Abstract
With the proposed Canadian July 2018 legalization of marijuana through the Cannabis Act, a thorough critical analysis of the current trials on the efficacy of medicinal marijuana (MM) as a treatment option is necessary. This review is particularly important for primary care physicians whose patients may be interested in using MM as an alternative therapy. In response to increased interest in MM, Health Canada released a document in 2013 for general practitioners (GPs) as an educational tool on the efficacy of MM in treating some chronic and acute conditions. Although additional studies have filled in some of the gaps since the release of the Health Canada document, conflicting and inconclusive results continue to pose a challenge for physicians. This review aims to supplement the Health Canada document by providing physicians with a critical yet concise update on the recent advancements made regarding the efficacy of MM as a potential therapeutic option. An update to the literature of 2013 is important given the upcoming changes in legislation on the use of marijuana. Also, we briefly highlight the current recommendations provided by Canadian medical colleges on the parameters that need to be considered prior to authorizing MM use, routes of administration as well as a general overview of the endocannabinoid system as it pertains to cannabis. Lastly, we outline the appropriate medical conditions for which the authorization of MM may present as a practical alternative option in improving patient outcomes as well as individual considerations of which GPs should be mindful. The purpose of this paper is to offer physicians an educational tool that provides a necessary, evidence-based analysis of the therapeutic potential of MM and to ensure physicians are making decisions on the therapeutic use of MM in good faith.
Introduction
In Canada, marijuana or cannabis (used interchangeably hereafter) has been used recreationally and medicinally for generations but was first legally available as medicinal marijuana (MM) in 2001 through the Medical Marijuana Access Regulations.Citation1 In its most recent form, the Access to Cannabis for Medical Purposes Regulations states that physicians have the responsibility of authorizing patients to access MM.Citation2,Citation3 Health Canada and the provincial medical colleges have published guidelines for physicians to follow and approve MM for their patient’s safety.Citation4 Despite these guidelines, physicians remain uncomfortable authorizing MM due to a lack of evidence-based literature and the perceived lack of education surrounding the subject.Citation5–Citation7 Many physicians feel that a robust understanding of cannabis would increase their comfort with MM.Citation6,Citation7 The basis of this knowledge is particularly relevant for a large demographic of the population presenting with chronic conditions that have reported to be self-medicating with marijuana where conventional therapies have failed in improving the overall quality of life (QOL).Citation7
Therefore, this review aims to serve as an educational tool that provides relevant information of which physicians must be mindful when authorizing MM. The information is primarily appropriate due to the expected increase in use following the proposed legalization of marijuana for recreational purposes by July 2018 through the Cannabis Act. Here, we provide a summary of the basic science behind cannabis and the endocannabinoid system, as well as the current Canadian laws and authorization guidelines for MM. We simplify and analyze new literature that has emerged since the 2013 release of the Canadian medical marijuana guidelines, delineate therapeutic uses of cannabis and its contraindications and outline gaps present in the current literature. We ultimately hope that this succinct review provides physicians with the necessary resources required for MM-related decision-making and improves the general practitioner’s level of comfort with MM and their capacity to attend to such patients.
Methods
This literature search identified articles using PubMed, EMBASE Ovid, and the Cochrane Library to determine high-quality, multicenter randomized controlled trials, systematic reviews, meta-analyses, and practice guidelines from February 2013 to August 2017. The assessment of the therapeutic potential of MM allowed in the identification of gaps including conflicting and inconclusive results in our knowledge since the release of the 2013 Health Canada guidelines that may pose a challenge for physicians. This review aims to supplement the Health Canada document by providing physicians with a critical yet concise update on the recent advancements for the prescribed use of MM. This paper presents an overview of previously published reviews and, as such, requires no ethics approval.
Canadian medical regulatory authorities’ policies and guidelines
Although the Cannabis Act is currently on track to its projected enactment taking place in July 2018, some challenges regarding MM use that are not addressed by the 2013 Health Canada guidelines remain. Specifically, under the ACMPR,Citation2 physicians are required to sign a medical document to authorize patients to access a specific quantity of cannabis. This medical report resembles a prescription; however, unlike all other prescribed medications, Health Canada has not reviewed data on the safety or efficacy of MM.
In light of the scarcity of data available to physicians, the medical regulatory authorities (colleges) have recently implemented policies on MM to assist physicians in making informed decisions that are most beneficial for their patients.Citation8–Citation16 Current guidelines and policies issued to date by these colleges repeatedly state that physicians should only sign the medical document if they have the necessary clinical knowledge; furthermore, physicians are not obligated to prescribe marijuana if they do not believe it is clinically appropriate for their patients.Citation17 Collectively, the colleges agree that MM is not appropriate in a number of circumstances including for patients under the age of 25 years, have a current or past substance use disorder, have personal or family history of mental illness (psychosis), have a history of chronic lung, cardiovascular, and/or kidney disease, and who are pregnant or breastfeeding. Moreover, all colleges recommend that informed consent should be obtained from patients before authorizing MM.Citation8–Citation16 During this process, physicians must discuss the risks and benefits of MM with their patients, including the necessary precautions that patients need to take when engaging in activities requiring mental alertness such as driving and operation of heavy machinery.
While the rules and restrictions that govern the authorization of MM may be challenging to interpret, determining the safe therapeutic dose for each patient will present a more significant challenge for physicians. Therefore, all colleges advise physicians to proceed cautiously where patients “start low and go slow” until a dose is reached that achieves symptom management while causing minimal euphoria or cognitive impairment.Citation18–Citation20 To ensure that these expectations are met, physicians must specify the quantity of marijuana to be dispensed to the patients as well as the (−)-Δ9-trans-(6aR,10aR)-tetrahydrocannabinol (Δ9-THC or THC) content (the relevance of this is discussed below) on every medical document. Furthermore, most colleges recommend that physicians follow up with their patients every 3 months to monitor for any emerging complications or risks of abuse, misuse, or diversion, even though the authorization of medical cannabis is valid for up to 1 year. To minimize risks, some medical regulatory authorities such as the Colleges of Physicians and Surgeons in Ontario, Saskatchewan, and Quebec require physicians to obtain a signed written treatment agreement from their patients before MM authorization.Citation8,Citation10,Citation11 This agreement must contain a statement from patients that they will not seek marijuana from another physician or any other source, will only use marijuana as prescribed, will store their marijuana safely and securely, and will not sell or give away their marijuana. Additional province-specific details can be found in Table S1.
Brief overview of the mechanisms of action of MM
Given the pending legalization of MM for recreational purposes through the enactment of the Cannabis Act, it is important for family physicians to understand the underlying effects of cannabis. This statute is to provide legal access to marijuana and to control and regulate its production, distribution, and sale.
The endocannabinoid system and MM
The endocannabinoid system is a naturally occurring communication network that plays a role in many physiological processes.Citation21 Currently, this system has been found to be implicated in gastrointestinal (GI) function,Citation22 appetite and metabolism,Citation23–Citation25 pain,Citation26,Citation27 memory,Citation28 movement,Citation29 immunity,Citation30 and inflammation.Citation31 The endocannabinoid system comprises two G-protein-coupled receptors (GPCRs): cannabinoid receptors 1 (CB1) and 2 (CB2).Citation32,Citation33 CB1 possesses psychoactive potential and is expressed in the central nervous system (CNS), Gastrointestinal (GI) system, adipocytes, liver tissue, and skeletal muscle.Citation32,Citation34,Citation35 In contrast, CB2 receptors are more restricted in their distribution and are primarily found on immune cells located in the tonsils, thymus, spleen, and bone marrow,Citation32,Citation34,Citation35 as well as in the enteric nervous system within the GI tract.Citation36 Activation of these receptors is dependent on endogenous endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG).Citation37,Citation38
Due to its abundance in the body, particularly in the nervous system, CB1 and its subsequent psychoactive effects have been extensively studied. As illustrated in , cannabinoid binding regulates presynaptic Ca++ levels generally leading to a reduced release of neurotransmitters. This mechanism plays an essential role in maintaining homeostasis, thereby implicating this system in several physiological and pathological conditions that have been previously reported in detail.Citation39
Figure 1 The endocannabinoid system and CB1/CB2 distribution. (A) The mechanism of action of the endocannabinoid system is depicted, with human endocannabinoids AEA or 2-AG binding to CB1 to initiate a signaling cascade through the release of neurotransmitters. THC is also able to bind to CB1, exerting its effects on the central nervous system and peripheral system. (B) Distribution of CB1 and CB2 in the body. CB1 is concentrated in the central and peripheral nervous systems. CB2 is more abundant in the immune system and, to a lesser degree, in the nervous system.
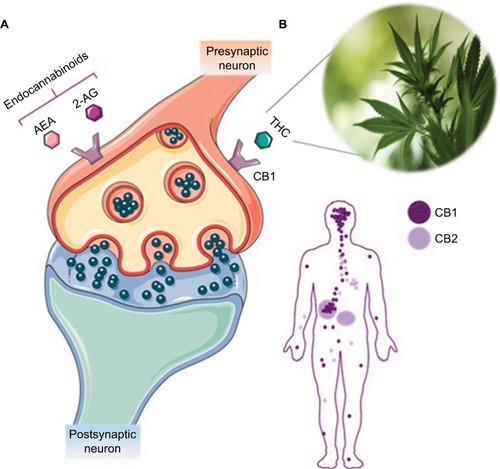
The medicinal properties of cannabis can be attributed primarily to phytocannabinoids Δ9-THC or THC and cannabidiol (CBD).Citation40–Citation42 THC and CBD are the most biologically active phytocannabinoids and are capable of mimicking human endocannabinoids AEA and 2-AG, respectively.Citation40–Citation42 Δ9-THC has been shown to bind to CB1 in the nervous system,Citation21 and the effects of THC on the CNS and peripheral body are outlined in and , respectively.
Figure 2 The effects of cannabis on the central nervous system. Brain areas in the central nervous system (in black) and their physiological functions (in red) are listed alongside potential effects of THC and CBD (in blue and green), respectively.
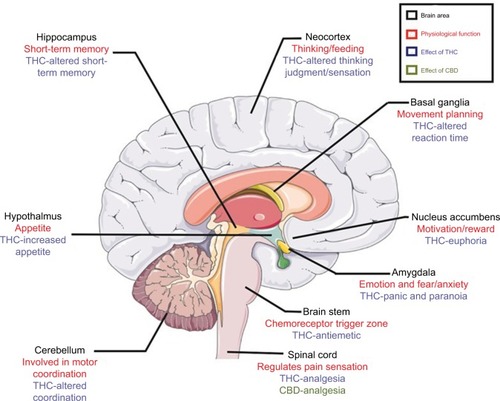
Table 1 Specific effects of THC in the peripheral system
In contrast, non-psychoactive CBD has high binding affinity to the CB2 receptor and exerts its effects on the immune system, resulting in its application for the treatment and management of neuropathic pain.Citation43 However, conflicting reports suggest that CBD indirectly mediates its effects by interacting with CB1 and CB2, the mechanism(s) of which is not well understood.Citation44 Given this inconclusive information, it is omitted from .
The potency of the mediating effects of THC on the endocannabinoid system depends on several factors that need to be considered before prescribing its use for treatment. In the unprocessed form, Δ9-THC and CBD concentrations depend on the species, strain, cultivation, and storage of the plant.Citation45,Citation46 Of the three species of cannabis identified (Cannabis sativa, C. sativa, C. indica, and C. ruderalis), C. sativa contains higher THC than CBD levels while the C. indica is richer in CBD compared to THC.Citation47 CBD attenuating the psychotropic actions of Δ9-THC on the body is thought to be due to affecting Δ9-THC metabolism and inhibiting the formation of 11-OH-THC, its more psychoactive metabolite.Citation47–Citation49 To summarize, a higher THC:CBD ratio is associated with more prominent psychoactive symptoms, whereas lower THC:CBD ratio suppresses psychoactive symptoms and has more sedative and relaxing effects.Citation50 Due to the varying effects of MM, pharmacokinetics is another critical aspect that physicians need to consider before authorizing the use of MM.
