Abstract
Advances in recombinant technology and knowledge about coagulation factor VIII (FVIII) are building a platform for new therapeutic options in patients with hemophilia A. The development of turoctocog alfa, a novel, high-purity, third-generation, B-domain truncated recombinant FVIII, has been produced and formulated without the use of animal-derived or human serum-derived components, in the wake of understanding of the new biochemical characteristics of FVIII, namely its protein structure, and glycosylation and sulfating patterns. Culture conditions and a five-step purification process have been developed to optimize the safety of turoctocog alfa. The results of two pilot clinical trials using turoctocog alfa confirmed high safety levels, with no patient developing inhibitors during the period of observation. The purpose of this review is to describe briefly the molecular and biological properties of turoctocog alfa, together with details of its clinical development, with emphasis on the needs of patients with hemophilia A.
Introduction
Coagulation factor VIII (FVIII) is the nonenzymatic cofactor of activated factor IX (FIXa) in the activation of factor X (FX).Citation1 When proteolytically activated, FVIIIa interacts with FIXa to form a tight noncovalent complex on the membrane phospholipids of activated platelets that binds to and converts FX to the activated proteinase form (FXa).Citation2,Citation3
Hemophilia A is an X chromosome-linked bleeding disorder caused by mutations in the gene coding for FVIII.Citation1 Patients with mild, moderate, and severe disease have a deficiency of FVIII activity in plasma with levels of 5%–40%,Citation3 1%–5%,Citation2,Citation3 and <1%,Citation2,Citation3 respectively. Patients with severe hemophilia A are at risk of uncontrolled and often spontaneous hemorrhages into joints, muscles, or internal organs, or excessive bleeding after injury or surgery.Citation1 Recurrent bleeding episodes may lead to progressive arthropathy and muscle contractures, often associated with chronic pain and disability.Citation1
FVIII replacement therapy has been the cornerstone in the treatment of hemophilia and has progressed over time from the use of blood transfusions to the use of cryoprecipitates in the 1960s, plasma-derived FVIII (pdFVIII) concentrates in the 1970s, and recombinant products in the 1990s.Citation4,Citation5 Manufacture of recombinant FVIII (rFVIII) evolved over decades and provided products that were classified depending on whether animal-derived or human-derived proteins were used during manufacturing and in the final formulation.Citation6
This article reviews the molecular aspects relevant for full functionality of rFVIII and translates the advances of a novel rFVIII, turoctocog alfa, in the setting of its specific pharmacological properties and safety profile as assessed in trials involving patients with hemophilia A.Citation7,Citation8
Recombinant FVIII products
rFVIII products were developed to improve the safety of pdFVIII concentrates. Three different generations of rFVIII products are currently available, including:Citation6 first-generation products using animal-derived proteins in the cell culture medium and human serum albumin in the final formulation to stabilize FVIII; second-generation products using human-derived proteins in the culture medium but with no albumin added in the final formulation; and third-generation products manufactured with no animal or human proteins other than FVIII either during processing or in the final formulation.
reports the characteristics of licensed rFVIII products compared with turoctocog alfa.Citation5–Citation15 rFVIII molecules may be full-length, B-domain-deleted, or B-domain-truncated. The B-domain is generally believed to be unnecessary for coagulant activity; however, the novel properties of this domain in the life cycle of FVIII and in the immune response of hemophilia patients have been gradually revealed.Citation16,Citation17 Moreover, the cellular host system and culture conditions are of the utmost importance for the pattern of post-translational modifications in gene therapy constructs.Citation1 also lists a number of methods for inactivation/removal of contaminating pathogens (eg, ultrafiltration, solvent/detergent, nanofiltration) that have been gradually added to successive generations of products to enhance their safety, not only as regards known pathogens but also unknown agents, eg, prions. As part of this progress, development of a new rFVIII molecule by engineering its physicochemical properties is of great interest in the improvement of clinical outcomes.
Table 1 Licensed recombinant factor VIII products
Molecular characterization of rFVIII
Protein structure
The human FVIII gene is localized on the long arm of the X chromosome and consists of 26 exons and introns, for a total length of 9 kbp in coding sequence.Citation18,Citation19 Sinusoidal endothelial and Kupffer cells in the liver are the major site of FVIII expression.Citation20,Citation21 The gene encodes a large precursor glycoprotein of 2,332 amino acid residues (the maximum length),Citation20 consisting of six structural domains and three acidic subdomains, organized in a heavy chain [A1(a1)A2(a2)B] and light chain [(a3)A3C1C2], as shown in .
Figure 1 Protein structures and post-translational modifications reported for factor VIII and turoctocog alfa, respectively.
Abbreviation: SP, signal peptide.
