Abstract
Purpose
The purpose of this study was to evaluate the effect and mechanism of quercetin on TGF-β1-induced retinal pigment epithelial (RPE) cell proliferation, migration, and extracellular matrix secretion.
Materials and methods
Cell counting kit-8, transwell, wound-healing assays, and ELISA were used to assess viability, migration, and collagen I secretion, respectively. Western blot analysis and qPCR were employed to detect mRNA and protein expression levels, respectively.
Results
Quercetin suppressed TGF-β1-induced cell proliferation, migration, and collagen I secretion. The results also showed that mRNA and protein expression of epithelial–mesenchymal transition (EMT)-related markers such as alpha-smooth muscle actin and N-cadherin was downregulated by quercetin in TGF-β1-treated RPE cells; conversely, quercetin upregulated the expression of E-cadherin and tight junction protein 1 (ZO-1). In addition, quercetin could inhibit mRNA and protein expression of matrix metalloproteinases. Quercetin may reverse the progression of EMT via the Smad2/3 pathway.
Conclusion
Our results demonstrate the protective effects of quercetin on RPE cell EMT, revealing a potential therapeutic agent for proliferative vitreoretinopathy treatment.
Introduction
Proliferative vitreoretinopathy (PVR) is a vision-threatening disease commonly associated with rhegmatogenous retinal detachment (RD). RD is the separation of the neurosensory retina from the linked retinal pigment epithelium. The intravitreal growth factors and cytokines after occurrence of RD may influence postoperative outcomes. Statistically, PVR occurs in 5%–10% of RD patients, especially postoperatively.Citation1 Progression of PVR involves several steps, such as the proliferation and migration of retinal pigment epithelial (RPE) and glial cells, the formation and contraction of the proliferative membrane, the production of extracellular collagen, and the formation of retinal folds.Citation2 The epithelial–mesenchymal transition (EMT) plays a vital role in the progression of PVR. Following the stimulation by several factors, polarized epithelial cells switch to a mesenchymal cell phenotype, producing an extracellular matrix (ECM) and exhibiting changes in morphological and molecular characteristics.Citation3
Some studies have indicated that multiple growth factors and cytokines are involved in the vitreous body of PVR patients, including tumor necrosis factor-α, interleukin-(IL-) 6, transforming growth factor-beta (TGF-β) and epidermal growth factor (EGF).Citation4 In our previous studies, TGF-β1 was found to have an essential role in EMT in RPE cell lines (ARPE-19).Citation5,Citation6 TGF-β1-induced EMT triggers epithelial cells to alter their epithelial phenotype to one with mesenchymal characteristics, and the proliferation, migration, and collagen generation of TGF-β1-induced RPE cells are enhanced, accelerating EMT progression. Some therapeutic methods have recently been proposed for reversing EMT development both in vitro and in vivo, such as the application of flavonoids or gene silencing. For example, Ren et al reported that curcumin inhibits RPE cell proliferation via downregulation of EGF and thus effectively inhibits the development of PVR.Citation7 In 2013, protein kinase Cα silencing was demonstrated to have effect on suppressing RPE cell proliferation and migration, which was crucial against PVR disease.Citation8 The degradations of collagen and other ECM proteins were closely associated with matrix metalloproteinases (MMPs). The MMP-2 and MMP-9 were expressed in higher levels in PVR patients, which played a vital role in the subretinal membrane formation and cell migration.Citation9,Citation10 However, despite the large number of studies of EMT in PVR treatment, no therapeutic drugs have been developed in the last few decades to effectively prevent PVR.
Quercetin is a natural polyphenolic flavonoid compound extracted from plants such as Phyllanthus emblica.Citation11 Some studies have reported that quercetin has many beneficial properties such as antioxidant,Citation12,Citation13 anti-inflammation,Citation14 antimicrobial,Citation15 anti-angiogenesis,Citation16,Citation17 and anticancer properties.Citation18,Citation19 Wang et al found that quercetin was able to upregulate certain oxidative stress-related genes such as Cu/Zn superoxide dismutase (SOD-1) and catalase (CAT) in vivo and in vitro.Citation13 Quercetin also downregulates vascular endothelial growth factor receptor (VEGFR) expression, blocking angiogenesis in retinoblastoma.Citation17 According to another report, quercetin conjugated with nanoparticles exhibited an anti-angiogenic effect on breast cancer via the epidermal growth factor receptor/VEGFR-2 (EGFR/VEGFR-2)-mediated pathway.Citation16 In addition, quercetin appears to have effects in several ocular diseases such as age-related macular degeneration,Citation20 diabetic cataract (DC),Citation21 dry eye,Citation22 and retinoblastoma.Citation17 Xu et al demonstrated that quercetin could protect oxidative damage via activation of the Nrf2 pathway.Citation23 In 2017, Du et al reported that quercetin had a potential therapeutic effect on DC, alleviating EMT by inhibiting the TGF-β/PI3K/Akt pathway.Citation21 In 2013, Stoddard et al indicated that quercetin could protect the corneal epithelium from oxidative damage by decreasing reactive oxygen species production.Citation24
Nonetheless, it remains unclear whether quercetin could inhibit TGF-β1-induced EMT progression and associated signaling in RPE cells.