Pharmacokinetics of MM
In addition to understanding the effects of phytocannabinoids on the endocannabinoid system, physicians should be mindful of the chemical composition and available routes of administration if considering the authorization of MM. Phytocannabinoids are lipophilic and require heat for activation, as accomplished through both the inhalation routes of administration.Citation51–Citation53 The course of administration also determines the absorption and metabolism of phytocannabinoids. The currently available routes of administration of cannabinoids are discussed as follows, with the most common forms summarized in detail in .
Table 2 Mode of administration of Δ9-THC
Inhalation: smoking or vaporization
Inhalation is the most commonly used route of administration with the quickest onset of actionCitation54 and shortest duration,Citation55 giving patients the capacity to titrate their dose through adaptive smoking behavior. Of the two inhalation options, vaporization is more discreet and has fewer toxic by-products,Citation56,Citation57 while inhalation is an appropriate option for patients requiring rapid relief for a shorter duration.
Oral
Oral cannabinoid administration offers a longer duration and a slower onset of action compared to inhalation, making titration challenging for patients attempting to achieve desired effects. Cannabinoids administered through the oral route can be taken as pills, such as nabilone (Cesamet®) and dronabinol (Marinol®) (which is no longer available in Canada), or mixed in with foods such as butter, oils, or teas. Administration of oral cannabis can be presented as a potential option for individuals in need of relief of symptoms such as chronic pain, arthritis, movement disorders, and select psychiatric disorders. At this time, there is no robust evidence to support cannabis as a treatment for any psychiatric disorders. The cannabis trials for acute anxiety, schizophrenia, and posttraumatic stress disorder (PTSD) are rather still preliminary, and cannabis is not standard of care treatment for any mental illness at this time.
Oromucosal
Oromucosal cannabinoid administration offers a balance between speed of onset and duration of action when compared to inhalation and oral routes. Nabiximol is currently the only oromucosal product approved for prescription, containing a combination of Δ9-THC and CBD in spray form allowing simple self-titration. Oromucosal cannabinoid administration is recommended for the symptomatic relief of spasticity in adults with multiple sclerosis (MS) but may also be a good option for patients in need of rapid relief for longer durations, such as in neuropathic pain.
Rectal
The rectal route of cannabinoid administration, though uncommon, has been shown to be efficacious in patients presenting with chemotherapy-related nausea and emesis.Citation58 Δ9-THC-hemisuccinate, a prodrug, is delivered as opposed to Δ9-THC because it is quickly absorbed, having a higher bioavailability than oral administration. Although rectal products are currently unavailable in Canada, they may be of future interest for patients unable to tolerate oral medications, for the pediatric population, for palliative use, and for patients unable to take oral medication or via inhalation.
Topical
Topical cannabinoid administration has been considered as a treatment for glaucoma.Citation59 However, due to its high lipophilicity, transport of Δ9-THC across aqueous layers in the body is a rate-limiting stepCitation55 but can be overcome through the use of Δ9-THC prodrugs resulting in improved penetration into the anterior eye, reducing intraocular pressure.Citation59
Metabolism, excretion, and long-term detection of THC
The metabolism and excretion of cannabinoids are highly regulated and affect many other metabolic processes that need to be considered if advising the medicinal use of cannabis. In brief, cannabinoids are mainly metabolized in liver by the cytochrome P450 (CYP 450) enzymes.Citation60 Once absorbed, ~97% of Δ9-THC and its metabolites bind to plasma proteinsCitation61–Citation63 and are incorporated into fatty tissue and highly perfused organs, such as heart, brain, lungs, and liver,Citation55 with the majority of Δ9-THC accumulating in cardiac and fat tissues.Citation64 Cannabinoids and their metabolites that are not absorbed are excreted in feces (65%) and, to a lesser extent, in urine (20%).Citation55 Given the complex processes involved in the metabolism and excretion of THC in addition to the prolonged detection of THC, it is essential to consider the underlying drug interactions and subsequent effects on patients presenting with additional chronic conditions.
Therapeutic options applicable for the authorization of MM use
As previously discussed, many physicians feel uncomfortable with authorizing MM use due to a lack of educational resources available. Although Health Canada released “Information for Healthcare Professionals, Cannabis (Marihuana, Marijuana) and the Cannabinoids” in February 2013 to educate health care professionals on cannabis, physicians continue to be apprehensive about recommending cannabis as a treatment option for patients who present with chronic conditions. A detailed summary of the Health Canada document can be found in Table S2. Updated evidence-based recommendations and short critical analyses on MM use for various chronic conditions are discussed below.
Multiple sclerosis
MS is a chronic inflammatory, demyelinating autoimmune disease of the CNS.Citation65 Current therapies decrease additional MS attacks and delay progression but are unsuccessful in improving patient QOL.Citation66 Patients with MS often seek psychoactive drugs to cope with their disabilities, with numerous studies showing increased rates of recreational and MM use in patients experiencing spasticity.Citation67–Citation69 In light of this, it is critical for primary care physicians to make educated assessments when deciding whether to authorize MM as a therapeutic option.
Recent studies on the use of MM in MS suggest that cannabinoid use is associated with improvements in spasticity, but they fail to show statistical significance.Citation70–Citation80 Nevertheless, clinical significance was observed where patients reported a subjective sense of a reduction in spasticity-related symptoms. Many observational open-labeled studies reported promising data on the role of cannabinoids in the treatment of MS in clinical practice.Citation75–Citation80 Overall, cannabinoids appear to be a well-tolerated add-on treatment associated with a more significant average improvement on the Ashworth Scale (is a measure of spasticity, as indicated by the amount of resistance encountered during passive stretching of soft-tissue, and the Modified Ashworth Scale (MAS) has an additional scoring category) or spasticity compared to placebo, although not statistically significant. However, there are conflicting studies that failed to demonstrate statistical significance in the efficacy of MM on the progression of MS after use for 36 months (95% CI, 2.0–0.2).Citation71,Citation72 Similarly, a study investigating the time to treatment failure and maintenance efficacy, an oromucosal spray which has an equal (1:1) ratio of THC:CBD (Sativex®), as an add-on treatment in the management of central neuropathic pain revealed conflicting results in the long-term efficacy maintenance of this treatment option.Citation70 The primary endpoint of time to treatment failure was statistically significant (P=0.04) in favor of THC:CBD spray, where 57% of the placebo group failed treatment, compared to only 24% of the THC:CBD group.
There is a scientific rationale for the role of MM in MS based on the understanding of the endocannabinoid system as well as improvements in subjective assessments of spasticity and other related symptoms. However, there is residual uncertainty about whether the effects of cannabinoids are real. These results may not be detected by “objective” outcome measures like the Ashworth scale, or if the perceived consequences are owing to the general psychoactive effect of THC on the CNS. Furthermore, although there were some promising findings in the Health Canada document, the fact remains that adverse effects of cannabis on cognition in people with MS does occur, as changes in cognitive function affects 40%–60% of patients with MS.Citation81 Therefore, changes in cognitive function should be appropriately monitored in individuals who begin a cannabis regimen. In addition, new clinical trials should explore other objective modalities such as the stretch reflex test which has demonstrated a statistically significant reduction in stretch reflex amplitude as well as statistically significant reductions in numeric rating scale (NRS) and MAS scores in assessing the improvement of MS-related spacticity.Citation74
Although there are indications that MM is effective in reducing patient-reported symptoms such as spasticity and pain, studies also show that cannabinoids have no proven overall effect on the progression of MS.Citation71,Citation72 Additional research on the long-term outcomes of MM in MS patients is required.
Epilepsy
As with MS patients who do not see an improvement in QOL following treatment, approximately one third of epileptic patients fail to respond to currently available antiepileptic drugs fully. Patients with treatment-resistant epilepsy have a higher prevalence of comorbidities,Citation82,Citation83 psychosocial and cognitive problems,Citation84 negative public attitudes,Citation82,Citation83 decreased QOL and increased risk of mortality.Citation85–Citation87 According to the 2013 Health Canada document on cannabinoids, the action of cannabinoid THC was too broad for therapeutic purposes, and there was insufficient evidence on CBDCitation4 to recommend MM as a potential treatment option for patients with epilepsy.
We identified five new trials published since 2013 investigating the therapeutic potential of CBD in the treatment of drug-resistant epilepsy in children or young adults failing to respond to conventional anticonvulsive medications.Citation88–Citation92 In addition to being administered CBD, these participants also continued their anticonvulsant drug regimen, most commonly clobazam (marketed under the brand names Frisium, Urbanol, Onfi, and Tapclob) and valproates, for the duration of the trials. Partial and atonic seizures had the most significant reduction in frequency followed by tonic and tonic–clonic seizures. CBD has shown some promise as a potential medical alternative in the treatment of drug-resistant epilepsy with minimal side effects. Based on the high-quality multicentered randomized controlled trials (RCTs) enrolling hundreds of patients to date,Citation90,Citation92,Citation93 there is evidence that CBD is effective on Lennox Gastaut, Dravet syndrome, and other types of childhood treatment-resistant epilepsy. In one study, a wide range of CBD is administered (from 0.5 to 50 mg/kg/day) with no correlation to the amount administered and adverse events.Citation90 Also, the mechanism(s) behind CBD therapy in the treatment of drug-resistant epilepsy is not well understood; thus, elucidating the pathway(s) of action is required to develop a more targeted treatment. Since CBD most potently inhibited the catalytic activity of human CYP3A4 and CYP3A5,Citation94,Citation95 co administered anticonvulsant medication needs to be monitored and adjusted on a regular basis.
Movement disorders
Given the location of cannabis receptors in the CNS, the scientific rationale for the use of MM to alleviate the symptoms associated with movement disorders is perhaps not surprising. Although several disorders could be considered, the therapeutic value of MM has only been investigated in Parkinson’s disease (PD) and Tourette’s syndrome (TS).Citation96
Parkinson’s Disease
PD is the second most common neurological illness in Canada following Alzheimer’s disease Citation97 and is characterized by the loss of nigrostriatal dopamine neurons leading to a tetrad of tremors, bradykinesia, rigidity, and postural instability.Citation98 Levodopa that replaces dopamine to improve motor symptoms is the current medication for PD, but fails to improve QOL, and is associated with many adverse effects such as dyskinesia.Citation98 Given the increasing evidence that suggests a prominent modulatory function of the endocannabinoids in the basal ganglia, the use of cannabinoids as a new therapeutic target has been recommended as a promising therapy for PD as well as for levodopa-induced dyskinesia.Citation99
In a double-blind clinical trial,Citation100 PD patients without dementia or comorbid psychiatric conditions were assigned to one of three groups: placebo, CBD 75 mg/day, and CBD 300 mg/day. There were no statistically significant differences in motor symptoms, neuroprotective effects, or magnetic resonance spectroscopy measures between the three groups; however, the 300 mg/day CBD group had a significantly different mean total score in well-being and QOL (P=0.05) compared to placebo, suggesting a possible effect of CBD in improving QOL in PD patients. In two open-label observational studiesCitation101,Citation102, PD patients demonstrated statistically significant improvements in their United Parkinson’s Disease Rating Scale (P<0.001), tremor (P<0.001), rigidity (P=0.004), and bradykinesia (P<0.001). They also demonstrated significant improvement in their sleep and pain scores just 30 minutes after smoking cannabis. Moreover, a case-series study that treated four PD patients suffering from “random eye movement” sleep behavior disorder (RBD) with 75–300 mg/day of CBD found that patients had a prompt and substantial reduction in the frequency of RBD-related events without side effects.Citation103
More extensive, controlled, randomized, and blinded clinical trials are required to better assess the role of cannabinoids in the treatment of PD and levodopa-associated dyskinesia, as small sample size and variability in study design limit our ability to draw definitive conclusions. Additional research is required to determine whether subsets of individuals with various neurological and psychiatric diseases derive the same therapeutic benefits from cannabis. However, these studies collectively demonstrate that marijuana plays a role in improving QOL measures in PD, with further studies being required to elucidate the exact effects/mechanisms of action.
