Abstract
The skeletal muscles play an essential role in life, providing the mechanical basis for respiration and movement. Skeletal muscle dysfunction is prevalent in all stages of chronic obstructive pulmonary disease (COPD), and significantly influences symptoms, functional capacity, health related quality of life, health resource usage and even mortality. Furthermore, in contrast to the lungs, the skeletal muscles are potentially remedial with existing therapy, namely exercise-training. This review summarizes clinical and laboratory observations of the respiratory and peripheral skeletal muscles (in particular the diaphragm and quadriceps), and current understanding of the underlying etiological processes. As further progress is made in the elucidation of the molecular mechanisms of skeletal muscle dysfunction, new pharmacological therapies are likely to emerge to treat this important extra-pulmonary manifestation of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is major health problem. By 2020, COPD is predicted to be the third leading cause of death and fifth leading cause of chronic disability worldwide.Citation1 Although a disease of the lungs, extra-pulmonary features of COPD are increasingly recognized as important contributors to morbidity and mortality.Citation2 Skeletal muscle dysfunction is of particular interest, as it directly influences exercise performance,Citation3 is associated with poor health status,Citation4 and is an independent predictor of health care utilizationCitation5 and mortality.Citation6 Furthermore, respiratory muscle function plays a key role in the pathogenesis of breathlessnessCitation7 and maximum inspiratory pressure is an independent predictor of survival in severe disease.Citation8
The most commonly studied skeletal muscles are the quadriceps and the diaphragm. Cross-sectional studies, with careful matching of patients with controls, have revealed the complexity of muscle dysfunction in COPD. In turn, these have provided insight into the possible etiological factors and pathophysiological processes. This review describes the distribution and nature of changes to skeletal muscle function in COPD and how this relates to lung function. Possible etiological factors and mechanisms underpinning COPD muscle dysfunction will be discussed and how this may inform emerging non-pharmacological and pharmacological treatment approaches in this field.
The peripheral muscles
Compared with healthy controls matched for age and gender, isometric quadriceps strength – whether assessed by volitionalCitation9,Citation10 or non-volitional measuresCitation11 – is reduced by about 20%–30% in patients with COPD. A marked increase in susceptibility to fatigue is also observed, with a more rapid decline in performance during continuousCitation12,Citation13 or repeated bouts of exercise.Citation14,Citation15
The reduction in strength can largely be explained by a comparable reduction in quadriceps cross-sectional area (CSA) and mass, the latter being assessed by magnetic resonance imagingCitation16 and the former by ultrasoundCitation17 or computed tomography.Citation9,Citation18 Microscopically, atrophy of single muscle fibers has been observed with a predilection for type IIX fibers.Citation19 Quadriceps endurance is more likely to be related to the relative loss of fatigue-resistant type I fibersCitation20,Citation21 and subsequent reduction in oxidative capacity. ***Matched case-control pairs of vastus lateralis samples reveal a shift in fiber type expression away from type I and toward type II/IIX fibers.Citation20 Concurrent structural changes include a reduction in capillary density,Citation22 number of capillary-muscle fiber contactsCitation21 and levels of oxidative enzyme activity.Citation23 Samples from patients with COPD consistently demonstrate reduced levels of aerobic enzyme activity – for example, citrate synthase and 3-hydroxyacyl CoA dehydrogenaseCitation23,Citation24 – together with lower concentrations of adenosine-5′-triphosphate and creatine phosphate.Citation25 The resultant impaired capacity for oxidative phosphorylation leads to a greater reliance on glycolysis during exercise and early accumulation of lactate that becomes limiting.Citation26 Indeed, the oxidative:glycolytic enzyme activity ratio correlates moderately with quadriceps endurance.Citation27
Where other peripheral muscles have been studied, a preferential distribution of muscle wasting and weakness to the lower limbs is observed. Mathur et al found reduced volumes and CSAs in the hamstrings and adductors (21% and 30%, respectively) of patients with moderate to severe disease.Citation16 In contrast, relatively preserved or maintained strength has been found in upper-limb muscles, such as the adductor pollicisCitation11 and elbow flexors,Citation28 and grip strength.Citation29 Structural and histochemical studies support this pattern of dysfunction. Biopsies of the bicepsCitation30 and deltoid musclesCitation29 reveal no differences between patients and controls in fiber type profile, nor single-fiber CSA. Gea et alCitation29 explored the metabolism in the deltoid muscle, with findings suggesting a preserved oxidative capacity with raised lactate dehydrogenase and citrate synthase activity and no differences in levels of phosphofructokinase or creatine kinase. In summary, distribution of peripheral muscle dysfunction is not uniform in COPD. Changes are most marked in the lower limbs, which are necessary for locomotion, providing evidence that local factors such as disuse/immobility may be more influential than any systemic process.
The respiratory muscles
Although the capacity of the diaphragm to generate transdiaphragmatic pressure is reduced in COPD, this is largely the product of hyperinflation, which places the muscle at a mechanical disadvantage. Indeed, when corrected for lung volume, the contractile strength of the diaphragm in COPD is not reduced compared with controlsCitation11 and may even be enhanced in some cases.Citation31 The maintenance of strength in this muscle is probably due to persistent involuntary training secondary to the increased work of breathing. As a result, the diaphragm adapts by remodeling its fiber type profile toward a fatigue-resistant phenotype with a relative increase the proportion of type I fibers. Relative to controls, samples reveal increases 20%–50% in the overall proportion of type I fibers,Citation32,Citation33 matched by reductions in type IIX fibers.Citation34
Less is known about change in single diaphragm fibers and debate exists as to whether their CSA or force-generating capacity is altered. Some studies report no change in fiber size,Citation35 while others observe selective atrophy of type I fibers.Citation36 Similarly, lower isometric force-generating capacity (normalized for CSA) has been reported among patient fibers tested in vitro,Citation36,Citation37 while others have found no difference between patient and control fibers.Citation38 More established is the intrinsic resistance to fatigue that occurs via an increased concentration of mitochondria,Citation38 capillary density,Citation34 and capacity to generate adenosine-5′-triphosphate through oxidative pathways, marked by an increased succinate dehydrogenase activity.Citation39 In COPD patients, no fatigue of the diaphragm is seen with maximum voluntary ventilation or exhaustive treadmill exercise.Citation40,Citation41 Diaphragm fibers from patients are also more efficient than those from controls, with a lower adenosine-5′-triphosphate cost to maintain a similar isometric force.Citation36 The reduced energy cost may be accounted for by the number of cross-bridge formations within each fiber, with COPD diaphragm muscle fibers having fewer active cross-bridges and each exerting a greater force than in control muscle.Citation35,Citation36
Where other accessory respiratory muscles have been studied, these appear to adapt in the same manner in response to the increased work of breathing. The shift in fiber type from II to I observed in the diaphragm is also seen in the parasternal intercostal muscles of patients with severe disease.Citation42 A contrasting shift in fiber type expression has been observed in the external intercostal muscles, which may reflect their postural role in this group.Citation43 Functionally, pectoralis major and latissimus dorsi strength are preserved relative to the quadriceps,Citation9 as is abdominal strength, presumably due to the additional activity of expiratory muscles in COPD.Citation10
In summary, the changes seen in the respiratory muscles are in stark contrast to those in quadriceps muscle in COPD. Whereas the quadriceps muscle is characterized by a reduced mass and loss of fatigue-resistant type I fibers and oxidative capacity, which impairs strength and endurance, the diaphragm remodels toward a fatigue-resistant profile, with a relative increase in type I fibers and resultant increase in oxidative capacity (), a pattern reflected in other accessory muscles of respiration. These observations support muscle disuse being a major etiological factor for the differential adaptation of peripheral and respiratory muscles in COPD.
