Abstract
The abuse of synthetic cathinones, widely known as bath salts, has been increasing since the mid-2000s. These substances are derivatives of the naturally occurring compound cathinone, which is the primary psychoactive component of khat. The toxicity of synthetic cathinones includes significant sympathomimetic effects, as well as psychosis, agitation, aggression, and sometimes violent and bizarre behavior. Mephedrone and methylenedioxypyrovalerone are currently the predominantly abused synthetic cathinones.
Introduction
A recent manifestation of the interaction between substance abusers, those seeking to profit by providing substances of abuse, and the desire of both parties to avoid legal censure, is the emergence of bath-salt abuse. Bath salts in this context refer to several synthetic cathinones sold as either bath salts or plant food, but not intended for any horticultural or personal hygiene use. Despite the ubiquitous packaging disclaimer “not intended for human consumption,” these substances are sold as “legal highs” and drugs of abuse.Citation1
History
Cathinone is a naturally occurring phenylalkylamine which is found in the leaves of khat (Catha edulis), a shrub or small tree indigenous to East Africa and the Arabian Peninsula.Citation2 Historical references to the chewing of khat leaves for their euphoric and stimulant effects dates back many centuries, and today this practice is prevalent in such countries as Somalia, Yemen, Kenya, and Ethiopia.Citation3 Khat leaves contain multiple compounds, notably phenylalkylamine alkaloids that include norpseudoephedrine, cathinone, and cathine.Citation4 Studies in the 1930s to determine the psychoactive components of khat initially erroneously identified norpseudoephedrine as the main psychoactive compound.Citation4 In 1975, cathinone was isolated from khat leaves and determined to be its principal psychoactive component.Citation5 Although cathinone is a chiral molecule, khat leaves contain only the S (−) enantiomer,Citation6 and synthesis of the racemate was not achieved until 1982.Citation7 Cathinone begins to decompose shortly after leaves are harvested, and thus fresh leaves are sought for chewing. This may explain why khat chewing has largely remained limited to geographic regions where Catha edulis grows.Citation3 In distinction to synthetic cathinone abuse, habitual khat chewing reportedly results in only mild psychological dependence and mild withdrawal symptoms.Citation8,Citation9
As reviewed by Kelly, the earliest report of synthetic cathinone synthesis is in 1928 with the synthesis of methcathinone in Germany, followed by the synthesis of mephedrone in 1929.Citation10 Today, there are approximately 30 known synthetic cathinones.Citation10
Although many synthetic cathinones have been investigated as anorectics, central nervous system stimulants, and antidepressants, clinical utility has been hindered by problems with abuse and dependence. For instance, methcathinone was initially used as an antidepressant in the USSR but removed from clinical use due to abuse.Citation11 Currently, the synthetic cathinone diethylpropion is available for use as an anorectic, but is infrequently prescribed due to abuse and dependence. In addition, diethylpropion has been shown to be neurotoxic in animal studies.Citation12 Pyrovalerone has been used in the past for chronic fatigue,Citation13 while the most clinically successful cathinone is bupropion, which is widely prescribed for depression and smoking cessation.Citation14
Patterns of abuse
Large-scale abuse of synthetic cathinones began with the use of methcathinone in the USSR in the 1970s and 1980s.Citation11 Clandestine methcathinone manufacture first appeared in the US in Michigan in 1991, followed by significant problems of abuse in the early 1990s.Citation11
