?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The assessment of percutaneous permeation of molecules is a key step in the evaluation of dermal or transdermal delivery systems. If the drugs are intended for delivery to humans, the most appropriate setting in which to do the assessment is the in vivo human. However, this may not be possible for ethical, practical, or economic reasons, particularly in the early phases of development. It is thus necessary to find alternative methods using accessible and reproducible surrogates for in vivo human skin. A range of models has been developed, including ex vivo human skin, usually obtained from cadavers or plastic surgery patients, ex vivo animal skin, and artificial or reconstructed skin models. Increasingly, largely driven by regulatory authorities and industry, there is a focus on developing standardized techniques and protocols. With this comes the need to demonstrate that the surrogate models produce results that correlate with those from in vivo human studies and that they can be used to show bioequivalence of different topical products. This review discusses the alternative skin models that have been developed as surrogates for normal and diseased skin and examines the concepts of using model systems for in vitro–in vivo correlation and the demonstration of bioequivalence.
Introduction
The skin is a major physical, immunological, and sensory barrier to our environment. While it has long been used as a portal for drug delivery, it is a formidable barrier that requires appropriate technology for successful delivery. It is particularly effective in preventing large (ie, molecular weight >500) or polar molecules from entering the body. It is also a heterogeneous organ, with several delivery routes and sites that could be targeted for desirable pharmacological and immune responses. A key challenge is to deliver to the target site sufficient quantities of the drugs, peptides, vaccines, and dyes that are mainly larger and polar to achieve these responses. This may require the design of a specific chemical or physical delivery system to enhance the permeation of the active substance.
The assessment of percutaneous permeation is key to the successful development of new formulations intended for human use. It is also an important quality-control measure to ensure batch-to-batch uniformity in the pharmaceutical industry.Citation1 Clinical end-point bioequivalence studies have generally been used for bioequivalence assessments of locally acting products. However, this is not the most feasible approach, due to the high costs involved, as well as the lack of sensitivity in highlighting formulation differences. Alternative methods for evaluating product performance include a range of models. More commonly used models to conduct skin-permeation studies are ex vivo human or animal skin. Through the standardization of protocols and techniques, the available skin models can be useful as surrogate models for in vivo human skin to evaluate the bioequivalence of topical products.
This review discusses the alternative skin models that have been developed as surrogates for normal and diseased skin, and examines the concepts of using model systems for in vitro–in vivo correlation (IVIVC) and the demonstration of bioequivalence. lists a range of appropriate skin models.
Table 1 Skin models
Human skin structure and function
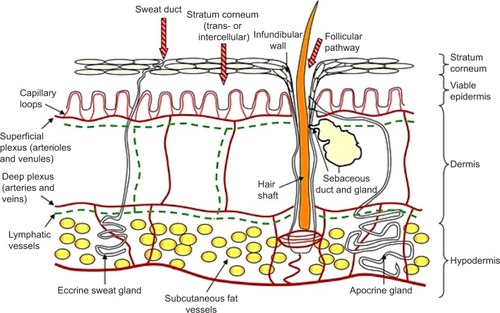
A comprehensive review of the structure and function of skin can be found in Monteiro-Riviere.Citation2 The skin accounts for approximately 16% of human body weight, with a surface area of approximately 2 m2 in adults.Citation3,Citation4 It provides a physical barrier to the environment, maintains homeostasis by limiting the loss of water, electrolytes, and heat, and protects against microorganisms, toxic agents, and ultraviolet radiation.Citation5 There are three basic layers: the epidermis, the dermis, and the subcutaneous layer. Hair, nails, sebaceous glands, and sweat glands (apocrine and eccrine) are considered to be skin derivatives or appendages. Even though it is structurally continuous throughout the body, skin varies in thickness according to the age of the individual and the anatomical site.
The epidermal layer is formed from squamous epithelium and is subdivided into separate layers, according to the degree of keratinization of the cells. The layers of the epidermis from the bottom to the surface are stratum basale (basal cell layer), the stratum spinosum (spinous or prickle-cell layer), the stratum granulosum (granular cell layer), and the stratum corneum (SC; horny layer) ().Citation6
The outermost layer of the epidermis, the SC, consists of denucleated, nonliving, flattened cells called corneocytes. There are ten to 25 layers of stacked corneocytes, which are nonhydrated cells lying parallel to the skin surface.Citation5 The SC layers are united by SC lipid bilayers assembled into a “brick and mortar” arrangement.Citation7
Below the SC, the remainder of the epidermis is viable tissue, called viable epidermis, containing nucleated cells called keratinocytes. The viable epidermis is a region for drug binding, metabolism, active transport, and surveillance. In addition to keratinocytes, it contains melanocytes (dendritic cells found on the basement membrane and in the basal layer), Merkel cells (functioning as mechanoreceptors involved in mediation of touch responses, found in the basal region), and Langerhans cells (dendritic cells playing a key role in protective immune function, present mainly in the stratum spinosum).Citation2
The viable epidermis is separated from the dermis at the dermal–epidermal junction. The dermis is rich in collagen. The subcutaneous (hypodermis) layer is the deepest layer of the skin and is formed from loose connective tissue and fat (50% of the body fat), which may be more than 3 cm thick on the abdomen. The dermis and subcutaneous layers contain blood vessels, lymphatics, and nerve cells, in addition to skin appendages.Citation8
The SC acts as the primary skin barrier, with the essential functions of protecting the body from the surrounding environment, providing an efficient obstacle to the permeation of exogenous moleculesCitation8 and microorganismsCitation9 and maintaining homeostasis by preventing excessive loss of water.Citation10 The surveillance, metabolic, and transport processes located in the deeper skin layersCitation11,Citation12 also contribute to the protective functions of the skin.
Models used to evaluate dermal absorption
Ex vivo human skin models
Measurement of dermal absorption for the purpose of targeted skin delivery, systemic delivery, or toxicological assessment should be done under the correct conditions, ideally using the gold-standard experimental model: in vivo human skin.Citation13 However, this is not always possible, due to the high cost of human trials and concerns over applying substances or materials with potentially toxic effects. As well, in vivo responses may be difficult to measure and interpret and subject to significant variability. Alternative methods are needed to derive data that are reproducible and reliable and which provide a meaningful prediction of the in vivo human situation.Citation14 There is a large body of work based on ex vivo human skin, and as we shall discuss in more detail later, some success has been achieved in correlating in vitro and in vivo dermal absorption, often driven by regulators seeking a standard, robust assessment method.Citation15,Citation16
Excised human skin is most commonly obtained from plastic surgery or cadavers, and in both cases, appropriate ethical approval is required to use the tissue. Abdominal, breast, or back skin is most convenient, due to the large areas that may be available. There are considerable differences in skin absorption across different body sites,Citation17 attributed to such factors as differences in SC thickness,Citation18 hydration,Citation19,Citation20 and lipid composition.Citation21 Clearly, this needs to be recognized when designing studies.