Tourette’s Syndrome
TS is a common neurobehavioral disorder characterized by multiple motor and phonic tics, generally starting in childhood.Citation104 There are a substantial number of TS patients who are unsatisfied with the current treatment strategies due to either minimal efficacy or significant adverse effects.Citation105 Moreover, there is a lack of medications effective against both behavioral disorders and the tics associated with TS, resulting in many TS patients seeking alternative or complementary treatments including special diets, nutritional supplements, and drugs such as nicotine, alcohol, and C. sativa to alleviate their symptoms.Citation106 Therefore, it is exceedingly important for physicians to understand the efficacy of MM when advising patients on alternate treatment options.
According to Health Canada,Citation18 anecdotal and case reports have suggested an improvement in symptoms associated with TS when smoking cannabis. The Health Canada document also cites two small RCTs that assessed the effects of short duration. To our knowledge since then, there have been no recent clinical trials that study the role of MM in TS except for two case reports, both investigating the role of Sativex® in the treatment of TS. In the first study,Citation107 the patient received 10.8 mg THS and 10 mg CBD daily, in the form of two oromucosal sprays of Sativex®, twice daily. In the second case report,Citation108 the patient started on at a dose of 1 puff per day and slowly increased up to a dosage of 3×3 puffs per day. Both the studies demonstrated a significant reduction in motor and vocal tic severity and frequency following MM treatment. Moreover, the second case report showed a substantial improvement in the QOL associated with MM treatment. More extensive clinical trials studying the effects of MM on alleviating TS symptoms are required for physicians to comfortably decide whether the use of MM would be an appropriate alternative option.
GI disorders
The endocannabinoid system is vastly integrated within the GI tract, particularly within the enteric nervous system.Citation109 A high expression of CB1 on epithelial cells, submucosal neurons, and myenteric neurons and elevated expression of CB2 on immune cells within the GI tract suggest that there is a therapeutic rationale for MM use as treatment options for patients with GI disorders.Citation110,Citation111
Inflammatory bowel disease
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and Ulcerative Colitis (UC), causing inflammation of the bowel.Citation112 Significant morbidity occurs in IBD patients whose symptoms are uncontrolled by conventional therapies. Trials reported in Health Canada’s document demonstrated that cannabinoids might attenuate intestinal inflammation and symptoms of IBD in animal models through the activation of cannabinoid receptors in the GI tract.Citation4 Although cannabis could be used in the treatment of refractory IBD, clinical data did not show a strong association between cannabis and symptom relief in IBD patients.
A significant portion of self-medicating IBD patients found cannabinoids helpful for symptoms such as abdominal pain,Citation113–Citation115 poor appetite,Citation113,Citation114 nausea,Citation113,Citation114 diarrhea,Citation113–Citation115 and joint pain.Citation115 It was also found that CD patients were more likely to be cannabis users compared to those with UC and IC.Citation114 RCT set out to examine the therapeutic effects of smoked cannabis,Citation116 and the effects of CBD in treatment-refractory CD,Citation116 as defined by the Crohn Disease Activity Index score.Citation117 While these reports initially demonstrated that THC was involved, the role of CBD was unclear. Furthermore, anecdotal data focused on the positive effects of cannabis use in the treatment of IBD, making it challenging to conclude the therapeutic efficacy of such compounds as treatment options. Due to the small sample sizes and the short course marked differences in the dose administered (115 mg Δ9-THC/negligible CBD and 10 mg CBD twice a day, respectively), there remains a lack of reliable clinical evidence to support the use of MM in the treatment of IBD. A concerning finding was the correlation between long-term cannabis use and increased rate of surgical procedures in IBD patients,Citation115 with cannabis use potentially masking disease activity leading to worsened disease outcomes. Future studies should focus on more substantial double-blinded RCTs to assess the efficiency and safety of MM treatment in IBD patients, focusing on optimal routes of administration and dosing.
Anorexia
Anorexia is often associated with a variety of chronic illnesses such as Anorexia Nervosa (AN), HIV infection, and cancer.Citation118 Health Canada’s 2013 document reported several promising findings on the use of MM as an alternative agent for patients presenting with anorexia as a result of HIV infection. Specifically, patients with HIV who were administered non-dronabinol were reported to have a statistically significant increase in caloric intake compared to placebo, particularly in patients with substantial cachexia.Citation119 Furthermore, most public anorexia trials published in the Health Canada document,Citation4 as well as one of two new trials we found published since,Citation120 have used dronabinol, a synthetic Δ9-THC compound that is no longer available in Canada. Theoretically, dronabinol studies may be applied to other forms of THC; however, the dosing, side effects, long- and short-term safety, and comparative efficacy against placebo or other appetite stimulants may differ among different formulations.
Since the Health Canada document, there have been only two ongoing trials using cannabinoids in anorexia, both in the context of cancer. A pilot study out of Israel is currently analyzing the use of “Cannabis Capsules” (THC extract) for cancer-related anorexia.Citation121 The second trial, a randomized double-blinded study in Mexico, is looking at the effect of nabilone as an appetite stimulant in terminal lung-cancer patients.Citation122 Both the trials may have future utility as they offer alternatives to dronabinol and new evidence in a cancer population. However, despite the potential of MM as a therapeutic option, the fact remains that there is little-to-no clinical trial evidence guiding the use of non-dronabinol cannabinoids in anorexia. For future trials, we suggest the use of available THC sources and incorporate cannabis naïve populations or use comparison against other appetite stimulants as opposed to placebo. With these tenets in mind, evidence can guide the use of cannabinoids in anorexia and potentially improve patient outcomes.
Nausea and vomiting
According to the Health Canada document, nabilone, dronabinol, and levonantradol perform significantly better than placebo and slightly better than conventional dopamine D2-receptor antagonist anti-emetics in suppressing chemotherapy-induced nausea and vomiting (CINV).Citation4 Ondansetron and dronabinol provided same relief of CINV, but there was no additive benefit.Citation4 The Δ9-THC capsule had an equivalent effect to smoked cannabis. Although cannabinoids were associated with higher incidence of adverse events such as dizziness, dysphoria, euphoria, and sedation, some participants expressed a preference for cannabinoids over other antiemetics. There is still limited information on the relative efficacy of cannabinoids over the newer antiemetics such as 5-HT3 (ondansetron and granisetron) or neurokinin-1 receptor antagonists.
Two placebo-controlled trials on the effect of cannabinoids on postoperative nausea and vomiting were identified. Participants have been pretreated with 0.5 mg nabilone before surgery,Citation123 or administered 0.125 mg/kg THC intravenously at the end of surgery.Citation124 There was no significant difference in nausea and vomiting reduction between cannabinoids and placebo groups in both the trials, and clinically relevant psychotropic THC side effects such as sedation and confusion that were deemed unacceptable were reported.Citation124 Therefore, while chemotherapy patients should be aware of cannabinoids as an alternative treatment of CINV, the side effects have been deemed unacceptable in the outpatient and acute settings.
Pain
The endocannabinoid system is a critical endogenous pain control systemCitation27,Citation125, as such, the targeting of this system with cannabis may provide a therapeutic advantage in the treatment of pain.Citation125 This system is present throughout several pain pathways, with cannabinoid receptor agonists demonstrating antinociceptive effects in animal models of acute, inflammatory, and neuropathic pain. The modulation of pain is thought to be due to inhibition of presynaptic neurotransmitter release and modulation of postsynaptic excitability.Citation39,Citation126
Acute pain
The Health Canada document presented mixed results in the efficacy of cannabinoids in acute, experimentally induced pain.Citation4 The variety of administration modes, such as nabiximol, smoked cannabis, and oral THC, as well as small sample sizes may explain this inconsistent result.Citation4 Since 2013, there has been one randomized, placebo-controlled, double-blind clinical trial on this subject, finding that smoked marijuana and dronabinol decreased pain sensitivity (3.56% THC, 20 mg, respectively) and increased pain tolerance (1.98% THC, 20 mg, respectively) when compared against placebo.Citation127 However, the small sample size (N=30), exclusion of naïve users, including only THC content, and use of dronabinol necessitate further research before commenting on the efficacy of cannabis in the treatment of acute pain.
Chronic neuropathic pain
Neuropathic pain is a complex, chronic pain state that affects over 2 million Canadians,Citation128,Citation129 with half of the sufferers failing to achieve adequate relief.Citation130,Citation131 In 2015, the Canadian Pain Society updated their guidelines for the management of neuropathic pain moving cannabis from the fourth- to a third-line medication.Citation132 In recognition of the growing body of evidence, the 2013 Health Canada document also indicated that the addition of cannabinoid medications to conventional therapy was a moderately active short-term treatment of neuropathic pain.Citation4 However, additional research needs to be done examining modes of administration further to inhalation, as well as the use of non-dronabinol to maintain consistency with currently available medications.
Since the publication of the document, 10 relevant studies were published about cannabinoids in neuropathic pain (see Table S3 for a detailed summary of trial data).Citation130,Citation131,Citation133–Citation140 These studies addressed several gaps present in the Health Canada document including examining both THCCitation131,Citation136–Citation138,Citation140 and THC/CBD blends at various concentrations and routes of administration,Citation130,Citation135,Citation139 such as oral tablets,Citation137,Citation138 oromucosal spray,Citation130,Citation135,Citation139 vaporizing,Citation131,Citation136 and metered-dose inhaler.Citation140 Specifically, two studies examined the mode of action of cannabinoids in neuropathic pain by using functional magnetic resonance imaging (fMRI), demonstrating that THC may act on the active qualities of chronic pain by reducing sensory limbic functional connectivity between the amygdala and the primary somatosensory cortex.Citation133,Citation134 Also, three long-term trials demonstrated long-term efficacy, safety, and tolerability.Citation130,Citation138,Citation139 Lastly, in the remaining eight studies, six studies were blinded, randomized-controlled trialsCitation130,Citation131,Citation135–Citation138 and two were open-label trials,Citation139,Citation140 all of which had differing experimental designs. They unanimously demonstrated statistical significance in at least one or more measurements of neuropathic pain, including some responders with 30% reduction in pain, visual analog scale, and (NRS).Citation130,Citation131,Citation135–Citation140 These data have strengthened the evidence for the use of cannabinoids as adjuvant therapy in chronic neuropathic pain; however, gaps remain that need to be addressed in future research, such as the use of other cannabinoids, terpenes, and additional investigations regarding modes of administration. Nonetheless, these gaps should not prevent health care professionals from using marijuana and its analogs to combat neuropathic pain.
Chronic non-cancer-related pain
Health Canada initially grouped chronic non-cancer pain with neuropathic pain; however, we believe that chronic non-cancer pain best fits into its category. Four trials found that causes of pain included functional chest pain,Citation141 chronic pancreatitis-related pain,Citation142 chronic abdominal pain,Citation143 and unspecified chronic non-cancer pain.Citation144 Each trial examined a different cause of pain, and the results were inconsistent with the neuropathic pain trials regarding efficacy. This contradiction of the findings creates a need for each case to be examined individually to determine the effectiveness and is the main reason for the separation of the data from the neuropathic pain section. The results of each trial have been summarized and included in Table S3.
Cancer-related pain
In Canada, it is estimated that in 2017 there will be over 200,000 newly diagnosed cancer patients.Citation145 Because pain is the most commonly experienced symptom by cancer patients,Citation146 Health Canada reviewed the therapeutic efficacy of dronabinol and nabiximols in the management of cancer-related pain and found them to be efficacious in providing relief, although not all results were statistically significant.Citation4 However, trials with larger sample sizes investigating alternative modes of administration of cannabinoids are required to comment on the efficacy of cannabis in cancer conclusively.
