?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Severe bleeding and perforation of the colon and rectum are complications of ulcerative colitis which can be treated by a targeted drug delivery system.
Purpose
Development of colon-targeted delivery usually involves a complex formulation process and coating steps of pH-sensitive methacrylic acid based Eudragit®. The current work was purposefully designed to develop dicalcium phosphate (DCP) facilitated with Eudragit-S100-based pH-dependent, uncoated mesalamine matrix tablets.
Materials and Methods
Mesalamine formulations were compressed using wet granulation technique with varying compositions of dicalcium phosphate (DCP) and Eudragit-S100. The developed formulations were characterized for physicochemical and drug release profiles. Infrared studies were carried out to ensure that there was no interaction between active ingredients and excipients. Artificial neural network (ANN) was used for the optimization of final DCP-Eudragit-S100 complex and the experimental data were employed to train a multi-layer perception (MLP) using quick propagation (QP) training algorithm until a satisfactory root mean square error (RMSE) was reached. The ANN-aided optimized formulation was compared with commercially available Masacol®.
Results
Compressed tablets met the desirability criteria in terms of thickness, hardness, weight variation, friability, and content uniformity, ie, 5.34 mm, 7.7 kg/cm2, 585±5 mg (%), 0.44%, and 103%, respectively. In-vitro dissolution study of commercially available mesalamine and optimized formulation was carried out and the former showed 100% release at 6 h while the latter released only 12.09% after 2 h and 72.96% after 12 h which was fitted to Weibull release model with b value of 1.3, indicating a complex release mechanism.
Conclusion
DCP-Eudragit-S100 blend was found explicative for mesalamine release without coating in gastric and colonic regions. This combination may provide a better control of ulcerative colitis.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition characterized by de-regulated immune response of intestinal microflora which ultimately limits the mucosal layer of the colon and rectum and is known for its remission and relapse throughout life.Citation1,Citation2 Untreated ulcerative colitis may cause severe bleeding, hole in the colon (perforated colon), severe dehydration, and increased risk of colon cancer. Oral treatment in UC treatment is preferred as compared to suppositories due to its ease of manufacturing, storing conditions, and patient compliance. 5-aminosalicylic acid (5-ASA), an anti-inflammatory agent has gained the status of drug of first choice in the treatment of active mild to moderate UC.Citation3 Among many advantages of oral dosage, dissolution and absorption of drugs in the small intestine reduces its concentration in the colon which results in failure of minimum effective concentration (MEC) of drugs.Citation4
UC can be treated successfully by targeted localized drug delivery to the colon, avoiding systemic concentration. It has been established that therapeutic effect of mesalamine is concentration-dependent, which led to the development of dosage forms which deliver the drug directly to the inflamed tissue with relatively low side effects.Citation5 For the past two decades, delayed release mesalamine has been used for treating UC.Citation6 But colon-targeted drug delivery systems involve difficult preparation procedures and certain management, thereby making the system complex and expensive. Different strategies like pro-drug, enteric coating and pH/time-dependent modified release, microflora-activated system, pressure controlled drug delivery system (PCDCS), osmotic controlled drug delivery to colon (OROS-CT), and pulsatile drug delivery have gained attention.Citation7 Based upon these strategies, the different products of 5-aminosalicylic acid (5-ASA) available are Pentasa® MR coated timed release microgranules, Asacol® and Aspiro® CR double layered enteric coated tablets, Lialda® XL Multimatrix SR hydrophilic/lipophilic matrix core tablets, Octasa® MR pH sensitive coated tablets and Salofalk® gastric-resistant granules coated by pH-independent polymer.Citation8–Citation13 But their polymer-dependent behavior and predicted drug release in the colon remained debatable. Besides taking large dose orally, prodrug has the disadvantage of linking moiety which may cause non-drug related side effects. In time-dependent systems, gastric emptying time and GI transit time limit predicted drug release in the colon. pH-dependent systems which involve enteric coating cannot be easily engineered due to interaction of enteric coated polymeric mixture with hydrophobic additives like magnesium stearate.
Therefore, a pH-dependent targeted drug delivery system was designed by simple wet granulation and without coating, with the advantages of minimum release of drug in upper GIT and enhanced local delivery of drug to the colon.Citation14 Eudragit-S100 is used as a coating agent in delayed release formulations and preferred in colon-targeted delivery due to its sensitivity to alkaline pH of 7.2. Eudragit-S100 is an anionic copolymer based on methacrylic acid and methyl methacrylate with the ratio of 1:2. Use of Eudragit-S100 alone for delayed release is restricted due to its gel forming and diffusion behavior. Dual nature of di-calcium phosphate (DCP), ie, as a binder and release retardant, can facilitate the combination of Eudragit-S100. The resultant complex of Eudragit-S100 and DCP can create the delayed release characteristic not only in the colon but can also prevent the GIT degradation of drugs.Citation14–Citation16
Therefore, attempts were made to develop Eudragit-S100 and DCP complex in uncoated delayed release matrix tablets of 5-ASA.Citation17 Two different types, un-milled and milled form, of DCP were used as diluent previously but presently only the latter was employed.Citation16 Drug release was controlled by varying compositions of the ingredients. Computer-aided technique; Artificial Neural Network (ANN) was employed to catechize the effect of different compositions in twenty-three formulations to optimize a delivery system with desired dissolution profile of mesalamine. ANN is an effective approach to estimate the quantity of the formulation ingredients which may meet the desirability criteria.Citation18 In-vitro dissolution study was carried out to note the drug release up to 70% until 12 h which ensured a delayed release dosage form. The designed delayed release tablets of mesalamine will provide the required concentration of the drug at the site of inflammation to treat UC successfully.
