Abstract
The importance of the kidney’s role in glucose homeostasis has gained wider understanding in recent years. Consequently, the development of a new pharmacological class of anti-diabetes agents targeting the kidney has provided new treatment options for the management of type 2 diabetes mellitus (T2DM). Sodium glucose co-transporter type 2 (SGLT2) inhibitors, such as dapagliflozin, canagliflozin, and empagliflozin, decrease renal glucose reabsorption, which results in enhanced urinary glucose excretion and subsequent reductions in plasma glucose and glycosylated hemoglobin concentrations. Modest reductions in body weight and blood pressure have also been observed following treatment with SGLT2 inhibitors. SGLT2 inhibitors appear to be generally well tolerated, and have been used safely when given as monotherapy or in combination with other oral anti-diabetes agents and insulin. The risk of hypoglycemia is low with SGLT2 inhibitors. Typical adverse events appear to be related to the presence of glucose in the urine, namely genital mycotic infection and lower urinary tract infection, and are more often observed in women than in men. Data from long-term safety studies with SGLT2 inhibitors and from head-to-head SGLT2 inhibitor comparator studies are needed to fully determine their benefit–risk profile, and to identify any differences between individual agents. However, given current safety and efficacy data, SGLT2 inhibitors may present an attractive option for T2DM patients who are failing with metformin monotherapy, especially if weight is part of the underlying treatment consideration.
Renal glucose handling in the kidney in glucose-tolerant individuals
The human kidney regulates glucose homeostasis via gluconeogenesis, glucose uptake from the circulation, and by glucose reabsorption from the urine filtered in the renal glomeruli.Citation1
Approximately 160–180 g/day of glucose is filtered by the kidneys.Citation1 In healthy (ie, glucose-tolerant) individuals, virtually all glucose filtered by the glomeruli is reabsorbed by the proximal renal tubule and returned into the circulation, so almost no glucose is excreted into the urine. The ability of the proximal tubule to reabsorb glucose increases as the filtered glucose load increases, which can occur by increasing plasma glucose concentration or glomerular filtration rate (GFR),Citation2 until the maximum glucose transport capacity (known as Tm glucose) is reached. Once this level is exceeded, surplus glucose cannot be reabsorbed and is excreted into the urine, resulting in urinary glucose excretion (UGE; ie, glucosuria). In a healthy adult, Tm glucose equates to a filtration rate of 260–350 mg/min/1.73 m,Citation2,Citation3 which is equivalent to a plasma glucose concentration of approximately 200 mg/dL (11.0 mmol/L).Citation4 The plasma glucose concentration at which Tm glucose is reached is known as the renal threshold for glucose excretion.
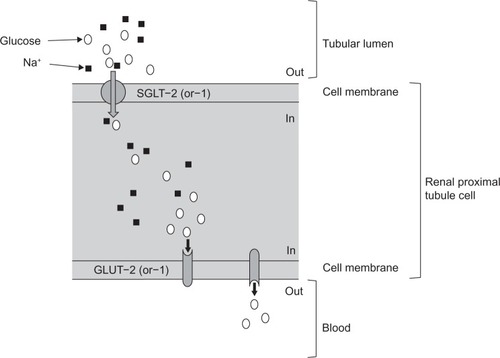
Glucose reabsorption from the glomerular filtrate is mediated by sodium glucose co-transporter (SGLT) proteins in a process that is independent of insulin ( and ), unlike the action of the major facilitative glucose transporter (GLUT) GLUT4 that is responsible for glucose uptake into insulin-sensitive tissues, such as adipose tissue and muscle. SGLTs are membrane-bound proteins that actively transport glucose against its concentration gradient and, thus, require an energy source to drive the sodium pump.Citation5 Details of the SGLT family are summarized in .Citation5 Around 90% of filtered renal glucose is reabsorbed in the brush-border of cells in the first segment of the proximal convoluted tubule by SGLT2, a low-affinity, high-capacity transporter, and the remaining 10% is removed in the distal straight segment by SGLT1, a related high-affinity, low-capacity transporter.Citation5,Citation6 SGLT1 is also extensively expressed in the small intestine where it has a significant role in glucose absorption.Citation5 A second group of glucose transporters, the facilitative glucose transporters or GLUTs, then enable the passive diffusion of glucose from the basolateral membrane of cells in the proximal convoluted tubule into the bloodstream, mainly via GLUT2 and to a minor degree via GLUT1.Citation5–Citation7
Table 1 Sodium-glucose co-transporter (SGLT) family
Figure 1 Renal tubular reabsorption of glucose.
Abbreviations: SGLT, sodium glucose co-transporter; T2DM, type 2 diabetes mellitus.
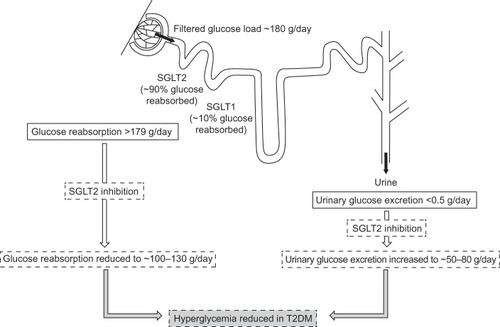
Figure 2 Renal glucose transport.
Abbreviations: GLUT, facilitative glucose transporter; Na+, sodium; SGLT, sodium glucose co-transporter.
SGLT2 is encoded by the SLC5A2 gene, and a range of loss-of-function mutations in this gene results in the rare disorder of familial renal glucosuria.Citation8 Familial renal glucosuria is characterized by UGE in the presence of normal plasma glucose concentrations, without any signs of renal tubular dysfunction.Citation8 Homozygous mutations in the gene encoding SGLT2 result in significant UGE (>10–100 g/1.73 m2/day), whereas heterozygous mutations generally result in lower degrees of UGE (<10 g/1.73 m2/day).Citation8 Nevertheless, most individuals affected by familial renal glucosuria are asymptomatic and only rarely suffer from hypoglycemia or hypovolemia,Citation8 and most of the commonly cited descriptions of this syndrome do not mention an increased risk of genito-urinary infections. In comparison, loss-of-function mutations in the gene encoding SGLT1, SLC5A1, cause glucose-galactose malabsorption in the gut,Citation9 with little or no glucosuria, which results in severe watery diarrhea in affected newborns;Citation9 however, dietary tolerance to glucose appears to develop in adulthood, possibly due to development of gastrointestinal flora that aid in its metabolization.Citation10
Renal glucose handling in the kidney of an individual with diabetes mellitus
Individuals with type 2 diabetes mellitus (T2DM) have increased renal glucose output in the post-absorptive state, causing increased release of glucose into the blood not only from the liver, but also with a significant contribution by the kidneys.Citation11 Greater postprandial elevation of renal glucose release is also observed in individuals with T2DM versus those with normal glucose tolerance.Citation12 Moreover, renal glucose uptake is increased in both post-absorptive and postprandial states in individuals with T2DM versus non-diabetic individuals.Citation11,Citation12
As demonstrated in an early study of individuals with type 1 DM (T1DM), hyperglycemia may occur without the expected degree of glucosuria, resulting from increased glucose reabsorption from the glomerular filtrate: the mean Tm glucose was reported to be up to 20% higher in individuals with T1DM than in healthy individuals.Citation13 In addition, increased expression and activity of SGLT2 mRNA and protein have been demonstrated in vitro.Citation14,Citation15 There may also be over-expression of SGLT1 in the gastrointestinal tract in patients with diabetes.Citation16 A recent study also demonstrated a change in renal glucose kinetics in response to SGLT2 inhibition in healthy subjects and those with T2DM,Citation17 whereby administration of the SGLT2 inhibitor dapagliflozin (10 mg/day for 7 days) reduced Tm glucose by approximately 55% in both groups.Citation17 Moreover, dapagliflozin reduced the plasma glucose threshold at which glucose excretion began to concentrations well below fasting levels (ie, 4.7–6.0 mmol/L [85–108 mg/dL]) in both groups: glucosuria threshold was reduced to 1.2±2.6 mmol/L (21±46 mg/dL) in subjects with T2DM and to 2.0±2.2 mmol/L (37±40 mg/dL) in healthy subjects (P<0.001 for both groups).Citation17
In healthy glucose-tolerant individuals, having a Tm glucose of approximately 200 mg/dL (11.0 mmol/L) that is well above the normal filtered glucose load of approximately 100 mg/dL (5.5 mmol/L) allows the kidney to conserve this energy source for future use when glucose availability is scarce; however, this process may become maladaptive in individuals with DM.Citation18 Instead of excreting excess glucose into the urine in the presence of hyperglycemia, the kidneys of a diabetic person continue to reabsorb glucose, due to an elevation of the Tm glucose.Citation18 Consequently, hyperglycemia remains uncorrected and contributes to the ensuing problem of glucose toxicity. Thus, if SGLT2 activity promotes glucose conservation and hinders normalization of plasma glucose levels in DM, it is postulated that inhibition of SGLT2 might decrease the threshold for UGE (glucosuria) and reduce hyperglycemiaCitation18,Citation19
Early SGLT2 inhibitors
Early investigations into renal glucose handling were carried out on phlorizin (), a naturally occurring glucoside found in the root bark of fruit trees.Citation20 Studies from the 1950s revealed that phlorizin blocked sugar transport in several tissues, including the kidney and small intestine.Citation21 This was later found to be due to inhibition of SGLT proteins: phlorizin is a competitive inhibitor of SGLT1 and SGLT2 but has greater affinity for SGLT2.Citation5,Citation20 In the 1980s, a rat model of diabetes was used to demonstrate that phlorizin-induced glucosuria was associated with normalization of plasma glucose without hypoglycemia.Citation22,Citation23 Phlorizin also normalized insulin sensitivity in partially pancreatectomized rats but did not affect insulin action in the control animals.Citation22 The ensuing glucosuria reversed insulin resistance, and discontinuation of phlorizin led to the return of hyperglycemia and insulin resistance.Citation22 However, phlorizin was unsuitable for clinical development in diabetes due to its poor oral bioavailability: phlorizin is metabolized to phloretin by glucosidase in the gut and, thus, must be given parenterally. Moreover, phloretin is a potent inhibitor of GLUT1,Citation20 the suppression of which could result in reduced glucose transport to other tissues, such as the central nervous system.Citation24
Figure 3 Structure of phlorizin and candidate SGLT2 inhibitors.