Since 2013, three studies have been published regarding cannabis use in cancer pain. An observational study demonstrated that 70% of patients who were prescribed marijuana for pain management reported subjective improvement in their pain control.Citation5 Similarly, an open-label extension study on the long-term efficacy and safety of Sativex spray reported a decrease from the mean baseline pain of 0.63 (P=0.014) in THC/CBD spray group versus placebo.Citation147 To further confirm these results, an extension of this study demonstrated a decrease in mean Brief Pain Inventory Short Form (BPI-SF) scores for pain, severity, worst pain, and pain interference domains with the THC/CBD spray.Citation147 Unfortunately, this study had a significant dropout rate (42/43 patients), with almost half citing adverse events as the reason for leaving the study, suggesting that the harmful effects may outweigh the benefits of cannabinoid use in cancer.Citation147 Finally, a blinded RCT study examining nabilone in head and neck cancers determined that there was no difference in pain between intervention and placebo groups (P=0.6048) and that nabilone did not alter the time required for progression of illness by 20% (P=0.46).Citation148
The study results, excluding the observational questionnaire, are in contrast to the trials analyzed in the Health Canada document and may be attributed to small sample sizes and significant dropout rates. Given the quality of the evidence reviewed, it can be concluded that these studies have not significantly added to the current knowledge on treatment of pain in cancer, and thus more research will be needed to clarify this. Future blinded RCT studies on the role of cannabis in the treatment of cancer pain should include examining a variety of modes of administration in large patient populations and examining both short-term and long-term efficacy and safety profiles of cannabis products.
Headaches
Since the Health Canada review, many survey studies,Citation149–Citation152 and a chart reviewCitation153 have studied the therapeutic efficacy of MM in the treatment of headaches, however only one controlled clinical trial was conducted.Citation154 In this trial, nabilone (0.5 mg) was used in a randomized, double-blind, crossover design against ibuprofen (400 mg) in 30 patients with a medication-overuse headache (MOH) and daily analgesic intake. Primary outcome measures included headache frequency, daily analgesic intake, pain intensity and duration, level of dependence, and pain-free days. While both the drugs resulted in statistically significant improvement in all primary outcomes, nabilone was superior to ibuprofen (greater effect size) in all parameters. In addition, subgroup analyses showed that patients who received ibuprofen in the second half of the study (crossing over from nabilone) did not demonstrate ibuprofen-associated improvements seen in the overall data. Furthermore, these patients did not experience continued improvement 2 weeks following the study endpoint, unlike patients who received nabilone following treatment with ibuprofen. This methodologically sound study makes a compelling case for the efficacy of nabilone compared to ibuprofen in the MOH population but is limited by a small sample size, patient dropout (four of 30 patients), missing controls for cannabis-experienced or naïve patients, and a lack of a psychoactive placebo (affecting patient blinding). However, given the subjective nature of pain, the psychoactive effects of cannabinoids may be considered a new part of the therapeutic profile of cannabis if they affect the perception of pain.
Special considerations
Although there are some promising therapeutic applications of MM in the treatment of several conditions outlined above, a thorough understanding of patient history and specific patient subpopulations presenting with other states should be considered. These contradictions are outlined in detail below.
Mental health
According to the 2013 Health Canada report,Citation4 there was a dose–response relationship between cannabis use and the risk of psychotic disorders. Early exposure and greater use were linked to initial symptom onset, particularly in those predisposed to mental illness. Furthermore, cannabis use after the first psychotic episode or schizophrenia diagnosis was associated with weak prognostic features, such as multiple relapses and worse symptoms.
Since the Health Canada report, literature has confirmed a dose-dependent relationship between cannabis use and the risk of psychotic disorders.Citation155–Citation157 Early exposure (ie, before the age of 15 yearsCitation158,Citation159 or during adolescenceCitation160), greater use,Citation158–Citation160 and escalation to daily useCitation160 have all been linked to an earlier initial psychotic episode relative to nonusers. Specifically, patients with a history of cannabis use experienced their first psychotic episode from 2.6Citation161 to 2.9 years earlier than nonusers.Citation162 This information is particularly relevant for individuals at a higher risk for psychiatric illness, with predictive factors for conversion to psychotic disorders including psychotic features with cannabis use,Citation159 high potency cannabis, and high frequency of use.Citation158,Citation163 Furthermore, studies on the effects of other substances in attenuating the relationship between cannabis use and mental health outcomes seem to be insignificant.Citation164,Citation165 In addition, these materials were not significant predictors of psychosis onset,Citation158,Citation159,Citation163 which could be due to the relatively low rate of other substance abuse.
Schizophrenia
Patients with schizophrenia have been found to bê10 times more likely to use cannabis than the general population.Citation166,Citation167 For schizophrenia, there is early evidence that CBD may be a helpful treatment, while THC seems to worsen psychosis. Eight recent correlational studies not included in Health Canada Report investigated the effects of marijuana on schizophrenia severity, including positive and negative symptoms and level of function. However, it is noteworthy to mention that these studies are meant to provide information on patients with psychosis who use recreational cannabis and are not treatment studies. Across all reviewed studies, cannabis use had no significant effect on negative symptoms based on the Positive and Negative Syndrome Scale (PANSS).Citation157,Citation168–Citation174 Some studies reported an increased prevalence of positive symptoms with cannabis use (PANSS-P),Citation157,Citation172,Citation173 while others reported no significant effect.Citation168–Citation171 In a meta-analysis, history of or current cannabis use had a moderate effect on positive symptoms when compared to cannabis naïve participants.Citation174 However, due to the high heterogeneity between the included studies, we advise interpreting results with caution.
Lastly, there was no significant difference between cannabis users and nonusers in the ability to adapt to various problems-in-living, based on the Global Assessment of Functioning (GAF) scale.Citation157,Citation169–Citation173 It is possible that an upper limit on the safe quantity of cannabis exists after which GAF declines. During a follow-up period, a change in cannabis use, whether escalation or de-escalation, exhibited a reverse relationship with GAF.Citation157,Citation170,Citation171 The change indicates that the effects of cannabis were reversible and corresponded to the amount used. As an alternative, there could have been confounding variables that were not accountable. Overall, these findings imply that not all people are affected equally by cannabis and that physicians should advocate against heavy and early cannabis use.
Treatment adherence
The majority of studies did not control for treatment adherence, which is an important confounding variable, as current cannabis users are less likely to adhere to psychiatric medical therapy than nonusers and former users by a factor of 4.8 and 4.5, respectively.Citation175 High potency (defined as a high ratio of THC:CBD), cannabis being particularly noxious, is a statistically better predictor of nonadherence than low potency or infrequent use.Citation176 Nonadherence to medical treatment is a significant risk for clinical and psychosocial remission.Citation177 Nonadherence can also partially confound the effect of cannabis use on the risk of relapse, some relapses, time until relapse, and care intensity.Citation178 Future studies need to control for a wide array of confounding variables including treatment adherence, other substance use, and baseline differences.
Cognition
People with psychotic illness develop a more significant decline in their cognitive abilities relative to other mood disorders.Citation179 We identified seven recent articles that addressed the relationship between cannabis use and cognitive skills in psychotic illness. Only one study detected a diminished cognitive performance in social cognition with a long-term cannabis use.Citation180 However, other cognitive domains were unaffected. After controlling for confounders, such as age, the age of illness onset, socioeconomic status, premorbid IQ, the effect of cannabis on cognitive function was not significant based on The Digit Symbol Coding Test.Citation181,Citation182 Paradoxically, some studies report that cannabis use was associated with small but statistically significant improvement in global cognitive index,Citation183,Citation184 attention and psychomotor speed,Citation184 verbal learning and memory,Citation184 processing speed,Citation183 executive function,Citation183 working memory,Citation183 and visual memory.Citation183 A reverse association was detected in control populations without psychiatric illness. It is possible that the disease itself exerts a stronger effect on cognitive performance than cannabis. Alternatively, a subpopulation of patients who uses marijuana could be functioning better relative to nonusers. This could explain that abstinence from cannabis resulted in statistically significant improvement in memory and learning.Citation185,Citation186 Cannabis users could also develop compensatory mechanisms. Based on the functional imaging studies between healthy cannabis users and nonusers, despite no difference in cognitive performance, cannabis users exhibited slightly different brain activity relative to nonusers, which was described as a “compensatory” effort.Citation187 Overall, there is no convincing evidence due to cannabis use for a diminished cognitive performance in patients with psychiatric illness.
Physicians should strongly advise against daily or high potency cannabis use, early onset of use, and any use if it is associated with subthreshold psychotic features to prevent future psychiatric complications. However, evidence around cannabis use during mental illness is conflicting. Currently, there is no evidence of active adverse effects for cannabis use, except for moderate exacerbation of positive symptoms, reversible effects on global function, and some cognitive domains. Additional longitudinal research is needed to examine various levels of cannabis use on psychiatric symptoms and cognitive function with better control for confounding variables.
Post-Traumatic Stress Disorder
PTSD can have a variety of triggers that affect multiple populations that are encountered within primary care, such as veterans and sexual assault victims.Citation188 Despite this, there has been limited research into the management of treatment-refractory PTSD.Citation189 Within Health Canada’s document, only one pilot study on PTSD was covered, showing a positive effect of nabilone on helping with PTSD-associated nightmares.Citation190 Of the patients with treatment-refractory nightmares, 60% reported a total cessation of nightmares, 13% reported a “satisfactory reduction” of nightmares, and 28% withdrew the study due to adverse events.
Since Health Canada’s review, we identified two studies exploring the effects of cannabis on PTSD-associated nightmares. A recent open-label pilot study administering 5 mg THC in oil daily for 3 weeks showed a reduction in nightmare frequency.Citation191 The blinded placebo-controlled trial conducted by the Canadian Forces randomized patients with PTSD to 7 weeks of placebo or nabilone in a crossover design with a 2-week washout period between regimens.Citation192 The nabilone group had significantly less frequent and intense distressing dreams compared to placebo (P=0.03). For these studies, the cognitive effects (acute or chronic) associated with cannabinoid use should be examined carefully in patients with cognitively demanding occupations such as active military duty, as PTSD is highly prevalent in this population. Currently, there are multiple ongoing trials, including two in Canada,Citation193,Citation194 which investigate smoked, vaporized, and ingested cannabis for use in PTSD which would help address the gaps in current knowledge and solidify the evidence for or against the use of marijuana in PTSD.
Cannabis and pregnancy
Cannabinoid receptors have been detected in the placenta,Citation195 and some cannabinoids, such as THC, can cross the placenta,Citation196,Citation197 accumulating in breast milk.Citation198 Concerns are raised about potential adverse effects of cannabinoid exposure on fetal development. According to the 2013 Health Canada report, the short-term effects of cannabis on neonatal outcomes were inconsistent, with some studies reporting reduced birth weight and length,Citation199–Citation201 as well as a non-statistically significant trends toward sudden death,Citation202 while others reported no effect.Citation203–Citation205 Long-term effects included poor attention, visual analysis, and executive function but no IQ changes.Citation206,Citation207 Exposure to cannabis in breast milk also transiently hindered motor development at 1 year of age.Citation208
Smoking marijuana during pregnancy had no direct effect on maternal health, labor complications, or postnatal problems; however, increased maternal anemia was reported.Citation209 It is possible that this finding is secondary to a confounding variable or type I error. Cannabis users are more likely to be single,Citation210,Citation211 have a low income,Citation211 or be unemployed,Citation212 which may predispose infants toward nutritional deficiency. It is possible that cannabis use during pregnancy has an equivalent effect on maternal health as on any other adult user. However, according to various reports, marijuana use during pregnancy falls between 3.1% to 29.6%,Citation213 thus sufficiently powered, and well-controlled matched cohort studies are warranted to identify adverse effects on maternal health.
Furthermore, maternal cannabis consumption was associated with a 109.42 g reduction in newborn birth weight.Citation209 However, that decline is not clinically significant and is not associated with a statistically significant increase in the risk of developing teratogenic effects, fetal deformities, fetal distress, fetal demise, or abnormal lab values among cannabis users.Citation209,Citation214 However, neonatal intensive care unit/intensive care unit (NICU/ICU) admissions significantly increased from 54% to 102% for newborns exposed to cannabis as compared to nonusers.Citation209,Citation214 Torri et alCitation213 reported a significant cumulative effect on morbidity and mortality for newborns of marijuana smokers, particularly in infection-related morbidity, such as sepsis, pneumonia, or bacterial meningitis, and neurological morbidity. However, the study is not sufficient to detect individual risks as it comprises only 48 marijuana users compared to 1562 nonusers. Large-scale trials, with sufficient power, are required to identify the underlying cause of NICU/ICU admissions and cumulative morbidity.