Table 1 Quadriceps and diaphragm structure and function in patients with COPD compared with controls
Muscle function and COPD severity
The traditional paradigm is that skeletal muscle dysfunction is a feature of severe or end-stage disease. Certainly, Bernard et al demonstrated a significant relationship between quadriceps strength and forced expiratory volume in 1 second (FEV1) percentage of predicted value, with the more flow-limited patients being weakerCitation9 and the prevalence of quadriceps weakness rises with increasing Global initiative for chronic Obstructive Lung Disease (GOLD) stage.Citation44 Others have also shown a moderate relationship between FEV1 and quadriceps endurance;Citation45 whereas, in the diaphragm, muscle-fiber proportion shift advances with increasing disease severity. Citation37 However, the literature is far from being unequivocal and several studies have not found any correlations between airflow obstruction and muscle dysfunction.Citation46,Citation47 Very recent data supports the presence of muscle dysfunction, even in the early stages of the disease. Seymour and colleagues showed that quadriceps weakness is common across all disease stages with a mean (95% confidence interval [CI]) prevalence of 31% (25%–38%) in GOLD stage I/II, rising (though not sufficiently to achieve statistical significance) to 38% (31%–46%) in GOLD stage IV.Citation44 Endurance is compromised even in patients with mild disease performing low-intensity tasks,Citation15 while invasive evaluation of diaphragm contractile function, structure, and biochemistry demonstrated that cellular and molecular alterations occur, even in GOLD I/II patients.Citation48 These data suggest that the relationship between airway obstruction and muscle dysfunction in COPD is modest at best and, certainly in some patients, muscle abnormalities may occur before any drop in FEV1 is detected.Citation49 This could be attributed to potential etiological factors such as smokingCitation49 or reductions in physical activity.Citation50,Citation51
Muscle function and physical inactivity in COPD
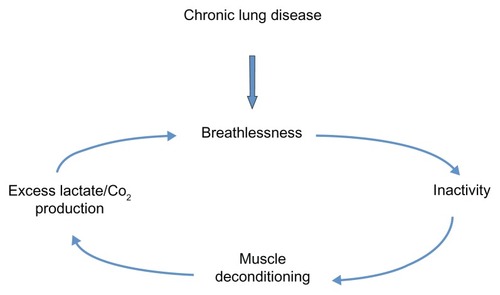
COPD patients often adopt sedentary lifestyles due to breathlessness and, in some, this can precipitate a downward spiral of disease ().Citation52 Anaerobic quadriceps metabolism results in lactate and carbon dioxide production, which stimulates ventilation and worsens breathlessness. Daily activity has been documented to be lower in COPD patients than in healthy controls and lower than recommended international guidelines for physical health maintenance.Citation53 Recently, Watz et alCitation50 demonstrated that physical activity and steps per day were reduced even in those with less severe disease. Individuals with early COPD were predominately (although not significantly) more sedentary than the control group of patients with “chronic bronchitis.”
Several lines of evidence support the important etiological role of physical inactivity in the development of COPD skeletal muscle dysfunction. With advancing airway obstruction severity, physical activity declines and matches the loss of muscle mass observed in COPD patients,Citation54 which correlates with muscle force.Citation55 Abnormalities in the quadriceps muscle in COPD patients are similarly observed in patients with other chronic diseases, such as heart failure,Citation56 suggesting a common etiological factor like physical inactivity and resultant deconditioning. Furthermore, as previously discussed, muscle dysfunction is most marked in the lower limb muscles of locomotion, again supporting the role of disuse. During hospitalization for an exacerbation of COPD, physical inactivity is marked and there is a corresponding reduction in quadriceps strength.Citation57 Exercise interventions, during or shortly after an exacerbation requiring hospitalization, result in significant improvements in quadriceps muscle strength.Citation58,Citation59
Large prospective population-based studies also support the relationships between physical inactivity and important clinical end-points in COPD. Physical activity is protective against hospital admissionsCitation60 and all-cause and respiratory mortalityCitation61 in COPD; furthermore, physical activity can also modify the smoking-related decline in lung function, therefore reducing the risk of COPD in those individuals.Citation62
Other etiological factors
Low-grade systemic inflammation is thought to be reflected by higher levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α); interleukin (IL)-6, -8, -18; and acute-phase proteins in COPD patients. These are postulated to originate either from the peripheral lung or the respiratory muscles. Quadriceps strength has been related to IL-6 and TNF-α in a stable cohort of aged individualsCitation63 and IL-8 in those with COPD during an exacerbation.Citation57 Elevated IL-6 levels are also associated with radiological evidence of quadriceps wasting in COPDCitation64 and reduced lean body mass.Citation65 A similar association was suggested in early studies for TNF-α; however, a possible confounding factor includes changes in assays used to quantify cytokine levels. Citation66 Moreover, quadriceps biopsy findings have not shown increased muscle levels of pro-inflammatory cytokines, including TNF-α, IL-6, IL-8, interferon-gamma, and transforming growth factor-beta, in COPD,Citation67–Citation69 and further work is required to determine the contribution of local inflammation to muscle dysfunction.
Hypoxemia and inflammation are thought to be the up-stream mediators of oxidative stress.Citation70 An increase in reactive oxygen species or reactive nitrogen species, and/or a reduction in antioxidant capacity, leads to local oxidative stress damaging cellular components. This can adversely affect muscle-fiber function via its contractile propertyCitation71 and mitochondria respiration. Furthermore, oxidative stress can alter protein catabolism and anabolism and induce cell death.Citation72 Peroxidation products from reactive oxygen speciesinduced lipid membrane damage can be detected peripherally and have been shown to be elevated in COPD patients at rest and during an exacerbation.Citation73 Studies of antioxidant capacity in COPD are less consistent. Some investigators have reported elevated antioxidant enzymes in patients with severe COPD with muscle wasting,Citation74,Citation75 while others have shown no differences in levels in the quadriceps muscle between COPD patients and controls.Citation72,Citation76,Citation77 Antioxidant enzyme function may be inadequate in the muscles of COPD patients and unable to respond to the increased oxidant stress after exercise.Citation76 However, given that exercise generally improves muscle function in COPD, the observation that whole-body and localized-limb exercise induces increased oxidative stress in COPD patientsCitation76,Citation78 questions whether oxidative stress is indeed pathological or simply a physiological reflection of the muscle repair cycle.
As more than 50% of very severe COPD patients have preserved quadriceps strength,Citation44 studies have sought to demonstrate a genetic predisposition to either loss of muscle mass or muscle resistance to the effects of long-term physical inactivity. The deletion (D) rather than the insertion (I) polymorphic variant of the angiotensin-converting enzyme (ACE) gene is associated with preserved quadriceps strength in COPD.Citation79 This is associated with higher tissue ACE and angiotensin II activity, which may affect muscle growth, and lower bradykinin levels. To establish whether the effects were mediated via increased ACE-related D allele kinin degradation, bradykinin receptor polymorphisms were later studied.Citation80 The +9/+9 (base pair repeat present) receptor polymorphism, which is associated with reduced gene transcription and lower mRNA, was more prevalent in COPD patients with low fat-free mass (FFM) index. However, this did not explain the previously identified ACE gene findings, as there was no interaction between the two genotypes on strength.Citation80 Polymorphisms of the vitamin D receptor are associated with reduced (FokI polymorphism) or greater quadriceps muscle strength (BsmI polymorphism) in COPD patients.Citation81 However, this association was not seen in healthy controls, suggesting a gene–environment interaction.