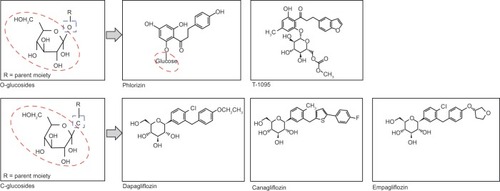
Consequently, pharmaceutical research has pursued phlorizin derivatives that possess increased stability/bioavailability and SGLT2 selectivity, and both O- and C-glucoside entities have been evaluated (). O-glucoside candidates, such as sergliflozin and T-1095,Citation25 were investigated first, but were discontinued in early clinical development for reasons probably related to nonselective SGLT2 inhibition,Citation26 and/or bioavailability issues.Citation27 C-glucoside candidates possessed increased resistance to enzymatic breakdownCitation28 and have fared more successfully during clinical development with a number of C-glucoside compounds progressing to marketing application and approval.
General characteristics of SGLT2 inhibitors
As the mode of action of SGLT2 is independent of insulin, SGLT2 inhibitors would be expected to act independently of pancreatic beta-cell function and insulin resistance. Consequently, there could be limited loss of potency in SGLT2 inhibitors (ie, maintained glucose lowering effect) when beta-cell function inevitably deteriorates over time, as is observed with other types of glucose-lowering agents. Furthermore, as inhibition of SGLT2 neither interferes with normal endogenous glucose production in response to hypoglycemia,Citation29 nor stimulates insulin release,Citation22,Citation30 the mode of action of SGLT2 inhibitor therapy should not increase the risk of hypoglycemic episodes. The novel mechanism of action of SGLT2 inhibitor therapy also suggests that it can be given in combination with any of the existing glucose-lowering agents, including insulin, as they share no common mechanistic pathways.
As well as these predicted benefits, several potential safety issues may be anticipated from the known pharmacodynamic effects of SGLT2 inhibitors. For example, as SGLT2 inhibitors cause a modest osmotic diuresis, there may be a risk of hypotension and hypovolemia; although, lowering of blood pressure (BP) may be of benefit in some individuals with T2DM. The ability of SGLT2 inhibition to increase UGE depends upon the presence of a normal GFR, so the glycemic effectiveness of an SGLT2 inhibitor would be expected to be lower in patients with chronic kidney disease (CKD) and a reduced GFR. The continual presence of glucose in the urine caused by SGLT2 inhibition theoretically increases the risk of urinary tract infections and mycotic genital tract infections. Furthermore, given the renal tubular mechanism of action of SGLT2 inhibitors, this class of compounds has the hypothetical ability to alter the absorption and excretion of calcium and phosphate and, in so doing, potentially affect bone metabolism. Although the various SGLT2 inhibitors in clinical development have a structural similarity, they differ in their respective selectivity profiles for SGLT2 over SGLT1: empagliflozin has the highest degree of selectivity (>2,500-fold), followed by tofogliflozin (>1,875-fold), dapagliflozin (>1,200-fold), ipragliflozin (>550-fold), and canagliflozin (>250-fold).Citation31 Inhibitors with lower selectivity for SGLT2 versus SGLT1 may incur safety issues arising from SGLT1 inhibition, such as diarrhea caused by glucose-galactose malabsorption. Although, recent data suggest that transient inhibition of SGLT1 by SGLT2 inhibitors may lower postprandial glucose by reducing intestinal glucose absorption.Citation32
Clinical data from SGLT2 inhibitor trials
A summary of SGLT2 inhibitors currently known to be in clinical development is presented in . Phase II through IV clinical trials with SGLT2 inhibitors are listed in . At the time of writing, dapagliflozin and canagliflozin are marketed in the US and EU and empagliflozin gained recent approval from the European Medicines Agency and the US Food and Drug Administration. Outside of the US and EU marketing applications for ipragliflozin, luseogliflozin, and tofogliflozin were submitted to Japan’s Pharmaceuticals and Medical Devices Agency, and ipragliflozin was recently approved. Developmental SGLT2 inhibitors are listed in . In addition, several fixed dose combination products utilizing SGLT2 inhibitors plus another class of oral anti-diabetes agents are currently in clinical development: dapagliflozin plus metformin (in 5 mg/850 mg and 5 mg/1,000 mg tablets) gained marketing authorization in the EU in early 2014,Citation33 and single-pill combination products containing dapagliflozin plus saxagliptin, canagliflozin plus metformin, and empagliflozin plus linagliptin or plus metformin, respectively, are in Phase III clinical trials.
Table 2 SGLT2 inhibitors in advanced clinical development
The SGLT2 inhibitors currently marketed are indicated as monotherapy for patients with T2DM and inadequate glycemic control from diet and exercise (US and EU indications),Citation34–Citation37 who are unable to use metformin (EU-specific),Citation35,Citation37 and as an add-on therapy with other glucose-lowering agents, including insulin (EU-specific).Citation35,Citation37 In Europe, the recommended dose of dapagliflozin is 10 mg once daily, whether given as a monotherapy or as an add-on therapy combined with other glucose-lowering agents.Citation35 In the US, the recommended starting dose is 5 mg once daily, which can be increased to 10 mg once daily in patients without renal impairment who tolerate the drug and who require additional glycemic control.Citation36 The use of dapagliflozin is generally not recommended when eGFR is below 60 mL/min/1.73 m2. The recommended starting dose of canagliflozin is 100 mg once daily, which can be increased to 300 mg once daily in patients (without renal impairment) who require additional glycemic control, provided the estimated glomerular filtration rate (eGFR) is 60 mL/min/1.73 m2 or greater.Citation34,Citation37 Canagliflozin is generally not recommended when eGFR is below 45 ml/min/1.73 m2. In pre-registration Phase III trials, empagliflozin was independently dosed at 10 mg and 25 mg once daily as monotherapy and as add-on combination therapy to other glucose-lowering agents, including insulin.
Clinical efficacy
A summary of efficacy data from key clinical trials of SGLT2 inhibitors (as registered in ClinicalTrials.gov) that are available, or expected to soon be available, in the US and EU is presented in . Selected efficacy data are also presented in . Dapagliflozin, canagliflozin, and empagliflozin are the most advanced of the SGLT2 inhibitors in terms of clinical development, and have the largest amount of published clinical data currently available. Pooled analyses of Phase III study data and data from US and EU regulatory reports were also available for dapagliflozin and canagliflozin, whereas data for empagliflozin were principally obtained from publications of individual Phase III studies. Other SGLT2 inhibitors were earlier in clinical development and had fewer publications available at the time of writing, or had no clinical trials registered in ClinicalTrials.gov due to their current development occurring outside the US.
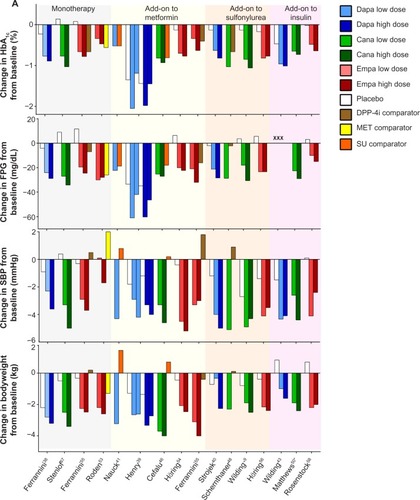
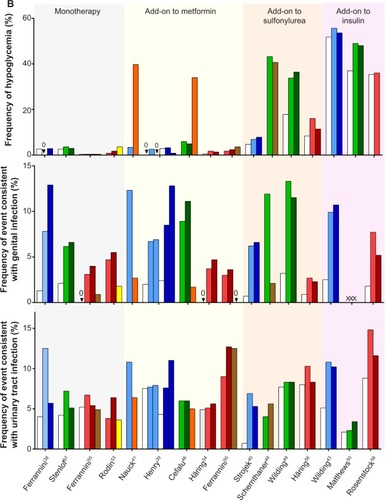
Figure 4 Efficacy and safety data from representative Phase III studies of dapagliflozin, canagliflozin, and empagliflozin.
