Abstract
Objective
To systematically evaluate the efficacy and safety of sodium cantharidinate and vitamin B6 (SC/B6) combined with conventional medical treatment (CMT) for the treatment of patients with advanced digestive system neoplasms (DSNs).
Methods
The Cochrane Library, Embase, PubMed, Web of Science, Chinese Scientific Journal Database (VIP), China National Knowledge Infrastructure, and Wanfang databases were searched for clinical trials using SC/B6 for DSNs. Outcome measures, including therapeutic efficacy, quality of life (QoL), and adverse events, were extracted and systematically evaluated.
Results
Data from 24 trials including 1,825 advanced DSN patients were included. Compared with CMT alone, its combination with SC/B6 significantly improved the patients’ overall response rate (OR =2.25, 95% CI =1.83–2.76, P<0.00001), disease control rate (OR =2.41, 95% CI =1.85–3.15, P<0.00001), and QoL improvement rate (OR =2.75, 95% CI =2.13–3.55, P<0.00001). Moreover, adverse events caused by chemotherapy, including leukopenia, nausea and vomiting, gastrointestinal side effects, hepatotoxicity, diarrhea, transaminase disorder, myelosuppression, anorexia, and anemia, were significantly alleviated (P<0.05) when SC/B6 was applied to DSN patients. Nephrotoxicity, thrombocytopenia, hand-foot syndrome, and oral mucositis were not significantly alleviated in patients receiving combination therapy (P>0.05).
Conclusion
The combination of SC/B6 and CMT is more effective in treating DSNs than CMT alone. This combination alleviates the adverse effects associated with chemotherapy and improves the QoL of DSN patients, and its application in the clinic is worth promoting.
Introduction
Digestive system neoplasms (DSNs) are the leading cause of cancer-related death worldwide, and cause 3,056,412 deaths in 2018, which accounts for 32% of all cancer deaths worldwide.Citation1–Citation3 This category comprises colorectal cancer, gastric cancer, liver cancer, esophageal cancer, and pancreatic cancer, which are the fourth, sixth, seventh, ninth, and fourteenth most common cancers, respectively.Citation1 Despite improvements in diagnostic and therapeutic methods in the past decades,Citation4 the prognosis of DSNs is still poor, because they are mostly diagnosed at advanced stages, which may be accompanied by extensive invasion and distant metastasis.Citation4–Citation6 Therefore, effective therapeutic approaches should be developed.
In recent years, traditional Chinese medicine has been more widely used as auxiliary treatment in tumor therapy and has shown promising therapeutic effects in many clinical studies.Citation7–Citation9 Sodium cantharidinate/vitamin B6 (SC/B6) is a combination of sodium cantharidinate (SC) and vitamin B6, and has the pharmacologic characteristics of both.Citation7,Citation8 SC is a derivative of cantharidin, which is extracted from the body of meloidae insects such as Mylabris phalerata pallas and Mylabris cichorii linnaeus.Citation10 SC preserves the unique anticancer activity of cantharidin and has lower toxicity and fewer adverse effects.Citation7,Citation10 Its combination with vitamin B6 can even further lower the side effects.Citation7 In recent years, SC has been used as a safe auxiliary antitumor drug for malignancies such as gastric cancer, liver cancer, and non-small-cell lung cancer.Citation7–Citation9,Citation11 Tao et alCitation12 indicated that SC induces HepG2 cells to undergo apoptosis through the LC3 autophagy pathway. Liang et alCitation13 showed that SC can inhibit tumor growth by downregulating vascular endothelial growth factor expression and blocking tumor angiogenesis. In addition, SC can also have an anticancer effect by blocking progression through the cell cycle, inhibiting invasion/metastasis, and improving the immunity of cancer patients.Citation14–Citation18
Several clinical studiesCitation8,Citation19–Citation41 have revealed the prominent therapeutic effects of SC/B6 and conventional medical treatment (CMT, including chemotherapy, symptomatic, and supporting therapy) for advanced DSNs but clinical efficacy and safety have not been systematically evaluated. In this study, we performed a meta-analysis to evaluate the efficacy and safety of SC/B6 for DSN treatment, with a comparison between SC/B6 and CMT combined therapy and CMT alone, in order to provide scientific reference for the design of future clinical trials.
Materials and methods
Search strategy and selection criteria
Publications were searched across the Cochrane Library, Embase, Pubmed, Web of Science, Chinese Scientific Journal Database (VIP), China National Knowledge Infrastructure, and Wanfang databases, using the search terms “sodium cantharidinate” or “disodium cantharidinate” and “vitamin B6” combined with “gastric cancer” or “colorectal cancer” or “gastrointestinal cancer” or “liver cancer” or “esophageal cancer” or “pancreatic cancer” or “digestive system neoplasms” without restriction on the language. The retrieval was initiated in May 2018 and updated in August 2018.