Since 2004, abuse of various synthetic cathinones has been reported in Asia, Israel, the EU, and the US, possibly fueled by a decrease in purity and availability of other stimulant drugs of abuse, including MDMA (3,4-methylenedioxy-N-methylamphetamine) and cocaine.Citation15 Data regarding synthetic cathinones seized in the EU since 2006 reveals ten different substances, including mephedrone, methylone, and MDPV (3,4-methylenedioxypyrovalerone), with mephedrone involved in 89% of seizures in the UK.Citation14 US Customs and Border Protection drug-seizure data report the seizure of multiple different synthetic cathinones between July 2009 and April 2011, including MDPV and mephedrone.Citation1 User surveys, poison center reports, and case series in the US and Europe indicate that current synthetic cathinone abuse involves primarily mephedrone and MDPV, with methylone, naphyrone, and flephedrone being less often implicated.Citation9,Citation16–Citation19
Law-enforcement data indicate that synthetic cathinone supply frequently originates in the People’s Republic of China, Pakistan, and India.Citation1 Substances are then sold to the public via the Internet and in retail establishments, including “head shops,” gas stations, convenience stores, and skateboard shops. Products are labeled as bath salts, plant food/fertilizer, vacuum freshener, pond cleaner, and insect repellent, and are typically sold as tablets or white powders.Citation1 While oral ingestion and nasal insufflation have been reported as the most common means of use,Citation9,Citation16 parenteral exposure has also been described, with a recent case series reporting injection as the most common means of use.Citation18
There is little epidemiologic data regarding synthetic cathinone use. Survey data imply that at least among certain demographic groups, use may be widespread. In a 2010 online survey sponsored by a magazine popular with UK clubbers, 41.7% of 2200 respondents reported having used mephedrone.Citation15,Citation20 A 2010 survey of 1006 high school and university students in Scotland reported a 20.3% prevalence of mephedrone use.Citation9 Evidence from poison center calls and drug seizures in the US support the concept that synthetic cathinone abuse is a recent and increasing phenomenon. Synthetic cathinone-related calls to US poison centers increased from zero in 2009 to 304 in 2010 and 6138 in 2011.Citation16,Citation21 Drug samples seized in the US and analyzed by state and local forensic laboratories reveal 34 reports of synthetic cathinones in 2009 and 628 in 2010.Citation17,Citation22
Coingestion of other drugs of abuse and alcohol frequently accompanies synthetic cathinone use.Citation19,Citation23,Citation24 While there are no data regarding people seeking treatment for synthetic cathinone dependence/addiction, users have reported a strong compulsion to redose, as well as addiction/dependence.Citation9
The legal status of cathinone analogues continues to evolve as new substances are produced in order to evade existing laws.Citation1 Mephedrone and several other substituted cathinones were banned in the EU in 2010,Citation23 and as of July 2012, mephedrone, MDPV, and methylone have been added to Schedule I of the Controlled Substances Act in the US.Citation25
Chemistry
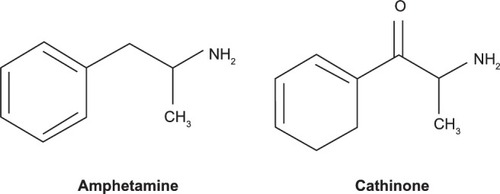
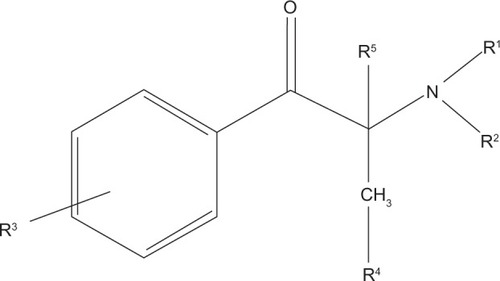
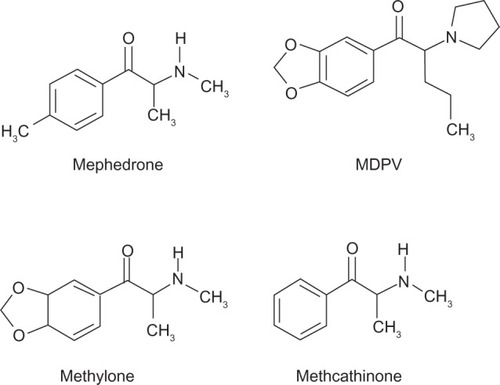
As seen in , cathinones are structurally related to amphetamines.Citation26 Both are substituted phenethylamines with cathinones possessing a ketone group at the β-carbon position. demonstrates the general structure of substituted cathinones. Various R-group substitutions give rise to the approximately 30 known cathinones, many of which have amphetamine analogues to which they are identical, save for the β-carbon ketone group.Citation26 Thus, replacing a hydrogen with a ketone converts amphetamine to cathinone, methamphetamine to methcathinone, and MDMA to methylone.Citation10 The structure of several cathinones recently implicated in recreational abuse can be seen in .