Abbreviations: SGLT, sodium glucose co-transporter; FPG, fasting plasma glucose; SBP, systolic blood pressure; DPP-4i, dipeptidyl peptidase-4 inhibitor; MET, metformin; SU, sulfonylurea; Dapa, dapagliflozin; Cana, canagliflozin; Empa, empagliflozin; HbA1c, glycosylated hemoglobin.
Glycemic efficacy
Several meta-analyses have demonstrated a significant improvement of glycemic control in patients with T2DM who were treated with SGLT2 inhibitors.Citation60–Citation62 The largest of these included data from 58 SGLT2 inhibitor trials, predominantly involving dapagliflozin and canagliflozin, and reported that this drug class had a favorable effect on reducing glycosylated hemoglobin (HbA1c; mean difference versus placebo, −0.7% [95% confidence interval {CI} −0.7, −0.6]; mean difference versus active comparator, −0.1% [95% CI −0.2, 0.05]).Citation60 Dapagliflozin 10 mg provided statistically significant and clinically relevant improvements in glycemic control compared with placebo (with mean placebo-corrected HbA1c decrease in the different studies ranging from −0.5% to −0.7% at 24 weeks), when given as monotherapy or as add-on therapy to metformin, sulfonylurea, thiazolidinediones, or insulin.Citation63 As add-on therapy to metformin, dapagliflozin 10 mg was shown to have non-inferior efficacy versus glipizide after 52 weeks.Citation41 Dapagliflozin 10 mg was also shown to have non-inferior efficacy versus metformin extended release when both were given as monotherapy for 24 weeks.Citation39 Furthermore, the glucose-lowering effect of dapagliflozin as add-on therapy was maintained over periods of 48–102 weeks.Citation42,Citation64,Citation65
Pooled data for canagliflozin 300 mg and 100 mg gave an overall mean change from baseline in HbA1c relative to placebo of −0.8% (95% CI −0.9, −0.8) and 0.7% (95% CI −0.75, −0.6), respectively.Citation66 Individual studies over 52 weeks using canagliflozin as monotherapy,Citation67 or with a background of metformin,Citation46,Citation47 or with metformin plus sulfonylurea,Citation48 reported that efficacy in terms of reduced HbA1c was maintained over this longer period. Furthermore, canagliflozin (300 mg) was superior in lowering HbA1c when compared to glimepiride,Citation46 or sitagliptin.Citation47,Citation48
Empagliflozin 10 mg and 25 mg also led to statistically significant and clinically meaningful improvements in HbA1c.Citation53–Citation57 In monotherapy and compared with placebo, adjusted mean differences in change from baseline HbA1c at week 24 were −0.7% (95% CI −0.9, −0.6; P<0·0001) for empagliflozin 10 mg, −0.9% (95% CI −1.0, −0.7; P<0.0001) for empagliflozin 25 mg, versus −0.7% (95% CI −0.9, −0.6; P<0·0001) for sitagliptin.Citation53 Placebo-corrected changes in HbA1c after 24 weeks for empagliflozin added to metformin were −0.6% (95% CI −0.7, −0.4; P<0.001) for empagliflozin 10 mg and −0.6% (95% CI −0.8, −0.5; P<0.001) for empagliflozin 25 mg.Citation54
Larger reductions in HbA1c were observed in patients with higher baseline levels of HbA1c for each of these three SGLT2 inhibitors.Citation38, Citation45,Citation53 Changes in HbA1c and fasting plasma glucose from individual key trials using dapagliflozin, canagliflozin, and empagliflozin are presented in .
Changes in body weight and composition
Meta-analysis demonstrated SGLT2 inhibitors reduced body weight compared with other anti-diabetes agents (mean difference −1.8 kg [95% CI −3.5, −0.1]).Citation60 Body weight reductions of approximately 2–3 kg were observed in most dapagliflozin Phase III studies, as stated in the European Medicines Agency (EMA) assessment report.Citation63 The effect was maintained over 102 weeks in a study of dapagliflozin 10 mg added to metformin therapy, with a body weight reduction −4.5 kg versus −2.1 kg for placebo plus metformin.Citation68 Dual-energy X-ray absorptiometry revealed this reduction in body weight was principally due to a reduction in body fat mass, rather than a loss of fluid or lean tissue.Citation68 For canagliflozin, the change in body weight from baseline was generally consistent across placebo-controlled Phase III studies, but was lower where sulfonylurea was a background therapy: the US Food and Drug Administration (FDA) briefing document stated the placebo-subtracted mean reduction in body weight (excluding sulfonylurea background) was −1.8% to −3.8% for the 300 mg dose and −1.6% to −2.4% for the 100 mg dose.Citation69 For empagliflozin monotherapy, mean placebo-corrected changes in body weight from baseline after 24 weeks were −1.9 kg (95% CI −2.4, −1.4; P<0.0001) and −2.1 kg (95% CI −2.6, −1.7; P<0.0001) for 10 mg and 25 mg groups, respectively, versus 0.5 kg (95% CI 0.04, 1.0; P=0.0355) for the sitagliptin comparator group.Citation53 When empagliflozin was added to metformin the mean change in body weight after 24 weeks was greater for empagliflozin groups versus placebo (mean change standard error [SE] −2.1 [0.2] kg and −2.5 [0.2] kg for 10 mg and 25 mg groups, respectively, versus −0.45 [0.2] kg for placebo; P<0.001 for each dose versus placebo).Citation54
Blood pressure-lowering effects
In a meta-analysis of six studies, SGLT2 inhibitors reduced systolic BP compared with other anti-diabetes agents (mean difference −4.5 mmHg [95% CI −5.7, −3.2 mmHg]).Citation60 A decrease in systolic BP was observed consistently across the dapagliflozin studies ().Citation63 In a small study (n=75) directly comparing dapagliflozin with an antihypertensive, treatment with placebo, dapagliflozin (10 mg/day), or hydrochlorothiazide (25 mg/day) resulted in adjusted changes from baseline in 24-hour ambulatory mean systolic BP of −0.9 (95% CI −4.2, 2.4), −3.3 (95% CI −6.8, 0.2), and −6.6 (95% CI −9.9, −3.2) mmHg, respectively, at week 12.Citation70 The study data suggest that dapagliflozin may have a diuretic-like capacity to lower BP in addition to beneficial effects on glycemic control.Citation70
Canagliflozin demonstrated a dose-dependent and significant placebo-subtracted mean reduction in systolic BP, except when used as an add-on to sulfonylurea, ranging from 2.6–5.7 mmHg and 3.5–7.9 mmHg for the 100 mg and 300 mg doses, respectively.Citation69 This was supported by a recent pooled analysis of six Phase III studies (n=4,158) using canagliflozin, in which modest reductions in systolic BP were observed relative to placebo (−3.3 and −4.5 mmHg for 100 mg and 300 mg, respectively).Citation71
A pooled analysis of data from four Phase III trials (n=2,477) investigating empagliflozin 10 mg or 25 mg given for 24 weeks as monotherapy or as add-on therapy (with metformin, or metformin plus sulfonylurea, or pioglitazone ± metformin) reported reductions in systolic blood pressure (SBP) for empagliflozin groups versus placebo (placebo-corrected change from baseline −3.4 mmHg and −3.8 mmHg for empagliflozin 10 mg and 25 mg, respectively).Citation72 A study of patients (n=823) with T2DM and hypertension found that empagliflozin 10 mg and 25 mg significantly reduced mean 24 hour SBP, measured via ambulatory BP monitoring, versus placebo (−2.95 and −3.68 mmHg versus 0.48 mmHg, respectively; P<0.001 versus placebo for each dose).Citation73
Clinical safety
As defined for , a summary of safety data from key clinical trials of SGLT2 inhibitors is presented in and selected safety data are presented in .
Urinary tract infections and genital tract infections
In a meta-analysis of eight studies using canagliflozin and dapagliflozin that compared the SGLT2 inhibitors with other anti-diabetes agents, urinary tract infections were more common with SGLT2 inhibitors (odds ratio, 1.42 [95% CI 1.06, 1.90]), as were genital tract infections (odds ratio, 5.06 [95% CI 3.44, 7.45]).Citation60 Safety data from a pooled retrospective analysis of data from the short-term, double-blind periods of 12 placebo-controlled trials (n=4,545) using dapagliflozin reported that genital tract infections and lower urinary tract infections were more common with dapagliflozin than placebo; however, between-group differences were less marked for urinary tract infections (genital tract infection 4.1%–5.7% dapagliflozin versus 0.9% placebo; urinary tract infection 3.6%–5.7% dapagliflozin versus 3.7% placebo).Citation74,Citation75 Similar findings were reported from pooled analyses of canagliflozin and empagliflozin.