Cachexia-associated polymorphisms of inflammatory cytokines such as TNF-α and IL-6 remain to be discovered. A -511 polymorphism of the IL-1β gene (the CC variant) has been shown to be associated with cachexiaCitation82 but functional implications are unknown. Cachexia or loss of muscle mass is well described in COPD, even in the presence of retained weight and fat mass.Citation83 Whether this process can be attributed directly to nutritional insufficiency or is secondary to a systemic inflammatory process remains unclear. Certainly, nutritional supplementation alone does not appear to improve measurements of FFM, lung function, or exercise capacity.Citation84 However, in combination with other anabolic stimuli, it can maintain or improve muscle mass but with undetermined effects on function.Citation85
Imbalance between anabolic and catabolic hormones has also been suggested as contributing to muscle dysfunction in COPD. Reduced circulating anabolic hormones such as testosterone and insulin-like growth factor-1 (IGF-1) have been reported in COPD patients.Citation64 Trials of testosterone have shown variable results; some have not been able to demonstrate an improvement in exercise capacity,Citation86,Citation87 but others report an increase in muscle mass and strength with a combination of testosterone and resistance training in male individuals with low baseline testosterone levels.Citation88 Insulin resistance and changes in glucose metabolism have also been found in COPD patients, but this remains poorly studied in the COPD population therefore the relationship with skeletal muscle dysfunction remains inconclusive.Citation89 Similarly, although the effect of long-term, low-dose systemic corticosteroids on the proximal muscles is well described,Citation90 short-term use of higher doses (prednisolone 30 mg for 2 weeks) does not cause significant skeletal muscle dysfunction nor alter metabolic parameters during exercise.Citation91
Potential molecular mechanisms and pathways
Cardinal molecular features of quadriceps muscle dysfunction include muscle-fiber atrophy and muscle-fiber shift. Muscle atrophy results in loss of muscle mass and can result from an imbalance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB), and/or individual fiber loss and gain. Muscle-fiber shift has been less extensively studied, but the loss of aerobic type I fibers classically results in a decrease in oxidative capacity, a reduction in mitochondria, and reduced muscle endurance. It is unclear at present whether these processes occur independently or are linked.
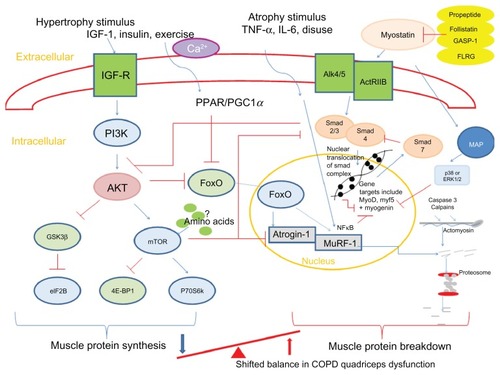
Animal models have identified key mediators in atrophy and hypertrophy signaling and therefore the control of muscle growth.Citation92 These are summarized in . The anabolic hormone and growth factor IGF-1 stimulates the phosphoinositide 3-kinase/Akt (protein kinase B) pathway in skeletal muscle cells. Upon phosphorylation, Akt is able to activate mammalian target of rapamycin (mTOR), which, in turn, activates 70-kD ribosomal S6 protein kinaseCitation93 and inhibits eukaryotic translation initiation factor 4E binding protein-1/PHAS-1;Citation94 these processes stimulate MPS. Another downstream regulator of anabolism, which is mTOR independent, is the phosphorylation of glycogen synthase kinase-3β (GSK-3β) by phosphorylated Akt.Citation95 This leads to the release of eukaryotic initiation factor 2B (eIF2B) which upregulates MPS. Phosphorylated Akt also plays a role in MPB pathways. It downregulates two muscle-specific E3 ligases, atrogin-1 (muscle atrophy F-box or MAFbx), and muscle-specific RING finger protein (MuRF)-1 via inactivation of the forkhead box class O (FoxO) family of transcription factors.Citation96 The muscle-specific ubiquitin ligases contribute to protein degradation via the ubiquitin-proteasome pathway.Citation97
Figure 2 Summary of pathways controlling muscle protein synthesis (MPS) and muscle protein breakdown (MPB). The role of myostatin in MPS and MPB has also been included. Myostatin is held in an inactive state by its pro-peptide, follistatin, and inhibitory binding proteins – growth and differentiation factor-associated serum protein-1 (GASP-1) and follistatin-like related gene, (FLRG) as shown. Upon activation, it binds to its transmembrane receptor activin receptor type IIB (ActRIIB), which then forms homodimers with activin receptor-like kinase 4 or 5 (Alk 4/5). The SMAD signaling pathway is then activated and translocation of this transcription factor complex to the nucleus occurs, where MyoD production and therefore myoblast proliferation and fusion are blocked. Myostatin is also proposed to increase proteosomal activity in a FoxO-dependent manner. Activation of MAP kinase is mediated via myostatin either via p38 or ERK1/2, which leads to the blocking of genes involved in myogenesis.
Abbreviations: 4E-BP1, eukaryotic translation initiation factor 4E binding protein-1; Akt, protein kinase B; ERK, extracellular signal-regulated kinase; eIF2B, eukaryotic initiation factor 2B; FoxO, forkhead box class O; GSK-3β, glycogen synthase kinase-3β; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; MAP, mitogen-activated protein; mTOR, mammalian target of rapamycin; MuRF, muscle-specific RING finger protein; p70S6k, 70-kD ribosomal S6 protein kinase; PGC1α, peroxisome proliferator-activated receptor gamma co-activator 1-alpha; PI3K, phosphoinositide 3-kinase; PPAR, peroxisome proliferator-activated receptor; SMAD,; TNF-α, tumor necrosis factor-alpha; GASP-1, growth and differentiation factor-associated serum protein-1; IGF-R, insulin-like growth factor-1 receptor; Ca2+, calcium ion; NFκB, nuclear factor κB.
