Abstract
Solid lipid nanoparticle (SLN) delivery systems have a wide applicability in the delivery of phyto-bioactive compounds to treat various chronic diseases, including diabetes, cancer, obesity and neurodegenerative diseases. The multiple benefits of SLN delivery include improved stability, smaller particle size, leaching prevention and enhanced lymphatic uptake of the bioactive compounds through oral delivery. However, the burst release makes the SLN delivery systems inadequate for the oral delivery of various phyto-bioactive compounds that can treat such chronic diseases. Recently, the surface-modified SLN (SMSLN) was observed to overcome this limitation for oral delivery of phyto-bioactive compounds, and there is growing evidence of an enhanced uptake of curcumin delivered orally via SMSLNs in the brain. This review focuses on different SLN and SMSLN systems that are useful for oral delivery of phyto-bioactive compounds to treat various chronic diseases.
Introduction
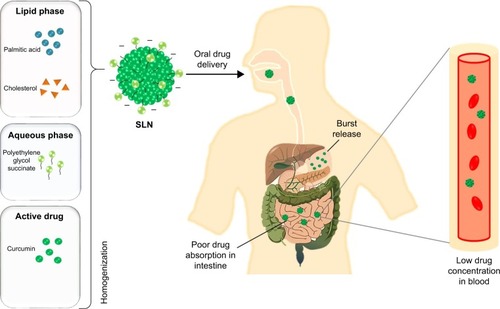
Solid lipid nanoparticles (SLNs) are lipid-based delivery systems that exist in numerous sizes, ranging from 30 to 1,000 nm. These can be developed using easily degradable lipids. SLNs have multiple advantages than other nano-delivery systems including bypassing the spleen or liver filtration with the particle size of 120–200 nm, lower chronic or acute toxicity due to physiological lipid, enhanced bioavailability and productivity, higher reproducibility, lower organic solvents usage in the preparation, protection of liable phytocompounds or drugs and possibility to incorporate both hydrophilic and hydrophobic compounds. Further, SLNs can be made with highly degradable lipids and hence are biologically safe systems which allow large-scale production, easy sterilization and long storage period. These advantages made the SLNs suitable for the oral delivery of various phyto-bioactive compounds, such as curcumin, resveratrol, quercetin and other polyphenols, to treat several types of chronic diseases.Citation1–Citation6 Even though conventional SLNs have several advantages, there is a challenge to oral delivery of bioactive compounds,Citation4,Citation7,Citation8 that is, the burst release of the phyto-bioactive compounds in the stomach at a lower pH of about 1–3. To overcome this problem, the SLNs are subjected to surface modification to enhance the delivery of the phyto-bioactive compounds and to prevent the higher release in the stomach.Citation7
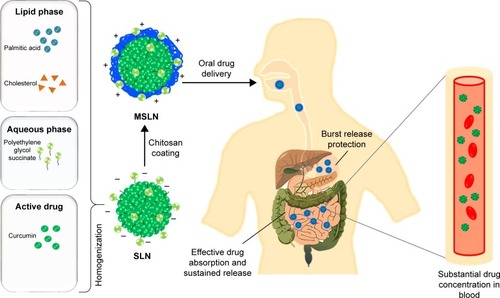
Surface-modified SLNs (SMSLNs) were recently produced using heparin, albumin, polyethylene glycol and polysaccharides to control the oral delivery of phyto-bioactive compounds. Chitosan is highly degradable, presents lower immunogenicity and is suitable for controlled oral delivery of the phyto-bioactive compounds under various pH conditions.Citation4,Citation9–Citation11 The fate of SLNs and modified SLNs (MSLNs) administered through an oral delivery system is shown in and . The surface coating of SLNs with chitosan along with modifications in chitosan has many advantages in reducing the pH, such as a sustained release of the bioactive compounds, and a higher positive charge leads to a lower burst release of the SMSLNs. Coating the modified chitosan on SLNs results in the controlled release of these phyto-bioactive compounds in harsh gastric environments, which will be helpful to treat chronic diseasesCitation7,Citation8,Citation12,Citation13 by improving the efficacy of the therapy. Some SMSLN delivery systems, such as trimethyl chitosan (TMC), showed enhanced delivery of the compounds to the brain in an Alzheimer’s mouse model. Further, modification of the chitosan and development of MSLNs are not cost effective. Other advantages of MSLNs including enhanced targeted delivery of the active compounds, ability to cross the blood–brain barrier in neuroinflammatory diseases and long-term storage with bulk production make them an appropriate choice among the other nano-delivery systems. The recent surface modification of SLNs using chitosan and their applicability in chronic diseases are discussed in this review, focusing on SLNs and SMSLNs for oral delivery of the phyto-bioactive compounds and treatment in various in vitro and in vivo chronic disease models.
Role of SLNs in the oral delivery of phyto-bioactive compounds
SLN is a first-generation nano-delivery system that has been extensively used for sustained release in oral delivery of phytocompounds to treat various chronic diseases.Citation7,Citation14–Citation19 Recently, many new nano-delivery systems have been developed for oral delivery.Citation15–Citation18 However, SLN has its own advantages in the bulk production, including a lower production cost, long-term stability and tolerability and biodegradability with lower toxic effects, along with enhanced oral delivery of phyto-bioactive compounds. Recently, sesamol-loaded SLN was developed with a particle size of about 120 nm, and it exhibited enhanced oral delivery for carbontetrachloride-induced hepatotoxicity in an animal model. The results confirmed that sesamol-loaded SLN has a higher protective effect than free sesamol, with lower irritation and no toxicity.Citation20 Further, the antioxidant potential of sesamol-loaded SLN was higher than that of free sesamol through oral delivery.Citation21 Similarly, curcumin-loaded SLN was studied in the cerebral ischemia rat model, and the results indicated 16.4 times greater bioavailability of curcumin in the brain than with free curcumin. The brain bioavailability greatly increased along with a 90% increase in the cognition of the cerebral ischemic rat group.Citation22 Resveratrol was also studied for its sustained bioavailability through oral delivery via resveratrol-loaded SLN with a particle size of about 241 nm in male Wistar rats. Compared to free resveratrol, lipid core-loaded resveratrol showed two times higher bioavailability in the brain, kidney and liver.Citation23 Recently, quercetin-loaded SLN was developed with a particle size of about 172 nm, and a single oral dose showed 3.2 times higher bioavailability than free quercetin along with enhanced osteoprotective effect in a postmenopausal rat model.Citation24 Similarly, many other flavonoid-loaded SLNs were studied to assess their efficacy in the delivery of bioactive compounds. Owing to the higher-release behavior of SLN, puerarin-loaded SLN was studied for the cardioprotective effect through intragastric delivery, and it showed 3.1 times higher bioavailability than free puerarin.Citation25 The association of resveratrol with lipids was also studied in a stimulated gastrointestinal environment, and it was found to be stable with efficient delivery. Even though the lipid association of other phyto-bioactive compounds may be different, researchers are now highly focusing on modified SLNs for the sustained release of the phyto-bioactive compounds through oral delivery.
SLN formulation and production strategies for the improvement of oral delivery of bioactive compounds
For the enhanced oral delivery and stability of the phyto-bioactive compounds through SLNs, their composition of the formulation and their production methods play a critical role. SLN formulation in turns depends on the type of surfactants, lipids, phyto-bioactive compounds, cosurfactant and cryoprotectant which determines the stability and target reachability of the loaded phyto-bioactive compounds.Citation26 Various range of lipids like triacylglycerols, waxes, hard fats, palmitic acid and stearic acid are used to fabricate SLNs which have their own advantages as well as disadvantages. In case of curcumin loaded in several types of lipids, the entrapment efficiency increases with increase in the chain length of the hydrocarbon chain. Recently, Aditya et al studied the entrapment efficiency of curcumin in SLNs made with different lipids including trimyristin, tristearin and glycerol monosterate and found that glycerol monosterate has greater entrapment efficacy than the other lipids.Citation26,Citation27 Further, the entrapment efficiency of phyto-bioactive compounds like curcumin, resveratrol, or genistein also depends on the molecular weight of the type of compounds involved. Increase in the molecular weight decreases the entrapment efficiency of the compounds which in turn leads to lower oral delivery of the phytocompounds. In addition to the lipids selection, surfactants also play a critical role in the formulation of SLNs, by avoiding coalescence during solidification which in turn depends on the type of surfactant involved and its concentration.Citation28–Citation30 Further, the production methods also determine the SLN loading capacity and stability of the phyto-bioactive compounds.Citation31,Citation32 Various methods like emulsification solvent diffusion, emulsification solvent evaporation, high-pressure homogenization and micro-fluidization are involved in the production of SLNs. Owing to the lower degradation of sensitive phyto-bioactive compounds like curcumin, lower toxicity, enhanced stability and bulk production of the SLNs, microfluidization and high-pressure homogenization techniques are generally recommended in the production of phyto-bioactive compounds-loaded SLNs.
