?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Human pancreatic tumors are known to be highly resistant to nutrient starvation, and this prolongs their survival in the hypovascular (austere) tumor microenvironment. Agents that retard this tolerance to nutrient starvation represent a novel antiausterity strategy in anticancer drug discovery. (+)-Grandifloracin (GF), isolated from Uvaria dac, has shown preferential toxicity to PANC-1 human pancreatic cancer cells under nutrient starvation, with a PC50 value of 14.5 μM. However, the underlying mechanism is not clear. In this study, GF was found to preferentially induce PANC-1 cell death in a nutrient-deprived medium via hyperactivation of autophagy, as evidenced by a dramatic upregulation of microtubule-associated protein 1 light chain 3. No change was observed in expression of the caspase-3 and Bcl-2 apoptosis marker proteins. GF was also found to strongly inhibit the activation of Akt, a key regulator of cancer cell survival and proliferation. Because pancreatic tumors are highly resistant to current therapies that induce apoptosis, the alternative cell death mechanism exhibited by GF provides a novel therapeutic insight into antiausterity drug candidates.
Introduction
Human pancreatic cancer is the most fatal form of cancer worldwide, with a 5-year survival rate of less than 5%.Citation1 Each year, approximately 29,000 people are diagnosed with pancreatic cancer in Japan.Citation2 The annual mortality rate from this malignancy closely approximates the annual incidence rate.Citation3,Citation4 Once diagnosed, the average life expectancy is 6 months. It is the fifth leading cause of cancer-related mortality in Japan and other industrialized countries.Citation4 Until now, no effective treatment has been available.Citation5,Citation6 Human pancreatic cancer shows resistance to most conventional chemotherapeutic drugs in clinical use, such as paclitaxel, doxorubicin, and cisplatin.Citation7 At present, gemcitabine and S-1 (tegafur + gimeracil + oteracil potassium) are the only standard regimens for advanced pancreatic cancer.Citation8–Citation11 Therefore, effective chemotherapeutic agents against this disease are urgently needed. Human pancreatic tumors are hypovascular in nature,Citation12 causing a limited supply of nutrients and oxygen to reach the aggressively proliferating tumor cells.Citation13 As tumor cells proliferate, the demand for essential nutrients and oxygen exceeds the supply. Consequently, large areas of tumor survive under the hostile environment characterized by nutrient and oxygen starvation. Yet, human pancreatic tumor cells show the extraordinary ability to tolerate such extreme states through the modulation of energy metabolism.Citation14 While normal human cells die within 24 hours under nutrient starvation, some human pancreatic cancer cell lines can survive up to 72 hours in the complete absence of nutrients such as glucose, amino acids, and serum.Citation14 This remarkable tolerance to nutrient starvation is one of the key factors for survival and progression of pancreatic tumors. Therefore, agents that retard the tolerance of cancer cells to nutrient starvation represent a novel approach in anticancer drug discovery.Citation15 Using this hypothesis, we established a novel antiausterity strategy for the discovery of anticancer agents that preferentially target tolerance to nutrient starvation by cancer cells. Previous work on this strategy has led to the discovery of a number of potent anticancer agents, such as arctigenin,Citation15 angelmarin,Citation16 kayeassamins A–I,Citation17,Citation18 and panduratins,Citation19,Citation20 from the medicinal plants used in Japanese Kampo medicine and Southeast Asian countries.Citation21 Interestingly, these compounds also strongly suppressed tumor growth in a xenograft model using pancreatic cancer cells.Citation15 In our continued work, we recently found that a dichloromethane extract of the stem of Uvaria dac preferentially inhibited PANC-1 human pancreatic cancer cell survival under nutrient deprivation.Citation22 Work-up of this bioactive extract led to the discovery of (+)-grandifloracin (GF) as a potent antiausterity agent that showed preferential toxicity to PANC-1 cells with a PC50 value of 14.5 μM. In this study, we explored the underlying mechanism of GF-induced modulation of key regulatory proteins involved in tolerance to nutrient starvation in PANC-1 cells.
