Abstract
Objective
Dapagliflozin was the first drug in a class of therapies that took a new approach to glycemic control in adults with type 2 diabetes (T2D). It is an inhibitor of the sodium glucose cotransporter, resident in the proximal nephron, which is responsible for the recovery of filtered glucose back into circulation. Inhibiting this cotransporter reduces glucose recovery, increases glucose excretion, and reduces hyperglycemia. Here, we review some of the literature relating to the action, efficacy, and clinical use of dapagliflozin.
Materials and methods
A Medline search was conducted within date, animal, and language limits, and relevant papers were selected for review. Conference proceedings were reviewed to obtain up-to-date literature on this drug. Clinical trial websites were reviewed for ongoing studies.
Results
On average, treatment with dapagliflozin results in improvement in glycated hemoglobin by 0.50%, fasting plasma glucose by 1 mmol/L, weight by 2 kg, body mass index by 1.1%, and systolic/diastolic blood pressure by 4/2 mmHg over 24–52 weeks. The weight benefit is greater when used in association with sulfonylureas. It is generally well tolerated, but comes with an increased risk of genitourinary and urinary tract infections. In addition, it is associated with reversible changes to renal function that need to be explored. Early reports of an association with cancer also need to be carefully monitored.
Conclusion
Dapagliflozin is a useful therapy for adult patients with T2D. It also holds potential for a broader range of patients with T2D (such as the elderly and pediatric populations), as well as those with other forms of diabetes, such as type 1 diabetes. While longer-term outcome studies of safety and efficacy are awaited, dapagliflozin forms a very useful and welcome addition to our armamentarium for managing patients with T2D.
Introduction
Diabetes is a significant international health care problem. There are currently 382 million people with diabetes, a staggering 8.3% or one in 12 of the adult population. This figure is estimated to rise to 592 million people by 2035.Citation1 The disease comes with a financial burden. An estimated US$548 billion was spent on this condition in 2013. The vast majority of this cost relates to the management of diabetic complications. These complications include renal replacement therapy, cardiovascular disease, diabetic retinopathy, and diabetic foot disease.
Optimized glucose control reduces the risk of diabetic complications.Citation2,Citation3 Several glycated hemoglobin (HbA1c) glycemic targets have been proposed for the management of diabetes, ranging from 6.0% to 8.5% (42–69 mmol/mol).Citation4–Citation10 While the last few years have seen an improvement in glucose control and generally in diabetes care, significant further improvement is urgently required. It is estimated that only about half of the diabetic population reach the proposed glycemic targets.Citation11 Reasons for failure are complex, but there is little doubt that identifying the right therapy for the patient will go some way to improve their glycemic control.
A number of factors need to be considered when individualizing first-line therapy. These are outlined in . Tolerability and side effects in particular significantly contribute to noncompliance and therapy failure. It is therefore important to have access to a range of therapies that can be trialed for individual patients.
Table 1 Some factors to consider while managing patients with diabetes
Furthermore, type 2 diabetes (T2D) is a progressive condition with a gradual and continuing loss of β-cell function.Citation13,Citation14 This results in deterioration in glycemic control and eventually for the need for insulin-replacement therapy.Citation15 This long-term requirement for the escalation of therapy provides additional pressure to have access to a range of therapies. Additionally, excess adiposity is seen in most T2D patientsCitation16 and is associated with insulin resistance,Citation17 and targeting it is an important consideration in diabetes management.
The twenty-first century has seen the emergence of several new classes of antidiabetic drugs. One is the sodium glucose cotransporter (SGLT)-2 inhibitors. Dapagliflozin is the first in this class of new therapies. It is currently licensed by the US Food and Drug Administration (FDA; in January 2014),Citation18 the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA; in April 2012)Citation19 and the Medicines and Healthcare Products Regulatory Agency in the UK, the Ministry of Health, Labour, and Welfare (MHLW) in Japan (March 2014),Citation20 and by the Scottish Medicines Consortium (in January 2013).Citation21
The aim of this article is to review the literature on dapagliflozin with regard to its mechanism of action, efficacy, side effects, drug interaction, contraindications, and cost. We also review its place in the treatment of T2D.
Materials and methods
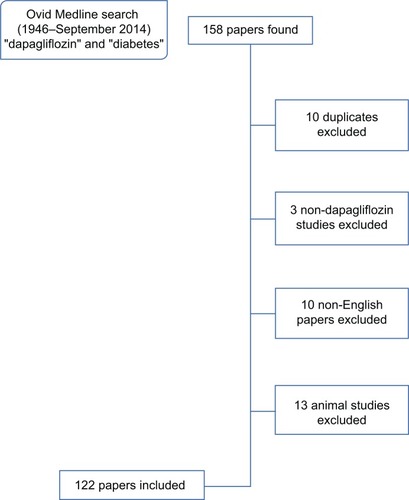
An Ovid Medline (1946 to September 2014) search was carried out using the terms “diabetes” and “dapagliflozin”. Papers and abstracts on animal studies and not in English were excluded, including any duplicates (). Any useful references cited in these papers/abstracts were also reviewed. A search on ClinicalTrials.gov using the search term “dapagliflozin” was also made to find information on past and ongoing studies. Other sources included the World Health Organization, the FDA, Centers for Disease Control and Prevention, clinical guidelines (including National Institute for Health and Care Excellence technology appraisal), FDA/EMA/UK labeling of summary of product characteristics, briefings, press releases, and Google searches. Conference abstracts were also used.