Absorption mechanisms of phyto-bioactive compounds loaded in SLNs and SMSLNs through oral delivery in various chronic disease models
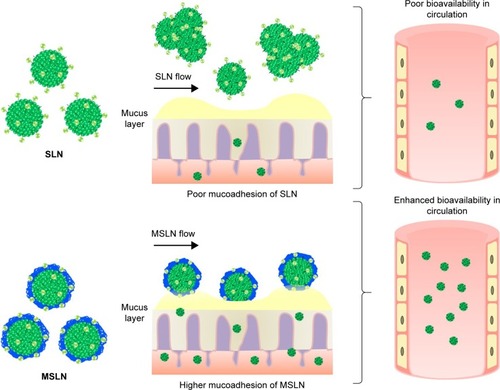
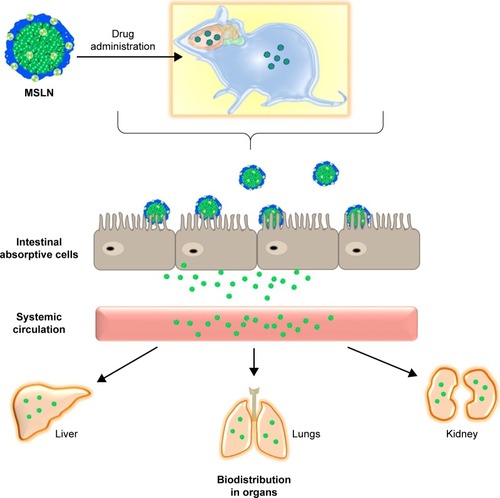
Phyto-bioactive compounds loaded in the SLNs and SMSLNs need to be solubilized before absorption in the gastrointestinal tract when chronic diseases are treated through oral delivery.Citation33–Citation38 The digestion of SLNs and SMSLNs by stomach enzymes results in SLN and SMSLN emulsion and formation of degradation products that form mixed micelles. These mixed micelles loaded with phyto-bioactive compounds can exhibit enhanced absorption due to their lower particle size.Citation18,Citation39,Citation40 In addition, surface modification results in the adhesion of SLNs to the intestine, which can result in longer or prolonged delivery to treat chronic disease. For the above reasons, SLNs loaded with phyto-bioactive compounds can pass through intervillar space or lymphatic system or Peyer’s Patch without much loss in the active site of the bioactive compounds. In addition to the transportation of the bioactive compounds, some amount of coated nanoparticles are also transported through ileum absorption.Citation41–Citation43 The absorption mechanisms and biodistribution in various organs are shown in . Many recent studies have confirmed the enhanced absorption of bioactive compounds through SLNs or SMSLNs to treat diseases including diabetes, cancers, neurological diseases and inflammations, and the effects of SLNs on a few of these diseases are discussed.
Figure 3 Biodistribution of phytocompounds loaded in MSLN through oral delivery.
Abbreviation: MSLN, modified solid lipid nanoparticle.
Anti-type 2 diabetic effect
Type 2 diabetes mellitus treatments with phyto-bioactive compounds are used in traditional medicinal systems in India, China and Korea. Various food-grade phytocompounds have shown an enhanced effect in preventing type 2 diabetes mellitus.Citation44–Citation48 Curcumin-treated prediabetic patients have shown a beneficial effect in reducing the development of diabetes with 9 months of intervention, along with a higher improvement in the β-cell functions.Citation49 Similarly, a resveratrol supplementation can enhance the antidiabetic effect in humans with a dose of 1 g for 45 days.Citation50 Quercetin is another flavonoid compound that showed a higher antidiabetic effect in streptozotocin-induced diabetic rats. Even though these phyto-bioactive compounds showed a higher antidiabetic effect, a longer duration of treatment was needed. In addition, their efficacy and bioavailability when administered through oral delivery systems were very low. To improve the bioavailability through oral delivery, several macro- or nano-sized colloidal systems have been studied. Among them, SLNs showed an enhanced effect in treating type 2 diabetes through oral delivery. Recently, berberine-loaded SLNs with a particle size of 76.8 nm showed an improved bioavailability with a higher antidiabetic effect in a diabetic mouse model.Citation51 This study also confirmed that berberine-loaded SLNs improved the islet function and can thereby effectively reduce diabetes progression. In addition, the same research group also found that the bioavailability of the berberine in the liver was 20 times higher than in blood, which led to a reduction in diabetes-associated complications such as lipolysis enhancement and lipogenesis inhibition.Citation52 These studies confirm that delivery of the bioactive compound to the systemic circulation in a highly active way can enhance not only specific activities but also improve the associated complications. The bioavailability of certain other compounds such as curcumin, resveratrol, or quercetin was effectively enhanced through SLN delivery systems, but their activity in a diabetic animal model remains limited. Recently, surface-modified SLNs loaded with cur-cumin showed a 9.5 times higher bioavailability through oral delivery,Citation7 and they can be potentially applied to treat type 2 diabetes.
Anticancer effect
Phytocompounds have been used to effectively treat various cancers for longer periods of time.Citation53–Citation56 However, this effect is not highly appreciable due to a higher loss of bioactivity during oral therapy. Several synthetic medicines have also faced limitations for oral therapy, so lipid-based delivery of their active compounds has been extensively used to develop various cancer treatments.Citation57,Citation58 SLNs have been extensively used in many studies to orally deliver bioactive compounds with an enhanced anticancer effect.Citation17,Citation57,Citation59–Citation62 Genistein is a phytoestrogen that is extensively used for hormone-related cancers, and it has limited bioavailability. Recently, genistein-loaded solid lipid microparticles (SLMs) with a particle size of 6 µm were compared with SLNs with a particle size of about 120 nm in terms of their bioavailability. Surprisingly, SLMs showed a greater anticancer effect than the SLNs due to a slow disintegration in the intestine as well as the particles reaching the colon. In addition, different sizes of the particles can be used to alter the surface area of genistein to improve its activity. Smaller SLNs can be extensively absorbed in mesenteric vessels, leading to a higher absorption of the bioactive compounds rather than reaching the colon.Citation63 In another study, curcumin-loaded SLNs were studied for their antitumor activity through intravenous administration, and curcumin showed a 1.25 times enhanced bioavailability.Citation62 Other research groups compared curcumin-loaded SLNs administered via intravenous or oral routes and showed 30 or 16.4 times higher bioavailability of curcumin, respectively.Citation22 Thereby, surface modification of SLNs with chitosan or modified chitosan could enhance the bioavailability of cur-cumin or other compounds in various organs through oral delivery. However, their applicability in various anticancer disease models is still limited. Many in vitro cell studies have shown an enhanced anticancer effect in various cancer models. Recently, berberine-loaded SLNs showed enhanced antitumor effect with a particle size of about 81 nm in MCF-7 cell lines.Citation64 Aloe-emodin is another phytocompound that can be loaded in SLNs, and when prepared with a particle size of about 88 nm, it showed an enhanced anticancer effect to treat breast and hepatoma cancer cell lines.Citation65 Resveratrol-loaded SLNs with a particle size of about 96 nm showed an enhanced anticancer effect in HepG2 cells.Citation66 Similarly, oridonin-loaded SLNs with a particle size of about 108 nm showed an enhanced antitumor effect in MCF-7 cells.Citation67 Various research studies confirm that SLNs could be a potential carrier for various anticancer phyto-bioactive compounds, and further research on oral delivery of those anticancer phyto-bioactive compounds is necessary.
