Abstract
Chronic obstructive pulmonary disease (COPD) is a complex condition defined by progressive airflow limitation in response to noxious stimuli, inflammation, and vascular changes. COPD exacerbations are critical events in the natural history of the disease, accounting for the majority of disease burden, cost, and mortality. Pulmonary vascular disease is an important risk factor for disease progression and exacerbation risk. Relative pulmonary artery enlargement on computed tomography scan, defined by a pulmonary artery to aortic (PA:A) ratio >1, has been evaluated as a marker of pulmonary vascular disease. The PA:A ratio can be measured reliably independent of electrocardiographic gating or the use of contrast, and in healthy patients a PA:A ratio >0.9 is considered to be abnormal. The PA:A ratio has been compared with invasive hemodynamic parameters, primarily mean pulmonary artery pressure in various disease conditions and is more strongly correlated with mean pulmonary artery pressure in obstructive as compared with interstitial lung disease. In patients without known cardiac or pulmonary disease, the PA:A ratio is predictive of mortality, while in COPD, an elevated PA:A ratio is correlated with increased exacerbation risk, outperforming other well established predictors of these events. Future studies should be aimed at determining the stability of the metric over time and evaluating the utility of the PA:A ratio in guiding specific therapies.
Background
Chronic obstructive pulmonary disease (COPD) is a major ongoing public health problem in the US with more than 16.3 million office visits and 672,000 hospitalizations each year.Citation1,Citation2 Mortality associated with COPD continues to increase, and the disease is now the third leading cause of death in the US, recently surpassing stroke.Citation3 COPD exacerbations are crucial events in the natural history of the disease, accounting for a majority of associated morbidity, mortality, and expense. These events can be predicted by a number of clinical factors, including prior exacerbations, airflow obstruction, symptom burden, gastroesophageal reflux, and leukocytosis.Citation4 Pulmonary hypertension is also associated with increased exacerbation risk and mortality.Citation5,Citation6 Recently, relative pulmonary arterial enlargement as measured by the pulmonary artery to ascending aortic (PA:A) ratio, a potential surrogate for pulmonary vascular disease, was shown to provide independent predictive information for both severe and nonsevere exacerbations.Citation7 In this paper, we discuss the pathophysiology and clinical impact of pulmonary vascular disease in COPD and in particular the utility of the PA:A ratio as a biomarker of this process and of exacerbation risk.
Pulmonary hypertension in patients with COPD
Pulmonary hypertension primarily occurs in advanced airflow limitation due to hypoxic vasoconstriction.Citation8–Citation10 However, it is increasingly recognized in milder disease and related to systolic and diastolic left ventricular failure, inflammation, and other comorbid conditions.Citation11–Citation13 Pulmonary hypertension in the setting of COPD has both functional and prognostic implications. Patients with increasing pulmonary artery pressures have a linear decline in 6-minute walk distance when controlled for demographics and the degree of airflow obstruction.Citation14 Patients with advanced airflow obstruction and a mean pulmonary artery pressure (mPAP) >20 mmHg by right heart catheterization have a worse 4-year and 7-year mortality.Citation15 In fact, mortality is directly related to the degree of pulmonary artery pressure. When patients with COPD are stratified based on the presence of mild-to-moderate pulmonary hypertension (defined as a mPAP 25–39 mmHg) or severe pulmonary hypertension (mPAP >40 mmHg), the patients with severe pulmonary hypertension had a worse mortality.Citation16 Additionally, there is a subset of patients with milder airflow obstruction who have “out of proportion” pulmonary hypertension.Citation5,Citation17,Citation18 These patients are characterized by mild-to-moderate airflow obstruction (forced expiratory volume in one second >50% predicted), very low spirometry diffusion capacity, hypoxemia, and hypercapnia.Citation17,Citation18 These patients have increased mortality compared with patients with similar degrees of airflow limitation. In this population, subjects with no pulmonary hypertension (mPAP <20 mmHg) and mild-to-moderate pulmonary hypertension (defined as mPAP 20–40 mmHg) had similar survival. However, patients with severe (mPAP >40 mmHg) had shorter cumulative survival times compared with those without or with mild-to-moderate pulmonary hypertension.Citation5 The average survival in the “out of proportion” pulmonary hypertension group was similar to that seen in patients with advanced airflow limitation and severe pulmonary hypertension.
Mechanisms in development of pulmonary hypertension in COPD
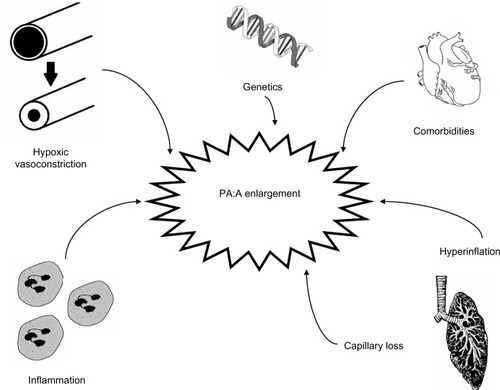
Hypoxic vasoconstriction drives much of the development of pulmonary hypertension in COPD, but other factors are in play as well. Patients who have the endothelial nitric oxide synthase (eNOS) polymorphism BB (BB homozygous genotype for intron 4 VNTR polymorphism of the eNOS gene) have higher mPAP compared with those without the mutation.Citation19 These patients have increased susceptibility to hypoxia and tobacco smoke, possibly through reduced eNOS activity.Citation20 Another factor associated with the development of pulmonary vascular disease in COPD is the emphysema-mediated destruction of alveolar units and accompanying capillaries. The loss of capillaries may be due in part to effects of vascular endothelial growth factorCitation21,Citation22 which in turn adds to the disruption of pulmonary parenchymal architecture. This is mediated by a combination of autophagy, senescence, and inflammation. This capillary loss can be detected by computed tomography (CT) scan with three-dimensional rendering of the small vasculature.Citation23,Citation24 This loss is accompanied by a decrease in thoracic blood volume in the small vessels and subsequent centralization of flow to larger pulmonary vessels.