As noted earlier, the SC represents the main barrier to penetration of exogenous substances into the skin,Citation8 as well as controlling the loss of water from inside the body.Citation10 It is believed that skin may be stored for up to 6 months without loss of SC barrier function,Citation22 particularly if 10% glycerol is used as a preservative.Citation23 Nielsen et al also found little effect of freezing at –20°C for 3 weeks, with or without polyethylene glycol as a preservative, but significant damage with storage at –80°C.Citation24 On the contrary, other evidence suggests significant loss of barrier function, causing increased skin permeation, with frozen storage of animal skin.Citation25,Citation26 Barbero and Frasch concluded that carefully handled frozen human skin was suitable for testing the passive permeation of chemicals, when skin viability and metabolic activity were not being investigated.Citation23 Cellular viability and metabolic activity within the human epidermis are likely to be reduced by frozen storage or heat.Citation27,Citation28 Our unpublished results from an MTT assay showed a complete elimination of epidermal viability following heat separation by the method of Kligman and Christophers.Citation29 For studies requiring the presence of viable epidermal tissue, such as imaging of endogenous skin autofluorescence by multiphoton tomography,Citation30 or investigations of skin metabolism,Citation28 fresh tissue is required.
Several different membrane types may be prepared from ex vivo human skin for use in permeation experiments. “Full-thickness skin” is prepared by removal of connective tissue and subcutaneous fat and consists of all layers down to and including the dermis.Citation31 To reduce variability while retaining significant dermal thickness, full-thickness skin may be cut to approximately 500–750 μm with a dermatome.Citation32 However, the presence of the hydrated dermis may introduce an additional, artificial barrier to permeation, particularly for more lipophilic molecules.
In contrast to the in vivo situation, where capillary circulation rapidly clears penetrated molecules, full-thickness or dermatomed skin mounted in a diffusion cell represents a situation analogous to vasoconstriction.Citation33 Consequently, the use of a membrane consisting of only the SC and the viable epidermis may be preferred. According to Cross and Roberts, this membrane represents a situation of “infinite dilatation”, since all material making its way past the SC barrier is immediately available to the receptor solution.Citation33 Atrux-Tallau et al found that dermatomed human skin and heat-separated epidermal membranes gave the same flux for caffeine, a hydrophilic compound.Citation34 Results from our group where steroids were applied to epidermal membranes, full-thickness skin, and isolated dermis showed that there was a minimal effect of increasing lipophilicity on epidermal maximum flux and a trend toward decreased dermal penetration rates.Citation35
Epidermal membranes are most commonly prepared by immersing full-thickness skin in hot water (~60°C) for approximately 1 minute.Citation29 Other techniques designed to separate the membrane at the dermal–epidermal junction include chemical treatments with ethylenediaminetetraacetic acid, ammonia, and enzymes.Citation32 Some researchers have used the isolated SC for permeation experimentsCitation36 and for desorption studies designed to study SC heterogeneity,Citation37 and the SC reservoir for water and other substances.Citation38 The SC is prepared by enzymatic methods, usually by incubation with aqueous trypsin solution, after which the digested epidermal material is rinsed and wiped off.Citation39
Ex vivo human skin: use in bioequivalence studies
For the majority of topical drug products, comparative clinical end-point studies are used to demonstrate bioequivalence to a reference drug. While this provides a direct in vivo assessment, it is also associated with a number of challenges. Clinical end points are associated with high variability (intra-subject) and low sensitivity (drug-related), which makes such studies less reliable and less efficient. The other clinical alternative is to use pharmacokinetic studies to demonstrate bioequivalence for topical products, but this is limited to particular cases where significant systemic absorption of the drug occurs. Recently, the use of techniques including in vitro permeation testing (IVPT), in vivo tape stripping, or dermatopharmacokinetics, and in vivo microdialysis or microperfusion, has been advocated for testing bioequivalence.Citation40
IVPT using human dermatomed skin mounted in diffusion cells is increasingly seen as a suitable tool for demonstrating bioequivalence of topical dosage forms.Citation40 Indeed, the generation of such data has been encouraged by regulatory agencies, such as the European Medicines Agency, the US Environmental Protection Agency, and the US Food and Drug Administration (FDA). Their utility is grounded on substantial evidence that 1) there is a good correlation between the in vitro and in vivo rates and extents of human skin absorption of a number of different substances and 2) there is good agreement on the bioequivalence of topical products seen with IVPT and in vivo clinical studies.
Currently, however, there are no approved protocols for carrying out IVPT studies. Franz et al pointed out that the demonstration of valid IVIVC is greatly dependent upon the protocols used, and they recommended that the in vitro and in vivo protocols followed should be as closely harmonized as possible to maximize the chance of achieving a good correlation.Citation15,Citation41 The work on which this conclusion was based was an analysis of historical literature data that was available to the researchers, and despite the wide variation in the way in which it was collected, their conclusion was that there was compelling evidence that it was possible to correlate IVPT data with human in vivo skin-absorption data. Others who have demonstrated good IVIVC include Hadgraft et al, who compared in vitro and in vivo delivery from nitroglycerin patches,Citation42 and more recently Yang et al, who compared their own IVPT data with literature reports of in vivo estradiol delivery from patches.Citation16
While the use of IVPT for bioequivalence has only recently been formalized, the design of in vitro permeation tests has been subject to consideration and validation for many years. In 1987, the FDA published a report on the important factors to be considered, which included the membrane type (dermatomed skin or heat-separated epidermis?), the receptor fluid, the cell design (static or flow-through?), application (finite or infinite dose?), and temperature.Citation43
The in vivo dermatopharmacokinetic (DPK) method uses tape stripping to remove SC layers. The FDA has investigated the possibility of introducing a DPK method for evaluating bioavailability and/or bioequivalence of topical dermatological drug products.Citation44,Citation45 In the DPK method, it is assumed that 1) in normal circumstances, the SC is the rate-determining barrier to percutaneous absorption, 2) the SC concentration of the drug is related to the amount that diffuses into the underlying viable epidermis, and 3) SC drug levels are more useful and relevant for assessing local, dermatological efficacy than plasma concentrations.Citation46 It is also possible to deduce partitioning and diffusion parameters that characterize the absorption process and which can subsequently be used to predict an entire absorption profile from a single short-contact-duration experiment.Citation44 The technique is very operator-dependent, and care needs to be taken to apply and remove the tapes reproducibly. The success of the method is equally dependent on the development of sensitive analytical methods to quantify the amount of drug in the tapes.