Ubiquitin-mediated protein degradation may play an important role in COPD skeletal muscle dysfunction. Two small studies found elevated atrogin-1Citation98,Citation99 and MuRF-1Citation98 in quadriceps from COPD patients, but, in one of these, it was alongside elevated hypertrophy signaling. Although larger studies are required to confirm these observations, this may suggest COPD patients actually fail to restore muscle mass and therefore have some form of synthetic resistance. Indeed, tracer studies from immobilized individuals would support this theory of resistance to MPS to protein nutrition (termed “anabolic resistance”).Citation100 MPS is greatly suppressed in the immobilized post-absorptive state,Citation101 while the contribution from MPB is minimal. Therefore, the relative contribution of MPB in the process of disuse-induced muscle atrophy remains under question.Citation102
Myostatin, or growth differentiation factor-8, is a member of the transforming growth factor-β (TGF-β) super-family and is a potent negative regulator of muscle mass, as demonstrated by naturally occurring mutations occurring in mice, cattle, and humans.Citation103–Citation105 Myostatin can influence muscle wasting by affecting the number and size of muscle cells and by inducing muscle atrophy pathways. Myostatin upregulates p21 (cyclin-dependent kinase inhibitor), which negatively affects activated satellite cell/myoblast proliferation,Citation106 and downregulates myogenic differentiation factors (MyoD, myf5, and myogenin), which inhibit myoblast differentiation.Citation107 It can also exert an effect on muscle catabolism by activating the ubiquitin proteolytic system in a FoxO1-dependent mannerCitation108 and may possibly inactivate Akt, affecting MPS.Citation108 Finally, myostatin has also been shown to inhibit satellite cell activation and self-renewal in a Pax7-dependent way.Citation109
Several lines of evidence implicate a role for myostatin in COPD quadriceps dysfunction. Myostatin mRNA was elevated in weak COPD patients.Citation99 Following an in-patient resistance training program, hospitalized COPD patients had reduced myostatin transcripts, with a trend toward an increase in MyoD and myogenin.Citation59 Following exercise training, non- cachectic COPD patients demonstrated a reduction in myostatin protein, with a reduction in MuRF-1 and atrogin-1,Citation110 while a modest reduction in myostatin was found following resistance training with or without testosterone.Citation111 Our group have also demonstrated a negative association between quadriceps muscle myostatin mRNA expression and quadriceps muscle strength in COPD patients.Citation112
Animal studies have shown that skeletal muscle-fiber phenotype appears to be regulated by several independent signaling pathways. Fiber type switching in mice can be induced by changes in nerve activity from differing electrical stimulations,Citation113 enabling the study of pathways that affect myosin gene expression and metabolic profiles.
Myofiber gene activation occurs via calcium signaling through calcineurin (Cn) and various kinases – for example, Ca2+/calmodulin-dependent protein kinases II. Cn is a calcium/calmodulin-regulated protein phosphatase that acts on transcription factors of the nuclear factor of activated T cells (NFAT) family. Ca2+/calmodulin-dependent protein kinases II regulate myocyte enhancer factor 2, via histone deacetylase (HDAC), and has been suggested to interact with NFAT.Citation114 Studies of transgenic miceCitation115 and those treated with Cn inhibitorCitation116 have implicated Cn signaling in activity-dependent maintenance of the slow gene program.Citation113
Cn-NFAT signaling may also upregulate the transcription factor peroxisome proliferator-activated receptor (PPAR)-β/-γ and the transcriptional co-activator PPAR-γ (PGC-1α),Citation117 both of which have been implicated in the muscle dysfunction of COPD patients. PPAR signaling can affect oxidative signaling and fiber type composition,Citation118 in addition to having inflammatory properties via effects on the nuclear factor kappa-light-chain-enhancer of activated B cells pathway.Citation119 The three isoforms of PPARS (α, β/δ, and γ) are all present in skeletal muscle. PPAR-δ regulates fatty acid utilization and energy homeostasisCitation120 and higher levels are expressed in type I muscle fibersCitation121 and can be induced by acute exercise in healthy young men.Citation122 A transgenic “marathon mouse” model, in which PPAR-δ expression is increased, shows fiber shift opposite to that seen in COPD.Citation121 PGC-1α is a co-activator of PPAR-δ that can interact with transcription factors and basal transcriptional machineryCitation123 and can stimulate mitochondrial and oxidative enzymes.Citation113 In one small study of 14 COPD patients and nine control subjects, PPAR-α and -δ protein levels and PGC-1α mRNA were significantly lower in the quadriceps of moderate/severe COPD patients than in controls with a similar smoking historyCitation124 and PPAR-α mRNA expression was lower still in cachectic patients. These findings suggest that PPAR-γ or -α content and/or function may in some way be involved in the change in oxidative gene program and mitochondrial dysfunction.Citation125 However, as type I fibers have higher expression of PPARs, these results may simply represent an association rather causation.
The mitogen-activated protein kinase (MAPK) pathway may have an influence on fiber shift via extracellular signal-regulated kinase (ERK) signaling. In muscle cell lines, a type I phenotype is induced when the ERK pathway is inhibited and a shift toward type I/IIa from IIx myosin heavy chain (MHC) results from MAPK phosphatase-1.Citation126 Recent data from COPD patients have been conflicting. Lemire and colleagues showed elevated ratios of phosphorylated to total level of p38 MAPK and ERK 1/2 in the quadriceps muscle compared with controls.Citation127 These ratios were negatively associated with mid-thigh muscle CSA, supporting the hypothesis that MAPK may contribute to the development of skeletal muscle dysfunction in COPD.Citation127 In contrast, data from a much larger cross-sectional study failed to show a role for p38 MAPK signaling.Citation128
Emerging data suggest that microRNAs (miRNAs), small polynucleotides that can decrease mRNA translation or directly destabilize mRNA, may also be implicated in the control of skeletal muscle phenotype.Citation129 Muscle-specific miRNAs include those which affect myocyte proliferation and differentiation, for example, miR-1 and miR-206,Citation130 and others, such as miR-208b and miR-499,Citation130,Citation131 that modulate the expression of slow MHC genes through regulation of transcriptional repressors.Citation132,Citation133 In COPD, we have recently shown that the miRNA profile of the quadriceps muscle in COPD patients differs from that of controls, with a downregulation in the myocardin-related transcription-serum response factor axis and reduced expression of muscle-specific miRNAs, particularly miR-1.Citation134 Reduction in miR-1 has been reported in other models of inactivity resulting from denervation, nerve entrapment, or space flight and targets include myostatinCitation135 and IGF-1.Citation136 We found IGF-1 was elevated in the COPD group consistent with previous reports of the overexpression of muscle hypertrophy pathways.Citation98 MiR-1 may also contribute toward reduction in MHC I and fiber shift, via an increase of HDAC4. HDAC4 inhibits serum response factor, an important regulator of MHC1 expression, and the expression of follistatin,Citation137 which may activate the myostatin pathway.