Multiple pathways of inflammation contribute to the development of pulmonary vascular disease and pulmonary hypertension in COPD. In explanted pulmonary arteries, increased muscular infiltration by CD8+ T-lymphocytes has been observed.Citation11,Citation25 This T-cell infiltrate correlates with the development of pulmonary hypertension and with airflow obstruction.Citation25 Levels of circulating interleukin (IL)-6 correlate with pulmonary artery pressures.Citation26,Citation27 While IL-6 levels are increased in patients with COPD, there are higher levels in those with COPD and pulmonary hypertension (mPAP >25 mmHg).Citation27 Other inflammatory markers have been implicated in the pathogenesis of pulmonary hypertension in COPD as well, including C-reactive protein, tumor necrosis factor-alpha, monocyte chemoattractant protein-1, soluble intercellular adhesion molecule-1, and platelet-derived growth factor.Citation28–Citation30 The novel phospholipid ceramide has been described as a unique player in the integration of several of these pathways.Citation31–Citation33 Other comorbidities contribute to the development of pulmonary hypertension in COPD including pulmonary thromboembolic disease,Citation34 diastolic dysfunction,Citation35,Citation36 sleep apnea,Citation37–Citation42 and obesity.Citation43 These contributors to pulmonary artery enlargement in COPD are illustrated in .
Detecting pulmonary vascular disease in COPD
Right heart catheterization remains the gold standard for evaluating pulmonary vascular disease and diagnosing pulmonary hypertension,Citation10 including in patients with COPD.Citation8 Although the procedure is often done in patients being considered for lung transplantation, it is otherwise rarely performed as it is relatively invasive and because the possibility of pulmonary hypertension in patients with less severe COPD is often not considered. In addition, although pulmonary hypertension is a poor prognostic marker in COPD, there are no vasodilator therapies that have been demonstrated to impact outcomes significantly and thus the clinical utility of invasive measurements of pulmonary pressures is viewed as limited.Citation5,Citation44,Citation45 However, given that the pathophysiology of pulmonary vascular disease in COPD is complex and that both right-sided and left-sided heart disease can contribute to its development, right heart catheterization remains an important tool to better define pathophysiology. This may be of particular importance for the detection of post-capillary pulmonary hypertension which directly contributes to mortality and for which treatment may improve outcomes. Positron emission tomography scanCitation46 and cardiac magnetic resonance imaging,Citation47–Citation49 techniques used in evaluating other World Health Organization classes of pulmonary hypertension, will not be reviewed here.
Echocardiography and pulmonary hypertension in COPD
Transthoracic echocardiography (echo) is the most widely used imaging technique for the evaluation of pulmonary vascular disease in COPD. Echo allows for detection of pulmonary artery pressure by the tricuspid regurgitant jet as well as evaluation of right ventricular characteristics and performance.Citation50–Citation52 Echo is advantageous because it is noninvasive, does not require radiation, and is widely available. However, the sensitivity and specificity of echo for the detection of pulmonary hypertension is not optimal, particularly in patients with underlying lung disease.Citation50,Citation53 In those with underlying COPD, Doppler echo has a sensitivity of 76% and specificity of 65% for the detection of pulmonary hypertension using estimated pulmonary artery systolic pressure (PASP, or right ventricular systolic pressure) and 84% and 56% using right ventricular findings (defined as right ventricular dilation, hypertrophy, or systolic dysfunction), respectively.Citation50 The major limitation of echo in this patient population is the inability to obtain an adequate echo window to perform these analyses. In fact, estimates could only be obtained in 44% of subjects evaluated in one study.Citation50 However, in those for whom adequate windows were available, there was a good, although moderately variable, correlation between right heart catheterization systolic pulmonary artery pressure and Doppler echo PASP, (r=0.69, P<0.001).Citation50 Second, Doppler echo PASP relies on a modified Bernoulli equation to estimate PASP, and these calculations depend on additional estimates of right atrial pressure, introducing another potential source of error.Citation54 Additionally, Doppler echo relies on accurate image acquisition, and this can be limited in instances of air trapping due to the properties of sound waves travelling through tissue versus air.Citation55 Finally, there is emerging evidence that echo detects right ventricular structural and functional changes even without significant increases in mPAP in patients with COPD.Citation56 This suggests that the markers traditionally used to diagnose pulmonary hypertension by echo may be insensitive for detection of pulmonary vascular disease in COPD.
CT scans and COPD
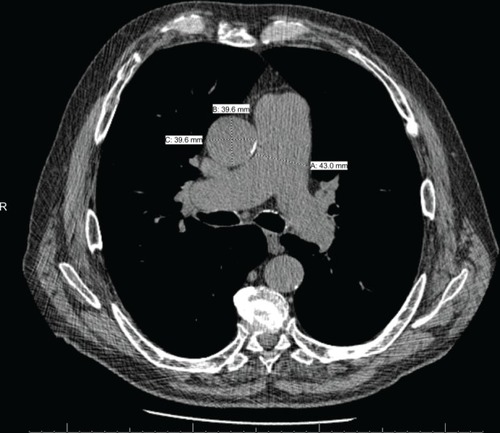
CT is a valuable tool in the evaluation of both intrinsic lung disease as well as the intrathoracic vasculature in patients with COPD. CT is able to detect changes to small vessels in patients with COPD and these abnormalities have important clinical implications,Citation23,Citation24 but this evaluation requires specialized software and expertise and is not routinely available. Evaluation of the central vasculature, including the main pulmonary artery, the right and left branches, and the ascending aorta, is much less problematic and these vessels can be evaluated using routine contrasted and noncontrasted CT scans.Citation57–Citation60 The diameter of the main pulmonary artery is measured proximal to the bifurcation into the left and right pulmonary arteries, as seen in . Measuring the ascending aorta on the same CT image allows for calculation of the PA:A ratio, a metric that (along with the pulmonary artery diameter) has good interobserver and intraobserver agreement, as depicted in . Discordant measurements can be due to the variable anatomy of the main pulmonary artery, particularly when it makes its oblique course at the base of the heart. In fact, Mahammedi et al investigated four separate sites of measurement for the pulmonary artery diameter and found that all were correlated with mPAP by right heart catheterization.Citation61
Table 1 Interobserver and intraobserver agreement in measuring the pulmonary artery and the PA:A ratio
Reference values for PA:A ratio
Lin et al were the first to evaluate the PA:A ratio by electrocardiography-gated multidetector CT in a healthy population of 103 patients over 18 years of age without cardiovascular or aortic disease, hypertension, obesity, known pulmonary hypertension, or COPD (by history or by CT appearance). The patients were of mean age 51 ± 14 years, 57% were male, and average body mass index was 25 ± 3 kg/m2. Only 8.8% of the study population currently smoked. The PA:A ranged considerably in this population, with a 5th to 95th percentile range of 0.66–1.13 and PA:A was inversely associated with age in multivariate analysis (−0.04 [0.06–0.02]/decade of age, P<0.001). This is partially explained because the pulmonary artery diameter is only independently correlated with body size and the aortic diameter correlates with both aging and body size, leading to a reduced PA:A ratio with aging. Sixteen percent of the subjects evaluated had a PA:A ratio >1, which suggests that factors other than pulmonary hypertension alone contribute to elevations in the PA:A value.Citation62
Truong et al expanded on these findings by defining reference values for the pulmonary artery diameter and the PA:A ratio in a large cohort using electrocardiography-gated multidetector CT. They evaluated 3,171 patients enrolled in the Offspring and Third Generation cohorts of the Framingham Heart Study. The patients were of mean age 51 ± 10 years, 51% were male, and average body mass index was 27.8 ± 5 kg/m2. Approximately 50% of the population were current or former smokers, with only 5.5% (n=159) having Global Initiative for Chronic Obstructive Lung Disease stage 2–4 COPD. In the entire cohort, the pulmonary artery diameter was 25.1 ± 2.8 mm and the PA:A ratio was 0.77 ± 0.09. Among the 159 patients with COPD evaluated, the pulmonary artery diameter was 25.3 ± 3.2 mm and the PA:A ratio was 0.75 ± 0.09. Further, they evaluated a subgroup of 706 never-smokers without known heart or lung disease. They found a normative pulmonary artery diameter of 29 mm in men and 27 mm in women and a PA:A ratio of 0.9 for both. Of patients with COPD, 2.7% had a PA:A ratio >0.9.Citation57 The reference values in healthy subjects are listed in .