Mechanism of action
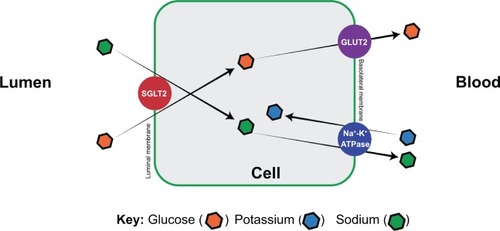
The human nephron reabsorbs almost all of the glucose present in the filtrate, and can do so from as early as 34 weeks of gestation.Citation22,Citation23 This comprises 180 g of glucose per day.Citation24,Citation25 Only 1% of the filtered glucose makes its way into the urine, and 90% of the filtered glucose is reabsorbed by SGLT2 expressed by epithelial cells lining the first segment of the proximal convoluted tubule. The remaining 10% is reabsorbed by SGLT1 lower in the nephron.Citation26,Citation27
The process of glucose reabsorbtionCitation28–Citation31 starts at the luminal surface of the proximal tubular epithelium, where SGLT2 actively moves glucose from the glomerular filtrate into the epithelial cells (). The cotransporters move glucose along with sodium, which is in turn driven by the active transport of sodium out of the basolateral cells by the Na+/K+-adenosine triphosphatase pump. Glucose transporters (GLUTs) are passive transporters that move glucose out of the cell across the basolateral membrane. This happens along the concentration gradient. This entire process results in glucose being moved from the proximal tubule back into the circulation.
Figure 2 S1 segment of the promixal convoluted tubule of the nephron.
Abbreviations: SGLT, sodium glucose cotransporter; GLUT, glucose cotransporter; ATPase, adenosine triphosphatase.
Two genetic disordersCitation23 have helped our understanding of the working of the SGLT2/GLUT2 transporter molecules: familial renal glycosuria and Fanconi–Bickel syndrome.
Familial renal glycosuria
This autosomal-recessive condition occurs due to mutations in the SLC5A2 gene, which encodes SGLT2. Persistent glycosuria is present, but without significant disturbance in plasma glucose, glucose tolerance, or insulin. This is considered to be a benign condition, and urinary tract infections are only occasionally seen in severe forms of this condition.Citation32
Fanconi–Bickel syndrome
This is a rare autosomal condition characterized by a mutation in the SLC2A2 gene, which encodes the GLUT2 transporter, and which results in glycosuria.Citation33
Phlorizin,Citation30,Citation34 first isolated from the root bark of the apple tree in 1835, is an inhibitor of SGLTs. However, this was not used therapeutically due to its nonspecificity for SGLT2. The use of this drug resulted in blockade of intestinal SGLT1, poor intestinal absorption of glucose, and thus led to diarrhea and dehydration. Dapagliflozin, however, is a highly selective competitive and reversible inhibitor of SGLT2,Citation35 and can achieve increased urinary excretion of glucose without the gastrointestinal side effects associated with nonspecific SGLT therapy.
While the mechanism of action of dapagliflozin is primarily through SGLT2 blockade, this may be complicated by the concurrent secretion of glucagon. Recent dataCitation36 indicate that SGLT2 is also expressed in human pancreatic α-cells, and therapy with dapagliflozin is described to increase circulating glucagon.Citation37 This increased glucagon potentially dampens the efficacy of SGLT2 inhibitors and needs further examination. This potentially is also protective against hypoglycemia.
Pharmacokinetics
Dapagliflozin () is rapidly and well absorbed after oral administration.Citation38,Citation39 Maximum dapagliflozin plasma concentrations occur within 2 hours of administration (in the fasted state). Bioavailability is 78% with the 10 mg once-daily (OD) dosing. It can be taken with or without food.Citation40 It is 91% protein-bound, and this is not affected by hepatic or renal disease.
Dapagliflozin is metabolized to its inactive metabolite – dapagliflozin 3-O-glucuronide – in the liver and kidney by the enzyme uridine diphosphate-glucuronosyltransferase 1A9. The mean plasma terminal half-life for dapagliflozin is 13 hours (10 mg dosing). Dapagliflozin and its metabolites are mainly excreted via the urine, and the excretion is impaired in the presence of renal disease;Citation41 15% is excreted unaltered via the feces and 2% excreted unaltered via the urine.
There do not appear to be any ethnic variations in the pharmacokinetics or pharmacodynamics of dapagliflozin, though to date this has only been examined in studies in ChineseCitation42 and JapaneseCitation43 populations.
Pharmacodynamics
Dapagliflozin is associated with dose-dependent glycosuria and increased diuresis averaging 375 mL/day.Citation38,Citation39,Citation44,Citation45 There is a transient increase in urinary sodium excretion, but this does not appear to affect serum sodium. There is a transient increase in urinary uric acid excretion, but a sustained reduction in serum uric acid levels. The mechanism of reduced serum uric acid levels is unclear, but was recently proposed to be due to glycosuria-induced uric acid secretion via GLUT9 isoform 2 in the proximal tubule or inhibition of uric acid uptake at the collecting duct of the renal tubule.Citation46 Additionally, this could be due to the weight reduction associated with dapagliflozin.
Place in management
Dapagliflozin can be used as monotherapy or in combination with other oral hypoglycemic agents and insulin.Citation47 It can be used at any stage of the natural history of T2D.Citation48 It has now been placed within clinical guidelinesCitation8 as monotherapy and in combination therapy where the renal function is not chronic kidney disease (CKD) stage 3 or lower (<60 mL/min/1.73 m2).
While studies have used various doses of dapagliflozin, for the purposes of this review we only report the results of the 5 and 10 mg doses, because only these are currently licensed for therapy.
Monotherapy
Dapagliflozin has been studied as a monotherapy against placeboCitation49–Citation53 and versus metformin or placebo.Citation54 In these studies, the mean HbA1c reduction compared to placebo at 24 weeks was 0.66–1.45%, and weight reduction was 1.0–2.73 kg. There was a reduction in fasting glucose, and more patients achieved an HbA1c <7% in at least one study. Genital and urinary infections are more common (3.7% and 2.3% difference, respectively) compared to placebo.Citation53
Dual therapy
In dual therapy, dapagliflozin has been studied (against placebo) with metformin,Citation55–Citation60 metformin slow/extended release,Citation51,Citation54 glimepiride,Citation61,Citation62 pioglitazone,Citation63 sitagliptin,Citation64 and exenatide.Citation65
The highest reduction in HbA1c was seen when dapagliflozin was combined with metformin. Here, HbA1c reduced by 0.8% following 102 weeks of therapy,Citation56 compared to 0.5%–0.68% when combined with the other agents. The highest reduction in weight was seen when combined with sulfonylureas (SUs). When combined with glipizide, an average 4.4 kg in weight was lost compared to glipizide alone.Citation58–Citation60 In comparison, weight loss of 1.74 kg with metforminCitation56 and 1.8 kg with sitagliptin has been reported.Citation64 Across all studies, a 5%–10% increase in genital infections was reported. There are no human studies of dapagliflozin with glucagon-like peptide-1 analog therapy.