A pooled analysis of four 26 week Phase III studies (n=2,313) of canagliflozin found higher proportions of subjects with urinary tract infections and genital tract infections occurred in the canagliflozin groups than with placebo (urinary tract infection 5.1% canagliflozin versus 4.0% placebo; genital tract infection 7.5% canagliflozin versus 1.9% placebo).Citation76,Citation77
A pooled analysis of four Phase III studies (n=2,477) using empagliflozin found that empagliflozin was associated with an increased frequency of genital tract infections compared with placebo (approximately 4% versus 1%, respectively), but this was not the case for urinary tract infections (frequency of approximately 8%–9% for each).Citation78
For dapagliflozin, canagliflozin, and empagliflozin studies, events of genital tract infections and urinary tract infections were more common in women than in men in all treatment groups (), and patients usually experienced only a single episode, which was usually mild in intensity and responded to standard treatment.Citation74–Citation78
Hypoglycemia
The incidence of hypoglycemia during SGLT2 inhibitor treatment was generally low, except for groups receiving background therapy of sulfonylureas or insulin. A meta-analysis of SGLT2 inhibitor (dapagliflozin and canagliflozin) trials concluded that hypoglycemic risk was similar to that of other agents (odds ratio versus placebo, 1.28 [95% CI 0.99, 1.65; I 2=0%]: odds ratio versus other anti-diabetes agents, 0.44 [95% CI 0.35, 0.54; I 2=93%]).Citation60 There were no major episodes of hypoglycemia when dapagliflozin was used as monotherapy, but an increased risk of hypoglycemic events, which were mainly minor in nature (defined as either a symptomatic episode with a capillary or plasma glucose measurement <3.5 mmol/L [<63 mg/dL] regardless of the need for external assistance or an asymptomatic capillary or plasma glucose measurement <3.5 mmol/L [<63 mg/dL], that does not qualify as a major episode), was observed when it was added to sulfonylurea or insulin.Citation40,Citation43,Citation63
Similar findings were observed with canagliflozin, with a low risk of hypoglycemia among subjects treated with canagliflozin taken as monotherapy, or in combination with other anti-hyperglycemic agents not associated with hypoglycemia. An increased incidence of hypoglycemia was observed when canagliflozin was used in combination with insulin or sulfonylureas.Citation34,Citation49,Citation50 The prescribing information for both canagliflozin and dapagliflozin recommend using a lower dose of insulin or insulin secretagogue to reduce the risk of hypoglycemia when used in combination with the respective SGLT2 inhibitor.Citation34,Citation36
The rate of hypoglycemia was also low with empagliflozin monotherapy and was comparable to placebo.Citation53 For empagliflozin added to metformin plus sulfonylurea, the frequency of confirmed hypoglycemia was greater for empagliflozin versus placebo, but none of these events required assistance.Citation56 When empagliflozin was added to basal insulin, no increased risk of hypoglycemia was reported versus placebo.Citation58
Renal safety and volume depletion events
Approximately 375 mL of extra urinary volume is produced per day with dapagliflozin 10 mg therapy.Citation35 A pooled safety analysis of dapagliflozin using data from the double-blind periods of 12 placebo-controlled trials (n>4,500) reported that volume depletion events occurred in 0.6%–1.2% for dapagliflozin groups (2.5–10 mg) versus 0.4% for placebo groups,Citation79 indicating a slightly elevated risk and a need to maintain an adequate fluid intake. Hypotension occurred more frequently in dapagliflozin-treated groups than placebo groups for subjects who were elderly, had moderate renal impairment, or were treated with loop diuretics.Citation63 Dapagliflozin treatment was not associated with increased risk of acute renal toxicity or deterioration of renal function.Citation80 The estimated GFR (eGFR) decreased initially then returned to baseline by week 24 and was maintained to week 102, while mean serum creatinine showed minimal change (± 0.01 mg/dL) from baseline to week 24 in all groups.Citation80 As a safety measure, the dapagliflozin Summary of Product Characteristics recommends against its use in patients receiving loop diuretics or who are volume depleted, or who have moderate to severe renal impairment (defined as patients with creatinine clearance <60 mL/min or eGFR <60 mL/min/1.73 m2), and encourages monitoring of volume status in cases where intercurrent conditions could lead to volume depletion.Citation35 A 104-week Phase III study of dapagliflozin treatment in T2DM patients with moderate renal impairment reported events of renal impairment or renal failure were uncommon (2.4% and 9.4% for dapagliflozin 5 mg and 10 mg, respectively; 7.1% for placebo), and volume depletion events were more frequent with dapagliflozin (9.6% and 12.9% for dapagliflozin 5 mg and 10 mg, respectively; 6.0% for placebo).Citation44
Analysis of a pooled dataset from the canagliflozin FDA briefing document stated that volume depletion-related adverse events, most commonly hypotension, occurred in 1.2% and 1.3% of canagliflozin 100 mg and 300 mg groups, respectively, versus 1.1% in placebo groups;Citation69 furthermore, none of these events in the canagliflozin groups were serious or led to study discontinuation.Citation69 In a pooled analysis of eight clinical trials (placebo- and active-controlled), volume depletion-related adverse events occurred in 2.3% and 3.4% of canagliflozin 100 mg and 300 mg groups, respectively, versus 1.5% in the comparator groups.Citation34 Risk factors for these events were similar to those identified for dapagliflozin (eg, patient’s age ≥75 years, eGFR <60 mL/min/1.73 m2, and use of loop diuretics).Citation34 A Phase III trial of canagliflozin use in T2DM patients with stage 3 CKD (eGFR ≥30 and <50 mL/min/1.73 m2) reported larger decreases in eGFR from baseline in canagliflozin treatment groups (least square mean change, −9.1% and −10.1% for 100 mg and 300 mg, respectively, versus −4.5% for placebo).Citation51 The reductions in eGFR with canagliflozin were largest at week 3 (the first post-baseline measurement) and then returned back toward baseline over the 26-week treatment period.Citation51 A lower proportion of subjects in the canagliflozin 100 mg and 300 mg groups progressed to albuminuria (ie, from normoalbuminuria to micro- or macro-albuminuria, or from micro- to macro-albuminuria) versus those in the placebo group (5.1%, 8.3%, and 11.8%, respectively; odds ratio [95% CI], 0.33 [0.08, 1.48] for canagliflozin 100 mg versus placebo, and 0.51 [0.14, 1.91] for canagliflozin 300 mg versus placebo).Citation51
A pooled analysis of empagliflozin data (>11,000 T2DM patients from Phase I, II, and III trials) reported that the percentage of patients with volume depletion events was similar with empagliflozin (10 mg dose group 1.4%; 25 mg dose group 1.5%) and placebo (1.4%).Citation81 More patients receiving diuretics reported these events than those not receiving diuretics (2.2%–2.7% versus 0.9%–1.0%, respectively).Citation81 Treatment with empagliflozin in patients with T2DM and stage 2 or 3 CKD (eGFR ≥60 to <90 mL/min/1.73 m2 and 30 to <60 mL/min/1.73 m2, respectively) significantly reduced mean HbA1c from baseline (placebo adjusted mean reduction in HbA1c at week 24 was −0.52% [95% CI −0.72, −0.32] and −0.68% [95% CI −0.88, −0.49] for stage 2 CKD receiving empagliflozin 10 mg and 25 mg, respectively, and −0.42% [95% CI −0.56, −0.28] for stage 3 CKD receiving empagliflozin 25 mg [empagliflozin 10 mg was not used]; P<0.0001 for each),Citation59 and the effect was sustained at week 52. However, empagliflozin 25 mg did not reduce HbA1c at week 24 or week 52 in patients with stage 4 CKD (eGFR ≥15 to <30 mL/min/1.73 m2).Citation59 In patients with stage 2, 3, or 4 CKD, small decreases in eGFR were noted in the empagliflozin groups, which returned to baseline by the end of the 3 week follow-up after treatment completion.Citation59 In patients with stage 3 CKD, fewer patients on empagliflozin 25 mg than placebo shifted from no albuminuria at baseline to microalbuminuria, or from microalbuminuria at baseline to macroalbuminuria, at end of treatment (12.2% with empagliflozin versus 22.2% with placebo, and 2.0% with empagliflozin versus 11.4% with placebo, respectively).Citation59
Venous thromboembolic events
As volume depletion may increase the risk of hemoconcentration and venous thromboembolism (VTE), VTE events were monitored in trials using SGLT2 inhibitors.