Table 2 Reference values for pulmonary artery diameter and PA:A ratio
PA:A and hemodynamics
Most interest in the PA:A ratio has been in determining its relationship to invasive hemodynamic measurements as summarized in . Ng et al were the first to correlate the PA:A ratio measured by CT with right heart catheterization in a heterogeneous group of 50 patients with underlying pulmonary and cardiac diseases. There is no mention of tobacco use, and 16% (n=8) of the patients had underlying COPD, although the severity is not reported. The authors found that the PA:A ratio correlated with mPAP (r=0.74, P<0.00005) and pulmonary vascular resistance (r=0.59, P<0.0001). There were stronger correlations in patients younger than 50 years of age compared with those over 50 years (r=0.77, P<0.001 versus r=0.63, P<0.005). The PA:A ratio had a stronger correlation with mPAP in pulmonary disease compared with cardiovascular disease (r=0.66, P<0.0001 versus r=0.51, P=0.05). Using an mPAP >20 mmHg to diagnose pulmonary hypertension (the accepted diagnostic threshold at the time), the PA:A ratio >1 is 70% sensitive, 92% specific, and has a positive predictive value of 96% and a negative predictive value of 52%. On multivariate analysis correcting for age, gender, body surface area, and total lung capacity, the PA:A ratio was independently associated with mPAP (r=0.33, P<0.0005).Citation63
Table 3 Correlation between PA diameter measured by CT, PA:A ratio, and hemodynamics
Mahammedi et al evaluated 298 patients with known pulmonary hypertension and 102 matched controls from a single center using high-resolution computed tomography. The mean PA:A ratio was 1.1 ± 0.2 in the pulmonary hypertension group and 0.9 ± 0.2 in the control group (P<0.0001), and the PA:A ratio was driven by increases in pulmonary artery diameter (31.7 ± 0.5 mm versus 26.7 ± 0.5 mm, P<0.00001). There were no differences in aortic diameter between groups. Using multiple linear regression, the authors found the strongest association between the PA:A ratio and mPAP (r=0.53, P<0.001), although they also found a similar association with pulmonary artery diameter and mPAP (r=0.51, P<0.001). The area under the receiver operating characteristic curve for the accuracy of the PA:A ratio in predicting pulmonary hypertension was 0.79 (0.74–0.84). A PA:A ratio >1 was 70.8% sensitive and 76.5% specific for pulmonary hypertension.Citation61
Another study evaluated whether changes to the pulmonary artery diameter and the PA:A ratio occur due to changes in intravascular pressure, cardiac output, or both in patients with pulmonary arterial hypertension using cardiac magnetic resonance imaging and right heart catheterization in 69 patients, of whom 51 had known pulmonary hypertension. Mean pulmonary artery diameter and PA:A ratio were larger in the pulmonary hypertension group compared with controls (33.7 ± 5.3 mm versus 25.0 ± 6.8 mm, P<0.001, and 1.26 ± 0.22 versus 0.87 ± 0.17, P<0.001, respectively). There was a strong correlation between the PA:A ratio and mPAP (r=0.71, P<0.001). The accuracy of the PA:A ratio in detecting pulmonary hypertension (mPAP >25 mmHg) by receiver operating characteristic analysis was 0.93 (95% confidence interval 0.86–0.99). Using cardiac magnetic resonance imaging, a PA:A ratio >1 has 92% sensitivity, 72% specificity, and a positive predictive value of 92% for detecting pulmonary hypertension. The patients were treated with various therapies for pulmonary arterial hypertension for a median of 942 days and during that time the pulmonary artery diameter increased to 35.7 ± 6.5 mm (P<0.001 from baseline) with an accompanying increase in cardiac output (5.2 ± 1.2 from 4.8 ± 1.65 L/minute at baseline, P=0.005) and a decline in pulmonary vascular resistance (730 ± 365 dyne/second/cm from 837 ± 401 dyne/second/cm, P=0.026) without changes to mPAP (P=0.15 from baseline).Citation64
The role of pulmonary artery dilation as a reliable indicator of pulmonary hypertension in patients with pulmonary fbrosis was investigated in a cohort of patients with fbrotic lung disease (n=30). Although the pulmonary artery diameter did not correlate with the mPAP in the fbrosis group (r=0.23, P=0.22), the PA:A ratio was correlated with mPAP in a heterogenous group without interstitial lung disease (r=0.54, P<0.005). Similarly, the PA:A ratio correlated with pulmonary vascular resistance (r=0.48, P=0.04). There were no correlations between pulmonary artery diameter and fbrosis scores, total lung capacity, and mPAP on multiple linear regression modeling (r2=0.09, P=0.50).Citation65 In a study examining the utility of the PA:A ratio in a cohort of patients with idiopathic pulmonary fbrosis, Zisman et al found no relationship between pulmonary artery diameter or PA:A ratio and mPAP in patients with idiopathic pulmonary fbrosis.Citation66 Another study examined the pulmonary artery diameter, PA:A ratio, and mPAP in a cohort of 100 patients with interstitial lung disease from different etiologies and a separate cohort of 34 patients without interstitial lung disease (eight of whom had COPD). They found that the pulmonary artery diameter and the PA:A ratio were increased in the group with pulmonary hypertension and interstitial lung disease (28.7 ± 3.7 mm versus 26.7 ± 3.4 mm, P=0.008 and 1.0 ± 0.2 versus 0.9 ± 0.1, P=0.008, respectively). In the group without interstitial lung disease (consisting primarily of COPD, lupus, obstructive sleep apnea, and bronchiectasis), the pulmonary artery diameter and PA:A ratio were associated with having an mPAP >25 mmHg (25.8 ± 4.9 mm versus 30.8 ± 6.7 mm, P=0.02, and 0.8 ± 0.1 versus 1.1 ± 0.3, P=0.006, respectively). Both the pulmonary artery diameter (r=0.701, P<0.0001) and the PA:A ratio (r=0.626, P<0.0001) had better correlations with mPAP than the same metrics in the group with interstitial lung disease (r=0.30, P=0.002 for pulmonary artery diameter and r=0.434, P<0.0001 for the PA:A ratio). For patients with obstructive lung disease without interstitial lung disease, a pulmonary artery diameter of 31.6 mm was 47.3% sensitive and 93.3% specific for diagnosing an mPAP >25 mmHg.Citation67