Microdialysis involves the insertion of an ultrathin hollow fiber as a probe into the dermis. The probe is semipermeable and perfused with sterile buffer using a microdialysis pump. This involves the exchange of the small diffusible molecules from the extracellular fluid into the probe and vice versa. This method is used to determine the concentration of the unbound drug or biomarkers at the site to establish the concentration-versus-time profile of the applied compound. There are several issues associated with microdialysis. Probe insertion in the skin can lead to inflammatory responses, as may interactions of the perfusing buffer with the tissue.Citation47,Citation48 Recovery is low for highly lipophilic molecules, which may be resolved to some extent by using albumin, cyclodextrins, and cosolvents such as ethanol and dimethyl sulfoxide in the buffer,Citation49 while highly protein-bound molecules may be difficult to detect, due to binding to the probe material. A major disadvantage of the method is the intrasubject variability.Citation50 The newer technique of dermal open-flow microperfusion (dOFM) differs from microdialysis, in that it gives continuous, membrane-free (ie, unfiltered) access to dermal fluid.Citation51 Like microdialysis, dOFM provides a direct estimate of the time course of delivery of the permeant near its site of application. Because of the lack of interaction with a membrane in dOFM, it can be used for a wider range of compounds than microdialysis.Citation40 The technical difficulty of microdialysis and dOFM means that significant operator expertise is required, and as such they are generally only available in a research setting.
Ex vivo animal skin models
The assessment of percutaneous absorption of molecules is an important step in the evaluation of any topical drug-delivery system or formulation. As we have already noted, if the dosage form is to be used in humans, the most relevant skin-absorption data should come from in vivo human studies. However, such studies are generally not feasible during the initial development of a novel pharmaceutical dosage form. Moreover, ex vivo human skin may not be readily available, and so researchers have relied on animal studies for much of the experimental data. This creates a major challenge in correlating results from ex vivo animal experiments with ex vivo and in vivo human studies for prediction of human percutaneous absorption.
A wide range of animal models has been used as alternatives to human skin to evaluate percutaneous permeation of substances. These include pig, mouse, rat, guinea pig, and snake models. Porcine (pig) skin is histologically similar to human skin,Citation52,Citation53 with a comparable SC thickness of 21–26 μm.Citation54,Citation55 In addition, the average hair-follicle density in porcine ear skin is 20/cm2 compared to 14–32/cm2 in human forehead skin.Citation54 As well as being similar to human skin, porcine ear skin is also convenient to obtain and has been widely used in skin-permeation studies.Citation56
The SC lipids are known to be important regulators of skin permeability. With this in mind, the conformational disordering and lateral packing of lipids in isolated porcine and human SC were compared using Fourier-transform infrared spectroscopy. The SC of both species differ markedly, with porcine SC lipids being arranged predominantly in a hexagonal lattice, while lipids in human SC were predominantly packed in the denser orthorhombic lattice.Citation57 In human as well as porcine SC, the main lipid classes are ceramide, cholesterol, and free fatty acid, and these lipid classes are present in an approximately equimolar ratio.Citation58 However, the compositions of free fatty acid and ceramide in the two species are different.
In a range of studies using both lipophilicCitation59,Citation60 and hydrophilicCitation59,Citation61 permeants, the permeability of pig skin was found to be similar to that of human skin, but to differ to a greater extent from dogCitation61 or rodent skin.Citation59,Citation61 Sato et al attributed the similarity in permeability to the similar SC lipids, barrier thickness, and morphological aspects of pig and human skin.Citation61 Nicoli et al further investigated the differences between pig skin and rabbit ear skin, finding that although they had similar SC thicknesses, pig skin was four to seven times more permeable to hydrophilic compounds than rabbit ear skin, most likely due to its different SC lipid composition.Citation62 The relationship between permeability and SC lipids is analogous to early findings by Lampe et al,Citation63 who showed the total lipid-weight percentage at various human body sites (face > abdomen > leg > plantar SC) was inversely proportional to the relative permeability of skin reported for those sites by Scheuplein and Blank.Citation64 Caussin et al also reported the similarity in SC lipid composition, as well as in lamellar organization, between pigs and humans.Citation63,Citation65 Interestingly, however, they also saw a substantial difference in lateral packing between the two species. As with human skin, permeation behavior was found to correlate with barrier function, as measured by transepidermal water loss in a study by Sekkat et al, who applied caffeine, lidocaine, and phenobarbital to tape-stripped pig skin.Citation66
Skin of rodents (mice, rat, and guinea pigs) is the most commonly used in in vitro percutaneous permeation studies, due to its availability, their small size, and relatively low cost. There are different hairless strains of each species that are reported to mimic the permeation properties of human skin better than the hairy variety.Citation67 Among rodents, rat skin is most structurally similar to human skin and it is the most frequently used rodent model. A large number of studies comparing permeation through human and rat skin have been carried out, showing that rat skin is generally more permeable than human skin across a range of permeants of different physicochemical properties,Citation68–Citation72 in some cases with differences of more than an order of magnitude. For example, for compounds with log-P-values ranging from 0.7 to 4.5, van Ravenzwaay and LeiboldCitation72 found that mean in vitro permeation flux through rat skin was around elevenfold greater than through human skin, while a similar comparison by Schmook et alCitation71 found flux increase of 50-fold for the relatively lipophilic molecules hydrocortisone and terbinafine.
Shed snake skin is another interesting membrane that was suggested as a suitable alternative to human skin.Citation73 This membrane, which can be obtained without killing the animal, has some similarity to human skin, in that it consists of thin, flat squamate cells surrounded by intercellular phospholipids, although it does lack hair follicles. Rigg and Barry compared permeation of fluorouracil (5-FU) through dermatomed human abdominal skin and shed skin from two snake species.Citation74 The permeability coefficients were similar between human and dorsal and ventral skin of one snake species, whereas there was a 30-fold increase in dorsal skin from Elaphe (Pantherophis) obsoleta. These authors found no changes in 5-FU permeability in human or snake skin after acetone pretreatment, whereas Megrab et alCitation75 found differential responses in human and dorsal snake skin with vehicles containing different ethanol concentrations. Apart from possible interspecies differences, it is likely that solvent effects in snake skin are influenced by both the lower water contentCitation75 and the nature of the intercellular lipids. While snake skin may be a reasonable model for human skin, it is not readily available, and doubts must exist over the quality and consistency. As Rigg and Barry noted, “[…] if at all possible, investigative problems should not be made more complex by selection of an animal tissue to represent human skin”.Citation74
It may be useful, particularly in the interpretation of dermal absorption for human risk assessment, to predict human in vivo dermal absorption from known in vitro human, in vivo animal, and in vitro animal data, the so-called triple-pack approach.Citation76 The animal in question is normally considered to be the rat. Human in vivo dermal absorption may be derived by the equation:
(1)
Here, it is assumed that 1) the factor between in vitro and in vivo dermal absorption is the same for rats and humans and 2) the factor between rat and human skin absorption is the same in vitro and in vivo, despite the morphological species differences.