Materials and methods
Cell culture and treatment
Human retinal pigment epithelium (ARPE-19) cells were purchased from iCell Bioscience Inc. (Shanghai, China) and cultured in DMEM/F-12 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS, 100 U/mL penicillin and streptomycin. The cells were grown at 37°C in 5% CO2-air. The cells with good shape and in good growth status were used in our experiments. The culture medium was changed every 2–3 days. Upon reaching 60%–70% confluence, the cells were treated with FBS-free DMEM/F-12 culture medium for 24 hours to simulate starvation conditions before experiments. The ARPE-19 cells were incubated with 0, 2.5, 5, 7.5, 10, 12.5, and 15 ng/mL TGF-β1 (PeproTech Inc., Rocky Hill, NJ, USA) and 0, 25, 50, 60, 75, and 100 µmol/L quercetin (Sigma-Aldrich Co., St Louis, MO, USA) for 24 and 48 hours. The following four groups were established: Group A, control; Group B, 10 ng/mL TGF-β1; Group C, 10 ng/mL TGF-β1+25 µmol/L quercetin; and Group D, 10 ng/mL TGF-β1+50 µmol/L quercetin.
Cell viability assay
Cell viability was measured using cell counting kit-8 (CCK-8 assay; Yeasen, Shanghai, China). ARPE-19 cells were seeded in 96-well plates at a density of 2,000 cells/well in 100 µL cultural medium and then starved in FBS-free DMEM/F-12 culture medium for 12 hours before stimulation with TGF-β1 at various concentrations of TGF-β1 and treatment with quercetin. After incubation as described above for 24 and 48 hours, 10 µL of CCK-8 reagent was added to each well for another 2 hours. Cell viability was analyzed spectrophotometrically at 450 nm, with the numbers of living, metabolically active cells being reflected in the absorbance values.
Wound healing assay
The above four groups of cells were plated in six-well plates at 1×105 cells per well. At 80% confluence, wounds were created with a 200 µL pipette tip in the middle of the mono-layer, and the wells were washed with PBS. Images were acquired at 0 hours as a starting point, and the cells were treated as described above. Wound closure was recorded after incubation for 24 and 48 hours by measuring the scratch widths, and migration rates were calculated based on the formula (distance/scratch width)×100%. Duplicated wells for each group were used, and the experiments were repeated three times.
Transwell assay
The four groups of cells were treated as mentioned above. A total of 5×104 cells in 200 µL of FBS-free DMEM/F-12 culture medium were seeded in the upper compartment of a transwell system (8 mm pore size; Corning Incorporated, Corning, NY, USA); culture medium with 10% FBS was added to the lower compartment. After incubation for 18 hours, non-migratory cells were removed with cotton swabs. After washing with PBS, the migrated cells attached to the bottom membrane were fixed with ethanol for 20 minutes and stained with 1% crystal violet for 15 minutes after drying in air. Images in three fields were acquired under phase-contrast microscopy (100×).
Type I collagen ELISA
The human Col I ELISA kit (Shanghai Saige Biotechnology Co Ltd, Shanghai, China) was used according to the manufacturer’s protocol to assess cell supernatant type I collagen (Col I) secretion. Cells were seeded in six-well plates at a density of 2×105 cells per well and exposed to four different treatments. All groups were cultured for 48 hours. The supernatants were collected, and Col I concentrations were measured at 492 nm; values were calculated using a standard curve.
RNA extraction and qRT-PCR
Total cellular RNA was extracted using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol, and a NanoDrop 2000 spectrophotometer was employed to quantify RNA concentrations. PrimerScript RT reagent Kit (TaKaRa Bio Inc., Osaka, Japan) was used to synthesize cDNA. The relative expression level of each gene was detected according to 7500 Fast Real-time PCR System (Thermo Fisher Scientific), as normalized with GAPDH in three replications. The primer sequences are presented in .