In another study, Devaraj et al compared alternate CT measurements (PA:vertebral body, segmental artery, segmental artery:bronchus) to the PA:A ratio and investigated whether the utility of the PA:A ratio in diagnosing pulmonary hypertension determined by right heart catheterization would be improved by addition of right ventricular systolic pressure from echocardiography in 77 patients with a wide spectrum of diseases associated with pulmonary hypertension, of which COPD accounted for 6% (n=5). The PA:A ratio had stronger associations with mPAP than other CT metrics (r2=0.45, P<0.001 for the PA:A and mPAP) and was similar to echocardiography (r2=0.44, P<0.001). When the PA:A ratio and right ventricular systolic pressure are combined, the association with mPAP is stronger than either metric alone (r2=0.55, P<0.001).Citation68
Chan et al investigated the role of the PA:A ratio as well as other CT metrics in detecting pulmonary hypertension by right heart catheterization in a heterogeneous population of 108 acutely hospitalized patients using non-gated CT scans, of which 52% (n=53) had pulmonary hypertension. There were only two patients (2%) with COPD as the primary cause of pulmonary hypertension. Patients with pulmonary hypertension had a higher body mass index and body surface area compared with those without pulmonary hypertension. There was no difference in the use of mechanical ventilation between groups (15.1% in the pulmonary hypertension group versus 8.3% in the control group, P=0.37). The mean PA:A ratio for those with pulmonary hypertension was 0.97 ± 0.2 compared with 0.86 ± 0.13 in the control group (P=0.0014). Using multiple logistic regression, a PA:A ratio >1 was independently associated with a diagnosis of pulmonary hypertension (odds ratio 9.1, P=0.0085) with an accuracy of 0.93 (area under the receiver operating curve), sensitivity of 86.8% and specificity of 79.2%.Citation69
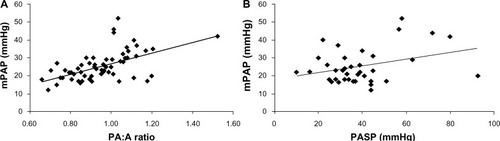
Iyer et al have compared the utility of the PA:A ratio and PASP measured on echo for detecting pulmonary hypertension in 60 patients with severe COPD. The patients were of mean age 55 ± 7 years, 83% were white, 43% were male, and the mean predicted forced expiratory volume in one second was 27% ± 12%. The pulmonary artery diameter and PA:A ratio correlated with mPAP (r=0.60, P<0.001 and r=0.56, P<0.001, respectively). Further, the PA:A ratio was found to be independently associated with mPAP in a multiple linear regression model (r=0.30, P=0.03) while echo-measured PASP did not correlate with mPAP. The simple linear associations between the PA:A ratio, echocardiography, and mPAP are shown in . A PA:A ratio >1 was found to be the most accurate in diagnosing an mPAP >25 mmHg with a sensitivity of 73%, a specificity of 84%, a positive predictive value of 73%, and a negative predictive value of 84%.Citation70 The capacity of the PA:A ratio to predict pulmonary hypertension is outlined in .
Table 4 Utility of the PA:A ratio in diagnosis of pulmonary hypertension
PA:A ratio and outcomes
Nakanishi et al evaluated the utility of the PA:A ratio in predicting mortality in patients undergoing evaluation for suspected coronary artery disease. They evaluated 1,326 patients from a single center without known cardiac disease using coronary CT angiography. The patients were of mean age 61 ± 13 years, 60% were male, and 14% were current smokers. There was no reported COPD or lung disease. Using this technique, 14% (n=182) of the patients had a PA:A ratio >0.9, and this group was younger, more likely to smoke (19% versus 13%, P=0.02), had more reported dyspnea (18% versus 11%, P=0.004), and had larger pulmonary artery diameters (2.9 ± 0.4 mm versus 2.4 ± 0.3 mm, P<0.0001). The patients with a PA:A ratio >0.9 had a higher prevalence of impaired left ventricular function (ejection fraction 64% ± 12.7% versus 67.5% ± 9.8%, P=0.008). During a follow-up period of 2.9 ± 1 years, 4.4% (n=58) died, including 43 (3.8%) in the control group compared with 15 (8.2%) in the PA:A >0.9 group (P=0.006). This correlates with a 2.5-fold greater annualized mortality (3.1% versus 1.3%, P=0.004) in patients with a PA:A ratio >0.9. In a multivariate Cox proportional model, a PA:A >0.9 was independently associated with increased mortality (hazards ratio 3.2, 95% confidence interval 1.6–6.6, P=0.001).Citation71
Other investigators have evaluated the prognostic impact of the PA:A ratio, among other CT metrics, in the setting of acute intermediate-to-high risk pulmonary thromboembolic events in 39 patients. Patients had an average pulmonary artery diameter of 3.1 ± 0.4 mm with a PA:A ratio of 0.9 ± 0.2. There were no differences between either the pulmonary artery diameter or the PA:A ratio in mortality. The authors found that the right ventricular/left ventricular ratio did have prognostic implications in this population.Citation72 Heinrich et al compared CT findings, including the pulmonary artery diameter and the PA:A ratio, to preoperative and postoperative hemodynamic measurements in 60 patients undergoing thrombo-endarterectomy. The pulmonary artery diameter was 3.9 ± 0.55 cm and the PA:A ratio was 1.14 ± 0.2. In 86% of patients with an mPAP >20 mmHg, the PA:A ratio was >1. Both the pulmonary artery and the PA:A ratio correlated with preoperative mPAP (r=0.42, P<0.001 and r=0.48, P<0.0001, respectively) and only the PA:A ratio was correlated with preoperative pulmonary vascular resistance (r=0.29, P=0.023). Neither the pulmonary artery diameter or the PA:A ratio correlated with postoperative pulmonary vascular resistance.Citation73 In a population with chronic thromboembolic pulmonary hypertension or pulmonary artery hypertension, Zylkowska et al evaluated the prognostic utility of pulmonary artery diameter obtained by multidetector CT angiography in 264 consecutive patients with a mean mPAP of 57.6 ± 16.5 mmHg. The average pulmonary artery diameter was 39 ± 8.6 mm and correlated weakly with mPAP (r=0.18, P=0.004). In multivariate Cox proportional hazard analysis, there was an increased risk of unexpected mortality for each increase in pulmonary artery diameter by 1 mm (hazards ratio 1.06, 95% confidence interval 1.03–1.08, P<0.001), although there was no correlation between pulmonary artery diameter and all-cause mortality.Citation74 The relationships between the PA:A ratio and clinical outcomes are outlined in .