Materials and Methods
Mesalamine was kindly gifted by Getz Pharma, Karachi. Eudragit-S100 was obtained from Highnoon Pharmaceuticals, Lahore. Dicalcium phosphate (DCP), polyvinyl-pyrrolidone-K30 (PVP-K30), magnesium stearate, isopropyl alcohol (IPA), potassium chloride, potassium monohydrogen phosphate, sodium hydroxide, and hydrochloric acid were all purchased from Sigma Aldrich, Germany, sodium starch glycolate (SSG) and ethyl cellulose (EC) were obtained from Merck® Darmstadt Germany. All the chemicals used in the study were of analytical grade.
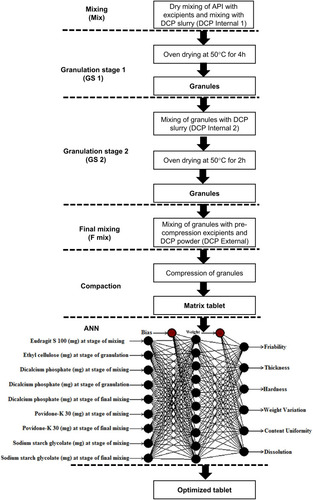
Wet granulation technique was used for the compression of mesalamine tablets as described elsewhere.Citation19 Briefly; compression was divided into five stages for the successful application of ANN. Stage 1 was mixing (Mix), stage 2 was granulation stage 1 (GS-1), stage 3 was granulation stage 2 (GS-2), stage 4 was final mixing (F-mix), and stage 5 was compaction. Total quantity of DCP used was divided into almost two equal parts, part A and B. In mix stage, mesalamine, Eudragit-S100, and PVP-K30 were geometrically mixed with a mortar and pestle for 10 min followed by addition of approximately half of DCP of part A as slurry with IPA (termed as DCP internal 1) until uniform wet mass was obtained. Wet mass was passed through sieve#16 followed by GS-1 which comprised only oven drying for a period of 4 h at 50°C.Citation20 In GS-2, dried granules were mixed again with the remaining half of DCP part A (termed as DCP internal 2) and were passed through sieve#22. The granules were dried in a tray drier (IMS corporation Lahore, Pakistan) at 50°C for 2 h.Citation21 In F-mix stage, these granules were mixed with part B of DCP as dry powder (termed as DCP external) and magnesium stearate.Citation22 These granules were then compressed into tablets and were stored in controlled airtight containers and compared with Masacol® (mesalamine by Getz Pharma Karachi, Pakistan). The five stages of compression along with details of each step have been shown in .
Figure 1 Scheme of study showing five stages of compression: Mix, GS-1, GS-2, F-mix and compression and structure of ANN applied using quick propagation method for data learning algorithm.
Characterization of Matrix Tablets
The prepared tablets were evaluated for their friability, thickness, hardness, weight variation, and drug content assay. Friability of twenty tablets was determined in Roche Friabilator (IMS Corporation, Lahore, Pakistan) for 4 min at 25 rpm. Thickness of tablets was measured by digital screw gauge (Galvano Scientific Ltd, Lahore, Pakistan) by taking 20 tablets from each combination.Citation23 Hardness of ten tablets was measured using digital hardness tester (Erweka, Germany). Weight variation was determined by taking average weight of twenty tablets using digital weight measuring balance (Sartorius Germany). For drug content assessment, 10 tablets were crushed and the aliquot of powdered tablet equivalent to 400 mg of drug was dissolved in 50 mL of phosphate buffer pH 7.2. The solution was filtered through 0.45 µm membrane filter. Sample was precisely diluted and analyzed on UV-spectrophotometer at 234 nm. Percentage of mesalamine present in solution was calculated from the standard calibration curve.Citation24
Drug Dissolution and Release Study
USP dissolution II paddle apparatus (Vision®, California) rotated at 100 rpm was used for two stage mesalamine delayed release studies.Citation4 Two different dissolution mediums, 0.2 M HCl pH 1.2 (simulation of stomach pH) for 2 h and phosphate buffer 0.2 M of pH 7.2 (simulation of colon pH) for further 6 h at 37 ± 0.5°C, were used.Citation25–Citation27 5 mL aliquot was withdrawn which was replaced with fresh acidic medium for first 2 h and with basic phosphate buffer pH 7.2 for remaining 6 h.Citation28 Collected samples were filtered through 0.45 µm Millipore filters and mesalamine concentration was measured at 234 nm after appropriate dilutions of samples.
Kinetics of Drug Release
Release kinetic modeling was carried out using Microsoft Excel based adds in program DD Solver Ver 1.0. The kinetic models were applied by using the following equations:Citation29
Where Qt and QH are the mean percentage of drug release, t is time in h, K0, K1 KH are zero, first and Higuchi order release constant expressed in concentration/time respectively.