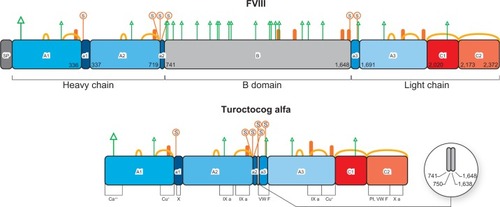
Every domain plays a physiological role (see below) throughout the life cycle of FVIII, from biosynthesis to clearance. A particular case is the 908 amino acid residue B-domain, which does not seem to be required for FVIII clotting activity but is important for processing, intracellular transport, and secretion of FVIII protein.Citation16 Today we could not definitely exclude potential post-secretion B-domain role during FVIII activation, platelet binding, and inactivation.Citation16 The B-domain is partially removed from mature FVIII, so that several truncated B-domain variants of FVIII circulate in the bloodstream. Complete or partial deletions of the B-domain are not associated with significant differences in procoagulant properties of these FVIII variants.Citation16
Twenty years of experimental research have shown that engineering B-domain deletions results in increased FVIII secretion in most expression systems.Citation1,Citation16 A variant chosen among progressively finer B-domain truncated forms with a linker peptide of 14 residues has shown useful pharmaceutical properties (ie, improved efficiency of manufacturing, long-term stability); this so-called B-domain-deleted FVIII was developed clinically as a second-generation and third-generation product.Citation22,Citation23
Physiological sulfation of rFVIII
Sulfation is required for full activity of FVIII. When rFVIII is expressed in the presence of chlorate, a potent inhibitor of protein sulfation, or when tyrosine residues are replaced by other amino acids, the functional activity of secreted FVIII is reduced 5-fold.Citation24
Post-translational cellular processing of the FVIII precursor enables sulfation of tyrosine residues in the Golgi apparatus, mediated by a sulfotransferase.Citation25 There are six potential tyrosine sulfation sites on the FVIII molecule, ie, four on the heavy chain (at amino acid residues 346, 718, 719, and 723) and two on the light chain (residues 1664 and 1680).Citation24 All the six sulfation sites are required to modulate FVIII activity ().Citation26,Citation27 Sulfation of key tyrosine residues is crucial not only for the function of FVIII, but also for its stability and binding to von Willebrand factor (VWF).Citation24,Citation26,Citation27
Table 2 Function specificity of sulfated tyrosine residues in factor VIII
When therapeutic FVIII concentrate (not containing VWF) is infused into patients, complex formation between FVIII and VWF occurs within seconds.Citation28 In the circulating complex, VWF serves as a chaperone, protecting FVIII from premature inactivation (cleavage by FXa and activated protein C), thereby helping to maintain a constant level of circulating FVIII Citation29,Citation30 Lack of interaction between FVIII and VWF may impair the pharmacokinetics of FVIII. Mutations in the binding sites for VWF (eg, Trp 2229) may induce a conformational change in FVIII, rendering the molecule antigenically distinct from wild-type FVIII.Citation31,Citation32 Therefore, physical interaction between FVIII and VWF may modulate the immunogenicity of FVIII, ie, mask epitopes and prevent entry of FVIII into antigen-presenting cells.Citation32,Citation33 In the absence of binding to WWF, infused rFVIII remains free in the circulation and is therefore more easily targeted by the immune system.Citation33
It has been reported that rFVIII products contain a variable proportion of protein that is unable to bind VWF.Citation34 Recent studies have shown that this may be attributable to the lack of Tyr-1680 sulfation.Citation35,Citation36 In summary, the potential impact of FVIII sulfation and, in particular of tyrosine 1680, on the functionality and immunogenicity of FVIII deserves further attention.
Molecular instability
The FVIII molecule is intrinsically unstable because it has inherently labile structural elements. An example is the amino acid backbone, which has the potential to undergo acid-base and redox reactions and is also prone to temperature-dependent, pH-dependent, and concentration-dependent precipitation.Citation19,Citation37 FVIII concentrates are prone to supramolecular aggregation as a result of their colloidal properties.Citation37 This latest aspect has caused speculation about differential immunogenicity of rFVIII products since baby hamster kidney cells produce more FVIII protein in aggregate form, which could affect tertiary structure and recognition by the immune system.Citation38,Citation39
If left unaddressed, molecular instability could impact the pharmacological properties of FVIII products. To address these issues, molecular level technologies can be employed to ameliorate its physicochemical properties, because design of rFVIII with improved efficacy can be achieved by optimization of drug molecular stability.
