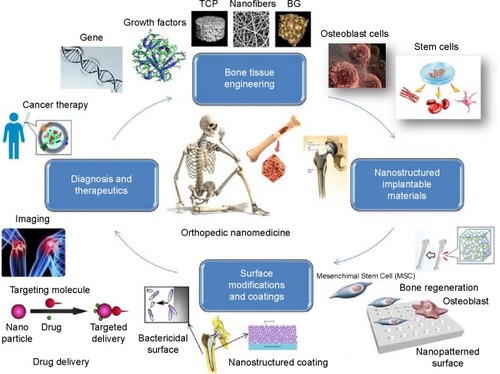
Abstract
The technological and clinical need for orthopedic replacement materials has led to significant advances in the field of nanomedicine, which embraces the breadth of nanotechnology from pharmacological agents and surface modification through to regulation and toxicology. A variety of nanostructures with unique chemical, physical, and biological properties have been engineered to improve the functionality and reliability of implantable medical devices. However, mimicking living bone tissue is still a challenge. The scope of this review is to highlight the most recent accomplishments and trends in designing nanomaterials and their applications in orthopedics with an outline on future directions and challenges.
Introduction
Nanomedicine, the application of nanotechnology to medicine, aims to overcome problems related to diseases at nanoscale, where most of the biological molecules exist and operate.Citation1 The response of host organisms at the protein and cellular level to nanomaterials is different from that observed for conventional materials.Citation2 In orthopedic applications, there is a significant need and demand for the development of a bone substitute that is bioactive and exhibits material properties comparable with those of natural and healthy bone.Citation3 For bone tissue engineering, nanostructured ceramics, polymers, metals, and composites have been receiving significant attention recently.Citation4 Nanostructured materials enhance osteoblast functions (such as adhesion, proliferation, synthesis of bone-related proteins, and deposition of calcium-containing mineral) and promote adequate osteointegration due to increased surface area and roughness.Citation5 Owing to the ability of nanomaterials to mimic the dimensions of constituent components of natural bone,Citation3 they are promising candidates as the future and alternative orthopedic materials. summarizes the potential of nanomedicine in orthopedic applications. We believe that there are a plenty of room for development and implantation of nanomaterials in orthopedic medicine because nanofunctionalized scaffolds can provide structural support for the cells and regulate cell proliferation, differentiation, and migration.Citation6 Many studies have shown that nanomaterials enable enhancement of osteointegration and promote healing of bone-related diseases.Citation7 Wang et alCitation8 have recently shown that nanostructured calcium phosphate scaffolds could support stem cell attachment/proliferation and induce osteogenic differentiation due to their chemical or crystallographic similarities to inorganic components of bone. Antimicrobial and drug-eluting coatings are other examples for the application of nanomaterials in orthopedic medicine. These coatings prevent infection risks of implants, which are the most common cause of reverse surgery.Citation9 Meanwhile, potential pitfalls or undesirable side effects associated with the use of nanomaterials should be considered. In this article, we review recent advances in utilizing nanomaterials for orthopedic medicine with a focus on implantable devices, functional coatings, surface modification techniques, diagnostics, and therapeutics. The potential risks of using nanomaterials are also presented.
Nanomaterials for orthopedic applications
The application of nanotechnology to bone substitutes is relatively a new frontier in orthopedic research. Nanotechnology offers novel materials that mimic the complex and hierarchical structure of the native bone tissue.Citation10 Since natural tissues are nanometer in dimensions and cells directly interact with nanostructured extracellular matrices, the biomimetic features and excellent physiochemical properties of nanomaterials play a crucial role in stimulating cell growth as well as tissue regeneration.Citation11 Some of the key characteristics that make nanomaterials attractive for orthopedic applications include high strength-to-weight ratio, wear/corrosion resistance, antimicrobial/drug release potentials, and tissue integration/regeneration capabilities.Citation12 This section overviews challenges in bone tissue engineering and elucidates how nanomaterials including ceramics, polymers, metals, and composites can be used to improve orthopedic implant by controlling their surface properties. summarizes typical materials in orthopedic medicine including nanostructures.
Table 1 Typical materials used in orthopedics, including nanostructures
Challenges in bone tissue engineering and requirements
The repair of large bone defects due to injury or disease is one of the major problems in orthopedic and maxillofacial surgery.Citation63 Bone is a vascular and highly specialized form of connective tissue composed of 10%–20% collagen, 60%–70% bone mineral (mainly hydroxyapatite [HA]), and 9%–20% water, by weight.Citation64 Conventional clinical treatments for bone repair and regeneration include autologous and allogeneic transplantations which have several limitations and complications, such as donor site morbidity and immunogenic response.Citation65 Tissue engineering emerged as a promising alternative for the reconstitution of lost or damaged organs and tissues, circumventing the problems associated with traditional transplants.Citation66 Although attempts have resulted in the development of novel biomaterials that support the capacity of the body to regenerate bone and integrate with the surrounding bone tissue,Citation67 more efforts are still required for the developed biomaterials to mimic living bone tissue. Specifically, these materials need to be biocompatible, biodegradable, osteoconductive, integrative, porous, and mechanically compatible with the native bone to fulfill the requirements of bone tissue reconstruction.Citation63 Current challenges are related to engineering materials that can match both the mechanical and biological context of real bone tissue matrix and support the vascularization of large tissue constructs while restoring its physiological function.Citation68–Citation70 Novel biomimetic scaffolds should be emerged to recreate nanoscale topographical and biofactor cues using biofunctionalization strategies.Citation71 Growth factors including bone morphogenetic proteins (BMP) as well as osteogenic/stem cells could also be loaded into the biomaterials in order to stimulate bone growth, collagen synthesis, and fracture repair in vivo.Citation72,Citation73
Implantable nanomaterials for orthopedics