Table 1 Nucleotide sequences of primers used for PCR
Western blotting
RIPA lysis buffer was used to lyse cells to extract total protein. The supernatant was collected after centrifugation (12,000 rpm, 4°C, 10 minutes), and the total protein concentration was quantified using a BCA Protein Assay kit (Beyotime, Shanghai, China). Cytosolic and nuclear protein were isolated using a Nuclear and Cytoplasmic Protein Extraction Kit, respectively, according to the manufacturer’s instructions (Beyotime) and also quantified using a BCA Protein Assay kit. Proteins were separated by 8%–10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked in non-fat milk dissolved in Tween-20/PBS buffer for 1 hour at room temperature and incubated overnight with primary antibodies including anti-ZO-1, -alpha-smooth muscle actin (−α-sma), -E-cadherin, -N-cadherin, -Smad2/3, -MMP-9, and -GAPDH antibodies (1:1,000, #8193, #19245, #3195, #13116, #3102, #8828, #2270, #5174, respectively, Cell Signaling Technology, Danvers, MA, USA) and anti-MMP-2 (1:1,000, ARG55236; Arigo Biolaboratories, Shanghai, China). The membranes were washed three times for 10 minutes each in Tween-20/PBS buffer, followed by incubation of secondary goat anti-rabbit antibodies for 45 minutes at room temperature in the dark. An Odyssey two-color infrared laser imaging system (LI-COR Biosciences, Lincoln, NE, USA) was used to scan the membranes.
Immunofluorescence staining
ARPE-19 cells were seeded and cultured in 35 mm confocal dishes (glass-bottom dishes) and then fixed with 4% paraformaldehyde for 10 minutes. The cells were permeabilized and blocked for 1 hour at room temperature with 0.1% Triton X-100 and 5% BSA dissolved in PBS and then incubated overnight at 4°C with primary antibodies (all 1:100 dilution). The cells were incubated with Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies for 40 minutes after washing three times with PBS. Next, nuclei were stained with DAPI (1:1,000 dilution) for 20 minutes. Images were obtained by confocal microscopy (LSM710; Carl Zeiss, Jena, Germany).
Statistical analyses
Statistical analyses were performed with GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 19.0 (IBM Corporation, Armonk, NY, USA) software packages. Data are expressed as mean ± standard deviation (SD), based on results from three separate experiments. One-way ANOVA was performed for multiple groups. P-values <0.05 were considered statistically significant.
Results
Effects of quercetin on TGF-β1-induced cell proliferation in ARPE-19 cells
ARPE-19 cells were treated with different concentrations of TGF-β1 (0, 2.5, 5, 7.5, 10, 12.5, and 15 ng/mL). As shown in , cell proliferation was stimulated, except at 12.5 ng/mL, by TGF-β1 at 24 and 48 hours in a concentration-dependent manner, especially after 48 hours (). Next, the cells were treated with various concentrations of quercetin to observe effects on cell viability. No cytotoxicity in ARPE-19 cells was found for quercetin for concentrations <100 µmol/L at 24 hours and 60 µmol/L at 48 hours, as shown in . Moreover, quercetin inhibited TGF-β1-induced cell proliferation at 25 and 50 µmol/L after 24 and 48 hours, respectively ().
Figure 1 Effects of quercetin on the cell viability of ARPE-19 cells stimulated by TGF-β1.
Abbreviations: CCK-8, cell counting kit-8; TGF-β, transforming growth factor-β.
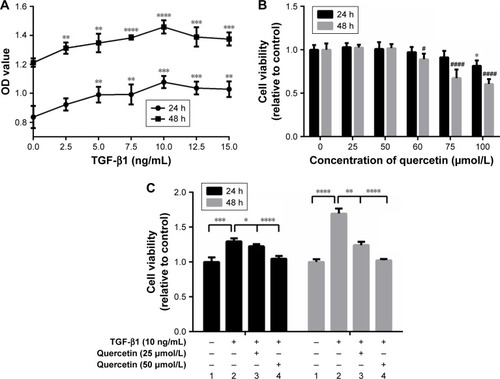
Effects of quercetin on TGF-β1-induced ARPE-19 cell migration
Quercetin-suppressed wound closure in TGF-β1-induced ARPE-19 cells
For the wound-healing test, the width of the opening was measured at 0, 24, and 48 hours, as shown in , and the ratios of (24 h-0 h)/0 hour and (48 h-0 h)/0 hour were calculated. Migration by TGF-β1-treated cells was greater than that of cells not treated with TGF-β1 at both 24 and 48 hours (***P<0.001 and ****P<0.0001, respectively). Quercetin attenuated TGF-β1-induced cell migration in a concentration-dependent manner. The migration rates at 24 hours decreased from 0.42±0.03 in TGF-β1-treated cells to 0.27±0.04 and 0.11±0.07 at 25 and 50 µmol/L in quercetin-treated cells, respectively. The migration rates of the four groups were 0.21±0.067, 0.99±0.02, 0.59±0.06, and 0.22±0.05 at 48 hours.
Figure 2 Effects of quercetin on wound closure in TGF-β1-treated RPE cells.
Abbreviations: que, quercetin; RPE, retinal pigment epithelial; TGF-β, transforming growth factor-β.