Materials and methods
Reagents
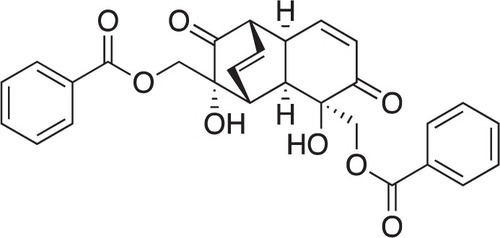
GF () was isolated from the stems of U. dac as described previously.Citation22 GF purity was determined to be 95% by high-performance liquid chromatography. Conventional anticancer agents, ie, gemcitabine, 5-fluorouracil, 2-deoxyglucose, paclitaxel, podophyllotoxin, and camptothecin, were purchased from Sigma-Aldrich (St Louis, MO, USA). Each reagent was dissolved in dimethyl sulfoxide as a 10 mM stock solution and stored at −30°C until use. Dilution to give the desired concentration was performed prior to treatment. Dulbecco’s phosphate-buffered saline was purchased from Nissui Pharmaceutical (Tokyo, Japan). Dulbecco’s Modified Eagle’s Medium. (DMEM) was purchased from Wako Pure Chemical (Osaka, Japan). Sodium bicarbonate, potassium chloride, magnesium sulfate, sodium dihydrogen phosphate, potassium dihydrogen phosphate, sodium chloride, and phenol red were purchased from Wako Pure Chemical. HEPES was purchased from Dojindo Laboratories (Kumamoto, Japan). Fetal bovine serum was purchased from Nichirei Biosciences Inc. (Tokyo, Japan). Antibiotic/antimycotic solution was purchased from Sigma-Aldrich. The WST-8 cell counting kit was purchased from Dojindo Laboratories. Cell culture flasks and 96-well plates were obtained from Falcon Becton Dickinson Labware (BD Biosciences, San Jose, CA, USA). Nutrient-deprived medium was prepared according to a previously described protocol.Citation14 Rabbit polyclonal antibodies to Akt, phosphoryl Akt (Ser473), mammalian target of rapamycin (mTOR), phosphoryl mTOR (Ser2448), Bcl-2, caspase 3, and LC3A/B were purchased from Cell Signaling Technology (Danvers, MA, USA). A goat polyclonal antibody to actin was purchased from Santa Cruz Biotechnologies (Dallas, TX, USA). Horseradish peroxidase-conjugated goat polyclonal anti-rabbit and rabbit polyclonal anti-goat immunoglobulins were purchased from DakoCytomation (Glostrup, Denmark).
Cell line
The PANC-1 (RBRC-RCB2095) cell line was purchased from the Riken BRC Cell Bank (Ibaraki, Japan) and maintained in standard DMEM with 10% fetal bovine serum supplement, 100 U/mL of penicillin G, 0.1 mg/mL of streptomycin, and 0.25 μg/mL of amphotericin B.
Preferential cytotoxic activity
The in vitro preferential cytotoxicity of GF was determined using a previously described procedure with a slight modification.Citation22 In brief, human pancreatic cancer cells were seeded in 96-well plates (1.5 × 10Citation4/well) and incubated in fresh DMEM at 37°C under humidified 5% CO2 and 95% air for 24 hours. After the cells were washed with Dulbecco’s phosphate-buffered saline, the medium was changed to serially diluted test samples in DMEM or nutrient-deprived medium, with the control and blank in each plate. After 24 hours of incubation, 100 μL of DMEM containing 10% WST-8 cell counting kit solution was directly added to each well. After 3 hours of incubation, absorbance at 450 nm was measured (EnSpire® Multilabel Reader, PerkinElmer, Waltham, MA, USA). Cell viability was calculated from the mean values for three wells using the following equation:
Morphologic assessment
Cells were seeded in 60 mm dishes (1 × 10Citation6 cells) and incubated in a humidified CO2 incubator for 24 hours to allow cell attachment. The cells were then washed twice with Dulbecco’s phosphate-buffered saline and treated with 25 μM GF in DMEM, nutrient-deprived medium, and the control. After 12 and 24 hours of incubation, the cells were treated with fluorescein-labeled annexin V and propidium iodide, and cell morphology was observed using an inverted Nikon Eclipse TS 100 microscope (40× objective) with phase-contrast and fluorescence modes. Microscopic images were taken using a Nikon DS-L-2 camera directly attached to the microscope.