Antiobesity effect
The antiobesity effect of various phyto-bioactive compounds is well known to function through the inhibition of various cell signaling mechanisms, but these are very complex. In general, obesity is characterized by an increase in the deposition of fat storage adipose cells. A diet rich in various phytochemicals has shown an extensive reduction in the deposition of fat through complex mechanisms.Citation68,Citation69 However, the concentration of certain phytochemicals reaching systemic circulation is very low. For instance, green tea catechin is a potential antiobesity compound, but its bioavailability is very low and is limited to 0.15 µM of the epigallocatechin gallate (EGCG).Citation70 In order to enhance the bioavailability of such phytocompounds, many lipid-based nanodelivery systems with SLNs or MSLNs have been developed to improve the delivery of the bioactive compounds with great potential for an antiobesity effect. Recently, EGCG was successfully loaded in SLNs with a particle size of about 300–400 nm, and it showed a higher stability and greater potential for oral delivery.Citation71 It could possibly be used in future as a delivery system to treat obesity-related complications. Zerumbone is another lipophilic compound that is most commonly found in ginger, and has shown an extensive antiobesity effect.Citation72 To date, there have been limited attempts to develop SLNs to deliver zerumbone for its antiobesity effect. Resveratrol is also a potential compound that has shown a higher antiobesity effect in various animal studies.Citation73 However, it requires a higher dose and prolonged supplementation. Recently, resveratrol-loaded SLNs and MSLNs were studied for their potential bioavailability. However, their roles in antiobesity have not yet been elucidated. Quercetin is another active compound that has shown significant potential for antiobesity in various animal studies. Recently, quercetin-loaded SLNs and chitosan-coated MSLNs were developed with a particle size of about 110 nm, and these showed an enhanced bioavailability of quercetin with a higher stability.Citation74 Further research is needed to focus on the oral delivery of SLNs and their antiobesity effect in animal models.
Anticardiovascular effect
Phyto-bioactive compounds such as resveratrol, curcumin, quercetin and diosgenins have shown an enhanced anticardiovascular effect through their cardioprotective activity. This cardioprotection is achieved through mechanisms such as antihyperlipidemia, antioxidation or platelet aggregation inhibition.Citation75–Citation77 However, most phyto-bioactive compounds taken with the diet or through an oral delivery system have exhibited a lower bioavailability in systemic circulation.Citation78–Citation80 Recently, many phyto-bioactive compounds showed an improved bioavailability through SLN or MSLN delivery systems, which can further improve their cardioprotective activity. Puerarin is among the most cardioprotective compounds, and its successful loading in SLNs resulted in a higher bioavailability in various organs, especially three times higher in the heart and the brain.Citation81 These studies confirm that a higher bioavailability and sustained release of these bioactive compounds can lead to a higher cardioprotective effect.Citation39,Citation194 Furthermore, there is no change in the production of the metabolite when given orally. Very recently, flavonoid from Dracocephalum moldavica L. loaded in the SLNs with a particle size of about 104 nm showed an improved protective effect against myocardial ischemic–reperfusion injury. This could be a base study to prepare another phyto-bioactive compound-loaded SLN with cardioprotective activities.Citation39 Hydroxycitric acid (HCA) is a cardioprotective agent that was found in Garcinia cowa, and it undergoes much degradation during processing, leading to the loss of its cardioprotective activity when administered orally. Recently, HCA was successfully loaded in SLNs, which showed 1.3 times higher bioavailability than its free form.Citation82 Many phytocompounds loaded in SLNs or MSLNs are yet to be studied for their efficacy in oral delivery along with cardioprotective activity. Many other studies were conducted with a low-molecular-weight heparin-loaded SLNs or MSLNs with a much higher oral bioavailability to improve the cardioprotective activities.
Anti-arthritic effect
Phyto-bioactive extracts and compounds, such as green tea extract, pomegranate extract, curcumin, resveratrol, celastrol and gamabogic acid, have been extensively used to treat rheumatoid arthritis (RA).Citation83–Citation85 These bioactive compounds showed an inhibitory mechanism against inflammatory mediators, thereby preventing cartilage destruction in various animal studies. Besides the protective effect of these bioactive compounds, their bioavailability through oral delivery system is a great challenge for their potential RA treatment.Citation86–Citation88 To overcome this, recently phytocompounds were loaded in SLNs and MSLNs which showed excellent bioavailability. Piperine-loaded SLNs with a particle size of about 128 nm showed excellent delivery of such compounds with potential anti-RA activity.Citation89 Similarly, hesperidin-loaded SLNs also showed a potential anti-RA effect with a particle size of about 279 nm in male Wistar rats. In another study, curcumin-loaded SLNs also showed an excellent delivery of curcumin in the RA-induced rats with potential anti-inflammatory or antioxidative mechanisms.Citation90 Other phytocompounds such as EGCG were also efficiently loaded in SLN systems and showed excellent bioavailability in animal models. This could also be potentially applied to treat RA. Many other bioactive compounds with potential anti-RA activities can also be efficiently loaded in SLN systemsCitation91 for use in future treatments with nanomedicines. Furthermore, to improve the sustained release of the bioactive compounds, MSLNs can also be developed with specific bioactive compounds with higher anti-RA activities.
Anti-Alzheimer’s effect
Phyto-bioactive compounds-loaded SLNs were recently used to treat Alzheimer’s disease (AD), overcoming conventional limitations in treating neurodegenerative diseases.Citation92–Citation96 Initially, SLN- or MSLN-loaded bioactive compounds were given intranasally or intravenously, and these showed extensive bioavailability in the brain, thereby preventing inflammation and further progression of AD.Citation97–Citation101 Quercetin-loaded SLN with a particle size of about 200 nm was studied for its efficacy in the AD model, and it showed excellent delivery of quercetin to the brain with a higher antioxidative effect in brain cells.Citation102 The transport of bioactive compounds to the brain occurs through the endocytosis of the brain capillaries, and these compounds can thereby cross the blood–brain barrier. In a recent study, piperine-loaded SLNs showed enhanced bioavailability in the brain cells, and can thereby prevent a further prognosis of the AD. The study also revealed that piperine can enhance acetyl cholinesterase activity, reducing the formation of plaques and thereby improving cognitive activity.Citation96 Another study investigating ferulic acid-loaded SLNs against neurotoxicity found that ferulic acid can be extensively delivered to brain cells and can thereby prevent oxidation without any toxicity.Citation103 Curcumin is another potential compound that showed excellent anti-Alzheimeric effect in various in vitro and in vivo studies,Citation104–Citation112 but it showed limitations in its bioavailability through oral delivery with a very low content in the brain, and could not achieve a significant potential effect. Therefore, many recent studies were intended to improve the bioavailability of curcumin to the brain through oral delivery via SLNs.
Recently, curcumin-loaded SLN was studied for its potential effect in an aluminum-induced AD model. Curcumin-loaded SLN showed excellent delivery of curcumin to the brain, and the bioavailability of curcumin varied from 32 to 155 times in a dose-dependent manner with an enhancement in cognition and the biochemical parameters associated with it. In comparison with free curcumin, treatment with curcumin-loaded SLN showed 73% higher recovery of the biochemical aspects. This confirmed that curcumin-loaded SLN will be a potential delivery system for the oral delivery of curcumin for AD treatment.Citation113 However, to further improve the bioavailability, curcumin-loaded MSLN was recently developed, and it showed improved delivery of the curcumin for AD treatment. Recently, resveratrol has gained more interest to treat AD due to its greater neuroprotective effect, and many studies confirmed that resveratrol treatment significantly improved the cognition and biochemical parameters.Citation114–Citation123 Recently, resveratrol-loaded SLN was studied for its bioavailability and brain delivery. Resveratrol was efficiently delivered to the brain and exhibited its potential bioactivity. To improve the sustained bioavailability, resveratrol-loaded chitosan-coated MSLN was also studied, which showed an enhanced and sustained delivery of resveratrol to the brain. In another study, resveratrol-loaded SLN with func-tionalized antibody showed excellent cellular uptake compared to normal SLN.Citation93 Many other phyto-bioactive compounds loaded onto SLNs or MSLNs are still in the development pipeline with a size suitable for effective transport through the blood–brain barrier, and these can result in higher protection of brain cells to overcome age-related degenerative diseases.Citation195
Anti-Parkinson’s effect
Parkinson’s disease (PD) is another neurodegenerative disease that occurs most commonly in the elderly due to the loss of the dopaminergic neurons and the activation of microglial cells.Citation124–Citation130 Phyto-bioactive compounds have shown an excellent anti-neuroinflammatory effect through various pathways.Citation131–Citation141 However, they have limitations in oral delivery due to their extensive first-pass metabolism and difficulty in crossing the blood–brain barrier. Recently, SLN-loaded phyto-bioactive compounds have shown excellent bioavailability through oral delivery and a higher brain bioavailability.Citation107,Citation132,Citation142,Citation143 Curcumin is a bioactive compound that has shown an anti-Parkinson effect in various in vitro and in vivo studies.Citation144–Citation152 However, its brain bioavailability is limited. Recently, curcumin-loaded SLN was studied for its efficacy and brain bioavailability. The bioavailability of curcumin-loaded SLNs or MSLNs in the brain was greatly enhanced,Citation7,Citation8,Citation12 which showed its potential for use in treating PD in future. Similarly, resveratrol-loaded SLNs or MSLNs also showed potential for delivery to the brain cells, and can also exert an anti-Parkinson effect. Many recent approaches assessed the brain bioavailability to exert a preventive effect against neuronal loss. Nevertheless, there is still a limited role for SLN- and MSLN-loaded phyto-compounds in PD animal models through oral delivery. Many new studies are currently in the pipeline to achieve an anti-Parkinson effect using SLN-loaded phyto-bioactive compounds to prevent neuronal loss and thereby ageing.