Table 5 Outcomes related to PA:A ratio
Implications of PA:A ratio in COPD
Wells et al investigated the utility of the PA:A ratio (specifically a PA:A ratio >1) to predict severe exacerbations in patients with Global Initiative for Chronic Obstructive Lung Disease stage 2–4 COPD.Citation7 They included 3,464 patients from the COPDGene study cohortCitation75 and 2,005 patients from the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) cohort.Citation76 Of the 3,464 patients in the COPDGene study, 819 (23.6%) had a PA:A ratio >1 and these patients were more often female, African-American, had higher body mass index, worse airflow obstruction, and a shorter 6-minute walk distance. Additionally, they had higher rates of congestive heart failure, thromboembolic disease, sleep apnea, and supplemental oxygen use. In multivariate logistic modeling, the PA:A ratio >1 had the strongest association with developing exacerbations over a median follow-up period of 2.1 years (odds ratio 3.44, 95% confidence interval 2.78–4.25, P<0.001), outperforming other metrics including history of prior exacerbations (odds ratio 2.01, 95% confidence interval 1.61–2.49, P<0.001). Patients with a PA:A ratio >1 had a 3.68-fold increase in severe exacerbation frequency. In the ECLIPSE cohort, the PA:A ratio was independently associated with severe exacerbations at one year (odds ratio 2.8, 95% confidence interval 2.11–3.71, P<0.001) and 3 years (odds ratio 3.81, 95% confidence interval 3.04–4.78, P<0.001) as listed in . A PA:A ratio <1 also predicted a 1.62-fold increase in developing an exacerbation of any severity in the COPDGene cohort and a 1.43-fold increase in developing an exacerbation of any severity in the ECLIPSE cohort.
The results of this study were unique in that this trial was the first to evaluate the PA:A ratio in a population of COPD patients. Additionally, it is the first to demonstrate a relationship between a CT-detected metric and severe exacerbation of COPD, which is a meaningful clinical outcome.Citation7
What contributes to pulmonary artery enlargement on CT scan?
As outlined above, resting pulmonary hypertension contributes to pulmonary artery enlargement seen on CT scan. However, other factors, including peripheral capillary destruction related to emphysema and subsequent centralization of blood flow and hyperinflation, may also play a role.Citation24 Interestingly, in patients from the COPDGene cohort who had lung volumes measured by CT scan, more patients with a PA:A ratio >1 had an inspiratory capacity to total lung capacity ratio <25% (28% versus 22%, P=0.001). Further, the PA:A ratio may also serve as a composite endpoint for various other comorbid conditions seen in COPD, including systolic and diastolic dysfunction,Citation22 sleep apnea,Citation77 thromboembolic disease, body habitus, and hyperinflation related to underlying parenchymal disease. These factors must play a role because elevated PA:A ratios occur in non-severe COPD and are associated with early right ventricular changes as seen on cardiac magnetic resonance imaging.Citation78
Therapeutic implications of PA:A ratio >1 in COPD
Other than predicting exacerbations,Citation7 the implications of a PA:A ratio >1 in COPD remain largely unknown. Pulmonary hypertension is associated with an increased risk of hospitalization from COPD exacerbationsCitation5 and is associated with an increased mortality (hazards ratio 1.36) in patients admitted to hospital.Citation6 If an elevated PA:A ratio is a reliable surrogate for pulmonary hypertension, then the metric may prove useful in screening patients for right heart catheterization to confirm the diagnosis and prior to considering therapy with vasodilators or other treatments aimed at the disorder.Citation79 Given that an elevated PA:A ratio may also be the result of left-sided heart disease, the use of right heart catheterization in this population may also improve the detection and management of diastolic dysfunction or other disorders which could improve outcomes. Although the correlation between the PA:A ratio and hemodynamics is reasonably robust, there is considerable variability between the absolute values, reinforcing the need for invasive measurements to confirm pathophysiology and select treatment.
