Abstract
The effects of Arabic gum (AG) against nephrotoxicity of mercury (Hg), an oxidative-stress inducing substance, in rats were investigated. A single dose of mercuric chloride (5 mg/kg intraperitoneal injection) induced renal toxicity, manifested biochemically by a significant increase in serum creatinine, blood urea nitrogen, thiobarbituric acid reactive substances, and total nitrate/nitrite production in kidney tissues. In addition, reduced glutathione, glutathione peroxidase, and catalase enzymes in renal tissues were significantly decreased. Pretreatment of rats with AG (7.5 g/kg/day per oral administration), starting 5 days before mercuric chloride injection and continuing through the experimental period, resulted in a complete reversal of Hg-induced increase in creatinine, blood urea nitrogen, thiobarbituric acid reactive substances, and total nitrate/nitrite to control values. Histopathologic examination of kidney tissues confirmed the biochemical data; pretreatment of AG prevented Hg-induced degenerative changes of kidney tissues. These results indicate that AG is an efficient cytoprotective agent against Hg-induced nephrotoxicity by a mechanism related at least in part to its ability to decrease oxidative and nitrosative stress and preserve the activity of antioxidant enzymes in kidney tissues.
Introduction
Mercury (Hg) is a hazardous environmental and industrial pollutant which induces severe alterations in the body tissues of both humans and animals.Citation1,Citation2 The toxicity of Hg depends on the form of the Hg compounds (elemental, inorganic, and organic). Inorganic Hg accumulates predominantly in the kidneys, causing acute renal failure.Citation3,Citation4 The uptake, accumulation, and toxicity of inorganic Hg in the kidney have been related to it binding to endogenous thiol-containing molecules.Citation5 Thiol-containing enzymes have been recognized as the targets of inorganic Hg.Citation5,Citation6 Moreover, binding of mercuric ions to thiol groups may cause decreased glutathione (GSH) levels, leading to increases in levels of reactive oxygen species (ROS), such as superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals, which provoke lipid, protein, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) oxidation.Citation7,Citation8 Considering that oxidative stress and endogenous thiol depletion are involved in inorganic Hg toxicity, it has been suggested that antioxidants could contribute to the treatment of Hg poisoning.Citation9,Citation10 In this way, melatonin, curcumin, and vitamin E have been found to play a protective effect against mercuric chloride (HgCl2)-induced acute renal toxicity.Citation2,Citation11–Citation13 Similarly, a number of plant extracts with antioxidant properties have been shown to inhibit HgCl2-induced renal toxicity.Citation14–Citation16
Arabic gum (AG) is a dried, gummy exudate from the stems and branches of Acacia senegal (Leguminosae), composed of calcium, magnesium, and potassium salts of the polysaccharide Arabic gum acid.Citation17 AG has been used in Arabic folk medicine to reduce both the frequency and the need for hemodialysis in chronic renal failure patients.Citation18 AG also has been shown to reduce urinary nitrogen excretion by increasing urea disposal in the cecum and lowering serum urea concentration in rats and humans.Citation19,Citation20 Additionally, we have recently reported that AG prevented gentamicin-induced nephrotoxicity. Co-treatment of AG significantly prevented gentamicin-induced lipid peroxidation in the kidney tissue, which was closely associated with protection of renal function and histological changes.Citation18
To the best of our knowledge, there are no studies concerning the nephroprotective effect of AG against Hg intoxication. Therefore, the present study was carried out to investigate: 1) the adverse effect of acute Hg intoxication on the kidneys based on serum biochemical parameters, oxidative stress, and histopathologic alterations; and 2) the possible mitigating effect of AG against acute Hg intoxication in rats.
Materials and methods
Chemicals
Hg in the form of HgCl2 was purchased from CHEMA TEC CO (Alexandria, Egypt). AG was purchased from Sigma-Aldrich (St Louis, MO, USA), and thiobarbituric acid was a product of Sigma-Aldrich. All other chemicals were of the highest grade commercially available.