Typical implantable biomaterials to provide structural support as bone substitutes encompass stainless steel alloys, cobalt–chrome alloys, titanium alloys, magnesium alloys, HA, alumina, zirconia, polymethylmethacrylate (PMMA), poly(lactic acid) (PLA), carbon fiber/polyetherether-ketone, and carbon fiber/ultra-high molecular weight polyethylene.Citation60,Citation74,Citation75 With recent advances in nanotechnology, nanostructured materials have emerged as novel orthopedic implants with greater potential to provide osseointegration while having cell-favorable surface properties to efficiently stimulate new bone growth as compared to common materials.Citation11 For instance, nanostructuring of metallic implantable devices enhances their mechanical properties and biocompatibility.Citation36 Nowadays, bulk nanocrystalline (NC; <100 nm) and ultrafine-grained (UFG; ~100–500 nm) metals including titanium (Ti) and their alloys can commercially be fabricated by severe plastic deformation (SPD) techniquesCitation76 and powder metallurgy (P/M)Citation36 routes. Herein, a bulk metal or powder material is subjected to high plastic strains with complex stress state, leading to breaking the coarse grains down into nanoscale range. Nanostructured Ti implants produced by SPD are bioinert without a potential toxic or allergic effects of alloying elements (such as Al and V) while having higher strength (>1,000 MPa) than conventional ones.Citation76 Very recently, Gain et alCitation55 have shown that UFG/NC P/M Ti implants have a higher strength and better ductility than common Ti–6Al–4V alloys and Ti parts processed by SPD. Estrin et alCitation77 prepared UFG Ti (170–200 nm) by equal channel angular pressing (ECAP) and compared the attachment of human bone marrow-derived mesenchymal stem cells (hMSCs) to the surface with a coarse-grained (CG) Ti specimen (4.5 µm). It was demonstrated that the attachment and spreading of hMSCs in the initial stages (up to 24 hours) of culture was enhanced. Wang et alCitation78 fabricated TiN-coated UFG Ti (~130 nm) by high-pressure torsion technique. The developed material showed a great potential as implants, owing to its high strength, reasonable ductility, good fatigue life, excellent abrasion resistance, and a nontoxic ion release. Park et alCitation79 investigated in vitro biocompatibility of UFG Ti produced by ECAP using MC3T3-E1 cells as compared to the commercially pure (CP) Ti and Ti–6Al–4V alloy. The specimens were grit-blasted with HA particles to produce microrough surfaces. The UFG material exhibited enhanced biological response including cell spreading, cell attachment, cell viability, Alkaline phosphatase (ALP) activity, osteopontin and osteocalcin mRNA levels in cells grown, and mineralization nodule formation. Another orthopedic material is elemental selenium, which has potential anticancer chemistry. Unlike titanium, selenium is an essential trace element in the human body. Selenium is important because mammalian selenoproteins play a role in antioxidant defense systems, thyroid hormone metabolism, and redox control of cell reactions.Citation80 In vitro research has shown the inhibitory effects of selenium on the growth of many cancerous cell lines.Citation81 Perla and WebsterCitation80 have demonstrated the positive effect of selenium on osteoblast growth. Tran and WebsterCitation82 created nanostructured roughness on selenium compacts for anticancer orthopedic applications and showed that healthy bone cell adhesion increased with greater nanometer selenium roughness. However, this mode of selenium addition can result in poor, unsuitable mechanical properties of the implant since selenium, being a metalloid, does not have sufficient mechanical strength. Moreover, considering that selenium is toxic at high doses, the stability and control over its release would be an extremely desirable attribute.Citation81 As an alternative strategy for using selenium as an anticancer orthopedic material, Tran et alCitation81 have fabricated a nanoselenium-coated titanium for improving orthopedic applications. They have demonstrated the potential of selenium nanoclusters as a chemopreventive Ti orthopedic material coating that can also promote healthy bone cell functions.
Besides, bioceramics are the most demanding materials for orthopedic applications, although their inherent brittleness prevented their use in some applications. Nanophased ceramics could offer advantages of improved fracture toughness with an ability to promote biofunctionality.Citation83 Recent advances include nanostructuring of various bioceramics including zirconia, titania, alumina, calcium phosphates, bioactive glass (BG), and HA.Citation84 Studies have shown that nanostructuring yields higher mechanical strength with improved ductility and toughness as the finer grains hinder dislocation slip and cause crack blunting.Citation85 Additionally, processing of nanoceramics at lower temperatures becomes feasible as the sintering activity is higher.Citation84 Meanwhile, restricting of grain growth upon high-temperature processing is challenging. Nanophased bioceramics also exhibit better functionality with cells both in vitro and in vivo. Zhou et alCitation46 have reported that NC HA provides a better substrate for cell viability and proliferation of rabbit MSCs compared to CG HA in vitro. Bosco et alCitation47 have shown an improved apoptosis of osteoclast-like cells of bone-like HA nanocrystals functionalized with alendronate in vitro. Nanostructured BG scaffolds are capable of guiding bone formation in a rabbit ulnar critical-sized-defect model with successful crack bridging.Citation86
Synthetic and natural polymers are also excellent candidates for bone/cartilage tissue engineering applications. Natural polymers such as collagen, fibrin, chitosan (CS), hyaluronic acid, and alginate are biocompatible and biologically active and hence promote cell adhesion and growth.Citation87 These natural materials have the advantage over synthetic ones in being similar to materials in the body and have potential to be used as implant surface scaffolds.Citation88,Citation89 Levengood and ZhangCitation15 highlighted recent advances in the development of CS-based scaffolds with enhanced bone regeneration capability. Mandal et alCitation90 fabricated silk-fiber-reinforced composite matrices with a high compressive strength (~13 MPa hydrated state) for bone engineering applications. Schiavi et alCitation14 introduced a new generation of collagen nanofiber implant functionalized with growth factor BMP-7 nanoreservoirs and equipped with human MSC microtissues for bone regenerative nanomedicine. Many different polymer nanofibers have also been investigated for bone tissue replacements.Citation29 These nanoporous or nanofibrous polymer matrices can be fabricated via electrospinning, phase separation, particulate leaching, chemical etching, and 3D printing techniques.Citation11 Xin et alCitation23 prepared electrospun PLGA nanofibrous scaffolds and studied the viability, growth, and differentiation of hMSCs as well as their osteogenic and chondrogenic derivatives. Results indicated that hMSCs continuously differentiated into chondrogenic cells and osteogenic cells after 2-week incubation in PLGA nanofibers. Park et alCitation24 reported enhanced chondrocyte functions on nanostructured 3D PLGA scaffolds.