Non-pharmacological treatments for muscle dysfunction in COPD
Exercise training remains the only known intervention to reverse some of the underlying skeletal muscle abnormalities seen in COPD, further supporting the notion that reduced daily physical activity is the major etiological factor. Exercise training, in the form of pulmonary rehabilitation (PR), has emerged as the most effective non-pharmacological intervention in improving exercise capacity, dyspnea, and health status in COPD patients, as evidenced by numerous randomized controlled trials and meta-analyses.Citation138 Given that PR does not directly improve lung mechanics or gas exchange,Citation139 it is likely that the main area of improvement with exercise lies in the skeletal muscle. Dysfunction of the locomotor muscles may limit exercise performance because of leg discomfort,Citation13 but also because early anaerobic metabolism leads to lactic acid production. Lactic acid, buffered by bicarbonate, causes production of carbon dioxide and an increased ventilatory stimulus. As expiratory flow limitation is commonly present in COPD, increased ventilation can exacerbate dynamic hyperinflation and promote premature exercise termination and dyspnea.Citation140
Quadriceps strength, endurance, and fatigability all improve significantly following exercise training.Citation58,Citation141,Citation142 Even in the acute setting, resistance training during an exacerbation can prevent muscle function deterioration,Citation59 while PR shortly following hospital discharge can significantly accelerate recovery of quadriceps muscle strength.Citation58 Debate remains as to the most effective mode of exercise to induce not only different skeletal muscle adaptations but also long-term improvements in clinically relevant health outcomes. Typically, chronic endurance training enhances the fatigue resistance of skeletal muscle by promoting a muscle-fiber type shift from fast-twitch fatigable type II fibers to slow-twitch fatigue-resistant type I fibers, increasing mitochondrial content and activity and improving skeletal muscle glucose transportation. However, resistance training reduces sarcopenia and promotes hypertrophy of muscle fibers, especially of type IIx.Citation143
Intensity of exercise training is an important determinant of the physiological training effect.Citation144 However, in patients with severe COPD, intolerable sensations of breathlessness may prevent sufficiently long periods of high-intensity training levels.Citation145 Strategies to augment exercise tolerance by reducing dyspnea sensation or ventilatory limitation have included noninvasive mechanical ventilation,Citation146 oxygen,Citation147 and/or heliox supplementation,Citation148 all of which have been demonstrated to increase exercise tolerance in the laboratory setting. However, these are rarely systematically used as part of clinical PR programs. An alternative approach, which may be particularly suitable for patients with more severe COPD, is interval training, which allows patients to complete short periods of high-intensity exercise not possible with classical aerobic exercise training.Citation149
Although the emphasis has so far been on the muscles of the lower limbs, there have been studies examining the effects of training the upper limbs or the respiratory muscles in COPD. A systematic review of upper-limb exercise-training studies in COPD showed improvements in arm exercise capacity, but the effects on symptoms, overall exercise capacity, and health-related quality of life were inconsistent. Citation150 Similarly, debate continues with regard to the role of inspiratory muscle training in the context of PR. Although most studies have demonstrated a positive effect on voluntary inspiratory muscle strength,Citation151 it remains unclear whether this is as a result of a genuine physiological improvement in the inspiratory muscles or a learning effect in performing the voluntary maneuver. Furthermore, the added benefit of inspiratory muscle training over a general exercise-training program seems relatively limited.Citation151
In patients unable or unwilling to adhere to existing forms of exercise, neuromuscular electrical stimulation (NMES) may offer an alternative way of enhancing leg muscle strength.Citation152 NMES uses a battery-powered stimulator unit to produce a controlled contraction of the muscles via skin electrodes. A typical program consists of 30–60 minutes of quadriceps stimulation, 3–5 times weekly for 4–6 weeks. NMES can lead to improvements in muscle strength and exercise performance, with pooled data revealing mean between-group differences in peak quadriceps torque and 6-minute walking distance of 9.7 Nm (95% CI 1.2, 18.1) and 48 m (95% CI 9, 86), respectively.Citation153 Recent studies have also demonstrated favorable changes in markers of anabolism/catabolismCitation154 and the quadriceps fiber type profile following NMES.Citation155 However, studies remain small, follow-up data are lacking, and the patient phenotypes most likely to benefit have yet to be identified.
Pharmacological treatments for muscle dysfunction in COPD
Despite the many benefits of PR, there are limitations. Firstly, exercise training does not fully reverse all of the abnormalities observed in the quadriceps muscle. Secondly, a proportion of patients either has limited accessibility to PR or has issues with uptake and completion. Thirdly, improvements following PR decline toward baseline level within 12–18 months.Citation156 Hence, there is interest in pharmacologically augmenting (or even replacing) exercise training to bring about structural and functional improvements in the skeletal muscles. However, as well as the technical problems involved in creating a drug that specifically benefits the muscles, there are regulatory hurdles to be overcome before such a compound can reach the market.Citation157
As previously discussed, systemic inflammation, oxidative stress, and anabolic/catabolic hormone imbalance have been postulated as etiological factors for muscle dysfunction in COPD. An early trial of infliximab, an anti-TNF-α therapy, found that it was largely ineffective in improving lung function, exercise capacity, or health-related quality of life,Citation158 although there did appear to be a trend for benefit in cachectic patients. Furthermore, antioxidant therapy with N-acetylcysteine led to a 25% increase in quadriceps endurance compared with placebo.Citation70 However, this was a very small study of nine COPD patients in a controlled laboratory setting. Studies of anabolic hormones have had mixed results. Anabolic steroids increase body weight and FFM in COPD, either aloneCitation159 or in conjunction with exercise training,Citation87 but not muscle strength or exercise capacity. However, the addition of testosterone to resistance training in hypogonadal COPD patients promotes anabolic pathways that can result in improved quadriceps strength and endurance.Citation88 Recombinant growth hormone (GH) improves FFM compared with placebo but does not improve muscle strength or exercise capacity.Citation86 Ghrelin is a novel GH-releasing peptide that induces a positive energy balance by decreasing fat utility and stimulating feeding through GH-independent mechanisms. In a small open-label study, ghrelin increased FFM, muscle strength, and 6-minute walk distance in cachectic COPD patients.Citation160 Systemic side effects with hormonal drugs are a concern, hence there is current interest in the development of anabolic drugs without the unwanted side effects. An example is the selective androgen-receptor modulator class of drugs that have the benefits of anabolic/androgenic steroids with a hypothetically reduced risk of prostate cancer in men and virilizing effects in women.Citation161
Another therapeutic approach is to use existing drugs for new indications. As previously discussed, common variations in the gene for the vitamin D receptorCitation81 and deletion of the allele of the ACECitation79 have been demonstrated to influence muscle strength. Vitamin D supplementation or the administration of ACE inhibitors may have a future role in treating muscle dysfunction in COPD, or at least augmenting the benefits of exercise training. Certainly, this approach has been used with positive results in elderly people with functional impairment. Citation162 Similarly, levosimendan, a calcium sensitizer used as a cardiac inotrope, has recently been shown to improve neuro-mechanical efficiency and contractile function of the human diaphragm in healthy subjects,Citation163 and conceivably it may also improve skeletal muscle dysfunction in COPD. However, with increasing understanding of the underlying molecular mechanisms, there is also hope that a number of novel pharmacological agents that address cachexia and skeletal muscle dysfunction in COPD will become available for clinical use. Already, prototype inhibitors of ubiquitin ligases and neutralizing antibodies to myostatin have been developed for cancer-related cachexia and muscle dystrophies. Citation164 PPAR-δ agonists have recently been shown to mimic and enhance exercise training and AICAR, an 5′ adenosine monophosphate-activated-kinase agonist, was sufficient to improve exercise endurance in mice alone.Citation165 Thus, there is much future promise that novel therapeutic agents will become available to address important extra-pulmonary manifestations of COPD such as skeletal muscle dysfunction.
Future directions
A greater understanding of the etiology and basic mechanisms of skeletal muscle dysfunction should continue to underpin developments in the field, informing the identification and testing of new pharmacological agents and strategies to augment or optimize PR across community and in-patient settings. Phenotyping of patients according to skeletal muscle dysfunction will also enhance the identification of those most likely to response to specific treatments, thus should be embraced in clinical trial design and practice, where possible.
Acknowledgments
This work is funded in part by the National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit, Royal Brompton and Harefield Foundation Trust and Imperial College. AVD and MIP are either fully or partly supported by the Biomedical Research Unit. MM is funded by an NIHR postdoctoral fellowship. WD-CM is supported by an NIHR Clinician Scientist Award and a Medical Research Council New Investigator Award. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, nor the Department of Health.
Disclosure
The authors declare no conflicts of interest in this work.