Animals
Male Swiss albino rats (Animal house of College of Pharmacy, King Saud University, Riyadh, Saudi Arabia) weighing 150–200 g were used in all experiments. Animals were maintained under standard conditions of temperature and humidity with regular light/dark cycles and allowed free access to food (Purina Chow, Gray Summit, MO, USA) and water. All animal experiments were conducted according to the regulations of the Committee on Bioethics for Animal Experiments of Riyadh Colleges of Dentistry and Pharmacy.
Animal treatment
The animals were divided at random into four groups of ten animals each. The first group (control) received vehicles used for Hg (physiological saline solution, intraperitoneal injection [IP]). The second group received AG by oral gavage (7.5 g/kg/day) for 1 week.Citation21 The third group was injected with HgCl2 (5 mg/kg IP).Citation22 The fourth group, received Ag per oral route (os) (7.5 g/kg/day) for 5 days, then injected with HgCl2 (5 mg/kg IP) and continued on Ag daily until the end of the experiment (1 week). Blood samples were taken by cardiac puncture, under light ether anesthesia, into non-heparinized tubes. Serum was separated by centrifugation for 5 minutes at 1,000 xg and stored at −20°C until analysis. Animals were sacrificed by cervical dislocation and the kidneys were quickly isolated, washed with saline, blotted dry on filter paper, and weighed, and 10% (% weight per volume [w/v]) homogenate of the left kidney was made in ice-cold saline.
Measurement of serum biochemical parameters
Serum creatinine and blood urea nitrogen (BUN) concentrations were determined colorimetrically as described by Bonsnes and Taussky, and Hallet and Cook, respectively, using commercially available diagnostic kits (bioMérieux-RCS, Lyon, France).Citation23,Citation24
Determination of lipid peroxides, GSH content, and enzyme activities of GSH peroxidase and catalase in kidney homogenate
GSH content and lipid peroxidation (malondialdehyde production) in the kidney tissues were determined according to Ellman, and Ohkawa et al, respectively.Citation25,Citation26 The enzyme activity of glutathione peroxidase (GSH-Px) and catalase were measured in the kidney homogenates according to Kraus and Ganther, and Higgins et al, respectively.Citation27,Citation28
Determination of total nitrate/nitrite concentrations in renal tissues
Total nitrate/nitrite (NOx) was measured as stable end product, nitrite, according to the method of Miranda et al.Citation29 The assay is based on the reduction of nitrate by vanadium trichloride combined with detection by the acidic Griess reaction. The diazotization of sulfanilic acid with nitrite at acidic pH and subsequent coupling with N-(10 naphthyl)-ethylenediamine produced an intensely colored product that is measured spectrophotometrically at 540 nm. The levels of total NOx were expressed as mol g−1 wet tissue.
Histopathology
Histopathologic examination was performed on the animals of each group. Right kidney samples were taken. The tissue samples were fixed for at least 48 hours in 10% formalin in phosphate buffer (pH 7). The samples were then embedded in paraffin wax, cut into 5 μm sections, and stained with hematoxylin and eosin. The slides were coded and were examined by a histopathologist who was unaware of the treated groups.
Statistical analysis
Data are expressed as mean ± standard error. Statistical comparison between different groups was conducted using one-way analysis of variance (ANOVA) followed by a Tukey–Kramer multiple comparison test to judge the difference between various groups. Significance was accepted at P<0.05.
Results
Effects of AG on Hg-induced changes in serum biochemical parameters
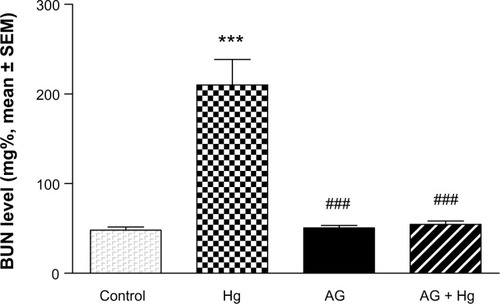
Serum creatinine and blood urea nitrogen (BUN) were significantly increased after injection of Hg as compared with the control group (P<0.001) ( and ). Pretreatment of animals with AG (7.5 g/kg/day per os) 5 days before and concomitantly with Hg significantly reduced the rise in the level of BUN and creatinine.