Annexin V/dead cell assay
The annexin V/dead cell assay was performed in a Muse™ cell analyzer (Merck Millipore, Billerica, MA, USA) utilizing a Muse annexin V and dead cell kit. The assay utilizes phycoerythrin-labeled annexin V to detect phosphatidylserine on the external membrane of apoptotic cells. The kit contains the DNA dye, 7-aminoactinomycin D (7-AAD) for the exclusion of nonviable cells. Four populations of cells can be distinguished in this assay: nonapoptotic cells, annexin V (−) and 7-AAD (−); early apoptotic cells, annexin V (+) and 7-AAD (−); late-stage apoptotic and dead cells, annexin V (+) and 7-AAD (+); and necrotic nuclear debris, annexin V (−) and 7-AAD (+). The assay was performed according to the manufacturer’s protocol. In brief, the cells were seeded in 60 mm dishes (1 × 10Citation6 cells) and incubated in a humidified CO2 incubator for 24 hours to allow cell attachment. The cells were then washed twice with Dulbecco’s phosphate-buffered saline and treated with 12.5 μM GF, 25 μM GF, or the control of nutrient-deprived medium for the indicated time periods. The cells were then harvested from the dish with trypsin to give single cell suspensions. Finally, 100 μL of annexin V/dead reagent and 100 μL of a single cell suspension were mixed in a microtube and incubated for 20 minutes at room temperature in the dark. The cells were then analyzed using the Muse cell analyzer, and 5,000 cell events were collected for each sample. The images were acquired as the screen-shots of the processed data and the text size was edited for clarity.
Western blot analysis
Proteins were separated by gel electrophoresis on a polyacrylamide gel containing 0.1% sodium dodecyl sulfate and transferred to polyvinylidene fluoride membranes. The membranes were blocked with Block Ace® (DS Pharma Medical, Suita, Japan), washed with Dulbecco’s phosphate-buffered saline containing 0.1% polyoxyethylene (20) sorbitan monolaurate (Wako Pure Chemical), and incubated overnight with primary antibodies diluted in Can Get Signal® (Toyobo, Osaka, Japan). After washing, the membranes were incubated for 45 minutes at room temperature with horseradish peroxidase-conjugated anti-rabbit or anti-goat immunoglobulins as the secondary antibody. The bands were detected with an enhanced chemiluminescence solution (PerkinElmer). The images were analyzed using Image Studio software version 3.1.4.
Statistical analysis
Statistical analysis was performed using the unpaired Student’s t-test. A P-value<0.05 was considered to be statistically significant.
Results
GF showed preferential cytotoxicity in a concentration-dependent manner
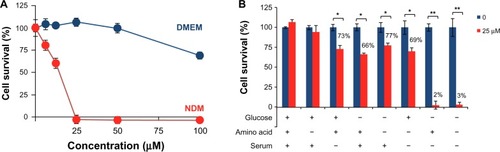
The PANC-1 cell line is highly resistant to nutrient deprivation and shows an extraordinary ability to survive for >48 hours even under complete nutrient starvation. GF remarkably diminished tolerance to nutrient starvation in a concentration-dependent manner (). Cells exposed to GF at 25 μM showed 100% cell death within 24 hours in nutrient-deprived medium, with a PC50 value of 14.5 μM; however, no toxicity was observed in nutrient-rich DMEM.