It should be noted, however, that at present there is no proven therapy for pulmonary vascular disease in COPD. Initiating supplemental oxygen for patients who meet the requirements for oxygen use results in a decrease in exercise-induced pulmonary hypertension.Citation80 Compared with placebo, there was little effect of supplemental oxygen in the setting of mild airflow obstruction, but during exercise in moderate and severe airflow obstruction, there was a reduction in mPAP (38.1 ± 2.1 mmHg to 32.0 ± 1.8 mmHg, P<0.05 in moderate and 44.4 ± 2.0 mmHg to 37.8 ± 1.9 mmHg, P<0.05 in severe airflow obstruction) and pulmonary vascular resistance index (4.83 ± 0.41 mmHg/L/minute/m2 to 4.17 ± 0.30 mmHg/L/minute/m2, P<0.05 in moderate and 4.75 ± 0.29 mmHg/L/minute/m2 to 3.85 ± 0.30 mmHg/L/minute/m2 in severe airflow obstruction, P<0.05). These changes were accompanied by increases in exercise capacity as measured by 6-minute walk distance (±12 m in mild airway obstruction, ±24 m in moderate airway obstruction, and ±32 m in severe airway obstruction, P<0.05 for each).Citation80
The most widely investigated pharmaceutical agent in COPD-related pulmonary hypertension is sildenafil. Acutely, sildenafil improves mPAP by −6 (95% CI −7 to −4) mmHg at rest and −11 (95% CI −14 to −8) mmHg during exercise, but this benefit is offset by worsening of oxygenation (PaO2 decrease by −6 mmHg, 95% CI −8 to −4) at rest due to increased ventilation/perfusion (V/Q) mismatch from loss of hypoxic vasoconstriction.Citation81 In a 12-week pulmonary rehabilitation study, sildenafil failed to improve pulmonary hypertension, exercise tolerance, symptoms, or 6-minute walk distance.Citation82 Bosentan, an endothelin-1 receptor antagonist, showed promise in preclinical models and has been shown to block endothelin-1 overexpression induced by cigarette smoke.Citation83 However, in a randomized controlled trial, there was no improvement in pulmonary artery pressure as measured by echocardiography at 12 weeks (systolic pulmonary artery pressure 32–30 mmHg, endothelin-1 =0.288), no change in 6-minute walk distance, and worsening of both symptoms and oxygenation.Citation84 Additionally, in subgroup analysis of ARIES-3 (A Phase III, Long-Term, Open-Label, Multi-center Safety and Efficacy Study of Ambrisentan in Subjects With Pulmonary Hypertension) using oral ambrisentan for 24 weeks, there was a mean decrease of 5 m on 6-minute walk distance compared with a mean increase of 21 m for the entire cohort.Citation85
There is interest in the use of inhaled iloprost due to the theoretical benefits of regional drug deposition in areas with better ventilation, minimizing the V/Q mismatch discrepancies that have been unmasked with the use of other vasodilators. The reports of its use are restricted to case series and case reports, limiting any conclusions.Citation86–Citation88 There are several other compounds and delivery methods with promising preclinical benefits, including 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors,Citation89–Citation91 Rho kinase inhibitors,Citation92,Citation93 inducible nitric oxide synthase inhibitors,Citation94 and myeloperoxidase inhibitors,Citation95 as well as the use of pulsed nitric oxide.Citation96
Using the PA:A ratio as a biomarker of disease progression and of exacerbation events may prove useful, independent of any information it provides about the pulmonary vasculature. Although azithromycin and roflumilast have been recently demonstrated to reduce exacerbations, these drugs have potentially important side effects and thus ideally should be targeted at those at greatest risk.Citation97–Citation102 The PA:A ratio could be used during stable disease to guide therapeutic decision-making and select patients most likely to benefit from these and other agents that reduce exacerbations.
Conclusion
COPD is a highly prevalent and complex disease characterized by multiple comorbidities and exacerbations. These acute events drive the excess cost and mortality associated with disease. Pulmonary hypertension is associated with severe acute exacerbations of COPD and provides independent and predictive and prognostic information additive to that obtained by spirometry alone. There are multiple pathways involved in the development of pulmonary vascular disease and pulmonary artery enlargement in COPD. There are several noninvasive imaging modalities available for evaluation of the pulmonary vasculature. Of these, only the PA:A ratio is associated with prognosticating disease progression by identifying patients at highest risk for acute exacerbations and related hospitalizations. The PA:A ratio outperforms other well established predictors of acute exacerbation risk, correlates with invasive measurements of pulmonary vascular disease, and is both sensitive and specific for the diagnosis of pulmonary hypertension in patients with advanced airflow disease. Future studies should be aimed at determining the stability of the metric over time and use of the PA:A ratio in guiding specific therapies.
Disclosure
JMW has no conflicts of interest to declare. MTD has served as a consultant to Boehringer Ingelheim, GlaxoSmithKline, and Ikaria and his institution has received research support from the National Institutes of Health, Aeris, Astra Zeneca, Boehringer Ingelheim, Boston Scientific, Centocor, Forest, GlaxoSmithKline, Ikaria, Medimmune, Olmstead Medical Center, Otsuka, Pfizer, and Pulmonx.
References
- HallMJDeFrancesCJWilliamsSNGolosinskiyASchwartzmanANational Hospital Discharge Survey: 2007 summaryNatl Health Stat Rep201029120
- Centers for Disease Control and PreventionChronic obstructive pulmonary disease among adults – United States, 2011MMWR Morb Mortal Wkly Rep20126146938943
- MininoAMMurphySLDeath in the United States, 2010NCHS Data Brief2012991823050606
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- KesslerRFallerMWeitzenblumE“Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung diseaseAm J Respir Crit Care Med2001164221922411463591
- McGhanRRadcliffTFishRSutherlandERWelshCMakeBPredictors of rehospitalization and death after a severe exacerbation of COPDChest200713261748175517890477
- WellsJMWashkoGRHanMKPulmonary arterial enlargement and acute exacerbations of COPDN Engl J Med20123671091392122938715
- SchulmanLLLennonPFWoodJAEnsonYPulmonary vascular resistance in emphysemaChest199410537988058131543
- McLaughlinVVArcherSLBadeschDBACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension AssociationJ Am Coll Cardiol200953171573161919389575
- McLaughlinVVArcherSLBadeschDBACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension AssociationCirculation2009119162250229419332472
- PeinadoVIBarberaJAAbatePInflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19991595 Pt 11605161110228134
- GordonCGudiKKrauseACirculating endothelial microparticles as a measure of early lung destruction in cigarette smokersAm J Respir Crit Care Med2011184222423221471087