Several functional properties can be ascribed to the carbohydrate moieties of a highly glycosylated protein such as FVIII. Glycosylation influences stability and modulates immunogenic properties.Citation40,Citation41 Some glycans carried by rFVIII but not by pdFVIII products may increase the level of antigenicity.Citation42 Stability is also attributed to the conservation of binding sites for metals. It was demonstrated that the stability of a new rFVIII is assured by two metal binding sites, one in domain A3 for Cu+ and one in domain A1 for Zn2+, determined by X-ray fluorescence and X-ray crystallography.Citation43 The intrinsic stability of rFVIII has to be preserved under a number of conditions, from the manufacturing process and lyophilization to storage and continuous infusion. A stable product without alterations in its protein characteristics and a very low propensity to form aggregates is desired.Citation43
Clinical issues with rFVIII treatment
Prophylaxis is considered gold standard for patients with severe hemophilia; even secondary or “tertiary” prophylaxis have been shown to have more beneficial effects than treatment on demand.Citation44 Research on newer rFVIII products is rapidly progressing to meet a number of needs, including but not limited to, the efficacy and safety of prophylactic treatment.Citation45–Citation47
In terms of the main adverse effects, the most serious complication of replacement therapy for hemophilia A is the occurrence of neutralizing alloantibodies (inhibitors) against administered FVIII. Approximately 25%–30% of severely affected patients (FVIII: C<1%) develop inhibitors, typically during the first 20–50 days of exposure.Citation48
Agents that bypass inhibitors, such as recombinant activated factor VII and activated prothrombin complex concentrates, are available to control and prevent bleeding;Citation49 however, their efficacy is suboptimal compared with FVIII replacement therapy. Induction of immune tolerance is the main treatment option for eradicating inhibitors and has high success rates (60%–80%), although it is extremely demanding and costly.Citation50,Citation51 Genetic background and acquired factors interplay and contribute to development of inhibitors.Citation52 Treatment-related determinants include age at first FVIII exposure, treatment intensity (dose and frequency of infusions) and modality (prophylactic or on-demand regimens), and factors linked to concentrate source and type (ie, rFVIII versus pdFVIII, B-domain-deleted versus full-length FVIII).Citation38,Citation51
In recent years, three systematic reviews were carried out to address the issue of immunogenicity of plasma-derived rFVIII and rFVIII in previously untreated patients. However, due to a number of methodological limitations, mainly differences in study design and patient populations, no conclusive and definite answer has been provided.Citation48,Citation53,Citation54
In addition, it has to be considered that the above-mentioned meta-analyses compared products grouped into two classes, although each class included heterogeneous FVIII derivatives; in particular, pdFVIII can be either of high, low, or intermediate purity, or with or without VWF content, while rFVIII can differ in terms of manufacturing process, cell line, and molecular structure (). Recently, in a large prospective study of previously untreated patients, the second-generation, full-length rFVIII derived from a baby hamster kidney cell line was associated with a higher risk of development of inhibitors than the third-generation, full-length rFVIII derived from a Chinese hamster ovary cell line,Citation55 and this unexpected finding has no plausible biological explanation to date.Citation38
Conflicting results were also provided by meta-analyses of studies carried out in previously treated patients. In this context, some authors have reported an increased incidence of de novo inhibitors with B-domain-deleted rFVIII in comparison with full-length rFVIII.Citation56 In contrast, a subsequent meta-analysis was not able to show any significant difference in the risk of inhibitors associated with use of different rFVIII products in previously treated patients.Citation57
Turoctocog alfa
Current rFVIII products have a high degree of safety and efficacy; however, improvement is still possible. New bioengineering technologies are being actively applied in the search for new recombinant products, and some of them are in preclinical or clinical development.Citation45 Turoctocog alfa is the first of these new products to be approved by the US Food and Drug Administration and European Medicines Agency. Turoctocog alfa is a novel, third-generation, B-domain truncated rFVIII produced and formulated without the use of animal-derived or human serum-derived components.Citation58
Cell line
Chinese hamster ovary cells were transfected with the turoctocog alfa-coding plasmid and selected with the dihydrofolate reductase system, leading to a clonal suspension of producer cells cultivated in medium free of animal components.Citation58 A chemically defined and animal component-free growth medium was used for large-scale fermentation.Citation58
Manufacturing process
The advanced purification techniques utilized for production of turoctocog alfa led to a final product formulated without albumin and animal-derived or human-derived materials. The manufacturing process involves a five-step procedure:Citation5,Citation58
Solvent/detergent inactivation to eliminate potential viral contaminants endowed with lipid envelopes.
