–Citation159 and is also associated with earlier hospital admission.Citation160,Citation161 Moreover, according to a recent meta-analysis, the presence of anxiety increases the likelihood of developing an exacerbation by 31%.Citation145
Anxiety also increases mortality rates,Citation145 emergency department visits, and readmission after exacerbation in the first 30 days after discharge in COPD patients,Citation145,Citation162 who have discrepancies in terms of lung function, with respect to the effect of anxiety on them. Some studies have shown a decline in FEV1,Citation151–Citation154 while others have found no correlation.Citation163 Because some of the symptoms of COPD and anxiety are similar (lack of energy, fatigue, sleep disturbances, or less enjoyment of pleasurable activities), it is very important to assess psychological health using questionnaires or scales validated in these patients.Citation153
The concomitant use of pharmacological treatments for anxiety and pulmonary rehabilitation programs may have additive therapeutic effects.Citation164 Physical exercise, as part of pulmonary rehabilitation programs, has a beneficial effect on anxiety associated with COPD.Citation165 In respect of treatments for anxiety in patients with COPD, the first line of treatment must be non-pharmacological interventions.Citation166,Citation167 In this respect, relaxation techniques showed a mildly positive effect on lung function and levels of anxiety, and a greater effect associated with an improvement in perceived quality of life.Citation168 In terms of pharmacological interventions, antidepressants alter patients’ perception of some symptoms, such as dyspnea, and improve quality of life.Citation85 Selective serotonin reuptake inhibitors have reduced anxiety symptoms in the short term (6 months’ follow-up), whereas no significant differences were found with tricyclic antidepressants or azapirones compared to placebo.Citation169–Citation173 The UK National Institute for Health and Care Excellence guidelines recommend the use of selective serotonin reuptake inhibitors as first-line treatment in patients with associated anxiety.Citation174 However, drug-drug interactions must be taken into account, as fluvoxamine reduces the metabolism of theophylline derivatives and interacts with roflumilast, so the use of alternative selective serotonin reuptake inhibitors is recommended.Citation4 The healthcare professional must be aware of and monitor potential interactions between antidepressants and beta-2 adrenergic agonists or anticholinergic bronchodilators.Citation175 Theophylline may decrease the anxiolytic effect of benzodiazepines, especially diazepam and alprazolam.Citation4 The recommendations about the therapy of anxiety in COPD patients are shown in Supplementary Table 4A.
Insomnia
Insomnia can affect up to 70% of COPD patients at some point during their disease,Citation176–Citation178 and COPD is a risk factor for insomnia.Citation179 The incidence of sleep disorders increases with the presence of respiratory symptoms in all age groups over 45 years.Citation180
The main consequences of poor sleep quality are reduced quality of life,Citation177,Citation181 increased states of anxiety or depression, the uncontrolled and possibly harmful use of hypnotics,Citation85 and hypoxemia and hypercapnia. The latter two may cause cardiac arrhythmias, pulmonary hypertension, and nocturnal death, especially during acute exacerbations.Citation182 Insomnia can predict COPD exacerbations, the use of emergency services, and all-cause mortality, which suggests that insomnia may be a significant risk factor for worsening or poor prognosis of COPD.Citation183 Asking about the quality of sleep in COPD patients is recommended (Supplementary Table 4B).
Insomnia in COPD patients must be treated (Supplementary Table 4B). In the treatment of insomnia, both a non-pharmacological approachCitation184 and drug treatment are used, principally with hypnotic drugs and benzodiazepines. Epidemiological studies have suggested that hypnotics increase the risk of respiratory adverse events, such as pneumonia, acute exacerbation, acute respiratory failure, and cardiorespiratory arrest in patients with COPD.Citation185 Benzodiazepines are currently the hypnotics of first choiceCitation184 in these patients, although they may cause respiratory depression.Citation186,Citation187 The anxiolytic effect of benzodiazepines can be reduced with theophylline,Citation4 and theophylline levels can increase with the use of St. John’s wort, requiring changes in dosing or suspension of the benzodiazepine.Citation4
Regarding the interactions between insomnia and COPD, no studies have shown that the treatment of a patient with COPD should differ according to the presence or absence of insomnia, with the exception of possible drug-drug interactions that may occur.
Depression
Depression is a common disorder in COPD patients, with a prevalence of around 25%,Citation188 which increases with COPD severity,Citation152 affecting between 50% and 90% of patients with advanced COPD. Thus, COPD increases the risk of depression.Citation145 The quality of life of patients with COPD and depression is reduced,Citation156,Citation189-191 and exacerbations, admissions and hospital readmissions, emergency room visits, and lengths of hospital stay are higher in these patients.Citation157,Citation192-194 Depression in COPD patients has also been associated with increased mortalityCitation145,Citation193 and with important clinical consequences, such as greater functional decline,Citation191 lower exercise tolerance,Citation195 dyspnea,Citation196 lower adherence to drug treatmentCitation147 and rehabilitation programs,Citation197 lower physical activity,Citation198 and higher healthcare costs in the subsequent 2 years.Citation157 As a result of this evidence, we recommend that the existence of depression in COPD patients should be ruled out at frequent intervals (Supplementary Table 4C).
The treatment of depression in patients with COPD should be similar to that of the general population,Citation3,Citation5 although patients with COPD generally do not receive treatment for depression.Citation151,Citation188 Studies that evaluated drug treatments for depression in COPD patients found that SSRIs improve depression,Citation199 whereas tricyclic antidepressants gave rise to great heterogeneity and high drop-out rates.Citation199 Treatment with paroxetine improved anxiety and depression in these patientsCitation171 and nortriptyline improved depression,Citation173 while paroxetine did not worsen respiratory symptoms.Citation170 Medicare data between 2006 and 2012 reported that patients with COPD and depression who complied with their antidepressant treatment made fewer visits to the emergency room and had fewer hospitalizations.Citation200 As for psychological treatment, 3 meta-analyses show that psychological and lifestyle interventions, multicomponent exercise training,Citation161 and behavioral therapyCitation201 are associated with a small reduction in depressive symptoms, and that relaxation techniques improved dyspnea and psychological well-being,Citation202 and also slightly improved respiratory function.Citation168
COPD treatment based on respiratory rehabilitation and physical intervention programs also improved depressive symptoms, dyspnea, and quality of life.Citation203,Citation204
There may be drug interactions between treatments for COPD and depression. For example, the combined use of beta-adrenergic agonists and antidepressants could prolong the QT interval and promote the emergence of ventricular arrhythmias.Citation175 Tricyclic antidepressants may also enhance the cardiovascular adverse effects of beta-adrenergic agonistsCitation175 and, moreover, their anticholinergic effect added to that of bronchodilators (such as ipratropium, tiotropium) can lead to adverse effects.Citation175 Despite these potential interactions, the combination of antidepressants with anticholinergic or beta-adrenergic bronchodilators is not contraindicated,Citation175 although recommendations have been made in this regard. For example, a type C interaction has been observed between beta-adrenergic bronchodilators and monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants,Citation4 so close monitoring of these patients is recommended. The interaction between roflumilast and fluvoxamine is also type C,Citation4 while a type D interaction has been reported between roflumilast, theophylline, fluvoxamine and St. John’s wort (the combination of these drugs is not recommended and treatment should be modified).Citation4
Cognitive Impairment
COPD patients often present cognitive dysfunction; in fact, they are twice as likely to develop cognitive impairment.Citation205 A systematic review estimated a prevalence of cognitive impairment of 2%-20% in COPD patients.Citation206 ExacerbationsCitation207 and chronic hypoxemia and hypercapnia in patients with COPD and obstructive sleep apnea syndromeCitation4,Citation208,Citation209 are associated with a greater cognitive impairment. Cognitive impairment is associated with longer hospital stays among patients hospitalized for acute COPD exacerbationCitation206 and an increased risk of hospital admission.Citation210 Patients with COPD and dementia have also shown an increased risk of death at 3 years,Citation210 and cognitive impairment has been associated independently with mortality at 32 months.Citation211 In another study, the presence of dementia was a predictor of 3-month mortality in patients hospitalized for COPD exacerbation.Citation79 Looking for and ruling out the existence of cognitive impairment is recommended in COPD patients (Supplementary Table 4D).
With regard to the treatment of patients with COPD and cognitive impairment, we have formulated several recommendations (Supplementary Table 4D), taking into account the following data. Chronic oxygen therapy in COPD patients with hypoxemia has been associated with a decreased risk of cognitive impairment,Citation212 and patients who followed pulmonary rehabilitation programs showed improved cognitive functionCitation213 in visual attention, verbal memory and visual-spatial functionsCitation214 and, in patients awaiting lung transplantation, an improved mental summary component of SF-36.Citation215 In the treatment of cognitive impairment, acetylcholinesterase inhibitors were not associated with more emergency department visits, more hospitalizations, or more exacerbations in patients with COPD and dementia.Citation216 Regarding the possible drug interactions, since anticholinergics counteract the therapeutic effect of central acetylcholinesterase inhibitors used in the treatment of dementia, the protocol of the Spanish Society of Internal Medicine on diagnostic and therapeutic management of comorbidities in COPDCitation4 recommend that the therapeutic effect of both groups is monitored during concomitant use.
Other Comorbidities
Pain
The prevalence of pain in COPD patients is between 45% and 60%,Citation217,Citation218 and reaches 70% in advanced stages of the disease,Citation219 although its incidence is underdiagnosed in these patients.Citation217,Citation220 Despite its importance, few clinical trials or meta-analysis have focused on pain associated with COPDCitation217,Citation218,Citation221-223 and pain management is barely mentioned in the national and international COPD guidelines. Pain is up to 2.6 times more common in COPD patients than in patients with other chronic diseases.Citation217,Citation223-225
The presence of pain does not alter lung function in COPD patients, but it is associated with increased dyspnea, worse quality of life, insomnia, fatigue, anxiety, depression, and a poor nutritional status. Pain has not been clearly associated with age, sex, or smoking status.Citation222,Citation226 No studies have evaluated pain and COPD disease course in terms of exacerbations, hospital admissions, and survival. For a proper evaluation of the intensity of pain associated with COPD and response to different analgesics, pain quantification scales, such as the Brief Pain Inventory,Citation227 the McGill Pain Questionnaire,Citation228 or the Visual Analogue Scale (VAS) are useful.Citation229 Patients with COPD and pain tend to score high in these scales. Thus, Lohne et al found that 38% of patients with severe COPD score over 6 in a numerical scale of 1 to 10,Citation220 (Supplementary Table 5A). No evidence is available on the influence of COPD treatment on the treatment of pain, although analgesics are widely consumed by patients with COPD, and used more than by patients without COPD.Citation230 Neither opioids at appropriate doses nor non-steroidal anti-inflammatory drugs confer a higher risk of respiratory depression in COPD patients,Citation231–Citation233 while NSAIDs induce less drowsiness than opioids.Citation234 The coadjuvant use of tricyclic antidepressants in the treatment of pain should be closely monitored in patients who use beta-2 agonists.Citation4 Concerning the interactions between COPD and pain, COPD patients with pain may develop worsening dyspnea, with increased use of bronchodilator medication.Citation220 Patients following pulmonary rehabilitation programs showed no improvement in pain,Citation235–Citation237 Supplementary Table 5A presents the recommendation about treatment of patients with COPD and pain.