Artificial and reconstructed skin models
Artificial and reconstructed skin models are useful tools in specific circumstances, driven by the need to find convenient, reproducible alternatives to in vivo and ex vivo tests with human and animal skin. The artificial skin models range from simple homogeneous polymer materials, such as poly(dimethoxysilane) or silicone membranes through to lipid-based parallel artificial membrane-permeability assay (PAMPA) or phospholipid vesicle-based permeation-assay membranes,Citation77 with the latter material designed to mimic the SC. By eliminating the complexity of human or animal skin, the simple homogeneous materials are particularly useful for studying the basic mechanisms controlling passive transport though a membrane.Citation78–Citation80 The main advantage they have in this regard is their relative reproducibility due to their simple standardized construction. However, they are not intended to represent, nor are they capable of, representing the multitude of in vivo skin properties.Citation81
The PAMPA can be used for rapid screening of passive transport.Citation82 The PAMPA assay is conducted in a 96-well filter plate coated with a liquid artificial membrane to separate two compartments: one containing a buffer solution of compounds to be tested (donor compartment) and the other containing an initial fresh buffer solution (acceptor compartment). Significant correlations with gastrointestinal absorption in humans were seen with PAMPA using filters impregnated with a solution of phospholipids or hexadecane.Citation82 To develop a new artificial membrane to be used in PAMPA for prediction of skin permeation, Ottaviani et al investigated the permeability coefficients of a number of compounds through human skin and the PAMPA-skin artificial membrane comprised of dimethylpolysiloxane (silicone) membranes. They reported a good correlation between the two skin models.Citation83
The FDA has encouraged the use of porous synthetic membranes for evaluating the performance of topical products, as they act as a support without posing a rate-limiting barrier.Citation84 Shah et al from the FDA used different microporous membranes, such as pure cellulose acetate, cellulose, and polysulfone, of similar pore sizes and thicknesses to examine the permeation of hydrocortisone from two commercial creams. They found that the hydrocortisone flux was consistent irrespective of the types of synthetic membrane.Citation85 Nitroglycerin drug release from commercial ointments was investigated by Wu et alCitation86 using ten types of commercial synthetic membranes, such as polysulfone, cellulose mixed esters, polytetrafluoroethylene, and polypropylene, with different pore size and thickness. From the results obtained in this study, the synthetic membranes may be classified into two groups: group 1, consisting of polysulfone, acrylic polymer, glass fiber, silicone, and mixed cellulose ester, showed higher drug permeation compared to group 2, which included polytetrafluoroethylene–polyethylene, mixed cellulose ester (of greater thickness), and polypropylene. The effect of membrane types upon ketoprofen drug release from a gel has also been studied. It was found that nylon exhibited the least rate-limiting effects, although it is a thicker synthetic membrane compared to others.Citation87
Reconstructed skin models are culture-based, with layers of human cells in culture laid down over a polymeric matrix. This allows different cell types to be incorporated to achieve a structure of the desired composition and complexity. Reconstructed models are generally designed to simulate the epidermis (reconstructed human epidermis [RHE] models) or the full human skin (living skin equivalents [LSEs]).Citation22,Citation77
Some reconstructed skin models are produced in-house for particular research purposes, such as drug-candidate or toxicological screeningCitation88 or the assessment of photodamage and photoprotection.Citation89 In one particular reconstructed model, consisting of layers of human dermal fibroblasts and human epidermal HaCaT cells, there was no change in the permeability coefficients of ibuprofen after freezing the membrane over liquid nitrogen for 24 hours or 6 months. Such a property would make reconstructed membranes attractive for general screening uses. In addition, there are commercially available RHEs (eg, EpiSkin®, SkinEthic®, and EpiDerm®) and LSEs (eg, GraftSkin®, EpiDermFT®, and Pheninon®) that have been suggested as suitable candidates for in vivo and ex vivo skin models in evaluating skin absorption, testing of cosmetic products, and for the toxicological screening of topically applied compounds. A number of studies have compared LSE and HRE models with animal and human skin.Citation71,Citation90,Citation91 Schmook et al studied salicylic acid, hydrocortisone, clotrimazole, and terbinafine permeation through ex vivo human (dermatomed), porcine, and rat skin, GraftSkin LSE, and SkinEthic RHE.Citation71 The fluxes and skin accumulation were generally in the order human ≤ porcine < rat < GraftSkin << SkinEthic. Comparing human and pig skin with two RHE models, Schreiber et alCitation91 found permeation coefficients of caffeine and testosterone were both in the order human < pig < EpiDerm << SkinEthic. Schäfer-Korting et al published an extensive comparison of human epidermal membranes, porcine skin, and three RHE models – EpiDerm, EpiSkin, and SkinEthic – with a series of hydrophilic and lipophilic permeants.Citation90 Their general conclusions were that the RHE models, particularly SkinEthic, were significantly more permeable than the ex vivo skins, although the ranking of the permeation of the compounds through pig skin and the RHEs mirrored that through human epidermis. Interestingly, they did not observe the expected improvement in reproducibility with the RHEs compared to the ex vivo skin.
As of 2013, reconstructed skin models had received Organization for Economic Co-operation and Development approval for testing of skin corrosion, acute skin irritation, and phototoxicity.Citation22 None is currently approved for testing of skin absorption. Further work is needed to validate the various models, particularly the LSEs, for this purpose, although they may be useful for in vitro screening. Interestingly, Schäfer-Korting et al concluded that the tested RHEs were applicable to both finite- and infinite-dose studies.Citation90
Models for skin diseases
The skin is not only a convenient portal to the systemic circulation but also a logical site of application for treatment of various localized skin disorders, such as skin cancers, inflammatory illnesses, and damaged skin. As the investigation of disease mechanisms and new therapies is usually difficult or impossible in humans, it is necessary to use alternative methods. Models representing normal or healthy skin are appropriate to test the delivery and targeting of topically applied drugs or other substances, often for the purpose of evaluating the delivery system used. However, models that are designed to mimic the effects of disease states can be used to study the delivery and effects of topical therapies or to gain insight into the molecular mechanisms responsible for particular diseases. In the following sections, we review some of the various animal and artificial models that have been applied to studies of skin diseases. Some recently published studies using animal and reconstructed human skin models for the study of skin diseases, including their use in therapeutic screening, are summarized in and .