Immunoaffinity chromatography that allows isolation of pure molecules of rFVIII utilizing a monoclonal F25 antibody directed against the integral A2 domain of the heavy chain, including residue 740 of the amino acid sequence; this step excludes incomplete molecules, particularly those with damage to the heavy chain in the position of greatest instability, 720, being a primary inactivating site by proteolysis.Citation58 A recombinant monoclonal antibody directed against the A2 domain is produced in the same cell line used for production of turoctocog alfa, and it is developed in an animal component- and serum- free process.Citation58
Ion-exchange chromatography to remove impurities produced by the cell line, and electrical charges to remove impurities in a further purification step.
Nanofiltration, where a 20 nm pore size filter is used to remove potential viruses, including those with a protein envelope, such as Parvovirus.
A gel filtration step to provide the pure protein, turoctocog alfa, eliminating aggregated/agglutinated forms of the protein.Citation59
Protein
The primary structure of the turoctocog alfa protein (also called N8) has been identified by a combination of amino acid sequencing, peptide mapping, and mass spectrometry.Citation58 In turoctocog alfa, the 908 amino acid residue wild-type B-domain was reduced to a 21 amino acid residue linker.Citation59 This has the sequence SFSQNSRHPSQNPPVLKRHQR.Citation58 The sequence represents ten amino residues from the N-terminal of the original B-domain linked to 11 amino acid residues from the C-terminal of the B-domain.Citation58 Attention must be paid to the last four amino acid residues (R), which represent the sequence RXXR (where X is a random residue).Citation58 This motif is the site where the protease furin cleaves the single-chain precursor of FVIII, giving rise to a light and heavy chain in the Golgi apparatus.Citation60 Thus, the short but functionally complete B-linker efficiently facilitates the expression of FVIII heavy (A1-A2-linker) and light (A3-C1-C2) chains indistinguishable from those formed from full-length FVIII.Citation58
In addition, the last 20 amino acid residues of the heavy chain represent an acidic subdomain, being the region required for optimal interaction with thrombin and other proteins. In particular, this is the point where FVIII is cleaved by thrombin.Citation1,Citation58 The light chain of N8 was found to consist of two similar species implicated in the formation of tenase.Citation43 Both of these light chain variants are known to be present in pdFVIII.Citation61 These specific features of the primary structure of turoctocog alfa highlight the fact that the molecule was developed with the goal of producing smaller but more efficient bioengineered FVIII.
Glycosylation
Manufacturing of turoctocog alfa was a key advance in bioengineering the glycosylation pathways of the expression system. The aim was to synthesize an rFVIII with the most “humanized” glycosylation possible. The glycoengineering efforts toward improvement of mammalian cell (Chinese hamster ovary) glycosylation has been directed to eliminating immunogenic glycotopes in turoctocog alfa.
Post-translational modifications of turoctocog alfa include N-linked and O-linked glycosylations. Prevalent N-linked glycosylation sites occur at the terminal amide groups of asparagine (Asn) residues present in the Asn-X-Thr/Ser sequence.Citation58,Citation62 O-linked glycosylation refers to linkage of carbohydrate structures at the serine (Ser) or threonine (Thr) residues through the Asn-X-Thr/Ser motif.Citation58,Citation63
Within the A and C domains of FVIII there are N-linked glycosylations at four Asn residues, ie, Asn41, Asn239, Asn1810, and Asn2118. The position of the four N-glycosylation sites known from pdFVIII were all confirmed in turoctocog alfa by mass spectrometry of peptide fragments derived from the tryptic map.Citation58
No significant differences were found between rFVIII and pdFVIII with regard to their oligosaccharide profiles. In both, the glycomap of N-linked glycans characterizes the chains linked to Asn41 and Asn1810 as core fucosylated and biantennary chains complex structures, with one or two sialic acids Asn239 carrying high-mannose, hybrid and complex structures and Asn2118 carrying only high-mannose structures.Citation35,Citation58
The O-linked glycosylation sites of FVIII are prevalent on the B-domain, where at least seven carbohydrate structures have been reported.Citation64 The linker N8 sequence includes one of these sites at Ser in position Ser750.Citation58,Citation60 Curiously, this is the only O-linked glycan for which the precise location and structure on FVIII is known.Citation64 The O-glycan composition of N8 is a doubly sialylated disaccharide, which is a common eukaryotic O-linked glycan.Citation58 This structure was confirmed by consecutive enzymatic digestion of N8. The site is glycosylated in approximately 65% of turoctocog alfa.Citation58
In brief, the oligosaccharide structures of the novel rFVIII and pdFVIII are very similar, with mainly small, quantitative differences, and heterogeneous glycosylation is present in both products.Citation35,Citation58
Sulfation
Analysis of turoctocog alfa for the degree of sulfation demonstrated that all six tyrosine sulfation sites are utilized.Citation58 Detection and quantification of tyrosine sulfation in turoctocog alfa was performed with care because sulfation is a potentially labile modification.Citation35,Citation58 Chromatographic and mass spectrometric techniques after tryptic digestion provided the following results:Citation5 a sulfated tyrosine residue in position 346 of the heavy chain in a single high performance liquid chromatography fraction; three other heavy chain sulfated tyrosines (residues 718, 719, and 723) in three different high-performance liquid chromatography fractions and mass analysis; and the presence of the last two sulfated residues (light chain tyrosine 1664 and 1680) in the individual peptide isolated from tryptic digestion of native N8.