Anemia
The prevalence of anemia in patients with COPD was estimated at between 7.5% and 34%Citation238 in a systematic review, rising to 43.9% in other studies,Citation239–Citation241 being chronic anemia and iron-deficiency anemia the most common in these patients.Citation242 Anemia is more common during exacerbations. A systematic reviewCitation243 found that the presence of anemia in patients with COPD leads to a reduction in quality of life, and a greater number of hospitalizations and readmissions. These findings have been corroborated in studies on administrative databases, in which more exacerbations, hospitalizations, pneumonia, and admissions in intensive care units were observed.Citation244,Citation245 Survival of patients with COPD and anemia during hospital admissions is lower,Citation241,Citation243,Citation245-249 with a mortality rate twice that of patients without anemia.Citation245 In addition, the presence of anemia in COPD is associated with a lower exercise tolerance,Citation243 greater dyspnea,Citation246 higher C-reactive protein values,Citation241 and greater use of healthcare resources.Citation243 It is also a risk factor for home oxygen therapy in these patients,Citation250 (Supplementary Table 5B). Anemia is a modifiable condition and correction of anemia in chronic diseases other than the COPD has been associated with improved health. No clinical trials are currently available on the treatment of anemia in patients with COPD, but it has been observed in case studies that blood transfusion helped in weaning from mechanical ventilationCitation251 and improved both the minute ventilation and work of breathing.Citation24 Treatment with intravenous iron and erythropoiesis-stimulating agents improved hemoglobin and dyspnea.Citation239 In patients with COPD, the etiological treatment of anemia is recommended (Supplementary Table 5B).
Osteoporosis
The prevalence of osteoporosis in patients with COPD ranges between 9% and 69%, depending on the study,Citation252 being higher in womenCitation253,Citation254 and 2 to 5 times higher than in the general population.Citation252 Osteoporosis is underdiagnosed and undertreated in COPD patientsCitation255 and has been associated with a lower quality of life.Citation256,Citation257 Osteoporosis appears to be a risk factor for COPD and vice versa. In several studies, osteoporosis, lower bone density, and vitamin D deficiency in COPD patients were associated with more acute exacerbations and hospitalizations.Citation258,Citation259 Vertebral fracture, lower bone density, osteoporosis, and vitamin D deficiency have also been associated with more hospitalizations.Citation258–Citation260 The number of exacerbations has been associated with an increased risk of osteoporosisCitation261 and a greater decrease in bone mass.Citation262 The degree of obstruction is also associated with an increased risk of bone fractures.Citation261 With regard to survival, vertebral fracture in patients with COPD has been associated with higher 2-year mortality.Citation260 Each vertebral fracture also reduces forced vital capacity by 9%.Citation263 A systematic review of rehabilitation programs in patients with COPD and osteoporosis found that exercise tolerance improved to a lesser extent than that of patients with other comorbidities.Citation264 The existence of osteoporosis should be ruled out in COPD patients. We recommended to check the lateral chest X-ray to find vertebral fractures (Supplementary Table 5C).
No specific treatments are available for osteoporosis in COPD patients, but corsets can be used for the treatment of unstable vertebral fractures. Systemic corticosteroids, used for the treatment of COPD exacerbations, increase the risk of osteoporosisCitation265 and their use has been associated with a higher incidence of fractures.Citation265 This is also true of ICS,Citation266 for which the association is dose-dependent.Citation267 Lung volume reduction surgeryCitation268 led to an improvement in bone density in 40 patients with emphysema,Citation268 and pulmonary rehabilitation programs have been shown to improve exercise tolerance in patients with COPD and osteoporosis.Citation264 Bisphosphonates were useful for the treatment of corticosteroid-induced osteoporosis.Citation269 Patients with COPD and osteoporosis should be referred for rehabilitation (Supplementary Table 5C).
The potential drug-drug interactions between treatments are not of particular importance, and only steroids show a type C interaction (it is recommended that patients receiving denosumab are closely monitored).Citation4
Gastroesophageal Reflux Disease
The prevalence of gastroesophageal reflux disease (GERD) in patients with COPD in Spain has been estimated at 28%-62%,Citation270–Citation273 higher than in the general population. The severity of GERD symptoms increases with the severity of COPD,Citation274,Citation275 and COPD patients diagnosed with GERD have poorer lung function.Citation276 The presence of reflux symptoms in COPD patients is related with more frequent and more severe exacerbations.Citation277–Citation279 The presence of GERD, then, is an independent risk factor for exacerbationsCitation276 and is associated with a worse quality of life,Citation280–Citation282 and may result in a COPD patient with an “infrequent exacerbator” phenotype developing a “frequent exacerbator” phenotype.Citation275 Pulmonary hyperinflation and dyspnea are risk factors associated with the incidence of symptoms of GERD.Citation283 The influence of GERD on mortality in COPD is not presently known.Citation271,Citation272,Citation276,Citation284 Supplementary Table 5D, shows the conclusions and recommendations referring to the treatment of patients with COPD and. Beta agonists and theophylline, used in the treatment of COPD, can produce reflux symptomsCitation285 while tiotropium may reduce the symptoms of GERD.Citation284,Citation286 Proton pump inhibitors are the most effective drugs in GERDCitation287,Citation288 and reduce chronic cough in these patients.Citation289 It has not been demonstrated that high-dose omeprazole improves the degree of pulmonary obstruction or bronchial hyperresponsiveness.Citation290 Oral lansoprazole has been effective in preventing exacerbations by inhibiting gastric secretion, reducing reflux, and decreasing levels of inflammatory markers in sputum.Citation291
Thromboembolic Disease
In Spain, the estimated prevalence of venous thromboembolism in hospitalized patients with COPD is between 8% and 25%,Citation292 and the incidence of deep venous thrombosis (DVT) is 10%-12%.Citation292 COPD patients are more likely to develop a venous thromboembolic event [DVT, pulmonary thromboembolism (PTE)], both during exacerbationsCitation293,Citation294 and in stable phase disease.Citation295 Clinical manifestations in patients with COPD and thromboembolic disease may be subtle and diagnosis is often delayed, while symptoms of PTE can be confused with a COPD exacerbation. With regard to the influence of thromboembolic disease on quality of life, data from studies vary widely,Citation296,Citation297 and in the course of COPD, the presence of DVT in patients with COPD exacerbation results in longer hospital stays, increased risk of PTE, a greater number of intensive care unit admissions, and a greater need for mechanical ventilation and placement of filters in the inferior vena cava.Citation293,Citation294,Citation298 (Supplementary Table 5E), Frequently PTE is the first presentation of thromboembolic disease in COPD patients, and is associated with higher mortality, more frequent hemorrhage, and more recurrence of thrombosis within 3 months.Citation299,Citation300
No evidence is available on the influence of COPD treatment on patients with thromboembolic disease. COPD patients present a greater number of major bleeds,Citation299 so other therapies such as temporary inferior vena cava filters have been used.Citation299,Citation300 In cases of spontaneous VTE, no significant differences were observed in the duration of anticoagulation therapy between patients with or without COPD.Citation301 Treatment with low molecular weight heparins during acute COPD exacerbations has proved useful in the prevention of DVT: nandroparine prophylaxis in patients with acute COPD exacerbations reduced the incidence of DVT.Citation302 The Supplementary Table 5E presents the recommendations about prophylaxis and treatment of venous thromboembolic disease in COPD patients.
Chronic Kidney Disease
The prevalence of chronic kidney disease (CKD) in patients with COPD ranges between 6.5% and 26.2%.Citation40,Citation78,Citation303,Citation304 Moderate CKD has been detected in 6.5% of the COPD population in SpainCitation78 and 22% of patients with multiple pathologies associated with COPD.Citation305 Frequently CKD is an unknown comorbidity.Citation303 The recommendation is to estimate the glomerular filtration rate (CKD-EPI or MDRD equations can be used) in COPD patients (Supplementary Table 5F). CKD is more common in COPD patients with serious lung function impairment and systemic inflammation,Citation306 and the risk increases significantly when obstructive sleep apnea syndrome occurs in COPD.Citation307 No studies have yet evaluated the impact of CKD on quality of life in COPD patients and patient progress. With regard to survival, CKD is an adverse prognostic factor related to short-term mortality in patients with COPD,Citation308 and moderate and severe COPD associated with CKD and acute CKD correlated with an increase in mortality,Citation309,Citation310 a finding corroborated in another longitudinal cohort study.Citation311
In respect of to the appropriate treatment of patients with COPD and CKD, there is no evidence that COPD treatments influence the treatment of CKD, nor that this comorbidity requires a change in COPD treatment, so it is advisable to follow clinical practice guideline recommendations,Citation312,Citation313 (Supplementary Table 5F).
Lung Cancer
The prevalence of COPD in patients with lung cancer (LC) is between 50% and 64%Citation314,Citation315 Moreover, 20%–25% of patients with COPD develop LC, primarily patients with mild or moderate airflow obstruction.Citation314,Citation315 According to other data, LC develops in around 3.8%–8.0% of COPD patients.Citation316,Citation317
Ample evidence is available to demonstrate the association between COPD and LC, the common etiologic factor of which is smoking.Citation6,Citation318 Also emphysema and airflow limitation are risk factors for LC development.Citation317 COPD is also an independent risk factor in the development of LC.Citation319 No studies have assessed the impact of LC on the quality of life of patients with COPD nor have any studies assessed the course of COPD in patients with LC. With regard to survival, various studies show that LC is one of the leading causes of mortality in COPD patients with mild-to-moderate obstruction and that the presence of COPD adversely affects the prognosis of the patient with LC.Citation6,Citation280
There is no evidence that the presence of LC requires a change of treatment in patients with COPD and vice versa. It has been suggested that, due to their anti-inflammatory properties, ICS have a possible protective effect on the development of LC, and high doses of these drugs reduce the LC risk.Citation320–Citation322 The treatment of LC requires a multidisciplinary approach with additional tests, since the decision to perform resection surgery is more complex due to worse lung function.Citation323 No drug-drug interactions have been described between treatments for COPD and LC (Supplementary Table 5G). In Supplementary Table 6 we present the recommendations in divergence after the Delphi rounds.
Conclusions
In summary, this document presents conclusions and recommendations on the consequences of various comorbidities in COPD patients and the treatment of these patients, proposed by the recommendations preparation group and validated by a group of experts after 2 Delphi rounds. Consensus was reached (agreement > 80%) for a total of 73 recommendations and 81 conclusions that are intended to be useful in the management of patients with COPD and comorbidities.