Antihepatic effect
Liver damage is associated with various chronic complications. The liver can be protected using dietary phyto-bioactive compounds,Citation45,Citation153–Citation166 but their potential for liver protection through oral delivery systems is very limited. However, SLN-loaded phytocompounds showed excellent bioavailability with enhanced liver protection.Citation1,Citation20,Citation52,Citation93,Citation167–Citation170 Recently, Ficus benjamina-loaded SLN was studied against the hepatotoxicity, and the results showed a higher delivery of bioactive compounds with enhanced hepatoprotectivity.Citation171 Similarly, sesamol-loaded SLN also showed excellent hepatoprotection along with lower irritation when administered through oral delivery.Citation20,Citation21,Citation95,Citation172 However, many phytocompounds that have shown excellent hepatoprotection have not yet been studied for their efficacy and bioavailability through SLN or MSLN delivery systems.
Chitosan-based surface modification of SLN delivery systems for the bioavailability of phytocompounds to the target organs
Owing to the higher release of SLN-loaded phyto-bioactive compounds in the stomach with an acidic pH, surface modification was effectively carried out with mucoadhesive polymers to enhance the sustained release of phyto-bioactive compounds in SLNs.Citation4,Citation173–Citation175 Chitosan has various advantages over other polymers including lower toxicity, enhanced absorption and high mucoadhesive and antimicrobial properties which enhance the oral delivery of the phyto-bioactive compounds. In order to further enhance the absorption properties of the chitosan-coated SLNs, grafting of chitosan moieties was done through conjugation of amine and hydroxyl groups leading to functional chitosan like TMC.Citation7 SLN coated with TMC has excellent properties compared with chitosan which include higher mucoadhesiveness, enhanced delivery and low toxicity. Further, modified chitosan grafted with lipids showed target-specific delivery of the core compounds. For example, palmitic acid-grafted TMC-coated SLNs showed enhanced delivery of the different phytocompounds through controlled release by providing excellent surface environment through nanomicelles. Recently, Ramalingam et al studied the delivery of curcumin to the brain compared to that of free curcumin, chitosan-coated, non-chitosan-coated and TMC-g-palmitic acid-coated SLNs.Citation7 Among those, TMC-coated SLNs showed enhanced bioavailability of the curcumin in the brain cells. The same research group also found that resveratrol-loaded TMC-g-palmitic acid-coated SLNs showed 3.8 times higher bioavailability than the resveratrol suspension. In another study, N-carboxymethyl chitosan-coated SLNs showed enhanced bioavailability of curcumin in lymphatic cells. The uptake by the lymphatic cells and the oral bioavailability of the curcumin were found to be 6.3 and 9.5 times higher than that of curcumin suspension.Citation176 Based on the above studies, we can confirm that chitosan derivatives can be extensively used to improve the delivery of the phyto-bioactive compounds against various chronic diseases ().
Table 1 Phyto-bioactive compounds loaded in chitosan-coated solid lipid nanoparticles used in various disease models
SLN modified with chitosan and its derivatives, and its bioavailability through oral delivery
Chitosan-coated SLNs
Chitosan-coated SLN is the first-generation modified SLN developed to enhance the delivery of phyto-bioactive compounds. Various properties of chitosan, such as high mucoadhesion, cationic nature, low toxicity and high bioavailability, have resulted in more researchers using this polysaccharide as a coating for SLNs to improve the delivery of bioactive compounds.Citation4,Citation19,Citation175,Citation177 Chitosan-coated SLN was also used in other delivery routes including nasal, vaginal and skin, due to its enhanced and sustained delivery.Citation19,Citation178–Citation180 Based on the type of chitosan and lipids involved, the application and delivery routes vary. In oral delivery, chitosan-coated SLN is preferred for its mucoadhesion and sustained release. Although many commercial drugs have been extensively studied for use with chitosan-coated SLNs for sustained oral delivery,Citation174,Citation177,Citation180,Citation181 there are few studies on phyto-bioactive compound loading. Recently, ferulic acid-loaded chitosan-coated SLNs were studied to treat pancreatic cancer, and these showed an enhanced effect via oral delivery. Similarly, chitosan-coated SLNs loaded with curcumin showed a sustained release of curcumin in various organs. Furthermore, toxicity studies were conducted for certain drugs in combination with curcumin in chitosan-coated SLNs, and the results indicated no toxicity during pancreatic cancer treatment.Citation182 In another study, resveratol loaded in chitosan-coated SLNs also showed a higher bioavailability in animal models. Similarly, caffeic acid-loaded SLNs coated with alginate chitosan showed higher antioxidant activity and sustained release.Citation183
TMC-coated SLNs
TMC-coated SLN is another modified SLN that overcomes the drawbacks of chitosan-coated SLN by increasing the solubility over a broad range of pH, improving the mucoadhesion and achieving a sustained release of the bioactive compounds in the SLN during oral delivery.Citation184–Citation186 Many early studies were conducted to deliver various drugs, including insulin, vaccines and proteins via sustained delivery with TMC-coated SLNs to enhance the biomedical effects in treating various chronic diseases.Citation187,Citation196–Citation198 Fewer studies were conducted on the delivery of phytocompounds through TMC-coated SLNs.Citation7,Citation12 A recent study report on the delivery of curcumin to the brain through TMC-coated SLNs showed sustained delivery to the brain through paracellular transport, and this presents a potential treatment for AD models.Citation7 The same research group also performed a study with resveratrol as a core compound and found 3.8 times higher delivery of the resveratrol to the target organ through oral delivery.Citation12 These studies show a pathway for future studies of various phyto-bioactive compounds for sustained release via oral delivery and improved bioavailability to treat various diseases.
Hydroxypropyl trimethyl ammonium chloride chitosan (HACC)-modified SLNs
HACC-modified SLN is another modified chitosan-loaded SLN that was recently developed to improve the stability in the gastrointestinal environment for sustained release.Citation188–Citation192 A recent study with docetaxel showed that HACC-modified chitosan administered orally exhibits a higher drug bioavailability via various absorption mechanisms including transcellular, paracellular and M cell uptake.Citation193 Interestingly, the study also found that HACC-modified SLNs showed a higher uptake of the drug in the Peyer’s Patches than normal cells. The same research group also showed that HACC-modified SLNs with a uniform particle size achieved enhanced bioavailability with around 2.45 times increase of the drug through oral delivery.Citation187 In addition, the toxicity of the HACC-modified SLNs was also studied in Caco-2 cells, and the results showed no toxic effect and no irritation in the mucosa of the rats. This study confirmed that there is a chance of increase in the bioavailability of phyto-bioactive compounds like curcumin, quercetin and resveratrol through HACC-modified SLNs, which will be the scope for future studies on enhanced delivery. shows the mucoadhesion and bioavailability of the phytocompounds loaded in the SLNs or MSLNs.