Pharmacokinetics
At present, there is a scarcity of data concerning the pharmacokinetics of synthetic cathinones in humans. However, insights can be gleaned from animal data as well as inferred from studies of naturally occurring cathinones.
The pharmacokinetics of cathinone have been studied in humans by multiple investigators since the initial isolation of this compound from khat leaves in 1975.Citation4 After chewing khat leaves, absorption takes place primarily in the oral mucosa, with a secondary contribution from absorption in the stomach and small intestine.Citation27 Extraction of khat alkaloids by mastication has been reported to be very efficient;Citation27 however, chewing results in delayed peak plasma concentrations when compared to administering oral cathinone. Following chewing khat leaves, time to peak plasma concentrations of 138 ± 39 minutesCitation27 and 127 ± 30 minutesCitation28 have been reported, while administration of gelatin cathinone capsules produced peak plasma concentrations in 72 minutes.Citation29 Orally ingested cathinone undergoes extensive first-pass hepatic metabolism primarily to norpseudoephedrine with a smaller fraction converted to norephedrine.Citation2,Citation27,Citation29,Citation30 Very little cathinone is excreted unchanged, with studies in humans reporting urinary excretion of unchanged cathinone to be between 2%Citation2 and 7%.Citation31 The half-life of cathine is reported to be 5.2 ± 3.4 hours, and cathinone 1.5 ± 0.8 hours in humans.Citation32
Research regarding synthetic cathinone pharmacokinetics in humans is lacking. There are no controlled studies of human in vivo synthetic cathinone pharmacokinetics; however, several studies examining urinary metabolites in people who claim to have ingested synthetic cathinones exist.Citation33–Citation36 In vivo animal studies have been published,Citation33,Citation35–Citation37 as have in vitro investigations utilizing animal hepatocytesCitation38,Citation39 and human liver microsomes.Citation36,Citation37,Citation40 Generalizing the findings of these studies is complicated not only by varying experimental models but also by the multiple different synthetic cathinones studied. However, within the framework of these limitations, several preliminary conclusions may be drawn. Similar to naturally occurring cathinone, synthetic cathinones appear to undergo extensive phase I and II metabolism,Citation33–Citation38,Citation40 with little of the drug excreted unchanged in urine.Citation35,Citation37 Commonly identified phase I reactions are demethylation and oxidation, as well as reduction of the β-keto moiety.Citation35,Citation36,Citation38 Glucuronidation of metabolites has been described by several investigators.Citation37,Citation38 Indirect evidence of sulfate conjugation has been reported;Citation33 however, other investigations have been unable to confirm this.Citation37,Citation38 Human liver microsomes have been used by Meyer et al in two separate studies, with the finding that human cytochrome P450 (CYP) enzymes CYP2B6, CYP2C19, CYP2D6, and CYP1A2 are involved in synthetic cathinone metabolism.Citation36,Citation37 While there are no studies examining synthetic cathinone half-lives in humans, surveys of users suggest that the duration of effects of mephedrone and MDPV are “short,” with users reporting frequent redosing at 1- to 2-hour intervals and a duration of effects of approximately 2–4 hours.Citation41,Citation42 This is not inconsistent with findings in rat hepatocytes of a half-life of approximately 1 hour for mephedrone.Citation38
Compared to amphetamines, the ketone group of cathinones confers a greater polarity and a predicted increased lipophilicity.Citation43 Thus, diffusion across the blood–brain barrier may be decreased.Citation43 However several pyrrolidine derivatives, including MDPV and MDPPP (3,4-methylenedioxy-α-pyrrolidinopropiophenone), have lower polarity and have shown high solubility in organic solvents.Citation15 In addition, recent in vitro studies of mephedrone, MDPV, methylone, ethylone, butylone, and naphyrone demonstrated high blood–brain barrier permeability of all these synthetic cathinones.Citation44
Mechanism of action