In general, nanoceramics and nanopolymers are mostly used as coating constituent materials for orthopedics or can be combined with other biomaterials to form nanocomposites that are suitable for implant applications. As mentioned earlier, bone is a true nanocomposite, so that nanocomposites are more beneficial than other nanostructured materials. Common nanocomposites for bone tissue regeneration consist of a ceramic nanophase in a ceramic matrix, a carbonaceous nanophase in a ceramic or polymer matrix, or a ceramic nanophase in a polymer matrix.Citation50,Citation54 Gain et alCitation55 fabricated porous HA/ZrO2 nanocomposites by P/M technique and showed that the nanocomposites exhibited better compressive strength and elastic modulus than that of porous monolithic HA due to the reinforcing effect of ZrO2 nanoparticles (NPs). Ceramic–polymer nanocomposites have also attracted particular attention for use as bone tissue regeneration materials because of their excellent combination of bioactivity and osteoconductivity of ceramics, and the flexibility and shape controllability of polymers.Citation91 Very recently, Hickey et alCitation56 have prepared PLLA-based nanocomposites reinforced with HA and MgO NPs. Their results indicate that MgO NPs significantly enhance osteoblast adhesion and proliferation on HA–PLLA nanocomposites while maintaining mechanical properties suitable for cancellous bone applications. Sadat-Shojai et alCitation92 synthesized 3D HA/gelatin hydrogel nanocomposites with enhanced stiffness that may be useful for treatment of cancellous bone defects or low load-bearing orthopedic applications. The encapsulation of MC3T3-E1 cells into the nanocomposites revealed that the whole process of composite formation was compatible with the bone cells. New alternative reinforcing materials are carbon nanomaterials. Due to their ultra-high mechanical strength over most other materials, carbon nanostructures (include carbon nanotubes, carbon nanofibers, graphene, nanodiamond (ND), and so forth) are effective additives to improve the mechanical properties of orthopedic materials.Citation50 Baradaran et alCitation93 fabricated reduced graphene oxide (rGO)-reinforced HA (nanotube) composites using a hydrothermal process. They showed improved elastic modulus and fracture toughness of the sintered samples with increasing of the rGO content. Enhanced osteoblast adhesion and proliferation were also reported. Wu et alCitation94 studied the biomimetic growth behavior of HA on CNFs functionalized with carboxylic groups and evaluated the structure and fracture strength of the resulting composites. Due to the strong interfacial bonding between HA and CNFs, improved mechanical strength with a potential of interfacial bonding of HA to host tissues was attained. In another study,Citation57 multiwalled carbon nanotubes (MWCNTs) and HA nanorods were incorporated into polypropylene to form biocomposites for bone replacements. The mechanical tests and 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay demonstrated that the mechanical properties including stiffness, tensile strength, and impact toughness were improved without a major side effect on the biocompatibility.
Surface modifications
The surface properties, surface chemistry, topography, and roughness of implantable materials influence their cell response and the absorption of biomolecules that regulate cell attachment and migration.Citation95 Various methods have been employed for the surface modification of implants to enhance their integration capacity with osseous tissue.Citation96 So far, techniques such as electrochemical processing, hydrothermal treatment, sandblasting, sol-gel, and chemical etching have been employed.Citation97–Citation99 In many attempts, formation of a dense or porous TiO2 film on the surface of Ti implants have been examined.Citation98,Citation100 Much work has also been performed on the preparation of TiO2 nanotubes in order to increase the surface roughness and to improve biological performance of implants.Citation41,Citation101 Metals (eg, Ag),Citation41 inorganic materials (like Ca, P),Citation102 or biomolecules (such as antibiotics)Citation103 may be incorporated in the surface layer to provide bactericidal capacity, increased ALP activity, and improved osseointegration. Surface modifications of Ti implants via different methods indicate that the rougher surfaces exhibit better early cell adhesive and proliferative abilities.Citation97 Rosales-Leal et alCitation104 investigated MG-63 cell culture on CP Ti with different surface treatments. It has been found that cell attachment is improved on rougher surfaces with irregular morphologies and more collagen are produced when cell grow on. Koller et alCitation96 have reported that BG abrasion can increase the roughness of CP Ti surfaces, leading to a bioactive and osteopromotive implant material. Although biological properties may be improved by surface roughening of implants, contact corrosion–fatigue can lead to surface instability and development of microcracks on the contacting surface.Citation38,Citation104 Ryu and ShrotriyaCitation38 showed that surface roughness of medical grade CoCrMo alloy exponentially accelerates the localized damage on the implant surfaces by contact corrosion–fatigue. Therefore, although the surface roughness has a beneficial effect on the implant biocompatibility, it may decrease the implant life time.
Surface nanostructuring
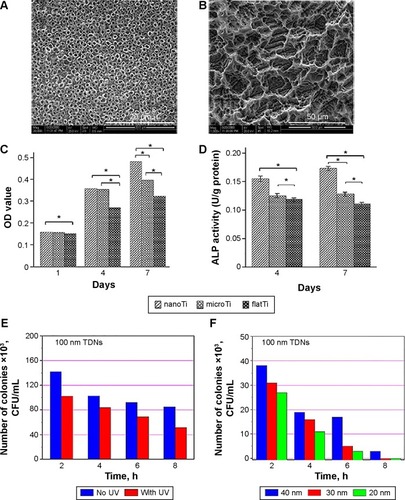
Microscale topography enhances biological events with indirect effect on cellular activity.Citation98 More direct outcomes may be obtained by nanoscale surface features because nanotopography can guide various molecular and biological processes at the implant/tissue interfaces while the provided large surface areas afford more binding sites to cell membrane receptors.Citation35,Citation101 Surface nanoarchitectures improve the in vivo and in vitro biocompatibility of implants.Citation105 shows the effect of surface nanostructuring on the properties of CP Ti. Xia et alCitation35 showed that the nanostructured topologies of Ti implants improve the proliferation, differentiation (), and development of the osteoblastic phenotype and increase the bone-implant interfacial strength. It is well documented that preparation of porous TiO2 film or TiO2 nanotubes improves the cell viability due to the higher surface energy, a larger number of particle-binding sites, and the topology mechanism.Citation35,Citation106 Cell attachment and proliferation are also improved. Furthermore, TiO2 nanotube films can provide antimicrobial activity particularly under ultraviolet (UV) radiation and/or in the presence of silver ions ().Citation41 Salou et alCitation107 studied the osseointegration of microstructured and nanostructured implants in rabbit femurs. They have shown that both the bone-to-implant contact and bone growth values are slightly higher for the nanostructured surface and it is better integrated into the bone. Experiments on the rabbit tibia using nanogrooved Ti implants indicated the dependency of bone response to the distribution of nanogrooves up to 8 weeks.Citation108
Figure 2 Effects of surface nanostructuring on the cell viability, differentiation, and bactericidal capacity of CP Ti.