All of the clinical trials brought into this analysis were randomized controlled trials with reference to advanced DSNs, in which patients in the experimental groups were treated by SC/B6 and CMT combined therapy, and patients in the control groups were treated by CMT alone.
Data extraction and quality assessment
Literature screening and data extraction were carried out by two independent investigators (Meirong Liu and Chunhong Xu) and verified by a third reviewer (Yingying Sun). All included studies were summarized as follows: first author name, year of publication, study location, Karnofsky Performance Score (KPS), number of cases, patient ages, study parameter type, treatment regimen and enrollment period, and administration route and dosage of SC/B6. The quality of the included trials was evaluated as described in the Cochrane Handbook.Citation42
Outcome definition
Clinical responses, including therapeutic effects, quality of life (QoL), and adverse events, were analyzed. Therapeutic effects were evaluated by overall survival (OS) rate, complete response (CR) rate, partial response (PR) rate, stable disease (SD) rate, progressive disease (PD) rate, overall response rate (ORR, ORR = CR + PR), and disease control rate (DCR = CR + PR + SD). OS was defined as the length of time from the start of treatment to death from any cause; QoL was assessed using KPS scales and the European Organization for Research and Treatment of Cancer core quality-of-life questionnaire. The QoL improvement rate (QIR) was defined as the improvement in QoL after treatment. Adverse events, including leukopenia, nausea and vomiting, gastrointestinal side effects, hepatotoxicity, nephrotoxicity, diarrhea, thrombocytopenia, transaminase disorder, myelosuppression, hand-foot syndrome, oral mucositis, anorexia, and anemia, were also assessed.
Statistical analysis
Review Manager 5.3 (Nordic Cochran Centre, Copenhagen, Denmark) and Stata 13.0 (Stata Corp., College Station, TX, USA) were the main statistical analysis tools in this study. P<0.05 indicated statistically significant differences. Cochran’s Q test was used to determine heterogeneity among studies,Citation43 and publication bias was analyzed by Begg’s and Egger’s regression asymmetry tests and presented by funnel plots.Citation44 I2<50% or P>0.1 indicated study homogeneity. Therapeutic effects were mainly represented by HRs and ORs presented with 95% CIs. HRs were collected for survival data. If HRs can neither be collected directly nor calculated, survival curve plots were extracted by Engauge Digitizer software and then transformed by specialized form.Citation45–Citation47
Pooled analysis with publication bias determined that the trim-and-fill method would be applied to coordinate the estimates of unpublished studies, and the adjusted results were compared with the original pooled OR.Citation48 Sensitivity analysis (subgroup analyses) was conducted to evaluate the impact of different cancer types, SC/B6 dosages, therapeutic regimens, sample sizes, and study types on clinical efficacy.
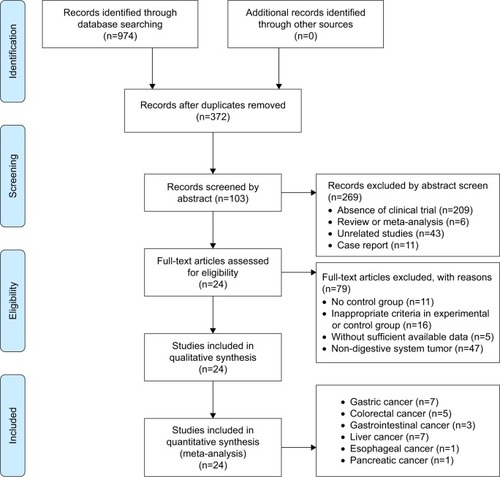
A total of 974 articles were identified with the initial search, and 602 papers were excluded due to duplication. After title and abstract review, 269 articles were further excluded because they did not include clinical trials (n=209), were reviews or meta-analyses (n=6), were unrelated studies (n=43), or were case reports (n=11), leaving 103 studies as potentially relevant. After detailed assessment of full texts, articles without a control group (n=11), studies with inappropriate criteria in the experimental or control group (n=16), studies with insufficient data (n=5), and studies including patients with non-digestive system tumors (n=47) were excluded. Finally, data from 24 trialsCitation8,Citation19–Citation41 (gastric cancer, n=7; colorectal cancer, n=5; gastrointestinal cancer, n=3; liver cancer, n=7; esophageal cancer, n=1; and pancreatic cancer, n=1) including 1,825 advanced DSN patients were included in the present analysis ().
Patient characteristics
All studies involved in this analysis were carried out in different hospitals in China. These trials include 1,825 patients with advanced DSNs; of these, 933 were treated by combined SC/B6 and CMT, and 892 were treated by CMT alone. Detailed information on the included trials and patients is presented in and .
Table 1 Clinical information from the eligible trials in the meta-analysis
Table 2 Information of SC/B6 combined with conventional medical treatment
Quality assessment
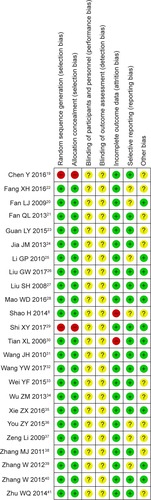
The evaluation of bias risk is presented in . Twenty-two studies had low risk, and the other two articles did not have a clear description of the randomization process. None of the included trials provided a clear description of the performance and detection risks. Two studies were regarded as high-risk due to the absence of follow-up and seven trials were considered as unclear risk owing to selective reporting.