In vitro studies utilizing human and animal cell preparations suggest that cathinones acutely increase extracellular dopamine, norepinephrine, and serotonin levels. Similar to amphetamines, cathinones inhibit dopamine, norepinephrine, and serotonin plasma membrane and vesicular monoamine transporters, resulting in increased neurotransmitter synaptic concentration.Citation45,Citation46 Different cathinones display varying ability to cause increases in extracellular dopamine, norepinephrine, and serotonin levels, which may account for the different mood-altering effects, toxicity, and potential for addiction related to these compounds. Mephedrone, for instance, has been shown to significantly inhibit norepinephrine reuptake.Citation46 While the expected sympathomimetic effects have not been specifically demonstrated in human studies, case reports do support a strong sympathomimetic effect of some synthetic cathinones, including mephedrone.Citation18,Citation24,Citation47 The reported addictive potential of several synthetic cathinones is likely related to effects on increasing extracellular dopamine, as has been demonstrated in animal models.Citation48
Toxicology
The current body of knowledge regarding the adverse effects of synthetic cathinones is based largely on case reports, data from poison centers, and surveys of users. Inherent flaws in these data are likely, as users may not be accurate regarding substances ingested, the amounts ingested, and the presence of coingestions. Compounding this is the limited ability to test accurately for synthetic cathinones,Citation15 and purchased products that do not contain the substances advertised or contain unlisted compounds in addition to the advertised drug. Inaccurate labeling of products has been reported, with products containing synthetic cathinones other than the listed compound, other substances of abuse, including MDMA and ketamine, and pharmaceuticals, including acetaminophen, caffeine, benzocaine, and lidocaine.Citation49,Citation50 Additionally, products sold as cocaine and MDMA have been found to contain synthetic cathinones.Citation51,Citation52 Finally, a review of 15 products sold as “legal highs” in the USA found no ingredients were listed on any of the packages, thus making it very difficult for patients to report exposures accurately.Citation19
Toxicity of naturally occurring cathinones
The reported toxicity of the natural substances cathinone and cathine appears less severe than many of their synthetic counterparts. This may be due to chewing khat representing the primary means of ingestion and the bulk of leaves that must be chewed in order to produce significant toxic effects.Citation29 Described toxic effects of chewing khat include depression, irritability, insomnia, anorexia, and paranoid psychosis. Adverse cardiovascular effects include hypertension, tachycardia, and an increased incidence of acute myocardial infarction and cerebral vascular accidents.Citation53 Chewing khat is also associated with an increased incidence of oral cancer.Citation54
Toxicity of synthetic cathinones
Toxic effects of synthetic cathinones include sympathomimetic effects, as well as psychological effects, including aggression, agitation, paranoia, and delusions.Citation16,Citation19,Citation55 Seizures, hyponatremia, hyperthermia, rhabdomyolysis, disseminated intravascular coagulation, renal failure, and hepatic failure have also been reported, as have several deaths.Citation19,Citation47,Citation56,Citation57 Several reports, primarily from Eastern Europe, document parkinsonism in patients following long-term parenteral use of methcathinone. Manganese contamination of homemade methcathinone has been identified as the cause.Citation58,Citation59
Chronic amphetamine use is known to be neurotoxic to dopaminergic neurons, resulting in long-term reductions in brain dopamine concentrations in chronic users.Citation60 Serotonergic neurotoxicity as a result of MDMA use occurs in animals, and possibly humans.Citation61 Whether synthetic cathinone abuse is related to dopaminergic or serotonergic neurotoxicity in humans is unknown; however, serotonin neurotoxicity has been described with methylone and mephedrone in rats.Citation62
User-reported adverse effects
User-reported adverse effects of synthetic cathinone use commonly include agitation, paranoia, bruxism, palpitations, headache, and depression.Citation9,Citation63,Citation64 A 2010 survey of 1006 students in Scotland with 205 mephedrone users found 56% reported at least one adverse effect, most commonly bruxism (28.3%) and paranoia (24.9%).Citation9 Mixmag is a publication popular with UK clubbers and host of the large Mixmag Drugs Survey, which in 2012 included online responses from over 15,500 respondents. Commonly reported adverse mephedrone effects included depression (41%), agitation (23%), “overheating” (26%), severe headache (12%), and chest pain (10%).Citation63 In a smaller study from Ireland utilizing privileged-access interviewing of eleven intravenous users of mephedrone, all users reported intense paranoia, and two reported extreme aggression and violence.Citation64