Triple therapy
Dapagliflozin has been used with metformin and sitagliptin,Citation64,Citation66 metformin and saxagliptin,Citation67 and metformin and an SU,Citation68 in triple combinations. HbA1c reduction of up to 0.6% and body weight reductions of 2.2 kg were reported. Genital and urinary infections were higher than in control groups.Citation60
With insulin
A number of studies of dapagliflozin in combination with insulin have been undertaken, and a further study is ongoing (ClinicalTrials.gov identifier: NCT02096705). Studies to date demonstrate benefits in HbA1c (−0.4%), weight (−3.33 kg), along with a stable insulin dose (−19.2 units when compared to increased requirements in the placebo group) at 104 weeks. Increased hypoglycemia rates were noted at 48 weeks, which leveled out by 104 weeks. There were higher rates of genital and urinary infections.Citation69–Citation71
Meta-analyses of dapagliflozin efficacy
There have been several meta-analyses on dapagliflozin to date.Citation72–Citation76 They suggest that treatment with dapagliflozin results in average improvement in HbA1c by 0.50%, fasting plasma glucose by 1.1 mmol/L, weight by 2 kg (4.5 kg when used with SUs), body mass index by 1.1%, and systolic/diastolic blood pressure (BP) by 4/2 mmHg, but is associated with an increased risk of genitourinary (odds ratio 3.50) and urinary tract (odds ratio 1.40) infections. These analyses are summarized in and show placebo-subtracted data from randomized controlled trials. By way of illustration, in one meta-analysis, study durations ranged between 12 and 104 weeks, with about 4,000 patients randomized across these randomized controlled trials (n=12).Citation72
Table 2 Meta-analyses of dapagliflozin
Benefits other than glucose
Weight
Weight loss is a benefit of therapy with dapagliflozin. It is thought to be through fluid loss in the initial phase of treatment, and then a more gradual loss through the net calorie deficit (typically 200–300 kcal per day). More recently, it has been shown that dapagliflozin therapy also reduces fat mass.Citation77,Citation78 Meta-analyses () show about a 1–2 kg weight reduction with this agent, and up to 5 kg when used in combination with SUs.
Hypertension
Investigators have noted a lowering of both systolic and diastolic BP in patients, and this is thought to be due to a combination of the diuretic effect of glycosuria, a natriuretic effect,Citation79 as well as via weight loss. The BP lowering is noted as early as 1–2 weeks of treatment initiation, and averages 4/2 mmHg.Citation29 The BP-lowering effects of dapagliflozin also manifest in patients on established antihypertensive therapy.Citation80
Sjöström et alCitation81 pooled safety data from 13 placebo-controlled Phase IIB/III studies, and found a slightly higher cumulative frequency of orthostatic reactions over 24 weeks with dapagliflozin (13.1% versus 11.3% with placebo), although adverse events due to orthostatic hypotension were rare (0.1%) and not serious.
Lipids
Dapagliflozin 10 mg OD has shown a mean change compared to placebo in total cholesterol of 2.5%, high-density lipoprotein of 3.3%, low-density lipoprotein of 3.9%, and triglycerides of −2.0%.Citation38 Meaningful long-term data are required to understand if this has any clinical impact.
Quality of life
Dapagliflozin-treated patients have been shown to have a high health-related quality-of-life (HRQOL, EQ-5D) score in several domains. This persisted for 102 weeks.Citation82 In a triple-therapy regimen, dapagliflozin showed greater improvement over placebo in weight change-related QOL, similar obesity-specific QOL, and greater treatment satisfaction.Citation83
Side effects
Genital (vulvovaginitis and balanitis) infections
Genital infections are by far the commonest side effect of dapagliflozin. Pooled data from 12 studiesCitation84 suggest infection rates of 5.7%, 4.8%, and 0.9% of study subjects treated with dapagliflozin 5 mg (n=1,145), 10 mg (n=1,193), or placebo (n=1,393), respectively. These were mainly mild or moderate infections that occurred in the first 6 months with low risk of recurrence or new emergent infections with prolonged therapy. Those with a previous history of these infections were at higher risk. These infections responded to standard oral antibiotic therapy. Therapy discontinuation was rare.
Urinary tract infections
Urinary tract infections have also been reported.Citation85 For dapagliflozin 5mg, 10 mg, or placebo, urinary tract infections were reported in 5.7%, 4.3%, and 3.7% of patients, respectively. Again, these were mild or moderate infections that responded to standard oral antibiotic therapy. Therapy discontinuation for this reason was also rare (0.3% with dapagliflozin and 0.1% with placebo). There was no increase in serious infections, eg, pyelonephritis.
Hypoglycemia
Hypoglycemia is not associated with dapagliflozin monotherapy, nor with dual therapy with metformin. It is associated with less hypoglycemia than SUs.Citation60 Dapagliflozin is however associated with an increased risk of hypoglycemia when used with other hypoglycemic agents, such as insulin and SUs.Citation73 It is advisable that the dose of these agents are downtitrated at initiation of dapagliflozin in order to reduce the risk of hypoglycemia at the onset of therapy.
Dehydration
Volume depletion (dehydration, hypotension, or hypovolemia) appears uncommon with dapagliflozin therapy. These were reported at 0.8% versus 0.4% for dapagliflozin 10 mg OD versus placebo (not statistically significant).