Where is a fraction of drug released at time t, k is the release rate constant and n is the release exponent.
In Peppas (Fickian diffusion) model, mechanisms of drug release are characterized using the release exponent (“n” value). An “n” value of 1 corresponds to zero-order release kinetics (case II transport); 0.5 < n < 1 means an anomalous (non-Fickian) diffusion release model; n= 0.5 indicates Fickian diffusion, and n > 1 indicates a super case II transport relaxational release.Citation30 The regression analysis data evaluate the kinetics of drug release from the prepared formulations.Citation31
In Hixson-Crowell model, is the initial amount of drug in pharmaceutical dosage form,
is the remaining amount of drug in pharmaceutical dosage form at time
and ƙ (kappa) is a constant incorporating the surface-volume relation.
In Weibull model, is the scale parameter which describes the time dependence,
describes the shape of dissolution curve dependence and
is the amount of drug dissolved. The model which showed the highest value of coefficient of determinants (R2) and lowest value of Akaike information criterion (AIC) was categorized as best model that described the release.
ANN-Assisted Colonic Delivery System of Mesalamine
ANN Neural Power® version 3.1 was used to study the relative effects of Eudragit-S100, DCP and PVP-K30 on hardness and in-vitro dissolution for 2–8 h.Citation32 Quick propagation (QP) method was employed for the data learning algorithm with Tanh as transfer function until a satisfactory root mean square error (RMSE), less than 1 was reached.Citation33 Multilayer perception (MLP) structure was used in the study where the class of structure was feed forward. The total number of inputs, outputs and hidden layers was 5, 8, and 1, respectively, shown in . Surface responses for the importance of excipients against hardness and in-vitro release were generated.Citation34 For the prediction of the best levels of polymers to further retard the drug release until 12 h “What if” approach of ANN was used. On the basis of ANN predicted composition, F24 was compressed with some adjustments as shown in to confirm the ANN prediction.
Table 1 Mesalamine Formulations by Wet Granulation Method
Characterization of ANN Assisted Formulation
The ANN assisted formulation was characterized in terms of friability, thickness, hardness, weight variation, dissolution and kinetics of release. Kinetics of drug release was studied by model independent approaches using already described DDSolver. The values of dissimilarity factor f1 and similarity factor f2 for F23 and F24 were calculated on the basis of release profile as reported in literatureCitation35 and compared with commercially available mesalamine, by using the following equations.
where n is the sample number, and Rt and Tt are the percentage of the reference and test drug release, respectively, at different time intervals.
Fourier Transform Infrared Spectroscopy (FTIR)
Infrared (IR) spectra for the pure drug, selected excipients and ANN assisted formulation were recorded on attenuated total reflectance (ATR) attached FTIR spectrophotometer (FTIR-Agilent Technologies). Spectrum was taken from the wavelength of 500–4000 cm−1.Citation36
Results
Different ratios of Eudragit-S100 and DCP were used successfully and 24 formulations were compressed by wet granulation method. Physicochemical properties of the powder blend and compressed tablets were within the limits (). Out of 24 formulations, F1 to F23 were prepared stepwise by controlling the composition of ingredients for desired outputs. The 24th formulation (F24) was prepared by the optimized levels of the ingredients predicted by ANN approach.
Table 2 Physicochemical Characteristics of Mesalamine Formulations (Mean ± S.D)
In-vitro Drug Release
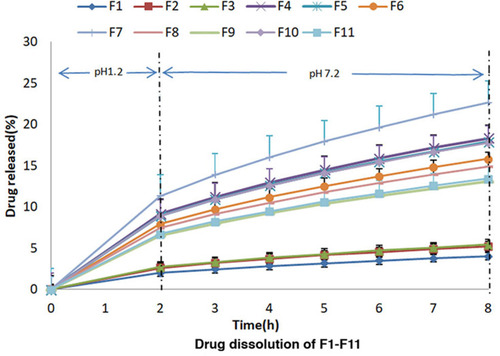
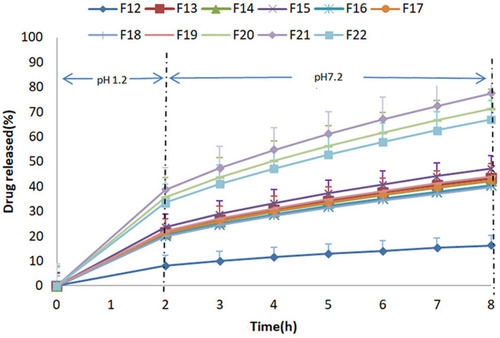
In-vitro dissolution of prepared formulations was conducted in acidic medium for initial 2 h followed by evaluation in basic medium for further 6 h, except F23 which was studied for 12 h using the same tablets due to its 71.66% release within the first 8 h. The graphical representation of drug release data of formulations F1-F22 due to the effect of formulation variables is shown in and . Prepared formulations did not show an adequate release in acidic media during first 2 h of this study, therefore, the release data for 1 h had not been given in any graphical and tabular representation. It was observed that formulations F1-F3 containing varying concentrations of DCP, Eudragit-S100, and EC had shown less than 9% drug release in 8 h. This high drug retardation might be attributed to the EC due to its binding effect that increased tablet hardness.Citation35 For this reason, EC was excluded from the next set of formulations F4-F12 which were prepared by varying concentrations of DCP and Eudragit-S100 in pre-decided ratio (). Non-desirable release pattern in F4 to F12 was observed throughout the study. The mesalamine release retardation might be attributed to the presence of Eudragit-S100.Citation37 Another set of formulations were prepared by excluding Eudragit-S100 and using DCP as bulking agent in F13-F15 to maintain the desired tablet weight. The findings of release of drug from F13-F15 demonstrated a breakthrough in the role of DCP, as release retardant. This set met the desirability of release criteria until 4 h but failed to exhibit required release until 8 h. For formulation F16 and onwards, Eudragit-S100 was included in combination with DCP which exaggerated a smooth drug release pattern to help obtain the desired sustained drug release characteristics which enabled the dosage form to target the colon without coating the dosage form. For the set of formulations F16-F19, PVP K30 was varied but desired release pattern was not achieved. It was noted that in this set of formulations, a small amount of Eudragit-S100 (2%) in combination with DCP helped to control the initial 2 h release and DCP External for smooth delayed release in 3–8 h. In this combination, the required amount of Eudragit-S100 was less than which has been reported.Citation37 Sodium starch glycolate was added as the superdisintegrant in F20-F23 to further enhance the smooth release pattern. Outcomes of all formulations were still not considered acceptable in terms of sustained drug delivery specifically from 2–5 h in small intestine, since drug release deviated from the desired profile.