Adverse effects reported to poison centers
Calls by physicians to the National Poisons Information Service in the UK from March 2009 to February 2010 included 188 calls regarding cathinones, with 131 of these concerning mephedrone. Reported adverse effects included agitation or aggression (24%), tachycardia (22%), confusion or psychosis (14%), chest pain (13%), and palpitations (11%).Citation16 A report documenting synthetic cathinone-related calls to Texas poison centers in 2010 and 2011 found 362 calls, with common adverse effects being tachycardia (45.9%), agitation (39.2%), hypertension (21.0%), and hallucinations (17.7%).Citation55 During an 8-month period in 2010 and 2011, 236 calls were received by poison centers in Kentucky and Louisiana regarding synthetic cathinone intoxication. Commonly reported toxicities included agitation (82%), combative behavior (57%), tachycardia (56%), hallucinations (40%), and paranoia (36%). This study also included descriptions of severe delusional behavior, including leaving a 2-year-old child in a highway “because she had demons,” firing guns at nonexistent people and “demons,” and destroying all the windows in a home and walking barefoot through the resulting broken glass. One patient died as a result of a self-inflicted gunshot wound while delusional.Citation19
Adverse effects reported in case series
Multiple case reports from emergency departments concerning patients presenting with synthetic cathinone toxicity, without analytical confirmation of substances ingested, have been published. Findings are summarized in .
Table 1 Synthetic cathinone toxicity: case reports with ≥15 patients without analytical confirmation of synthetic cathinone exposure
Case reports in which the presence of synthetic cathinones was confirmed by body-fluid or tissue analysis include multiple single-patient case reports, a series of three deaths involving methylone, and a series of seven patients with mephedrone toxicity.Citation17,Citation51,Citation56,Citation57,Citation65–Citation70 A series of 13 patients with confirmed MDPV intoxication has been published, but clinical findings in patients with confirmed and unconfirmed exposures are grouped together.Citation19 In the report of seven patients with confirmed mephedrone ingestion, five of the patients had only synthetic cathinones detected on drug screening, while the other two patients also tested positive for cocaine. Adverse effects included heart rate >100 (five patients), systolic blood pressure >160 (three), agitation (four), and seizure (one). One patient had hyponatremia with a serum sodium concentration of 125 mmol/L, and one patient had rhabdomyolysis. No patients had significant hyperpyrexia.Citation65 In the three reported deaths from methylone intoxication, all patients had hyperpyrexia and seizures, with metabolic acidosis, disseminated intravascular coagulation, and acute renal failure also reported.Citation66 Single-patient case reports have also reported hyperpyrexia, seizures, hyponatremia, rhabdomyolysis, and metabolic acidosis.Citation51,Citation56,Citation57,Citation69
Conclusion
The recreational use of synthetic cathinones, widely known as bath salts, has been the subject of much recent interest. Synthetic cathinones are derivatives of the naturally occurring compound cathinone, which is the primary psychoactive component of khat. Cathinones are structurally related to amphetamines, and their mechanisms of action are thought to be similar. Although the first synthetic cathinones were synthesized in the 1920s, and recreational abuse can be traced back many decades, rapid increase in use began in the mid-2000s, likely fueled by the legality of these substances. Mephedrone and MDPV have been the dominant synthetic cathinones of abuse in recent years, and are implicated in an increasing number of emergency department visits due to adverse effects. Case series and poison center data indicate that toxicity includes significant sympathomimetic effects, as well as psychosis, agitation, aggression, and sometimes violent and bizarre behavior. Multiple deaths attributed to synthetic cathinone use have been reported. There is a paucity of data concerning the pharmacodynamics and pharmacokinetics of synthetic cathinones in humans, with current understanding based primarily on in vitro and animal studies. Long-term effects of synthetic cathinone use, including the potential for addiction/dependence, are largely unknown. Although mephedrone and MVPV are now illegal in many countries, the current landscape of synthetic cathinone abuse will likely continue to shift as new substances are developed and marketed, and as legal pressures change.