Notes: (A, B) SEM images of TiO2 nanotube layer and microporous titanium, respectively. (C, D) The nanostructuring effect on the MG-63 cell proliferation and ALP activity. Reproduced with permission of Dove Medical Press, from Xia L, Feng B, Wang P, et al. In vitro and in vivo studies of surface-structured implants for bone formation. Int J Nanomedicine. 2012;7:4873; permission conveyed through Copyright Clearance Center, Inc.Citation35 Antibacterial activity of TiO2 nanotubes. *P<0.05, n=9; . *P<0.05, n=7. (E) under UV radiation and (F) in the presence of silver NPs of different sizes. Copyright © 2014. John Wiley & Sons, Inc. Reproduced from Esfandiari N, Simchi A, Bagheri R. Size tuning of Ag-decorated TiO2 nanotube arrays for improved bactericidal capacity of orthopedic implants. J Biomed Mater Res A. 2014;102(8):2625–2635.Citation41
Abbreviations: CP, commercially pure; SEM, scanning electron microscope; ALP, alkaline phosphatase; UV, ultraviolet; NPs, nanoparticles; h, hours; TDN, titanium dioxide nanotubes.
Nanostructured coatings
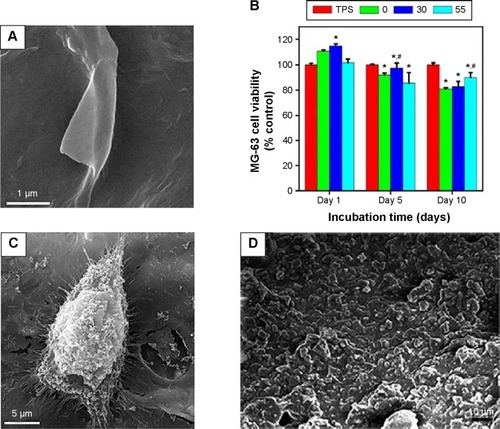
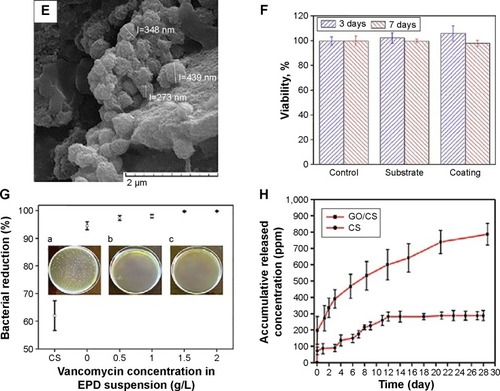
Surface functionalization of orthopedic implants by nanostructured coatings and nanocomposites is an alternative way to affect their integration to bone while tailoring their biological responses. Many studies have been devoted to the preparation and characterizations of nanoceramics, nanopolymers, nanocomposites, and carbon nanomaterials on implantable materials. Safonov et alCitation109 have shown that nanostructured Al2O3 coating on the surface of implantable metals such as Ti alloys and stainless steel improves in vivo and in vitro biocompatibility due to hydrophilic nature of the coated surface. Deposition of glycidyl methacrylate (GMA) nanolayer on the surface of Ti improves cellular attachment.Citation110 Various polymer-based nanocomposites such as PCL/TiO2,Citation26 PCL/n-BG,Citation27 CS/BG,Citation111 polymer/HA,Citation56 and polymer/calcium phosphateCitation8 have been examined and shown to possess improved bioactivity, enhanced mechanical properties, and better osteoconductivity. Many studies have also focused on utilizing carbon nanostructures as orthopedic implants coatings.Citation50 Prodana et alCitation106 developed a complex ceramic coating on Ti plates with TiO2 nanotubes, HA NPs, and functionalized MWCNTs. The complex coating showed a better biological adhesion and osteoblasts response. Ahmed alCitation112 prepared CS/MWCNTs/CaCO3 nanocomposites on the surface of Ti–6Al–4V alloy and reported improved bioactivity and corrosion resistance of the orthopedic implant. Ordikhani et alCitation113 fabricated novel GO/CS nanocomposite coatings on the surface of Ti foils by electrophoretic deposition (EPD) technique (). In vitro viability assay by human osteosarcoma cells (MG-63) demonstrated that the nanocomposite films were highly biocompatible up to 30 wt% GO (). The GO/CS films also supported the initial attachment, proliferation, and growth of the cells (). Mansoorianfar et alCitation53 incorporated NDs and BG NPs in alginate films by EPD technique to prepare functional coatings on Ti implants (). In vitro bioactivity assessment in simulated body fluid (SBF) and MTT assay using MG-63 and L929 cells exhibited enhanced biocompatibility and bioactivity of the composite films (). summarizes the surface modification methods for titanium and its alloys as the most widely used implantable materials in orthopedic medicine.
Table 2 Surface modification methods for titanium and its alloys implants
Figure 3 Effect of carbon nanostructures on the performance of electrodeposited polysaccharide coatings on Ti foils.
Notes: (A) SEM image of CS/GO (30 wt%) coating.Citation113 (B) MTT viability and (C) SEM morphology of MG-63 cells cultured on the surface of the CS/GO coating. (D) A SEM image of alginate/BG/ND film. Copyright © 2013. Elsevier B.V. Reproduced from Mansoorianfar M, Shokrgozag MA, Mehrjoo M, Tamjid E, Simchi A. Nanodiamonds for surface engineering of orthopedic implants: enhanced biocompatibility in human osteosarcoma cell culture. Diam Relat Mater. 2013;40(0):107–114.Citation53 (E) Formation of apatite phases on the surface of the alginate coating after 28 days of incubation in the SBF and (F) its MG-63 cell viability response. (G) The antibacterial performance of the CS/GO coating containing vancomycin against Staphylococcus aureus. Insets: plate counting images showing S. aureus bacteria colonies after 120 min incubation for the CS-30GO film containing (a) 0, (b) 0.5 and (c) 1 g/l antibiotics. (H) Cumulative drug release of the CS/GO (30 wt%) coating. Copyright © 2015. Elsevier B.V. Reproduced from Ordikhani F, Ramezani Farani M, et al. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon. 2015;84(0):91–102.Citation113 *Denotes significant difference between TPS and EPD coatings (P<0.05). #Denotes significant difference between CS and composite coatings (P<0.05).