Therapeutic efficacy assessments
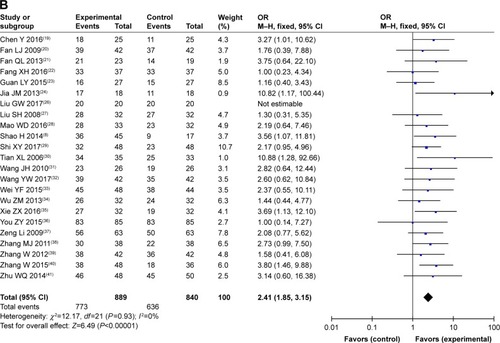
As shown in and , , and , patients who underwent combined therapy had a significantly improved CR rate (OR =2.06, 95% CI =1.41–3.00, P=0.0002), PR rate (OR =1.85, 95% CI =1.50–2.29, P<0.00001), ORR (OR =2.25, 95% CI =1.83–2.76, P<0.00001), and DCR (OR =2.41, 95% CI =1.85–3.15, P<0.00001), and significantly decreased SD and PD rates (SD, OR =0.77, 95% CI =0.63–0.93, P=0.009; PD, OR =0.45, 95% CI =0.35–0.59, P<0.00001) compared to patients receiving CMT alone. The OS rates of patients who received combination treatment (HR =0.74, 95% CI =0.47–1.17, P=0.20) did not differ significantly from those in patients who received CMT alone.
Table 3 Comparison of CR, PR, SD, PD, ORR, and DCR between the SC/B6 + CMT and SC/B6 group
Figure 3 Forest plot of the comparison of overall survival between the experimental and control groups.
Abbreviations: CMT, conventional medical treatment; IV, intravenous.
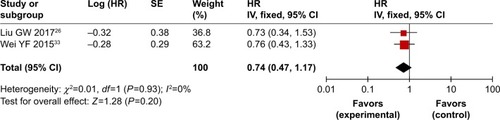
Figure 4 Forest plot of the comparison of overall response rate (A) and disease control rate (B) between the experimental and control groups.
Abbreviations: CMT, conventional medical treatment; M–H, Mantel–Haenszel.
QoL assessment
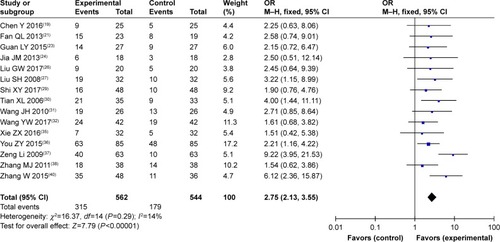
QoL evaluation demonstrated that SC/B6 and CMT combined therapy-treated DSN patients had improved QoL compared to those treated by CMT alone (, OR =2.75, 95% CI =2.13–3.55, P<0.00001).
Figure 5 Forest plot of the comparison of quality-of-life improved rate between the experimental and control groups.
Abbreviations: CMT, conventional medical treatment; M–H, Mantel–Haenszel; SC/B6, sodium cantharidinate and vitamin B6 injection.
Adverse events assessment
As shown in and , patients treated by SC/B6 and CMT combined therapy had lower incidences of leukopenia, nausea and vomiting, gastrointestinal side effects, hepatotoxicity, diarrhea, transaminase disorder, myelosuppression, anorexia, and anemia than those treated with CMT alone (leukopenia: OR =0.29, 95% CI =0.21–0.39, P<0.00001; nausea and vomiting: OR =0.30, 95% CI =0.22–0.40, P<0.00001; gastrointestinal side effects: OR =0.42, 95% CI =0.29–0.62, P<0.00001; hepatotoxicity: OR =0.49, 95% CI =0.30–0.78, P=0.003; diarrhea: OR =0.37, 95% CI =0.23–0.60, P<0.0001; transaminase disorder: OR =0.23, 95% CI =0.09–0.62, P=0.003; myelosuppression: OR =0.33, 95% CI =0.18–0.60, P=0.0003; anorexia: OR =0.37, 95% CI =0.20–0.68, P=0.001; anemia: OR =0.54, 95% CI =0.32–0.91, P=0.02). No significant difference was found in the occurrence of nephrotoxicity, thrombocytopenia, hand-foot syndrome, and oral mucositis (nephrotoxicity: OR =0.70, 95% CI =0.38–1.30, P=0.26; thrombocytopenia: OR =0.77, 95% CI =0.31–1.92, P=0.57; hand-foot syndrome: OR =0.75, 95% CI =0.40–1.40, P=0.36; oral mucositis: OR =0.45, 95% CI =0.13–1.62, P=0.22) between patients receiving combination treatment and those receiving CMT alone.