Author Contributions
All the authors of this document made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Jesús Recio Iglesias has received speaker or consulting fees from Boehringer Ingelheim, Chiesi, Ferrer and Novartis unrelated to this manuscript. Jesús Díez Manglano has received speaker or consulting fees from Bayer, Bristol-Myers-Squibb, Chiesi, and Novartis unrelated to this manuscript. Francisco López García has received speaker or consulting fees from Boehringer Ingelheim, Chiesi, GSK and Novartis unrelated to this manuscript. José Antonio Díaz Peromingo has received speaker or clinical trials fees from Boehringer, Chiesi, Bayer, Bristol, Novartis, Faes, GSK and Sanofi. Pere Almagro Mena has received grants from Astra Zeneca and SEPAR, personal fees from Chiesi, Boehringer Ingelheim and GSK and travel support from Rovi and Esteve, outside the submitted work. Jose Manuel Varela Aguilar has received speaker or consulting fees from Astra Zeneca, Boehringer Ingelheim, Chiesi, GSK, Menarini, Novartis, Novo Nordisk and Rovi. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank Heather Hamilton, specialized biomedical translator, for her translation of the manuscript into English. The authors would like to thank Boehringer Ingelheim for their continued and constant support for this project, and GOC Networking team for their support and collaboration in the development of this document and for editorial assistance. The authors would also like to thank the 23 experts who participated in the Delphi process for the validation of the conclusions and recommendations prepared by the RPG: Dr. Fernando Javier Sánchez Lora (Hospital Virgen de la Victoria, Malaga); Dr. Jesús Castiella Herrero (Hospital Fundación de Calahorra, La Rioja), Dr. Joaquín Antón Martínez (Hospital San Pedro Alcántara, Cáceres); Dr. Manuel Jesús Menduiña Guillén (Hospital Clínico San Cecilio, Granada); Dr. José Portillo Sánchez (Hospital General De Ciudad Real, Ciudad Real); Dr. Ramón Boixeda i Viu (Hospital De Mataró, Barcelona); Dr. Xoel Pena Pérez (Hospital Sant Joan De Deu, Barcelona); Dr. Elena Zubillaga Azpiroz (Hospital De Zumárraga, Guipúzcoa); Dr. María del Carmen Romero Pérez (Hospital Severo Ochoa, Madrid); Dr. María Gómez Antúnez (Hospital Gregorio Marañón, Madrid); Dr. Mercedes Racionero Moreno (Hospital Virgen De La Luz, Cuenca); Dr. Núria Galofré Álvaro (Hospital Municipal de Badalona, Barcelona); Dr. Olga H. Torres Bonafonte (Hospital Santa Creu i Sant Pau, Barcelona); Dr. Meritxell Salvadó Soro (Hospital de Mollet, Barcelona); Dr. Jorge Cabello Carro (Hospital Clínico San Carlos, Madrid); Dr. Robert Hurtado García (Hospital de la Vega Baja de Orihuela, Alicante); Dr. Lorena Montero Rivas (Hospital Infanta Margarita De Cabra, Córdoba); Dr. María Luz Calero Bernal (Hospital de San Juan de Dios de Bormujos, Seville); Dr. Pilar Martínez Heras (Hospital Miguel Servet, Zaragoza); Dr. José Luis Fernández Reyes (Complejo Hospitalario de Jaén, Jaén); Dr. María Belén Alonso Ortiz (Hospital Juan Negrín, Las Palmas de Gran Canaria); Dr. Carlos Trescolí* (Hospital de la Ribera, Valencia), and Dr. Enrique Peral Gutiérrez de Ceballos* (Hospital Virgen de la Macarena, Seville) *These panelists only participated in the first of the 2 Delphi rounds.
References
- Organización Mundial de la Salud (OMS). Descriptive note : chronic obstructive pulmonary disease (COPD). 2016, November. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 2017.
- MiravitllesM, SorianoJB, Garcia-RioF, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi:10.1136/thx.2009.11572519553233
- Grupo de Trabajo de GesEPOC. Guía de Práctica Clínica para el Diagnóstico y Tratamiento de Pacientes con Enfermedad Pulmonar Obstructiva Crónica (EPOC) – guía Española de la EPOC (GesEPOC). Versión 2017 [Clinical Practice Guideline for the Diagnosis and Treatment of Patients with Chronic Obstructive Pulmonary Disease (COPD) – Spanish Guideline for COPD (GesEPOC). 2017 version]. Arch Bronconeumol. 2017;53(Suppl 1):2–64. Spanish.
- Díez-ManglanoJ, López-GarcíaF. Protocolos: manejo Diagnóstico y Terapéutico de las Comorbilidades en la EPOC [Protocols: Diagnostic and Therapeutic management of Comorbidities in COPD]. Sociedad Española de Medicina Interna (SEMI) y Elsevier SL. 2014;1–259. Spanish.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). From the global strategy for the diagnosis, management and prevention of COPD. 2017 Available from: http://goldcopd.org. Accessed 316, 2020.
- SinDD, AnthonisenNR, SorianoJB, AgustiAG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. doi:10.1183/09031936.0013380517138679
- SmithMC, WrobelJP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888. doi:10.2147/COPD25210449
- BrookR. The RAND/UCLA Appropriateness Method. Rockville, MD: AHCPR Pub; 1994.
- Scottish Intercollegiate Guidelines Network. A Guideline Developers’ Handbook (Publication Nº 50). Edinburgh: SIGN; 2001 Disponible en: http://www.sign.ac.uk/guidelines/fulltext/50/index.html. Accessed 316, 2020.
- Oxford Centre for Evidence-Based Medicine. Levels of Evidence Document. (Consultado Enero 2017). 2011 Disponible en: http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed 316, 2020.
- ScholsAM, BuurmanWA, Staal van den BrekelAJ, DentenerMA, WoutersEF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51(8):819–824. doi:10.1136/thx.51.8.8198795671
- Almagro MenaP, Rodríguez CarballeiraM. Afectación extrapulmonar en la EPOC.¿ Qué es relevante y cómo debemos evaluarla [Extrapulmonary involvement in COPD: what is relevant and how should we evaluate it?] Med Clin (Barc). 2011;18–23. Spanish.
- Garcia-RioF, SorianoJB, MiravitllesM, et al. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e105220. doi:10.1371/journal.pone.010522025153331
- SteutenLM, CreutzbergEC, VrijhoefHJ, WoutersEF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91. doi:10.1016/j.pcrj.2005.09.00116701766
- FranssenFM, O’DonnellDE, GoossensGH, BlaakEE, ScholsAM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63(12):1110–1117. doi:10.1136/thx.2007.08682719020276
- CecereLM, LittmanAJ, SlatoreCG, et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD. 2011;8(4):275–284. doi:10.3109/15412555.2011.58666021809909
- VozorisNT, O’DonnellDE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–24. doi:10.1155/2012/73261822679617
- CurtisJR, WestfallAO, AllisonJ, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–426. doi:10.1002/(ISSN)1529-013116739208
- YuanL, DaiX, YangM, CaiQ, ShaoN. Potential treatment benefits and safety of roflumilast in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:1477–1483. doi:10.2147/COPD.S10637027418821
- SavaF, LavioletteL, BernardS, BretonMJ, BourbeauJ, MaltaisF. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10(1):55. doi:10.1186/1471-2466-10-5521054892
- RamachandranK, McCuskerC, ConnorsM, ZuwallackR, LahiriB. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5(4):205–209. doi:10.1177/147997230809671119029231
- SabinoPG, SilvaBM, BrunettoAF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics (Sao Paulo). 2010;65(6):599–605. doi:10.1590/S1807-5932201000060000720613936
- FarsangC, KissI, TykarskiA, NarkiewiczK. Treatment of hypertension in patients with chronic obstructive pulmonary disease (COPD). Eur Soc Hypertension Sci Newsletter. 2016;17:62.
- SchonhoferB, WenzelM, GeibelM, KohlerD. Blood transfusion and lung function in chronically anemic patients with severe chronic obstructive pulmonary disease. Crit Care Med. 1998;26(11):1824–1828. doi:10.1097/00003246-199811000-000229824074
- MillerJ, EdwardsLD, AgustiA, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi:10.1016/j.rmed.2013.05.00123791463
- SchnabelE, NowakD, BrascheS, WichmannHE, HeinrichJ. Association between lung function, hypertension and blood pressure medication. Respir Med. 2011;105(5):727–733. doi:10.1016/j.rmed.2010.12.02321276721
- WakabayashiK, GonzalezMA, DelhayeC, et al. Impact of chronic obstructive pulmonary disease on acute-phase outcome of myocardial infarction. Am J Cardiol. 2010;106(3):305–309. doi:10.1016/j.amjcard.2010.03.02620643237
- WorthH, ChungKF, FelserJM, HuH, RueeggP. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med. 2011;105(4):571–579. doi:10.1016/j.rmed.2010.11.02721227674
- WiseRA, AnzuetoA, CottonD, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi:10.1056/NEJMoa130334223992515
- WhiteWB, CookeGE, KoweyPR, et al. Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest. 2013;144(3):758–765. doi:10.1378/chest.12-233223412642
- Di DanieleN. Therapeutic approaches of uncomplicated arterial hypertension in patients with COPD. Pulm Pharmacol Ther. 2015;35:1–7. doi:10.1016/j.pupt.2015.09.00426363278
- ManciniGB, EtminanM, ZhangB, LevesqueLE, FitzGeraldJM, BrophyJM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47(12):2554–2560. doi:10.1016/j.jacc.2006.04.03916781387
- SalpeterS, OrmistonT, SalpeterE. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;4:Cd003566.