Figure 1 Effects of AGon elevated levels of serum creatinine induced by Hg.
P<0.05, ###
P<0.001.
Abbreviations: AG, Arabic gum; Hg, mercury; SEM, standard error of the mean; po, per oral.
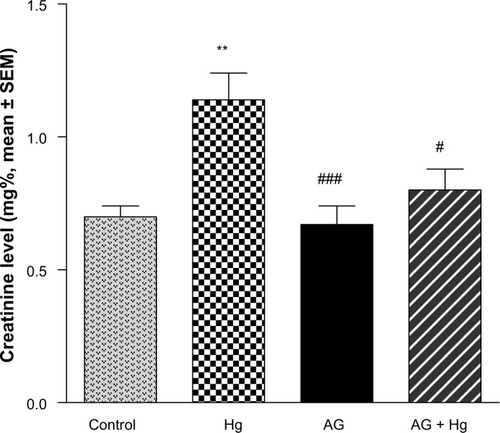
Figure 2 Effects of AG on elevated levels of BUN induced by Hg.
Abbreviations: AG, Arabic gum; BUN, blood urea nitrogen; Hg, mercury; SEM, standard error of the mean; po, per oral.
Oxidative and nitrosative stress biomarkers
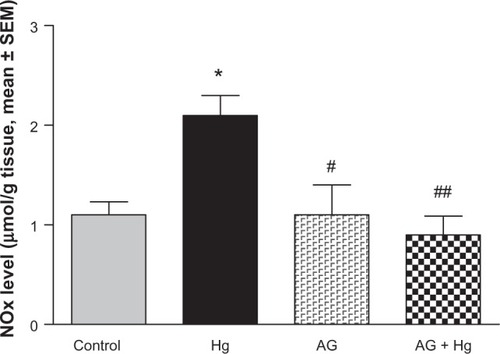
and show the effects of AG, Hg, and their combination on oxidative stress biomarkers in renal tissues, namely thiobarbituric acid reactive substance (TBARS) and reduced GSH, respectively. Hg resulted in a significant decrease in GSH content to reach only 75% of control group. Also, it leads to a significant 73% increase in TBARS as compared to the control group. Combined AG treatment with Hg significantly decreased TBARS (P<0.001) and restored GSH level in renal tissues compared to the control values. shows the effects of AG, Hg, and their combination on the level of NOx levels in rat renal tissues. Hg resulted in a significant 91% increase of NOx in renal tissues as compared to the control group. Combined AG treatment with Hg significantly decreased NOx in renal tissues (P<0.05) compared to the control values.
Figure 3 Effect of Hg, AG, and their combination on the levels of TBARS in rat renal tissues.
Abbreviations: AG, Arabic gum; Hg, mercury; SEM, standard error of the mean; TBARS, thiobarbituric acid reactive substances; po, per oral.
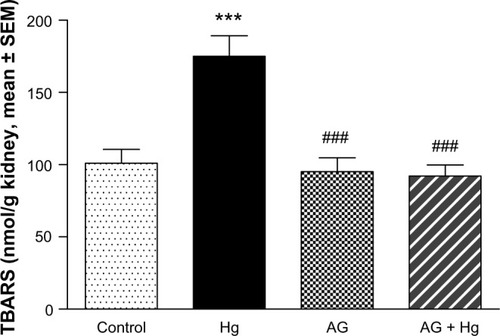
Figure 4 Effect of Hg, AG, and their combination on the levels of reduced GSH in rat renal tissues.
P<0.05.
Abbreviations: AG, Arabic gum; GSH, glutathione; Hg, mercury; SEM, standard error of the mean; po, per oral.
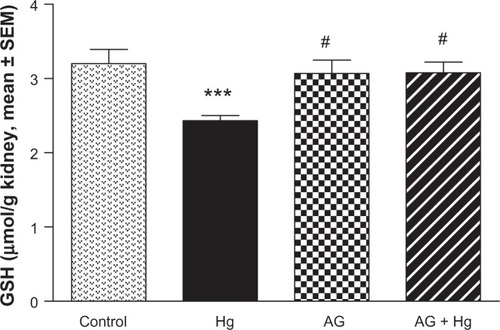
Figure 5 Effect of Hg, AG, and their combination on total NOx levels in rat renal tissues.