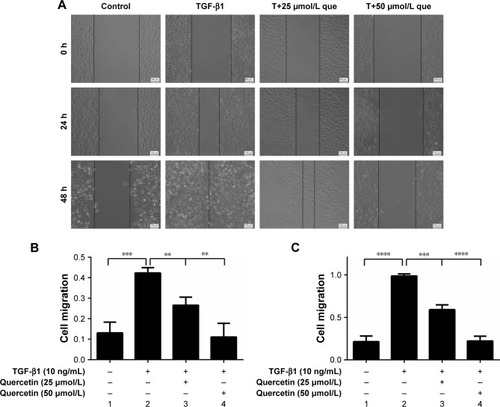
Quercetin-suppressed TGF-β1-induced cell migration
The transwell assay was employed to evaluate cell migration. The numbers of cell migrating after stimulation with TGF-β1 were increased from 164.00±28.48 to 457.00±22.11 at 24 hours (***P<0.001), and quercetin reduced these numbers to 316.67±30.55 (**P<0.01) and 161.33±32.96 (***P<0.001) at concentrations of 25 and 50 µmol/L, respectively. The numbers for the four groups after incubation for 48 hours were 259.67±29.09, 422.00±38.00, 186.00±55.57, and 152.33±20.65. These results showed that quercetin significantly inhibited TGF-β1-induced cell migration in a concentration-dependent and time-dependent manner ().
Figure 3 Effects of quercetin on cell migration in TGF-β1-treated RPE cells.
Abbreviations: que, quercetin; RPE, retinal pigment epithelial; TGF-β1, transforming growth factor-β1.
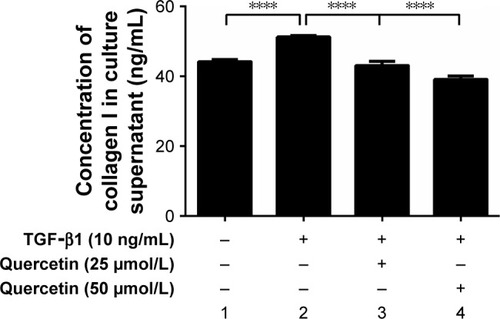
Effects of quercetin on TGF-β1-treated cell collagen I secretion in ARPE-19 cells
As shown in , 10 ng/mL of TGF-β1 promoted cell collagen I secretion at 48 hours. The concentration of collagen I in the culture supernatant increased from 44.16±0.54 to 51.20±0.48 ng/mL but was significantly reduced after treatment with quercetin.
Figure 4 Effect of quercetin on collagen I secretion in TGF-β1-treated RPE cells.
Abbreviations: RPE, retinal pigment epithelial; TGF-β1, transforming growth factor-β1.
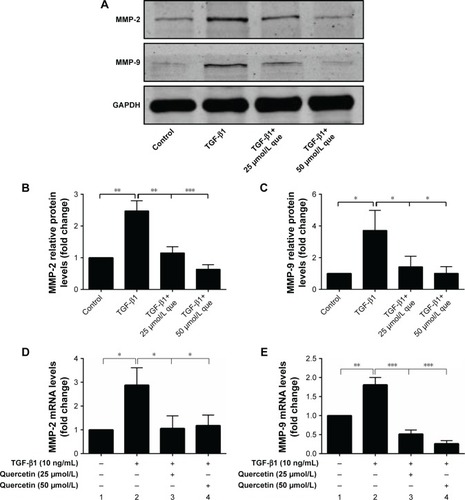
Effects of quercetin on TGF-β1-induced MMP-2 and MMP-9 expressions in ARPE-19 cells
As shown in , 10 ng/mL of TGF-β1 promoted the mRNA and protein expression of MMP-2 and MMP-9 at 48 hours. Meanwhile, quercetin significantly decreased the levels of expression of MMP-2 and MMP-9 in a concentration-dependent manner.
Figure 5 Quercetin suppressed TGF-β1-induced MMP expression in RPE cells.
Abbreviations: MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; que, quercetin; RPE, retinal pigment epithelial; TGF-β1, transforming growth factor-β1.
Effects of quercetin on expression of EMT markers in TGF-β1-induced RPE cells
Quercetin reversed mRNA and protein expression of ZO-1, E-cadherin, N-cadherin, and α-SMA during TGF-β1-induced EMT in ARPE-19 cells
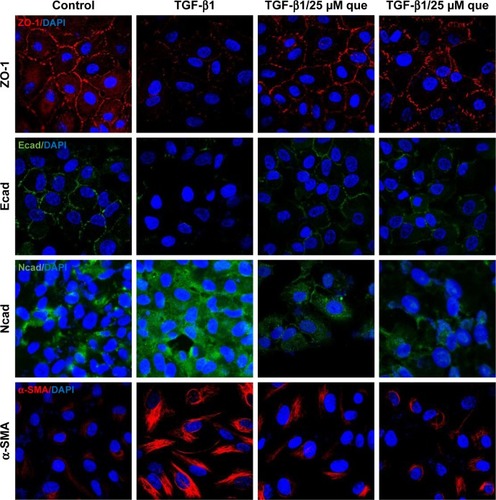
As shown in , qPCR and Western blot analyses demonstrated an increase in the expression levels of the mesenchymal markers N-cadherin and α-SMA after TGF-β1 incubation; however, quercetin significantly decreased the levels of expression of mesenchymal markers in a concentration-dependent manner. Moreover, expression of the epithelial marker ZO-1 and E-cadherin was significantly decreased after treatment with TGF-β1, whereas that of ZO-1 and E-cadherin was increased by exposure to quercetin. Based on immunofluorescence staining, quercetin significantly upregulated the expression of ZO-1 and E-cadherin while downregulating N-cadherin and α-SMA ().