- CazzolaM, NoscheseP, D’AmatoG, MateraMG. The pharmacologic treatment of uncomplicated arterial hypertension in patients with airway dysfunction. Chest. 2002;121(1):230–241. doi:10.1378/chest.121.1.23011796456
- CazzolaM, NoscheseP, D’AmatoM, D’AmatoG. Comparison of the effects of single oral doses of nebivolol and celiprolol on airways in patients with mild asthma. Chest. 2000;118(5):1322–1326. doi:10.1378/chest.118.5.132211083681
- DransfieldMT, RoweSM, JohnsonJE, BaileyWC, GeraldLB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63(4):301–305. doi:10.1136/thx.2007.08189317951276
- RuttenFH, ZuithoffNP, HakE, GrobbeeDE, HoesAW. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(10):880–887. doi:10.1001/archinternmed.2010.11220498416
- HillNS. Fluid and electrolyte considerations in diuretic therapy for hypertensive patients with chronic obstructive pulmonary disease. Arch Intern Med. 1986;146(1):129–133. doi:10.1001/archinte.1986.003601301590222867747
- ManninoDM, ThornD, SwensenA, HolguinF. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi:10.1183/09031936.0001240818579551
- AlmagroP, LopezF, CabreraFJ, et al. Comorbilidades en pacientes hospitalizados por enfermedad pulmonar obstructiva crónica. Análisis comparativo de los estudios ECCO y ESMI [Comorbidities in hospitalized patients for chronic obstructive pulmonary disease. Comparative analysis of the ECCO and ESMI studies]. Rev Clin Esp. 2012;212(6):281–286. doi:10.1016/j.rce.2012.02.014. Spanish.22521437
- EhrlichSF, QuesenberryCP Jr., Van Den EedenSK, ShanJ, FerraraA. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi:10.2337/dc09-088019808918
- WackerME, HungerM, KarraschS, et al. Health-related quality of life and chronic obstructive pulmonary disease in early stages - longitudinal results from the population-based KORA cohort in a working age population. BMC Pulm Med. 2014;14:134. doi:10.1186/1471-2466-14-13425107380
- JansonC, MarksG, BuistS, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472–1483. doi:10.1183/09031936.0015371223722617
- Lopez VarelaMV, Montes de OcaM, HalbertRet al,. Comorbidities and health status in individuals with and without COPD in five Latin American cities: the PLATINO study. Arch Bronconeumol. 2013;49(11):468–474. doi:10.1016/j.arbr.2013.09.00923856439
- KoskelaJ, KilpelainenM, KupiainenH, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. 2014;14:102. doi:10.1186/1471-2466-14-10224946786
- PutchaN, PuhanMA, HanselNN, DrummondMB, BoydCM. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001–2008. COPD. 2013;10(3):324–332. doi:10.3109/15412555.2012.74496323713595
- KinneyGL, Black-ShinnJL, WanES, et al. Pulmonary function reduction in diabetes with and without chronic obstructive pulmonary disease. Diabetes Care. 2014;37(2):389–395. doi:10.2337/dc13-143524026562
- BakerEH, JanawayCH, PhilipsBJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. doi:10.1136/thx.2005.05102916449265
- BurtMG, RobertsGW, Aguilar-LozaNR, FrithP, StranksSN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011;96(6):1789–1796. doi:10.1210/jc.2010-272921411550
- ChakrabartiB, AngusRM, AgarwalS, LaneS, CalverleyPM. Hyperglycaemia as a predictor of outcome during non-invasive ventilation in decompensated COPD. Thorax. 2009;64(10):857–862. doi:10.1136/thx.2008.10698919454410
- YoungRP, HopkinsRJ, ChristmasT, BlackPN, MetcalfP, GambleGD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi:10.1183/09031936.0014420819196816
- CazzolaM, BettoncelliG, SessaE, CricelliC, BiscioneG. Prevalence of comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(2):112–119. doi:10.1159/00028188020134148
- GudmundssonG, UlrikCS, GislasonT, et al. Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: a prospective observational study in the Nordic countries. Int J Chron Obstruct Pulmon Dis. 2012;7:571–576. doi:10.2147/COPD.S3446623055707
- van den BorstB, GoskerHR, ZeegersMP, ScholsAM. Pulmonary function in diabetes: a metaanalysis. Chest. 2010;138(2):393–406. doi:10.1378/chest.09-262220348195
- Wood-BakerRR, GibsonPG, HannayM, WaltersEH, WaltersJA. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;1:Cd001288.
- SuissaS, KezouhA, ErnstP. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001–1006. doi:10.1016/j.amjmed.2010.06.01920870201
- O’ByrnePM, RennardS, GersteinH, et al. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir Med. 2012;106(11):1487–1493. doi:10.1016/j.rmed.2012.07.01122902134
- WoutersEF, BredenbrokerD, TeichmannP, et al. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):E1720–1725. doi:10.1210/jc.2011-288622723325
- Garcia-OlmosL, AlberquillaA, AyalaV, et al. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: a cross sectional study. BMC Fam Pract. 2013;14:11. doi:10.1186/1471-2296-14-1123324308
- de Lucas-ramosP, Izquierdo-AlonsoJL, Rodriguez-Gonzalez MoroJM, et al. [Cardiovascular risk factors in chronic obstructive pulmonary disease: results of the ARCE study]. Arch Bronconeumol. 2008;44(5):233–238. doi:10.1157/1311993718448013
- de Lucas-ramosP, Izquierdo-AlonsoJL, Rodriguez-Gonzalez MoroJM, FrancesJF, LozanoPV, Bellon-CanoJM. Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study). Int J Chron Obstruct Pulmon Dis. 2012;7:679–686. doi:10.2147/COPD.S3622223055717
- BasiliS, FerroniP, VieriM, et al. Lipoprotein(a) serum levels in patients affected by chronic obstructive pulmonary disease. Atherosclerosis. 1999;147(2):249–252. doi:10.1016/S0021-9150(99)00192-610559510
- EttingerWH, KlinefelterHF, KwiterovitchPO. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis. 1987;63(2–3):167–172. doi:10.1016/0021-9150(87)90117-13493784
- HooperPL, WooW, ViscontiL, PathakDR. Terbutaline raises high-density-lipoprotein-cholesterol levels. N Engl J Med. 1981;305(24):1455–1457. doi:10.1056/NEJM1981121030524066946283
- de Miguel DiezJ, Chancafe MorganJ, Jimenez GarciaR. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305–312. doi:10.2147/COPD.S3123623847414
- TsiligianniIG, KosmasE, Van der MolenT, TzanakisN. Managing comorbidity in COPD: a difficult task. Curr Drug Targets. 2013;14(2):158–176. doi:10.2174/138945011131402000423256716
- LeeTM, LinMS, ChangNC. Usefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am J Cardiol. 2008;101(4):530–535. doi:10.1016/j.amjcard.2007.09.10218312772
- CrinerGJ, ConnettJE, AaronSD, et al. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201–2210. doi:10.1056/NEJMoa140308624836125
- ChogtuB, KuriachanS, MagazineR, et al. A prospective, randomized study: evaluation of the effect of rosuvastatin in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Indian J Pharmacol. 2016;48(5):503–508. doi:10.4103/0253-7613.19072127721534
- IngebrigtsenTS, MarottJL, NordestgaardBG, LangeP, HallasJ, VestboJ. Statin use and exacerbations in individuals with chronic obstructive pulmonary disease. Thorax. 2015;70(1):33–40. doi:10.1136/thoraxjnl-2014-20579525349333
- HuangCC, ChanWL, ChenYC, et al. Statin use and hospitalization in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study in Taiwan. Clin Ther. 2011;33(10):1365–1370. doi:10.1016/j.clinthera.2011.08.01021962452
- AjmeraM, ShenC, SambamoorthiU. Association between statin medications and COPD-specific outcomes: a real-world observational study. Drugs Real World Outcomes. 2017;4(1):9–19. doi:10.1007/s40801-016-0101-627943058
- LahousseL, LothDW, JoosGF, et al. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm Pharmacol Ther. 2013;26(2):212–217. doi:10.1016/j.pupt.2012.10.00823142156
- RaymakersAJN, SadatsafaviM, SinDD, De VeraMA, LyndLD. The impact of statin drug use on all-cause mortality in patients with COPD: a population-based cohort study. Chest. 2017;152(3):486–493. doi:10.1016/j.chest.2017.02.00228202342
- CitgezE, van der PalenJ, Koehorst-Ter HuurneK, MovigK, van der ValkP, Brusse-KeizerM. Statins and morbidity and mortality in COPD in the COMIC study: a prospective COPD cohort study. BMJ Open Respir Res. 2016;3(1):e000142. doi:10.1136/bmjresp-2016-000142
- ZhangW, ZhangY, LiCW, JonesP, WangC, FanY. Effect of statins on COPD: a meta-analysis of randomized controlled trials. Chest. 2017;152(6):1159–1168. doi:10.1016/j.chest.2017.08.01528847550
- BhattSP, DransfieldMT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162(4):237–251. doi:10.1016/j.trsl.2013.05.00123727296
- AlmagroP, Lopez GarciaF, CabreraFJ, et al. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir Med. 2010;104(2):253–259. doi:10.1016/j.rmed.2009.09.01919879744
- AlmagroP, CabreraFJ, DiezJ, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC en Servicios de medicina interna (ESMI) study. Chest. 2012;142(5):1126–1133. doi:10.1378/chest.11-241323303399
- FonarowGC, HeywoodJT, HeidenreichPA, LopatinM, YancyCW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007;153(6):1021–1028. doi:10.1016/j.ahj.2007.03.01217540205
- MentzRJ, FiuzatM, WojdylaDM, et al. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: findings from OPTIMIZE-HF. Eur J Heart Fail. 2012;14(4):395–403. doi:10.1093/eurjhf/hfs00922302663
- MatamisD, TsagouriasM, PapathanasiouA, et al. Targeting occult heart failure in intensive care unit patients with acute chronic obstructive pulmonary disease exacerbation: effect on outcome and quality of life. J Crit Care. 2014;29(2):315.e317–314. doi:10.1016/j.jcrc.2013.11.011
- SuzukiT, LyonA, SaggarR, et al. Editor’s choice-biomarkers of acute cardiovascular and pulmonary diseases. Eur Heart J Acute Cardiovasc Care. 2016;5(5):416–433. doi:10.1177/204887261665230927221957
- PavasiniR, TavazziG, BiscagliaS, et al. Amino terminal pro brain natriuretic peptide predicts all-cause mortality in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Chron Respir Dis. 2017;14(2):117–126. doi:10.1177/147997231667439327956645
- CavaillesA, Brinchault-RabinG, DixmierA, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi:10.1183/09059180.0000861224293462
- LeeH, JhunBW, ChoJ, et al. Different impacts of respiratory symptoms and comorbidities on COPD-specific health-related quality of life by COPD severity. Int J Chron Obstruct Pulmon Dis. 2017;12:3301–3310. doi:10.2147/COPD29180860
- HenochI, StrangS, LofdahlCG, Ekberg-JanssonA. Health-related quality of life in a nationwide cohort of patients with COPD related to other characteristics. Eur Clin Respir J. 2016;3(1):31459. doi:10.3402/ecrj.v3.3145927238360
- JacobsDM, NoyesK, ZhaoJ, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thorac Soc. 2018;15(7):837–845. doi:10.1513/AnnalsATS.201712-913OC29611719
- ShahT, ChurpekMM, Coca PerraillonM, KonetzkaRT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi:10.1378/chest.14-218125539483
- AlmagroP, CalboE, Ochoa de EchaguenA, et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448. doi:10.1378/chest.121.5.144112006426
- TavazziL, SwedbergK, KomajdaM, et al. Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: an efficacy and safety analysis of SHIFT study. Int J Cardiol. 2013;170(2):182–188. doi:10.1016/j.ijcard.2013.10.06824225201
- CanepaM, Straburzynska-MigajE, DrozdzJ, et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail. 2018;20(1):100–110. doi:10.1002/ejhf.96428949063
- VogelmeierCF, CrinerGJ, MartinezFJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP28128970
- VestboJ, AndersonJA, BrookRD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/S0140-6736(16)30069-127203508