All these six sulfated residues are located in the acidic regions of domains A1, A2, and A3.
Despite technical difficulties with high molecular weight proteins like FVIII, particular attention has been paid to sulfation of tyrosine 1680, because it could impact on binding to VWF.Citation26,Citation36,Citation65 Recently, it has become possible to determine the degree of Tyr1680 sulfation in different rFVIII products (by nanoliquid chromatography coupled with electrospray tandem mass spectrometry).Citation36 The percentage of nonsulfated Tyr1680 ranged from 1% to 6.5% for second-generation rFVIII to up to 15% for third-generation rFVIII, while the percentage of nonsulfated pdFVIII was negligible.Citation26 These results were confirmed in a second study using a proteomics analysis technique ().Citation35
Table 3 Levels of nonsulfated tyrosine in rFVIII
In summary, turoctocog alfa is sulfated in all tyrosine moieties. This full sulfation was stable for a year in an end-of-shelf life analysis, an indication for physiological stability in circulation.Citation36,Citation66 Moreover, a number of molecular interventions have been shown to stabilize turoctocog alfa against almost all of the major physicochemical instability factors encountered during manufacturing, storage, and clinical use.Citation22,Citation58,Citation65 Improved long-term stability was obtained for turoctocog alfa to accommodate a variety of everyday living conditions in hemophiliac patients and make treatment feasible with ease in any situation.
Clinical development
The clinical development plan for turoctocog alfa, known as the Guardian™ program, has involved several pharmacokinetic, field, efficacy, and safety trials.Citation67–Citation70 Two Phase III trials investigated the safety and efficacy of turoctocog alfa in previously treated patients, ie, Guardian 1 in adult/adolescent patients ≥12 years and Guardian 3 in pediatric patients aged <12 years.Citation69,Citation70 Overall, the two trials included 210 previously treated patients with severe hemophilia A, involving 150 adults exposed to turoctocog alfa for a mean of 85 (11–172) days and 60 pediatric patients treated for a mean of 60 (range 20–104) exposure days.Citation69,Citation70
The two Phase III Guardian trials specifically evaluated the incidence of inhibitors in previously treated patients, potential immunogenicity and neoantigenicity of rFVIII leading to neutralizing antibodies being the main objective of the studies.Citation69,Citation70 No patient developed inhibitors during either trial.Citation71,Citation72 One adult patient was excluded from the analysis because of a positive inhibitor test at baseline. Interestingly, this patient received 13 infusions of turoctocog alfa with no recurrence of inhibitors.Citation69 Patients in the two trials could continue turoctocog alfa in the ongoing long-term safety and efficacy Guardian 3 extension trial.Citation71
With regard to adverse events other than development of inhibitors, a total of six nonserious events were evaluated by the investigators as possibly or probably related to turoctocog alfa, comprising four in the adult trial (hypertension, sinus tachycardia, and insomnia in a 27-year-old patient; raised liver enzymes in a 37-year-old patient) and two in the pediatric trial (contusion and incorrect dose administration in one patient).Citation69,Citation70
Over and above these pivotal studies in previously treated patients, and as required by regulatory authorities, the Guardian 4 trial is ongoing to assess the safety and efficacy of turoctocog alfa in previous untreated patients.Citation71
Conclusion
The development of turoctocog alfa as a new option for the treatment of hemophilia A was based on advanced bioengineering techniques to address unresolved molecular issues concerning rFVIII products. The novelty of turoctocog alfa required adequate and extensive documentation of clinical safety and efficacy.Citation71 Phase III trials met their primary endpoint regarding the safety of turoctocog alfa, with no induction of FVIII inhibitors.Citation69,Citation70 In the ongoing extension phase of about 3 years, the prophylactic turoctocog alfa regimen resulted in stabilization of bleeding rates at a low level.Citation72 Future studies will add further important information to the database regarding the long-term safety and efficacy of turoctocog alfa in specific patient populations.Citation71
Disclosure
The author acted as a paid consultant and received an unrestricted research grant from Novo Nordisk, and wrote and reviewed the manuscript. Editorial assistance was provided by Airon Communications, Milan, Italy, with financial support from Novo Nordisk, in compliance with international guidelines for good publication practice.