Figure 6 Quercetin suppressed TGF-β1-induced EMT in RPE cells.
Abbreviations: α-SMA, α-smooth muscle actin; EMT, epithelial–mesenchymal transition; que, quercetin; RPE, retinal pigment epithelial; ZO-1, zonula occludens-1.
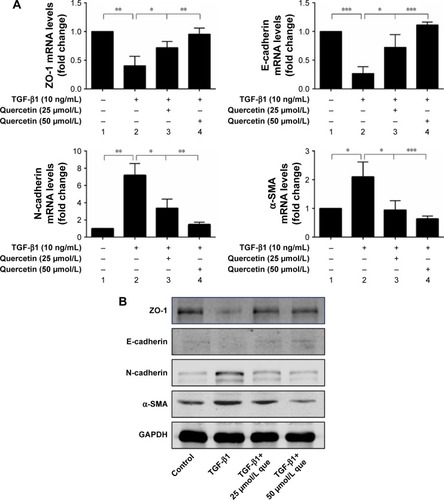
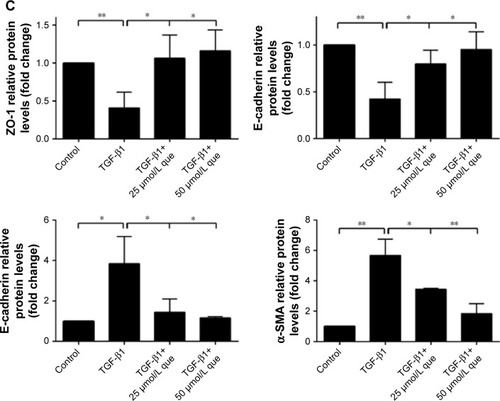
Figure 7 Immunofluorescence analysis of EMT-related proteins in ARPE-19 cells.
Abbreviations: α-SMA, α-smooth muscle actin; Ecad, E-cadherin; EMT, epithelial–mesenchymal transition; Ncad, N-cadherin; que, quercetin; RPE, retinal pigment epithelial; TGF-β1, transforming growth factor-β1; ZO-1, zonula occludens-1.
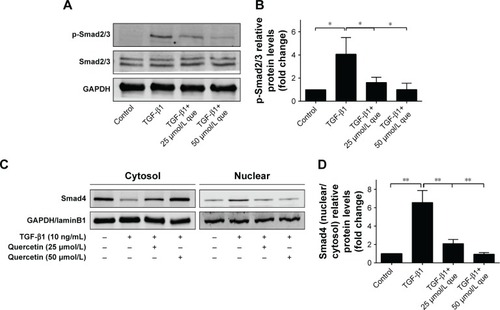
Quercetin-suppressed expression of phosphorylated-Smad2/3 and nuclear translocation of Smad4 during TGF-β1-induced EMT in ARPE-19 cells
We then investigated the effect of quercetin on the Smad signaling pathway, which has been identified as an important factor for EMT. Phosphorylation of Smad2/3 was significantly increased after TGF-β1 treatment, contributing to EMT progression. In addition, cytosolic protein of Smad4 was decreased while nuclear protein of Smad4 was increased after TGF-β1 incubation, which indicated that Smad4 was transported into the nucleus. Interestingly, incubation with quercetin inhibited Smad2/3 phosphorylation and translocation of Smad4. In summary, these results suggest that quercetin inhibits TGF-β1-induced EMT in ARPE-19 cells by suppressing phosphorylation of Smad2/3 ().
Figure 8 Quercetin attenuates TGF-β1-induced Smad2/3 phosphorylation and nuclear translocation of Smad4 in RPE cells.
Abbreviations: que, quercetin; RPE, retinal pigment epithelial; TGF-β1, transforming growth factor-β1.
Discussion
The results of our study show that quercetin inhibits TGF-β1-induced EMT in RPE cells by regulating phosphorylation of Smad2/3 pathway components. Based on our data, we propose that quercetin might serve as a therapeutic agent in the treatment of PVR.