Abbreviations: SEM, scanning electron microscope; CS, chitosan; GO, graphene oxide; BG, bioactive glass; ND, nanodiamond; SBF, simulated body fluid; TPS, tissue culture polystyrene; EPD, electrophoretic deposition; MTT, 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide.
Orthopedic infections, diagnosis, and therapeutics
Drug-eluting and antibacterial coatings
Postoperation infections of Ti implants including bacterial adhesion and biofilm formation remain the most common and serious complications in orthopedic surgery.Citation40 A simple and promising approach to reduce the risk of these infections would be local administration of antibacterial agents through implants coatings. Recent studies have demonstrated the potential of drug-eluting CS-based coatings to prevent implant-associated infections.Citation111,Citation116 Microbial examinations against Gram-positive Staphylococcus aureus exhibited a diminished bacterial behavior without impairing cell attachment and proliferation (). Results also showed that embedding of GO in the CS matrix affects the drug releasing profile with a longer release potential (). The results determine that GO controls the release rate but reduces osteogenesis.Citation113 Patel et alCitation48 have developed drug-eluting composite coatings of CS/BG NPs containing ampicillin. The drug was eluted from the coatings continuously over 10–11 weeks, confirming long-term drug delivering capacity. Antibacterial tests using agar diffusion assay against Streptococcus mutants also approved the positive effect of released ampicillin. Radin and DucheyneCitation117 used silica sol-gel technology to obtain thin antibacterial nanostructure coatings on the metallic substrates. Mattioli-Belmonte et alCitation118 developed a ciprofloxacin-loaded CS NP-based coating on Ti substrates. This antibacterial coating was able to inhibit the growth of two nosocomial S. aureus strains in vitro without impairing the MG-63 viability, adhesion, and gene expression after several days. In another study, TiO2 nanotubes were used as cefuroxime carriers to prevent periprosthetic infections.Citation119 Silver NPs have also been used as antibacterial agents in many studies.Citation40–Citation42,Citation60 Recently, composite orthopedic coatings with antibacterial capability containing CS/BG/Ag NPs have been fabricated by employing a single-step EPD process.Citation60 Studying of the structural and preliminary in vitro bactericidal and cellular properties showed that the composite coatings containing 342 µg of Ag NPs were cytotoxic on MG-63 cells. Liu et alCitation58 prepared a silver incorporated HA nanocomposite coating on implant surface and showed a significant enhancement in the antibacterial property in vitro. Zhao et alCitation40 fabricated TiO2 nanotubes incorporated with Ag NPs on Ti implants. The bactericidal potential of the coating up to 30 days was shown. Anyway, the cytotoxicity of silver-loaded coatings is a great concern that can be reduced by controlling the Ag release rate. Esfandiari et alCitation41 prepared Ag-decorated TiO2 nanotubes with different sizes by a combined electrochemical and UV-assisted reduction method. They showed synergetic effect of Ag NPs and TiO2 nanotubes on the bactericidal activity. The highest antibacterial activity of the coating was obtained for TiO2 nanotubes with opening diameter of ~100 nm and silver NPs of 20 nm. Yan et alCitation59 synthesized HA films containing Ag+ ions via electrochemical deposition on anodized Ti. In vitro examinations revealed that a HA coating containing 2.03 wt% Ag had significant antibacterial and osteogenic properties.
Diagnosis
Another important issue in orthopedic therapies is detection of the healing sites by versatile tools in order to effectively detect bone-related diseases such as osteoporosis, Paget’s disease, and renal osteodystrophy in the earliest stage and monitor orthopedic therapies.Citation120 Implantable wireless bio-sensors have recently been developed for this purpose.Citation121 Particularly, CNTs have been found of great potential for the fabrication of bone sensorsCitation51,Citation122 due to their excellent electrical conductivity and mechanical strength as well as unique chemical–biological properties. The high electrical conductivity of CNTs promotes bone growth as bone regenerates under electrical conduction. Supronowicz et alCitation123 reported a 46% increase in osteoblast proliferation as a result of adding MWCNTs into nanocomposites of PLA, and more than 300% rise in calcium production when an alternating current was applied to the substrate in vitro.
It is noteworthy that for such sensor design, cell responses transduce and transmit a variety of chemical and physical signals to produce specific substances and proteins within specific tissues and organs. Sirinrath and ThomasCitation124 showed the redox of proteins could be enhanced on MWCNTs grown from an anodized nanotubular Ti electrode in order to sense bone growth, promote osteoblast proliferation, and differentiation after 21 days.
Another idea to monitor the bone turnover is detecting bone-related degradation products. A label-free electrochemical impedance spectroscopy immunosensor for detecting C-terminal teleopeptides from Type-1 collagen was developed by Yun et al.Citation120,Citation125 Such sensing methods are based on the principal idea that the cycle of bone remodeling consists of three consecutive phases: preexisting bone resorption by osteoclasts, a reversal phase that is characterized by mono-nuclear cells on the bone surface, and new bone formation by osteoblasts to fill in the cavities after resorption. These types of sensors can also be prepared on gold electrode but recent advances suggest utilizing CNT electrodes to enhance sensitivity due to improved electrical conductivity. For instance, CNT-TiN nanocomposites, composed of 12% CNTs by volume, showed a 45% increased electrical conductivity over TiN materials.Citation126
Meanwhile, coupling drug delivery to implantable wireless sensors enables on-command diffusion-controlled drug delivery systems by using radio frequency, as a new approach in the orthopedic field.Citation121 Taking advantage of nanotechnology, the upcoming future sensors can sense new bone formation, and if that is not happening, release drugs to promote new bone growth.