Table 2 In vivo animal disease models
Table 3 Reconstructed skin-disease models
Ex vivo animal models for skin diseases
A plethora of in vivo animal models employing fish, guinea pigs, mice, rats, rabbits, and pigs have been developed to mimic human skin diseases. Some recent reviews have focused on the most widely studied areas of melanoma,Citation92 atopic dermatitis,Citation93 and psoriasis.Citation94 Other applications include skin infections (eg, acne, viral infections), damaged skin (eg, wounding, photo-damaged skin), hair disorders (eg, different types of alopecia), and skin cancers, such as basal and squamous cell carcinomas. A significant number of models use genetically engineered mice, due to the fact that many human skin diseases are cause by gene mutations.Citation95 These animal models have been extensively used for the understanding of disease mechanisms and to a lesser extent for the clinical evaluation of drug candidates. For example, epidermal VEGF-knockout mice were used to identify a specific role for epidermal VEGF in the maintenance of epidermal permeability-barrier homeostasis and pointed to the disruption of VEGF pathways in the development of psoriasis.Citation96 In very recent work by Rossbach et al,Citation97 histamine H4 receptor (H4R)-knockout mice showed significant reductions in ovalbumin-induced skin lesions analogous to those caused by atopic dermatitis. Their findings suggested that H4R could be a new therapeutic target in allergic skin diseases like atopic dermatitis.
In addition to melanoma, mouse models have been used particularly for the other common skin cancers, squamous cell carcinoma,Citation98–Citation103 and basal cell carcinoma.Citation104–Citation106 An overview of animal models for a wide range of skin conditions, with an emphasis on their application to drug discovery, has been published by Avci et al.Citation107
Of particular interest are the chimeric models, in which living human skin is grafted on to the skin of severe combined immunodeficient (SCID) mice. In this way, responses or treatments can be studied in living human skin. For example, targeted Kv1.3-cell immunotherapy was shown to be effective in reducing human epidermal thickness and the number of CD3+ lymphocytes in an SCID mouse–human psoriatic skin xenograft model, leading the authors to propose the investigated therapy for treatment of psoriasis and possibly other inflammatory skin conditions.Citation108 Similar investigative work in psoriasis used the SCID mouse–human psoriatic skin xenograft model to identify a role for Hsp90 in signaling pathways that are upregulated in psoriasis. Mice treated orally with the Hsp90 inhibitor Debio 0932 showed a reduction in xenograft epidermal thickness.
The xenograft model has also been used for investigation of cancer targets and therapies. Targeted oralCitation109 or intravenousCitation110 treatments in SCID mouse–human melanoma xenografts caused significant reductions in tumor proliferation and size in BRAF- and ALDH+-specific melanomas.Citation109,Citation110
Reconstructed skin models for diseases
Today, there are increasing regulatory restrictions on the use of animals, and the availability of excised human diseased skin is limited. For these reasons and following the advances in tissue engineering, the development of artificial in vitro human skin models to mimic both healthy and diseased skin has intensified. Another important benefit of using artificial skin models is that they allow the incorporation of specific disease characteristics in a controlled and relatively reproducible manner. In vitro models have been developed for a wide range of skin diseases, such as inflammatory disorders, fungal infections, skin cancer, photodamaged skin, and wounding. A general review has recently been published by Küchler et al.Citation22 The models are generally developed in-house by researchers, with the goals of understanding disease mechanisms and progression, or less commonly to use as screening tools for the assessment of therapeutic modalities. A major challenge in the use of these models is to assess whether they are relevant to and predictable of the in vivo situation.
Inflammatory and autoimmune diseases for which artificial skin models have been developed include psoriasis,Citation111,Citation112 atopic dermatitis,Citation113,Citation114 and eczematous dermatitis.Citation115 In most cases, the specific pathway leading to expression of the disease state was induced by suitable interventions, such as stimulation by psoriasis-associated cytokines,Citation112 or in the generation of atopic dermatitis by downregulation of filaggrinCitation113 or treatment with an inflammatory cocktail.Citation114 Some of the models were developed as potential screening tools for drugs to treat the expressed disease states.Citation112,Citation114
Skin-cancer models were constructed by incorporating various tumor entities within the three-dimensional (3-D) matrix, including cultured melanomaCitation116 cells, an A375 metastatic melanoma cell line,Citation117 and melanoma-tumor spheroids,Citation118 as well as various cutaneous squamous cell carcinoma cell lines.Citation119 Like the inflammatory models, these were used to study disease progression and targeted therapeutic interventions. Mohapatra et alCitation117 used the cyclin-dependent kinase inhibitor roscovitine to inhibit growth of the A375 cells within the dermal layer of the 3-D matrix. Using combination therapies, Vörsmann et alCitation118 showed significant advantages in using their 3-D melanoma model to deliver in vivo-like responses compared to a standard 2-D monolayer culture.
A novel chimeric model consisting of a human artificial 3-D skin construct grafted onto the back of SCID mice has also been reported.Citation120 The bioengineered skin, containing human keratinocytes and fibroblasts isolated from skin biopsies of healthy donors or scleroderma patients, was generated ex vivo and then grafted onto the back of SCID mice. Results implicated the involvement of a PDGF receptor-mediated pathway in the disease and confirmed the suitability for testing in vivo the disease progression-screening antifibrotic drugs.
Summary and conclusion
Despite ethical concerns, the use of animals or isolated animal skin models to assess percutaneous absorption of molecules is frequently reported. These models are generally more widely available than human skin, and prove important in basic research to improve our understanding of the processes, pathways, and driving forces of various agents across the skin barrier. However, because of a large number of animal skin models described in the literature, it may be difficult to compare the results obtained across various species, in addition to the variations in experimental methodology used with a specific skin model, such as type of diffusion cells, body site, skin temperature, receiver media, application dose, and diffusion area. Therefore, it is important to emphasize that in vitro and animal models provide important tools for screening a series of drug formulations, evaluation of skin permeation-enhancing properties and mechanism of action of the carrier systems, and estimation of rank of skin transport for a series of drug molecules. Also, the majority of the work on synthetic membranes for transdermal and topical delivery studies has been focused on the use of polymeric materials, usually silicone based. Such membranes are ideal for replacing ex vivo skin, as they can be prepared with a defined thickness, are easy to handle and store, are comparatively cheap, inert, and provide reproducible results. Despite all of these advantages, they cannot completely replace human or animal skin for prediction of skin absorption in vivo. These membranes generally lack the type of barrier normally provided by the SC in ex vivo or in vivo skin, and this may lead to some false-positive results in toxicity studies and permeation studies. Therefore, we recommend that where possible, human skin should be used in skin-permeation studies.
A wide range of skin models for testing skin absorption for cutaneous and transdermal delivery has been developed. There is an increasing need, largely driven by regulatory authorities and industry, to ensure that the models and testing protocols are standardized and reproducible, and are validated to show that they accurately reflect the in vivo situation.
Disclosure
The authors report no conflicts of interest in this work.