Table 4 Comparison of adverse events between the SC/B6 + CMT and SC/B6 group
Publication bias
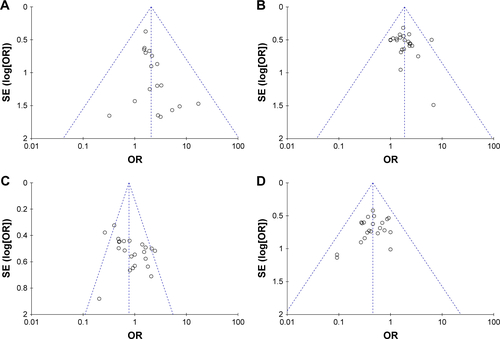
Publication bias of primary outcomes (CR, PR, SD, PD, ORR, DCR, QIR, and adverse events) was evaluated and presented by funnel plots. All plots were approximately symmetrical, indicating generally controlled publication bias ( and ).
Figure 6 Funnel plot of percentage of overall response rate (A), disease control rate (B), quality-of-life improved rate (C), leukopenia (D), nausea and vomiting (E), gastrointestinal side effects (F), and hepatotoxicity (G).
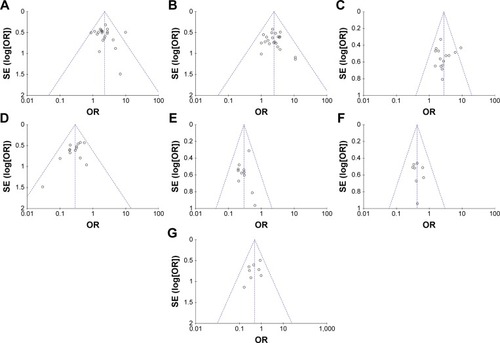
We also assessed the publication bias by Begg’s and Egger’s regression asymmetry tests, and SD and leukopenia were found to have bias (SD, Egger: 0.024, Begg: 0.039; leukopenia, Egger: 0.041, Begg: 0.080; ). To determine whether the bias affected the pooled risk, we conducted trim-and-fill analysis. The adjusted OR indicated the same trend as the primary analysis (SD, before: P=0.010, after: P<0.0001; leukopenia, before: P<0.0001, after: P<0.0001), reflecting the reliability of our primary conclusions, except those based on a small number of trials.
Table 5 Publication bias on therapeutic efficacy and adverse events
Sensitivity analysis
Subgroup analysis was performed for ORR and DCR heterogeneity assessment concerning cancer types, SC/B6 dosages, therapeutic regimens, sample sizes, and study types of involved trials. No significant difference was observed in the sample sizes, study types, or SC/B6 dosages (). SC/B6 combined with CMT was more effective in treating gastric cancer, colorectal cancer, and liver cancer. Moreover, SC/B6 combined with oxaliplatin and capecitabine (XELOX) or capecitabine regimens was more effective for DSN treatment.
Table 6 Subgroup analyses of ORR and DCR between the SC/B6 + CMT and SC/B6 groups
Discussion
The chemotherapeutic regimens commonly used to treat DSNs cause serious side effects, such as myelosuppression, hepatotoxicity, and gastrointestinal side effects, which severely affect the QoL of DSN patients.Citation7,Citation9 Therefore, seeking a therapy that can improve treatment outcomes and decrease the adverse effects of chemotherapy is a major direction in the development of tumor treatment. Traditional Chinese medicine plays a unique role in improving host immunity and lowering the toxic effects of chemotherapy.Citation7,Citation9,Citation49–Citation52 In recent decades, SC/B6 has been clinically applied as an adjuvant therapy for malignancies and has been beneficial for advanced DSN patients in several trials.Citation7–Citation9,Citation11 Despite the published reports on clinical trials using SC/B6, its therapeutic effects have not been systematically demonstrated. In the present study, we performed an extensive literature search followed by rigorous contrasting and combining data analysis for categorization to provide clear and systematic conclusions.
Our meta-analysis revealed that SC/B6 and CMT combined therapy for DSN patients achieved more beneficial effects than CMT alone. Combined therapy-treated patients exhibited markedly improved ORR and DCR (P<0.05 for all) and also significantly improved QoL. These results indicated that intravenous infusion of SC/B6 improved the curative effects of CMT for advanced DSNs.
Our analysis indicates that most of the adverse events caused by chemotherapy, including leukopenia, nausea and vomiting, gastrointestinal side effects, and hepatotoxicity, were alleviated with SC/B6 combination therapy (P<0.05). Therefore, SC/B6 is a safe auxiliary antitumor medicine for DSN and can effectively alleviate the adverse events associated with chemotherapy.