References
- Orlova NA Kovnir SV Vorobiev II Gabibov AG Vorobiev AI Blood clotting factor VIII: from evolution to therapy Acta Naturae 2013 5 2 19 39 23819034
- Fay PJ Activation of factor VIII and mechanisms of cofactor action Blood Rev 2004 18 1 1 15 14684146
- Sichler K Kopetzki E Huber R Bode W Hopfner KP Brandstetter H Physiological fIXa activation involves a cooperative conformational rearrangement of the 99-loop J Biol Chem 2003 278 6 4121 4126 12444082
- Shapiro AD Anti-hemophilic factor (recombinant), plasma/albumin-free method (octocog-alpha; ADVATE) in the management of hemophilia A Vasc Health Risk Manag 2007 3 5 555 565 18078007
- NovoEight (antihemophilic factor [recombinant], prescribing information) Novo Nordisk Bagsværd, Denmark 2011 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/002719/WC500157553.pdf Accessed June 30, 2014
- Franchini M Mannucci PM Hemophilia A in the third millennium Blood Rev 2013 27 4 179 184 23815950
- Haddley K Turoctocog alfa for the treatment of hemophilia A Drugs Today (Barc) 2014 50 2 121 131 24619589
- Lentz SR Seremetis S Staber J Kulkarni R Turoctocog alfa and drug development for hemophilia A Expert Opin Orphan Drugs 2014 2 4 419 431
- Brooker M Registry of clotting factor concentrates 9th ed Montréal, QC, Canada World Federation of Hemophilia 2012 Available from: http://www1.wfh.org/publication/files/pdf-1227.pdf Accessed October 24, 2014
- Recombinate (antihemophilic factor [recombinant], prescribing information) Deerfield, IL, USA Baxter Healthcare Corporation 2010 Available from: http://www.baxter.com/downloads/healthcare_professionals/products/recombinate_pi_5ml.pdf Accessed June 30, 2014
- Kogenate FS (antihemophilic factor [recombinant], prescribing information) Berlin, Germany Bayer Schering Pharma AG 2006 Available from: http://www.abopharmaceuticals.com/ProductSheets/KogenateFS.pdf Accessed June 30, 2014
- Helixate FS (antihemophilic factor [recombinant], prescribing information) Kankakee, IL, USA CSL Behring 2013 Available from: http://labeling.cslbehring.com/PI/US/HelixateFS/EN/HelixateFS-Prescribing-Information.pdf Accessed June 30, 2014
- ReFacto (antihemophilic factor [recombinant], prescribing information) Madison, NJ, USA Wyeth Pharmaceuticals Inc 2005 Available from: http://www.abopharmaceuticals.com/ProductSheets/Refacto.pdf Accessed June 30, 2014
- Advate [antihemophilic factor (recombinant)] [prescribing information] Deerfield, IL, USA Baxter Healthcare Corporation 2013 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000520/WC500022467.pdf Accessed June 30, 2014
- Refacto AF (antihemophilic factor [recombinant], prescribing information) New York, NY, USA Pfizer 2010 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000232/WC500049008.pdf Accessed June 30, 2014
- Pipe SW Functional roles of the factor VIII B domain Haemophilia 2009 15 6 1187 1196 19473417
- Grushin K Miller J Dalm D Lack of recombinant factor VIII B-domain induces phospholipid vesicle aggregation: implications for the immunogenicity of factor VIII Haemophilia 2014 20 5 723 731 24750465
- Gitschier J Wood WI Goralka TM Characterization of the human factor VIII gene Nature 1984 312 5992 326 330 6438525
- Solá RJ Griebenow K Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy Bio Drugs 2010 24 1 9 21
- Hollestelle MJ Thinnes T Crain K Tissue distribution of factor VIII gene expression in vivo – a closer look Thromb Haemost 2001 86 3 855 861 11583319
- Shahani T Covens K Lavend’homme R Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII J Thromb Haemost 2014 12 1 36 42 24118899
- Sandberg H Almstedt A Brandt J Structural and functional characteristics of the B-domain-deleted recombinant factor VIII protein, r-VIII SQ Thromb Haemost 2001 85 1 93 100 11204595
- Recht M Nemes L Matysiak M Clinical evaluation of moroctocog alfa (AF-CC), a new generation of B-domain deleted recombinant factor VIII (BDDrFVIII) for treatment of haemophilia A: demonstration of safety, efficacy, and pharmacokinetic equivalence to full-length recombinant factor VIII Haemophilia 2009 15 4 869 880 19473411
- Pittman DD Wang JH Kaufman RJ Identification and functional importance of tyrosine sulfate residues within recombinant factor VIII Biochemistry 1992 31 13 3315 33325 1554716