Challenges associated with SLNs and MSLNs in the food systems
Even though SLN and MSLN delivery systems try to accomplish the criteria required for the enhanced delivery of the phyto-bioactive compounds, it is not possible to use a single delivery system for all the phyto-bioactive compounds. However, both systems have unique advantages in both food and pharmaceutical applications like use of high food-grade lipids, bulk production with lower production cost and higher loading capacity in comparison to the food bioactive compounds. The incorporation of SLNs or MSLNs in the food particles, their physiological changes in the food systems during storage, and toxicity of these systems to the target organs are yet to be studied. The research in these aspects will increase the utilization of the SLNs or MSLNs in the food products. Food-based medicine will be a greater demand soon owing to the toxicity of various synthetic medicines. These systems could efficiently deliver the phyto-bioactive compounds along with the nutrients, and they will be helpful in the development of fortified food products or functional foods in future. In addition, MSLN development is not cost effective, and further research is necessary in the development of low-cost chitosan and modified chitosan for their effective usage and research in the toxicological aspects could widen their applications in many other associated industries.
Conclusion
SLNs and MSLNs are promising colloidal delivery systems that help deliver phytocompounds to various organs, including the brain, via oral delivery. The bioavailability of these phytocompounds loaded in SLNs has been found to be about 5–10 times greater than that of their native form. Furthermore, the sustained release of these phyto-bioactive compounds through oral delivery can also be achieved through surface modification of the SLNs, which opens the way for development of many new phytocompounds loaded onto SLNs or MSLNs to treat various chronic diseases. The sustained and improved delivery of phyto-bioactive compounds via oral delivery is a focus of future development in nanomedicine.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2017R1C1B2010276 and NRF-2017R1A2A2A07001035).
Disclosure
The authors report no conflicts of interest in this work.
References
- AyanAKYenilmezAErogluHEvaluation of radiolabeled curcumin-loaded solid lipid nanoparticles usage as an imaging agent in liver-spleen scintigraphyMater Sci Eng C Mater Biol Appl20177566367028415513
- ComogluTArisoySAkkusZBNanocarriers for effective brain drug deliveryCurr Top Med Chem201717131490150628017157
- CoutoRAlvarezVTemelliFEncapsulation of Vitamin B2 in solid lipid nanoparticles using supercritical CO2J Supercrit Fluids2017120432442
- SandriGMottaSBonferoniMCChitosan-coupled solid lipid nanoparticles: tuning nanostructure and mucoadhesionEur J Pharm Biopharm2017110131827989765
- PistollatoFBremer-HoffmannSBassoGTargeting glioblastoma with the use of phytocompounds and nanoparticlesTarget Oncol2015111116
- PistollatoFGiampieriFBattinoMThe use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironmentFood Chem Toxicol201575587025445513
- RamalingamPKoYTEnhanced oral delivery of curcumin from N-trim-ethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluationsPharm Res201532238940225082210
- RamalingamPYooSWKoYTNanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcuminFood Res Int201684113119
- LeeWHLooCYYoungPMTrainiDMasonRSRohanizadehRRecent advances in curcumin nanoformulation for cancer therapyExpert Opin Drug Deliv20141181183120124857605
- NiuZConejos-SanchezIGriffinBTO’DriscollCMAlonsoMJLipid-based nanocarriers for oral peptide deliveryAdv Drug Deliv Rev2016106Pt B33735427080735
- RajaMAZeenatSArifMLiuCSelf-assembled nanoparticles based on amphiphilic chitosan derivative and arginine for oral curcumin deliveryInt J Nanomedicine2016114397441227660435
- RamalingamPKoYTImproved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticlesColloids Surf B Biointerfaces2016139526126700233
- RamalingamPKoYTValidated LC-MS/MS method for simultaneous quantification of resveratrol levels in mouse plasma and brain and its application to pharmacokinetic and brain distribution studiesJ Pharm Biomed Anal2016119717526657178
- Ikeuchi-TakahashiYIshiharaCOnishiHFormulation and evaluation of morin-loaded solid lipid nanoparticlesBiol Pharm Bull20163991514152227320782
- GrandhiBKThakkarAWangJPrabhuSA novel combinatorial nanotechnology-based oral chemopreventive regimen demonstrates significant suppression of pancreatic cancer neoplastic lesionsCancer Prev Res (Phila)20136101015102524072676
- KakkarVMishraAKChuttaniKKaurIPProof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brainInt J Pharm2013448235435923558314
- LiuJChenSLvLSongLGuoSHuangSRecent progress in studying curcumin and its nano-preparations for cancer therapyCurr Pharm Des201319111974199323116308
- ShomeSTalukdarADChoudhuryMDBhattacharyaMKUpadhyayaHCurcumin as potential therapeutic natural product: a nanobiotechnological perspectiveJ Pharm Pharmacol201668121481150027747859
- VijayakumarABaskaranRJangYSOhSHYooBKQuercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptakeAAPS PharmSciTech201718387588327368922
- SinghNKhullarNKakkarVKaurIPSesamol loaded solid lipid nanoparticles: a promising intervention for control of carbon tetrachloride induced hepatotoxicityBMC Complement Altern Med20151514225935744
- SinghNKhullarNKakkarVKaurIPHepatoprotective effects of sesamol loaded solid lipid nanoparticles in carbon tetrachloride induced sub-chronic hepatotoxicity in ratsEnviron Toxicol201631552053225410024
- KakkarVMuppuSKChopraKKaurIPCurcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in ratsEur J Pharm Biopharm2013853 Pt A33934523454202
- FrozzaRLBernardiAPaeseKCharacterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in ratsJ Biomed Nanotechnol20106669470321361135
- AhmadNBanalaVTKushwahaPQuercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: a preventive strategy for post-menopausal osteoporosisRSC Adv201661009761397628
- LuoCFYuanMChenMSPharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administrationInt J Pharm20114101–213814421392565
- AdityaNPKoSSolid lipid nanoparticles (SLNs): delivery vehicles for food bioactivesRSC Adv20155393090230911
- AdityaNPPatankarSMadhusudhanBMurthyRSRSoutoEBArthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activityEur J Pharm Sci201040544845520493255
- AdityaNPAdityaSYangHKimHWParkSOKoSCo-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsionFood Chem201517371325465989
- AdityaNPShimMYangHLeeYKoSAntiangiogenic effect of combined treatment with curcumin and genistein on human prostate cancer cell lineJ Funct Foods20148204213
- ChoiKOAdityaNPKoSEffect of aqueous pH and electrolyte concentration on structure, stability and flow behavior of non-ionic surfactant based solid lipid nanoparticlesFood Chem201414723924424206712
- AdityaNPAdityaSYangHJCurcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage applicationJ Funct Foods2015153543
- AdityaNPMacedoASDoktorovovSDevelopment and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE)LWT Food Sci Technol2014591115121
- JiPYuTLiuYNaringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokineticsDrug Des Devel Ther201610911925
- KumarPSharmaGKumarRVitamin-derived nanolipoidal carriers for brain delivery of dimethyl fumarate: a novel approach with preclinical evidenceACS Chem Neurosci2017861390139628157295
- LinglingGYuanZWeigenLPreparation, optimization, characterization and in vivo pharmacokinetic study of asiatic acid tromethamine salt-loaded solid lipid nanoparticlesDrug Dev Ind Pharm20164281325133326821124
- MadureiraARCamposDGullonBFermentation of bioactive solid lipid nanoparticles by human gut microfloraFood Funct20167151652926606879
- NevesARMartinsSSegundoMAReisSNanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticalsNutrients20168313126950147
- SiafakaPIUstundag OkurNKaravasEBikiarisDNSurface modified multifunctional and stimuli responsive nanoparticles for drug targeting: current status and usesInt J Mol Sci2016179
- TanMEHeCHJiangWDevelopment of solid lipid nanoparticles containing total flavonoid extract from Dracocephalum moldavica L. and their therapeutic effect against myocardial ischemia-reperfusion injury in ratsInt J Nanomedicine2017123253326528458544