Patients receiving dapagliflozin had a similar rate of VTE events to those in the comparator group (0.3% for both groups).Citation63 For canagliflozin, the rate of VTE in Phase III trials was also low (0.2% and 0.3% for canagliflozin 100 mg and 300 mg groups, respectively, versus 0.2% for non-canagliflozin groups).Citation69 VTE data have not yet been reported for empagliflozin.Citation82,Citation83
Bone safety
There was no clear evidence that dapagliflozin induced bone demineralization or increased fracture rates in people with diabetes and normal or mildly impaired renal function (eGFR >90 mL/min/1.73 m2 and ≥60 to <90 mL/min/1.73 m2, respectively),Citation63,Citation84 but bone fractures were more common in dapagliflozin-treated patients with moderate renal impairment (eGFR >30 to <60 mL/min/1.73 m2; 4.8% and 9.4% for 5 mg and 10 mg groups, respectively, versus 0% for placebo-treated subjects).Citation63 A 102 week study (n=140) did not identify any meaningful changes from baseline in markers of bone turnover or bone mineral density in patients receiving dapagliflozin added to metformin, when compared with placebo.Citation68 No meaningful changes in bone density were observed with canagliflozin treatment over 26 weeks, according to the FDA briefing report,Citation69 but there was an increase in overall bone fracture events with canagliflozin (2.5% for 100 mg and 2.3% for 300 mg) compared to control (1.7%; includes placebo and active comparators, both with various background therapies). A 104-week trial (26-week double-blind phase + 78-week double-blind extension phase) evaluating canagliflozin in older patients (aged 55–80 years) with T2DM (ClinicalTrials.gov identifier: NCT01106651) included an assessment of bone density, which will be reported separately from the main efficacy/safety analysis.Citation52 However, no discernible changes in bone density were observed at 26 weeks.Citation85 In a pooled analysis of data from more than 11,000 patients with T2DM from Phase I, II, and III trials, empagliflozin was not associated with an increased frequency of bone fractures versus placebo (1.6% and 1.1% for empagliflozin 10 mg and 25 mg, respectively, versus 1.6% for placebo).Citation86
Cardiovascular safety
SGLT2 inhibitors have favorable effects on cardiovascular (CV) risk factors by reducing hyperglycemia, body weight, and BP,Citation87 but changes in lipid profiles have caused some concern,Citation88 and information on major CV outcomes such as stroke, heart attack, and other vascular complications is currently limited.Citation89 Several large, long-term studies with CV endpoints are ongoing and will provide data in the next 2–6 years ().Citation90,Citation91 Results from a meta-analysis on CV outcomes and death with SGLT2 inhibitors showed overall no evidence for an increased CV risk with SGLT2 inhibitor treatment.Citation60 The EMA assessment report on dapagliflozin stated that an independently confirmed meta-analysis of Phase IIb/III studies did not show an increased CV risk in dapagliflozin-treated patients.Citation63 The estimated hazard ratio for the primary composite endpoint (time to first event of the following adjudicated events: CV death, myocardial infarction, stroke, and hospitalization for unstable angina) using a Cox proportional hazards method was 0.674 (95% CI 0.421, 1.078).Citation63 Similarly, a meta-analysis to assess CV safety for canagliflozin was presented in the FDA report,Citation69 and included all Major Adverse Cardiovascular Events Plus (MACE-Plus; defined as a composite endpoint consisting of the following adjudicated events: CV death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina) in nine Phase III trials (including interim data from the canagliflozin cardiovascular assessment study [CANVAS]). The estimated hazard ratio was 0.91 (95% CI 0.68, 1.22) for the risk of MACE-Plus comparing canagliflozin to all comparators (via the pre-specified primary Cox proportional hazards model fit to all trials including CANVAS).Citation69
Table 3 Registered cardiovascular clinical trials of SGLT2 inhibitors
Changes in lipid profiles observed with SGLT2 inhibitor therapy have caused some concern.Citation88 Dose-related increases in low-density lipoprotein cholesterol (LDL-C) were observed with canagliflozin, as shown in a pooled analysis of data from four 26-week placebo-controlled trials in which the mean percentage increases from baseline in LDL-C were 4.5% and 8.0% for 100 mg and 300 mg canagliflozin, respectively, relative to placebo.Citation92 Canagliflozin labeling information recommends LDL-C should be monitored and treated according to standard care after initiating canagliflozin therapy.Citation92 Statistically significant increases in high-density lipoprotein cholesterol (HDL-C) from baseline were observed with canagliflozin in four of eight placebo-controlled Phase III trials, but decreases in triglyceride levels with canagliflozin were small and were generally not statistically significant.Citation93 For patients receiving dapagliflozin in Phase III trials, overall small mean changes in HDL-C (+2.1% to +9.3%), triglyceride (−0.9% to −10.6%), and LDL-C (−0.5% to +9.5%) were observed, but there was no clinically significant effect on lipid levels in the individual dapagliflozin studies concerned.Citation94 For empagliflozin, a pooled analysis of four placebo-controlled Phase III trials reported small increases in HDL-C and LDL-C and small decreases in triglycerides with empagliflozin versus placebo after 24 weeks.Citation72
Malignancies
A pooled analysis of data for all dapagliflozin doses from 19 Phase IIb/III trials revealed that the incidence rates for malignancies were similar for dapagliflozin (1.4%) and placebo/comparator (1.3%),Citation79 and there was no carcinogenicity or mutagenicity signal in animal data.Citation35 However, breast and bladder cancer adverse events were numerically greater with dapagliflozin than placebo/comparator.Citation35,Citation63,Citation79,Citation95 The US prescribing information for dapagliflozin states that the drug should not be used in patients with active bladder cancer and should be used with caution in patients with a history of this disease.Citation36 Furthermore, the dapagliflozin Summary of Product Characteristics does not recommend the use of dapagliflozin in patients being treated with pioglitazone, as epidemiological data suggest a small increased risk of bladder cancer with pioglitazone.Citation35 Adverse events for breast and bladder cancer, plus renal cell cancer, were also monitored in the clinical studies for canagliflozin.Citation69 The incidences of these tumor events were low and they occurred at a similar rate across treatment groups (breast cancer 0.38%–0.46% versus 0.4%; bladder cancer 0.06%–0.09% versus 0.11%; renal cell cancer 0.06%–0.09% versus 0.08% for canagliflozin 100 mg and 300 mg groups versus non-canagliflozin groups, respectively).Citation69 No data on malignancy rates from trials using empagliflozin (or any of the other SGLT2 inhibitors) have been reported to date. Nevertheless, these safety signals raised concerns and further data are required to exclude the possibility of an elevated risk of certain types of cancer occurring with SGLT2 inhibitor treatment.
Current and future roles for SGLT2 inhibitors
Currently available published clinical trial data for SGLT2 inhibitors document their use as add-on therapy with metformin, insulin, sulfonylureas, dipeptidyl peptidase (DPP-4) inhibitors, or thiazolidinediones. SGLT2 inhibitors may also have a role as monotherapy; for example, in patients who are intolerant to metformin due to ensuing gastrointestinal side effects. Data from published trials indicate that various SGLT2 inhibitors have a similar ability to improve glucose control with a low risk of hypoglycemia, together with promoting modest reductions in BP and body weight. The properties of SGLT2 inhibitors present for the first time the possibility of a triple combination (ie, metformin, DPP-4 inhibitor, and SGLT2 inhibitor), with the expected net effect of weight reduction and freedom from hypoglycemic episodes. This could be particularly attractive in Europe, where triple oral combinations have not been popular (presumably, because at least one of the combination components introduced undesired adverse events, such as weight gain and/or hypoglycemia). At present, there is no evidence suggesting preference of any one SGLT2 inhibitor over another: any differences between individual SGLT2 inhibitors may be revealed when clinical head-to-head comparator studies are carried out, although no such studies are currently reported to be underway. A Phase I study comparing the pharmacodynamics of canagliflozin and dapagliflozin was recently completed and publication of the data is awaited (ClinicalTrials.gov identifier: NCT01877889), the primary outcome measure was the between-treatment difference in 24-hour mean renal threshold for glucose.
The effect of SGLT2 inhibition on preserving beta-cell function and improving insulin sensitivity has also been reported. Data from a study using an insulin-resistant animal model of T2DM found that sustained glucose lowering with dapagliflozin improved insulin sensitivity and pancreatic islet function and morphology.Citation96 The authors suggested that reduction of hyperglycemia by dapagliflozin, through an insulin-independent mechanism, may improve core defects present in T2DM; however, further research is needed before firm conclusions can be drawn.Citation96 Recently published and independent studies using dapagliflozin and empagliflozin in patients with T2DM reported increased insulin sensitivity following SGLT2 inhibitor therapy,Citation97,Citation98 and empagliflozin-induced UGE also improved beta-cell function.Citation98 SGLT2 inhibition with either of these agents increased to some extent endogenous glucose production, despite reducing fasting plasma glucose, and this may be at least partially explained by concentration change in the insulin to glucagon ratio which has been observed with SGLT2 inhibitor therapy.Citation89,Citation90 There is also preliminary evidence to suggest that SGLT2 inhibitors with lower selectivity towards SGLT1 (ie, canagliflozin) achieve intra-intestinal levels after oral dosing that may be sufficiently high to transiently inhibit intestinal SGLT1 and reduce intestinal glucose absorption,Citation32,Citation99 resulting in increased release of glucagon-like peptide-1 and peptide YY.Citation32,Citation100 These factors together may make SGLT2 inhibitors an attractive choice for T2DM patients who are failing with metformin and who need to lose weight.