References
- ClowesHMScottRCHeylingsJRSkin absorption: flow-through or static diffusion cellsToxicol In Vitro19948482783020693022
- Monteiro-RiviereNAStructure and function of skinMonteiro-RiviereNAToxicology of the SkinNew YorkInforma Healthcare2010118
- BensouilahJBuckPAromadermatology: Aromatherapy in the Treatment and Care of Common Skin ConditionsLondonRadcliffe2006
- HadgraftJSkin, the final frontierInt J Pharm20012241–211811512545
- BarryBWDermatological Formulations: Percutaneous Absorption18New YorkMarcel Dekker1983
- MontagnaWParakkalPFThe Structure and Function of Skin3rd edNew YorkAcademic Press2012
- RobertsMSCrossSEPellettMAWaltersKASkin transportWaltersKADermatological and Transdermal FormulationsNew YorkMarcel Dekker200289196
- JeppsOGDancikYAnissimovYGRobertsMSModeling the human skin barrier: towards a better understanding of dermal absorptionAdv Drug Deliv Rev201365215216822525516
- DuckneyPWongHKSerranoJYaradouDOddosTStamatasGNThe role of the skin barrier in modulating the effects of common skin microbial species on the inflammation, differentiation and proliferation status of epidermal keratinocytesBMC Res Notes2013647424245826
- BoerMDuchnikEMaleszkaRMarchlewiczMStructural and biophysical characteristics of human skin in maintaining proper epidermal barrier functionPostepy Dermatol Alergol20163311526985171
- DancikYAnissimovYGJeppsOGRobertsMSConvective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical applicationBr J Clin Pharmacol201273456457821999217
- OeschFFabianEGuthKLandsiedelRXenobiotic-metabolizing enzymes in the skin of rat, mouse, pig, guinea pig, man, and in human skin modelsArch Toxicol201488122135219025370008
- LaiJMaibachHIExperimental models in predicting topical antifungal efficacy: practical aspects and challengesSkin Pharmacol Physiol200922523123919729988
- WaltersKAWatkinsonACBrainKRIn vitro skin permeation evaluation: the only realistic optionInt J Cosmet Sci199820530731618505515
- FranzTJLehmanPARaneySGUse of excised human skin to assess the bioequivalence of topical productsSkin Pharmacol Physiol200922527628619707043
- YangYMandaPPavuralaNKhanMAKrishnaiahYSDevelopment and validation of in vitro-in vivo correlation (IVIVC) for estradiol transdermal drug delivery systemsJ Control Release2015210586625979329
- RougierALotteCMaibachHIIn vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping methodJ Pharm Sci19877664514543625489
- Sandby-MøllerJPoulsenTWulfHCEpidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habitsActa Derm Venereol200383641041314690333
- MarrakchiSMaibachHIBiophysical parameters of skin: map of human face, regional, and age-related differencesContact Dermatitis2007571283417577354
- ShrinerDLMaibachHIRegional variation of nonimmunologic contact urticaria: functional map of the human faceSkin Pharmacol1996953123218990506
- LampeMABurlingameALWhitneyJHuman stratum corneum lipids: characterization and regional variationsJ Lipid Res19832421201306833889
- KüchlerSStrüverKFriessWReconstructed skin models as emerging tools for drug absorption studiesExpert Opin Drug Metab Toxicol20139101255126323829446
- BarberoAMFraschHFEffect of Frozen human epidermis storage duration and cryoprotectant on barrier function using two model compoundsSkin Pharmacol Physiol2016291314026606593
- NielsenJBPlasenciaISørensenJABagatolliLAStorage conditions of skin affect tissue structure and subsequent in vitro percutaneous penetrationSkin Pharmacol Physiol20112429310221196813
- AbdayemRRousselLZamanNPirotFGilbertEHaftekMDeleterious effects of skin freezing contribute to variable outcomes of the predictive drug permeation studies using hydrophilic moleculesExp Dermatol2015241297297426268618
- AhlstromLACrossSEMillsPCThe effects of freezing skin on transdermal drug penetration kineticsJ Vet Pharmacol Ther200730545646317803739
- MessagerSHannACGoddardPADettmarPWMaillardJYAssessment of skin viability: is it necessary to use different methodologies?Skin Res Technol20039432133014641882
- WesterRCChristoffelJHartwayTPobleteNMaibachHIForsellJHuman cadaver skin viability for in vitro percutaneous absorption: storage and detrimental effects of heat-separation and freezingPharm Res199815182849487551
- KligmanAMChristophersEPreparation of isolated sheets of human stratum corneumArch Dermatol196388670270514071437
- SanchezWYProwTWSanchezWHGriceJERobertsMSAnalysis of the metabolic deterioration of ex vivo skin from ischemic necrosis through the imaging of intracellular NAD(P)H by multiphoton tomography and fluorescence lifetime imaging microscopyJ Biomed Opt201015404600820799810
- CrossSEMagnussonBMWinckleGAnissimovYRobertsMSDetermination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layersJ Invest Dermatol2003120575976412713577
- HaighJMSmithEWThe selection and use of natural and synthetic membranes for in-vitro diffusion experimentsEur J Pharm Sci199425–6311330
- CrossSERobertsMSUse of in vitro human skin membranes to model and predict the effect of changing blood flow on the flux and retention of topically applied solutesJ Pharm Sci20089783442345018064682
- Atrux-TallauNPirotFFalsonFRobertsMSMaibachHIQualitative and quantitative comparison of heat separated epidermis and dermatomed skin in percutaneous absorption studiesArch Dermatol Res20072991050751117901965
- MagnussonBMCrossSEWinckleGRobertsMSPercutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layersSkin Pharmacol Physiol200619633634216931901
- TäuberAMüller-GoymannCCIn vitro model of infected stratum corneum for the efficacy evaluation of poloxamer 407-based formulations of ciclopirox olamine against Trichophyton rubrum as well as differential scanning calorimetry and stability studiesInt J Pharm2015494130431126276254
- AnissimovYGRobertsMSDiffusion modeling of percutaneous absorption kinetics: 3. Variable diffusion and partition coefficients, consequences for stratum corneum depth profiles and desorption kineticsJ Pharm Sci200493247048714705203
- AnissimovYGRobertsMSDiffusion modelling of percutaneous absorption kinetics: 4. Effects of a slow equilibration process within stratum corneum on absorption and desorption kineticsJ Pharm Sci200998277278118543296
- ZhangQLiPLiuDRobertsMSEffect of vehicles on the maximum transepidermal flux of similar size phenolic compoundsPharm Res2013301324022923350
- RaneySGFranzTJLehmanPALionbergerRChenMLPharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug productsClin Pharmacokinet201554111095110626063051