P<0.05, ##
P<0.01.
Abbreviations: AG, Arabic gum; Hg, mercury; NOx, nitrate/nitrite; SEM, standard error of the mean; po, per oral.
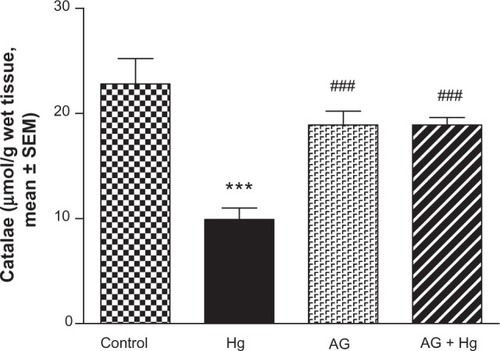
Antioxidant enzymes activities
and show the effects of AG, Hg, and their combination on the activity of antioxidant enzymes GSH-Px and catalase in renal tissues, respectively. Hg resulted in a significant decrease in both GSH-Px and catalase enzyme activities as compared to the control group (P<0.001 and P<0.001, respectively). Combined AG treatment with Hg significantly improved both enzymes’ activity (P<0.001) in renal tissues compared to the control values.
Figure 6 Effects of AGon changes in GSH-Px enzyme activities induced by Hg.
Abbreviations: AG, Arabic gum; GSH-Px, glutathione peroxidase; Hg, mercury; SEM, standard error of the mean; po, per oral.
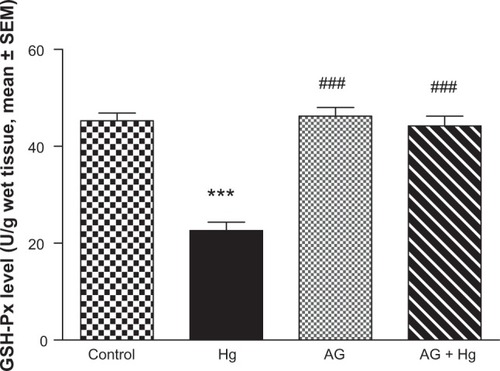
Figure 7 Effect of Hg, AG, and their combination on the catalase activity in rat renal tissues.
Abbreviations: AG, Arabic gum; Hg, mercury; SEM, standard error of the mean; po, per oral.
Kidney pathology
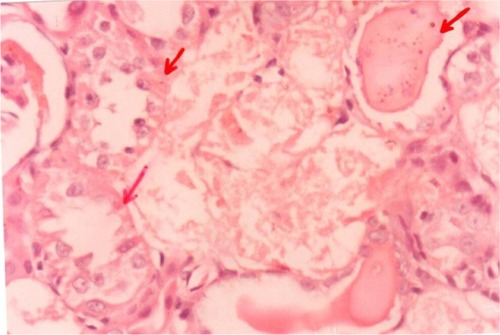
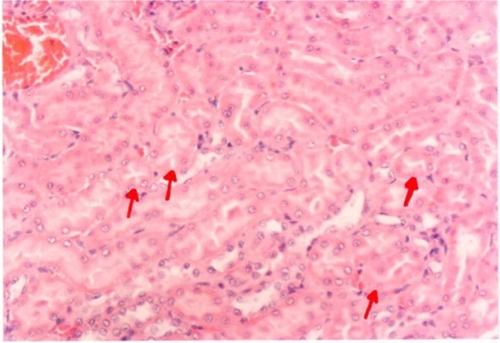
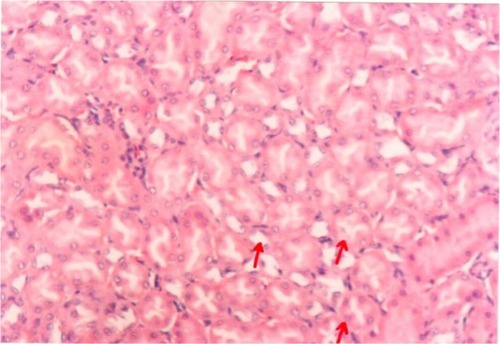
Pathological examination of the kidneys of control and AG groups showed normal morphology of the renal parenchyma with well-defined glomeruli and tubules with non-significant changes ( and ). However, animals treated with Hg showed clear signs of glomerular and tubular necrosis, interstitial nephritis, and desquamation of the tubular epithelial cells in the renal cortex (). Interestingly, kidney specimens from rats treated with AG and Hg revealed significant improvement in glomeruli and renal tubules, evidenced by less vacuolization and more preservation of tubular histology ().