Furthermore, SGLT2 inhibitors may have the potential to be used as an insulin-sparing agent in T2DM patients using insulin.Citation43,Citation58,Citation64 A long-term study of dapagliflozin in T2DM patients using insulin reported the mean insulin dose increased by 18.3 IU/day and body weight increased by 1.8 kg in the placebo group after 104 weeks, whereas insulin dose was stable and body weight decreased by 0.9 kg in the dapagliflozin groups.Citation64 A similar trend was reported after 78 weeks of empagliflozin treatment.Citation58 SGLT2 inhibitors could possibly be used transiently instead of insulin treatment in patients who are otherwise well controlled but who develop temporary acute hyperglycemia, due to factors such as short-term immobility, infectious diseases, etc. Additionally, SGLT2 inhibitors may have a role in improving glucose tolerance in pre-diabetic individuals. However, to allow the use of these agents in patients without established disease, clinical trials with SGLT2 inhibitors would need to show a reduced risk for relevant clinical endpoints (eg, CV, etc) as well as robust safety data.
Pilot studies using SGLT2 inhibitors in patients with T1DM are also in progress (ClinicialTrials.gov identifiers: NCT01498185, NCT01392560, NCT01742208), and preliminary results have been presented.Citation101,Citation102 A further possible use of SGLT2 inhibitors in T1DM is the concept that SGLT2 inhibition may have renal effects by lowering intra-glomerular pressure, which has recently been demonstrated with empagliflozin in patients with T1DM.Citation103 This observation could explain the reduction of albuminuria with SGLT2 treatment described in Phase III studies. In addition, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE; ClinicialTrials.gov identifier: NCT02065791) study has just commenced, and is a renal outcome study to investigate whether SGLT2 inhibition has renal potential beyond its glucose-lowering properties.
As a final point, it is of interest to note that current SGLT2 inhibitors only inhibit 30%–50% of the filtered glucose load, ie, 50–80 g of the ~180 g filtered per day. The possible pharmacokinetic reasons for this imbalance are discussed in a report by Liu et al,Citation104 and a novel hypothesis to explain this conundrum was recently postulated by Abdul-Ghani et al.Citation105 Namely, complete inhibition of SGLT2 causes SGLT1 to reabsorb glucose at full capacity; therefore, only the fraction of filtered glucose that escapes SGLT1 will be excreted in the urine.Citation105 A better understanding of renal SGLT2 inhibitor handling may help to develop future agents that can inhibit a larger proportion of filtered glucose and further reduce HbA1c levels,Citation104 for example, agents with the capacity to also partially inhibit renal SGLT1 and produce a more vigorous UGE than those that are highly specific for SGLT2 inhibition only.Citation105
There is potentially much more to come from this novel class of drugs, and we wait with interest to see what further developments and therapeutic applications may arise.
Author contributions
The author was fully responsible for all content and editorial decisions, was involved at all stages of manuscript development, and has approved the final version of the manuscript that reflects the author’s interpretation and conclusions.
Disclosure
The author has received research grants to his institution from Berlin-Chemie/Menarini, Eli Lilly, Merck Sharp and Dohme, Novartis, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Lilly Deutschland, MetaCure, Roche Pharma, Novo Nordisk, and Tolerx for participation in multicenter clinical trials. He has received consulting fees and/or honoraria for membership in advisory boards and/or honoraria for speaking from Amylin, AstraZeneca, Berlin-Chemie/Menarini, Boehringer Ingelheim, Bristol-Myers Squibb, Diartis Pharmaceuticals, Eli Lilly, Hoffmann-LaRoche, GlaxoSmithKline, Intarcia Therapeutics, MannKind, Merck Sharp and Dohme, Novartis, Novo Nordisk, Sanofi, Takeda, Versartis, and Wyeth Research, including reimbursement for travel expenses.
Acknowledgments
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Debra Brocksmith, MB ChB, PhD, of Envision Scientific Solutions during the preparation of this manuscript. Boehringer Ingelheim was given the opportunity to check the data used in the manuscript for factual accuracy only.
Supplementary material
Table S1 SGLT2 inhibitor clinical trials (Phase 11+)
Table S2 SGLT2 and SGLTI inhibitors currently in the development pipeline
Table S3 Efficacy data from pivotal clinical trials of SGLT2 inhibitorsTable Footnotea
Table S4 Safety data from pivotal clinical trials of SGLT2 inhibitorsTable Footnotea
References
- List JF Woo V Morales E Tang W Fiedorek FT Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes Diabetes Care 2009 32 4 650 657 19114612
- Wilding JP Norwood P T’Joen C Bastien A List JF Fiedorek FT A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment Diabetes Care 2009 32 9 1656 1662 19528367
- Ferrannini E Ramos SJ Salsali A Tang W List JF Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial Diabetes Care 2010 33 10 2217 2224 20566676
- Bailey CJ Iqbal N T’Joen C List JF Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range Diabetes Obes Metab 2012 14 10 951 959 22776824
- Bailey CJ Gross JL Pieters A Bastien A List JF Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial Lancet 2010 375 9733 2223 2233 20609968
- Bolinder J Ljunggren O Kullberg J Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin J Clin Endocrinol Metab 2012 97 3 1020 1031 22238392
- Henry RR Murray AV Marmolejo MH Hennicken D Ptaszynska A List JF Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial Int J Clin Pract 2012 66 5 446 456 22413962
- Strojek K Yoon KH Hruba V Elze M Langkilde AM Parikh S Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial Diabetes Obes Metab 2011 13 10 928 938 21672123
- Nauck MA Del Prato S Meier JJ Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial Diabetes Care 2011 34 9 2015 2022 21816980
- Rosenstock J Vico M Wei L Salsali A List JF Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy Diabetes Care 2012 35 7 1473 1478 22446170
- Wilding JP Woo V Soler NG Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial Ann Intern Med 2012 156 6 405 415 22431673
- Kohan DE Fioretto P Tang W List JF Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control Kidney Int 2014 85 4 962 971 24067431
- Jabbour SA Hardy E Sugg J Parikh S Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study Diabetes Care 2014 37 3 740 750 24144654
- Stenlöf K Cefalu WT Kim KA Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise Diabetes Obes Metab 2013 15 4 372 382 23279307
- Cefalu WT Leiter LA Yoon KH Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial Lancet 2013 382 9896 941 950 23850055
- Lavalle-González FJ Januszewicz A Davidson J Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial Diabetologia 2013 56 12 2582 2592 24026211
- Schernthaner G Gross JL Rosenstock J Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial Diabetes Care 2013 36 9 2508 2515 23564919
- Wilding JP Charpentier G Hollander P Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial Int J Clin Pract 2013 67 12 1267 1282 24118688
- Forst T Guthrie R Goldenberg R Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone Diabetes Obes Metab 2014 16 5 467 477 24528605
- Matthews DR Fulcher G Perkovic V Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy +/− oral agents in type 2 diabetes. Abstract 764 Diabetologia 2012 55 Suppl 1 S314
- Rosenstock J Aggarwal N Polidori D Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes Diabetes Care 2012 35 6 1232 1238 22492586
- Yale JF Bakris G Cariou B Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease Diabetes Obes Metab 2013 15 5 463 473 23464594
- Bode B Stenlöf K Sullivan D Fung A Usiskin K Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial Hosp Pract (1995) 2013 41 2 72 84 23680739
- Roden M Weng J Eilbracht J Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial Lancet Diabetes Endocrinol 2013 1 3 208 219 24622369
- Häring HU Merker L Seewaldt-Becker E Weimer M Meinicke T Empagliflozin as add-on to metformin for 24 weeks improves glycemic control in patients with type 2 diabetes (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1092-P
- Ferrannini E Berk A Hantel S Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes Diabetes Care 2013 36 12 4015 4021 24186878
- Häring HU Merker L Seewaldt-Becker E Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes. A 24-week, randomized, double-blind, placebo-controlled trial Diabetes Care 2013 36 11 3396 3404 23963895
- Kovacs CS Seshiah V Swallow R Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial Diabetes Obes Metab 2014 16 2 147 158 23906415
- Rosenstock J Jelaska A Kim G Broedl UC Woerle HJ Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1102-P