The analysis of therapeutic effects may be influenced by several factors. In our study, no difference was found between sample sizes, study types, and SC/B6 dosages. SC/B6 combined with CMT was more effective in treating gastric cancer, colorectal cancer, and liver cancer than it was in treating esophageal cancer and pancreatic cancer. Moreover, our subgroup analysis showed that SC/B6 combined with XELOX/capecitabine was more effective for DSN treatment. However, recent studies on the impact of these factors on the curative effect of SC/B6 adjuvant therapy remain insufficient, and further investigations should be performed.
There are some limitations in our analysis. First, the follow-up durations of the included studies were not long enough. Second, as a traditional medicine, SC/B6 was mainly applied in China, which may bring an unavoidable regional bias and subsequently influence the clinical application of SC/B6 worldwide. Furthermore, treatment/medical history is very important for evaluating the efficacy of SC/B6-mediated therapy. However, our data were extracted from published papers rather than from the original patient records; therefore, analytical bias may possibly exist. More original data would be valuable to achieve a higher reliability of statistical analysis on SC/B6 for DSN treatment.
In summary, this meta-analysis indicated that SC/B6 and CMT combined therapy was effective in treating advanced DSNs. Intravenous infusion of SC/B6 not only greatly improved the therapeutic effects of CMT but also effectively alleviated the toxicity and most of the side effects associated with chemotherapy. Therefore, SC/B6 has potential for development as a new adjuvant therapy for the treatment of DSN.
Supplementary materials
Figure S1 Forest plot of the comparison of complete response rates (A), partial response rates (B), stable disease rates (C), and progressive disease rates (D) between the experimental and control groups. Control group, CMT alone group; Experimental group, sodium cantharidinate and vitamin B6 injection (SC/B6) + CMT. The fixed-effects meta-analysis model (M–H method) was used.
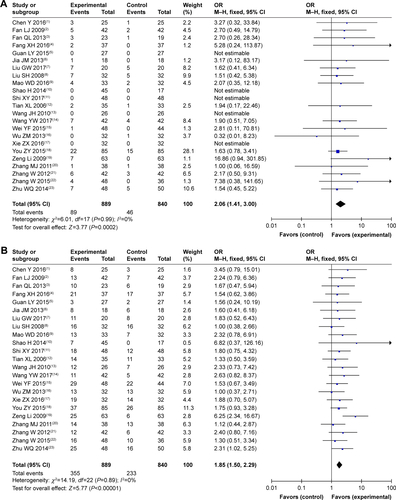
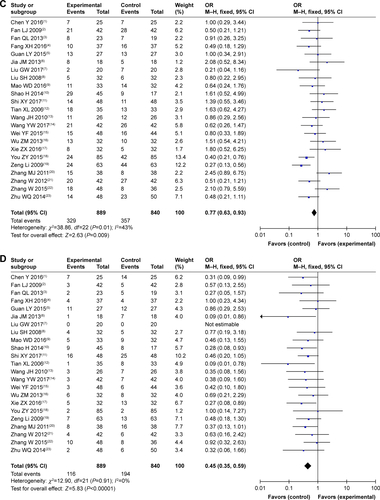
Figure S2 Forest plot of the comparison of adverse effects including leukopenia (A), nausea and vomiting (B), gastrointestinal side effects (C), hepatotoxicity (D), nephrotoxicity (E), diarrhea (F), thrombocytopenia (G), transaminase disorder (H), myelosuppression (I), hand foot syndrome (J), oral mucositis (K), anorexia (L), and anemia (M) between the experimental and control groups. Control group, CMT-alone group; Experimental group, sodium cantharidinate and vitamin B6 injection (SC/B6) + CMT.
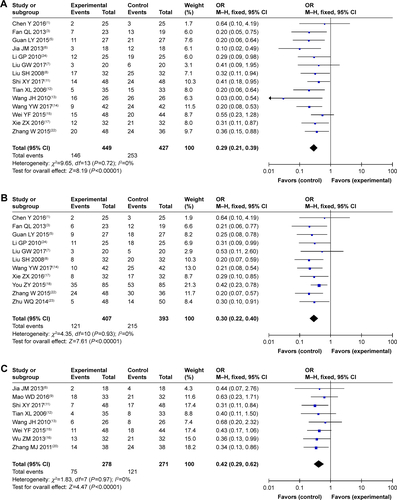
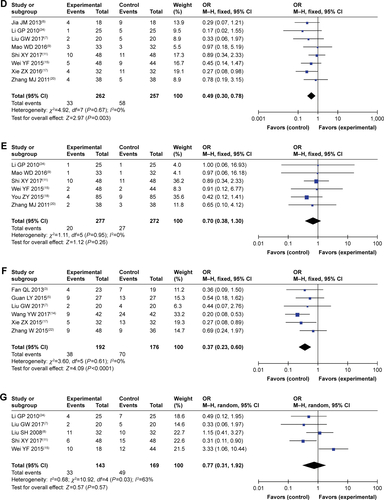
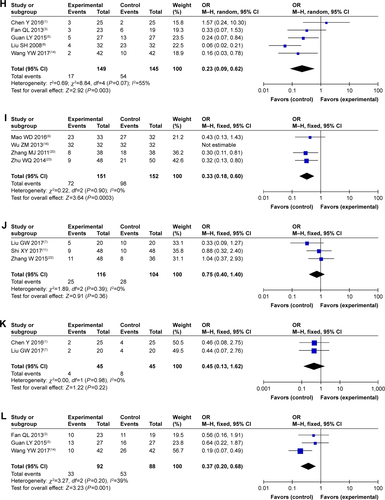
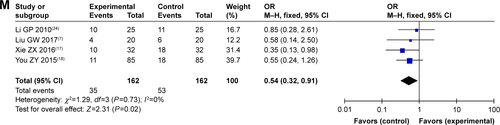
Figure S3 Funnel plot of percentage of complete response rates (A), partial response rates (B), stable disease rates (C), and progressive disease rates (D).