Stem cell therapies by hMSCs have exceptional regenerative potential and thus are promising candidates for bone regeneration and fracture healing.Citation127,Citation128 To monitor these processes, hMSCs have been labeled with diverse NPs such as quantum dots (QDs), fluorescence-labeled mesoporous silica NPs, gold NPs, and superparamagnetic iron oxide (SPIO) NPs.Citation7 The fluorescent labeling of osteoblast cells using HA grown with nucleating seed of hydrophilic CdSe/ZnS QDs allows real-time observation of cell under confocal microscope. A study performed on MC3T3-E1 osteoblast cells showed that the cells could engulf HA with surface-tailored QDs showing fluorescent spots in the cytoplasm, while HA and QDs NPs were not engulfed. Interestingly, the fluorescence was only displayed in the cytoplasm of MC3T3-E1 osteoblast cells.Citation129 Gold NPs have recently been investigated as contrast agents for micro-CT applicationsCitation130,Citation131 and advanced X-ray imaging technologies.Citation132 Since Au has a higher absorption coefficient than Iodine and experiences less interference with bones and tissues, Au NPs improved contrast at lower doses while prolonging the imaging time.Citation132 The SPIO NPs are also promising materials as contrast agents for MRI. Nevertheless, it should be noted that bone represents a formidable target organ, which poses a particular challenge with regard to cell labeling (due to its high mineralization grade); hence, making the visualization of labeled cells in MRI difficult.Citation7 In a very recent study, the incorporation of a multimodal contrast agent based on HA nanocrystals within a PCL nanofibrous scaffold by electrospinning have been reported. This preliminary study was performed to eventually exploit the MR contrast imaging capability of nanofibrous scaffolds for real-time imaging of the changes in the tissue engineered construct.Citation133 In a clinical trial performed on osteoblasts, SPIO NPs bounded to RGD receptors enabled stimulation of cells over a 3-week period. Following cyclical magnetic stimulation, labeled cells demonstrated upregulation of osteopontin and increased osteo-related protein production. These results indicate that culture of SPIONs with osteoblasts does not inhibit osteogenic behavior. Furthermore, SPIONs can be bound to selected membrane receptors and then subjected to oscillating magnetic fields via a magnetic force bioreactor in order to stimulate tagged receptors and provide mechanotransduction through the cell membrane.Citation128
Similar attempts are being struggled for decreasing orthopedic implant infection and inflammation. As mentioned in the previous section, implant-associated infections are a serious health threat for patients, and their clinical management is expensive. Diagnosis of these infections is hampered by intracellular bacteria, formation of biofilms, and aseptic and posttraumatic changes. There is a clinical niche for improved diagnostic tools to uncover pathogenic forms of infections. Taking the advantages of NPs as contrast agents in CT and MRI imaging, nanotechnology can be an indispensable tool for infection diagnostic and eradication. Nuclear medicine using radiolabeled infection tracers is also a promising method for implant-associated infection diagnostic in clinics. Development of multiplexed imaging modalities together with identification of specific infection probes is progressing rapidly.Citation134
Biological properties such as temperature and pH values at the border of the implant are key parameters that can be utilized for the characterization of the implant-bone interface infections.Citation135 Gou et alCitation136 developed a solid-state sensor based on oxidized single-walled carbon nanotubes functionalized with poly(1-aminoanthracene). By attaching to a passively powered radio frequency identification tag, they could transmit pH data through simulated skin. This device had a Nernstian response over a wide pH range (2–12) and retained sensitivity over 120 days. It is therefore expected that new achievements in diagnostic imaging will significantly decrease the risk of late and falsely diagnosed implant-associated infections.
Cancer therapy
Skeletal complications resulting from bone cancer are an important health care problem. Osteosarcoma is the most common primary tumor of bone and considered to be the third most common malignancy in children and adolescents.Citation137 Bone is the most common site of cancer metastasis and is particularly important in breast and prostate cancers because these diseases have a high prevalence of bone metastases.Citation85 Bone metastases are mainly treated through surgery, radiotherapy, systemic chemotherapy, bisphosphonates, and radioisotopes.Citation138 Recent advances have led to the development of multifunctional bionanomaterials that can target a bone tumor and deliver therapeutic drugs or genes.Citation139 Various composite materials with different content of magnetite are used in order to induce hyperthermia.Citation140,Citation141 Andronescu et alCitation13 developed a magnetite-enriched collagen/HA composite material as a bone graft material and also hyperthermia generator for bone cancer therapy. Hu et alCitation141 prepared 3D nanomagnetite/CS rod, which could be useful for local hyperthermia in bone tumors. In another study, Murakami et alCitation142 developed a magnetite/HA composite that facilitates direct bonding to bones through HA and generation of heat from magnetite (exposed to AC magnetic field) for hyperthermia therapies of cancer in bones. This composite had micro-sized pores of approximately 400 µm and submicron-sized pores of approximately 0.2 µm in size. They showed that magnetite aggregates were strongly trapped in the cages of rod-shaped HA particles at 30 mass% or less concentrations.
Nanotoxicology
By rapidly emerging science of nanotechnology and the development of new products in a wide range of applications, concerns have been raised regarding potential risks of nanomaterials on human health and environment that may result from exposure during their life cycle.Citation143 Nanotoxicology, the science of engineered nanostructures that deals with the health threats or adverse effects on living organisms, has attracted a lot of attention recently.Citation144 In practice, the diversity of engineered nanomaterials and their unlimited potential applications have posed major challenges for safety assessment. The analytical methods to detect and quantify concentrations of nanoscale materials in the environment and human body are still under development as well.Citation145 Research on humans and animals indicates that some NPs are able to enter the body, and then translocate into different organs via the circulatory and lymphatic systems.Citation150 Toxic responses to NPs generated from the degradation of implanted nanomaterials, via wear debris from artificial joints with nanofeatures, and heavy metals (iron, nickel, and cobalt catalysts) remaining in CNTs, have recently been reported.Citation11,Citation146 Even though nanophase materials have increased wear fatigue properties, debris may form from articulating components of nanostructured orthopedic implants when subjected to physiological loading properties.Citation147 Potential adverse effects may range from inflammation, exacerbation of asthma and metal fume fever to fibrosis, chronic inflammatory lung diseases, and carcinogenesis.Citation148 For instance, silica NPs have been shown to have a low toxicity in vivo when administered in moderate doses due to oxidative stress. Lung and embryonic toxicity has also been observed for CNTs.Citation149 It should be noted that very little is known about the underlying toxicity mechanisms responsible for the possible toxic actions of nanomaterials.Citation150 Production of increased reactive oxygen species is considered as the major cause of toxicity in nanoscale materials.Citation148 It has been shown that the tendency for toxicity increases with decreasing particles size, even when the same material is inert in bulkier form, such as carbon and Ti dioxide.Citation151 Therefore, the interactions between nanomaterials and living organisms as well as the biological effects of these materials should be exclusively studied. Particularly, the relationship between nanomaterial characteristics (size, shape, surface area, etc) and their toxic responses should be illustrated.Citation152 Besides technological advancement that expands the nanomedicine market,Citation153,Citation154 we believe that much more in vivo evaluations, toxicological surveys, and clinical trials are needed before nanomaterials can be widely commercialized for orthopedic applications.