Figure 2 Effect of (+)-grandifloracin on PANC-1 cell survival after 24 hours in NDM and normal medium (DMEM). (A) Effect of (+)-grandifloracin concentration on cell survival in NDM and DMEM. (B) Effects of medium components, ie, glucose, amino acids, and serum. Data are expressed as the mean ± standard deviation, n=3. *P<0.05; **P<0.01 indicate significant difference from the control.
GF sensitized PANC-1 cell death under glucose/serum-deprived conditions
To determine the conditions under which GF induces sensitivity to nutrient starvation resulting in cell death, the PANC-1 cells were treated with 25 μM GF under various nutrient conditions of glucose, amino acids, and serum. Cell viability was measured 24 hours after treatment. As shown in , GF was found to be toxic during glucose or serum deprivation, irrespective of the presence or absence of amino acids. In the presence of glucose and serum, cell viability was 100%. However, removal of serum led to a decrease in cell viability to 73% and 69% in the presence or absence of amino acids, respectively. Similarly, removal of glucose also led to a significant decrease in cell viability to 66%. Removal of both glucose and serum decreased cell viability to 2%.
Conventional anticancer agents are ineffective against PANC-1 cells in nutrient-deprived medium
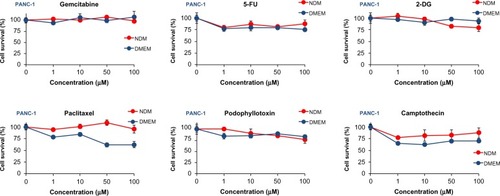
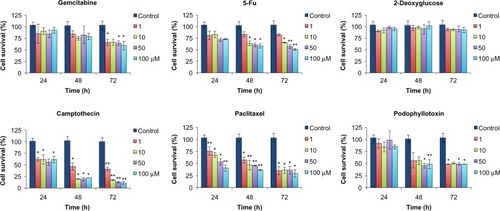
The preferential cytotoxicity of GF was compared with that of several conventional anticancer agents, including gemcitabine, 5-fluorouracil, 2-deoxyglucose, paclitaxel, camptothecin, and podophyllotoxin, using PANC-1 cells grown in nutrient-deprived medium versus DMEM (). All tested agents were virtually inactive in nutrient-deprived medium; however, paclitaxel and camp-tothecin showed weak activity in nutrient-rich DMEM at the maximum tested dose of 100 μM after 24 hours. Because some of the conventional anticancer agents showed weak activity in DMEM, their effects during prolonged treatment were also evaluated by monitoring their cytotoxicity after 24, 48, and 72 hours. As shown in , gemcitabine and 5-fluorouracil weakly decreased cell viability 72 hours after treatment. However, these compounds did not show a clear concentration-dependent effect. 2-Deoxyglucose was completely inactive. Paclitaxel and podophyllotoxin were found to reduce cell viability after 72 hours, but the effect was not concentration-dependent. On the other hand, camptothecin exhibited strong activity with cell viability of <25% at 10 μM 48 hours after treatment.