Release Kinetics and Mechanism
The kinetic mechanisms for the drug release from all the formulations were plotted, and the coefficients of the drug release (R2) were calculated as presented in . In the present study, most of the formulations exhibited n value greater than 1, indicative of super case II transport release. R2 for Hixson Crowell cube root indicated uniform exhaustion of the drug delivery system.Citation38 The drug release profiles with highest R2 and lowest AIC values among all kinetics models indicated that the release data for majority of the formulations were best fitted to Weibull model.
Table 3 Dissolution Kinetic Modeling of All Selected Formulations
ANN-Assisted Colon-Targeted Formulation
Based on the inputs given, ANN foretold five sets of the compositions with their characteristics (outputs) predicted (). Among these five sets, set 4 was selected for preparing optimized formulation (F24). The composition of this set was close to the composition of F23 which had shown best release () against the desirability criteria.Citation35 F24 was prepared after minor adjustments to amounts such as 3 mg of Eudragit-S100, 5 mg of DCP-Internal 2 and 20 mg of DCP-External as mentioned ().
Table 4 Optimized Formulations Predicted by ANN
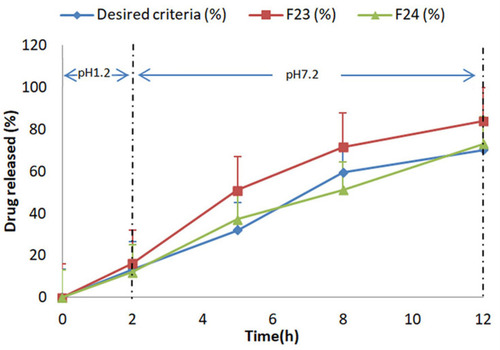
Figure 4 Comparative % release studies of mesalamine from formulation F23-F24 with reference release data using 0.2 M HCl for 2 h at pH 1.2 and 0.2 M phosphate buffer at pH 7.2 for further 10 h.
The adjustment in composition regarding DCP-External was based on the maintenance of the tablet weight in accordance with the previous formulations, regardless of the final weight of the tablet formulations. The physicochemical and release profile for formulation F24 were measured. The release profile and characteristics of the colon-targeted formulation matched the predicted outputs given ().
Characteristics of ANN Predicted Formulation
The physicochemical characteristics of the ANN predicted mesalamine formulation (F24) showed friability 0.44%, thickness 5.34 mm, hardness 7.73 kg/cm2, weight variation 585±3.42 and drug content 103%. The release kinetic analysis of all formulations and comparative release of F23 and F24 have been given in and respectively.
Comparative Release Profiles
Desired released criteria for Colon-Targeted Drug Delivery System from delayed release matrix tablets have been cited in literature.Citation35 In the present study, it was found that F23 was close to the desired release of 70% but this release occurred earlier in 8 h. ANN predicted composition F24 produced further retardation in mesalamine release and clearly indicated a sustained smooth drug release from 0–12 h. The drug release pattern of F23 and F24 along with desired release criteria have been illustrated in .
In terms of release, dissimilarity (f1) and similarity (f2) data from DDSolver showed that formulation 24 was similar to that of the desired release profile of the delayed release formulations having f1 10.14 and f2 63.86. Formulation F23 lost its closeness from the desired profile due to difference of release at time intervals of 3–7 h. F24 was also compared with mesalamine in terms of release and it showed f1 68.12 and f2 23.32 which proved to be different from the available market product.
Fourier Transform Infrared Studies
FTIR study indicated that the drug maintained its integrity; mesalamine displayed all the major peaks inside the fingerprint region of 500–2000 cm−1.