- WangTMaXLeiYLuoYSolid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcuminColloids Surf B Biointerfaces201614811127588376
- DeningTJRaoSThomasNPrestidgeCAOral nanomedicine approaches for the treatment of psychiatric illnessesJ Control Release201622313715626739547
- MaYHeHXiaFIn vivo fate of lipid-silybin conjugate nanoparticles: implications on enhanced oral bioavailabilityNanomedicine20171362643265428778838
- NunesSMadureiraARCamposDSolid lipid nanoparticles as oral delivery systems of phenolic compounds: overcoming pharmacokinetic limitations for nutraceutical applicationsCrit Rev Food Sci Nutr20175791863187326192708
- AnandKTilokeCNaidooPChuturgoonAAPhytonanotherapy for management of diabetes using green synthesis nanoparticlesJ Photochem Photobiol B201717362663928709077
- BulleSReddyvariHNallanchakravarthulaVVaddiDRTherapeutic potential of Pterocarpus santalinus L.: an updatePharmacogn Rev20161019434927041873
- ChenCYChiuFYLinYHuangWJHsiehPSHsuFLChemical constituents analysis and antidiabetic activity validation of four fern species from TaiwanInt J Mol Sci20151622497251625622260
- DeyPSahaMRChowdhuriSRAssessment of anti-diabetic activity of an ethnopharmacological plant Nerium oleander through alloxan induced diabetes in miceJ Ethnopharmacol201516112813725498854
- SinghAKJatwaRPurohitARamHSynthetic and phytocompounds based dipeptidyl peptidase-IV (DPP-IV) inhibitors for therapeutics of diabetesJ Asian Nat Prod Res201719101036104528351157
- ChuengsamarnSRattanamongkolgulSLuechapudipornRPhisalaphongCJirawatnotaiSCurcumin extract for prevention of type 2 diabetesDiabetes Care201235112121212722773702
- MovahedANabipourILieben LouisXAntihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patientsEvid Based Complement Alternat Med2013201385126724073011
- XueMYangMXZhangWCharacterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticlesInt J Nanomedicine201384677468724353417
- XueMZhangLYangMXBerberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db miceInt J Nanomedicine2015105049505726346310
- ChenSSMichaelAButler-ManuelSAAdvances in the treatment of ovarian cancer: a potential role of antiinflammatory phytochemicalsDiscov Med2012136871722284780
- MahataSMaruSShuklaSAnticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cellsBMC Complement Altern Med2012121522405256
- NieQXingMHuJHuXNieSXieMMetabolism and health effects of phyto-estrogensCrit Rev Food Sci Nutr201757112432245426558495
- RaniNVelanLPVijaykumarSArunachalamAAn insight into the potentially old-wonder molecule-quercetin: the perspectives in foreseeChin J Integr Med Epub201599
- Davatgaran-TaghipourYMasoomzadehSFarzaeiMHPolyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspectiveInt J Nanomedicine2017122689270228435252
- ParkJSAhnEYParkYAsymmetric dumbbell-shaped silver nanoparticles and spherical gold nanoparticles green-synthesized by mangosteen (Garcinia mangostana) pericarp waste extractsInt J Nanomedicine2017126895690829066885
- HazzahHAFaridRMNasraMMA new approach for treatment of precancerous lesions with curcumin solid-lipid nanoparticle-loaded gels: in vitro and clinical evaluationDrug Deliv20162341409141926146889
- LiCZhangJZuYJBiocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicalsChin J Nat Med201513964165226412423
- NaksuriyaOOkonogiSSchiffelersRMHenninkWECurcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatmentBiomaterials201435103365338324439402
- SunJBiCChanHMSunSZhangQZhengYCurcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailabilityColloids Surf B Biointerfaces201311136737523856543
- KimJTBaruaSKimHAbsorption study of genistein using solid lipid microparticles and nanoparticles: control of oral bioavailability by particle sizesBiomol Ther (Seoul)201725445245928605834
- WangLLiHWangSEnhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulationAAPS PharmSciTech201415483484424696391
- ChenRWangSZhangJChenMWangYAloe-emodin loaded solid lipid nanoparticles: formulation design and in vitro anti-cancer studyDrug Deliv201422566667424512431
- ZhangQHXiongQPShiYYZhangDYStudy on preparation and characterization of resveratrol solid lipid nanoparticles and its anticancer effects in vitroZhong Yao Cai2010331219291932 Chinese [with English abstract]21548373
- WangLWangSChenROridonin loaded solid lipid nanoparticles enhanced antitumor activity in MCF-7 cellsJ Nanomater20142014903646
- JinQYuHLiPThe evaluation and utilization of marine-derived bioactive compounds with anti-obesity effectCurr Med Chem Epub201761
- PanMHTungYCYangGLiSHoCTMolecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffeeFood Funct20167114481449127722362
- MerelesDHunsteinWEpigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises?Int J Mol Sci20111295592560322016611
- FriasINevesARPinheiroMReisSDesign, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementationDrug Des Devel Ther20161035193528
- HemnHONoordinMMRahmanHSHazilawatiHZukiAChartrandMSAntihypercholesterolemic and antioxidant efficacies of zerumbone on the formation, development, and establishment of atherosclerosis in cholesterol-fed rabbitsDrug Des Devel Ther2015941734208
- ChangCCLinKYPengKYDayYJHungLMResveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cellsEndocr J201663216917826698690
- VijayakumarABaskaranRJangYSOhSHYooBKQuercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptakeAAPS PharmSciTech201618387588327368922
- OlasBSea buckthorn as a source of important bioactive compounds in cardiovascular diseasesFood Chem Toxicol20169719920427616182
- Rangel-HuertaODPastor-VillaescusaBAguileraCMGilAA systematic review of the efficacy of bioactive compounds in cardiovascular disease: phenolic compoundsNutrients2015775177521626132993
- SolaRVallsRMPuzoJEffects of poly-bioactive compounds on lipid profile and body weight in a moderately hypercholesterolemic population with low cardiovascular disease risk: a multicenter randomized trialPLoS One201498e10197825084280
- MalagutiMAngeloniCHreliaSNutraceutical bioactive compounds promote healthspan counteracting cardiovascular diseasesJ Am Coll Nutr201534Suppl 1222726400430
- Pastor-VillaescusaBRangel-HuertaODAguileraCMGilAA systematic review of the efficacy of bioactive compounds in cardiovascular disease: carbohydrates, active lipids and nitrogen compoundsAnn Nutr Metab2015662–316818126045206
- ScolaroBSoo Jin KimHde CastroIABioactive compounds as an alternative for drug co-therapy: overcoming challenges in cardiovascular disease preventionCrit Rev Food Sci Nutr Epub20161110114
- LuoCFChenMSProfessorYMetabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticlesInt J Nanomedicine2013893394023486407
- EzhilarasiPNMuthukumarSPAnandharamakrishnanCSolid lipid nanoparticle enhances bioavailability of hydroxycitric acid compared to a microparticle delivery systemRSC Adv20166595378453793
- GuptaSCTyagiAKDeshmukh-TaskarPHinojosaMPrasadSAggarwalBBDownregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenolsArch Biochem Biophys2014559919924946050
- KhalifeSZafarullahMMolecular targets of natural health products in arthritisArthritis Res Ther201113110221345249
- RahmanMBegSVermaAPhytoconstituents as pharmacotherapeutics in rheumatoid arthritis: challenges and scope of nano/submicromedicine in its effective deliveryJ Pharm Pharmacol201769111427774648
- MobasheriAHenrotinYBiesalskiHKShakibaeiMScientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint healthInt J Mol Sci20121344202423222605974
- RecioMCAndujarIRiosJLAnti-inflammatory agents from plants: progress and potentialCurr Med Chem201219142088210322414101
- ShakibaeiMMobasheriABuhrmannCCurcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitroGenes Nutr20116217117921484156
- BhalekarMRMadgulkarARDesalePSMariumGFormulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritisDrug Dev Ind Pharm20174361003101028161984
- AroraRKuhadAKaurIPChopraKCurcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in ratsEur J Pain201519794095225400173
- ChenJWeiNLopez-GarciaMDevelopment and evaluation of resveratrol, Vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applicationsEur J Pharm Biopharm201711728629128411056
- Fonseca-SantosBGremiaoMPChorilliMNanotechnology-based drug delivery systems for the treatment of Alzheimer’s diseaseInt J Nanomedicine2015104981500326345528
- LoureiroJAAndradeSDuarteAResveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s diseaseMolecules2017222