Assessment of GF-induced apoptosis
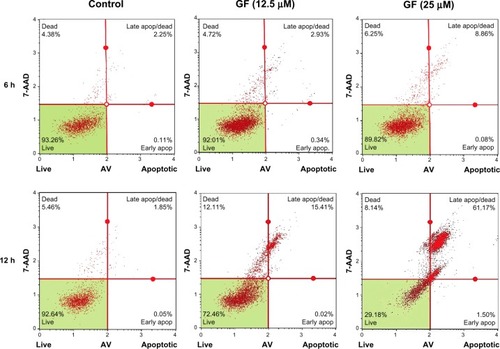
To investigate whether GF-induced cell death in nutrient-deprived medium involves apoptosis, the cell morphology was examined. As shown in , at 25 μM, GF induced a marked change in PANC-1 cell morphology within 8 hours. However, the cells lacked the classical signs of apoptosis, such as shrinkage or fragmentation into membrane-bound apoptotic bodies. Instead, swelling and rupture of cell membranes and disruption of cellular organelles appeared to be closer to a necrotic-type cell death. Staining with annexin V/propidium iodide reagent showed an increased population of cells containing Annexin V (green fluorescence) and propidium iodide (red fluorescence). Annexin V is a Ca2+-dependent phospholipid-binding protein with high affinity for phosphatidylserine. Translocation of phosphatidylserine to the external cell surface occurs both in apoptosis and necrosis. We further performed flow cyto-metric analysis of cells treated with GF utilizing the Muse Annexin V and dead cell kit, which contains 7-AAD as a dye for exclusion of nonviable cells. 7-AAD is impermeable to viable cells and does not stain viable or early apoptotic cells. In late apoptotic and necrotic cells, the integrity of the cell membrane decreases, which allows 7-AAD to pass through the membranes, intercalate into nucleic acids and DNA, and display red fluorescence. As shown in , the cells are predominantly stained with both Annexin V and 7-AAD within 12 hours in a concentration-dependent manner. In the control of nutrient-deprived medium, more than 90% of the cells survived. After treatment with GF, this cell population decreased markedly to 72% (12.5 μM) and 29% (25 μM), with an increase in the late apoptotic/necrotic cell population from 1% (control) to 15% (12.5 μM) and 61% (25 μM), respectively (). We further performed Western blot analysis to examine GF-induced apoptosis. Treatment with GF neither led to cleavage of caspase-3 nor showed Bcl-2 inhibition (data not shown).
Figure 5 Effect of GF (25 μM) on PANC-1 cell morphology after 8 hours in N Phase-contrast (upper left), fluorescent (lower left), and merged (lower images of PANC-1 cells.
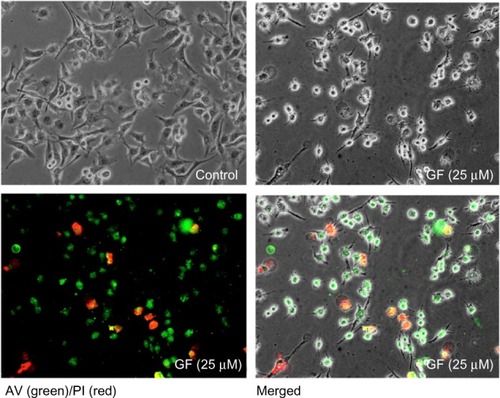
Figure 6 Assessment of apoptosis by GF. PANC-1 cells were treated with vehicle or GF (12.5 μM and 25 μM) in nutrient-deprived medium. After treatment (6 hours and 12 hours), the cells were treated with Annexin V/7-AAD reagent and cytometric analysis was performed.
GF inhibits Akt/mTORactivation
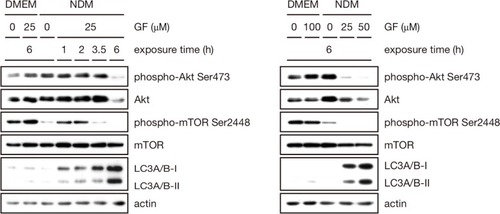
Akt is a prosurvival factor that is activated in a majority of tumors and regulates cellular functions such as cell cycle progression, cell migration, invasion, and angiogenesis. High Akt activation has been associated with tolerance to nutrient starvation and survival in an austerity environment.Citation14 Therefore, the effect of GF on Akt activation was investigated by Western blot analysis. As shown in , Akt phosphorylation at Ser473 was completely inhibited by GF in a concentration-dependent as well as time-dependent manner in nutrient-deprived medium. GF also strongly suppressed total Akt. mTOR is a downstream effector of Akt and is frequently activated in various cancer types, where it is involved in tumor progression and metastasis.Citation23 Therefore, we tested whether GF has any modulatory activity against mTOR activation. As shown in , addition of 25 μM GF completely inhibited mTOR phosphorylation at Ser2448 6 hours after treatment.