Discussion
On average, an oral dosage form may remain in the stomach for 2 h and in the intestine for 4 h, thus the average time for a formulation to arrive at the colon is 6 h.Citation39 The rapid and extensive absorption of mesalamine in the small intestine causes a minor local action on mucosa and substantial systemic side effects.Citation9 Therefore, a delivery system was designed to retard drug release until 6 h and to liberate at least 70% drug by 12 h. In the present study, the milled form of DCP was used during three stages, ie, Mix, GS-2, and F mix according to the literature.Citation40 The weights of ingredients in a pharmaceutical formulation are relative to each other, ie, when weight of one is changed, at least weight of one other ingredient has to be changed to keep the final weight of the tablet constant. The change in the relative weights in formulation of the individual ingredients is usually compensated with that of the bulking agent to achieve a fixed weight of the formulation. In this study, though DCP was used as a bulking agent, when it was combined with Eudragit-S100, it was found to act as a modulator for release, in line with literature, according to which the DCP, despite acting as a diluent in direct compression, might also retard drug release, a potential of DCP that had not been well explored.Citation16 Hence, the amount of DCP was not adjusted to make up for achieving a fixed weight of all formulations. Thus, the final weight of the tablet formulations depended upon the amount of DCP and Eudragit-S100 in the blend. shows the different final weights of the tablets. Keeping the foregoing discussion in mind, another bulking agent should have to be added in formulations, but keeping the formulation with least numbers and quantity of ingredients was also an intention. The formulation F24 was the optimized tablet, its final weight was 575.2 mg. Unlike statistical approach such as design of experiment, the ANN is based on the learning of the patterns in the data, it is capable of capturing the effect of the change, parallel or unparallel in the ratios of the inputs on the final properties of the formulation. In this study, tablets’ final weights were not entered as the properties so prediction of the optimized formulation was not affected by the different weights of the tablet formulations. Moreover DCP can be a source of Ca++ and Heemskerk et alCitation41 and Jing et alCitation42 have separately shown that blood clotting is expedited when cytosol Ca++ is increased. This increased cytosolic Ca++ in µM range, even for minutes, can evoke platelet membrane blebbing and phosphatidylserine (PS) exposure leading to coagulation of bleeding scars.
Being sans statistical-intensive approach, the predictivity and suitability of the ANN model is assessed through RMSE; RMSE < 1 indicates appropriateness of data training and adequacy of model for a reliable prediction.Citation18 ANN predicted the relative importance of each of the ingredients shown in for overall properties of formulation which were ranked as Eudragit-S100> DCP internal 1> used in pre-granulation stage DCP internal 2 > used in intra-granulation stage DCP external > PVP internal used in extra-granulation stage as explained earlier. Thus, the summary of combined effect of Eudragit-S100, DCP internal 1 (mixed during GS1), DCP internal 2 (mixed during GS2), DCP-external and PVP-Internal only has been shown in .
Table 5 Summary of Effect of Ingredients on Parameters
Figure 5 Relative significance of ingredients for the mesalamine colonic system for overall properties of formulation which were ranked as Eudragit-S100> DCP internal 1 used in pre-granulation or Mix stage> DCP internal 2 used in intra-granulation stage or GS-2> DCP external used in F-Mix stage > PVP internal used in extra-granulation stage.
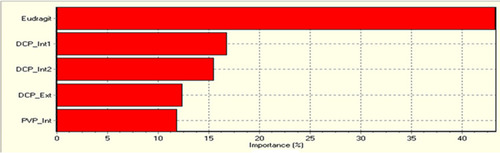
DCP, being an insoluble filler, was mainly focused on because of its earlier reported release retardant property,Citation43 good flowability, and its action by reducing Fickian diffusion with increasing erosion rate of matrix for sustained release drug delivery from a simple uncoated matrix tablet.Citation43,Citation44 The desired hardness of less than 8 kg/cm2 was obtained by varying amounts of Eudragit-S100 and DCP-internal 1 (), showing that the concentrations of both excipients played a critical role in hardness parameter. The maximum amount of drug was released with the highest concentration of DCP-internal 1. The desired 30% release was obtained at a point with the minimum amount of Eudragit- and maximum amount of DCP-internal 1. This showed the least effect of Eudragit- on release at 2 h ().
Figure 6 Response surface plots of: (A) hardness: showing that desired hardness of less than 8 kg/cm2 was obtained by varying amount of Eudragit-S100 and DCP-Internal1. (B) Release at 2 h: showing the least effect of Eudragit-S 100 on release at 2 h (C) and (D) release at 3 h and 4 h: showing that Eudragit-S100 and DCP Internal 1 did not impart a significant effect on drug release at 3 h and 4 h. (E) and (F) release at 5 h and 6 h: showing the least effect of DCP-Internal 1 on drug release at 5 h and 6 h. (G, H) Release at 7 h and 8 h: showing the maximum amount of drug was released with the higher amount of Eudragit-S100 and minimum amount of DCP-Internal 1 at 7 h and 8 h.