We could not identify any recent research on the effect of cannabis use on breastfeeding or long-term outcomes since the 2013 Health Canada report. Such research is challenging due to the extended follow-up period needed and the presence of many confounding variables, such as parental cannabis use, socioeconomic status, family dynamic, and neighborhood influence. Although studies have reported no or transient effects of early cannabinoids exposure on growth,Citation215 motor,Citation216–Citation218 and cognitive development,Citation216–Citation219 these earlier findings have limited applications today, given that new cannabis strains are more potent than before.Citation220
Since cannabis use during pregnancy has a noticeable effect on early childhood morbidity, physicians should strongly advise against its recreational use during pregnancy. Pregnant women refusing or incapable of stopping cannabis use should be encouraged to obtain cannabis from approved sources where the exact amount of marijuana used can be monitored. Such information could be used in future research to quantify better cannabinoids consumed and identify dose-dependent outcomes. The new study should also consider various routes of cannabis administration, whether edible, smoked, or vaporized, and control confounding variables such as maternal health and socioeconomic status.
Cannabis and opioids
The widespread abuse of opioids has led to a spike in opioid-related death to 8.8 per 100,000 in Canada.Citation221 The increased prescribing practices of these drugs and the introduction of highly addictive, potent synthetics such as fentanyl may be attributed to the rampant spread of this epidemic. Methadone, buprenorphine, and naltrexone are the only three US Food and Drug Administration-approved drugs for long-term treatment of opiate addiction.Citation222 Several studies have hypothesized the potential use of cannabis for the treatment of opioid addiction; however, results from studies conducted on these proposed uses have shown conflicting results. Cannabis smoking during a methadone taper demonstrated no evidence for cannabis smoking reducing opioid-withdrawal symptoms (P=0.52).Citation223 Although smoked cannabis was not shown to be successful in reducing opioid withdrawal symptoms, it is yet to be seen if isolated cannabinoids such as CBD or different concentrations of cannabinoids have a role in opioid withdrawal. CBD may play a role due to its anti-anxiety effect,Citation224 curbing the extreme anxiety associated with opioid withdrawal.Citation225
Additional considerations
First, it is essential to remark that a single dose of Δ9-THC in chronic smokers can be detected up to 13 days following intake,Citation226 while in others, 80%–90% of a total Δ9-THC dose will be excreted within 5 days.Citation227 Additional evidence has shown that both Δ9-THC and 11-OH-THC (an active Δ9-THC metabolite) can be detected in circulation for up to 1 month after intake, causing neurocognitive impairment in the first weeks of abstinence.Citation228
Second, it has been shown that cannabinoids can cause increased glucose intake and lipogenesis.Citation229 Therefore, if authorizing the use of medicinal cannabis for obese diabetic patients who are receiving insulin injections, the effects of MM on blood glucose levels and the patients’ response to their current treatment regimen should be examined with these underlying impacts in mind, particularly when considering a change in treatment or dose.
Cannabis abuse
Tolerance to THC is theorized to be due to downregulation and desensitization of CB1Citation230,Citation231 and has been documented in heavy and therapeutic users, but not in social users.Citation232,Citation233 Physical and psychological dependence also occurs with heavy usage.Citation234,Citation235 However, according to National Epidemiological Survey on Alcohol and Related Conditions, the rate of transition to dependence for cannabis is 8.9%, which is small percentage relatively to 22.7% and 67.5% for alcohol and nicotine, respectively.Citation236 Moreover, the withdrawal symptoms of marijuana are milder than other drugs,Citation231 such as alcohol, cocaine, heroin, and include anger, depressed mood, irritability, anxiety, restlessness, insomnia, strange dreams, weight loss, and decreased appetite.Citation40 The delayed onset of withdrawal due to THC’s relatively long half-life and relative mildness of symptoms compared to other substances contributes to apprehensions of its clinical implications.Citation231,Citation237
A few studies examined agonist therapy with synthetic cannabinoids to attenuate withdrawal symptoms and promote cannabis use cessation. In the placebo-controlled trial, dronabinol suppressed cannabis withdrawal symptoms in a dose-dependent manner based on the withdrawal discomfort score (P<0.05).Citation238 Another study using nabiximols significantly attenuated withdrawal symptoms relative to placebo (P=0.01) but did not have a better effect than placebo on a complete cessation of cannabis use (P=0.75).Citation239 A similar study using Sativex was found to reduce withdrawal symptoms (P<0.01) with high fixed doses but was also unable to demonstrate long-term cessation.Citation240 The attenuation of withdrawal could be due to the tapering off effect created by supplementing cannabis with synthetic cannabinoids. However, because opioids and cannabinoids have been shown to interact synergistically with each other, if a patient is prescribed both opioid and cannabis, care providers should know that opioid may need to be reduced to avoid dependency.Citation241 Further research needs to be done on the amount and THC/CBD ratio of cannabinoids necessary to safely taper withdrawal.
Other studies investigated vilazadone,Citation242 escitalopram,Citation243 buspirone,Citation244 lithium carbonate,Citation245 and a combination of lofexidine and dronabinol,Citation246 to treat cannabis dependence, but none showed any significant results. Only gabapentin significantly reduced the amount of marijuana smoked per week based on patient self-report (P=0.004) and the biochemical urine analysis (P=0.001).Citation247 However, gabapentin also carries abuse potential.Citation248–Citation250 The addiction potential of cannabis is a concern to clinicians and should be discussed with patients. The risk of addiction must be weighed against the benefit on a case-by-case basis. Currently, an accepted pharmacological treatment for cannabis-use disorders does not exist.
Conclusion
In summary, the effect of cannabis has been intensely studied in several disease states, as previously discussed; however, gaps in our knowledge remain. Although recent research has advanced our understanding from the release of the 2013 Health Canada document, there is a need for additional research that addresses different modes of administration, controlling for cannabis users and cannabis naïve individuals, as well as for other contraindications. Bearing this in mind, our current knowledge on cannabis use suggests that cannabis presents as an appropriate alternative therapy option for patients who have epilepsy, movement disorders, and pain. For individuals with MS, GI disorders, anorexia, and headaches, further research is recommended to improve our understanding of the effects of MM, and caution is advised when considering the authorization of MM use. For patients who are under the age of 25 years, pregnant, or present with a history of mental health and substance use, it is safe to err on the side of caution and avoid MM authorization. Overall, MM is an exciting field of exploration, and the diverse range of receptor expression in the human body offers many therapeutic benefits, yet additional research is required for a more robust understanding and characterization of the mechanism of action of MM to achieve maximal therapeutic efficacy.
Author information
YM (MBBCh), AV (MD), SMSF (MBBS), CN (MSc, MD), AL (MD), and UB (MBBS) are postgraduate medical trainees in the Graduate Diploma and Professional Master in Medical Sciences (GDPM), Postgraduate Medical Education, School of Medicine, Faculty of Health Sciences, Queen’s University, Kingston, ON, K7L 3N6, Canada. BQ (BSc) and MS (MSc) are graduate research trainees in the laboratory of Prof. Dr. Myron R Szewczuk, Department of Biomedical and Molecular Sciences (DBMS), Faculty of Health Science, Queen’s University. MRS (BSc, MSc, PhD) is Full Professor in DBMS and Academic and Research Lead of the GDPM program.
Data sharing statement
The data sets supporting the conclusions of this article are included within the article and in the supplementary material.
Author contributions
MRS conceived the review of the article and obtained funding; YM, AV, BQ, MS, SMSF, CN, AL, and UB designed the study, carried out the searches, refined the study design, selected studies and extracted data, and conducted the thematic analyses; YM, AV, BQ, MS, and MRS led the writing of the draft manuscript as contributing first authorship. All Authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to the initial internal peer-reviewers with expertise in medicinal marijuana for their comments and suggestions for the improvement of this review article. The internal reviewers are from the Faculty of Arts & Sciences, University of Toronto, Toronto; Pharmacology and Toxicology, the Department of Biomedical and Medical Sciences, Queen’s University; the Department of Psychiatry, School of Medicine, Queen’s University; and the Department of Oncology and Undergraduate Medical Education for Palliative Care, School of Medicine, Queen’s University, Kingston, ON, Canada. The authors acknowledge support from the Graduate Diploma & Professional Master in Medical Sciences, School of Medicine, Queen’s University, Kingston, ON, Canada. This review was an independent research study funded in part by Postgraduate Medical Education, Queen’s University, Kingston, ON, Canada, and the Natural Sciences and Engineering Research Council of Canada and a private sector cancer funding from the Josefowitz Family to MRS. The views expressed in this article are those of the authors and not the funding bodies.
Disclosure
B Qorri is a recipient of the Queen’s Graduate Award (QGA) and the 2017 Terry Fox Research Institute Transdisciplinary Training Program in Cancer Research. M Sambi is a recipient of the QGA. The authors report no other conflicts of interest in this work.