- PiconePBondiMLMontanaGFerulic acid inhibits oxidative stress and cell death induced by Ab oligomers: improved delivery by solid lipid nanoparticlesFree Radic Res200943111133114519863373
- SachdevaAKMisraSPal KaurIChopraKNeuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: behavioral and biochemical evidenceEur J Pharmacol201574713214025449035
- YusufMKhanMKhanRAAhmedBPreparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease modelJ Drug Target2012213300311
- BarbuEMolnarETsibouklisJGoreckiDCThe potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrierExpert Opin Drug Deliv20096655356519435406
- FilipovaMRusinaRHoladaKNanoparticle-based drug delivery systems crossing blood-brain barrier – hope for future treatment of neurodegenerative disorders?Cesk Slov Neurol N2016792160167
- ShahBKhuntDBhattHMisraMPadhHApplication of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parametersEur J Pharm Sci201578546626143262
- WilsonCMMagnaudeixANavesTVincentFLalloueFJauber-teauMOThe ins and outs of nanoparticle technology in neurodegenerative diseases and cancerCurr Drug Metab201516860963226264207
- ZhangGLChenLKGuoXYKhanAAGuYCGuNNanoparticle-mediated drug delivery systems (DDS) in the central nervous systemCurr Org Chem2017213272283
- DhawanSKapilRSinghBFormulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain deliveryJ Pharm Pharmacol201163334235121749381
- BondiMLMontanaGCraparoEFFerulic acid-loaded lipid nanostructures as drug delivery systems for Alzheimer’s disease: preparation, characterization and cytotoxicity studiesCurr Nanosci2009512632
- BarbaraRBellettiDPederzoliFNovel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt Abeta aggregatesInt J Pharm20175261–241342428495580
- BattistiAPiccionelloAPSgarbossaACurcumin-like compounds designed to modify amyloid beta peptide aggregation patternsRSC Adv20177503171431724
- ChandraBMithuVSBhowmikDCurcumin dictates divergent fates for the central salt bridges in amyloid-β40 and amyloid-β42Biophys J201711281597160828445751
- Di MartinoPCensiRGigliobiancoMRNano-medicine improving the bioavailability of small molecules for the prevention of neurodegenerative diseasesCurr Pharm Des201723131897190828025942
- JaroonwitchawanTChaicharoenaudomrungNNatnkaewJNoisaPCurcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagyNeurosci Lett2017636404727793699
- KarimianMSPirroMJohnstonTPMajeedMSahebkarACurcumin and endothelial function: evidence and mechanisms of protective effectsCurr Pharm Des201723172462247328228072
- McClureROngHJanveVAerosol delivery of curcumin reduced amyloid-β deposition and improved cognitive performance in a transgenic model of Alzheimer’s diseaseJ Alzheimers Dis201755279781127802223
- UllahFLiangARangelAGyengesiENiedermayerGMunchGHigh bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammationArch Toxicol20179141623163428204864
- ZengZShenZLZhaiSTransport of curcumin derivatives in Caco-2 cell monolayersEur J Pharm Biopharm201711712313128396278
- KakkarVKaurIPEvaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brainFood Chem Toxicol201149112906291321889563
- AhmedTJavedSJavedSResveratrol and Alzheimer’s disease: mechanistic insightsMol Neurobiol20175442622263526993301
- ChengJRuiYQinLVitamin D combined with resveratrol prevents cognitive decline in SAMP8 miceCurr Alzheimer Res201714882083328176624
- FameniniSRigaliEAOlivera-PerezHMIncreased intermediate M1–M2 macrophage polarization and improved cognition in mild cognitive impairment patients on omega-3 supplementationFASEB J201731114816027677546
- HeXLiZRizakJDResveratrol attenuates formaldehyde induced hyperphosphorylation of Tau protein and cytotoxicity in N2a cellsFront Neurosci20161059828197064
- JerabekJUliassiEGuidottiLTacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s diseaseEur J Med Chem201712725026228064079
- MoussaCHebronMHuangXResveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s diseaseJ Neuroinflammation2017141128086917
- NoratiqahSBNaina-MohamedIZulfarinaMSQodriyahHMNatural polyphenols in the treatment of Alzheimer’s diseaseCurr Drug Targets Epub2017328
- OmarSHScottCJHamlinASObiedHKThe protective role of plant biophenols in mechanisms of Alzheimer’s diseaseJ Nutr Biochem20174712028301805
- WightmanELPotential benefits of phytochemicals against Alzheimer’s diseaseProc Nutr Soc201776210611228143625
- XuPZhangMShengRMaYSynthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Abeta1-42 aggregation inhibitors and metal-chelating agents for Alzheimer’s diseaseEur J Med Chem201712717418628061347
- CuryRGFraixVCastriotoAThalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystoniaNeurology201789131416142328768840
- KanegusukuHSilva-BatistaCPecanhaTPatients with Parkinson disease present high ambulatory blood pressure variabilityClin Physiol Funct Imaging201737553053528776928
- ParkJLeeJWCooperSCBroxmeyerHECannonJRKimCHParkinson disease-associated LRRK2 G2019S transgene disrupts marrow myelopoiesis and peripheral Th17 responseJ Leukoc Biol201710241093110228751472
- RamirezAIde HozRSalobrar-GarciaEThe role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and glaucomaFront Aging Neurosci2017921428729832
- Saez-AtienzarSSingletonABParkinson disease and clathrin coat dynamics at synapses, why not?Mov Disord2017328116328681957
- SonHJJeongYJYoonHJParkinson disease-related cortical and striatal cognitive patterns in dual time F-18 FP CIT: evidence for neural correlates between the caudate and the frontal lobeQ J Nucl Med Mol Imaging Epub2017727
- StruppJKundeAGalushkoMVoltzRGollaHSeverely affected by Parkinson disease: the patient’s view and implications for palliative careAm J Hosp Palliat Care Epub201711
- da CostaIMCavalcantiJRLPde QueirozDBSupplementation with herbal extracts to promote behavioral and neuroprotective effects in experimental models of Parkinson’s disease: a systematic reviewPhytother Res201731795997028544038
- GanesanPKoHMKimISChoiDKRecent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease modelsInt J Nanomedicine2015106757677226604750
- GokulKMuralidharaOral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: relevance to Parkinson’s diseaseNeurochem Res20143971382139424831121
- GuoHShiFLiMLiuQYuBHuLNeuroprotective effects of Eucommia ulmoides Oliv. and its bioactive constituent work via ameliorating the ubiquitin-proteasome systemBMC Complement Altern Med20151515125994206
- KlionskyDJAbdelmohsenKAbeAGuidelines for the use and interpretation of assays for monitoring autophagy (3rd edition)Autophagy2016121122226799652
- MazoNAEcheverriaVCabezasRMedicinal plants as protective strategies against Parkinson’s diseaseCurr Pharm Des201723284180418828302024
- MorshediDAliakbariFTayaranian-MarvianAFassihiAPan-MontojoFPérez-SánchezHCuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicityJ Food Sci20158010H2336H234526351865
- MythriRBHarishGBharathMMTherapeutic potential of natural products in Parkinson’s diseaseRecent Pat Endocr Metab Immune Drug Discov20126318120022827714
- NabaviSFBraidyNHabtemariamSSuredaAManayiANabaviSMNeuroprotective effects of fisetin in Alzheimer’s and Parkinson’s diseases: from chemistry to medicineCurr Top Med Chem201616171910191526845554
- PathakASrivastavaAKSingourPKGoudaPSynthetic and natural monoamine oxidase inhibitors as potential lead compounds for effective therapeuticsCent Nerv Syst Agents Med Chem2016162819726104056
- SolankiIPariharPMansuriMLPariharMSFlavonoid-based therapies in the early management of neurodegenerative diseasesAdv Nutr201561647225593144
- CacciatoreICiullaMFornasariEMarinelliLDi StefanoASolid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseasesExpert Opin Drug Deliv20161381121113127073977
- CunhaSAmaralMHLoboJMSilvaACTherapeutic strategies for Alzheimer’s and Parkinson’s diseases by means of drug delivery systemsCurr Med Chem201623313618363127554805
- ChenTDengYLiaoXEffect of curcumin on oligomer formation and mitochondrial ATP-sensitive potassium channels induced by overexpression or mutation of α-synucleinZhonghua Yi Xue Yi Chuan Xue Za Zhi2015324462467 Chinese [with English abstract]26252085
- CuiQLiXZhuHCurcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathwayMol Med Rep20161321381138826648392
- DuXXXuHMJiangHSongNWangJXieJXCurcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s diseaseNeurosci Bull201228325325822622825
- Guerrero-MuñozMJCastillo-CarranzaDLKayedRTherapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteinsBiochem Pharmacol201488446847824406245