References
- LopezADMurrayCCThe global burden of disease, 1990–2020Nat Med1998411124112439809543
- ManWDKempPMoxhamJPolkeyMISkeletal muscle dysfunction in COPD: clinical and laboratory observationsClin Sci (Lond)2009117725126419681758
- GosselinkRTroostersTDecramerMPeripheral muscle weakness contributes to exercise limitation in COPDAm J Respir Crit Care Med199615339769808630582
- SimpsonKKillianKMcCartneyNStubbingDGJonesNLRandomised controlled trial of weightlifting exercise in patients with chronic airflow limitationThorax199247270751549826
- DecramerMGosselinkRTroostersTVerschuerenMEversGMuscle weakness is related to utilization of health care resources in COPD patientsEur Respir J19971024174239042643
- SwallowEBReyesDHopkinsonNSQuadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary diseaseThorax200762211512017090575
- ManWMustfaNNikoletouDEffect of salmeterol on respiratory muscle activity during exercise in poorly reversible COPDThorax200459647147615170026
- Gray-DonaldKGibbonsLShapiroSHMacklemPTMartinJGNutritional status and mortality in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199615339619668630580
- BernardSLeBlancPWhittomFPeripheral muscle weakness in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199815826296349700144
- ManWDHopkinsonNSHarrafFNikoletouDPolkeyMIMoxhamJAbdominal muscle and quadriceps strength in chronic obstructive pulmonary diseaseThorax200560971872215923239
- ManWSolimanMNikoletouDNon-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary diseaseThorax200358866566912885979
- AllaireJMaltaisFDoyonJFPeripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPDThorax200459867367815282387
- ManWDSolimanMGGearingJSymptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003168556256712829456
- MadorMJDenizOAggarwalAKufelTJQuadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003168110210812689846
- CoronellCOrozco-LeviMMendezRRamirez-SarmientoAGáldizJBGeaJRelevance of assessing quadriceps endurance in patients with COPDEur Respir J200424112913615293615
- MathurSTakaiKPMacintyreDLReidDEstimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary diseasePhys Ther200888221923018056754
- SeymourJMWardKSidhuPSUltrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPDThorax200964541842319158125
- MarquisKDebigaréRLacasseYMidthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2002166680981312231489
- GoskerHREngelenMPvan MamerenHMuscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary diseaseAm J Clin Nutr200276111311912081824
- GoskerHRZeegersMPWoutersEFScholsAMMuscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysisThorax2007621194494917526675
- WhittomFJobinJSimardPMHistochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary diseaseMed Sci Sports Exerc19983010146714749789845
- JobinJMaltaisFDoyonJFChronic obstructive pulmonary disease: capillarity and fiber type characteristics of skeletal muscleJ Cardiopulm Rehabil19981864324379857275
- MaltaisFSimardAASimardCJobinJDesgagnésPLeBlancPOxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPDAm J Respir Crit Care Med199615312882938542131
- JakobssonPJorfeldtLHenrikssonJMetabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19951512 Pt 13743777842194
- KutsuzawaTShioyaSKuritaDHaidaMOhtaYYamabayashiHMuscle energy metabolism and nutritional status in patients with chronic obstructive pulmonary disease. A 31P magnetic resonance studyAm J Respir Crit Care Med199515226476527633721
- MaltaisFJobinJSullivanMJMetabolic and hemodynamic responses of lower limb during exercise in patients with COPDJ Appl Physiol1998845157315809572801
- SwallowEBGoskerHRWardKAA novel technique for nonvolitional assessment of quadriceps muscle endurance in humansJ Appl Physiol2007103373974617569771
- NewellSZMcKenzieDKGandeviaSCInspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitationThorax198944119039122595630
- GeaJGPastoMCarmonaMAOrozco-LeviMPalomequeJBroquetasJMetabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary diseaseEur Respir J200117593994511488330
- SatoYAsohTHondaYFujimatsuYHiguchiIOizumiKMorphologic and histochemical evaluation of muscle in patients with chronic pulmonary emphysema manifesting generalized emaciationEur Neurol19973721161219058068
- SimilowskiTYanSGauthierAPMacklemPTBellemareFContractile properties of the human diaphragm during chronic hyperinflationN Engl J Med1991325139179231881417
- LevineSKaiserLLeferovichJTikunovBCellular adaptations in the diaphragm in chronic obstructive pulmonary diseaseN Engl J Med199733725179918069400036
- MercadierJJSchwartzKSchiaffinoSMyosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflationAm J Physiol19982744 Pt 1L5275349575870
- DoucetMDebigaréRJoanisseDRAdaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPDEur Respir J200424697197915572541
- OttenheijmCAHeunksLMSieckGCDiaphragm dysfunction in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005172220020515849324
- StubbingsAKMooreAJDusmetMPhysiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary diseaseJ Physiol2008586Pt 102637265018372305
- LevineSNguyenTKaiserLRHuman diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implicationsAm J Respir Crit Care Med2003168670671312857719
- Orozco-LeviMGeaJLloretaJLSubcellular adaptation of the human diaphragm in chronic obstructive pulmonary diseaseEur Respir J199913237137810065684
- LevineSGregoryCNguyenTBioenergetic adaptation of individual human diaphragmatic myofibers to severe COPDJ Appl Physiol20029231205121311842060
- PolkeyMIKyroussisDHamnegardCHDiaphragm performance during maximal voluntary ventilation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199715526426489032207
- PolkeyMIKyroussisDKeiltySEExhaustive treadmill exercise does not reduce twitch transdiaphragmatic pressure in patients with COPDAm J Respir Crit Care Med199515239599647663810
- LevineSNguyenTFrisciaMParasternal intercostal muscle remodeling in severe chronic obstructive pulmonary diseaseJ Appl Physiol200610151297130216777998
- GeaJOrozco-LeviMAguarMCAdaptive changes concerning the types of fibres and isoforms of myosin in the external intercostal muscle of COPD patientsEur Respir J19969160S
- SeymourJMSpruitMAHopkinsonNSThe prevalence of quadriceps weakness in COPD and the relationship with disease severityEur Respir J2010361818819897554
- SerresIGautierVVarrayAPréfautCImpaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patientsChest199811349009059554623
- GoskerHRKubatBSchaartGvan der VusseGJWoutersEFScholsAMMyopathological features in skeletal muscle of patients with chronic obstructive pulmonary diseaseEur Respir J200322228028512952261
- DegensHSanchez HornerosJMHeijdraYFDekhuijzenPNHopmanMTSkeletal muscle contractility is preserved in COPD patients with normal fat-free massActa Physiol Scand2005184323524215954991
- OttenheijmCAHeunksLMDekhuijzenRPDiaphragm adaptations in patients with COPDRespir Res2008911218218129
- van den BorstBKosterAYuBIs age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking?Thorax2011661196196921724748
- WatzHWaschkiBMeyerTMagnussenHPhysical activity in patients with COPDEur Respir J200933226227219010994
- KonSSManWDMuscle mass and strength in obstructive lung disease: a smoking gun?Thorax2011661193393521813620
- PolkeyMIMoxhamJAttacking the disease spiral in chronic obstructive pulmonary diseaseClin Med20066219019616688981
- BossenbroekLde GreefMHWempeJBKrijnenWPTen HackenNHDaily physical activity in patients with chronic obstructive pulmonary disease: a systematic reviewCOPD20118430631921728804
- DecramerMRennardSTroostersTCOPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early interventionCOPD20085423525618671149