References
- Belle-IsleLWalshZCallawayRBarriers to access for Canadians who use cannabis for therapeutic purposesInt J Drug Policy201425469169924947993
- Minister of JusticeAccess to Cannabis for Medical Purposes RegulationsCanadaMinister of Justice2016 Available from: http://www.gazette.gc.ca/rp-pr/p2/2016/2016-08-24/html/sor-dors230-eng.htmlAccessed November 1, 2017
- The College of Physicians and Surgeons of OntarioMarijuana for Medical PurposesToronto, ONThe College of Physicians and Surgeons of Ontario2015 Available from: http://www.cpso.on.ca/Policies-Publications/Policy/Marijuana-for-Medical-PurposesAccessed November 1, 2017
- Health CanadaInformation for Health Care Professionals: Cannabis (Marihana, Marijuana) and the CannabinoidsCanadaGovernment of Canada2013 Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-use-marijuana/information-medical-practitioners/information-health-care-professionals-cannabis-marihuana-marijuana-cannabinoids.htmlAccessed November 1, 2017
- WaissengrinBUrbanDLeshamYGartyMWolfIPatterns of use of medical cannabis among Israeli cancer patients: a single instituition experienceJ Pain Sympt Manag2015492223230
- ZiemianskiDCaplerRTekanoffRLacasseALuconiFWareMACannabis in medicine: a national educational needs assessment among Canadian physiciansBMC Med Educ20151515225888752
- KondradEReidAColorado family physicians’ attitudes toward medical marijuanaJ Am Board Fam Med2013261526023288281
- The College of Physicians and Surgeons of OntarioPolicy Statement on Marijuana for Medical Purposes2015 Available from: http://policyconsult.cpso.on.ca/?page_id=4090Accessed May 1, 2018
- College of Physicians and Surgeons of Nova ScotiaPolicy Regarding the Authorization of Marijuana for Medical Purposes2014 Available from: http://www.cpsns.ns.ca/DesktopModules/Bring2mind/DMX/Download.aspx?PortalId=0&TabId=129&EntryId=52
- College of Physicians and Surgeons of SaskatchewanRegulatory Bylaws2015 Available from: http://cps.sk.ca/Documents/Legislation/Legislation/2015_Regulatory_Bylaws_-_June_2015.pdf
- Collège Des Médecins, QuébecGuidelines Concerning the Prescription of Dried Cannabis for Medical Purposes2015 Available from: http://www.cmq.org/publications-pdf/p-1-2014-04-01-en-directives-concernant-ordonnance-cannabis-seche-fins-medicales.pdf?t=1455740574019
- The College of Physicians and Surgeons of ManitobaStandards of Practice of Medicine (Bylaw 11)2016 Available from: http://cpsm.mb.ca/cjj39alckF30a/wp-content/uploads/ByLaws/By-Law-11.pdf
- The College of Physicians and Surgeons of Prince Edward IslandPolicy on Prescribing of Medical Marijuana2016 Available from: http://cpspei.ca/wp-content/uploads/2017/03/Marijuana-Prescribing-Nov-3016.pdf
- The College of Physicians and Surgeons of Newfoundland and LabradorAdvisory and Interim Guideline2014 Available from: http://www.cpsnl.ca/default.asp?com=Policies&m=340&y=&id=98
- The College of Physicians and Surgeons of British ColumbiaProfessional Standards and Guidelines on Marijuana for Medical Purposes2015 Available from: https://www.cpsbc.ca/files/pdf/PSG-Marijuana-for-Medical-Purposes.pdf
- The College of Physicians and Surgeons of AlbertaStandard of Prac-tice on Cannabis for Medical Purposes2014 Available from: http://www.cpsa.ca/standardspractice/cannabis-for-medical-purposes/
- Canadian Medical Protective AssociationMedical Marijuana: Considerations for Canadian Doctors2016 Available from: https://www.cmpa-acpm.ca/en/advice-publications/browse-articles//medical-marijuana-new-regulations-new-college-guidance-for-canadian-doctors2014
- Government of CanadaInformation for Health Care Professionals: Cannabis (marihuana, marijuana) and the cannabinoids [Health Canada, 2013]2013 Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-use-marijuana/information-medical-practitioners/information-health-care-professionals-cannabis-marihuana-marijuana-cannabinoids.htmlAccessed November 1, 2017
- CAMHCannabis Policy Framework2014 Available from: https://www.ijdp.org/article/S0955-3959(16)30114-1/fulltextAccessed May 1, 2017
- The College of Family Physicians of CanadaAuthorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance2014 Availale from: http://www.cfpc.ca/Release_Dried_Cannabis_Prelim_Guidance/
- Ligresti A, De Petrocellis L, Di Marzo V. from phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacologyPhysiol Rev20169641593165927630175
- IzzoAAMascoloNCapassoFThe gastrointestinal pharmacology of cannabinoidsCurr Opin Pharmacol20011659760311757815
- Di MarzoVMelckDBisognoTDe PetrocellisLEndocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory actionTrends Neurosci199821125215289881850
- NavarroMCarreraMRAFrattaWFunctional interaction between opioid and cannabinoid receptors in drug self-administrationJ Neurosci20012114534411438610
- GómezRNavarroMFerrerBA peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feedingJ Neurosci20022221961212417686
- CalignanoALa RanaGLoubet-LescouliéPPiomelliDA role for the endogenous cannabinoid system in the peripheal control of pain initiationProg Brain Res200012947148211098711
- PertweeRGCannabinoid receptors and painProg Neurobiol200163556961111164622
- CastellanoCRossi-ArnaudCCestariVCostanziMCannabinoids and memory: animal studiesCurr Drug Targets CNS Neurol Disord20032638940214683467
- Rodríguez de FonsecaFDel ArcoIMartín-CalderónJLGorritiMANavarroMRole of the endogenous cannabinoid system in the regulation of motor activityNeurobiol Dis1998564835019974180
- CabralGAMarijuana and cannabinoidsJ Cannabis Ther200113–46185
- RobsonPJTherapeutic potential of cannabinoid medicinesDrug Test Anal201461–2243024006213
- MunroSThomasKLAbu-ShaarMMolecular characterization of a peripheral receptor for cannabinoidsNature1993365644161657689702
- MatsudaLALolaitSJBrownsteinMJYoungACBonnerTIStructure of a cannabinoid receptor and functional expression of the cloned cDNANature199034662845615642165569
- HerkenhamMLynnABLittleMDCannabinoid receptor localization in brainProc Natl Acad Sci U S A1990875193219362308954
- MackieKCannabinoid receptors as therapeutic targetsAnnu Rev Pharmacol Toxicol20064610112216402900
- DuncanMMouihateAMackieKCannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated ratsAm J Physiol Gastrointestinal Liver Physiol20082951G78G87
- DevaneWAHanusLBreuerAIsolation and structure of a brain constituent that binds to the cannabinoid receptorScience (New York, NY)1992258509019461949
- StellaNSchweitzerPPiomelliDA second endogenous cannabinoid that modulates long-term potentiationNature199738866447737789285589
- Aizpurua-OlaizolaOElezgaraiIRico-BarrioIZarandonaIEtxebarriaNUsobiagaATargeting the endocannabinoid system: future therapeutic strategiesDrug Discov Today201722110511027554802
- BaronEPComprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: what a long strange trip it’s beenHeadache201555688591626015168
- HillAJWilliamsCMWhalleyBJStephensGJPhytocannabinoids as novel therapeutic agents in CNS disordersPharmacol Ther20121331799721924288
- ElsohlyMASladeDChemical constituents of marijuana: the complex mixture of natural cannabinoidsLife Sci200578553954816199061
- RaczINadalXAlferinkJCrucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic painJ Neurosci20082846121251213519005077
- PertweeRGThe diverse CB(1) and CB(2) receptor pharmacology of three plant cannabinoids: Δ(9)-tetrahydrocannabinol, cannabidiol and Δ(9)-tetrahydrocannabivarinBr J Pharmacol2008153219921517828291
- HilligKWMahlbergPGA chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae)Am J Bot200491696697521653452
- MehmedicZChandraSSladeDPotency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008J Forensic Sci20105551209121720487147
- FasinuPSPhillipsSElSohlyMAWalkerLACurrent status and prospects for cannabidiol preparations as new therapeutic agentsPharmacotherapy201636778179627285147
- MorganCJAFreemanTPSchaferGLCurranHVCannabidiol attenuates the appetitive effects of [delta]9-tetrahydrocannabinol in humans smoking their chosen cannabisNeuropsychopharmacology20103591879188520428110
- YucelMLorenzettiVSuoCHippocampal harms, protection and recovery following regular cannabis useTransl Psychiatry20166e71026756903
- RussoEGuyGWA tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiolMed Hypotheses20066623424616209908
- HuestisMAPharmacokinetics and metabolism of the plant cannabinoids, Δ9-tetrahydrocannibinol, cannabidiol and cannabinolPertweeRGCannabinoidsBerlin, HeidelbergSpringer Berlin Heidelberg2005657690
- WhittleBAGuyGWDevelopment of cannabis-based medicines: risk, benefit and serendipityGuyGWRobsonPJWhittleBAThe Medicinal Uses of Cannabis and CannabinoidsLondonPharmaceutical Press2004427463
- DussyFEHambergCLuginbühlMSchwerzmannTBriellmannTAIsolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis productsForensic Sci Int2005149131015734104
- IversenLThe pharmacology of THC, the psychoactive ingredient in CannabisThe Science of MarijuanaNew York, NYOxford University Press20002937
- HuestisMAHuman cannabinoid pharmacokineticsChem Biodivers2007481770180417712819
- AbramsDIVizosoHPShadeSBJayCKellyMEBenowitzNLVaporization as a smokeless cannabis delivery system: a pilot studyClin Pharmacol Ther200782557257817429350
- PomahacovaBVan der KooyFVerpoorteRCannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativaInhal Toxicol200921131108111219852551
- MattesRDShawLMEdling-OwensJEngelmanKElsohlyMABypassing the first-pass effect for the therapeutic use of cannabinoidsPharmacol Biochem Behav19934437457478383856
- AdelliGRBhagavPTaskarPDevelopment of a Δ9-tetrahydrocannabinol amino acid-dicarboxylate prodrug with improved ocular bioavailability Δ9-THC prodrugs for improved ocular bioavailabilityInvest Ophthalmol Vis Sci20175842167217928399267
- KerstrinIFranjoGAn update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studiesCannabis Cannabinoid Res20172113915428861514
- GarrettERHuntCAPharmacokinetics of Δ9-tetrahydrocannabinol in dogsJ Pharm Sci1977663395407845807
- WidmanMAgurellSEhrneboMJonesGBinding of (+)- and (−)-Δ1-tetrahydrocannabinols and (−)-7-hydroxy-Δ1-tetrahydrocannabinol to blood cells and plasma proteins in manJ Pharm Pharmacol197426119149164156569
- WahlqvistMNilssonIMSandbergFAgurellSGranstrandBBinding of δ1-tetrahydrocannabinol to human plasma proteinsBiochem Pharmacol1970199257925825478282
- TruittEBBiological disposition of tetraydrocannabinolsPharmacol Rev19712342732784399986
- Public Health Agency of CanadaMultiple sclerosis Available from: http://www.phac-aspc.gc.ca/cd-mc/ms-sp/index-eng.phpAccessed November 1, 2017
- OlsenSAA review of complementary and alternative medicine (CAM) by people with multiple sclerosisOccup Ther Int2009161577019222053
- PryceGBakerDEndocannabinoids in multiple sclerosis and amyotrophic lateral sclerosisPertweeRGEndocannabinoidsChamSpringer International Publishing2015213231
- KindredJHLiKGKetelhutNBCannabis use in people with Parkinson’s disease and multiple sclerosis: a web-based investigationComplement Ther Med2017339910428735833
- BrentonJNSchreinerTKaroscikKAttitudes, perceptions, and use of marijuana in youth with multiple sclerosisJ Neurol2018265241742329273844
- LangfordRMMaresJNovotnaAA double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosisJ Neurol2013260498499723180178
- BallSVickeryJHobartJThe cannabinoid use in progressive inflammatory brain disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosisHealth Technol Assess20151912viiviiixxvxxxi1187
- ZajicekJBallSWrightDEffect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trialLancet Neurol129857865
- LeocaniLNuaraAHoudayerESativex® and clinical–neurophysiological measures of spasticity in progressive multiple sclerosisJ Neurol2015262112520252726289497
- MarinelliLMoriLCannevaSThe effect of cannabinoids on the stretch reflex in multiple sclerosis spasticityInt Clin Psychopharmacol201631423223927003093
- FlacheneckerPHenzeTZettlUKNabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice – results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticityEur Neurol2014715–627127924525548
- FlacheneckerPHenzeTZettlUKLong-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practiceEur Neurol2014721–29510224943098
- FernándezLLBoquetEMPérez-MirallesFClinical experiences with cannabinoids in spasticity management in multiple sclerosisNeurología (English Edition)2014295257260
- KoehlerJFenebergWMeierMPöllmannWClinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticityInt J Neurosci2014124965265624392812
- FlacheneckerPA new multiple sclerosis spasticity treatment option: effect in everyday clinical practice and cost–effectiveness in GermanyExpert Rev Neurother201313Suppl 11519
- García-MerinoAEndocannabinoid system modulator use in everyday clinical practice in the UK and SpainExpert Rev Neurother201313Suppl 191323369054
- HonarmandKTierneyMCO’ConnorPFeinsteinAEffects of cannabis on cognitive function in patients with multiple sclerosisNeurology201176131153116021444900