Figure 8 A photomicrograph of the renal cortex of a control rat. The red arrows showing parenchyma with normal glomeruli and tubules, ×200 magnification.
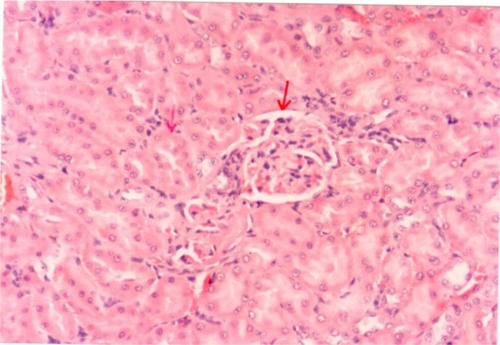
Figure 9 A photomicrograph of kidney of an Arabic gum-treated rat. The red arrows showing cortical tubules and peritubular capillaries with no pathogenic changes, ×200 magnification.
Discussion
Mercuric ion, one of strongest thiol-binding agents, increases the intracellular levels of ROS and induces oxidative stress, resulting in tissue damage.Citation30–Citation32 Hg toxicity is associated with superoxide radical generation and GSH reduction.Citation33,Citation34 Our study demonstrates that the treatment of rats with HgCl2 revealed a significant enhancement in TBARS levels, indicative of the generation of lipid peroxides. Enhanced lipid peroxidation levels were also reported in Hg toxicity by Agarwal et al and Sener et al.Citation13,Citation35 HgCl2 is known to increase the production of many ROS, such as superoxide and H2O2, which cause lipid peroxidation and subsequently oxidative tissue damage.Citation36–Citation38 Endogenous GSH has a specific role in protecting the body from Hg toxicity due to its function as a carrier of Hg and its antioxidant properties. GSH binds with Hg and forms a complex that prevents Hg from binding to cellular proteins and subsequently causing damage to both enzymes and tissue.Citation39 Hg poisoning leads to a reduction of intracellular GSH content and decreases the antioxidant potential of the cells. The present study revealed that Hg-treated rats showed a significant depletion of serum GSH levels. Agarwal et al reported a significant reduction of GSH levels in liver, kidney and brain tissues.Citation12,Citation13
Alterations observed in the activity of GSH-Px and catalase in kidney tissues of Hg-exposed animals indicate the generation of ROS (O–2 or H2O2). Inhibition in the activity of renal antioxidant enzymes, such as superoxide dismutase (SOD), GSH-Px, and catalase, in addition to depletion of GSH levels was also reported earlier.Citation40,Citation41 Enhanced creatinine and BUN levels indicate nephrotoxicity, as reported by Rumbeiha et al.Citation42 Histopathologic alterations in kidney tissues after Hg exposure were revealed. Rumbeiha et al, Al-Saleh et al, Sarwar Alam et al, and Augusti et al have also reported similar histopathologic alterations in Hg-induced nephrotoxicity.Citation42–Citation45
Pretreatment with AG attenuated the Hg-induced oxidative damage. Hence, pretreatment with AG significantly restored the increased TBARS and decreased GSH levels to the normal values. This could be attributed to the excellent antioxidant properties of AG.Citation46 These properties seems to be due to its ability to scavenge free radicals. The kidneys are the primary target organ for accumulation and toxicity of inorganic Hg.Citation5 In fact, in as little as l hour, 50% of an administered dose of inorganic Hg is present in the kidney.Citation47 Within the kidney, the majority of mercuric ions were detected in the cortex and outer stripe of the outer medulla. This finding was expected considering that the proximal tubule, which spans these two renal zones, is the primary site of accumulation of mercuric ions.Citation5 The histopathologic findings in the kidney tissue of Hg-treated rats include severe diffuse acute necrosis of the tubular epithelium, fragmentation and shedding of tubular epithelium in the lumina of the renal tubules, and