- Ferrannini E Seman L Seewaldt-Becker E Hantel S Pinnetti S Woerle H A phase IIb, randomised, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes Diabetes Obes Metab 2013 15 8 721 728 23398530
- Rosenstock J Seman LJ Jelaska A Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia Diabetes Obes Metab 2013 15 12 1154 1160 23906374
- Barnett AH Mithal A Manassie J Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial Lancet Diabetes Endocrinol 2014 2 5 369 384 24795251
- Yamamoto Y Kawanishi E Koga Y N-Glucosides as human sodium-dependent glucose cotransporter 2 (hSGLT2) inhibitors Bioorg Med Chem Lett 2013 15 23 20 5641 5645 23999047
- Powell DR Smith M Doree D LX2761, an SGLT1 inhibitor restricted to the intestine, improves glycaemic control in mice Diabetologia 2013 56 1 S399 Abstract 995
- Young AA Liu Y McNulty D Synergistic glucose-lowering effects of SGLT1- and ASBT-inhibitor combinations in ZDF rats Diabetologia 2013 56 Suppl 1 S399 Abstract 994
- hang L Wang Y Xu H Discovery of 6-deoxydapagliflozin as a highly potent sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes Med Chem 2014 10 304 317 24059684
References
- Gerich JE Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications Diabet Med 2010 27 2 136 142 20546255
- Mather A Pollock C Glucose handling by the kidney Kidney Int Suppl 2011 120 S1 S6 21358696
- Zelikovic I Aminoaciduria and glycosuria Avner ED Harmon WE Niaudet P Pediatric Nephrology 5th edition Philadelphia Lippincott Williams & Wilkins 2004 701 728
- Moe OW Wright SH Palacín M Renal handling of organic solutes Brenner BM Rector Brenner and Rector’s The Kidney Philadelphia Saunders Elsevier 2008 214 247
- Wright EM Loo DD Hirayama BA Biology of human sodium glucose transporters Physiol Rev 2011 91 2 733 794 21527736
- Hediger MA Rhoads DB Molecular physiology of sodium-glucose cotransporters Physiol Rev 1994 74 4 993 1026 7938229
- Dominguez JH Camp K Maianu L Garvey WT Glucose transporters of rat proximal tubule: differential expression and subcellular distribution Am J Physiol 1992 262 5 Pt 2 F807 F812 1590425
- Santer R Kinner M Lassen CL Molecular analysis of the SGLT2 gene in patients with renal glucosuria J Am Soc Nephrol 2003 14 11 2873 2882 14569097
- Turk E Zabel B Mundlos S Dyer J Wright EM Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter Nature 1991 350 6316 354 356 2008213
- Xin B Wang H Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish Clin Genet 2011 79 1 86 91 20486940
- Meyer C Stumvoll M Nadkarni V Dostou J Mitrakou A Gerich J Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus J Clin Invest 1998 102 3 619 624 9691098
- Meyer C Woerle HJ Dostou JM Welle SL Gerich JE Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes Am J Physiol Endocrinol Metab 2004 287 6 E1049 E1056 15304374
- Mogensen CE Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects Scand J Clin Lab Invest 1971 28 1 101 109 5093515
- Rahmoune H Thompson PW Ward JM Smith CD Hong G Brown J Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes Diabetes 2005 54 12 3427 3434 16306358
- Vestri S Okamoto MM de Freitas HS Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat J Membr Biol 2001 182 2 105 112 11447502
- Dyer J Wood IS Palejwala A Ellis A Shirazi-Beechey SP Expression of monosaccharide transporters in intestine of diabetic humans Am J Physiol Gastrointest Liver Physiol 2002 282 2 G241 G248 11804845
- Defronzo RA Hompesch M Kasichayanula S Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes Diabetes Care 2013 36 10 3169 3176 23735727
- Abdul-Ghani MA Norton L Defronzo RA Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes Endocr Rev 2011 32 4 515 531 21606218
- Abdul-Ghani MA DeFronzo RA Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus Endocr Pract 2008 14 6 782 790 18996802
- Ehrenkranz JR Lewis NG Kahn CR Roth J Phlorizin: a review Diabetes Metab Res Rev 2005 21 1 31 38 15624123
- Alvarado F Crane RK Phlorizin as a competitive inhibitor of the active transport of sugars by hamster small intestine, in vitro Biochim Biophys Acta 1962 56 170 172 13860792
- Rossetti L Smith D Shulman GI Papachristou D DeFronzo RA Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats J Clin Invest 1987 79 5 1510 1515 3571496
- Rossetti L Shulman GI Zawalich W DeFronzo RA Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats J Clin Invest 1987 80 4 1037 1044 3308956
- Thorens B Mueckler M Glucose transporters in the 21st Century Am J Physiol Endocrinol Metab 2010 298 2 E141 E145 20009031
- Oku A Ueta K Arakawa K T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes Diabetes 1999 48 9 1794 1800 10480610
- Isaji M SGLT2 inhibitors: molecular design and potential differences in effect Kidney Int Suppl 2011 120 S14 S19 21358697
- Chao EC Henry RR SGLT2 inhibition – a novel strategy for diabetes treatment Nat Rev Drug Discov 2010 9 7 551 559 20508640
- Hardman TC Dubrey SW Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes Diabetes Ther 2011 2 3 133 145 22127823
- McCrimmon RJ Evans ML AICAR and phlorizin reverse the hypoglycemia-specific defect in glucagon secretion in the diabetic BB rat Am J Physiol Endocrinol Metab 2002 283 5 E1076 E1083 12376337
- Han S Hagan DL Taylor JR Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats Diabetes 2008 57 6 1723 1729 18356408
- Grempler R Thomas L Eckhardt M Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors Diabetes Obes Metab 2012 14 1 83 90 21985634
- Polidori D Sha S Mudaliar S Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study Diabetes Care 2013 36 8 2154 2161 23412078
- European Medicines Agency [homepage on the internet] Xigduo (dapagliflozin/metformin) authorisation details (EMEA/H/C/002672) 2014 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002672/human_med_001721.jsp&mid=WC0b01ac058001d124 Accessed February 10, 2014
- INVOKANA™ (canagliflozin) tablets, for oral use [prescribing information] Janssen Pharmaceuticals Inc 2013 Available from: http://www.invokanahcp.com/prescribing-information.pdf Accessed March 31, 2014
- Bristol-Myers Squibb-AstraZeneca EEIG [homepage on the Internet] Summary of Product Characteristics: Forxiga 5 mg film coated tablets 2012 Available from: http://ec.europa.eu/health/documents/community-register/2012/20121112124487/anx_124487_en.pdf Accessed March 31, 2014
- AstraZeneca Pharamceuticals LP, Bristol-Myers Squibb Company Highlights of prescribing information: FARXIGA (dapagliflozin) tablets, for oral use 2014 Available from: http://packageinserts.bms.com/pi/pi_farxiga.pdf Accessed February 11, 2014
- Janssen-Cilag International NV Summary of Product Characteristics: Invokana 100 mg film-coated tablets 2013 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002649/WC500156456.pdf Accessed January 20, 2014
- Ferrannini E Ramos SJ Salsali A Tang W List JF Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial Diabetes Care 2010 33 10 2217 2224 20566676
- Henry RR Murray AV Marmolejo MH Hennicken D Ptaszynska A List JF Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial Int J Clin Pract 2012 66 5 446 456 22413962
- Strojek K Yoon KH Hruba V Elze M Langkilde AM Parikh S Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial Diabetes Obes Metab 2011 13 10 928 938 21672123
- Nauck MA Del Prato S Meier JJ Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial Diabetes Care 2011 34 9 2015 2022 21816980
- Rosenstock J Vico M Wei L Salsali A List JF Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy Diabetes Care 2012 35 7 1473 1478 22446170
- Wilding JP Woo V Soler NG Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial Ann Intern Med 2012 156 6 405 415 22431673
- Kohan DE Fioretto P Tang W List JF Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control Kidney Int 2014 85 4 962 971 24067431
- Stenlöf K Cefalu WT Kim KA Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise Diabetes Obes Metab 2013 15 4 372 382 23279307
- Cefalu WT Leiter LA Yoon KH Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial Lancet 2013 382 9896 941 950 23850055
- Lavalle-González FJ Januszewicz A Davidson J Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial Diabetologia 2013 56 12 2582 2592 24026211
- Schernthaner G Gross JL Rosenstock J Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial Diabetes Care 2013 36 9 2508 2515 23564919
- Wilding JP Charpentier G Hollander P Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial Int J Clin Pract 2013 67 12 1267 1282 24118688
- Matthews DR Fulcher G Perkovic V Efficacy and safety of canagliflozin (CANA), an inhibitor of sodium glucose co-transporter 2 (SGLT2), added-on to insulin therapy +/− oral agents in type 2 diabetes. Abstract 764 Diabetologia 2012 55 Suppl 1 S314
- Yale JF Bakris G Cariou B Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease Diabetes Obes Metab 2013 15 5 463 473 23464594
- Bode B Stenlöf K Sullivan D Fung A Usiskin K Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial Hosp Pract (1995) 2013 41 2 72 84 23680739
- Roden M Weng J Eilbracht J Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial Lancet Diabetes Endocrinol 2013 1 3 208 219 24622369