References
- ChenYZhuWMZhouDXShanYFClinical study on disodium cantharidinate and vitamin B6 combined with raltitrexed and oxaliplatin in treatment of advanced colorectal cancerDrugs Clinic2016311117841787
- FanLJEfficacy of sodium cantharidinate and vitamin B6 combined with 5-fluorouracil and calcium folinate in treatment of advanced colorectal cancerMod Med J China200911106465
- FanQLWangYZhaoKXKaoJClinical observation on effects of sodium cantharidate vitamin B6 injection combined with S-1 treatment of advanced gastrointestinal tract tumor patientsGuangming J Chin Med2013284786788
- FangXHXuJCantharidin sodium vitamin B6 injection as assistant therapy for treating liver cancer in 37 casesChina Pharm20162512527
- GuanLYMaJYuanSFQuJDisodium cantharidinate and vitamin B6 combined with tegafur, gimeracil and oteracil porassium for treating stage IV pancreatic cancer in 27 casesTradit Med20152420124125
- JiaJMZhLEfficacy of sodium cantharidinate and vitamin B6 comwith intravenous chemotherapy for advanced esophageal cancerMed Inf2013269188189
- LiuGWRenWDCantharis acid sodium vitamin B6 injection comwith maintain capecitabine synchronization for the treatment of advanced colorectal cancerWorld Latest Med Inf201717261214
- LiuSHFengWZhuRXFengZCComparative study of disodium cantharidinate and vitamin B6 injection combined with oxaliplatin and tegafur in the treatment of advanced gastric cancerPract Clin J Integr Tradit Chinese West Med20088434
- MaoWDClinical study of sodium cantharidinate and vitamin B6 combined with capecitabine in the treatment of advanced gastric cancerJ Clin Med201634590349036
- ShaoHHongGLuoXEvaluation of sodium cantharidinate/vitamin B6 in the treatment of primary liver cancerJ Cancer Res Ther201410Suppl 1757825207897
- ShiXYZhangJMengWMaFLiZLZhaoJFClinical observation of sodium cantharidinate and vitamin B6 injection combined with XELOX protocol in treatment of patients with advanced colorectal carcinomaMed & Pharm J Chin PLA20172963639
- TianXLYangPClinical efficacy of treatment of liver cancer with combined sodium cantharidate vitamin B6 injection and TACE therapyChin J Clin Oncol Rehabil2006134351353
- WangJHLiGPWuJHApplication of sodium cantharidinate and vitamin B6 injection in patients with advanced colorectal cancerJ Mod Med Health2010266844845
- WangYWYangRLThe effect observation of sodium cantharivitamin B6 combined with capecitabine in the treatment of advanced stomach and colorectal carcinomaMed Innov China201714362326
- WeiYFWuJSHuoZGJiaZYQiSSWangXXEfficacy evaluation of sodium cantharidinate and vitamin B6 injection combined with TACE in the treatment of 48 patients with intermediate and advanced primary liver cancerChina Pharm20152457274
- WuZMLiuQShiSMYuSYClinical study of ai yishu combined with FOLFIRI regimen in the treatment of recurrent gastric cancerMed Inf2013266256
- XieZXWangJSLiuYBShort-term efficacy and adverse effects of sodium cantharidinate and vitamin B6 injection combined with chein the treatment of advanced gastric cancerHebei Med J2016381015541559
- YouZYLiuYShuXHLiuBNLiuXFMalignant ascites of gas-tumor treated with sodium cantharidinate and vitamin B6 injection and hyperthermic intraperitoneal chemotherapyWorld J Integr Tradit West Med201510912491255
- ZengLLiuYLLiuXTYangZFClinical observation of sodium cantharidinate and vitamin B6 injection in the treatment of liver cancerJ Hebei Med Univ2009306596597
- ZhangMJZuoCFClinical efficacy of sodium cantharidate vitamin B6 injection combined with chemotherapy in treatment of liver cancerJ Pract Oncol20112615052
- ZhangWYangJZhongCSEfficacy of sodium cantharidinate vitamin B6 combined with xeloda in the treatment of advanced gastric cancerJ Huaihai Med2012305446447
- ZhangWLaoWWEfficacy of sodium cantharidinate vitamin B6 combined with CapeOX in the treatment of advanced gastric cancerMed Front2015516108110
- ZhuWQSodium cantharindinate and vitamin B6 injection in combinawith TACE for primary liver cancerInt Med Health Guid News2014201421302132
- LiGPLiXRWangJHClinical observation on sodium cantharidate vitamin B6 injection combined with postoperative chemotherapy in treatment of gastric cancerPract Prev Med2010171105106
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrayFFerlayJSoerjomataramISiegelRLTorreLAJemalAGlobal cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin201868639442430207593