interstitial edema as a result of tubular leakage. The interaction of Hg with protein thiol groups is thought to play an important role in nephrotoxicity induced by Hg at the cellular level.Citation5 The results of this study indicate that AG improved Hg-induced nephrotoxicity, manifested by a decrease in both serum creatinine and urea levels, and minimized the intensity of the renal lesions. The nephroprotective effect of AG against many nephrotoxic agents was noted in several reports.Citation18,Citation21, Citation48–Citation53 The antioxidation induced by AG might be one of the most likely mechanisms contributing to its beneficial effect against renal injury. This antioxidant effect of AG was confirmed previously by in vitro studies, which showed that AG had a dose-dependent scavenging of superoxide radicals generated enzymatically and nonenzymatically.Citation54 It could be suggested that AG scavenges Hg free-radical generation and, in turn, inhibits lipid peroxidation–induced injury in renal tissues, which has been suggested to protect renal structure and function. Therefore, the protective effect is provided by AG on renal tissue through antioxidants as well as by scavenging free radicals in vivo.
Conclusion
In summary, our data indicate that Hg-induced nephrotoxicity is related to lipid peroxidation. Coadministration of AG provided protection against HG-induced nephrotoxicity, possibly by inhibiting the free radical mediated process. These protective effects of AG on renal injury induced by Hg might have a considerable impact on developing clinically feasible strategies to treat patients with toxin-induced renal failure.
Disclosure
The authors report no conflicts of interest in this work.
References
- Mahboob M Shireen KF Atkinson A Khan AT Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury J Environ Sci Health B 2001 36 5 687 697 11599730
- Sener G Sehirli AO Avanoglu-Dülger G Melatonin protects against mercury (ll)-induced oxidative tissue damage in rats Pharmacol Toxicol 2003 93 6 290 296 14675463
- Emanuelli T Rocha JB Pereira ME Effect of mercuric chloride intoxication and dimercaprol treatment on delta-aminolevulinate dehydratase from brain, liver and kidney of adult mice Pharmacol Toxicol 1996 79 3 136 143 8884872
- Tanaka-Kagawa T Suzuki M Naganuma A Yamanaka N Imura N Strain difference in sensitivity of mice to renal toxicity of inorganic mercury J Pharmacol Exp Ther 1998 285 1 335 341 9536029
- Zalups RK Molecular interactions with mercury in the kidney Pharmacol Rev 2000 52 1 113 143 10699157
- Nogueira CW Soares FA Nascimento PC Muller D Rocha JB 2,3-Dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid increase mercury- and cadmium-induced inhibition of delta-aminolevulinate dehydratase Toxicology 2003 184 2–3 85 95 12499112
- Li Z Wu J Deleo CJ RNA damage and surveillance under oxidative stress IUBMB Life 2006 58 10 581 588 17050375
- Clarkson TW The toxicology of mercury Crit Rev Clin Lab Sci 1997 34 4 369 403 9288445
- Patrick L Mercury toxicity and antioxidants. Part 1: Role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity Altern Med Rev 2002 7 6 456 471 12495372
- Pillai A Gupta S Antioxidant enzyme activity and lipid peroxidation in liver of female rats co-exposed to lead and cadmium: effects of vitamin E and Mn2+ Free Radic Res 2005 39 7 707 712 16036349
- Nava M Romero F Quiroz Y Parra G Bonet L Rodríguez-Iturbe B Melatonin attenuates acute renal failure and oxidative stress induced by mercuric chloride in rats Am J Physiol Renal Physiol 2000 279 5 F910 F918 11053052