- WeitzenblumESchrijenFMohan-KumarTColas des FrancsVLockhartAVariability of the pulmonary vascular response to acute hypoxia in chronic bronchitisChest19889447727783139371
- SimsMWMargolisDJLocalioARPanettieriRAKawutSMChristieJDImpact of pulmonary artery pressure on exercise function in severe COPDChest2009136241241919318664
- WeitzenblumEHirthCDucoloneAMirhomRRasaholinjanaharyJEhrhartMPrognostic value of pulmonary artery pressure in chronic obstructive pulmonary diseaseThorax198136107527587330793
- HurdmanJCondliffeRElliotCAPulmonary hypertension in COPD: results from the ASPIRE registryEur Respir J20134161292130123018917
- ChaouatANaeijeRWeitzenblumEPulmonary hypertension in COPDEur Respir J20083251371138518978137
- ChaouatABugnetASKadaouiNSevere pulmonary hypertension and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2005172218919415831842
- YildizPOflazHCineNErginel-UnaltunaNErzenginFYilmazVGene polymorphisms of endothelial nitric oxide synthase enzyme associated with pulmonary hypertension in patients with COPDRespir Med200397121282128814682408
- SuYHanWGiraldoCDe LiYBlockEREffect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cellsAm J Respir Cell Mol Biol19981958198259806747
- TuderRMFlookBEVoelkelNFIncreased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxideJ Clin Invest1995954179818077706486
- TuderRMZhenLChoCYOxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockadeAm J Respir Cell Mol Biol2003291889712600822
- WashkoGRParragaGCoxsonHOQuantitative pulmonary imaging using computed tomography and magnetic resonance imagingRespirology201217343244422142490
- San Jose EsteparRKinneyGLBlack-ShinnJLComputed tomographic measures of pulmonary vascular morphology in smokers and their clinical implicationsAm J Respir Crit Care Med2013188223123923656466
- SaettaMBaraldoSCorbinoLCD8+ve cells in the lungs of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1999160271171710430750
- EddahibiSChaouatATuLInterleukin-6 gene polymorphism confers susceptibility to pulmonary hypertension in chronic obstructive pulmonary diseaseProc Am Thorac Soc20063647547616921112
- ChaouatASavaleLChouaidCRole for interleukin-6 in COPD-related pulmonary hypertensionChest2009136367868719349390
- JoppaPPetrasovaDStancakBTkacovaRSystemic inflammation in patients with COPD and pulmonary hypertensionChest2006130232633316899829
- AmsellemVGary-BoboGMarcosETelomere dysfunction causes sustained inflammation in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2011184121358136621885626
- GoncharovaEAKhavinISGoncharovDAKrymskayaVPDifferential effects of formoterol on thrombin- and PDGF-induced proliferation of human pulmonary arterial vascular smooth muscle cellsRespir Res20121310923186269
- PetracheINatarajanVZhenLCeramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in miceNat Med200511549149815852018
- PetracheINatarajanVZhenLCeramide causes pulmonary cell apoptosis and emphysema: a role for sphingolipid homeostasis in the maintenance of alveolar cellsProc Am Thorac Soc20063651016921130
- PetracheIPetruscaDNBowlerRPKamockiKInvolvement of ceramide in cell death responses in the pulmonary circulationProc Am Thorac Soc20118649249622052925
- CarsonJLTerrinMLDuffAKelleyMAPulmonary embolism and mortality in patients with COPDChest19961105121212198915223
- OzerNTokgozogluLCopluLKesSEchocardiographic evaluation of left and right ventricular diastolic function in patients with chronic obstructive pulmonary diseaseJ Am Soc Echocardiogr200114655756111391283
- AcikelMKoseNAribasAThe effect of pulmonary hypertension on left ventricular diastolic function in chronic obstructive lung disease: a tissue Doppler imaging and right cardiac catheterization studyClin Cardiol2010338E13E1820589939
- FletcherECSchaafJWMillerJFletcherJGLong-term cardiopulmonary sequelae in patients with sleep apnea and chronic lung diseaseAm Rev Respir Dis198713535255333826878
- Levi-ValensiPWeitzenblumERidaZSleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patientsEur Respir J1992533013071572442
- WeitzenblumEKriegerJOswaldMChaouatABachezPKesslerRChronic obstructive pulmonary disease and sleep apnea syndromeSleep199215Suppl 6S33S351470805
- KesslerRChaouatAWeitzenblumEPulmonary hypertension in the obstructive sleep apnoea syndrome: prevalence, causes and therapeutic consequencesEur Respir J1996947877948726947
- WeitzenblumEChaouatAKesslerRCanuetMOverlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary diseaseProc Am Thorac Soc20085223724118250217
- MarinJMSorianoJBCarrizoSJBoldovaACelliBROutcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndromeAm J Respir Crit Care Med2010182332533120378728
- LeungCCMoondraVCatherwoodEAndrusBWPrevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fractionAm J Cardiol2010106228428620599017
- ElwingJPanosRJPulmonary hypertension associated with COPDInt J Chron Obstruct Pulmon Dis200831557018488429
- DoiMNakanoKHiramotoTKohnoNSignificance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemiaRespir Med200397891592012924518
- BasuSAlzeairSLiGDadparvarSAlaviAEtiopathologies associated with intercostal muscle hypermetabolism and prominent right ventricle visualization on 2-deoxy-2[F-18]fluoro-D-glucose-positron emission tomography: significance of an incidental finding and in the setting of a known pulmonary diseaseMol Imaging Biol20079633333917610018
- HueperKParikhMAPrinceMRQuantitative and semiquantitative measures of regional pulmonary microvascular perfusion by magnetic resonance imaging and their relationships to global lung perfusion and lung diffusing capacity: the Multi-Ethnic Study of Atherosclerosis chronic obstructive pulmonary disease studyInvest Radiol201348422323023385398
- KawutSMBarrRGLimaJARight ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA) – right ventricle studyCirculation2012126141681168822932258
- LeySKreitnerKFFinkCHeusselCPBorstMMKauczorHUAssessment of pulmonary hypertension by CT and MR imagingEur Radiol200414335936814740163
- ArcasoySMChristieJDFerrariVAEchocardiographic assessment of pulmonary hypertension in patients with advanced lung diseaseAm J Respir Crit Care Med2003167573574012480614
- BachDSCurtisJLChristensenPJPreoperative echocardiographic evaluation of patients referred for lung volume reduction surgeryChest199811449729809792564
- BurgessMIMogulkocNBright-ThomasRJBishopPEganJJRaySGComparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary diseaseJ Am Soc Echocardiogr200215663363912050605
- LaabanJPDieboldBZelinskiRLafayMRaffoulHRochemaureJNoninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary diseaseChest1989966125812622582830
- RudskiLGLaiWWAfilaloJGuidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of EchocardiographyJ Am Soc Echocardiogr201023768571320620859
- MorpurgoMNon-invasive assessment of pulmonary arterial hypertension in chronic lung disease (WHO study)Eur Respir J Suppl19897666s668s2679613
- HildeJMSkjortenIGrottaOJRight ventricular dysfunction and remodeling in COPD without pulmonary hypertensionJ Am Coll Cardiol201362121103111123831444