- PittaFTroostersTSpruitMAProbstVSDecramerMGosselinkRCharacteristics of physical activities in daily life in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171997297715665324
- GoskerHRLencerNHFranssenFMvan der VusseGJWoutersEFScholsAMStriking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPDChest200312351416142412740256
- SpruitMGosselinkRTroostersTMuscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-IThorax200358975275612947130
- SeymourJMMooreLJolleyCJOutpatient pulmonary rehabilitation following acute exacerbations of COPDThorax201065542342820435864
- TroostersTProbstVSCrulTResistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2010181101072107720133927
- Garcia-AymerichJFarreroEFélezMAIzquierdoJMarradesRMAntóJMEstudi del Factors de Risc d’Agudització de la MPOC investigatorsRisk factors of readmission to hospital for a COPD exacerbation: a prospective studyThorax200358210010512554887
- Garcia-AymerichJLangePBenetMSchnohrPAntoJMRegular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort studyThorax200661977277816738033
- Garcia-AymerichJLangePBenetMSchnohrPAntóJMRegular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort studyAm J Respir Crit Care Med2007175545846317158282
- YendeSWatererGWTolleyEAInflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjectsThorax2006611101616284220
- DebigaréRMarquisKCôtéCHCatabolic/anabolic balance and muscle wasting in patients with COPDChest20031241838912853506
- EidAAIonescuAANixonLSInflammatory response and body composition in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20011648 Pt 11414141811704588
- WagnerPDPossible mechanisms underlying the development of cachexia in COPDEur Respir J200831349250118310396
- BarreiroEScholsAMPolkeyMIENIGMA in COPD projectCytokine profile in quadriceps muscles of patients with severe COPDThorax200863210010717875568
- PetersenAMPenkowaMIversenMElevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary diseaseLung2007185316117117436040
- CrulTSpruitMAGayan-RamirezGMarkers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patientsEur J Clin Invest2007371189790417883420
- KoechlinCCouillardASimarDDoes oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease?Am J Respir Crit Care Med200416991022102715001462
- ReidMBNitric oxide, reactive oxygen species, and skeletal muscle contractionMed Sci Sports Exerc200133337137611252061
- BarreiroEde la PuenteBMinguellaJOxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005171101116112415735057
- SupinskiGSCallahanLAFree radical-mediated skeletal muscle dysfunction in inflammatory conditionsJ Appl Physiol200710252056206317218425
- BarreiroERabinovichRMarin-CorralJBarberàJAGeaJRocaJChronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPDThorax2009641131918835959
- GoskerHRBastAHaenenGRAltered antioxidant status in peripheral skeletal muscle of patients with COPDRespir Med200599111812515672860
- CouillardAMaltaisFSaeyDExercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167121664166912672647
- BarreiroEGeaJCorominasJMHussainSNNitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol200329677177812816735
- CouillardAKoechlinCCristolJPVarrayAPrefautCEvidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patientsEur Respir J20022051123112912449164
- HopkinsonNSNickolAHPayneJAngiotensin converting enzyme genotype and strength in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170439539915117739
- HopkinsonNSEleftheriouKIPayneJ+9/+9 Homozygosity of the bradykinin receptor gene polymorphism is associated with reduced fat-free mass in chronic obstructive pulmonary diseaseAm J Clin Nutr200683491291716600946
- HopkinsonNSLiKWKehoeAVitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary diseaseAm J Clin Nutr200887238539018258629
- BroekhuizenRGrimbleRFHowellWMPulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta-511 single nucleotide polymorphismAm J Clin Nutr20058251059106416280439
- EngelenMPScholsAMBakenWCWesselingGJWoutersEFNutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPDEur Respir J1994710179317977828687
- FerreiraIMBrooksDLacasseYGoldsteinRSNutritional support for individuals with COPD: a meta-analysisChest2000117367267810712990
- Op den KampCMLangenRCHaegensAScholsAMMuscle atrophy in cachexia: can dietary protein tip the balance?Curr Opin Clin Nutr Metab Care200912661161619741519
- BurdetLde MuraltBSchutzYPichardCFittingJWAdministration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled studyAm J Respir Crit Care Med19971566180018069412558
- CreutzbergECWoutersEFMostertRPluymersRJScholsAMA role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trialChest200312451733174214605042
- CasaburiRBhasinSCosentinoLEffects of testosterone and resistance training in men with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2004170887087815271690
- ScholsAMPulmonary cachexiaInt J Cardiol200285110111012163214
- DecramerMLacquetLMFagardRRogiersPCorticosteroids contribute to muscle weakness in chronic airflow obstructionAm J Respir Crit Care Med1994150111168025735
- HopkinsonNSManWDDayerMJAcute effect of oral steroids on muscle function in chronic obstructive pulmonary diseaseEur Respir J200424113714215293616
- NaderGAMolecular determinants of skeletal muscle mass: getting the “AKT” togetherInt J Biochem Cell Biol200537101985199616125108
- RommelCBodineSCClarkeBAMediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathwaysNat Cell Biol20013111009101311715022
- HaraKYonezawaKKozlowskiMTRegulation of eIF-4E BP1 phosphorylation by mTORJ Biol Chem19972724226457264639334222
- CrossDAAlessiDRCohenPAndjelkovichMHemmingsBAInhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase BNature199537865597857898524413
- SandriMSandriCGilbertAFoxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophyCell2004117339941215109499
- BodineSCLatresEBaumhueterSIdentification of ubiquitin ligases required for skeletal muscle atrophyScience200129455471704170811679633
- DoucetMRussellAPLégerBMuscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007176326126917478621
- PlantPJBrooksDFaughnanMCellular markers of muscle atrophy in chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol201042446147119520920
- RennieMJAnabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnoverAppl Physiol Nutr Metab200934337738119448702
- de BoerMDSelbyAAthertonPThe temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuseJ Physiol2007585Pt 124125117901116
- MarimuthuKMurtonAJGreenhaffPLMechanisms regulating muscle mass during disuse atrophy and rehabilitation in humansJ Appl Physiol2011110255556021030670
- McPherronACLawlerAMLeeSJRegulation of skeletal muscle mass in mice by a new TGF-beta superfamily memberNature1997387662883909139826
- McPherronACLeeSJDouble muscling in cattle due to mutations in the myostatin geneProc Natl Acad Sci U S A1997942312457124619356471
- SchuelkeMWagnerKRStolzLEMyostatin mutation associated with gross muscle hypertrophy in a childN Engl J Med2004350262682268815215484
- ThomasMLangleyBBerryCMyostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferationJ Biol Chem200027551402354024310976104
- LangleyBThomasMBishopASharmaMGilmourSKambadurRMyostatin inhibits myoblast differentiation by down-regulating MyoD expressionJ Biol Chem200227751498314984012244043
- McFarlaneCPlummerEThomasMMyostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB- independent, FoxO1-dependent mechanismJ Cell Physiol2006209250151416883577
- McFarlaneCHennebryAThomasMMyostatin signals through Pax7 to regulate satellite cell self-renewalExp Cell Res2008314231732917949710
- VogiatzisISimoesDCStratakosGEffect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPDEur Respir J201036230131020110400