- SampJC, JooMJ, SchumockGT, CalipGS, PickardAS, LeeTA. Risk of cardiovascular and cerebrovascular events in COPD patients treated with long-acting beta2-agonist combined with a long-acting muscarinic or inhaled corticosteroid. Ann Pharmacother. 2017;51(11):945–953. doi:10.1177/106002801771971628677404
- RodrigoGJ, PriceD, AnzuetoA, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/COPD28360514
- RoversiS, FabbriLM, SinDD, HawkinsNM, AgustiA. Chronic obstructive pulmonary disease and cardiac diseases. an urgent need for integrated care. Am J Respir Crit Care Med. 2016;194(11):1319–1336. doi:10.1164/rccm.201604-0690SO27589227
- LainscakM, PodbregarM, KovacicD, RozmanJ, von HaehlingS. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med. 2011;105(Suppl 1):S44–49. doi:10.1016/S0954-6111(11)70010-522015086
- CoiroS, GirerdN, RossignolP, et al. Association of beta-blocker treatment with mortality following myocardial infarction in patients with chronic obstructive pulmonary disease and heart failure or left ventricular dysfunction: a propensity matched-cohort analysis from the high-risk myocardial infarction database initiative. Eur J Heart Fail. 2017;19(2):271–279. doi:10.1002/ejhf.64727774703
- DuQ, SunY, DingN, LuL, ChenY. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One. 2014;9(11):e113048.25427000
- EtminanM, JafariS, CarletonB, FitzGeraldJM. Beta-blocker use and COPD mortality: a systematic review and meta-analysis. BMC Pulm Med. 2012;12(1):48. doi:10.1186/1471-2466-12-4822947076
- MiravitllesM, Soler-CatalunaJJ, CalleM, et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.01828477954
- PutchaN, DrummondMB, WiseRA, HanselNN. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(4):575–591. doi:10.1055/s-0000007526238643
- MapelDW, DedrickD, DavisK. Trends and cardiovascular co-morbidities of COPD patients in the veterans administration medical system, 1991–1999. COPD. 2005;2(1):35–41. doi:10.1081/COPD-20005067117136959
- MullerovaH, AgustiA, ErqouS, MapelDW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–1178. doi:10.1378/chest.12-284723722528
- MapelD. Insights into COPD comorbidities from the OLIN study and other large databases. COPD. 2011;8(6):397–399. doi:10.3109/15412555.2011.63655522149398
- ChenW, ThomasJ, SadatsafaviM, FitzGeraldJM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi:10.1016/S2213-2600(15)00241-626208998
- Soriano OrtizJB, AlmagroP, Sauleda RoigJ. [Causes of mortality in COPD]. Arch Bronconeumol. 2009;45(Suppl 4):8–13. doi:10.1016/S0300-2896(09)72857-120116743
- Miguel-DíezJ, Carrasco-GarridoP, Rejas-GutierrezJ, et al. The influence of heart disease on characteristics, quality of life, use of health resources, and costs of COPD in primary care settings. BMC Cardiovasc Disord. 2010;10(1):8. doi:10.1186/1471-2261-10-820167091
- Black-ShinnJL, KinneyGL, WiseAL, et al. Cardiovascular disease is associated with COPD severity and reduced functional status and quality of life. COPD. 2014;11(5):546–551. doi:10.3109/15412555.2014.89802924831864
- PatelARC, DonaldsonGC, MackayAJ, WedzichaJA, HurstJR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141(4):851–857. doi:10.1378/chest.11-085321940771
- DalalAA, ShahM, LunacsekO, HananiaNA. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med. 2011;105(10):1516–1522. doi:10.1016/j.rmed.2011.04.00521684731
- PatelAR, KowlessarBS, DonaldsonGC, et al. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(9):1091–1099. doi:10.1164/rccm.201306-1170OC24033321
- CampoG, PavasiniR, MalagùM, et al. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther. 2015;29(2):147–157. doi:10.1007/s10557-014-6569-y25645653
- CalverleyPM, AndersonJA, CelliB, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa06307017314337
- ContoliM, CampoG, PavasiniR, et al. Inhaled corticosteroid/long-acting bronchodilator treatment mitigates STEMI clinical presentation in COPD patients. Eur J Intern Med. 2018;47:82–86. doi:10.1016/j.ejim.2017.08.01628821412
- LipsonDA, BarnhartF, BrealeyN, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa171390129668352
- VestboJ, FabbriL, PapiA, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52(6):1801230. doi:10.1183/13993003.01230-201830209195
- XiaN, WangH, NieX. Inhaled long-acting beta2-agonists do not increase fatal cardiovascular adverse events in COPD: a meta-analysis. PLoS One. 2015;10(9):e0137904. doi:10.1371/journal.pone.013790426378450
- CazzolaM, CalzettaL, MateraMG, MuscoliS, RoglianiP, RomeoF. Chronic obstructive pulmonary disease and coronary disease: cOPDCoRi, a simple and effective algorithm for predicting the risk of coronary artery disease in COPD patients. Respir Med. 2015;109(8):1019–1025. doi:10.1016/j.rmed.2015.05.02126111914
- MortensenEM, CopelandLA, PughMJ, et al. Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res. 2009;10(1):45. doi:10.1186/1465-9921-10-4519493329
- StefanMS, RothbergMB, PriyaA, PekowPS, AuDH, LindenauerPK. Association between beta-blocker therapy and outcomes in patients hospitalised with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertension. Thorax. 2012;67(11):977–984. doi:10.1136/thoraxjnl-2012-20194522941975
- PavasiniR, BiscagliaS, d’AscenzoF, et al. Antiplatelet treatment reduces all-cause mortality in COPD patients: a systematic review and meta-analysis. COPD. 2016;13(4):509–514. doi:10.3109/15412555.2015.109962026678708
- Houben-WilkeS, JorresRA, BalsR, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and systemic consequences-comorbidities network study. Am J Respir Crit Care Med. 2017;195(2):189–197. doi:10.1164/rccm.201602-0354OC27532739
- AzizF, LehmanEB. Preexisting conditions determine the occurrence of unplanned readmissions after procedures for treatment of peripheral arterial disease. Ann Vasc Surg. 2018;50:60–72. doi:10.1016/j.avsg.2018.01.07529481929
- WaschkiB, KirstenA, HolzO, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-252121273294
- PepinJL, CockcroftJR, MidwinterD, SharmaS, RubinDB, AndreasS. Long-acting bronchodilators and arterial stiffness in patients with COPD: a comparison of fluticasone furoate/vilanterol with tiotropium. Chest. 2014;146(6):1521–1530. doi:10.1378/chest.13-285925058845
- van GestelYR, HoeksSE, SinDD, et al. Beta-blockers and health-related quality of life in patients with peripheral arterial disease and COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:177–183. doi:10.2147/copd.s551119516916
- MiraultT, GalloulaA, CambouJP, et al. Impact of betablockers on general and local outcome in patients hospitalized for lower extremity peripheral artery disease: the COPART registry. Medicine (Baltimore). 2017;96(5):e5916. doi:10.1097/MD.000000000000591628151868
- Gerhard-HermanMD, GornikHL, BarrettC, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135(12). e686-e725. doi:10.1161/CIRCULATIONAHA.117.027305
- OnishiK. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol. 2017;70(2):128–134. doi:10.1016/j.jjcc.2017.03.00128325523
- BarrettTW, SelfWH, JenkinsCA, et al. Predictors of regional variations in hospitalizations following emergency department visits for atrial fibrillation. Am J Cardiol. 2013;112(9):1410–1416. doi:10.1016/j.amjcard.2013.07.00523972347
- SteerJ, GibsonJ, BourkeSC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. doi:10.1136/thoraxjnl-2012-20210322895999
- EchevarriaC, SteerJ, Heslop-MarshallK, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016;71(2):133–140. doi:10.1136/thoraxjnl-2015-20777526769015
- SangwanV, ChaudhryD, DyspneaMR, EosinopeniaC. Acidemia and atrial fibrillation score and BAP-65 score, tools for prediction of mortality in acute exacerbations of chronic obstructive pulmonary disease: a comparative pilot study. Indian J Crit Care Med. 2017;21(10):671–677. doi:10.4103/ijccm.IJCCM_148_1729142379
- GoudisCA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J Cardiol. 2017;69(5):699–705. doi:10.1016/j.jjcc.2016.12.01328188041
- KirchhofP, BenussiS, KotechaD, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88.27663299
- AnthonisenNR, ConnettJE, EnrightPL, ManfredaJ. Lung health study research G. Hospitalizations and mortality in the lung health study. Am J Respir Crit Care Med. 2002;166(3):333–339. doi:10.1164/rccm.211009312153966
- OgaleSS, LeeTA, AuDH, BoudreauDM, SullivanSD. Cardiovascular events associated with ipratropium bromide in COPD. Chest. 2010;137(1):13–19. doi:10.1378/chest.08-236719363211
- WilcheskyM, ErnstP, BrophyJM, PlattRW, SuissaS. Bronchodilator use and the risk of arrhythmia in COPD: part 1: saskatchewan cohort study. Chest. 2012;142(2):298–304. doi:10.1378/chest.10-249922871755
- HuertaC, LanesSF, Garcia RodriguezLA. Respiratory medications and the risk of cardiac arrhythmias. Epidemiology. 2005;16(3):360–366. doi:10.1097/01.ede.0000158743.90664.a715824553
- WilcheskyM, ErnstP, BrophyJM, PlattRW, SuissaS. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest. 2012;142(2):305–311. doi:10.1378/chest.11-159722871756
- van der HooftCS, HeeringaJ, BrusselleGG, et al. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166(9):1016–1020. doi:10.1001/archinte.166.9.101616682576
- WillgossTG, YohannesAM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858–866. doi:10.4187/respcare.0186222906542
- AtlantisE, FaheyP, CochraneB, SmithS. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766–777. doi:10.1378/chest.12-191123429910
- von LeupoldtA, KennK. The psychology of chronic obstructive pulmonary disease. Curr Opin Psychiatry. 2013;26(5):458–463. doi:10.1097/YCO.0b013e328363c1fc23867658
- QianJ, Simoni-WastilaL, RattingerGB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2014;29(1):49–57. doi:10.1002/gps.v29.123606418
- PapaioannouAI, BartziokasK, TsikrikaS, et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823. doi:10.1183/09031936.0001311222878874
- LaurinC, MoullecG, BaconSL, LavoieKL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918–923. doi:10.1164/rccm.201105-0939PP22246177
- CafarellaPA, EffingTW, UsmaniZA, FrithPA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627–638. doi:10.1111/j.1440-1843.2012.02148.x22309179
- KunikME, RoundyK, VeazeyC, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. doi:10.1378/chest.127.4.120515821196
- NgTP, NitiM, TanWC, CaoZ, OngKC, EngP. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60–67. doi:10.1001/archinte.167.1.6017210879
- MaurerJ, RebbapragadaV, BorsonS, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43s–56s. doi:10.1378/chest.08-034218842932
- EisnerMD, BlancPD, YelinEH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229–234. doi:10.1136/thx.2009.12620120335292
- BakasT, McLennonSM, CarpenterJS, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012;10(1):134. doi:10.1186/1477-7525-10-13423158687
- BlakemoreA, DickensC, GuthrieE, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501–512. doi:10.2147/COPD24876770
- DalalAA, ShahM, LunacsekO, HananiaNA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293–299. doi:10.3109/15412555.2011.58665921827298
- SinghG, ZhangW, KuoYF, SharmaG. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905–915. doi:10.1378/chest.15-044926204260
- ZwerinkM, Brusse-KeizerM, van der ValkPD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD002990.