- Agarwal R Goel SK Behari JR Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury J Appl Toxicol 2010 30 5 457 468 20229497
- Agarwal R Goel SK Chandra R Behari JR Role of vitamin E in preventing acute mercury toxicity in rat Environ Toxicol Pharmacol 2010 29 1 70 78 21787585
- Ahn CB Song CH Kim WH Kim YK Effects of Juglans sinensis Dode extract and antioxidant on mercury chloride-induced acute renal failure in rabbits J Ethnopharmacol 2002 82 1 45 49 12169405
- Oda SS El-Ashmawy IM Adverse effects of the anabolic steroid, boldenone undecylenate, on reproductive functions of male rabbits Int J Exp Pathol 2012 93 3 172 178 22583130
- Sarwar Alam M Kaur G Jabbar Z Javed K Athar M Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity Food Chem Toxicol 2007 45 6 910 920 17207565
- Rehan A Johnson KJ Kunkel RG Wiggins RC Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury Kidney Int 1985 27 3 503 511 3999539
- Al-Majed AA Mostafa AM Al-Rikabi AC Al-Shabanah OA Protective effects of oral Arabic gum administration on gentamicin-induced nephrotoxicity in rats Pharmacol Res 2002 46 5 445 451 12419649
- Ramsammy L Ling KY Josepovitz C Levine R Kaloyanides GJ Effect of gentamicin on lipid peroxidation in rat renal cortex Biochem Pharmacol 1985 34 21 3895 3900 4062965
- Salahudeen AK Clark EC Nath KA Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo J Clin Invest 1991 88 6 1886 1893 1752950
- Al-Majed AA Abd-Allah AR Al-Rikabi AC Al-Shabanah OA Mostafa AM Effect of oral administration of Arabic gum on cisplatin-induced nephrotoxicity in rats J Biochem Mol Toxicol 2003 17 3 146 153 12815610
- Augusti PR Conterato GM Somacal S Effect of lycopene on nephrotoxicity induced by mercuric chloride in rats Basic Clin Pharmacol Toxicol 2007 100 6 398 402 17516994
- Bonsnes RW Taussky HN On the colorimetric determination of creatinine by the Jaffe reaction J Biol Chem 1945 158 581 591
- Hallett CJ Cook JG Reduced nicotinamide adenine dinucleotide-coupled reaction for emergency blood urea estimation Clin Chim Acta 1971 35 1 33 37 4331345
- Ellman GL Tissue sulfahydryl groups Arch Biochem Biophys 1959 82 1 70 77 13650640
- Ohkawa H Ohish N Yagi K Assay for lipid peroxides in animal tissues by thiobarbituric acid Anal Biochem 1979 95 2 351 358 36810
- Kraus RJ Ganther HE Reaction of cyanide with glutathione peroxidase Biochem Biophys Res Commun 1980 96 3 1116 1122 7437059
- Higgins CP Baehner RL McCallister J Boxer LA Polymorphonuclear leukocyte species differences in the disposal of hydrogen peroxide (H2O2) Proc Soc Exp Biol Med 1978 158 3 478 481 210469
- Miranda KM Espey MG Wink DA A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite Nitric Oxide 2001 5 1 62 71 11178938
- Zahir F Rizwi SJ Haq SK Khan RH Low dose mercury toxicity and human health Environ Toxicol Pharmacol 2005 20 2 351 360 21783611
- Hussain S Atkinson A Thompson SJ Khan AT Accumulation of mercury and its effect on antioxidant enzymes in brain, liver, and kidneys of mice J Environ Sci Health B 1999 34 4 645 660 10390852
- Reus IS Bando I Andrés D Cascales M Relationship between expression of HSP70 and metallothionein and oxidative stress during mercury chloride induced acute liver injury in rats J Biochem Mol Toxicol 2003 17 3 161 168 12815612
- Girardi G Elías MM Mercuric chloride effects on rat renal redox enzymes activities: SOD protection Free Radic Biol Med 1995 18 1 61 66 7896172