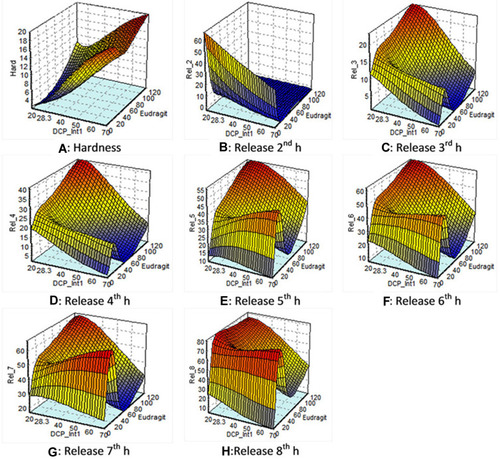
The desired 35 and 45% release at 3 h and release at 4 h were not obtained with this amount of excipients. The predicted pattern gives the maximum amount of drug released 25 and 40% at 3 and 4 h at the highest concentration of Eudragit- and least effect of DCP-Internal 1. This showed that Eudragit-S100 and DCP-Internal 1 have not imparted a significant effect on drug release at 3 and 4 h ().
The maximum amount of drug was released with the highest concentration of Eudragit- and minimum concentration of DCP-Internal 1 for release at 5 and 6 h. The desired 50 and 55% release at 5 and 6 h respectively were obtained at the minimum level of DCP-Internal 1 and maximum level of Eudragit-. This shows the least effect of DCP-Internal 1 on Release 5 & 6 ().
The desired 65 and 70% drug release at 7 and 8 h, respectively were obtained at highest concentration of Eudragit-. The maximum amount of drug was released with the higher amount of Eudragit- and minimum amount of DCP-Internal 1 ().
The predicted graphical representation of hardness and 2–8 h release study from ANN,Citation34 showed that Eudragit-S100 has a critical effect on physicochemical factor, ie, hardness and in-vitro drug dissolution release profile at 2, 3, 4, 5, 6, 7, and 8 h. The effect of DCP-Internal 1 showed a more pronounced effect on hardness of tablets rather than on the drug release, as summarized in .
Dissolution profile of commercially available mesalamine have shown 100% release until 6 h, instead, F23 and F24 () have shown a marked difference among their respective release data at different time intervals and retarded the drug release until 12 h. Pulsatile release pattern of commercially available mesalamine validates our delayed release formulation which showed 70% release until 12 h.Citation45
Table 6 Dissolution Profile of Commercially Available Mesalamine, F-23 and F-24
Weibull model is more useful in comparing the release profiles of matrix type drug delivery that has been described for different types of dissolution processes.Citation30
Weibull model, log [-ln (1-m)] = b log [t – Ti] – log a, fitted the data of optimized formulation. This model explains the dissolution process of matrix type of drug delivery in the following Equationequation 9(8)
(8) :
where M is the amount of drug dissolved as a function of time t. M0 is total amount of drug being released, and T is the lag time. The equation parameter “a” is a scale parameter that describes the time dependence and “b” determines the progression of shape of the dissolution curve. If b = 1, the rise in curve corresponds to an exponential profile with a constant k = 1/a and the previous equation could be rewritten as:
The b value higher than 1 yields a sigmoidal curve with a turning point, while b lower than 1 produces a steeper increase. Using the inverse function of the previous equation, T50% and T90% is calculated. The b value of the formulation F24 was found to be 1.3, greater than 1 generating a sigmoidal curve with a minor lag time.
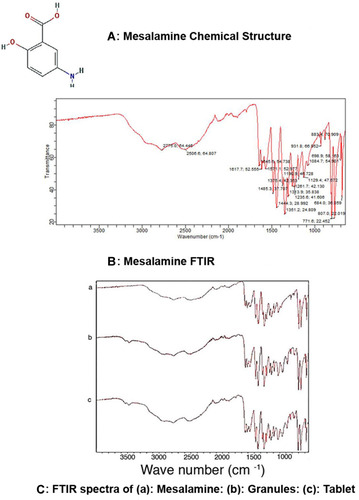
The characteristic peaks of mesalamine functional groups were found in both spectra of mesalamine matrix tablets and granules. The –NH2 functional group bending peak and C=O functional group stretching was observed at 1615–1700 cm−1, along with C-O stretch at 1215 cm−1, indicating that mesalamine was stable during granulation and tableting procedure and there was no indication of any excipient-drug incompatibility nor denaturation of drug during manufacturing procedure. The chemical structure of mesalamine and its chemical peaks have been shown () respectively. The FTIR spectra of mesalamine, mesalamine granules and tablets are shown ( (a–c)).
Figure 7 FTIR spectra. (A) Mesalamine chemical structure. (B) Mesalamine FTIR peaks showing the –NH2 functional group bending peak and C=O functional group stretching at 1615–1700 cm−1, along with C-O stretch at 1215 cm−1. (C) FTIR spectra of (a) mesalamine; (b) mesalamine granules; (c) mesalamine tablet showing characteristic peaks of mesalamine functional groups in spectra of mesalamine, mesalamine matrix tablets, and granules with indication of no excipient-drug incompatibility.