PVR, which involves a wound-healing process, is the most common cause of anatomic failure in RD surgery and a challenge in the clinic. Several cells such as RPE cells, Müller glia cells, macrophages, and fibroblasts are known to participate during the development of PVR.Citation25 RPE cells have been reported to de-differentiate, proliferate, and migrate through retinal holes onto the retinal surface.Citation26,Citation27 Müller glia release inflammatory factors and cytokines such as Granulocyte colony-stimulating factor (Platelet-derived growth factor-bb G-CSF), Platelet-derived growth factor-bb (PDGF-bb), and VEGF,Citation28 and macrophages release inflammatory cytokines, which trigger cell proliferation and migration.Citation29 Fibroblasts might be involved in the contraction of epiretinal membranes, which would accelerate the progression of PVR.Citation30 Various studies have illuminated that certain growth factors, cytokines, MMPs, and chemokines are present in the vitreous or subretinal fluid of PVR patients,Citation31 and Hoerster et al indicated that several factors, such as TGF-β1 and 2, IL-6 and 8, and CC-chemokine ligand 2/monocyte chemoattractant protein, are involved in PVR development.Citation32
There have been many efforts to prevent the occurrence or progression of PVR in the last few decades.Citation2 Some therapeutic strategies, such as vitreous surgery or the use of pharmacologic agents, have been studied in PVR treatment.Citation1 In 1984, corticosteroids were first studied in the rabbit vitreous in an experimental model of PVR.Citation33 However, complications with secondary ocular hypertension after intravitreal injection of triamcinolone have been reported,Citation34 and patients under PVR treatment had a poor response.Citation35 Pharmacologic agents such as anti-inflammatory, anti-proliferative, or antioxidant agents have been investigated for PVR treatment. Anti-proliferative agents used in previous studies include curcumin, resveratrol, and epigallocatechin gallate.Citation36–Citation38 However, none of these compounds have been incorporated into clinical treatments. Thus, it is necessary to investigate additional pharmacologic agents in the treatment of PVR.
Quercetin exhibits beneficial properties such as antioxidative, anti-inflammatory, anti-angiogenic, and anticarcinogenic activities in various diseases. In 2011, Kviecinski et al reported that by acting as a free radical scavenger, quercetin effectively inhibited oxidative stress.Citation39 Feng et al found that quercetin could restrain EMT by regulating E-cadherin expression, as enhancing E-cadherin expression restored cell–cell adhesion to prevent the progression of EMT.Citation40 In ocular diseases, quercetin can protect the lens from oxidative damage and prevent cataract development due to its strong antioxidant and chelating properties.Citation41
In our study, we investigated the effect of quercetin on ameliorating TGF-β1-induced RPE cell proliferation, migration, and collagen secretion. First, RPE cells were exposed to various concentrations of TGF-β1 for 24 and 48 hours to determine the most appropriate concentrations. As 10 ng/mL of TGF-β1 could effectively accelerate proliferation, this was used in our experiments. Next, we treated these cells with quercetin to observe the effects on proliferation, and the results showed that quercetin could effectively inhibit TGF-β1-induced cell proliferation at both 24 and 48 hours. TGF-β1 caused the epithelial cells to transform their epithelial morphology to a mesenchymal morphology. RPE cell migration has a crucial role in the progression of PVR, and TGF-β1 promoted cell migration, whereas quercetin suppressed migration in a concentration-dependent manner.
MMP-2 and MMP-9 were correlated with collagen and ECM protein production, which induced the development and progression of PVR. Wang et al reported that repression of MMP-2 and MMP-9 could markedly reverse EMT in human non-small-cell lung cancer (NSCLC).Citation42 Lin et al showed that inhibition of MMP-9 could reduce RPE cell migration so that ameliorated the PVR progression.Citation10 In our studies, quercetin could markedly suppress the MMP-2 and MMP-9 expression. Thus, quercetin effectively decreased extracellular collagen production.
TGF-β/Smad signaling pathway is involved in many diseases such as PVR,Citation43 multiorgan fibrosis,Citation44–Citation46 and cancers.Citation47,Citation48 TGF-β induces the activation and phosphorylation of Smad2/3, forming trimers with Smad4. Then the complex is transported into the nucleus. Wu et al have shown that quercetin could inhibit Smad signaling pathway in the hepatic fibrosis animal model, and our results were consistent with it.Citation49 Xin et al reported that incubation with quercetin of 1, 3, and 10 µM concentration for 48 hours could effectively inhibit Smad2/3 activation, which protected renal fibrosis of diabetic rats.Citation50 Lu et al found that quercetin ameliorated kidney injury via the inactivation of TGF-β1/Smad2/3 signaling, which decreased the ECM production.Citation51
In the present study, expression of the tight junction protein ZO-1 and E-cadherin was upregulated after incubation with quercetin, which contributed to maintaining epithelial cell properties. Loss of cell–cell adhesion plays a vital role in the progression of EMT, and E-cadherin is involved in EMT as a key mediator of cell–cell adhesion. The mesenchymal markers α-SMA and N-cadherin were upregulated during EMT progression, and the ability of quercetin to modulate these markers would promote EMT. Our results show significant increases in phosphorylated Smad2/3 under TGF-β1 stimulation, which was restrained by quercetin. These findings indicate that quercetin may prevent the development of EMT by regulating the Smad2/3 pathway.