- SpitzerC, GlaserS, GrabeHJ, et al. Mental health problems, obstructive lung disease and lung function: findings from the general population. J Psychosom Res. 2011;71(3):174–179. doi:10.1016/j.jpsychores.2011.03.00521843753
- CoventryPA, BowerP, KeyworthC, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta-analysis. PLoS One. 2013;8(4):e60532. doi:10.1371/journal.pone.006053223585837
- AbramsTE, Vaughan-SarrazinM, Van der WegMW. Acute exacerbations of chronic obstructive pulmonary disease and the effect of existing psychiatric comorbidity on subsequent mortality. Psychosomatics. 2011;52(5):441–449. doi:10.1016/j.psym.2011.03.00521907063
- TselebisA, KosmasE, BratisD, et al. Prevalence of alexithymia and its association with anxiety and depression in a sample of Greek chronic obstructive pulmonary disease (COPD) outpatients. Ann Gen Psychiatry. 2010;9:16. doi:10.1186/1744-859X-9-1620398249
- AlexopoulosGS, SireyJA, RauePJ, KanellopoulosD, ClarkTE, NovitchRS. Outcomes of depressed patients undergoing inpatient pulmonary rehabilitation. Am J Geriatr Psychiatry. 2006;14(5):466–475. doi:10.1097/01.JGP.0000199381.98971.d116670251
- DursunogluN, KokturkN, BahaA, et al. Comorbidities and their impact on chronic obstructive pulmonary disease. Tuberk Toraks. 2016;64(4):289–298. doi:10.5578/tt.224528393718
- National Collaborating Centre for Mental H. National Institute for Health and Clinical Excellence: guidance In: Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester (UK): British Psychological SocietyCopyright (c) The British Psychological Society & The Royal College of Psychiatrists; 2010:2010.
- BritainG; Excellence NIfC. Anxiety: Management of Anxiety (Panic Disorder, with or Without Agoraphobia, and Generalised Anxiety Disorder) in Adults in Primary, Secondary and Community Care. National Institute for Clinical Excellence; 2004.
- VolpatoE, BanfiP, RogersSM, PagniniF. Relaxation techniques for people with chronic obstructive pulmonary disease: a systematic review and a meta-analysis. Evid Based Complement Alternat Med. 2015;2015:628365. doi:10.1155/2015/62836526339268
- YohannesAM, ConnollyMJ, BaldwinRC. A feasibility study of antidepressant drug therapy in depressed elderly patients with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2001;16(5):451–454. doi:10.1002/gps.46111376459
- LacasseY, BeaudoinL, RousseauL, MaltaisF. Randomized trial of paroxetine in end-stage COPD. Monaldi Arch Chest Dis. 2004;61(3):3. doi:10.4081/monaldi.2004.692
- EiserN, HarteR, SpirosK, PhillipsC, IsaacMT. Effect of treating depression on quality-of-life and exercise tolerance in severe COPD. Copd. 2005;2(2):233–241. doi:10.1081/COPD-5759617136950
- LightRW, MerrillEJ, DesparsJ, GordonGH, MutalipassiLR. Doxepin treatment of depressed patients with chronic obstructive pulmonary disease. Arch Intern Med. 1986;146(7):1377–1380. doi:10.1001/archinte.1986.003601901550223521524
- BorsonS, McDonaldGJ, GayleT, DeffebachM, LakshminarayanS, VanTuinenC. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190–201. doi:10.1016/S0033-3182(92)71995-11557484
- Depression in adults: recognition and management | guidance and guidelines | NICE. 2018; Available from: https://www.nice.org.uk/guidance/cg90. Accessed 316, 2020.
- YohannesAM, AlexopoulosGS. Pharmacological treatment of depression in older patients with chronic obstructive pulmonary disease: impact on the course of the disease and health outcomes. Drugs Aging. 2014;31(7):483–492. doi:10.1007/s40266-014-0186-024902934
- PriceD, SmallM, MilliganG, HigginsV, GilEG, EstruchJ. Impact of night-time symptoms in COPD: a real-world study in five European countries. Int J Chron Obstruct Pulmon Dis. 2013;8:595–603. doi:10.2147/COPD.S4857024348032
- NunesDM, MotaRM, de Pontes NetoOL, PereiraED, de BruinVM, de BruinPF. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187(3):159–163. doi:10.1007/s00408-009-9147-519399553
- ValipourA, LavieP, LothallerH, MikulicI, BurghuberOC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12(4):367–372. doi:10.1016/j.sleep.2010.08.01721388878
- BudhirajaR, SiddiqiTA, QuanSF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. doi:10.5664/jcsm.454025700872
- DodgeR, ClineMG, QuanSF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med. 1995;155(16):1797–1800. doi:10.1001/archinte.1995.004301601450147654114
- ScharfSM, MaimonN, Simon-TuvalT, Bernhard-ScharfBJ, ReuveniH, TarasiukA. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;6:1–12. doi:10.2147/COPD21311688
- McNicholasWT, VerbraeckenJ, MarinJM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–375. doi:10.1183/09059180.0000321323997063
- OmachiTA, BlancPD, ClamanDM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. doi:10.1016/j.sleep.2011.12.00722429651
- RothT. Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med. 2009;10(1):19–25. doi:10.1016/j.sleep.2008.06.00518693067
- ChungWS, LaiCY, LinCL, KaoCH. Adverse respiratory events associated with hypnotics use in patients of chronic obstructive pulmonary disease: a population-based case-control study. Medicine (Baltimore). 2015;94(27):e1110. doi:10.1097/MD.000000000000111026166105
- VozorisNT, FischerHD, WangX, et al. Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Drugs Aging. 2013;30(3):183–192. doi:10.1007/s40266-013-0056-123371396
- VozorisNT, FischerHD, WangX, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332–340. doi:10.1183/09031936.0000801424743966
- ZhangMW, HoRC, CheungMW, FuE, MakA. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217–223. doi:10.1016/j.genhosppsych.2011.03.00921601717
- HuberMB, WackerME, VogelmeierCF, LeidlR. Comorbid influences on generic health-related quality of life in COPD: a systematic review. PLoS One. 2015;10(7):e0132670. doi:10.1371/journal.pone.013267026168154
- OmachiTA, KatzPP, YelinEH, et al. Depression and health-related quality of life in chronic obstructive pulmonary disease. Am J Med. 2009;122(8):778.e779–715. doi:10.1016/j.amjmed.2009.01.036
- NgTP, NitiM, FonesC, YapKB, TanWC. Co-morbid association of depression and COPD: a population-based study. Respir Med. 2009;103(6):895–901. doi:10.1016/j.rmed.2008.12.01019136238
- SalteK, TitlestadI, HallingA. Depression is associated with poor prognosis in patients with chronic obstructive pulmonary disease - a systematic review. Dan Med J. 2015;62(10):A5137.26441395
- LechelerL, RichterM, FranzenDP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):144. doi:10.1183/16000617.0026-2017
- PoolerA, BeechR. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315–330. doi:10.2147/COPD24729698
- Al-shairK, DockryR, Mallia-MilanesB, KolsumU, SinghD, VestboJ. Depression and its relationship with poor exercise capacity, BODE index and muscle wasting in COPD. Respir Med. 2009;103(10):1572–1579. doi:10.1016/j.rmed.2008.11.02119560330
- RegvatJ, ZmitekA, VegnutiM, KosnikM, SuskovicS. Anxiety and depression during hospital treatment of exacerbation of chronic obstructive pulmonary disease. J Int Med Res. 2011;39(3):1028–1038. doi:10.1177/14732300110390033821819737
- KeatingA, LeeA, HollandAE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8(2):89–99. doi:10.1177/147997231039375621596892
- LeeSH, KimKU, LeeH, KimYS, LeeMK, ParkHK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease. Korean J Intern Med. 2018;33(1):130–137. doi:10.3904/kjim.2016.09028602061
- FritzscheA, ClamorA, von LeupoldtA. Effects of medical and psychological treatment of depression in patients with COPD–a review. Respir Med. 2011;105(10):1422–1433. doi:10.1016/j.rmed.2011.05.01421680167
- AlbrechtJS, KhokharB, HuangT-Y, et al. Adherence and healthcare utilization among older adults with COPD and depression. Respir Med. 2017;129:53–58. doi:10.1016/j.rmed.2017.06.00228732836
- SmithSM, SonegoS, KetchesonL, LarsonJL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042. doi:10.1136/bmjresp-2014-000042
- DevineEC, PearcyJ. Meta-analysis of the effects of psychoeducational care in adults with chronic obstructive pulmonary disease. Patient Educ Couns. 1996;29(2):167–178. doi:10.1016/0738-3991(96)00862-29006233
- TselebisA, PachiA, IliasI, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297–328. doi:10.2147/NDT26929625
- Torres-SanchezI, ValenzaMC, Saez-RocaG, Cabrera-MartosI, Lopez-TorresI, Rodriguez-TorresJ. Results of a multimodal program during hospitalization in obese COPD exacerbated patients. COPD. 2016;13(1):19–25. doi:10.3109/15412555.2015.104342826418629
- SinghB, MielkeMM, ParsaikAK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol. 2014;71(5):581–588. doi:10.1001/jamaneurol.2014.9424637951
- DoddJW, CharltonRA, van den BroekMD, JonesPW. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest. 2013;144(1):119–127. doi:10.1378/chest.12-209923349026
- ZhangX, CaiX, ShiX, et al. Chronic obstructive pulmonary disease as a risk factor for cognitive dysfunction: a meta-analysis of current studies. J Alzheimers Dis. 2016;52(1):101–111. doi:10.3233/JAD-15073526967208
- OrtapamukH, NaldokenS. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med. 2006;20(2):99–106. doi:10.1007/BF298562116615418
- ZhengGQ, WangY, WangXT. Chronic hypoxia-hypercapnia influences cognitive function: a possible new model of cognitive dysfunction in chronic obstructive pulmonary disease. Med Hypotheses. 2008;71(1):111–113. doi:10.1016/j.mehy.2008.01.02518331781
- ChangSS, ChenS, McAvayGJ, TinettiME. Effect of coexisting chronic obstructive pulmonary disease and cognitive impairment on health outcomes in older adults. J Am Geriatr Soc. 2012;60(10):1839–1846. doi:10.1111/j.1532-5415.2012.04171.x23035917
- RaffaeleA-I, AndreaC, ClaudioP, et al. Drawing impairment predicts mortality in severe COPD. Chest. 2006;130(6):1687–1694. doi:10.1378/chest.130.6.168717166983
- ThakurN, BlancPD, JulianLJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–269. doi:10.2147/copd.s1068420856825
- PereiraED, VianaCS, TaunayTC, SalesPU, LimaJW, HolandaMA. Improvement of cognitive function after a three-month pulmonary rehabilitation program for COPD patients. Lung. 2011;189(4):279–285. doi:10.1007/s00408-011-9303-621656143
- KozoraE, TranZV, MakeB. Neurobehavioral improvement after brief rehabilitation in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2002;22(6):426–430. doi:10.1097/00008483-200211000-0000812464831
- KennK, GloecklR, SoennichsenA, et al. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–1077. doi:10.1097/TP.000000000000047225393161
- StephensonA, SeitzDP, FischerHD, et al. Cholinesterase inhibitors and adverse pulmonary events in older people with chronic obstructive pulmonary disease and concomitant dementia: a population-based, cohort study. Drugs Aging. 2012;29(3):213–223. doi:10.2165/11599480-000000000-0000022332932
- RobertsMH, MapelDW, HartryA, Von WorleyA, ThomsonH. Chronic pain and pain medication use in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2013;10(4):290–298. doi:10.1513/AnnalsATS.201303-040OC23952846