Conclusion and future remarks
Preliminary investigations support the potential of nanobiomaterials in orthopedic applications; however, advancements are still necessary to achieve clinical use. The goal is to fabricate bioactive scaffolds designed for bone regeneration that will temporarily substitute for natural tissues while interacting with their surroundings, respond to environmental changes, and actively direct cellular events for faster bone formation, reduced healing time, and rapid recovery to function. Future work will likely develop enhanced design methodologies to take advantage of nanomaterials and new fabrication technology. It is critical to understand molecular mechanisms of cell–nanobiomaterial interactions. Besides, validating the biosafety of nanomaterials and minimizing their impacts should be taken into consideration seriously.
Disclosure
The authors report no conflict of interest in this work.
References
- WangMThanouMTargeting nanoparticles to cancerPharmacol Res2010622909920380880
- LiuHWebsterTJNanomedicine for implants: a review of studies and necessary experimental toolsBiomaterials200728235436921898921
- LiXWangLFanYFengQCuiF-ZWatariFNanostructured scaffolds for bone tissue engineeringJ Biomed Mater Res A2013101A82424243523377988
- McMahonREWangLSkorackiRMathurABDevelopment of nanomaterials for bone repair and regenerationJ Biomed Mater Res B Appl Biomater2012101B238739723281143
- TranNWebsterTJNanotechnology for bone materialsWiley Interdiscip Rev Nanomed Nanobiotechnol20091333635120049801
- McMahonREWangLSkorackiRMathurABDevelopment of nanomaterials for bone repair and regenerationJ Biomed Mater Res B Appl Biomater2013101238739723281143
- TautzenbergerAKovtunAIgnatiusANanoparticles and their potential for application in boneInt J Nanomedicine201274545455722923992
- WangPZhaoLLiuJWeirMDZhouXXuHHBone tissue engineering via nanostructured calcium phosphate biomaterials and stem cellsBone Res20142 Article 14017
- SimchiATamjidEPishbinFBoccacciniARRecent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applicationsNanomedicine201171223921050895
- BoseSVahabzadehSBandyopadhyayABone tissue engineering using 3D printingMater Today20131612496504
- ZhangLWebsterTJNanotechnology and nanomaterials: promises for improved tissue regenerationNano Today2009416680
- ShirwaikerRASambergMECohenPHWyskRAMonteiro-RiviereNANanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devicesWiley Interdiscip Rev Nanomed Nanobiotechnol20135319120423335493
- AndronescuEFicaiMVoicuGFicaiDMaganuMFicaiASynthesis and characterization of collagen/hydroxyapatite: magnetite composite material for bone cancer treatmentJ Mater Sci Mater Med20102172237224220372983
- SchiaviJKellerLMorandD-NActive implant combining human stem cell microtissues and growth factors for bone-regenerative nanomedicineNanomedicine201510575376325816878
- LevengoodSKLZhangMChitosan-based scaffolds for bone tissue engineeringJ Mater Chem B201422131613184
- VenkatesanJKimS-KChitosan composites for bone tissue engineering – an overviewMar Drugs2010882252226620948907
- KimJKimISChoTHBone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cellsBiomaterials200728101830183717208295
- PattersonJSiewRHerringSWLinASPGuldbergRStaytonPSHyaluronic acid hydrogels with controlled degradation properties for oriented bone regenerationBiomaterials201031266772678120573393
- LeePTranKChangWShelkeNBKumbarSGYuXInfluence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: cartilage regenerationJ Biomed Nanotechnol20141081469147925016647
- CorreiaCBhumiratanaSYanL-PDevelopment of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cellsActa Biomater2012872483249222421311
- ZhangWZhuCYeDPorous silk scaffolds for delivery of growth factors and stem cells to enhance bone regenerationPloS One201497e10237125050556
- ZhangYFanWNothdurftLIn vitro and in vivo evaluation of adenovirus combined silk fibroin scaffolds for bone morphogenetic protein-7 gene deliveryTissue Eng Part C Methods201117878979721506685
- XinXHussainMMaoJJContinuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffoldBiomaterials200728231632517010425
- ParkGEPattisonMAParkKWebsterTJAccelerated chondrocyte functions on NaOH-treated PLGA scaffoldsBiomaterials200526163075308215603802
- GentilePChionoVCarmagnolaIHattonPVAn overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineeringInt J Mol Sci20141533640365924590126
- TamjidEBagheriRVossoughiMSimchiAEffect of TiO2 morphology on in vitro bioactivity of polycaprolactone/TiO2 nanocompositesMater Lett20116515–1625302533
- TamjidEBagheriRVossoughiMSimchiAEffect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone compositesMater Sci Eng C201131715261533
- KamathMSAhmedSSSJDhanasekaranMSantoshSWPolycaprolactone scaffold engineered for sustained release of resveratrol: therapeutic enhancement in bone tissue engineeringInt J Nanomedicine2014918319524399875
- StreicherRMSchmidtMFioritoSNanosurfaces and nanostructures for artificial orthopedic implants2007
- XingZCHanSJShinYSEnhanced osteoblast responses to poly(methyl methacrylate)/hydroxyapatite electrospun nanocomposites for bone tissue engineeringJ Biomater Sci Polym Ed2013241617622289639
- JaeblonTPolymethylmethacrylate: properties and contemporary uses in orthopaedicsJ Am Acad Orthop Surg201018529730520435880
- LopesMSJardiniALFilhoRMPoly (lactic acid) production for tissue engineering applicationsProcedia Eng201242014021413
- MaRTangTCurrent strategies to improve the bioactivity of PEEKInt J Mol Sci20141545426544524686515
- EvansNTTorstrickFBLeeCSDHigh-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implantsActa Biomater201513015916725463499
- XiaLFengBWangPIn vitro and in vivo studies of surface-structured implants for bone formationInt J Nanomedicine20127487323028216
- Mishnaevsky JrLLevashovEValievRZNanostructured titanium-based materials for medical implants: Modeling and developmentMater Sci Eng R Rep2014810119