Disclosure
The author reports no conflicts of interest in this work.
References
- US Department of Justice National Drug Intelligence CenterSituation report: Synthetic cathinones (bath salts) – an emerging domestic threat2011 Available from: http://www.justice.gov/archive/ndic/pubs44/44571/44571p.pdfAccessed December 10, 2012
- KalixPBraendenOPharmacological aspects of the chewing of khat leavesPharmacol Rev19853721491642864707
- [No authors listed]Review of the pharmacology of khat. Report of a WHO advisory groupBull Narc198032383936266563
- SzendreiKThe chemistry of khatBull Narc19803235356911031
- United NationsStudies on the chemical composition of khat. III. Investigations on the phenylalkylamine fraction UN document MNAR/11/1975
- PetersonDWMaitaiCKSparberSBRelative potencies of two phenylalkylamines found in the abused plant Catha edulis, khatLife Sci19802722214321476111010
- BerrangBDLewinAHCarrollFIEnantiomeric alpha-aminopropiophenones (cathinone): preparation and investigationJ Org Chem1982471326432647
- LuqmanWDanowskiTSThe use of khat (Catha edulis) in Yemen. Social and medical observationsAnn Intern Med1976852246249942147
- DarganPIAlbertSWoodDMMephedrone use and associated adverse effects in school and college/university students before the UK legislation changeQJM20101031187587920675396
- KellyJPCathinone derivatives: a review of their chemistry, pharmacology and toxicologyDrug Test Anal201137–843945321755607
- GlennonRAYoungRMartinBRDal CasonTAMethcathione (“cat”): an enantiomeric potency comparisonPharmacol Biochem Behav19955046016067617707
- Galvan-ArzateSSantamariaANeurotoxicity of diethylpropion: neurochemical and behavioral findings in ratsAnn N Y Acad Sci200296521422412105097
- GardosGColeJOEvaluation of pyrovalerone in chronically fatigued volunteersCurr Ther Res Clin Exp197113106316354402508
- Advisory Council on the Misuse of DrugsACMD report on the consideration of the cathinones2010 Available from: http://www.homeoffice.gov.uk/acmd1/acmd-cathinodes-report-2010?view=BinaryAccessed November 1, 2012
- CoppolaMMondolaRSynthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food.”Toxicol Lett2012211214414922459606
- JamesDAdamsRDSpearsRClinical characteristics of mephedrone toxicity reported to the UK National Poisons Information ServiceEmerg Med J201128868668920798084
- ThorntonSLGeronaRRTomaszewskiCAPsychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantificationJ Med Toxicol20128331031322528592
- Centers for Disease Control and Prevention (CDC)Emergency department visits after use of a drug sold as “bath salts” – Michigan, November 13, 2010–March 31, 2011MMWR Morb Mortal Wkly Rep2011601962462721597456
- SpillerHARyanMLWestonRGJansenJClinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United StatesClin Toxicol2011496499505
- DickDTorranceCMixmag drugs surveyMixmag20102254453
- American Association of Poison Control Centers (AAPCC)Bath salts data2012 Available from: https://aapcc.s3.amazonaws.com/files/library/Bath_Salts_Data_for_Website_1.09.2013.pdfAccessed December 15, 2012
- Drug Enforcement AdministrationSpecial Report: Synthetic Cannabinoids and Synthetic Cathinones Reported in NFLIS, 2009–2010Springfield (VA)DEA2011 Available from: http://www.deadiversion.usdoj.gov/nflis/2010rx_synth.pdfAccessed December 10, 2012
- DarganPISedefovRGallegosAWoodDMThe pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone)Drug Test Anal201137–845446321755604