Other side effects
Some common side effects of dapagliflozin include back pain, dyslipidemia, dizziness, and hematocrit increase.Citation38
Potential for “off-target” effects that need further monitoring
Renal dysfunction
Mean estimated glomerular filtration rate (GFR) and creatinine clearance appear to fall in the first week with dapagliflozin therapy, but remain stable thereafter up to 104 weeks and return back to baseline after 2–6 months, whereas there is a continual slow decline seen with placebo. The changes in the first week are felt to be due to the diuretic and antihypertensive effect and a possible enhanced tubuloglomerular feedback.Citation86,Citation87 The creatinine changes are reversible,Citation88 and therefore do not appear to demonstrate an irreversible damage to the nephrons.
Cancer
The FDA had highlighted increased signals for cancer seen in early studies.Citation89 These were bladder and breast cancer. For bladder cancer, there were nine patients on dapagliflozin and one on placebo (0.3% versus 0.05%, respectively). All patients were male, and most were aged ≥60 years. There was a current or past smoking history in six patients, and five had microscopic hematuria at baseline from the dapagliflozin group.
There were nine patients who developed breast cancer (compared to one in the control group; 0.4% versus 0.1%, respectively). All were aged over 50 years (seven were over 60 years in age), all diagnoses were made within the first year of exposure, and two diagnoses were made within the first 8 weeks of exposure to dapagliflozin.
The duration of exposure to dapagliflozin was too short to be attributable to these cancers. The cancer risk was subsequently reevaluatedCitation89 and license-authorized by the FDA.Citation18
The safety of dapagliflozin has been examined in animal studies and also the effects of dapagliflozin and increased glucose on human bladder transitional cell carcinoma cell lines and in vivo xenograft models. There was no increased cancer with dapagliflozin in either of these models.Citation90 Furthermore, canagliflozin, a comparator medication, has not shown a higher incidence in these cancers, but this could be due to different monitoring precautions. However, longer-term human safety data are awaited.
Dosage
Dapagliflozin is marketed under the trade names of Forxiga® in the EUCitation91 and in the US as Farxiga®.Citation18 It is available in 5 mg or 10 mg strengths. It is also available in combination with metformin, under the trade name of Xigduo®, in 5 mg/850 mg or 10 mg/850 mg (dapagliflozin/metformin) strengths.Citation92
Contraindications
Age
Due to comorbidities, concurrent medication, and risk of volume depletion, dapagliflozin is to be used with caution in the ≥65-year age group; it is not recommended in those aged ≥75 years (). Barring these considerations, a placebo-controlled pool of nine double-blind Phase IIB/III studies shows dapagliflozin is safe and well tolerated in old patients with T2D up to 104 weeks of studied data.Citation93
Table 3 Differentiating features between dapa-/empa-/canagliflozin
Pregnancy
There are no trials to support the use of dapagliflozin in pregnancy. The manufacturers advise to discontinue treatment if pregnancy is detected.Citation38 In rat studies, toxicity to the developing kidney was observed in the time period corresponding to the second and third trimesters.
Use of dapagliflozin in the presence of renal, liver, and cardiovascular disease
Renal disease
The glycemic benefit of dapagliflozin persists in renal disease at least down to the level of CKD stage 3 (≥60 mL/min/1.73 m2).Citation87 It has not been tested in patients with more significant renal disease. Therapy also induces weight loss (−1.33 and −1.68 kg for 5 and 10 mg, respectively) and lowers BP. Importantly, there is no further deterioration in renal function in these patients. Therefore, there is potentially some benefit in CKD stage 3A (45–59 mL/min/1.73 m2) patients, but only of a modest nature. Longer-term studies are required.
Liver disease
T2D is associated with liver disease.Citation94,Citation95 Dapagliflozin has been tested in patients with varying degrees of liver disease at a dose of 10 mg. It was well tolerated, and there was no change in liver-function tests.Citation96 In clinical practice, no dosage modification is necessary with mild or moderate hepatic impairment. In patients with severe hepatic impairment, a starting dose of 5 mg is recommended by the manufacturers, and if the medication is well tolerated, the dose may be increased to 10 mg.Citation38
Cardiovascular disease
Dapagliflozin has been shown to be safe and efficacious in a large (964 patients) study of older T2D patients with coexistent cardiovascular disease.Citation97 Dapagliflozin improved glycemic control without an increase in hypoglycemia, promoted weight loss, and was well tolerated in the study subjects, and these benefits persisted for 2 years.Citation98
There is an ongoing study (Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events [DECLARE-TIMI58], ClinicalTrials.gov identifier: NCT01730534), further evaluating this subject and due to end in April 2019.
There is a 20-year simulation studyCitation99 estimating the long-term cardiovascular and microvascular outcomes that found relative reductions in the incidence of myocardial infarction, stroke, cardiovascular death, and all-cause death of 13.8%, 9.1%, 9.6%, and 5.0%, respectively, and relative reductions in the incidence of end-stage renal disease, foot amputation, and diabetic retinopathy of 18.7%, 13.0%, and 9.8%, respectively, when compared with standard care, thereby potentially showing additional benefits with adding dapagliflozin to standard therapy in reducing diabetes-related complications.
Use of dapagliflozin in other contexts
Ethnicity
Studies have now shown efficacy of dapagliflozin in ChineseCitation42,Citation53 and JapaneseCitation41,Citation43,Citation100 populations. These studies demonstrated that it is as effective at lowering glucose in these populations as it is in the white Caucasian population. There is, however, a need for studies in other ethnicities.
Type 1 diabetes
There is currently one study that has examined dapagliflozin in type 1 diabetes (T1D). This was a randomized, double-blind, placebo-controlled, parallel-group, Phase II trial (ClinicalTrials.gov identifier: NCT01498185). It was designed to explore dapagliflozin (1, 2.5, 5, 10 mg OD) as an add-on to insulin therapy in subjects with T1D. A dose-dependent increase in urinary glucose and a reduction in glycemic levels/variability and total daily dose of insulin was noted with dapagliflozin. Hypoglycemia was noted to be common in all treatment groups, and led to discontinuation in one patient on dapagliflozin 10 mg OD due to a major hypoglycemic event.Citation101
Another gliflozin, empagliflozin, has been studiedCitation102 in an open-label 8-week trial at a dose of 25 mg OD on renal hyperfiltration in T1D. Patients were divided into those with renal hyperfiltration (GFR ≥135 mL/min/1.73m2, n=27) or normal GFR (GFR 90–134 mL/min/1.73m2, n=13). A statistically significant reduction in total daily dose of insulin and in HbA1c was observed in both study groups. There is therefore proof of concept for the use of SGLT2 inhibitors for the therapy of T1D.