- Miura K Naganuma A Himeno S Imura N Mercury toxicity Goyer RA Cherian MG Toxicology of Metals: Biochemical Aspects Berlin Springer-Verlag 1995 163 187
- Sener G Sehirli O Tozan A Velioğlu-Ovunç A Gedik N Omurtag GZ Ginkgo biloba extract protects against mercury(II)-induced oxidative tissue damage in rats Food Chem Toxicol 2007 45 4 543 550 17267089
- Miller DM Lund BO Woods JS Reactivity of Hg(II) with superoxide: evidence for the catalytic dismutation of superoxide by Hg(II) J Biochem Toxicol 1991 6 4 293 298 1663557
- Huang YL Cheng SL Lin TH Lipid peroxidation in rats administrated with mercuric chloride Biol Trace Elem Res 1996 52 2 193 206 8773760
- Linden A Gülden M Martin HJ Maser E Seibert H Peroxide-induced cell death and lipid peroxidation in C6 glioma cells Toxicol In Vitro 2008 22 5 1371 1376 18346863
- Kromidas L Trombetta LD Jamall IS The protective effects of glutathione against methylmercury cytotoxicity Toxicol Lett 1990 51 1 67 80 2315960
- Agarwal R Raisuddin S Tewari S Goel SK Raizada RB Behari JR Evaluation of comparative effect of pre- and posttreatment of selenium on mercury-induced oxidative stress, histological alterations, and metallothionein mRNA expression in rats J Biochem Mol Toxicol 2010 24 2 123 135 20143455
- Gstraunthaler G Pfaller W Kotanko P Glutathione depletion and in vitro lipid peroxidation in mercury or maleate induced acute renal failure Biochem Pharmacol 1983 32 19 2969 2972 6226293
- Rumbeiha WK Fitzgerald SD Braselton WE Roth RA Kaneene JB Potentiation of mercury-induced nephrotoxicity by endotoxin in the Sprague-Dawley rat Toxicology 2000 149 2–3 75 87 10967405
- Al-Saleh I El-Doush I Shinwari N Al-Baradei R Khogali F Al-Amodi M Does low mercury containing skin-lightening cream (fair and lovely) affect the kidney, liver, and brain of female mice? Cutan Ocul Toxicol 2005 24 1 11 29 17040886
- Sarwar Alam M Kaur G Jabbar Z Javed K Athar M Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity Food Chem Toxicol 2007 45 6 910 920 17207565
- Augusti PR Conterato GM Somacal S Effect of lycopene on nephrotoxicity induced by mercuric chloride in rats Basic Clin Pharmacol Toxicol 2007 100 6 398 402 17516994
- Ali BH Does gum Arabic have an antioxidant action in rat kidney? Ren Fail 2004 26 1 1 3 15083914
- Zalups RK Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride Toxicology 1993 79 3 215 228 8316951
- Mahmoud MF Diaai AA Ahmed F Evaluation of the efficacy of ginger, Arabic gum, and Boswellia in acute and chronic renal failure Ren Fail 2012 34 1 73 82 22017619
- Ali BH Al-Husseni I Beegam S Effect of gum Arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats PLoS ONE 2013 8 2 e55242 23383316
- Ali BH Ziada A Al Husseni I Beegam S Al-Ruqaishi B Nemmar A Effect of Acacia gum on blood pressure in rats with adenine-induced chronic renal failure Phytomedicine 2011 18 13 1176 1180 21741228
- Ali BH Al-Salam S Al Husseni I Effects of Gum Arabic in rats with adenine-induced chronic renal failure Exp Biol Med (Maywood) 2010 235 3 373 382 20404056
- Ali AA Ali KE Fadlalla AE Khalid KE The effects of gum Arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan Nat Prod Res 2008 22 1 12 21 17999333
- Nasir O Umbach AT Rexhepaj R Effects of gum Arabic (Acacia senegal) on renal function in diabetic mice Kidney Blood Press Res 2012 35 5 365 372 22473073
- Abd-Allah AR Al-Majed AA Mostafa AM Al-Shabanah OA Din AG Nagi MN Protective effect of Arabic gum against cardiotoxicity induced by doxorubicin in mice: a possible mechanism of protection J Biochem Mol Toxicol 2002 16 5 254 259 12439867