- ReganLMitchelsonMMacdonaldCMephedrone toxicity in a Scottish emergency departmentEmerg Med J201128121055105821183522
- Drug Enforcement AdministrationSchedules of controlled substances: placement of ezogabine into Schedule V. Final ruleFed Regist201176241778957789922175093
- KalixPCathinone, a natural amphetaminePharmacol Toxicol199270277861508843
- ToennesSWHarderSSchrammMNiessCKauertGFPharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leavesBr J Clin Pharmacol200356112513012848785
- WidlerPMathysKBrenneisenRKalixPFischHUPharmacodynamics and pharmacokinetics of khat: a controlled studyClin Pharmacol Ther19945555565627910126
- BrenneisenRFischHUKoelbingUGeisshuslerSKalixPAmphetamine-like effects in humans of the khat alkaloid cathinoneBr J Clin Pharmacol19903068258282288828
- GuantaiANMaitaiCKMetabolism of cathinone to d-norpseudoephedrine in humansJ Pharm Sci19837210121712186644577
- ToennesSWKauertGFExcretion and detection of cathinone, cathine, and phenylpropanolamine in urine after kath chewingClin Chem200248101715171912324488
- KalixPPharmacological properties of the stimulant khatPharmacol Ther19904833974161982180
- MeyerMRWilhelmJPetersFTMaurerHHBeta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometryAnal Bioana Chem2010397312251233
- ZaitsuKKatagiMKamataHTDetermination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urineForensic Sci Int20091881–313113919406592
- KamataHTShimaNZaitsuKMetabolism of the recently encountered designer drug, methylone, in humans and ratsXenobiotica200636870972316891251
- MeyerMRDuPSchusterFMaurerHHStudies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MSJ Mass Spectrom201045121426144221053377
- MeyerMRVollmarCSchwaningerAEWolfEMaurerHHNew cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urineJ Mass Spectrom201247225326222359337
- KhreitOIGrantMHZhangTHendersonCWatsonDGSutcliffeOBElucidation of the phase I and phase II metabolic pathways of (+/−)-4′-methylmethcathinone (4-MMC) and (+/−)-4′-(trifluoromethyl) methcathinone (4-TFMMC) in rat liver hepatocytes using LC-MS and LC-MS(2)J Pharm Biomed Anal20137217718522985528
- PawlikEPlässerGMahlerHDaldrupTStudies on the phase I metabolism of the new designer drug 3-fluoromethcathinone using rabbit liver slicesInt J Legal Med2012126223124021785905
- MuellerDMRentschKMGeneration of metabolites by an automated online metabolism method using human liver microsomes with subsequent identification by LC-MS(n), and metabolism of 11 cathinonesAnal Bioanal Chem201240262141215122231510
- Psychonaut WebMapping Research GroupMephedrone ReportLondonInstitute of Psychiatry, King’s College London2009
- NewcombeRMephedrone: The Use of Mephedrone (M-cat, Meow) in MiddlesbroughManchesterLifeline Publications and Research2009
- GibbonsSZlohMAn analysis of the ‘legal high’ mephedroneBioorg Med Chem Lett201020144135413920542690
- SimmlerLDBuserTADonzelliMPharmacological characterization of designer cathinones in vitroBr J Pharmacol2013168245847022897747
- CozziNVSievertMKShulginATJacobP3rdRuohoAEInhibition of plasma membrane monoamine transporters by beta-ketoamphetaminesEur J Pharmacol19993811636910528135
- Lopez-ArnauRMartinez-ClementeJPubillDEscubedoECamarasaJComparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methyloneBr J Pharmacol2012167240742022509960