Drug–drug interactions
Dapagliflozin and loop diuretics are advised not to be used together, in order to avoid hypotension and dehydration. Lack of interaction has been shown between dapagliflozin and simvastatin, valsartan, warfarin, digoxin, rifampin, or mefenamic acid.Citation103,Citation104
Other considerations
Cost
Dapagliflozin is a cost-effective treatment when used in combination with insulin in patients with T2D. Van Haalen et alCitation105 used the Cardiff Diabetes Model to show a lower incidence of both micro- and macrovascular complications, a greater life expectancy, and an incremental benefit of 0.42 quality-adjusted life-years (QALYs). They conclude that the lifetime incremental cost per patient in those taking dapagliflozin was €2,293, with a €27,779 incremental cost-effectiveness ratio per life-year gained and a €5,502 incremental cost-utility ratio per QALY gained.
Sabale et alCitation106 studied the cost-effectives of dapagliflozin added to metformin compared to an SU added to metformin in patients with T2D and inadequate diabetes control. In this 52-week Scandinavian study, there was a gain in cost per QALY ranging between €4,769 and €7,944. The QALY gains for the dapagliflozin group were reported to be between 0.236 and 0.278, with incremental costs between €1,125 and €1,962.
In the UK, the National Institute for Health and Care Excellence has undertaken a technology appraisal (TA288) to evaluate the cost-effectiveness of dapagliflozin.Citation107
What does the future hold for the SGLT2 inhibitors?
Competition within this class of drug is proving to be fierce,Citation108 with several other SGLT2 inhibitors on the market: canagliflozin, developed by Janssen-Cilag (approved by the FDA in March 2013 and EMA in November 2013); empagliflozin, developed by Boehringer Ingelheim and Eli Lilly (approved by the EMA in March 2014 and the FDA in August 2014); ipragliflozin,Citation109 developed by Astellas Pharma and Kotobuki Pharmaceutical (approved by the MHLW, Japan in January 2014); tofogliflozin,Citation110 developed by Chugai Pharmaceutical (approved by the MHLW, Japan in March 2014), and luseogliflozin,Citation111 developed by Taisho Pharmaceutical (approved by the MHLW, Japan in March 2014).
There are yet more molecules, including ertugliflozinCitation112 and remogliflozin,Citation113 that have undergone development through Phase I, II, and III trials, with some, including remogliflozin, that have been discontinued. While the currently available SGLT2 inhibitors are broadly similar, there are some differentiating features ().
Conclusion
Dapagliflozin is the first class SGLT2 inhibitor licensed for use in adult patients with T2D. Large studies have demonstrated glycemic benefit with this therapy without significant hypoglycemia. There are also benefits with BP, weight, and lipids that may translate to cardiovascular benefit.
However, a number of important clinical questions remain to be answered, and we require further reassurance on issues relating to safety. Specifically, we see these as being:
the safety profile in patients over the age of 75 years and in pediatric patients
the efficacy profile in estimated GFR <60 mL/min/1.73 m2, particularly when other agents have these data already available
the long-term cardiovascular safety and risk-modification profile
cancer safety data, particularly bladder cancer, in view of the increased glucose load passing through the urinary system
combination use with glucagon-like peptide-1 agonists
use in T1D, pregnancy, monogenic forms of diabetes, and other conditions resulting in hyperglycemia
efficacy across different ethnicities.
Regardless, dapagliflozin makes a significant contribution to the range of therapies we require for the optimal management of patients with T2D. Its action is not affected by where a patient lies in the natural history of diabetes and regardless of residual β-cell function. It is also independent of the mechanisms through which other oral hypoglycemic agents work, such as increasing insulin or decreasing glucagon secretion, reducing gluconeogenesis or insulin resistance, slowing intestinal carbohydrate digestion/absorption, or increasing glucose deposition into liver, muscle or fat tissues. This allows for a versatile therapy that can be used in patients who are drug-naïve, as well as those on established blood glucose-lowering therapy.
Acknowledgments
The authors would like to thank Professor Clifford Bailey (Aston University, Birmingham, UK) for critical review of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- International Diabetes Federation (IDF) IDF Diabetes Atlas 6th ed Brussels IDF 2013 Available from: http://www.idf.org/diabetesatlas Accessed August 18, 2014
- Stratton IM Adler AI Neil HA Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study BMJ 2000 321 7258 405 412 10938048
- Gregg EW Li Y Wang J Changes in diabetes-related complications in the United States, 1990–2010 N Engl J Med 2014 370 16 1514 1523 24738668
- National Institute for Health and Care Excellence (NICE) Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period London NICE 2008 Available from: http://www.nice.org.uk/guidance/CG63 Accessed August 18, 2014
- National Institute for Health and Care Excellence (NICE) Type 2 Diabetes: The Management of Type 2 Diabetes London NICE 2009 Available from: http://www.nice.org.uk/Guidance/CG87 Accessed August 18, 2014
- International Diabetes Federation (IDF) Working Group Global Guideline for Type 2 Diabetes Brussels IDF 2012 Available from: http://www.idf.org/global-guideline-type-2-diabetes-2012 Accessed August 18, 2014
- Scottish Intercollegiate Guidelines Network (SIGN) Management of Diabetes: A National Clinical Guideline Edinburgh SIGN 2010 Available from: http://www.sign.ac.uk/guidelines/fulltext/116 Accessed August 18, 2014