- Niehrs C Huttner WB Purification and characterization of tyrosylprotein sulfotransferase EMBO J 1990 9 1 35 42 2295314
- Michnick DA Pittman DD Wise RJ Kaufman RJ Identification of individual tyrosine sulfation sites within factor VIII required for optimal activity and efficient thrombin cleavage J Biol Chem 1994 269 31 20095 20102 8051097
- Kaufman RJ Post-translational modifications required for coagulation factor secretion and function Thromb Haemost 1998 79 6 1068 1079 9657426
- Gilbert GE Drinkwater D Barter S Clouse SB Specificity of phosphatidylserine-containing membrane binding sites for factor VIII. Studies with model membranes supported by glass microspheres (lipospheres) J Biol Chem 1992 267 22 15861 15868 1639816
- Fay PJ Coumans JV Walker FJ von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation J Biol Chem 1991 266 4 2172 2177 1846615
- Nogami K Shima M Nishiya K A novel mechanism of factor VIII protection by von Willebrand factor from activated protein C-catalyzed inactivation Blood 2002 99 11 3993 3998 12010799
- Hay CR Factor VIII inhibitors in mild and moderate-severity haemophilia A Haemophilia 1998 4 4 558 563 9873794
- Dasgupta S Repessé Y Bayry J VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors Blood 2007 109 2 610 612 16985172
- Gringeri A Ofosu FA Grancha S Understanding FVIII/VWF complex – report from a symposium of XXIX WFH meeting 2010 Haemophilia 2012 18 3 469 475 21943193
- Lin Y Yang X Chevrier MC Relationships between factor VIII: Ag and factor VIII in recombinant and plasma-derived factor VIII concentrates Haemophilia 2004 10 5 459 469 15357771
- Kannicht C Ramström M Kohla G Characterisation of the post-translational modifications of a novel, human cell line-derived recombinant human factor VIII Thromb Res 2013 131 1 78 88 23058466
- Grancha S Navajas R Marañón C Incomplete tyrosine 1680 sulphation in recombinant FVIII concentrates Haemophilia 2011 17 4 709 710 21299741
- Chi EY Krishnan S Kendrick BS Roles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony stimulating factor Protein Sci 2003 12 5 903 913 12717013
- Kessler CM Iorio A The Rodin (Research Of Determinants of INhibitor Development among PUPs with haemophilia) study: the clinical conundrum from the perspective of haemophilia treaters Haemophilia 2013 19 3 351 354 23577743
- Pahl S Pavlova A Driesen J In vitro characterization of recombinant factor VIII concentrates reveals significant differences in protein content, activity and thrombin activation profile Haemophilia 2013 19 3 392 398 23252674
- Kosloski MP Miclea RD Balu-Iyer SV Role of glycosylation in conformational stability, activity, macromolecular interaction and immunogenicity of recombinant human factor VIII AAPS J 2009 11 3 424 431 19499345
- Lenting PJ Pegon JN Christophe OD Denis CV Factor VIII and von Willebrand factor – too sweet for their own good Haemophilia 2010 16 Suppl 5 194 199 20590881
- Schilow WF Schoerner-Burkhardt E Seitz R Charge analysis of N-glycans from human recombinant coagulation factor VIII and human FVIII standards Thromb Haemost 2004 92 2 427 428 15269842
- Svensson LA Thim L Olsen OH Nicolaisen EM Evaluation of the metal binding sites in a recombinant coagulation factor VIII identifies two sites with unique metal binding properties Biol Chem 2013 394 6 761 765 23435097
- Gringeri A Lambert T Street A Aledort L on behalf of the Adolescent/Adult Prophylaxis Expert Working Group of the International Prophylaxis Study Group Tertiary prophylaxis in adults: is there a rationale? Haemophilia 2012 18 5 722 728 22639786
- Peyvandi F Garagiola I Seregni S Future of coagulation factor replacement therapy J Thromb Haemost 2013 11 Suppl 1 84 98 23809113
- Dimichele DM Blanchette V Berntorp E Clinical trial design in haemophilia Haemophilia 2012 18 Suppl 4 18 23 22726077
- Makris M Calizzani G Fischer K EUHASS: The European Haemophilia Safety Surveillance System Thromb Res 2011 127 Suppl 2 S22 S25 21193110
- Wight J Paisley S The epidemiology of inhibitors in haemophilia A: a systematic review Haemophilia 2003 9 4 418 435 12828678
- Teitel JM Sholzberg M Current status and future prospects for the prophylactic management of hemophilia patients with inhibitor antibodies Blood Rev 2013 27 2 103 109 23452718