- LehmanPAFranzTJAssessing topical bioavailability and bioequivalence: a comparison of the in vitro permeation test and the vasoconstrictor assayPharm Res201431123529353725005736
- HadgraftJBeutnerDWolffHMIn vivo-in vitro comparisons in the transdermal delivery of nitroglycerinInt J Pharm1993891R1R4
- SkellyJPShahVPMaibachHIFDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies: relevance to bioavailability and bioequivalencePharm Res198743265267
- ShahVTopical Dermatological Drug Product NDAs and ANDAs: In Vivo Bioavailability, Bioequivalence, In Vitro Release and Associated StudiesRockville (MD)US Dept of Health and Human Services1998119
- AlbertiIKaliaYNNaikABonnyJDGuyRHIn vivo assessment of enhanced topical delivery of terbinafine to human stratum corneumJ Control Release200171331932711295224
- NicoliSBungeALDelgado-CharroMBGuyRHDermatopharmacokinetics: factors influencing drug clearance from the stratum corneumPharm Res200926486587119034629
- StenkenJAChurchMKGillCACloughGFHow minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studiesAAPS J2010121737819950008
- NarkarYBioequivalence for topical products: an updatePharm Res201027122590260120859663
- SunLStenkenJAImproving microdialysis extraction efficiency of lipophilic eicosanoidsJ Pharm Biomed Anal20033351059107114656597
- BenfeldtEHansenSHVølundAMennéTShahVPBioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic methodJ Invest Dermatol2007127117017816874309
- BodenlenzMAignerBDragatinCClinical applicability of dOFM devices for dermal samplingSkin Res Technol201319447448323581539
- GrayGYardleyHLipid compositions of cells isolated from pig, human, and rat epidermisJ Lipid Res19751664344401194786
- WesterRCMelendresJSedikLMaibachHRiviereJEPercutaneous absorption of salicylic acid, theophylline, 2, 4-dimethylamine, diethyl hexyl phthalic acid, and p-aminobenzoic acid in the isolated perfused porcine skin flap compared to man in vivoToxicol Appl Pharmacol199815111591659705899
- JacobiUKaiserMTollRPorcine ear skin: an in vitro model for human skinSkin Res Technol2007131192417250528
- WesterRCMaibachHIIn vivo methods for percutaneous absorption measurementsJ Toxicol Cutaneous Ocul Toxicol2001204411422
- LademannJRichterHMeinkeMSterryWPatzeltAWhich skin model is the most appropriate for the investigation of topically applied substances into the hair follicles?Skin Pharmacol Physiol2010231475220090408
- CaussinJGoorisGSJanssensMBouwstraJALipid organization in human and porcine stratum corneum differs widely, while lipid mixtures with porcine ceramides model human stratum corneum lipid organization very closelyBiochim Biophys Acta2008177861472148218381060
- WertzPDowningDGoldsmithLPhysiology, Biochemistry, and Molecular Biology of the SkinOxfordOxford University Press1991
- DickIPScottRCPig ear skin as an in-vitro model for human skin permeabilityJ Pharm Pharmacol19924486406451359086
- SinghSZhaoKSinghJIn vitro permeability and binding of hydrocarbons in pig ear and human abdominal skinDrug Chem Toxicol2002251839211850972
- SatoKSugibayashiKMorimotoYSpecies differences in percutaneous absorption of nicorandilJ Pharm Sci19918021041071828835
- NicoliSPadulaCAversaVCharacterization of rabbit ear skin as a skin model for in vitro transdermal permeation experiments: histology, lipid composition and permeabilitySkin Pharmacol Physiol200821421822618509256
- LampeMAWilliamsMLEliasPMHuman epidermal lipids: characterization and modulations during differentiationJ Lipid Res19832421311406833890
- ScheupleinRJBlankIHPermeability of the skinPhysiol Rev19715147027474940637
- CaussinJGoorisGSJanssensMBouwstraJALipid organization in human and porcine stratum corneum differs widely, while lipid mixtures with porcine ceramides model human stratum corneum lipid organization very closelyBiochim Biophys Acta2008177861472148218381060
- SekkatNKaliaYNGuyRHPorcine ear skin as a model for the assessment of transdermal drug delivery to premature neonatesPharm Res20042181390139715359573
- SimonGAMaibachHIRelevance of hairless mouse as an experimental model of percutaneous penetration in manSkin Pharmacol Physiol19981128086
- BarberETeetselNMKolbergKFGuestDA comparative study of the rates of in vitro percutaneous absorption of eight chemicals using rat and human skinFundam Appl Toxicol19921944934971426706
- ChowhanZTPritchardREffect of surfactants on percutaneous absorption of naproxen I: comparisons of rabbit, rat, and human excised skinJ Pharm Sci197867912721274690833
- HughesMFEdwardsBCIn vitro dermal absorption of pyrethroid pesticides in human and rat skinToxicol Appl Pharmacol20102461293720398685
- SchmookFPMeingassnerJGBillichAComparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorptionInt J Pharm20012151–2515611250091
- van RavenzwaayBLeiboldEA comparison between in vitro rat and human and in vivo rat skin absorption studiesHum Exp Toxicol200423942143015497817
- RobertsJBUse of squamate epidermis in percutaneous absorption studies: a reviewJ Toxicol Cutaneous Ocul Toxicol198654319324
- RiggPCBarryBWShed snake skin and hairless mouse skin as model membranes for human skin during permeation studiesJ Invest Dermatol19909422352402299198
- MegrabNAWilliamsABarryBOestradiol permeation through human skin and silastic membrane: effects of propylene glycol and supersaturationJ Control Release1995363277294
- Organization for Economic Co-operation and DevelopmentGuidance Notes on Dermal Absorption2011 Available from: https://www.oecd.org/chemicalsafety/testing/48532204.pdfAccessed April 25, 2016
- FlatenGEPalacZEngeslandAFilipović-GrčićJVani掊kalko-BasnetNIn vitro skin models as a tool in optimization of drug formulationEur J Pharm Sci201575102425746955
- ZhangJSunMFanAWangZZhaoYThe effect of solute-membrane interaction on solute permeation under supersaturated conditionsInt J Pharm20134411–238939423178214
- OliveiraGHadgraftJLaneMEThe role of vehicle interactions on permeation of an active through model membranes and human skinInt J Cosmet Sci201234653654522928552
- NgSFRouseJJSandersonFDEcclestonGMThe relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion studyArch Pharm Res201235457959322553050
- DabrowskaAKRotaruGMDerlerSMaterials used to simulate physical properties of human skinSkin Res Technol201622131426096898
- KansyMSennerFGubernatorKPhysicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processesJ Med Chem1998417100710109544199
- OttavianiGMartelSCarruptPAParallel artificial membrane permeability assay: a new membrane for the fast prediction of passive human skin permeabilityJ Med Chem200649133948395416789751