- BentsenSB, RustoenT, MiaskowskiC. Differences in subjective and objective respiratory parameters in patients with chronic obstructive pulmonary disease with and without pain. Int J Chron Obstruct Pulmon Dis. 2012;7:137–143. doi:10.2147/COPD22419861
- ElkingtonH, WhiteP, Addington-HallJ, HiggsR, EdmondsP. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19(6):485–491. doi:10.1191/0269216305pm1056oa16218161
- LohneV, HeerHC, AndersenM, MiaskowskiC, KongerudJ, RustoenT. Qualitative study of pain of patients with chronic obstructive pulmonary disease. Heart Lung. 2010;39(3):226–234. doi:10.1016/j.hrtlng.2009.08.00220457343
- van Dam van IsseltEF, Groenewegen-SipkemaKH, Spruit-van EijkM, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898. doi:10.1136/bmjopen-2014-005898
- BlindermanCD, HomelP, BillingsJA, TennstedtS, PortenoyRK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115–123. doi:10.1016/j.jpainsymman.2008.07.00619232893
- BorgeCR, WahlAK, MoumT. Pain and quality of life with chronic obstructive pulmonary disease. Heart Lung. 2011;40(3):e90–101. doi:10.1016/j.hrtlng.2010.10.00921444112
- BentsenSB, RustoenT, MiaskowskiC. Prevalence and characteristics of pain in patients with chronic obstructive pulmonary disease compared to the Norwegian general population. J Pain. 2011;12(5):539–545. doi:10.1016/j.jpain.2010.10.01421549316
- HajGhanbariB, GarlandSJ, RoadJD, ReidWD. Pain and physical performance in people with COPD. Respir Med. 2013;107(11):1692–1699. doi:10.1016/j.rmed.2013.06.01023845881
- BorgeCR, WahlAK, MoumT. Association of breathlessness with multiple symptoms in chronic obstructive pulmonary disease. J Adv Nurs. 2010;66(12):2688–2700. doi:10.1111/j.1365-2648.2010.05447.x20825511
- TanG, JensenMP, ThornbyJI, ShantiBF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.00515042521
- RashiqS, DickBD. Factors associated with chronic noncancer pain in the Canadian population. Pain Res Manage. 2009;14(6):454–460. doi:10.1155/2009/919628
- JanssenDJ, SpruitMA, Uszko-LencerNH, ScholsJM, WoutersEF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743. doi:10.1089/jpm.2010.047921510771
- MapelDW, DutroMP, MartonJP, WoodruffK, MakeB. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res. 2011;11(1):43. doi:10.1186/1472-6963-11-4321345188
- VerberktCA, van den Beuken-van EverdingenMHJ, ScholsJ, et al. Respiratory adverse effects of opioids for breathlessness: a systematic review and meta-analysis. Eur Respir J. 2017;50(5):5. doi:10.1183/13993003.01153-2017
- EkstromM, BajwahS, BlandJM, CurrowDC, HussainJ, JohnsonMJ. One evidence base; three stories: do opioids relieve chronic breathlessness? Thorax. 2018;73(1):88–90. doi:10.1136/thoraxjnl-2016-20986828377491
- EkstromMP, Bornefalk-HermanssonA, AbernethyAP, CurrowDC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g44524482539
- GruberEM, TschernkoEM. Anaesthesia and postoperative analgesia in older patients with chronic obstructive pulmonary disease: special considerations. Drugs Aging. 2003;20(5):347–360. doi:10.2165/00002512-200320050-0000412696995
- KaelinM, SwankA, BarnardK, AdamsK, BeachP, NewmanJ. Physical fitness and quality of life outcomes in a pulmonary rehabilitation program utilizing symptom limited interval training and resistance training. J Exerc Physiol Online. 2001;4:3.
- BoueriFM, Bucher-BartelsonBL, GlennKA, MakeBJ. Quality of life measured with a generic instrument (Short Form-36) improves following pulmonary rehabilitation in patients with COPD. Chest. 2001;119(1):77–84. doi:10.1378/chest.119.1.7711157587
- De TorresJP, Pinto-PlataV, IngenitoE, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4):1092–1098. doi:10.1378/chest.121.4.109211948037
- YohannesAM, ErshlerWB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care. 2011;56(5):644–652. doi:10.4187/respcare.0100221276321
- SilverbergDS, MorR, WeuMT, SchwartzD, SchwartzIF, CherninG. Anemia and iron deficiency in COPD patients: prevalence and the effects of correction of the anemia with erythropoiesis stimulating agents and intravenous iron. BMC Pulm Med. 2014;14:24. doi:10.1186/1471-2466-14-2424564844
- KrishnanG, GrantBJ, MutiPC, et al. Association between anemia and quality of life in a population sample of individuals with chronic obstructive pulmonary disease. BMC Pulm Med. 2006;6(1):23. doi:10.1186/1471-2466-6-2316953872
- ChambellanA, ChailleuxE, SimilowskiT. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128(3):1201–1208. doi:10.1378/chest.128.3.120116162707
- PortilloK, BeldaJ, AntonP, CasanP. [High frequency of anemia in COPD patients admitted in a tertiary hospital]. Rev Clin Esp. 2007;207(8):383–387. doi:10.1157/1310875517688864
- VasquezA, LogomarsinoJV. Anemia in chronic obstructive pulmonary disease and the potential role of iron deficiency. Copd. 2016;13(1):100–109. doi:10.3109/15412555.2015.104351926418826
- BarbaR, de CasasolaGG, MarcoJ, et al. Anemia in chronic obstructive pulmonary disease: a readmission prognosis factor. Curr Med Res Opin. 2012;28(4):617–622. doi:10.1185/03007995.2012.67531822409165
- HalpernMT, ZilberbergMD, SchmierJK, LauEC, ShorrAF. Anemia, costs and mortality in chronic obstructive pulmonary disease. Cost Eff Resour Alloc. 2006;4(1):17. doi:10.1186/1478-7547-4-1717042950
- CoteC, ZilberbergMD, ModySH, DordellyLJ, CelliB. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007;29(5):923–929. doi:10.1183/09031936.0013710617251227
- Haja MydinH, MurphyS, ClagueH, SridharanK, TaylorIK. Anemia and performance status as prognostic markers in acute hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:151–157. doi:10.2147/COPD.S3940323658480
- Martinez-RiveraC, PortilloK, Munoz-FerrerA, et al. Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. Copd. 2012;9(3):243–250. doi:10.3109/15412555.2011.64713122360381
- RasmussenL, ChristensenS, Lenler-PetersenP, JohnsenSP. Anemia and 90-day mortality in COPD patients requiring invasive mechanical ventilation. Clin Epidemiol. 2010;3:1–5. doi:10.2147/CLEP.S1288521326654
- CopurAS, FulambarkerA, MolnarJ, et al. Role of anemia in home oxygen therapy in chronic obstructive pulmonary disease patients. Am J Ther. 2015;22(5):361–366. doi:10.1097/MJT.0b013e3182785f7c23567789
- SchonhoferB, BohrerH, KohlerD. Blood transfusion facilitating difficult weaning from the ventilator. Anaesthesia. 1998;53(2):181–184. doi:10.1046/j.1365-2044.1998.00275.x9534644
- Graat-VerboomL, WoutersEF, SmeenkFW, van den BorneBE, LundeR, SpruitMA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34(1):209–218. doi:10.1183/09031936.5013040819567604
- AlmagroP, Lopez GarciaF, CabreraFJ, et al. [Study of the comorbidities in hospitalized patients due to decompensated chronic obstructive pulmonary disease attended in the Internal Medicine Services. ECCO Study]. Rev Clin Esp. 2010;210(3):101–108. doi:10.1016/j.rce.2009.12.00220226938
- SilvaDR, CoelhoAC, DumkeA, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56(7):961–968. doi:10.4187/respcare.0105621352667
- Graat-VerboomL, SpruitMA, van den BorneBE, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009;103(8):1143–1151. doi:10.1016/j.rmed.2009.02.01419304474
- LeeSH, KwonHY. Prevalence of Osteoporosis in Korean patients with chronic obstructive pulmonary disease and their health-related quality of life according to the Korea National Health and Nutrition Examination Survey 2008–2011. J Bone Metab. 2017;24(4):241–248. doi:10.11005/jbm.2017.24.4.24129259964
- LinC-W, Chen-Y-Y, ChenY-J, LiangC-Y, LinM-S, ChenW. Prevalence, risk factors, and health-related quality of life of osteoporosis in patients with COPD at a community hospital in Taiwan. Int J Chron Obstruct Pulmon Dis. 2015;10:1493–1500. doi:10.2147/COPD.S8543226251589
- RommeEA, MurchisonJT, EdwardsLD, et al. CT-measured bone attenuation in patients with chronic obstructive pulmonary disease: relation to clinical features and outcomes. J Bone Miner Res. 2013;28(6):1369–1377. doi:10.1002/jbmr.187323361992
- MalinovschiA, MasoeroM, BellocchiaM, et al. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res. 2014;15:131. doi:10.1186/s12931-014-0131-025496239
- Pascual-GuardiaS, Badenes-BonetD, Martin-OntiyueloC, et al. Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. Int J Chron Obstruct Pulmon Dis. 2017;12:1837–1845. doi:10.2147/COPD28684906
- HattiholiJ, GaudeGS. Prevalence and correlates of osteoporosis in chronic obstructive pulmonary disease patients in India. Lung India. 2014;31(3):221–227. doi:10.4103/0970-2113.13575925125807
- KiyokawaH, MuroS, OgumaT, et al. Impact of COPD exacerbations on osteoporosis assessed by chest CT scan. COPD. 2012;9(3):235–242. doi:10.3109/15412555.2011.65024322360380
- HarrisonRA, SiminoskiK, VethanayagamD, MajumdarSR. Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res. 2007;22(3):447–457. doi:10.1359/jbmr.06120217181402
- HornikxM, Van RemoortelH, DemeyerH, et al. The influence of comorbidities on outcomes of pulmonary rehabilitation programs in patients with COPD: a systematic review. Biomed Res Int. 2013;2013:146148. doi:10.1155/2013/14614824490146
- AmicheMA, AlbaumJM, TadrousM, et al. Fracture risk in oral glucocorticoid users: a Bayesian meta-regression leveraging control arms of osteoporosis clinical trials. Osteoporos Int. 2016;27(5):1709–1718. doi:10.1007/s00198-015-3455-926694595
- MattishentK, ThavarajahM, BlancoP, GilbertD, WilsonAM, LokeYK. Meta-review: adverse effects of inhaled corticosteroids relevant to older patients. Drugs. 2014;74(5):539–547. doi:10.1007/s40265-014-0202-z24659375
- LokeYK, CavallazziR, SinghS. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66(8):699–708. doi:10.1136/thx.2011.16002821602540
- MineoTC, AmbrogiV, MineoD, FabbriA, FabbriniE, MassoudR. Bone mineral density improvement after lung volume reduction surgery for severe emphysema. Chest. 2005;127(6):1960–1966. doi:10.1378/chest.127.6.196015947308
- AllenCS, YeungJH, VandermeerB, HomikJ. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:Cd001347.27706804
- CasanovaC, BaudetJS, Del Valle VelascoM, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23(6):841–845. doi:10.1183/09031936.04.0010700415218995
- SakaeTM, PizzichiniMM, TeixeiraPJ, SilvaRM, TrevisolDJ, PizzichiniE. Exacerbations of COPD and symptoms of gastroesophageal reflux: a systematic review and meta-analysis. J Bras Pneumol. 2013;39(3):259–271. doi:10.1590/S1806-3713201300030000223857694
- KimJ, LeeJH, KimY, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013;13:51. doi:10.1186/1471-2466-13-5123927016
- KempainenRR, SavikK, WhelanTP, DunitzJM, HerringtonCS, BillingsJL. High prevalence of proximal and distal gastroesophageal reflux disease in advanced COPD. Chest. 2007;131(6):1666–1671. doi:10.1378/chest.06-226417400682
- DonaldsonGC, MullerovaH, LocantoreN, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res. 2013;14(1):79. doi:10.1186/1465-9921-14-7923899210
- HurstJR, VestboJ, AnzuetoA, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa090988320843247
- López GarcíaFPLF. Enfermedades digestivas y enfermedad pulmonar obstructiva crónica. [Digestive diseases and chronic obstructive pulmonary disease]. Med Clin (Barc). 2010;11:13–16. Spanish.