- GaitatzisAJohnsonALChadwickDWShorvonSDSanderJWLife expectancy in people with newly diagnosed epilepsyBrain2004127112427243215371287
- TrinkaEEpilepsy: comorbidity in the elderlyActa Neurol Scand2003108s1803336
- McCaghJFiskJEBakerGAEpilepsy, psychosocial and cognitive functioningEpilepsy Res200986111419616921
- MohanrajRNorrieJStephenLJKellyKHitirisNBrodieMJMortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative studyLancet Neurol20065648148716713919
- TrinkaEBauerGOberaignerWNdayisabaJPSeppiKGranbichlerCACause-specific mortality among patients with epilepsy: results from a 30-year cohort studyEpilepsia201354349550123167828
- GaitatzisASisodiyaSMSanderJWThe somatic comorbidity of epilepsy: a weighty but often unrecognized burdenEpilepsia20125381282129322691064
- HessEJMoodyKAGeffreyALCannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complexEpilepsia201657101617162427696387
- PorterBEJacobsonCReport of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsyEpilepsy Behav201329357457724237632
- DevinskyOMarshEFriedmanDCannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trialLancet Neurol201615327027826724101
- TzadokMUliel-SiboniSLinderICBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experienceSeizure201635414426800377
- DevinskyOCrossJHLauxLTrial of cannabidiol for drug-resistant seizures in the dravet syndromeN Engl J Med2017376212011202028538134
- ThieleEAMarshEDFrenchJACannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trialLancet2018391101251085109629395273
- YamaoriSEbisawaJOkushimaYYamamotoIWatanabeKPotent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moietyLife Sci2011881573073621356216
- JiangRYamaoriSTakedaSYamamotoIWatanabeKIdentification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomesLife Sci201189516517021704641
- LimKSeeYMLeeJA systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disordersClin Psychopharm Neurosci2017154301312
- WongSLGilmourHRamage-MorinPLParkinson’s disease: prevalence, diagnosis and impact2015 Available from: http://www.statcan.gc.ca/pub/82-003-x/2014011/article/14112-eng.pdfAccessed May 1, 2018
- BabayevaMAssefaHBasuPChumkiSLoewyZMarijuana compounds: a nonconventional approach to Parkinson’s disease therapyParkinsons Dis2016201619
- MoreSVChoiDKPromising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotectionMol Neurodegener20151011725888232
- ChagasMHNZuardiAWTumasVEffects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trialJ Psychopharmacol201428111088109825237116
- LotanITrevesTARoditiYDjaldettiRCannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational studyClin Neuropharmacol2014372414424614667
- ShohetAKhlebtovskyARoizenNRoditiYDjaldettiREffect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson’s diseaseEur J Pain201721348649327723182
- ChagasMEckeliAZuardiACannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case seriesJ Clin Pharm Ther201439556456624845114
- JankovicJKurlanRTourette syndrome: evolving conceptsMov Disord20112661149115621484868
- Müller-VahlKRTreatment of Tourette syndrome with cannabinoidsBehav Neurol201327111912423187140
- Müller-VahlKKolbeHDenglerRGilles de la Tourette syndrome. Effect of nicotine, alcohol and marihuana on clinical symptomsDer Nervenarzt199768129859465342
- TrainorDEvansLBirdRSevere motor and vocal tics controlled with Sativex®Australas Psychiatry201624654154427558217
- KanaanASJakubovskiEMüller-VahlKSignificant tic reduction in an otherwise treatment-resistant patient with Gilles de la Tourette syndrome following treatment with nabiximolsBrain Sci20177547
- DiPatrizioNVEndocannabinoids in the gutCannabis Cannabinoid Res201611677727413788
- MackieKCannabinoid receptors: where they are and what they doJ Neuroendocrinol200820101418426493
- IzzoAACouttsAACannabinoids and the digestive tractHandb Exp Pharmacol2005168573598
- RocchiABenchimolEIBernsteinCNInflammatory bowel disease: a Canadian burden of illness reviewCan J Gastroenterol2012261181181723166905
- PhatakUPRojas-VelasquezDPortoAPashankarDSPrevalence and patterns of marijuana use in young adults with inflammatory bowel diseaseJ Pediatr Gastroenterol Nutr201764226126427846066
- AllegrettiJRCourtwrightALucciMKorzenikJRLevineJMarijuana use patterns among patients with inflammatory bowel diseaseInflamm Bowel Dis20131913280924185313
- StorrMDevlinSKaplanGGPanaccioneRAndrewsCNCannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s diseaseInflamm Bowel Dis201420347248024407485
- NaftaliTMechulamRMariiALow-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trialDig Dis Sci20176261615162028349233
- NaftaliTBar-Lev SchleiderLDotanILanskyEPSklerovsky BenjaminovFKonikoffFMCannabis induces a clinical response in patients with crohn’s disease: a prospective placebo-controlled studyClin Gastroenterol Hepatol2013111012761280.e127123648372
- Controlled Substances and Tobacco DirectorateInformation for health care professionals: cannabis (marihuana, marijuana) and the cannabinoids2013 Available from: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/marihuana/med/infoprof-eng.pdfAccessed November 1, 2017
- HaneyMRabkinJGundersonEFoltinRWDronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and moodPsychopharmacology2005181117017815778874
- AndriesAFrystykJFlyvbjergAStovingRKDronabinol in severe, enduring anorexia nervosa: a randomized controlled trialInt J Eat Disord2014471182324105610
- AbbracchioMPDi LucaMDi GiulioAMCattabeniFTenconiBGorioADenervation and hyperinnervation in the nervous system of diabetic animals: III. Functional alterations of G proteins in diabetic encephalopathyJ Neurosci Res19892445175232513414
- ClinicalTrials.govIdentifier NCT02802540, Nabilone effect on the attenuation of anorexia, nutritional status and quality of life in lung cancer patients2016 Available from: https://clinicaltrials.gov/show/NCT02802540Accessed November 1, 2017
- LevinDNDulbergZChanA-WHareGMMazerCDHongAA randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgeryCan J Anesth201764438539528160217
- Kleine-BrueggeneyMGreifRBrenneisenRUrwylerNStueberFTheilerLGIntravenous delta-9-tetrahydrocannabinol to prevent postoperative nausea and vomiting: a randomized controlled trialAnesth Analg201512151157116426426861
- WoodhamsSGChapmanVFinnDPHohmannAGNeugebauerVThe cannabinoid system and painNeuropharmacology2017124Suppl C10512028625720
- StarowiczKFinnDPChapter thirteen – cannabinoids and pain: sites and mechanisms of actionKendallDAlexanderSPHAdvances in Pharmacology80Cambridge, MAAcademic Press2017437475
- CooperZDSandraDCHaneyMComparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokersNeuropsychopharmacology2013381019821992
- BouhassiraDLanteri-MinetMAttalNLaurentBTouboulCPrevalence of chronic pain with neuropathic characteristics in the general populationPain2008136338038717888574
- TorranceNSmithBHBennettMILeeAJThe epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population surveyJ Pain20067428128916618472
- SerpellMRatcliffeSHovorkaJA double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatmentEur J Pain2014187999101224420962
- WilseyBMarcotteTDeutschRGouauxBSakaiSDonagheHLow-dose vaporized cannabis significantly improves neuropathic painJ Pain201314213614823237736
- MoulinDEBoulangerAClarkAJPharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain SocietyPain Res Manag201419632833525479151
- WalterCOertelBGFeldenLBrain mapping-based model of [delta]9-tetrahydrocannabinol effects on connectivity in the pain matrixNeuropsychopharmacology20164161659166926514581
- LeeMCPlonerMWiechKAmygdala actviity contributes to the dissociative effect of cannabis on pain perceptionPain2013154112413423273106
- LynchMECesar-RittenbergPHohmannAA double-blind, placebo-controlled, crossover pilot trial with extension using and oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic painJ Pain Symptom Manage201447116617323742737
- WallaceMSMarcotteTDUmlaufAGouauxBAtkinsonJHEfficacy of inhaled cannabis on painful diabetic neuropathyJ Pain201516761662725843054
- van AmerongenGKanhaiKBaakmanACEffects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosisClin Ther2017 Epub29
- TurcotteDDoupeMTorabiMNabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trialPain Med201516114915925288189
- HoggartBRatcliffeSEhlerEA multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic painJ Neurol20152621274025270679
- EisenbergEOgintzMAlmogSThe pharmacokinetics, efficacy, safety, and ease of use of a novel portable metered-dose cannabis inhaler in patients with chronic neuropathic pain: a phase 1a studyJ Pain Palliat Care Pharmacother201428321622525118789
- MalikZBaymanLValestinJRizvi-TonerAHashmiSScheyRDronabinol increases pain threshold in patients with functional chest pain: a pilot double-blind placebo-controlled trialDis Esophagus201730218
- de VriesMVan RijckevorselDCMVissersKCPWilder-SmithOHGVan GoorHSingle dose delta-9-tetrahydrocannabinol in chronic pancreatitis patients: analgesic efficacy, pharmacokinetics and tolerabilityBr J Clin Pharmacol201681352553726505163
- de VriesMvan RijckevorselDCMVissersKCPWilder-SmithOHGvan GoorHTetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled studyClin Gastroenterol Hepatol201715710791086.e107427720917
- WareMAWangTShapiroSColletJPCannabis for the management of pain: assessment of safety study (COMPASS)J Pain201516121233124226385201
- Canadian Cancer SocietyCanadian cancer statistics2017 Available from: http://www.cancer.ca/~/media/cancer.ca/CW/publications/Cana-dian%20Cancer%20Statistics/Canadian-Cancer-Statistics-2017-EN.pdfAccessed November 1, 2017
- DeandreaSMontanariMMojaLApoloneGPrevalence of under-treatment in cancer pain. A review of published literatureAnn Oncol200819121985199118632721
- JohnsonJRLossignolDBrunell-NugentMFallonMTAn open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractrory to strong opioid analgesicsJ Pain Sympt Manage2013462207218
- CôtéMTrudelMWangCFortinAImproving quality of life with nabilone during radiotherapy treatments for head and neck cancersAnn Otol Rhinol Laryngol2016125431732426503964
- Beckmann YY, Seçkin M, Manavgat Aİ, Zorlu N. Headaches related to psychoactive substance useClin Neurol Neurosurg2012114799099922424726
- RossiPAllenaMTassorelliCIllicit drug use in cluster headache patients and in the general population: a comparative cross-sectional surveyCephalalgia201232141031104022967729
- LorenzoCDCoppolaGLorenzoGDBracagliaMRossiPPierelliFThe use of illicit drugs as self-medication in the treatment of cluster headache: results from an Italian online surveyCephalalgia201636219419825903763
- PearceDDMitsourasKIrizarryKJDiscriminating the effects of Cannabis sativa and Cannabis indica: a web survey of medical cannabis usersJ Altern Complement Med2014201078779125191852
- RhyneDNAndersonSLGeddeMBorgeltLMEffects of medical marijuana on migraine headache frequency in an adult populationPharmacotherapy201636550551026749285
- PiniLAGuerzoniSCainazzoMMNabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trialJ Headache Pain201213867768423070400
- MarconiADi FortiMLewisCMMurrayRMVassosEMetaanalysis of the association between the level of cannabis use and risk of psychosisSchizophr Bull20164251262126926884547
- DavisGPComptonMTWangSLevinFRBlancoCAssociation between cannabis use, psychosis, and schizotypal personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related ConditionsSchizophr Res2013151119720224200416
- StoneJFisherHMajorBCannabis use and first-episode psychosis: relationship with manic and psychotic symptoms, and with age at presentationPsychol Med201444349950623701858
- Di FortiMMarconiACarraEProportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control studyLancet Psychiatry20152323323826359901
- McHughMMcGorryPYungACannabis-induced attenuated psychotic symptoms: implications for prognosis in young people at ultra-high risk for psychosisPsychol Med201747461662627821204
- KelleyMEWanCRBroussardBMarijuana use in the immediate 5-year premorbid period is associated with increased risk of onset of schizophrenia and related psychotic disordersSchizophr Res20161711626726785806
- MylesNNewallHNielssenOLargeMThe association between cannabis use and earlier age at onset of schizophrenia and other psychoses: meta-analysis of possible confounding factorsCurr Pharm Des201218325055506922716150
- HelleSRingenPAMelleICannabis use is associated with 3years earlier onset of schizophrenia spectrum disorder in a naturalistic, multi-site sample (N=1119)Schizophr Res2016170121722126682958
- ValmaggiaLDayFJonesCCannabis use and transition to psychosis in people at ultra-high riskPsychol Med201444122503251225055169
- PowerBDDragovicMJablenskyAStefanisNCDoes accumulating exposure to illicit drugs bring forward the age at onset in schizophrenia?Aust N Z J Psychiatry2013471515823042939
- van der MeerFMeijerJMeijerCvan den BrinkWVelthorstECognitive functioning associated with stimulant use in patients with non-affective psychosis, their unaffected siblings and healthy controlsPsychol Med20144491901191124267407
- HasinDSSahaTDKerridgeBTPrevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013JAMA Psychiatry201572121235124226502112
- KoskinenJLöhönenJKoponenHIsohanniMMiettunenJRate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysisSchizophr Bull20103661115113019386576
- van DijkDKoeterMWHijmanRKahnRSvan den BrinkWEffect of cannabis use on the course of schizophrenia in male patients: a prospective cohort studySchizophr Res20121371505722313726
- TosatoSLasalviaABonettoCThe impact of cannabis use on age of onset and clinical characteristics in first-episode psychotic patients. Data from the Psychosis Incident Cohort Outcome Study (PICOS)J Psychiatr Res201347443844423290558
- BarrowcloughCEmsleyREisnerEBeardmoreRWykesTDoes change in cannabis use in established psychosis affect clinical outcome?Schizophr Bull201339233934822037770
- BarrowcloughCGreggLLobbanFBucciSEmsleyRThe impact of cannabis use on clinical outcomes in recent onset psychosisSchizophr Bull201541238239025011381
- SeddonJLBirchwoodMCopelloACannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK national EDEN studySchizophr Bull201542361962526536902
- ClausenLHjorthøjCThorupAChange in cannabis use, clinical symptoms and social functioning among patients with first-episode psychosis: a 5-year follow-up study of patients in the OPUS trialPsychol Med201444111712623590927
- SzokeAGalliotA-MRichardJ-RAssociation between cannabis use and schizotypal dimensions–a meta-analysis of cross-sectional studiesPsychiatry Res20142191586624878296
- FogliaESchoelerTKlamerusEMorganKBhattacharyyaSCannabis use and adherence to antipsychotic medication: a systematic review and meta-analysisPsychol Med201747101691170528179039
- SchoelerTPetrosNContinued cannabis and substance use in the first 2 years following onset of psychosisSchizophr Bull201743Suppl_1S80S81
- BernardoMCañasFHerreraBDoradoMGAdherence predicts symptomatic and psychosocial remission in schizophrenia: naturalistic study of patient integration in the communityRev Psiquiatr Salud Ment201710314915927291833
- SchoelerTPetrosNDi FortiMPoor medication adherence and risk of relapse associated with continued cannabis use in patients with first-episode psychosis: a prospective analysisLancet Psychiatry20174862763328705600
- BarchDMSheffieldJMCognitive impairments in psychotic disorders: common mechanisms and measurementWorld Psychiatry201413322423225273286
- Sánchez-TorresAMBasterraVRosaALifetime cannabis use and cognition in patients with schizophrenia spectrum disorders and their unaffected siblingsEur Arch Psychiatry Clin Neurosci2013263864365323580110
- PowerBDDragovicMBadcockJCNo additive effect of cannabis on cognition in schizophreniaSchizophr Res2015168124525126235754
- WaterreusABadcockJCDi PrinzioPMartin-IversonMMorganVAThe impact of current cannabis use on general cognitive function in people with psychotic illnessSchizophr Res201719016417128381332
- YücelMBoraELubmanDIThe impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sampleSchizophr Bull201238231633020660494
- DonoghueKDoodyGAEffect of illegal substance use on cognitive function in individuals with a psychotic disorder, a review and meta-analysisNeuropsychology201226678580122924618
- RabinRABarrMSGoodmanMSEffects of extended cannabis abstinence on cognitive outcomes in cannabis dependent patients with schizophrenia vs non-psychiatric controlsNeuropsychopharmacology201742112259227128443616
- SchoelerTKambeitzJBehlkeIMurrayRBhattacharyyaSThe effects of cannabis on memory function in users with and without a psychotic disorder: findings from a combined meta-analysisPsychol Med201646117718826353818
- BatallaABhattacharyyaSYücelMStructural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findingsPLoS One201382e5582123390554
- Van AmeringenMManciniCPattersonBBoyleMHPost-traumatic stress disorder in CanadaCNS Neurosci Ther200814317118118801110
- IpserJCSteinDJEvidence-based pharmacotherapy of post-traumatic stress disorder (PTSD)Int J Neuropsychopharmacol201215682584021798109
- FraserGAThe use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD)CNS Neurosci Ther2009151848819228182
- RoitmanPMechoulamRCooper-KazazRShalevAPreliminary, open-label, pilot study of add-on oral Delta9-tetrahydrocannabinol in chronic post-traumatic stress disorderClin Drug Investig2014348587591
- JetlyRHeberAFraserGBoisvertDThe efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design studyPsychoneuroendocrinology20155158558825467221
- ClinicalTrials.govIdentifier: NCT02517424, Evaluating Safety and Efficacy of Cannabis in Participants With Chronic Posttraumatic Stress Disorder2015 Available from: https://clinicaltrials.gov/show/NCT02517424Accessed November 1, 2017
- Identifier: 192675, Placebo-Controlled, Triple-Blind, Crossover Study of the Safety and Efficacy of Three Different Potencies of Vaporized Cannabis in 42 Participants with Chronic, Treatment-Resistant Posttraumatic Stress Disorder (PTSD)2016 Available from: https://health-products.canada.ca/ctdb-bdec/search-recherche.do;jsessionid=831B8B6717B50580890DCCBED62CBCCEAccessed November 1, 2017
- ParkBGibbonsHMitchellMGlassMIdentification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placentaPlacenta2003241099099514580383
- HutchingsDEMartinBRGamagarisZMillerNFicoTPlasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in ratsLife Sci198944116977012538691
- BaileyJCunnyHPauleMSlikkerWFetal disposition of Δ9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkeyToxicol Appl Pharmacol19879023153212820086
- Perez-ReyesMWallMPresence of AMetrahydrocannabinol in human milkN Engl J Med1982307138198206287261
- HurdYWangXAndersonVBeckOMinkoffHDow-EdwardsDMarijuana impairs growth in mid-gestation fetusesNeurotoxicol Teratol200527222122915734273
- El MarrounHTiemeierHSteegersEAIntrauterine cannabis exposure affects fetal growth trajectories: the Generation R StudyJ Am Acad Child Adolesc Psychiatry200948121173118119858757
- GrayTREidenRDLeonardKEConnorsGJShislerSHuestisMAIdentifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growthClin Chem20105691442145020628142
- ScraggRMitchellEFordRThompsonJTaylorBStewartAMaternal cannabis use in the sudden death syndromeActa Paediatr2001901576011227335
- ShionoPHKlebanoffMANugentRPThe impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter studyAm J Obstet Gynecol1995172119277847533
- FriedPAWatkinsonBGrayRGrowth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuanaNeurotoxicol Teratol199921551352510492386
- van GelderMMReefhuisJCatonARWerlerMMDruschelCMRoeleveldNCharacteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based studyDrug Alcohol Depend2010109124324720171023
- FriedPConceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposureJ Child Psychol Psychiatry20024318110211848338
- RichardsonGARyanCWillfordJDayNLGoldschmidtLPrenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 yearsNeurotoxicol Teratol200224330932012009486
- AstleySJLittleREMaternal marijuana use during lactation and infant development at one yearNeurotoxicol Teratol19901221611682333069
- GunnJRosalesCCenterKPrenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysisBMJ open201664e009986
- ChabarriaKCRacusinDAAntonyKMMarijuana use and its effects in pregnancyAm J Obstet Gynecol20162154506.e501506.e50727263998
- HasinDSKerridgeBTSahaTDPrevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions–IIIAm J Psychiatry2016173658859926940807
- MarkKDesaiATerplanMMarijuana use and pregnancy: prevalence, associated characteristics, and birth outcomesArch Womens Ment Health201619110511125895138
- MetzTDAllshouseAAHogueCJRMaternal marijuana use, adverse pregnancy outcomes and neonatal morbidityAm J Obstet Gynecol20172174478.e1478.e828578174
- WarshakCReganJMooreBMagnerKKritzerSVan HookJAssociation between marijuana use and adverse obstetrical and neonatal outcomesJ Perinatol2015351299126401751
- FriedPAJamesDSWatkinsonBGrowth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuanaNeurotoxicol Teratol200123543143611711245
- Fried P, Watkinson B. 12-and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcoholNeurotoxicol Teratol19881043053133226373
- RichardsonGADayNLGoldschmidtLPrenatal alcohol, marijuana, and tobacco use: infant mental and motor developmentNeurotoxicol Teratol19951744794877565494
- El MarrounHTiemeierHSteegersEAA prospective study on intrauterine cannabis exposure and fetal blood flowEarly Hum Dev201086423123620451334
- FriedPAWatkinsonBGrayRDifferential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuanaNeurotoxicol Teratol19982032933069638687
- CasciniFAielloCDi TannaGIncreasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysisCurr Drug Abuse Rev201251324022150622
- Health CanadaNational report: apparent opioid-related deaths 2016–2017 Available from: https://www.canada.ca/en/health-canada/services/substance-abuse/prescription-drug-abuse/opioids/national-report-apparent-opioid-related-deaths.htmlAccessed November 1, 2017
- BartGMaintenance medication for opiate addiction: the foundation of recoveryJ Addict Dis201231320722522873183
- EpsteinDHPrestonKLNo evidence for reduction of opioid-withdrawal symptoms by cannabis smoking during a methadone dose taperAm J Addict201524432332825846329
- ZuardiAWCannabidiol: from an inactive cannabinoid to a drug with wide spectrum of actionRev Bras Psiquiatr20083027128018833429
- Health CanadaBest Practices: Methadone Maintenance Treatment2002 Available from: http://www.hc-sc.gc.ca/hc-ps/alt_formats/hecs-sesc/pdf/pubs/adp-apd/methadone-bp-mp/methadone-bp-mp-eng.pdfAccessed November 1, 2017
- JohanssonEVAAgurellSHollisterLEHalldinMMProlonged apparent half-life of Δ1-tetrahydrocannabinol in plasma of chronic marijuana usersJ Pharm Pharmacol19884053743752899638
- WallMESadlerBMBrineDTaylorHPerez-ReyesMMetabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and womenClin Pharmacol Ther19833433523636309462
- BergamaschiMMKarschnerELGoodwinRSImpact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving lawsClin Chem201359351952623449702
- MatiasIBelluomoICotaDThe fat side of the endocannabinoid system: role of endocannabinoids in the adipocyteCannabis Cannabinoid Res201611176185
- Di MarzoVBerrenderoFBisognoTEnhancement of anandamide formation in the limbic forebrain and reduction of endocannab-inoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant ratsJ Neurochem20007441627163510737621
- GonzalezSCebeiraMFernandez-RuizJCannabinoid tolerance and dependence: a review of studies in laboratory animalsPharmacol Biochem Behav200581230031815919107
- HaneyMWardASComerSDFoltinRWFischmanMWAbstinence symptoms following oral THC administration to humansPsychopharmacology1999141438539410090646
- JonesRTBenowitzNLHerningRIClinical relevance of cannabis tolerance and dependenceJ Clin Pharmacol1981218–9 Suppl143s152s6271820
- HallWSolowijNAdverse effects of cannabisLancet19983529140161116169843121
- VandreyRHaneyMPharmacotherapy for cannabis dependence: how close are we?CNS Drugs200923754355319552483
- Lopez-QuinteroCde los CobosJPHasinDSProbability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)Drug Alcohol Depend20111151–212013021145178
- BudneyAJRoffmanRStephensRSWalkerDMarijuana dependence and its treatmentAddict Sci Clin Pract20074141618292704
- VandreyRStitzerMLMintzerMZHuestisMAMurrayJALeeDThe dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis usersDrug Alcohol Depend20131281–2647022921474
- AllsopDJCopelandJLintzerisNNabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trialJAMA Psychiatry201471328129124430917
- TrigoJMLagzdinsDRehmJEffects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravingsDrug Alcohol Depend201616129830626925704
- AbramsDICoueyPShadeSBKellyMEBenowitzNLCannabinoid-opioid interaction in chronic painClin Pharmacol Ther201190684485122048225
- McRae-ClarkALBakerNLGrayKMKilleenTHartwellKJSimonianSJVilazodone for cannabis dependence: a randomized, controlled pilot trialAm J Addict2016251697526685701
- WeinsteinAMMillerHBluvsteinITreatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled studyAm J Drug Alcohol Abuse2014401162224359507
- McRae-ClarkALBakerNLGrayKMBuspirone treatment of cannabis dependence: a randomized, placebo-controlled trialDrug Alcohol Depend2015156293726386827
- JohnstonJLintzerisNAllsopDJLithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient settingPsychopharmacology2014231244623463624880749
- LevinFRMarianiJJPavlicovaMDronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trialDrug Alcohol Depend2016159536026711160
- MasonBJCreanRGoodellVA proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adultsNeuropsychopharmacology20123771689169822373942
- SchifanoFMisuse and abuse of pregabalin and gabapentin: cause for concern?CNS Drugs201428649149624760436
- SmithRVHavensJRWalshSLGabapentin misuse, abuse and diversion: a systematic reviewAddiction201611171160117427265421
- MersfelderTLNicholsWHGabapentin: abuse, dependence, and withdrawalAnn Pharmacother201650322923326721643