- US Food and Drug AdministrationGuidance for Industry: Nonsterile Semisolid Dosage Forms Scale-Up and Postapproval Changes – Chemistry, Manufacturing, and Controls: In Vitro Release Testing and In Vivo Bioequivalence DocumentationRockville (MD)FDA1997
- ShahVPElkinsJLamSYSkellyJPDetermination of in vitro drug release from hydrocortisone creamsInt J Pharm19895315359
- WuSTShiuGKSimmonsJEBronaughRLSkellyJPIn vitro release of nitroglycerin from topical products by use of artificial membranesJ Pharm Sci19928112115311561491329
- GallagherSJTrottetLCarterTPHeardCMEffects of membrane type and liquid/liquid phase boundary on in vitro release of ketoprofen from gel formulationsJ Drug Target200311637337914668058
- van DrongelenVDansoMOMulderABarrier properties of an N/TERT-based human skin equivalentTissue Eng Part A20142021–223041304924819925
- BernerdFAsselineauDAn organotypic model of skin to study photodamage and photoprotection in vitroJ Am Acad Dermatol2008585 Suppl 2S155S15918410802
- Schäfer-KortingMBockUDiembeckWThe use of reconstructed human epidermis for skin absorption testing: results of the validation studyAltern Lab Anim200836216118718522484
- SchreiberSMahmoudAVuiaAReconstructed epidermis versus human and animal skin in skin absorption studiesToxicol In Vitro200519681382215913948
- van der WeydenLPattonEEWoodGACross-species models of human melanomaJ Pathol2016238215216526354726
- JinHHeROyoshiMGehaRSAnimal models of atopic dermatitisJ Invest Dermatol20091291314019078986
- SchönMPAnimal models of psoriasis: a critical appraisalExp Dermatol200817870371218557923
- ScharfenbergerLHennericiTKirályGKitzmüllerSVernooijMZielinskiJGTransgenic mouse technology in skin biology: generation of complete or tissue-specific knockout miceJ Invest Dermatol201413411524352072
- EliasPMArbiserJBrownBEEpidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasisAm J Pathol2008173368969918688025
- RossbachKSchaperKKlothCHistamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitisAllergy201671218919726440543
- BurnsEMToberKLRiggenbachJAPreventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinomaCarcinogenesis201334237037723125227
- CozziSJLeTTOgbourneSMJamesCSuhrbierAEffective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 miceArch Dermatol Res20133051798322871992
- SinghASinghASandJMTopically applied Hsp90 inhibitor 17AAG inhibits UVR-induced cutaneous squamous cell carcinomasJ Invest Dermatol201513541098110725337691
- SonavaneKPhillipsJEkshyyanOTopical curcumin-based cream is equivalent to dietary curcumin in a skin cancer modelJ Skin Cancer2012201214786323316365
- VirosAHaywardRMartinMTopical 5-fluorouracil elicits regressions of BRAF inhibitor-induced cutaneous squamous cell carcinomaJ Invest Dermatol2013133127427622895366
- WangHLiJLvTTuQHuangZWangXTherapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless miceExp Dermatol201322536236323614746
- FilocamoGBrunettiMColaceciFMK-4101 – a potent inhibitor of the Hedgehog pathway – is highly active against medulloblastoma and basal cell carcinomaMol Cancer Ther20161561177118926960983
- LarsimontJCYoussefKKSanchez-DanesASox9 controls self-renewal of oncogene targeted cells and links tumor initiation and invasionCell Stem Cell2015171607326095047
- TangTTangJYLiDTargeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of SmoothenedClin Cancer Res201117103378338721558397
- AvciPSadasivamMGuptaAAnimal models of skin disease for drug discoveryExpert Opin Drug Discov20138333135523293893
- Kundu-RaychaudhuriSChenYJWulffHRaychaudhuriSPKv1.3 in psoriatic disease: PAP-1, a small molecule inhibitor of Kv1.3 is effective in the SCID mouse psoriasis – xenograft modelJ Autoimmun201455637225175978
- SaltonMKasprzakWKVossTShapiroBAPoulikakosPIMisteliTInhibition of vemurafenib-resistant melanoma by interference with pre-mRNA splicingNat Commun20156710325971842
- YueLHuangZMFongSTargeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanomaMelanoma Res201525213814825643237
- ChiricozziANogralesKEJohnson-HuangLMIL-17 induces an expanded range of downstream genes in reconstituted human epidermis modelPLoS One201492e9028424587313
- TjabringaGBergersMvan RensDde BoerRLammeESchalkwijkJDevelopment and validation of human psoriatic skin equivalentsAm J Pathol2008173381582318669614
- PendariesVMalaisseJPellerinLKnockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiationJ Invest Dermatol2014134122938294624940654
- Rouaud-TinguelyPBoudierDMarchandLFrom the morphological to the transcriptomic characterization of a compromised three-dimensional in vitro model mimicking atopic dermatitisBr J Dermatol201517341006101426147950
- EngelhartKEl HindiTBiesalskiHKPfitznerIIn vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin modelsArch Dermatol Res200529711915952007
- LiLFukunaga-KalabisMHerlynMThe three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progressionJ Vis Exp201154293721847077
- MohapatraSCoppolaDRikerAIPledgerWJRoscovitine inhibits differentiation and invasion in a three-dimensional skin reconstruction model of metastatic melanomaMol Cancer Res20075214515117314272
- VörsmannHGroeberFWallesHDevelopment of a human three-dimensional organotypic skin-melanoma spheroid model for in vitro drug testingCell Death Dis201347e71923846221
- CommandeurSDrongelenVGruijlFREl GhalbzouriAEpidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinomaCancer Sci2012103122120212622974223
- LuchettiMMMoronciniGEscamezMJInduction of scleroderma fibrosis in skin-humanized mice by anti-platelet-derived growth factor receptor agonistic autoantibodiesArthritis Rheumatol Epub2016425
- KogaHNanjohYKanedaHYamaguchiHTsuboiRShort-term therapy with luliconazole, a novel topical antifungal imidazole, in guinea pig models of tinea corporis and tinea pedisAntimicrob Agents Chemother20125663138314322391525
- AndersenFHedegaardKPetersenTKBindslev-JensenCFullertonAAndersenKEThe hairless guinea-pig as a model for treatment of cumulative irritation in humansSkin Res Technol2006121606716420540
- SchröderHKomljenovicDHeckerMKorffTTransdermal drug targeting and functional imaging of tumor blood vessels in the mouse auricleFASEB J201630292393226546130
- ChenYWuQZhangZYuanLLiuXZhouLPreparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamicsMolecules20121755972598722609787
- YuXDuLZhuLMelanoma therapy with transdermal mitoxantrone cubic phasesDrug Deliv20162351565157025835224