- RizkallahJ, ManSFP, SinDD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786–793. doi:10.1378/chest.08-151618812453
- TeradaK, MuroS, SatoS, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63(11):951–955. doi:10.1136/thx.2007.09285818535116
- TakadaK, MatsumotoS, KojimaE, et al. Prospective evaluation of the relationship between acute exacerbations of COPD and gastroesophageal reflux disease diagnosed by questionnaire. Respir Med. 2011;105(10):1531–1536. doi:10.1016/j.rmed.2011.03.00921454063
- Lopez-EncuentraA, AstudilloJ, CerezalJ, et al. Prognostic value of chronic obstructive pulmonary disease in 2994 cases of lung cancer. Eur J Cardiothorac Surg. 2005;27(1):8–13. doi:10.1016/j.ejcts.2004.09.01015736303
- MartinezC, OkajimaY, MurrayS, et al. Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir Res. 2014;15:62. doi:10.1186/1465-9921-15-6224894541
- IngebrigtsenTS, MarottJL, VestboJ, NordestgaardBG, HallasJ, LangeP. Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology. 2015;20(1):101–107. doi:10.1111/resp.1242025297724
- LiangBM, FengYL. Association of gastroesophageal reflux disease symptoms with stable chronic obstructive pulmonary disease. Lung. 2012;190(3):277–282. doi:10.1007/s00408-011-9365-522258420
- Garcia RodriguezLA, RuigomezA, Martin-MerinoE, JohanssonS, WallanderM-A. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest. 2008;134(6):1223–1230. doi:10.1378/chest.08-090218689591
- Del GrandeLM, HerbellaFA, BigataoAM, JardimJR, PattiMG. Inhaled beta agonist bronchodilator does not affect trans-diaphragmatic pressure gradient but decreases lower esophageal sphincter retention pressure in patients with Chronic Obstructive Pulmonary Disease (COPD) and Gastroesophageal Reflux Disease (GERD). J Gastrointest Surg. 2016;20(10):1679–1682. doi:10.1007/s11605-016-3192-127350150
- CuiY, DevillierP, KuangX, et al. Tiotropium reduction of lung inflammation in a model of chronic gastro-oesophageal reflux. Eur Respir J. 2010;35(6):1370–1376. doi:10.1183/09031936.0013990919926736
- Fernández-RuizM, Guerra-ValesJ. Comorbilidad digestiva y hepática en la EPOC [Digestive and liver comorbidity in COPD]. Rev Clin Esp. 2007;207(Supl1):40–46. Spanish
- KatzPO, GersonLB, VelaMF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308–328; quiz 329. doi:10.1038/ajg.2012.444
- BoereeMJ, PetersFT, PostmaDS, KleibeukerJH. No effects of high-dose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. Eur Respir J. 1998;11(5):1070–1074. doi:10.1183/09031936.98.110510709648957
- PoeRH, KallayMC. Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment. Chest. 2003;123(3):679–684. doi:10.1378/chest.123.3.67912628862
- XiongW, ZhangQS, ZhaoW, DingW, LiuJM, ZhaoYF. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exp Pathol. 2016;97(2):107–113. doi:10.1111/iep.1217327135904
- PenaX, Van Den EyndeE, MeneE, RecioJ. EPOC y enfermedad cardiovascular [COPD and cardiovascular disease]. Rev Clin Esp. 2007;207(Supl1):14–21. Spanish.
- GunenH, GulbasG, InE, YetkinO, HacievliyagilSS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35(6):1243–1248. doi:10.1183/09031936.0012090919926740
- DuanSC, YangYH, LiXY, et al. Prevalence of deep venous thrombosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J (Engl). 2010;123(12):1510–1514.20819502
- BørvikT, BrækkanSK, EngaK, et al. COPD and risk of venous thromboembolism and mortality in a general population. Eur Respir J. 2016;47(2):473–481. doi:10.1183/13993003.00402-201526585434
- KimV, GoelN, GangarJ, et al. Risk factors for venous thromboembolism in chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2014;1(2):239–249. doi:10.15326/jcopdf.1.2.2014.0133#sthash.pvwPnxaI.dpuf25844397
- BurgelPR, EscamillaR, PerezT, et al. Impact of comorbidities on COPD-specific health-related quality of life. Respir Med. 2013;107(2):233–241. doi:10.1016/j.rmed.2012.10.00223098687
- WangTS, MaoYM, SunYM, LouYJ. [Pulmonary embolism in patients with chronic obstructive pulmonary disease exacerbations of unknown origin: clinical characteristics and risk factors]. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35(4):259–263.22781197
- BertolettiL, QuenetS, LaporteS, et al. Pulmonary embolism and 3-month outcomes in 4036 patients with venous thromboembolism and chronic obstructive pulmonary disease: data from the RIETE registry. Respir Res. 2013;14:75. doi:10.1186/1465-9921-14-7523865769
- SteinPD, MattaF. Vena cava filters in hospitalised patients with chronic obstructive pulmonary disease and pulmonary embolism. Thromb Haemost. 2013;109(5):897–900. doi:10.1160/TH13-01-000623467701
- PiazzaG, GoldhaberSZ, KrollA, GoldbergRJ, EmeryC, SpencerFA. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med. 2012;125(10):1010–1018. doi:10.1016/j.amjmed.2012.03.00722884176
- FraisseF, HolzapfelL, CoulandJM, et al. Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. The Association of Non-University Affiliated Intensive Care Specialist Physicians of France. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1109–1114. doi:10.1164/ajrccm.161.4.980702510764298
- IncalziRA, CorsonelloA, PedoneC, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest. 2010;137(4):831–837. doi:10.1378/chest.09-171019903974
- TerzanoC, ContiV, Di StefanoF, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung. 2010;188(4):321–329. doi:10.1007/s00408-009-9222-y20066539
- Diez ManglanoJ, Bernabeu-WittelM, Escalera-ZalvideA, et al. [Comorbidity, discapacity and mortality in patients with multiple conditions and chronic obstructive pulmonary disease]. Rev Clin Esp. 2011;211(10):504–510. doi:10.1016/j.rce.2011.04.00621982043
- MapelDW, MartonJP. Prevalence of renal and hepatobiliary disease, laboratory abnormalities, and potentially toxic medication exposures among persons with COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:127–134. doi:10.2147/COPD23515180
- HanlyP. Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial. 2004;17(2):109–114. doi:10.1111/j.0894-0959.2004.17206.x15043611
- SinganayagamA, SchembriS, ChalmersJD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. doi:10.1513/AnnalsATS.201208-043OC23607835
- van GestelYR, ChoncholM, HoeksSE, et al. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrol Dial Transplant. 2009;24(9):2763–2767. doi:10.1093/ndt/gfp17119369691
- FabbianF, De GiorgiA, ManfrediniF, et al. Impact of renal dysfunction on in-hospital mortality of patients with severe chronic obstructive pulmonary disease: a single-center Italian study. Int Urol Nephrol. 2016;48(7):1121–1127. doi:10.1007/s11255-016-1272-527020445
- FedeliU, De GiorgiA, GennaroN, et al. Lung and kidney: a dangerous liaison? A population-based cohort study in COPD patients in Italy. Int J Chron Obstruct Pulmon Dis. 2017;12:443–450. doi:10.2147/COPD28184156
- IncalziRA, CorsonelloA, PedoneC, BattagliaS, PaglinoG, BelliaV. Chronic renal failure: a neglected comorbidity of COPD. Chest. 2010;137(4):831–837. doi:10.1378/chest.09-171019903974
- AlcazarR, EgocheagaMI, OrteL, et al. [SEN-SEMFYC consensus document on chronic kidney disease]. Nefrologia. 2008;28(3):273–282.18590493
- Diez HerranzA. [COPD and lung cancer: practical implications]. Arch Bronconeumol. 2001;37(5):240–247. doi:10.1016/s0300-2896(01)75061-211412516
- Wasswa-KintuS, GanWQ, ManSF, ParePD, SinDD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60(7):570–575. doi:10.1136/thx.2004.03713515994265
- ManninoDM, AguayoSM, PettyTL, ReddSC. Low lung function and incident lung cancer in the United States: data from the first national health and nutrition examination survey follow-up. Arch Intern Med. 2003;163(12):1475–1480. doi:10.1001/archinte.163.12.147512824098
- WilsonDO, WeissfeldJL, BalkanA, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi:10.1164/rccm.200803-435OC18565949
- FabbriLM, LuppiF, BegheB, RabeKF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31(1):204–212. doi:10.1183/09031936.0011430718166598
- de-TorresJP, WilsonDO, Sanchez-SalcedoP, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191(3):285–291. doi:10.1164/rccm.201407-1210OC25522175
- ParimonT, ChienJW, BrysonCL, McDonellMB, UdrisEM, AuDH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712–719. doi:10.1164/rccm.200608-1125OC17185647
- KiriVA, FabbriLM, DavisKJ, SorianoJB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103(1):85–90. doi:10.1016/j.rmed.2008.07.02418793832
- YaoR, WangY, LemonWJ, LubetRA, YouM. Budesonide exerts its chemopreventive efficacy during mouse lung tumorigenesis by modulating gene expressions. Oncogene. 2004;23(46):7746–7752. doi:10.1038/sj.onc.120798515361829
- LimE, BaldwinD, BecklesM, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(Suppl 3):iii1–27. doi:10.1136/thx.2010.145938.20940263