- LewisMIFournierMStorerTWSkeletal muscle adaptations to testosterone and resistance training in men with COPDJ Appl Physiol200710341299131017673568
- D-C ManWNatanekSARiddoch-ContrerasJQuadriceps myostatin expression in COPDEur Respir J201036368668820930205
- SchiaffinoSSandriMMurgiaMActivity-dependent signaling pathways controlling muscle diversity and plasticityPhysiology (Bethesda)20072226927817699880
- WuHNayaFJMcKinseyTAMEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber typeEMBO J20001991963197310790363
- NayaFJMercerBSheltonJRichardsonJAWilliamsRSOlsonENStimulation of slow skeletal muscle fiber gene expression by calcineurin in vivoJ Biol Chem200027574545454810671477
- ChinEROlsonENRichardsonJAA calcineurin-dependent transcriptional pathway controls skeletal muscle fiber typeGenes Dev19981216249925099716403
- LongYCGlundSGarcia-RovesPMZierathJRCalcineurin regulates skeletal muscle metabolism via coordinated changes in gene expressionJ Biol Chem200728231607161417107952
- LuquetSLopez-SorianoJHolstDPeroxisome proliferator-activated receptor delta controls muscle development and oxidative capabilityFASEB J200317152299230114525942
- BeckerJDelayre-OrthezCFrossardNPonsFRegulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases?Fundam Clin Pharmacol200620542944716968414
- LuquetSGaudelCHolstDRoles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetesBiochim Biophys Acta20051740231331715949697
- WangYXZhangCLYuRTRegulation of muscle fiber type and running endurance by PPARdeltaPLoS Biol2004210e29415328533
- MahoneyDJPariseGMelovSSafdarATarnopolskyMAAnalysis of global mRNA expression in human skeletal muscle during recovery from endurance exerciseFASEB J200519111498150015985525
- PuigserverPSpiegelmanBMPeroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulatorEndocr Rev2003241789012588810
- RemelsAHSchrauwenPBroekhuizenRPeroxisome proliferator- activated receptor expression is reduced in skeletal muscle in COPDEur Respir J200730224525217459894
- RemelsAHGoskerHRSchrauwenPLangenRCScholsAMPeroxisome proliferator-activated receptors: a therapeutic target in COPD?Eur Respir J200831350250818310397
- ShiHSchefflerJMPleitnerJMModulation of skeletal muscle fiber type by mitogen-activated protein kinase signalingFASEB J20082282990300018417546
- LemireBBDebigareRDubéAThériaultMECoteCHMaltaisFMAPK signalling in the quadriceps of patients with chronic obstructive pulmonary diseaseJ Appl Physiol2012Epub April19
- Riddoch-ContrerasJGeorgeTNatanekSAp38 mitogen-activated protein kinase is not activated in the quadriceps of patients with stable chronic obstructive pulmonary diseaseCOPD20129214215022409248
- WilliamsAHLiuNvan RooijEOlsonENMicroRNA control of muscle development and diseaseCurr Opin Cell Biol200921346146919278845
- ChenJFMandelEMThomsonJMThe role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiationNat Genet200638222823316380711
- GüllerIRussellAPMicroRNAs in skeletal muscle: their role and regulation in development, disease and functionJ Physiol2010588Pt 214075408720724363
- van RooijEQuiatDJohnsonBAA family of microRNAs encoded by myosin genes governs myosin expression and muscle performanceDev Cell200917566267319922871
- McCarthyJJEsserKAPetersonCADupont-VersteegdenEEEvidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophyPhysiol Genomics200939321922619690046
- LewisARiddoch-ContrerasJNatanekSADownregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPDThorax2012671263421998125
- ClopAMarcqFTakedaHA mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheepNat Genet200638781381816751773
- EliaLContuRQuintavalleMReciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditionsCirculation2009120232377238519933931
- SunYGeYDrnevichJZhaoYBandMChenJMammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesisJ Cell Biol201018971157116920566686
- LacasseYGoldsteinRLassersonTJMartinSPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20064CD00379317054186
- CasaburiRZuWallackRPulmonary rehabilitation for management of chronic obstructive pulmonary diseaseN Engl J Med2009360131329133519321869
- O’DonnellDELamMWebbKAMeasurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981585 Pt 1155715659817708
- MadorMJKufelTJPinedaLAEffect of pulmonary rehabilitation on quadriceps fatiguability during exerciseAm J Respir Crit Care Med2001163493093511282768
- O’DonnellDEMcGuireMSamisLWebbKAGeneral exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitationAm J Respir Crit Care Med19981575 Pt 1148914979603128
- ManWDKempPMoxhamJPolkeyMIExercise and muscle dysfunction in COPD: implications for pulmonary rehabilitationClin Sci (Lond)2009117828129119689433
- CasaburiRPatessioAIoliFZanaboniSDonnerCFWassermanKReductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung diseaseAm Rev Respir Dis199114319181986689
- MaltaisFLeBlancPJobinJIntensity of training and physiologic adaptation in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199715525555619032194
- van’t HulAGosselinkRHollanderPPostmusPKwakkelGTraining with inspiratory pressure support in patients with severe COPDEur Respir J2006271657216387937
- SomfayAPórszászJLeeSMCasaburiREffect of hyperoxia on gas exchange and lactate kinetics following exercise onset in nonhypoxemic COPD patientsChest2002121239340011834648
- PalangePValliGOnoratiPEffect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patientsJ Appl Physiol20049751637164215234959
- VogiatzisIStrategies of muscle training in very severe COPD patientsEur Respir J201138497197521737548
- Janaudis-FerreiraTHillKGoldsteinRWadellKBrooksDArm exercise training in patients with chronic obstructive pulmonary disease: a systematic reviewJ Cardiopulm Rehabil Prev200929527728319935139
- GosselinkRDe VosJvan den HeuvelSPSegersJDecramerMKwakkelGImpact of inspiratory muscle training in patients with COPD: what is the evidence?Eur Respir J201137241642521282809
- MaddocksMMurtonAJWilcockAImproving muscle mass and function in cachexia: non-drug approachesCurr Opin Support Palliat Care20115436136421934503
- RoigMReidWDElectrical stimulation and peripheral muscle function in COPD: a systematic reviewRespir Med2009103448549519091537
- VivodtzevIDebigaréRGagnonPFunctional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trialChest2012141371672522116795
- AbdellaouiAPréfautCGouziFSkeletal muscle effects of electrostimulation after COPD exacerbation: a pilot studyEur Respir J201138478178821349913
- GriffithsTLBurrMLCampbellIAResults at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trialLancet2000355920136236810665556
- SteinerMCRoubenoffRTal-SingerRPolkeyMIProspects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: a translational reviewThorax2012Epub May5
- RennardSIFogartyCKelsenSCOPD InvestigatorsThe safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2007175992693417290043
- YehSSDeGuzmanBKramerTM012 Study GroupReversal of COPD-associated weight loss using the anabolic agent oxandroloneChest2002122242142812171812
- NagayaNItohTMurakamiSTreatment of cachexia with ghrelin in patients with COPDChest200512831187119316162705
- NarayananRMohlerMLBohlCEMillerDDDaltonJTSelective androgen receptor modulators in preclinical and clinical developmentNucl Recept Signal20086e01019079612
- SumukadasDWithamMDStruthersADMcMurdoMEEffect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trialCMAJ2007177886787417923654
- DoorduinJSinderbyCABeckJThe calcium sensitizer levosimendan improves human diaphragm functionAm J Respir Crit Care Med20121851909521960535
- AndeSRChenJMaddikaSThe ubiquitin pathway: an emerging drug target in cancer therapyEur J Pharmacol20096251–319920519835866
- NarkarVADownesMYuRTAMPK and PPARdelta agonists are exercise mimeticsCell2008134340541518674809