- Coppola A Di Minno MN Santagostino E Optimizing management of immune tolerance induction in patients with severe haemophilia A and inhibitors: towards evidence-based approaches Br J Haematol 2010 150 5 515 528 20573153
- Ettingshausen CE Kreuz W The immune tolerance induction (ITI) dose debate: does the International ITI Study provide a clearer picture? Haemophilia 2013 19 Suppl 1 12 17 23278995
- Kruse-Jarres R Inhibitors: our greatest challenge. Can we minimize the incidence? Haemophilia 2013 19 Suppl 1 2 7 23278993
- Iorio A Halimeh S Holzhauer S Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma-derived or recombinant factor VIII concentrates: a systematic review J Thromb Haemost 2010 8 6 1256 1265 20345722
- Franchini M Coppola A Rocino A Italian Association of Hemophilia Centers (AICE) Working Group Systematic review of the role of FVIII concentrates in inhibitor development in previously untreated patients with severe hemophilia A: a 2013 update Semin Thromb Hemost 2013 39 7 752 766 24022806
- Gouw SC van der Bom JG Ljung R PedNet and RODIN Study Group Factor VIII products and inhibitor development in severe hemophilia A N Engl J Med 2013 368 3 231 239 23323899
- Aledort LM Navickis RJ Wilkes MM Can B-domain deletion alter the immunogenicity of recombinant factor VIII? A meta-analysis of prospective clinical studies J Thromb Haemost 2011 9 11 2180 2192 21848690
- Xi M Makris M Marcucci M Santagostino E Mannucci PM Iorio A Inhibitor development in previously treated hemophilia A patients: a systematic review, meta-analysis, and meta-regression J Thromb Haemost 2013 11 9 1655 1662 23802542
- Thim L Vandahl B Karlsson J Purification and characterization of a new recombinant factor VIII (N8) Haemophilia 2010 16 2 349 359 19906157
- Gagnon P Cheung CW Lepin EJ Minibodies and multimodal chromatography methods: a convergence of challenge and opportunity Bioprocess Int 2010 8 2 26 35 21984873
- Lenting PJ van Mourik JA Mertens K The life cycle of coagulation factor VIII in view of its structure and function Blood 1998 92 11 3983 3996 9834200
- Lind P Larsson K Spira J Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization Eur J Biochem 1995 232 1 19 27 7556150
- Medzihradszky KF Characterization of protein N-glycosylation Methods Enzymol 2005 405 116 138 16413313
- Peter-Katalinic J Methods in enzymology: O-glycosylation of proteins Methods Enzymol 2005 405 139 171 16413314
- Mazsaroff I Yu W Kelley BD Vath JE Quantitative comparison of global carbohydrate structures of glycoproteins using LC-MS and in-source fragmentation Anal Chem 1997 69 13 2517 2524 9212710
- Leyte A van Schijndel HB Niehrs C Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor J Biol Chem 1991 266 2 740 746 1898735
- Nielsen PF Bak S Vandahl B Characterization of tyrosine sulphation in rFVIII (turoctocog alfa) expressed in CHO and HEK-293 cells Haemophilia 2012 18 5 e397 e398 22681385
- Martinowitz U Bjerre J Brand B Bioequivalence between two serum-free recombinant factor VIII preparations (N8 and ADVATE®) – an open-label, sequential dosing pharmacokinetic study in patients with severe haemophilia A Haemophilia 2011 17 6 854 859 21443634
- Viuff D Barrowcliffe T Saugstrup T Ezban M Lillicrap D International comparative field study of N8 evaluating factor VIII assay performance Haemophilia 2011 17 4 695 702 21426445
- Lentz SR Misgav M Ozelo M Results from a large multinational clinical trial (Guardian™1) using prophylactic treatment with turoctocog alfa in adolescent and adult patients with severe haemophilia A: safety and efficacy Haemophilia 2013 19 5 691 697 23647704
- Kulkarni R Karim FA Glamocanin S Results from a large multinational clinical trial (Guardian™3) using prophylactic treatment with turoctocog alfa in paediatric patients with severe haemophilia A: safety, efficacy and pharmacokinetics Haemophilia 2013 19 5 698 705 23651313
- Laguna P Vdovin V Rageliene L Abad-Franch L Lindblom A Overview of a global clinical trial programme with turoctocog alfa, a new recombinant factor VIII: the Guardian™ programme Presented at the XXIV International Society on Thrombosis and Haemostasis Congress Amsterdam, The Netherlands June 29 to July 4, 2013
- Ozelo M Misgav M Abdulkarim F Reductions in annualized bleeding rates over time with turoctocog alfa prophylaxis: 3-year interim results of the Guardian™ 2 extension trial Haemophilia 2014 20 Suppl 3 84