- Garber AJ Abrahamson MJ Barzilay JI AACE comprehensive diabetes management algorithm 2013 Endocr Pract 2013 19 2 327 336 23598536
- Inzucchi SE Bergenstal RM Buse JB Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care 2012 35 6 1364 1379 22517736
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Imran SA Rabasa-Lhoret R Ross S Targets for glycemic control Can J Diabetes 2013 37 Suppl 1 S31 S34 24070959
- Cheung BM Ong KL Cherny SS Sham PC Tso AW Lam KSL Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006 Am J Med 2009 122 5 443 453 19375554
- Grant RW Devita NG Singer DE Meigs JB Polypharmacy and medication adherence in patients with type 2 diabetes Diabetes Care 2003 26 5 1408 1412 12716797
- Holman RR Assessing the potential for alpha-glucosidase inhibitors in prediabetic states Diabetes Res Clin Pract 1998 40 Suppl S21 S25 9740498
- Prentki M Nolan CJ Islet beta cell failure in type 2 diabetes J Clin Invest 2006 116 7 1802 1812 16823478
- Matthews DR Cull CA Stratton IM Holman RR Turner RC UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group Diabet Med 1998 15 4 297 303 9585394
- Diabetes UK Diabetes and obesity rates soar 2009 Available from: http://www.diabetes.org.uk/About_us/News_Landing_Page/Diabetes-and-obesity-rates-soar Accessed September 2, 2014
- Kahn SE Hull RL Utzschneider KM Mechanisms linking obesity to insulin resistance and type 2 diabetes Nature 2006 444 7121 840 846 17167471
- US Food and Drug Administration FDA approves Farxiga to treat type 2 diabetes [press release] Silver Spring, MD FDA 2014 [January 8]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829 Accessed October 13, 2014
- European Medicines Agency European Medicines Agency recommends authorisation of novel treatment for type-2 diabetes [press release] London EMA 2012 [April 20]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/04/news_detail_001499.jsp&mid=WC0b01ac058004d5c12012 Accessed October 13, 2014
- AstraZeneca Forxiga® receives regulatory approval in Japan for the treatment of type 2 diabetes [press release] London AstraZeneca 2014 [March 24]. Available from: http://www.astrazeneca.com/Media/Press-releases/Article/20140324--forxiga-japan-approval Accessed October 13, 2014
- Scottish Medicines Consortium Archived: dapagliflozin (Forxiga) 2013 Available from: http://www.scottishmedicines.org.uk/SMC_Advice/Advice/799_12_dapagliflozin_Forxiga/dapagliflozin_Forxiga Accessed October 13, 2014
- Arant BSJr Developmental patterns of renal functional maturation compared in the human neonate J Pediatr 1978 92 5 705 712 641617
- Alpern RJ Moe O Caplan M Seldin DW Seldin and Giebisch’s The Kidney: Physiology and Pathophysiology Oxford Academic 2012
- Wright EM Hirayama BA Loo DF Active sugar transport in health and disease J Intern Med 2007 261 1 32 43 17222166
- Wright EM Renal Na(+)-glucose cotransporters Am J Physiol Renal Physiol 2001 280 1 F10 F18 11133510
- Hediger MA Rhoads DB Molecular physiology of sodium-glucose cotransporters Physiol Rev 1994 74 4 993 1026 7938229
- Bakris GL Fonseca VA Sharma K Wright EM Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications Kidney Int 2009 75 12 1272 1277 19357717
- Wright EM Loo DD Hirayama BA Biology of human sodium glucose transporters Physiol Rev 2011 91 2 733 794 21527736
- Oliva RV Bakris GL Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors J Am Soc Hypertens 2014 8 5 330 339 24631482
- Chao EC Henry RR SGLT2 inhibition – a novel strategy for diabetes treatment Nat Rev Drug Discov 2010 9 7 551 559 20508640
- Jung CH Jang JE Park JY A novel therapeutic agent for type 2 diabetes mellitus: SGLT2 inhibitor Diabetes Metab J 2014 38 4 261 273 25215272
- Santer R Calado J Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target Clin J Am Soc Nephrol 2010 5 1 133 141 19965550
- McKusick VA Fanconi-Bickel syndrome 2006 Available from: http://www.omim.org/entry/227810 Accessed August 18, 2014
- Ehrenkranz JR Lewis NG Kahn CR Roth J Phlorizin: a review Diabetes Metab Res Rev 2005 21 1 31 38 15624123
- Anderson SL Marrs JC Dapagliflozin for the treatment of type 2 diabetes Ann Pharmacother 2012 46 4 590 598 22433611
- Bonner C Popescu I Queniat G The glucose transporter SGLT 2 is expressed in human pancreatic alpha cells and is required for proper control of glucagon secretion in type 2 diabetes Diabetes 2014 63 Suppl 1 A101
- Merovci A Solis-Herrera C Daniele G Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production J Clin Invest 2014 124 2 509 514 24463448
- Electronic Medicines Compendium Forxiga 5 mg and 10 mg film coated tablets 2014 Available from: http://www.medicines.org.uk/emc/medicine/27188/SPC/Forxiga+5+mg+%26+10+mg+film+coated+tablets Accessed August 18, 2014
- Kasichayanula S Liu X Lacreta F Griffen SC Boulton DW Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2 Clin Pharmacokinet 2014 53 1 17 27 24105299
- Kasichayanula S Liu X Zhang W Effect of a high-fat meal on the pharmacokinetics of dapagliflozin, a selective SGLT2 inhibitor, in healthy subjects Diabetes Obes Metab 2011 13 8 770 773 21435141
- Kasichayanula S Liu X Pe Benito M The influence of kidney function on dapagliflozin exposure, metabolism and pharmacodynamics in healthy subjects and in patients with type 2 diabetes mellitus Br J Clin Pharmacol 2013 76 3 432 444 23210765
- Yang L Li H Li H Pharmacokinetic and pharmacodynamic properties of single- and multiple-dose of dapagliflozin, a selective inhibitor of SGLT2, in healthy Chinese subjects Clin Ther 2013 35 8 1211.e2 1222.e2 23910664
- Kasichayanula S Chang M Hasegawa M Pharmacokinetics and pharmacodynamics of dapagliflozin, a novel selective inhibitor of sodium-glucose co-transporter type 2, in Japanese subjects without and with type 2 diabetes mellitus Diabetes Obes Metab 2011 13 4 357 365 21226818
- Komoroski B Vachharajani N Feng Y Li L Kornhauser D Pfister M Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus Clin Pharmacol Ther 2009 85 5 513 519 19129749