- WoodDMGreeneSLDarganPIClinical pattern of toxicity associated with the novel synthetic cathinone mephedroneEmerg Med J201128428028220581379
- KehrJIchinoseFYoshitakeSMephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake ratsBr J Pharmacol201116481949195821615721
- DaviesSWoodDMSmithGPurchasing ‘legal highs’ on the Internet – is there consistency in what you get?QJM2010103748949320413562
- European Monitoring Centre for Drugs and Drug AddictionReport on the Risk Assessment of Mephedrone in the framework of the Council Decision on New Psychoactive SubstancesLisbonEMCDDA2011
- SauerCHoffmannKSchimmelUPetersFTAcute poisoning involving the pyrrolidinophenone-type designer drug 4′-methyl-alpha-pyrrolidinohexanophenone (MPHP)Forensic Sci Int20112081–3e20e2521444164
- BruntTMPoortmanANiesinkRJvan den BrinkWInstability of the ecstasy market and a new kid on the block: mephedroneJ Psychopharmacol201125111543154720826554
- Al-MotarrebABrianconSAl-JaberNKhat chewing is a risk factor for acute myocardial infarction: a case-control studyBr J Clin Pharmacol200559557458115842556
- BalintEEFalkayGBalintGAKhat – a controversial plantWien Klin Wochenschr200912119–2060461419921126
- ForresterMBSynthetic cathinone exposures reported to Texas poison centersAm J Drug Alcohol Abuse201238660961522541001
- BorekHAHolstegeCPHyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovaleroneAnn Emerg Medicine2012601103105
- SammlerEMFoleyPLLauderGDWilsonSJGoudieARO’RiordanJIA harmless high?Lancet2010376974274220801405
- IqbalMMonaghanTRedmondJManganese toxicity with ephedrone abuse manifesting as parkinsonism: a case reportJ Med Case Rep2012615222313512
- YildirimEAEsşsizoğluAKöksalADoğuBBaybasşSGökalpP[Chronic manganese intoxication due to methcathinone (ephedron) abuse: a case report]Turk Psikiyatri Derg2009203294298 Turkish19757228
- EllisonGNeural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitryEur Neuropsychopharmacol200212428729712126867
- PuertaEHerviasIAguirreNOn the mechanisms underlying 3,4-methylenedioxymethamphetamine toxicity: the dilemma of the chicken and the eggNeuropsychobiology2009603–411912919893329
- den HollanderBRozovSLindenAMUusi-OukariMOjanperaIKorpiERLong-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedronePharmacol Biochem Behav2012103350150923099177
- MixmagMixmag’s drug survey: the results2012 Available from: http://www.mixmag.net/drugssurveyAccessed December 1, 2012
- Van HoutMCBinghamT“A costly turn on”: patterns of use and perceived consequences of mephedrone based head shop products amongst Irish injectorsInt J Drug Policy201223318819722342322
- WoodDMDaviesSGreeneSLCase series of individuals with analytically confirmed acute mephedrone toxicityClin Toxicol2010489924927
- PearsonJMHargravesTLHairLSThree fatal intoxications due to methyloneJ Anal Toxicol201236644445122589523
- AdamowiczPTokarczykBStanaszekRSlopiankaMFatal mephedrone intoxication – a case reportJ Anal Toxicol2013371374223134715
- WoodDMDaviesSPuchnarewiczMRecreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicityJ Med Toxicol20106332733020358417
- MurrayBLMurphyCMBeuhlerMCDeath following recreational use of designer drug “bath salts” containing 3,4-methylenedioxypyrovalerone (MDPV)J Med Toxicol201281697522271565
- DerungsASchietzelSMeyerMRMaurerHHKrahenbuhlSLiechtiMESympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone)Clin Toxicol2011497691693