Conclusion
The combination of Eudragit-S100 and dicalcium phosphate, was used to develop the delayed release matrix tablet with the aid of artificial neural network (ANN) which helped to control the release of the mesalamine. The optimized uncoated matrix tablet surpass the stomach and intestinal conditions without any major drug release for 2 h and 6 h respectively and the drug maintained its integrity until 12 h up to 70%. FTIR spectra showed that mesalamine did not show any interaction with the previously mentioned novel combination. DCP is used as a critical excipient in terms of multiple purposes, ie, as bulking agent and mainly as release retardant which has not been used in previous colon-specific drug delivery systems. Formulations F-23 and F-24 are the candidates for in-vivo evaluation in comparison to any of the products available on the market. Comparative dissolution study of the commercially available mesalamine and our newly developed matrix tablet showed a sustained drug release until 12 h of the latter. This sustainability of mesalamine along with increased residence time in the colon with comfortable dosage form signify its novelty as compared to the enteric coated products. The role of Ca++ provided by DCP needs further exploration in reducing blood clotting time of bleeding scars in the colon in-vivo.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
The authors are thankful to the management of Lahore Medical and Dental College and Department of Pharmaceutics, Faculty of Pharmacy, Bahauddin Zakariya University, Multan for their kind support in completion of this project.
References
- CuffariC, PierceD, KorczowskiB, et al. Randomized clinical trial: pharmacokinetics and safety of multimatrix mesalamine for treatment of pediatric ulcerative colitis. Drug Des Devel Ther. 2016;10:593. doi:10.2147/DDDT.S95316
- PerrottaC, PellegrinoP, MoroniE, et al. Five-aminosalicylic acid: an update for the reappraisal of an old drug. Gastroenterol Res Pract. 2015;2015:9. doi:10.1155/2015/456895
- PierceD, CorcoranM, MartinP, et al. Effect of MMX® mesalamine coadministration on the pharmacokinetics of amoxicillin, ciprofloxacin xr, metronidazole, and sulfamethoxazole: results from four randomized clinical trials. Drug Des Devel Ther. 2014;8:529. doi:10.2147/DDDT.S55373
- KhanAM, BashirS, HanifM, AbbasN, AhsanM. Colon specific formulation of mesalamine: preparation and in vitro evaluation. Lat Am J Pharm. 2016;35(6):1391–1398.
- JinL, DingY-C, ZhangY, XuX-Q, CaoQ. A novel pH–enzyme-dependent mesalamine colon-specific delivery system. Drug Des Devel Ther. 2016;10:2021.
- JakateA, McNameeB, BurkindineD. Bioavailability and swallowability of an age-appropriate, delayed-release mesalamine formulation in healthy volunteers. Clin Pharmacol. 2019;11:93.31372067
- TrivediDH, PuranikKP. Colon targeted delivery system (CODESTM): propitious approaches in targeting colon. World J Pharm Pharm Sci. 2017;6(4):768–789.
- ElbaryAA, AboelwafaAA, Al SharabiIM. Once daily, high-dose mesalazine controlled-release tablet for colonic delivery: optimization of formulation variables using box–behnken design. AAPS PharmSciTech. 2011;12(4):1454–1464. doi:10.1208/s12249-011-9708-922038474
- DeckD, WinstonL, KatzungB, MastersS, TrevorA. Basic & Clinical Pharmacology. McGraw-Hill Medical; 2012.
- GoyanesA, HattonGB, MerchantHA, BasitAW. Gastrointestinal release behaviour of modified-release drug products: dynamic dissolution testing of mesalazine formulations. Int J Pharm. 2015;484(1–2):103–108. doi:10.1016/j.ijpharm.2015.02.05125721685
- FeaganBG, ChandeN, MacDonaldJK. Are there any differences in the efficacy and safety of different formulations of oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? Evidence from cochrane reviews. Inflamm Bowel Dis. 2013;19(9):2031–2040. doi:10.1097/MIB.0b013e318292010823811638
- NagarajuR, SwapnaY, BabuRH, KazaR. Design and evaluation of delayed and extended release tablets of mesalamine. J Pharm Technol. 2010;2(1):103–110.
- EdmundsMW. Introduction to Clinical Pharmacology-e-Book. Elsevier Health Sciences; 2015.
- BourgeoisS, HarveyR, FattalE. Polymer colon drug delivery systems and their application to peptides, proteins, and nucleic acids. Am J Adv Drug Deliv. 2005;3(3):171–204. doi:10.2165/00137696-200503030-00003
- HerrlichS, SpiethS, MessnerS, ZengerleR. Osmotic micropumps for drug delivery. Adv Drug Deliv Rev. 2012;64(14):1617–1627. doi:10.1016/j.addr.2012.02.00322370615
- RoweRC, SheskeyPJ, QuinnM. Handbook of pharmaceutical excipients–7th edition. Pharm Dev Technol. 2013;18:544.
- AultonME, TaylorKM. Aulton’s Pharmaceutics e-Book: The Design and Manufacture of Medicines. Elsevier Health Sciences; 2017.
- BukhariNI, JuliantoT, Valente PereiraRE, et al. Computer-aided prediction of cefotaxime sodium stability in aqueous solution at different pH from sparse data. Lat Am J Pharm. 2018;37(3):571–578.
- Patel JayvadanK, Patel NiravV, Shah ShreerajH. Formulation and in-vitro evaluation of mesalamine matrix tablets using chitosan for colonic drug delivery. J Pharm Res. 2009;2(7):1319–1323.
- ChiesiP, VenturaP, MezzadriR, BrambillaG, AcerbiD. Pharmaceutical Compositions Containing an Effervescent Acid-Base Couple. Google Patents; 2004.