- Komoroski B Vachharajani N Boulton D Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects Clin Pharmacol Ther 2009 85 5 520 526 19129748
- Chino Y Samukawa Y Sakai S SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria Biopharm Drug Dispos 2014 35 7 391 404 25044127
- Aylsworth A Dean Z VanNorman C Nkemdirim Okere A Dapagliflozin for the treatment of type 2 diabetes mellitus Ann Pharmacother 2014 48 9 1202 1208 24951310
- Zhang L Feng Y List J Kasichayanula S Pfister M Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight Diabetes Obes Metab 2010 12 6 510 516 20518806
- Ferrannini E Ramos SJ Salsali A Tang W List JF Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial Diabetes Care 2010 33 10 2217 2224 20566676
- Bailey CJ Iqbal N T’Joen C List JF Dapagliflozin monotherapy in drug-naive patients with diabetes: a randomized-controlled trial of low-dose range Diabetes Obes Metab 2012 14 10 951 959 22776824
- Henry RR Murray AV Marmolejo MH Hennicken D Ptaszynska A List JF Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial Int J Clin Pract 2012 66 5 446 456 22413962
- Kaku K Inoue S Matsuoka O Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial Diabetes Obes Metab 2013 15 5 432 440 23194084
- Ji L Ma J Li H Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study Clin Ther 2014 36 1 84.e9 100.e9 24378206
- List JF Woo V Morales E Tang W Fiedorek FT Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes Diabetes Care 2009 32 4 650 657 19114612
- Bailey CJ Gross JL Pieters A Bastien A List JF Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial Lancet 2010 375 9733 2223 2233 20609968
- Bailey CJ Gross JL Hennicken D Iqbal N Mansfield TA List JF Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial BMC Med 2013 11 43 23425012
- Mudaliar S Henry RR Boden G Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin Diabetes Technol Ther 2014 16 3 137 144 24237386
- Nauck MA Del Prato S Meier JJ Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial Diabetes Care 2011 34 9 2015 2022 21816980
- Nauck MA Del Prato S Duran-Garcia S Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus was inadequately controlled with metformin Diabetes Obes Metab 2014 16 11 1111 1120 24919526
- Langkilde AM Nauck MA Prato SD Durability of dapagliflozin vs glipizide as add-on therapies in T2DM inadequately controlled on metformin: 4-year data Diabetologia 2013 56 1 S374
- Strojek K Yoon KH Hruba V Elze M Langkilde AM Parikh S Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial Diabetes Obes Metab 2011 13 10 928 938 21672123
- Strojek K Yoon KH Hruba V Sugg J Langkilde AM Parikh S Dapagliflozin added to glimepiride in patients with type 2 diabetes mellitus sustains glycemic control and weight loss over 48 weeks: a randomized, double-blind, parallel-group, placebo-controlled trial Diabetes Ther 2014 5 1 267 283 24920277
- Rosenstock J Vico M Wei L Salsali A List JF Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy Diabetes Care 2012 35 7 1473 1478 22446170
- Jabbour SA Hardy E Sugg J Parikh S Study G Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study Diabetes Care 2014 37 3 740 750 24144654
- Tatarkiewicz K Polizzi C Villescaz C Combined antidiabetic benefits of exenatide and dapagliflozin in diabetic mice Diabetes Obes Metab 2014 16 4 376 380 24251534
- Jabbour S Hardy E de Bruin TW Dapagliflozin helps reduce HbA1c and body weight in patients with type 2 diabetes as part of triple combination therapy: a subanalysis of 4 clinical studies Poster presented at: European Association for the Study of Diabetes Meeting September 23–27, 2013 Barcelona, Spain
- Rosenstock J Hansen L Zee PY Cook W Hirshberg B Iqbal N Dual add-on therapy in poorly controlled type 2 diabetes on metformin: randomized, double-blind trial of saxagliptin + dapagliflozin vs saxagliptin and dapagliflozin alone Diabetes 2014 63 Suppl 1A LB32 LB33
- Matthaei S Bowering K Rohwedder K Grohl A Johnsson E Improvement in glycemic control and reduction in body weight over 52 weeks with dapagliflozin as add-on therapy to metformin plus sulfonylurea Diabetes 2014 63 Suppl 1 A70 A71
- Wilding JP Norwood P T’Joen C Bastien A List JF Fiedorek FT A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment Diabetes Care 2009 32 9 1656 1662 19528367
- Wilding JP Woo V Soler NG Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial Ann Intern Med 2012 156 6 405 415 22431673
- Wilding JP Woo V Rohwedder K Sugg J Parikh S Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years Diabetes Obes Metab 2014 16 2 124 136 23911013
- Sun YN Zhou Y Chen X Che WS Leung SW The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta-analysis of randomised controlled trials BMJ Open 2014 4 4 e004619
- Zhang M Zhang L Wu B Song H An Z Li S Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials Diabetes Metab Res Rev 2014 30 3 204 221 24115369
- Goring S Hawkins N Wygant G Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis Diabetes Obes Metab 2014 16 5 433 442 24237939
- Musso G Gambino R Cassader M Pagano G A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials Ann Med 2012 44 4 375 393 21495788
- Vasilakou D Karagiannis T Athanasiadou E Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis Ann Intern Med 2013 159 4 262 274 24026259
- Bolinder J Ljunggren O Kullberg J Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin J Clin Endocrinol Metab 2012 97 3 1020 1031 22238392
- Bolinder J Ljunggren O Johansson L Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin Diabetes Obes Metab 2014 16 2 159 169 23906445
- Lambers Heerspink HJ de Zeeuw D Wie L Leslie B List J Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes Diabetes Obes Metab 2013 15 9 853 862 23668478