GF-induced autophagy in PANC-1 cells
Because no apoptotic cell death was observed in cells treated with GF, we speculated that GF might have induced autophagy. Therefore, expression of the autophagic marker microtubule-associated protein-light chain 3 (LC3), the cytoplasmic form of LC3-I (16 kDa), and the preautophagosomal and autophagosomal membrane-bound form of LC3-II (14 kDa) was examined by Western blot. The PANC-1 cells were cultured for varying time periods at different GF concentrations. As shown in , no apparent differences were observed in LC3-I and LC3-II expression in the controls of both DMEM and nutrient-deprived medium. However, treatment with GF led to an enhancement in the expression of both LC3-I and LC3-II in a concentration-dependent as well as time- dependent manner. In nutrient-deprived medium, treatment with 25 μM GF led to incremental increases of eight-fold, 13-fold, and 22-fold in LC3-I expression with respect to the control after 2, 3.5, and 6 hours, respectively. Similarly, increases of 141-fold, 146-fold, and 659-fold in LC3-II expression were observed with respect to the control after 2, 3.5, and 6 hours, respectively.
Discussion
Pancreatic cancer is associated with the lowest 5-year survival rate of any known cancer and is largely resistant to conventional chemotherapeutic agents. Although the median survival rate of the disease is only 6 months, some recent progress has been reported with FOLFIRINOX (folinic acid + 5-fluorouracil + irinotecan + oxaliplatin) and erlotinib.Citation24,Citation25 However, new alternatives are urgently needed to improve the clinical outcome for patients diagnosed with pancreatic cancer. Pancreatic tumors are hypovascular and supply only a limited amount of essential nutrients and oxygen to aggressively proliferating cells. Consequently, these cells live in a hostile microenvironment under chronic metabolic stress conditions. For survival, these cells activate adaptive mechanisms such as autophagy.Citation26,Citation27
Autophagy is a homeostatic and evolutionarily conserved cellular pathway whereby cellular proteins and organelles are engulfed by autophagosomes, digested in lysosomes, and recycled in order to sustain cellular metabolism.Citation28 The process is activated in response to nutrient and energy starvation and acts as a survival mechanism to cope with diverse stresses in the tumor microenvironment.Citation28 Autophagy has been reported to be activated in colorectal cancer cells and to contribute to the tolerance to nutrient deprivation.Citation29 However, in the clinical setting, autophagy has been reported to serve as an alternative mechanism of programmed cell death that leads to tumor suppression.Citation30 One of the notable examples of a proautophagic cytotoxic drug that has demonstrated therapeutic benefits in several apoptosis-resistant cancer types in a clinical trial is temozolomide.Citation30 Several mechanisms have been suggested to explain the role of autophagy in suppression of tumorigenesis. Maintenance of genomic stability by clearance of damaged mitochondria and protein aggregates is considered one of the major mechanisms of tumor suppression by autophagy.Citation31 Further, excessive metabolic stresses in the tumor microenvironment often lead to necrotic cell death. Activation of autophagy under such circumstances prevents necrotic cell death and suppresses inflammation, which is known to increase tumor growth. Because the therapeutic goal of cancer treatment has been to trigger tumor-selective cell death, accelerating autophagy in apoptosis-resistant cancer cells would be an attractive alternative strategy in cancer therapy.
In the present study, GF does not appear to induce apoptosis but rather to operate by an alternative mechanism of programmed cell death, ie, autophagy. A marked activation of the autophagy marker LC3-II was observed after treatment with GF in a concentration-dependent and time-dependent manner. This was observed not only under nutrient-deprived conditions but also under nutrient-rich conditions, suggesting that GF is indeed an activator of autophagy. However, the effect of GF in nutrient-deprived medium was found to be highly significant compared with that in the control of nutrient-deprived medium at concentrations of 25 μM and 50 μM within 6 hours. Although a basal level of LC3-II protein is observed in the control of nutrient-deprived medium, it is activated within one hour after treatment with GF, which was found to be hyperactivated with respect to time as shown in . This suggests that GF-induced autophagy mediates the death of PANC-1 cells preferentially during nutrient starvation.