- KawakamiY, SatoN, HoshinoM, KouyamaT, ShiikiZ. Controlled Release Formulations Using Intelligent Polymers. Google Patents; 2005.
- HarishG, BhargaviC, RiyajuneA, et al. Effect of different disintegrants on ciprofloxacin conventional tablets. IJRPB. 2013;1(3):281.
- RatnaparkhiMP, MohantaG, UpadhyayL. Review on: fast dissolving tablet. J Pharm Res. 2015;2:5–12.
- PawarKP, GautamC. Design, optimization and evaluation of mesalamine matrix tablet for colon drug delivery system. Int J Pharm Investig. 2016;46(1):67–78. doi:10.1007/s40005-015-0214-z
- United States Pharmacopeia 35 - National Formulary 30 (USP 35-NF 30) Rockville: United States Pharmacopeial Convention; 2011. Available from: https://www.uspnf.com/purchase-usp-nf/usp-nf-archive.
- BendasER, ChristensenJM, AyresJW. Development and in vitro evaluation of mesalamine delayed release pellets and tableted reservoir-type pellets. Drug Dev Ind Pharm. 2010;36(4):393–404. doi:10.3109/0363904090321371719740039
- StolkL, RietbroekR, WiltinkE, TukkerJ. Dissolution profiles of mesalazine formulations in vitro. Pharm Weekbl Sci. 1990;12(5):200–204. doi:10.1007/BF019800472255590
- JainS, YadavS, PatilU. Preparation and evaluation of sustained release matrix tablet of furosemide using natural polymers. Res J Pharm Technol. 2008;1(4):374–376.
- DashS, MurthyPN, NathL, ChowdhuryP. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–223.20524422
- LokhandwalaH, DeshpandeA, DeshpandeS. Kinetic modeling and dissolution profiles comparison: an overview. Int J Pharm Bio Sci. 2013;4(1):728–773.
- ZhangY, HuoM, ZhouJ, et al. Ddsolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–271. doi:10.1208/s12248-010-9185-120373062
- HussainA, SyedMA, AbbasN, et al. Development of ann optimized mucoadhesive buccal tablet containing flurbiprofen and lidocaine for dental pain. Acta Pharm. 2016;66(2):245–256. doi:10.1515/acph-2016-002027279067
- MandaA, WalkerRB, KhamangaSM. An artificial neural network approach to predict the effects of formulation and process variables on prednisone release from a multipartite system. Pharmaceutics. 2019;11(3):109. doi:10.3390/pharmaceutics11030109
- IbricS, JovanovicM, DjuricZ, et al. Artificial neural networks in the modeling and optimization of aspirin extended release tablets with Eudragit- l 100 as matrix substance. AAPS PharmSciTech. 2003;4(1):62–70. doi:10.1208/pt040109
- RehmanK, AminMC, MudaS. Influence of beta-cyclodextrin and chitosan in the formulation of a colon-specific drug delivery system. Drug Res. 2013;63:657–662. doi:10.1055/s-0033-1349129
- ElzeinT, Nasser-EddineM, DelaiteC, BistacS, DumasP. FTIR study of polycaprolactone chain organization at interfaces. J Colloid Interface Sci. 2004;273(2):381–387. doi:10.1016/j.jcis.2004.02.00115082371
- MehtaR, ChawlaA, SharmaP, PawarP. Formulation and in vitro evaluation of Eudragit- s-100 coated naproxen matrix tablets for colon-targeted drug delivery system. JAPTR. 2013;4(1):31.23662280
- ChenS, ZhuJ, ChengJ. Preparation and in vitro evaluation of a novel combined multiparticulate delayed-onset sustained-release formulation of diltiazem hydrochloride. Pharmazie. 2007;62(12):907–913.18214341
- Tugcu-DemirozF, AcarturkF, TakkaS, Konus-BoyunagaO. In-vitro and in-vivo evaluation of mesalazine–guar gum matrix tablets for colonic drug delivery. J Drug Target. 2004;12(2):105–112. doi:10.1080/1061186041000169375115203904
- LachmanL, LiebermanHA, KanigJL. The Theory and Practice of Industrial Pharmacy. Lea & Febiger Philadelphia; 2010.
- HeemskerkJW, BeversEM, LindhoutT. Platelet activation and blood coagulation. Thromb Haemost. 2002;88(08):186–193. doi:10.1055/s-0037-161320912195687
- JingJ, SunY. An αIIbβ3-and phosphatidylserine (PS)-binding recombinant fusion protein promotes ps-dependent anticoagulation and integrin-dependent antithrombosis. J Biol Chem. 2019;294(17):6670–6684. doi:10.1074/jbc.RA118.00604430803987
- BharateSS, BharateSB, BajajAN. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients: a comprehensive review. J Excip Food Chem. 2016;1(3):1131.
- JiminiM, KothariHA. Sustained release matrix type drug delivery system: a review. J Drug Deliv Ther. 2012;2(6).
- SchellekensRC, StuurmanFE, van der WeertFH, KosterinkJG, FrijlinkHW. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur J Pharm Sci. 2007;30(1):15–20. doi:10.1016/j.ejps.2006.09.00417085024