- Häring HU Merker L Seewaldt-Becker E Weimer M Meinicke T Empagliflozin as add-on to metformin for 24 weeks improves glycemic control in patients with type 2 diabetes (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1092-P
- Ferrannini E Berk A Hantel S Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes Diabetes Care 2013 36 12 4015 4021 24186878
- Häring HU Merker L Seewaldt-Becker E Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes. A 24-week, randomized, double-blind, placebo-controlled trial Diabetes Care 2013 36 11 3396 3404 23963895
- Kovacs CS Seshiah V Swallow R Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial Diabetes Obes Metab 2014 16 2 147 158 23906415
- Rosenstock J Jelaska A Kim G Broedl UC Woerle HJ Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1102-P
- Barnett AH Mithal A Manassie J Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial Lancet Diabetes Endocrinol 2014 2 5 369 384 24795251
- Vasilakou D Karagiannis T Athanasiadou E Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis Ann Intern Med 2013 159 4 262 274 24026259
- Clar C Gill JA Court R Waugh N Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes BMJ Open 2012 2 5 e001007
- Berhan A Barker A Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized double-blind controlled trials BMC Endocr Disord 2013 13 1 58 24341330
- European Medicines Agency [homepage on the Internet] Forxiga (Dapagliflozin). EMA Assessment Report. Procedure no. EMEA/H/C/002322 2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002322/WC500136024.pdf Accessed September 17, 2013
- Wilding JP Woo V Rohwedder K Sugg J Parikh S Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years Diabetes Obes Metab Epub 2013 8 1
- Bailey CJ Gross JL Hennicken D Iqbal N Mansfield TA List JF Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial BMC Med 2013 11 1 43 23425012
- European Medicines Agency [homepage on the Internet] Canagliflozin. EMA Assessment Report. Procedure no. EMEA/H/C/002649/0000 2013 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002649/WC500156457.pdf Accessed December 3, 2013
- Stenlöf K Cefalu WT Kim KA Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-Week CANTATA-M study Curr Med Res Opin 2014 30 2 163 175 24073995
- Bolinder J Ljunggren O Johansson L Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin Diabetes Obes Metab Epub 2011 8 1
- US Food and Drug Administration [homepage on the Internet] FDA Briefing Document. NDA 204042. Invokana (Canagliflozin) tablets 2013 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf Accessed March 31, 2014
- Lambers Heerspink HJ de Zeeuw D Wie L Leslie B List J Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes Diabetes Obes Metab 2013 15 9 853 862 23668478
- Weir MR Januszewicz A Gilbert RE Lavalle Gonzalez FJ Meininger G Lower blood pressure (BP) with canagliflozin (cana) in subjects with type 2 diabetes mellitus (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1077-P
- Hach T Gerich J Salsali A Empagliflozin improves glycemic parameters and cardiovascular risk factors in patients with type 2 diabetes (T2DM): pooled data from four pivotal phase III trials Diabetes 2013 62 Suppl 1 Abstract 69-LB
- Tikkanen I Narko K Zeller C Empagliflozin improves blood pressure in patients with type 2 diabetes (T2DM) and hypertension. Abstract 942 Diabetologia 2013 56 Suppl S1 S377
- Johnsson KM Ptaszynska A Schmitz B Sugg J Parikh SJ List JF Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin J Diabetes Complications 2013 27 5 479 484 23806570
- Johnsson KM Ptaszynska A Schmitz B Sugg J Parikh SJ List JF Urinary tract infections in patients with diabetes treated with dapagliflozin J Diabetes Complications 2013 27 5 473 478 23849632
- Nicolle LE Capuano G Fung A Usiskin K Urinary tract infection (UTI) with canagliflozin (CANA) in subjects with type 2 diabetes mellitus (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1139-P
- Nyirjesy P Sobel J Fung A Gassmann-Meyer C Ways K Usiskin K Genital mycotic infections with canagliflozin (CANA) in subjects with type 2 diabetes mellitus (T2DM) Diabetes 2013 62 Suppl 1 Abstract 1069-P
- Kim G Gerich JE Salsali A Empagliflozin (EMPA) increases genital infections but not urinary tract infections (UTIs) in pooled data from four pivotal phase III trials Diabetes 2013 62 Suppl 1 Abstract 74-LB
- Ptaszynska A Johnsson KM Apanovitch A-M Sugg J Parikh S List J Safety of dapagliflozin in clinical trials for T2DM Diabetes 2012 61 Suppl 1 Abstract 1011-P
- Ptaszynska A Chalamandaris AG Sugg JE Johnsson KM Parikh S List JL Effect of dapagliflozin on renal function Diabetes 2012 61 Suppl 1 Abstract 1098-P
- Toto RD Wanner C Gerich J No overall increase in volume depletion events with empagliflozin (EMPA) in a pooled analysis of more than 11,000 patients with type 2 diabetes (T2DM) J Am Soc Nephrol 2013 24 Suppl Abstract SA-PO373
- Liakos A Karagiannis T Athanasiadou E Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis Diabetes Obes Metab Epub 2014 4 26
- Gangadharan Komala M Mather A Empagliflozin for the treatment of Type 2 diabetes Expert Rev Clin Pharmacol 2014 7 3 271 279 24716752
- Ljunggren Ö Bolinder J Johansson L Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin Diabetes Obes Metab 2012 14 11 990 999 22651373
- Bode B Stenlof K Sullivan D Fung A Usiskin K Meininger G Efficacy and safety of canagliflozin (CANA), a sodium glucose cotransporter 2 inhibitor (SGLT2), in older subjects with type 2 diabetes mellitus Diabetologia 2012 55 Suppl 1 S315 Abstract 765
- Wanner C Toto RD Gerich J No increase in bone fractures with empagliflozin (EMPA) in a pooled analysis of more than 11,000 patients with type 2 diabetes (T2DM) J Am Soc Nephrol 2013 24 Suppl Abstract TH-PO452
- Basile JN The potential of sodium glucose cotransporter 2 (SGLT2) inhibitors to reduce cardiovascular risk in patients with type 2 diabetes (T2DM) J Diabetes Complications 2013 27 3 280 286 23375850
- Rodríguez-Gutiérrez R Gonzalez-Saldivar G Canagliflozin Cleve Clin J Med 2014 81 2 87 88 24493488
- Foote C Perkovic V Neal B Effects of SGLT2 inhibitors on cardiovascular outcomes Diab Vasc Dis Res 2012 9 2 117 123 22381403
- Neal B Perkovic V de Zeeuw D Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)-A randomized placebo-controlled trial Am Heart J 2013 166 2 217 223 23895803
- Zinman B Inzucchi SE Lachin J Design of the empagliflozin cardiovascular (CV) outcome event trial in type 2 diabetes (T2D) Can J Diabetes 2013 37 Suppl 4 S29 S30
- Janssen Pharmaceuticals Inc. [homepage on the Internet] INVOKANA™ (canagliflozin) tablets, for oral use. Highlights of Prescribing Information (Revised 11/2013) 2013 Available from: http://www.invokanahcp.com/prescribing-information.pdf Accessed February 27, 2014
- US Food and Drug Administration [homepage on the Internet] Invokana (Canagliflozin) Tablets. NDA 204042. FDA Briefing Document (January 10, 2013) FDA Briefing Document 2013 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf Accessed April 10, 2014
- Ptaszynska A Hardy E Johnsson E Parikh S List J Effects of dapagliflozin on cardiovascular risk factors Postgrad Med 2013 125 3 181 189 23748519
- US Food and Drug Administration [homepage on the Internet] FDA Briefing Document. NDA 202293. Dapagliflozin tablets, 5 and 10 mg 2011 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm262994.pdf Accessed March 31, 2014
- Macdonald FR Peel JE Jones HB The novel sodium glucose transporter 2 inhibitor dapagliflozin sustains pancreatic function and preserves islet morphology in obese, diabetic rats Diabetes Obes Metab 2010 12 11 1004 1012 20880347
- Merovci A Solis-Herrera C Daniele G Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production J Clin Invest 2014 124 2 509 514 24463448
- Ferrannini E Muscelli E Frascerra S Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients J Clin Invest 2014 124 2 499 508 24463454
- Powell DR Smith M Greer J LX4211 Increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose J Pharmacol Exp Ther 2013 345 2 250 259 23487174
- Zambrowicz B Freiman J Brown PM LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial Clin Pharmacol Ther 2012 92 2 158 169 22739142
- Henry RR Rosenstock J Chalamandaris AG Kasichayanula S Bogle A Griffen SC Exploring the potential of dapagliflozin in type 1 diabetes: Phase 2a pilot study Diabetes 2013 62 Suppl 1 Abstract 70-LB
- Perkins BA Cherney DZI Partridge H Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: Results of an 8-week open-label proof-of-concept trial Diabetes Care 2014 37 5 1480 1483 24595630
- Cherney DZ Perkins BA Soleymanlou N Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus Circulation 2014 129 5 587 597 24334175
- Liu JJ Lee T DeFronzo RA Why Do SGLT2 inhibitors inhibit only 30%–50% of renal glucose reabsorption in humans? Diabetes 2012 61 9 2199 2204 22923645
- Abdul-Ghani MA Defronzo RA Norton L Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30%–50% of filtered glucose load in humans Diabetes 2013 62 10 3324 3328 24065789