- TruongQAMassaroJMRogersISReference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart StudyCirc Cardiovasc Imaging20125114715422178898
- BurgerIAHusmannLHerzogBAMain pulmonary artery diameter from attenuation correction CT scans in cardiac SPECT accurately predicts pulmonary hypertensionJ Nucl Cardiol201118463464121688066
- Perez-EnguixDMoralesPTomasJMVeraFLloretRMComputed tomographic screening of pulmonary arterial hypertension in candidates for lung transplantationTransplant Proc20073972405240817889203
- TanRTKuzoRGoodmanLRSiegelRHaaslerGBPresbergKWUtility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant GroupChest19981135125012569596302
- MahammediAOshmyanskyAHassounPMThiemannDRSiegelmanSSPulmonary artery measurements in pulmonary hypertension: the role of computed tomographyJ Thorac Imaging20132829610323096163
- LinFYDevereuxRBRomanMJThe right sided great vessels by cardiac multidetector computed tomography: normative reference values among healthy adults free of cardiopulmonary disease, hypertension, and obesityAcad Radiol200916898198719394871
- NgCSWellsAUPadleySPA CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameterJ Thorac Imaging199914427027810524808
- BoerrigterBMauritzGJMarcusJTProgressive dilatation of the main pulmonary artery is a characteristic of pulmonary arterial hypertension and is not related to changes in pressureChest201013861395140120495109
- DevarajAWellsAUMeisterMGCorteTJHansellDMThe effect of diffuse pulmonary fibrosis on the reliability of CT signs of pulmonary hypertensionRadiolog y2008249310421049
- ZismanDAKarlamanglaASRossDJHigh-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosisChest2007132377377917573485
- AlhamadEHAl-BoukaiAAAl-KassimiFAPrediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findingsRadiology2011260387588321613438
- DevarajAWellsAUMeisterMGCorteTJWortSJHansellDMDetection of pulmonary hypertension with multidetector CT and echocardiography alone and in combinationRadiology2010254260961620093532
- ChanALJuarezMMSheltonDKNovel computed tomographic chest metrics to detect pulmonary hypertensionBMC Med Imaging201111721447184
- IyerASWellsJMVishinSBhattSPWilleKMDransfieldMTCT measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPDChest Epub20131010
- NakanishiRRanaJSShalevAMortality risk as a function of the ratio of pulmonary trunk to ascending aorta diameter in patients with suspected coronary artery diseaseAm J Cardiol201311191259126323415638
- BaptistaRSantiagoIJorgeEOne-shot diagnostic and prognostic assessment in intermediate- to high-risk acute pulmonary embolism: the role of multidetector computed tomographyRev Port Cardiol201332171323245344
- HeinrichMUderMTschollDGrgicAKramannBSchafersHJCT scan findings in chronic thromboembolic pulmonary hypertension: predictors of hemodynamic improvement after pulmonary thromboendarterectomyChest200512751606161315888835
- ZylkowskaJKurzynaMFlorczykMPulmonary artery dilatation correlates with the risk of unexpected death in chronic arterial or thromboembolic pulmonary hypertensionChest201214261406141622797193
- ReganEAHokansonJEMurphyJRGenetic epidemiology of COPD (COPDGene) study designCOPD201071324320214461
- VestboJAndersonWCoxsonHOEvaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE)Eur Respir J200831486987318216052
- KawanoYTamuraAWatanabeTKadotaJSevere obstructive sleep apnoea is independently associated with pulmonary artery dilatationRespirology5212013 [Epub ahead of print.]
- WellsJMBhattSPGuptaHCardiac MRI detects alterations in right ventricular preload in subjects with COPD and a PA:A ratio >1Am J Respir Crit Care Med2013187A2421
- RobbinsIMMooreTMBlaisdellCJAbmanSHImproving outcomes for pulmonary vascular diseaseAm J Respir Crit Care Med201218591015102022335936
- FujimotoKMatsuzawaYYamaguchiSKoizumiTKuboKBenefits of oxygen on exercise performance and pulmonary hemodynamics in patients with COPD with mild hypoxemiaChest2002122245746312171817
- BlancoIGimenoEMunozPAHemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertensionAm J Respir Crit Care Med2010181327027819875684
- BlancoISantosSGeaJSildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trialEur Respir J2212013 [Epub ahead of print.]
- MilaraJGabardaEJuanGBosentan inhibits cigarette smoke-induced endothelin receptor expression in pulmonary arteriesEur Respir J201239492793821828025
- StolzDRaschHLinkaAA randomised, controlled trial of bosentan in severe COPDEur Respir J200832361962818448495
- BadeschDBFeldmanJKeoghAARIES-3: ambrisentan therapy in a diverse population of patients with pulmonary hypertensionCardiovasc Ther2012302939921884013
- HegewaldMJElliottCGSustained improvement with iloprost in a COPD patient with severe pulmonary hypertensionChest2009135253653719201716
- LasotaBSkoczynskiSMizia-StecKPierzchalaWThe use of iloprost in the treatment of ‘out of proportion’ pulmonary hypertension in chronic obstructive pulmonary diseaseInt J Clin Pharm201335331331523549775
- DernaikaTABeavinMKinasewitzGTIloprost improves gas exchange and exercise tolerance in patients with pulmonary hypertension and chronic obstructive pulmonary diseaseRespiration201079537738219786728
- YaoJXiongMTangBSimvastatin attenuates pulmonary vascular remodelling by down-regulating matrix metalloproteinase-1 and -9 expression in a carotid artery-jugular vein shunt pulmonary hypertension model in ratsEur J Cardiothoracic Surg2012425e121e127
- ReedRMIaconoADeFilippisAStatin therapy is associated with decreased pulmonary vascular pressures in severe COPDCOPD2011829610221495837
- WrightJLZhouSPreobrazhenskaOStatin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodelingAm J Respir Crit Care Med20111831505820709821
- MouchaersKTSchalijIde BoerMAFasudil reduces monocrotaline-induced pulmonary arterial hypertension: comparison with bosentan and sildenafilEur Respir J201036480080720351034
- Duong-QuySBeiYLiuZDinh-XuanATRole of Rho-kinase and its inhibitors in pulmonary hypertensionPharmacol Ther2013137335236423261521
- SeimetzMParajuliNPichlAInducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in miceCell2011147229330522000010
- ChurgAMarshallCVSinDDLate intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20121851344321997333
- VonbankKZiescheRHigenbottamTWControlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPDThorax200358428929312668787
- AlbertRKConnettJBaileyWCAzithromycin for prevention of exacerbations of COPDN Engl J Med2011365868969821864166
- FabbriLMCalverleyPMIzquierdo-AlonsoJLRoflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trialsLancet2009374969169570319716961
- RabeKFBatemanEDO’DonnellDWitteSBredenbrokerDBethkeTDRoflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trialLancet2005366948556357116099292
- RayWAMurrayKTHallKArbogastPGSteinCMAzithromycin and the risk of cardiovascular deathN Engl J Med2012366201881189022591294
- WallaceMRMillerLKNguyenMTShieldsAROtotoxicity with azithromycinLancet199434388912417904701
- GuptaSSide-effects of roflumilastLancet2012379981771071122364756