- Weber MA Mansfield T List JF Ptaszynska A Dapagliflozin lowered ambulatory blood pressure in patients with T2DM and hypertension inadequately controlled by a renin-angiotensin system blocker with or without another agent Diabetes 2014 63 Suppl 1 A266
- Sjöström D Ptaszynska A List JF Johnsson E Dapagliflozin lowers blood pressure in patients with type 2 diabetes Diabetes 2014 63 Suppl 1 A613
- Grandy S Hashemi M Langkilde AM Parikh S Sjöström CD Changes in weight loss-related quality of life among type 2 diabetes mellitus patients treated with dapagliflozin Diabetes Obes Metab 2014 16 7 645 650 24443876
- Grandy S Ryden A Sugg JE Rohwedder K Weight-related quality of life and treatment satisfaction among type 2 diabetes mellitus patients treated with dapagliflozin in triple-therapy regimen Diabetes 2014 63 Suppl 1 A204 A205
- Johnsson KM Ptaszynska A Schmitz B Sugg J Parikh SJ List JF Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin J Diabetes Complications 2013 27 5 479 484 23806570
- Johnsson KM Ptaszynska A Schmitz B Sugg J Parikh SJ List JF Urinary tract infections in patients with diabetes treated with dapagliflozin J Diabetes Complications 2013 27 5 473 478 23849632
- Thomson SC Rieg T Miracle C Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat Am J Physiol Regul Integr Comp Physiol 2012 302 1 R75 R83 21940401
- Kohan DE Fioretto P Tang W List JF Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control Kidney Int 2014 85 4 962 971 24067431
- Ptaszynska A Mansfield T Johnsson E Parikh SJ Yavin Y List JF Long-term renal safety with dapagliflozin treatment Diabetes 2014 63 Suppl 1 A267
- US Food and Drug Administration Background document: dapagli-flozin (BMS-512148, NDA 202293) 2013 Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm378079.pdf Accessed August 18, 2014
- Reilly TP Graziano MJ Janovitz EB Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus Diabetes Ther 2014 5 1 73 96 24474422
- European Medicines Agency Forxiga (dapagliflozin) 2014 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002322/human_med_001546.jsp&mid=WC0b01ac058001d124 Accessed August 18, 2014
- European Medicines Agency Xigduo (dapagliflozin/metformin) 2014 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002672/human_med_001721.jsp&mid=WC0b01ac058001d124 Accessed August 18, 2014
- Mansfield T Fioretto P Ptaszynska A Dapagliflozin is safe and well tolerated in older patients with T2DM Diabetes 2014 63 Suppl 1 A71
- Erbey JR Silberman C Lydick E Prevalence of abnormal serum alanine aminotransferase levels in obese patients and patients with type 2 diabetes Am J Med 2000 109 7 588 590 11063962
- Harris EH Elevated liver function tests in type 2 diabetes Clin Diabetes 2005 23 3 115 119
- Kasichayanula S Liu X Zhang W Pfister M LaCreta FP Boulton DW Influence of hepatic impairment on the pharmacokinetics and safety profile of dapagliflozin: an open-label, parallel-group, single-dose study Clin Ther 2011 33 11 1798 1808 22030444
- Leiter LA Cefalu WT de Bruin TW Gause-Nilsson I Sugg J Parikh SJ Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension J Am Geriatr Soc 2014 62 7 1252 1262 24890683
- Gause-Nilsson I Bruin TW Sugg JE Parikh SJ Johnsson E Leiter LA Two-year efficacy and safety of dapagliflozin for T2DM patients with a history of cardiovascular disease Diabetes 2014 63 Suppl 1 A271
- Dziuba J Alperin P Racketa J Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes Diabetes Obes Metab 2014 16 7 628 635 24443793
- Kaku K Inoue S Matsuoka O Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial Diabetes Obes Metab 2013 15 5 432 440 23194084
- Henry RR Rosenstock J Chalamandaris AG Kasichayanula S Bogle A Griffen SC Exploring the potential of dapagliflozin in type 1 diabetes: phase 2A pilot study Diabetes 2013 62 Suppl 1A LB70
- Cherney DZ Perkins BA Soleymanlou N Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus Circulation 2014 129 5 587 597 24334175
- Kasichayanula S Chang M Liu X Lack of pharmacokinetic interactions between dapagliflozin and simvastatin, valsartan, warfarin, or digoxin Adv Ther 2012 29 2 163 177 22271159
- Kasichayanula S Liu X Griffen SC Lacreta FP Boulton DW Effects of rifampin and mefenamic acid on the pharmacokinetics and pharmacodynamics of dapagliflozin Diabetes Obes Metab 2013 15 3 280 283 23061428
- van Haalen HG Pompen M Bergenheim K McEwan P Townsend R Roudaut M Cost effectiveness of adding dapagliflozin to insulin for the treatment of type 2 diabetes mellitus in the Netherlands Clin Drug Investig 2014 34 2 135 146
- Sabale U Ekman M Granström O Bergenheim K McEwan P Cost-effectiveness of dapagliflozin (Forxiga) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries Prim Care Diabetes Epub 2014 5 16
- National Institute for Health and Care Excellence (NICE) Dapagliflozin in Combination Therapy for Treating Type 2 Diabetes London NICE 2013 Available from: http://www.nice.org.uk/Guidance/TA288 Accessed August 18, 2014
- Kurosaki E Ogasawara H Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data Pharmacol Ther 2013 139 1 51 59 23563279
- Poole RM Dungo RT Ipragliflozin: first global approval Drugs 2014 74 5 611 617 24668021
- Poole RM Prossler JE Tofogliflozin: first global approval Drugs 2014 74 8 939 944 24848755
- Markham A Elkinson S Luseogliflozin: first global approval Drugs 2014 74 8 945 950 24848756
- Diamant M Morsink LM SGLT2 inhibitors for diabetes: turning symptoms into therapy Lancet 2013 382 9896 917 918 23850056
- Kapur A O’Connor-Semmes R Hussey EK First human dose-escalation study with remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2 (SGLT2), in healthy subjects and in subjects with type 2 diabetes mellitus BMC Pharmacol Toxicol 2013 14 26 23668634
- Yale JF Bakris G Cariou B Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease Diabetes Obes Metab 2013 15 5 463 473 23464594
- Barnett AH Mithal A Manassie J Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial Lancet Diabetes Endocrinol 2014 2 5 369 384 24795251