There are some limitations to our study. First, we investigated the effect and mechanism of quercetin in cultured RPE cells. However, we did not establish an animal model of PVR, such as RPE cell-induced PVR in pigmented rabbits. Second, we elucidated the therapeutic effect of quercetin on TGF-β1-induced EMT via the Smad-dependent signaling pathway, although other signaling pathways are involved in EMT. Thus, further studies should be applied to reveal the mechanism of quercetin in cell lines and PVR animal models to determine the underlying effects.
Conclusion
We found that quercetin significantly suppressed TGF-β1-induced proliferation and migration without influencing cell viability. Our findings suggest that quercetin is a potential therapeutic agent in PVR therapy. Further studies are needed to investigate the mechanisms in detail before the clinical application of quercetin.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China in 2014 (project number: 81470648) and the Fundamental Research Funds for the Central Universities.
Disclosure
The authors report no conflicts of interest in this work.
References
- SadakaAGiuliariGPProliferative vitreoretinopathy: current and emerging treatmentsClin Ophthalmol201261325133322942638
- PastorJCRojasJPastor-IdoateSDi LauroSGonzalez-BuendiaLDelgado-TiradoSProliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequencesProg Retin Eye Res20165112515526209346
- ShuDYLovicuFJMyofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosisProg Retin Eye Res201760446528807717
- MorescalchiFDuseSGambicortiERomanoMRCostagliolaCSemeraroFProliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloidMediators Inflamm2013201326978724198445
- CaiWWeiQLiuQEffect of bradykinin on TGF-β1-induced retinal pigment epithelial cell proliferation and extracellular matrix secretionBMC Ophthalmol201616119927832751
- WeiQLiuQRenCEffects of bradykinin on TGF-β1-induced epithelial-mesenchymal transition in ARPE-19 cellsMol Med Rep20181745878588629436636
- RenYXMaJXZhaoFAnJBGengYXLiuLYEffects of curcumin on epidermal growth factor in proliferative vitreoretinopathyCell Physiol Biochem20184752136214629975931
- QiuSJiangZHuangZMigration of retinal pigment epithelium cells is regulated by protein kinase Cα in vitroInvest Ophthalmol Vis Sci201354107082709024084091
- González-AvilaGMéndezDLozanoDRamosCDelgadoJIturriaCRole of retinal detachment subretinal fluid on extracellular matrix metabolismOphthalmologica20042181495614688436
- LinHYChenYSWangKChienHWHsiehYHYangSFFisetin inhibits epidermal growth factor-induced migration of ARPE-19 cells by suppression of AKT activation and Sp1-dependent MMP-9 expressionMol Vis20172390091029296070
- SrinivasanPVijayakumarSKothandaramanSPalaniMAnti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approachesJ Pharm Anal20188210911829736297
- CaiHDSuSLQianDWRenal protective effect and action mechanism of Huangkui capsule and its main five flavonoidsJ Ethnopharmacol201720615215928408246
- WangJQianXGaoQQuercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitroJ Ovarian Res20181115129929541
- MengLQYangFYWangMSQuercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathwaysProstate2018781179080029654614
- Şeker KaratoprakGAydinGAltinsoyBAltinkaynakCKoşarMOcsoyIThe effect of pelargonium endlicherianum Fenzl. root extracts on formation of nanoparticles and their antimicrobial activitiesEnzyme Microb Technol201797212628010769
- BalakrishnanSBhatFARaja SinghPGold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancerCell Prolif201649667869727641938
- SongWZhaoXXuJZhangHQuercetin inhibits angiogenesis-mediated human retinoblastoma growth by targeting vascular endothelial growth factor receptorOncol Lett20171433343334828927086
- YuDYeTXiangYQuercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cellsOnco Targets Ther2017104719472929026320
- PatelDHSharmaNInhibitory effect of quercetin on epithelial to mesenchymal transition in SK-MEL-28 human melanoma cells defined by in vitro analysis on 3D collagen gelsOnco Targets Ther201696445645927799792
- KookDWolfAHYuALThe protective effect of quercetin against oxidative stress in the human RPE in vitroInvest Ophthalmol Vis Sci20084941712172018385095
- DuLHaoMLiCQuercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and SRA01/04 cells through transforming growth factor-β2/phosphoinositide 3-kinase/Akt pathwayMol Cell Endocrinol2017452445628501572
- OhHNKimCELeeJHYangJWEffects of Quercetin in a Mouse Model of Experimental Dry EyeCornea20153491130113626203745
- XuXRYuHTYangYHangLYangXWDingSHQuercetin phospholipid complex significantly protects against oxidative injury in ARPE-19 cells associated with activation of Nrf2 pathwayEur J Pharmacol20167701826643168