The serine/threonine kinase Akt/mTOR pathway is constitutively activated in a majority of human pancreatic cancer cell lines. Activation of this pathway has been attributed to the survival of cancer cells in the heterogeneous tumor microenvironment, which confers resistance to chemotherapy and radiotherapy.Citation14 Akt has been found to be overexpressed in pancreatic cancer cells during extreme nutrient deprivation. Increased Akt expression is one of the austerity markers that enables tumor cells to survive and proliferate in the hostile hypovascular tumor microenvironment.Citation14 Therefore, inhibition of the Akt pathway might have therapeutic value in cancer patients. A number of antiausterity agents such as arctigenin, kigamicin D, and pyrvinium pamoate have been found to strongly suppress Akt activation, which suggests that inhibition of Akt phosphorylation by these compounds is partially responsible for the preferential cytotoxicity observed under nutrient deprivation.Citation15,Citation32,Citation33 However, the manner in which Akt inhibition affects downstream signaling under austerity conditions remains largely unknown. In the present study, GF suppressed both total Akt and phospho(Ser473) Akt in a time-dependent as well as concentration-dependent manner. It has been reported that mTOR is frequently inappropriately activated in many cancer types, and development of drugs that inhibit mTOR is an alluring therapeutic target in cancer therapy. mTOR is a downstream effector of the PI3K/AKT pathway and is composed of two distinct complexes, ie, mTORC1 and mTORC2. In the present study, although the effects of GF on each multiprotein complex were not elucidated, complete inhibition of mTOR phosphorylation at Ser2448 was observed. mTOR inhibitors, such as temsirolimus and everolimus, have been approved by the US Food and Drug Administration for the treatment of renal cell carcinoma, primitive neuroectodermal tumor, and giant cell astrocytoma.Citation34 In this regard, GF is a dual inhibitor of the principal survival factors, Akt and mTOR, in tumors. Because pancreatic tumors are highly resistant to current chemotherapeutic agents that induce apoptosis, induction of an alternative cell death mechanism exhibited by GF represents a novel attractive candidate for preclinical evaluation.
Acknowledgments
This work was supported by a grant from the Toyama Support Center for Young Principal Investigators in Advanced Life Sciences, Japan, and a Grant in Aid for Scientific Research (No 24510314) from the Japan Society for the Promotion of Science.
Disclosure
The authors report no conflict of interest in this work.
References
- Asuthkar S Rao JS Gondi CS Drugs in preclinical and early-stage clinical development for pancreatic cancer Expert Opin Investig Drugs 2012 21 143 152
- The Editorial Board of the Cancer Statistics in Japan Cancer Statistics in Japan 2012 Tokyo, Japan Foundation for Promotion Cancer Research 2012
- Hidalgo M Pancreatic cancer N Engl J Med 2010 362 1605 1617 20427809
- Jemal A Bray F Center MM Ferlay J Ward E Forman D Global cancer statistics CA Cancer J Clin 2011 61 69 90 21296855
- Lionetto R Pugliese V Bruzzi P Rosso R No standard treatment is available for advanced pancreatic cancer Eur J Cancer 1995 31 882 887 7646915
- Philip PA Benedetti J Corless CL Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest oncology group-directed intergroup trial S0205 J Clin Oncol 2010 28 3605 3610 20606093
- Arumugam T Ramachandran V Fournier KF Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer Cancer Res 2009 69 5820 5828 19584296
- Qiu MT Ding XX Hu JW Tian HY Yin R Xu L Fixed-dose rate infusion and standard rate infusion of gemcitabine in patients with advanced non-small-cell lung cancer: a meta-analysis of six trials Cancer Chemother Pharmacol 2012 70 861 873 23053260