- FidlerMMBrayFSoerjomataramIThe global cancer burden and human development: a reviewScand J Public Health20184612736
- GongJLvLHuoJRoles of F-box proteins in human digestive system tumors (review)Int J Oncol20144562199220725270675
- LiMXBiXYHuangZPrognostic role of phospho-STAT3 in patients with cancers of the digestive system: a systematic review and meta-analysisPLoS One2015105e012735626024373
- DaiCWangMLuJPrognostic and predictive values of PD-L1 expression in patients with digestive system cancer: a meta-analysisOnco Targets Ther2017103625363428769571
- FuMZouCPanLLong noncoding RNAs in digestive system cancers: Functional roles, molecular mechanisms, and clinical implications (review)Oncol Rep20163631207121827431376
- WangBCuiJTreatment of mid-late stage NSCLC using sodium cantharidinate/vitamin B6/GP regimen in clinicJ Cancer Res Ther201410Suppl 1C79C8125207898
- ShaoHHongGLuoXEvaluation of sodium cantharidinate/vitamin B6 in the treatment of primary liver cancerJ Cancer Res Ther201410Suppl 1757825207897
- ShiZSongTWanYA systematic review and meta-analysis of traditional insect Chinese medicines combined chemotherapy for nonsurgical hepatocellular carcinoma therapySci Rep201771435528659623
- WenSQChenQHuMExperimental study on the inhibitory effect of sodium cantharidinate on human hepatoma HepG2 cellsAfrJ Tradit Complement Altern Med2014111131134
- ZhangDWuJWangKDuanXLiuSZhangBWhich are the best Chinese herbal injections combined with XELOX regimen for gastric cancer?: A PRISMA-compliant network meta-analysisMedicine20189712e012729561411
- TaoRSunWYYuDHSodium cantharidinate induces HepG2 cell apoptosis through LC3 autophagy pathwayOncol Rep20173821233123928677738
- LiangFWangMYHuangWBLiAJEffect of sodium cantharidinate on the angiogenesis of nude mice with human gastric cancerZhong Yao Cai201134334334621823448
- ZhaoRLChenMJZhaoFMXuDQZhangXZhouKFStudy on synergistic effect of sodium cantharidinate combined with chemotherapeutic drugs on hepatic carcinoma and its effective mechanismZhong Yao Cai201437111938194626027111
- TaoRWangZFQiuWRole of S100A3 in human hepatocellular carcinoma and the anticancer effect of sodium cantharidinateExp Ther Med20171362812281828588665
- ZangGHLiRZhouRSEffects of disodium cantharidinate on dendritic cells of patients with bladder carcinomaOncol Lett20181522273227729434934
- VermaAKPrasadSBChanges in glutathione, oxidative stress and mitochondrial membrane potential in apoptosis involving the anticancer activity of cantharidin isolated from redheaded blister beetles, epicauta hirticornisAnticancer Agents Med Chem20131371096111423343079
- LiWXieLChenZCantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosisCancer Sci201010151226123320331621
- ChenYZhuWMZhouDXShanYFClinical study on disodium cantharidinate and vitamin B6 combined with raltitrexed and oxaliplatin in treatment of advanced colorectal cancerDrugs Clinic2016311117841787
- FanLJEfficacy of sodium cantharidinate and vitamin B6 combined with 5-fluorouracil and calcium folinate in treatment of advanced colorectal cancerMod Med J China200911106465
- FanQLWangYZhaoKXKaoJClinical observation on effects of sodium cantharidate vitamin B6 injection combined with S-1 treatment of advanced gastrointestinal tract tumor patientsGuangming J Chin Med2013284786788
- FangXHXuJCantharidin sodium vitamin B6 injection as assistant therapy for treating liver cancer in 37 casesChina Pharm20162512527
- GuanLYMaJYuanSFQuJDisodium cantharidinate and vitamin B6 combined with tegafur, gimeracil and oteracil porassium for treating stage IV pancreatic cancer in 27 casesTradit Med20152420124125
- JiaJMLiZHEfficacy of sodium cantharidinate and vitamin B6 combined with intravenous chemotherapy for advanced esophageal cancerMed Inf2013269188189
- LiGPLiXRWangJHClinical observation on sodium cantharidate vitamin B6 injection combined with postoperative chemotherapy in treatment of gastric cancerPract Prev Med2010171105106
- LiuGWRenWDCantharis acid sodium vitamin B6 injection combined with maintain capecitabine synchronization for the treatment of advanced colorectal cancerWorld Latest Med Inf201717261214
- LiuSHFengWZhuRXFengZCComparative study of disodium cantharidinate and vitamin B6 injection combined with oxaliplatin and tegafur in the treatment of advanced gastric cancerPract Clin J Integr Tradit Chinese West Med20088434