- StoddardARKoetjeLRMitchellAKSchotanusMPUbelsJLBioavailability of antioxidants applied to stratified human corneal epithelial cellsJ Ocul Pharmacol Ther201329768168723634787
- YuJLiuFCuiSJVitreous proteomic analysis of proliferative vitreoretinopathyProteomics20088173667367818752205
- SuCCChanCMChenHMLutein inhibits the migration of retinal pigment epithelial cells via cytosolic and mitochondrial Akt pathways (lutein inhibits RPE cells migration)Int J Mol Sci2014158137551376725110866
- KhanMABradyCJKaiserRSClinical management of proliferative vitreoretinopathy: an updateRetina201535216517525602631
- EastlakeKBanerjeePJAngbohangACharterisDGKhawPTLimbGAMüller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathyGlia201664449550626556395
- MorescalchiFDuseSGambicortiERomanoMRCostagliolaCSemeraroFProliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloidMediators Inflamm2013201326978724198445
- GarwegJGTappeinerCHalberstadtMPathophysiology of proliferative vitreoretinopathy in retinal detachmentSurv Ophthalmol201358432132923642514
- CiprianDThe pathogeny of proliferative vitreoretinopathyRom J Ophthalmol2015592889226978867
- HoersterRFauserSCursiefenCKirchhofBHeindlLMThe influence of systemic renin-angiotensin-inhibition on ocular cytokines related to proliferative vitreoretinopathyGraefes Arch Clin Exp Ophthalmol201725591721172528600710
- SunalpMWiedemannPSorgenteNRyanSJEffects of cytotoxic drugs on proliferative vitreoretinopathy in the rabbit cell injection modelCurr Eye Res1984346196236425019
- JonasJBKreissigIDegenringRFTreatment of oedematous, proliferative and neovascular diseases by intravitreal triamcinolone acetonideKlin Monbl Augenheilkd20032206384390 German12830391
- MoysidisSNThanosAVavvasDGMechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedsideMediators Inflamm2012201281593723049173
- IshikawaKHeSTerasakiHResveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathySci Rep201551638626552368
- AlexAFSpitznasMTittelAPKurtsCEterNInhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitroCurr Eye Res201035111021103320958191
- ZhouXKuangXLongCCurcumin Inhibits Proliferation and Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells Via Multiple PathwaysCurr Mol Med201717431231929110611
- KviecinskiMRFelipeKBCorreiaJFBrazilian Bidens pilosa Linné yields fraction containing quercetin-derived flavonoid with free radical scavenger activity and hepatoprotective effectsLibyan J Med201165651
- FengJSongDJiangSQuercetin restrains TGF-β1-induced epithelial-mesenchymal transition by inhibiting Twist1 and regulating E-cadherin expressionBiochem Biophys Res Commun2018498113213829425820
- FerlemiAVMakriOEMermigkiPGLamariFNGeorgakopoulosCDQuercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating propertiesExp Eye Res201614525826826808488
- WangZLuYShengBDingYChengXCatalpol inhibits TGF-β1-induced epithelial-mesenchymal transition in human non-small-cell lung cancer cells through the inactivation of Smad2/3 and NF-κB signaling pathwaysJ Cell Biochem Epub2018117
- ChenCLChenYHTaiMCLiangCMLuDWChenJTResveratrol inhibits transforming growth factor-β2-induced epithelial-to-mesenchymal transition in human retinal pigment epithelial cells by suppressing the Smad pathwayDrug Des Devel Ther201711163173
- JiXWangHWuZSpecific Inhibitor of Smad3 (SIS3) Attenuates Fibrosis, Apoptosis, and Inflammation in Unilateral Ureteral Obstruction Kidneys by Inhibition of Transforming Growth Factor β (TGF-β)/Smad3 SignalingMed Sci Monit2018241633164129555895
- ZhangZHLiMHLiuDRhubarb Protect Against Tubulointerstitial Fibrosis by Inhibiting TGF-β/Smad Pathway and Improving Abnormal Metabolome in Chronic Kidney DiseaseFront Pharmacol20189102930271345
- MaLZengYWeiJKnockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylationBiomed Pharmacother20181071728173530257391
- ZengYZhuJShenDRepression of Smad4 by miR-205 moderates TGF-β-induced epithelial-mesenchymal transition in A549 cell linesInt J Oncol201649270070827279345
- TaoSLiuMShenDZhangWWangTBaiYTGF-β/Smads Signaling Affects Radiation Response and Prolongs Survival by Regulating DNA Repair Genes in Malignant GliomaDNA Cell Biol Epub2018919
- WuLZhangQMoWQuercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathwaysSci Rep201771928928839277
- XinXLiXHWuJZPentamethylquercetin ameliorates fibrosis in diabetic Goto-Kakizaki rat kidneys and mesangial cells with suppression of TGF-β/Smads signalingEur J Pharmacol20137131–361523665496
- LuHWuLLiuLQuercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarizationBiochem Pharmacol201815420321229753749