- Conroy T Desseigne F Ychou M FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer N Engl J Med 2011 364 1817 1825 21561347
- Von Hoff DD Ramanathan RK Borad MJ Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial J Clin Oncol 2011 29 4548 4554 21969517
- Ko AH Venook AP Bergsland EK A Phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer Cancer Chemother Pharmacol 2010 66 1051 1057 20130876
- Sakamoto H Kitano M Suetomi Y Maekawa K Takeyama Y Kudo M Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas Ultrasound Med Biol 2008 34 525 532 18045768
- Feig C Gopinathan A Neesse A Chan DS Cook N Tuveson DA The pancreas cancer microenvironment Clin Cancer Res 2012 18 4266 4276 22896693
- Izuishi K Kato K Ogura T Kinoshita T Esumi H Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy Cancer Res 2000 60 6201 6207 11085546
- Awale S Lu J Kalauni SK Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation Cancer Res 2006 66 1751 1757 16452235
- Awale S Nakashima EMN Kalauni SK Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation Bioorg Med Chem Lett 2006 16 581 583 16288865
- Win NN Awale S Esumi H Tezuka Y Kadota S Novel anticancer agents, kayeassamins A and B from the flower of Kayea assamica of Myanmar Bioorg Med Chem Lett 2008 18 4688 4691 18640837
- Win NN Awale S Esumi H Tezuka Y Kadota S Novel anticancer agents, kayeassamins C–I from the flower of Kayea assamica of Myanmar Bioorg Med Chem 2008 16 8653 8660 18725180
- Win NN Awale S Esumi H Tezuka Y Kadota S Bioactive secondary metabolites from Boesenbergia pandurata of Myanmar and their preferential cytotoxicity against human pancreatic cancer PANC-1 cell line in nutrient-deprived medium J Nat Prod 2007 70 1582 1587 17896818
- Win NN Awale S Esumi H Tezuka Y Kadota S Panduratins D–I, novel secondary metabolites from rhizomes of Boesenbergia pandurata Chem Pharm Bull 2008 56 491 496 18379096
- Awale S Linn TZ Than MM Swe T Saiki I Kadota S The healing art of traditional medicines in Myanmar J Trad Med 2006 23 47 68
- Awale S Ueda J Athikomkulchai S Antiausterity agents from Uvaria dac and their preferential cytotoxic activity against human pancreatic cancer cell lines in a nutrient-deprived condition J Nat Prod 2012 75 1177 1183 22676269
- Guertin DA Sabatini DM Defining the role of mTOR in cancer Cancer Cell 2007 12 9 22 17613433
- Conroy T Gavoille C Samalin E Ychou M Ducreux M The role of the FOLFIRINOX regimen for advanced pancreatic cancer Curr Oncol Rep 2013 15 182 189 23341367
- Saif MW Advancements in the management of pancreatic cancer: 2013 JOP 2013 14 112 118 23474549
- Schönthal AH Endoplasmic reticulum stress and autophagy as targets for cancer therapy Cancer Lett 2009 275 163 169 18692955
- Schönthal AH Gliomas: Aggravating endoplasmic reticulum stress by combined application of bortezomib and celexoib as a novel therapeutic strategy for glioblastoma Hayat MA Tumors of the Central Nervous System Dordrecht, The Netherlands Springer 2011 1
- Yang ZJ Chee CE Huang S Sinicrope FA The role of autophagy in cancer: therapeutic implications Mol Cancer Ther 2011 10 1533 1541 21878654
- Sato K Tsuchihara K Fujii S Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation Cancer Res 2007 67 9677 9684 17942897
- Choi KS Autophagy and cancer Exp Mol Med 2012 44 109 120 22257886
- Gozuacik D Kimchi A Autophagy as a cell death and tumor suppressor mechanism Oncogene 2004 23 2891 2906 15077152
- Esumi H Lu J Kurashima Y Hanaoka T Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation Cancer Sci 2004 95 685 690 15298733
- Lu J Kunimoto S Yamazaki Y Kaminishi M Esumi H Kigamicin D, a novel anticancer agent based on a new anti-austerity strategy targeting cancer cells’ tolerance to nutrient starvation Cancer Sci 2004 95 547 552 15182438
- Yardley DA Combining mTOR inhibitors with chemotherapy and other targeted therapies in advanced breast cancer: rationale, clinical experience, and future directions Breast Cancer Basic Res Treat 2013 7 7 22