- MaoWDClinical study of sodium cantharidinate and vitamin B6 combined with capecitabine in the treatment of advanced gastric cancerJ Clin Med201634590349036
- ShiXYZhangJMengWMaFLiZLZhaoJFClinical observation of sodium cantharidinate and vitamin B6 injection combined with XELOX protocol in treatment of patients with advanced colorectal carcinomaMed & Pharm J Chin PLA20172963639
- TianXLYangPClinical efficacy of treatment of liver cancer with combined sodium cantharidate vitamin B6 injection and TACE therapyChin J Clin Oncol Rehabil2006134351353
- WangJHLiGPWuJHApplication of sodium cantharidinate and vitamin B6 injection in patients with advanced colorectal cancerJ Mod Med Health2010266844845
- WangYWYangRLThe effect observation of sodium canthari-date vitamin B6 combined with capecitabine in the treatment of advanced stomach and colorectal carcinomaMed Innov China201714362326
- WeiYFWuJSHuoZGJiaZYQiSSWangXXEfficacy evaluation of sodium cantharidinate and vitamin B6 injection combined with TACE in the treatment of 48 patients with intermediate and advanced primary liver cancerChina Pharm20152457274
- WuZMLiuQShiSMYuSYClinical study of ai yishu combined with FOLFIRI regimen in the treatment of recurrent gastric cancerMed Inf2013266256
- XieZXWangJSLiuYBShort-term efficacy and adverse effects of sodium cantharidinate and vitamin B6 injection combined with chemotherapy in the treatment of advanced gastric cancerHebei Med J2016381015541559
- YouZYLiuYShuXHLiuBNLiuXFMalignant ascites of gastrointestinal tumor treated with sodium cantharidinate and vitamin B6 injection and hyperthermic intraperitoneal chemotherapyWorld J Integr Tradit West Med201510912491255
- ZengLLiuYLLiuXTYangZFClinical observation of sodium cantharidinate and vitamin B6 injection in the treatment of liver cancerJ Hebei Med Univ2009306596597
- ZhangMJZuoCFClinical efficacy of sodium cantharidate vitamin B6 injection combined with chemotherapy in treatment of liver cancerJ Pract Oncol20112615052
- ZhangWYangJZhongCSEfficacy of sodium cantharidinate vitamin B6 combined with xeloda in the treatment of advanced gastric cancerJ Huaihai Med2012305446447
- ZhangWLaoWWEfficacy of sodium cantharidinate vitamin B6 combined with CapeOX in the treatment of advanced gastric cancerMed Front2015516108110
- ZhuWQSodium cantharindinate and vitamin B6 injection in combination with TACE for primary liver cancerInt Med Health Guid News2014201421302132
- ZengXZhangYKwongJSWThe methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic reviewJ Evid Based Med20158121025594108
- JacksonDWhiteIRRileyRDQuantifying the impact of between-study heterogeneity in multivariate meta-analysesStat Med201231293805382022763950
- ZhangLMuYZhangACytokine-induced killer cells/dendritic cells-cytokine induced killer cells immunotherapy combined with chemotherapy for treatment of colorectal cancer in China: a meta-analysis of 29 trials involving 2,610 patientsOncotarget2017828451644517728404886
- WilliamsonPRSmithCTHuttonJLMarsonAGAggregate data meta-analysis with time-to-event outcomesStat Med200221223337335112407676
- ZhouLWangXLDengQLDuYQZhaoNQThe efficacy and safety of immunotherapy in patients with advanced NSCLC: a systematic review and meta-analysisSci Rep2016613202027558285
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials2007811617555582
- LiangMChenQZhangYImpact of diabetes on the risk of bedsore in patients undergoing surgery: an updated quantitative analysis of cohort studiesOncotarget201789145161452428036285
- GuYJiangLMiaoJHLiangTSKanQCYangDKClinical effects of thermotherapy in combination with intracavitary infusion of traditional Chinese medicine in the treatment of malignant pleural effusionJ Biol Regul Homeost Agents20163041023102828078848
- YanZLaiZLinJAnticancer properties of traditional Chinese medicineComb Chem High Throughput Screen201720542342928093974
- XuJSongZGuoQLiJSynergistic effect and molecular mechanisms of traditional Chinese medicine on regulating tumor microenvironment and cancer cellsBiomed Res Int201620165114
- LiaoYHLiCILinCCLinJGChiangJHLiTCTraditional Chinese medicine as adjunctive therapy improves the long-term survival of lung cancer patientsJ Cancer Res Clin Oncol2017143122425243528803328