- PhomLAchumiBAloneDPMuralidharaYenisettiSCCurcumin’s neuroprotective efficacy in Drosophila model of idiopathic Parkinson’s disease is phase specific: implication of its therapeutic effectivenessRejuvenation Res201417648148925238331
- SongSNieQLiZDuGCurcumin improves neurofunctions of 6-OHDA-induced parkinsonian ratsPathol Res Pract2016212424725126922613
- SpinelliKJOsterbergVRMeshulCKSoumyanathAUnniVKCurcumin treatment improves motor behavior in α-synuclein transgenic micePLoS One2015106e012851026035833
- UğuzACÖzANaziroğluMCurcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cellsJ Recept Signal Transduct Res201636439540126608462
- YuSYZhangMLuoJZhangLShaoYLiGCurcumin ameliorates memory deficits via neuronal nitric oxide synthase in aged miceProg Neuropsychopharmacol Biol Psychiatry201345475323665290
- Abdel-WahabWMThymoquinone attenuates toxicity and oxidative stress induced by bisphenol A in liver of male ratsPak J Biol Sci201417111152116026027160
- BabuPRBhuvaneswarCSandeepGRamaiahCVRajendraWHepatoprotective role of Ricinus communis leaf extract against d-galactosamine induced acute hepatitis in albino ratsBiomed Pharmacother20178865866628152474
- DongiovanniPLantiCRisoPValentiLNutritional therapy for nonalcoholic fatty liver diseaseJ Nutr Biochem20162911126895659
- El-HadaryAERamadan HassanienMFHepatoprotective effect of cold-pressed Syzygium aromaticum oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in ratsPharm Biol20165481364137226440388
- FuYQHuaCZhouJChengBRZhangJProtective effects of ginseng total saponins against hepatic ischemia/reperfusion injury in experimental obstructive jaundice ratsPharm Biol201351121545155124004049
- HuangHPOuTTWangCJMulberry (sang shen zi) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivoJ Tradit Complement Med20133171524716151
- InadaACFigueiredoPSSantos-EichlerRADMorinda citrifolia Linn. (Noni) and its potential in obesity-related metabolic dysfunctionNutrients201796
- KowalskaKOlejnikACurrent evidence on the health-beneficial effects of berry fruits in the prevention and treatment of metabolic syndromeCurr Opin Clin Nutr Metab Care201619644645227583706
- MopuriRIslamMSMedicinal plants and phytochemicals with anti-obesogenic potentials: a reviewBiomed Pharmacother2017891442145228372259
- Rodriguez-RamiroIVauzourDMinihaneAMPolyphenols and non-alcoholic fatty liver disease: impact and mechanismsProc Nutr Soc2016751476026592314
- SamoutNEttayaABouzennaHNcibSElfekiAHfaiedhNBeneficial effects of Plantago albicans on high-fat diet-induced obesity in ratsBiomed Pharmacother2016841768177527876214
- ValentiLRisoPMazzocchiAPorriniMFargionSAgostoniCDietary anthocyanins as nutritional therapy for nonalcoholic fatty liver diseaseOxid Med Cell Longev2013201314542124282628
- XuWHHuHGTianYBioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosisHepatology201460264866024668691
- YuJYHaJYKimKMJungYSJungJCOhSAnti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liverMolecules2015207130411305426205049
- AbdelwahabSISheikhBYTahaMMThymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administrationInt J Nanomedicine201382163217223818776
- NaseriNValizadehHZakeri-MilaniPSolid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and applicationAdv Pharm Bull20155330531326504751
- RaoMPManjunathKBhagawatiSTThippeswamyBSBixin loaded solid lipid nanoparticles for enhanced hepatoprotection – preparation, characterisation and in vivo evaluationInt J Pharm20144731–248549225066077
- ShuhendlerAJPrasadPLeungMRauthAMDacostaRSWuXYA novel solid lipid nanoparticle formulation for active targeting to tumor α(v) β(3) integrin receptors reveals cyclic RGD as a double-edged swordAdv Healthc Mater20121560060823184795
- SharmaAKKumarAKumarSPreparation and therapeutic evolution of Ficus benjamina solid lipid nanoparticles against alcohol abuse/antabuse induced hepatotoxicity and cardio-renal injuryRSC Adv20177573593835949
- KakkarVMishraAKChuttaniKChopraKKaurIPDelivery of sesamol-loaded solid lipid nanoparticles to the brain for menopause-related emotional and cognitive central nervous system derangementsRejuvenation Res201114659760421978086
- ElzoghbyAOAbd-ElwakilMMAbd-ElsalamKElsayedMTHashemYMohamedONatural polymeric nanoparticles for brain-targeting: implications on drug and gene deliveryCurr Pharm Des201622223305332326845323
- FontePAndradeFAraújoFAndradeCNevesJDSarmentoBChitosan-coated solid lipid nanoparticles for insulin deliveryMethods Enzymol201250829531422449932
- LuoYTengZLiYWangQSolid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptakeCarbohyd Polym2015122221229
- BaekJSChoCWSurface modification of solid lipid nanoparticles for oral delivery of curcumin: improvement of bioavailability through enhanced cellular uptake, and lymphatic uptakeEur J Pharm Biopharm201711713214028412471
- CamposJVaras-GodoyMHaidarZSPhysicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: a proof-of-concept study in breast cancer cellsNanomedicine (Lond)201712547349028181464
- NguyenHMHwangICParkJWParkHJPhotoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticlesPest Manag Sci20126871062106822383397
- RassuGSodduEPosadinoAMNose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapyColloids Surf B Biointerfaces201715229630128126681
- WangJYWangYMengXChitosan nanolayered cisplatin-loaded lipid nanoparticles for enhanced anticancer efficacy in cervical cancerNanoscale Res Lett201611152427888498
- ThakkarAChenreddySWangJPrabhuSFerulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticlesCell Biosci201554626301084
- ThakkarAChenreddySThioAKhamasWWangJPrabhuSPreclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c miceInt J Nanomedicine2016113265327627499621
- FathiMMirlohiMVarshosazJMadaniGNovel caffeic acid nanocarrier: production, characterization, and release modelingJ Nanomater20132013434632
- ChenFZhangZRYuanFQinXWangMHuangYIn vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein deliveryInt J Pharm20083491–222623317825506
- HanLTangCYinCOral delivery of shRNA and siRNA via multifunctional polymeric nanoparticles for synergistic cancer therapyBiomaterials201435154589460024613049
- ZhangJTangCYinCGalactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophagesBiomaterials201334143667367723419643
- ShiLLLuJCaoYGastrointestinal stability, physicochemical characterization and oral bioavailability of chitosan or its derivative-modified solid lipid nanoparticles loading docetaxelDrug Dev Ind Pharm201743583984627487431
- CaiJDangQLiuCPreparation, characterization and antibacterial activity of O-acetyl-chitosan-N-2-hydroxypropyl trimethyl ammonium chlorideInt J Biol Macromol20158081526093196
- LiGFWangJCFengXMLiuZDJiangCYYangJDPreparation and testing of quaternized chitosan nanoparticles as gene delivery vehiclesAppl Biochem Biotechnol201517573244325725686559
- XiaoBMaPMaLEffects of tripolyphosphate on cellular uptake and RNA interference efficiency of chitosan-based nanoparticles in Raw 264.7 macrophagesJ Colloid Interface Sci201749052052827918990
- YangZShangYHuangXCationic content effects of biodegradable amphoteric chitosan-based flocculants on the flocculation propertiesJ Environ Sci (China)20122481378138523513678
- ZhaoKSunYChenGBiological evaluation of N-2-hydroxypropyl trimethyl ammonium chloride chitosan as a carrier for the delivery of live Newcastle disease vaccineCarbohydr Polym2016149283927261727
- ShiLLXieHLuJPositively charged surface-modified solid lipid nanoparticles promote the intestinal transport of docetaxel through multifunctional mechanisms in ratsMol Pharm20161382667267627379550
- RezzaniRRodellaLFFraschiniFMelatonin delivery in solid lipid nanoparticles: prevention of cyclosporine A induced cardiac damageJ Pineal Res200946325526119196438
- KarthivashanGGanesanPParkSKimJChoiDTherapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s diseaseDrug delivery201825130732029350055
- SayinBSomavarapuSLiXWSesardicDSenelSAlparOHTMC–MCC (N-trimethyl chitosan–mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccinesEur J Pharm Sci200938436236919733658
- YinLDingJHeCCuiLTangCYinCDrug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin deliveryBiomaterials200930295691570019615735
- SlütterBSoemaPCDingZVerheulRHenninkWJiskootWConjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigenJ Control Release2010143220721420074597