- WebsterTJEjioforJUIncreased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMoBiomaterials200425194731473915120519
- RyuJJShrotriyaPInfluence of roughness on surface instability of medical grade cobalt–chromium alloy (CoCrMo) during contact corrosion–fatigueAppl Surf Sci2013273536541
- YamanakaKMoriMChibaANanoarchitectured Co–Cr–Mo orthopedic implant alloys: Nitrogen-enhanced nanostructural evolution and its effect on phase stabilityActa Biomater2013946259626723253619
- ZhaoLWangHHuoKAntibacterial nanostructured titania coating incorporated with silver nanoparticlesBiomaterials201132245706571621565401
- EsfandiariNSimchiABagheriRSize tuning of Ag-decorated TiO2 nanotube arrays for improved bactericidal capacity of orthopedic implantsJ Biomed Mater Res A201410282625263523982977
- PaukschLHartmannSRohnkeMBiocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblastsActa Biomater201410143944924095782
- KrawczynskaATGlocMLublinskaKIntergranular corrosion resistance of nanostructured austenitic stainless steelJ Mater Sci2013481345174523
- CaiYZhangKZhangZDongJLeiYZhangTSurface nanostructure formations in an AISI 316L stainless steel induced by pulsed electron beam treatmentJ Nanomater201520155
- MohandasGOskolkovNMcMahonMTWalczakPJanowskiMPorous tantalum and tantalum oxide nanoparticles for regenerative medicineActa Neurobiol Exp201474188196
- ZhouCDengCChenXMechanical and biological properties of the micro-/nanograin functionally graded hydroxyapatite bioceramics for bone tissue engineeringJ Mech Behav Biomed Mater20150
- BoscoRIafiscoMTampieriAJansenJALeeuwenburghSCGvan den BeuckenJJJPHydroxyapatite nanocrystals functionalized with alendronate as bioactive components for bone implant coatings to decrease osteoclastic activityAppl Surf Sci20153280516524
- PatelKDEl-FiqiALeeH-HH-YChitosan–nanobioactive glass electrophoretic coatings with bone regenerative and drug delivering potentialJ Mater Chem201222472494524956
- PriceRLHaberstrohKMWebsterTJEnhanced functions of osteoblasts on nanostructured surfaces of carbon and aluminaMed Biol Eng Comput200341337237512803305
- YangLZhangLWebsterTJCarbon nanostructures for orthopedic medical applicationsNanomedicine2011671231124421929458
- NewmanPMinettAEllis-BehnkeRZreiqatHCarbon nanotubes: their potential and pitfalls for bone tissue regeneration and engineeringNanomedicine2013981139115823770067
- ZhaoCLuXZandenCLiuJThe promising application of graphene oxide as coating materials in orthopedic implants: preparation, characterization and cell behaviorBiomed Mater201510101501925668049
- MansoorianfarMShokrgozarMAMehrjooMTamjidESimchiANanodiamonds for surface engineering of orthopedic implants: enhanced biocompatibility in human osteosarcoma cell cultureDiam Relat Mater2013400107114
- GarmendiaNOlaldeBObietaI16 – Biomedical applications of ceramic nanocompositesBanerjeeRMannaICeramic NanocompositesCambridge, UKWoodhead Publishing2013530547
- GainAKZhangLLiuWMicrostructure and material properties of porous hydroxyapatite-zirconia nanocomposites using polymethyl methacrylate powdersMater Des2015670136144
- HickeyDJErcanBSunLWebsterTJAdding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applicationsActa Biomater201514017518425523875
- LiaoCZLiKWongHMTongWYYeungKWKTjongSCNovel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacementsMater Sci Eng C201333313801388
- LiuXMouYWuSManHCSynthesis of silver-incorporated hydroxyapatite nanocomposites for antimicrobial implant coatingsAppl Surf Sci20132730748757
- YanYZhangXHuangYDingQPangXAntibacterial and bioactivity of silver substituted hydroxyapatite/TiO2 nanotube composite coatings on titaniumAppl Surf Sci20143140348357
- PishbinFMourinoVGilchristJBSingle-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nanosilver composite systemActa Biomater2013977469747923511807
- ZhouCShiQGuoWElectrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLAACS Appl Mater interfaces2013593847385423590943
- ChengYRamosDLeePLiangDYuXKumbarSGCollagen functionalized bioactive nanofiber matrices for osteogenic differentiation of mesenchymal stem cells: bone tissue engineeringJ Biomed Nanotechnol201410228729824738337
- El-GhannamABone reconstruction: from bioceramics to tissue engineeringExpert Rev Med Devices2005218710116293032
- WuSLiuXYeungKWKLiuCYangXBiomimetic porous scaffolds for bone tissue engineeringMater Sci Eng R Rep2014800136
- HoexterDLBone regeneration graft materialsJ Oral Implantol200228629029412498538
- SalgadoAJOliveiraJMMartinsATissue engineering and regenerative medicine: past, present, and futureInt Rev Neurobiol201310813324083429
- ThibaultRAMikosAGKasperFKScaffold/extracellular matrix hybrid constructs for bone-tissue engineeringAdv Healthc Mater201321132423184883
- StevensMMBiomaterials for bone tissue engineeringMater Today20081151825
- LaurencinCTAmbrosioAMBordenMDCooperJAJrTissue engineering: orthopedic applicationsAnnu Rev Biomed Eng19991194611701481
- ChristensonEMAnsethKSvan den BeuckenJJJPNanobiomaterial applications in orthopedicsJ Orthop Res2007251112217048259
- QuDMosherCZBoushellMKLuHHEngineering complex orthopaedic tissues via strategic biomimicryAnn Biomed Eng201433
- de GuzmanRCSaulJMEllenburgMDBone regeneration with BMP-2 delivered from keratose scaffoldsBiomaterials20133461644165623211447
- ReddiAHCunninghamNSBone induction by osteogenin and bone morphogenetic proteinsBiomaterials19901133342204436
- LiuCWanPTanLLWangKYangKPreclinical investigation of an innovative magnesium-based bone graft substitute for potential orthopaedic applicationsJ Orthop Translat201423139148
- PompaLRahmanZUMunozEHaiderWSurface characterization and cytotoxicity response of biodegradable magnesium alloysMater Sci Eng C2015490761768
- SerraGMoraisLEliasCNNanostructured severe plastic deformation processed titanium for orthodontic mini-implantsMater Sci Eng C201333741974202
- EstrinYIvanovaEPMichalskaATruongVKLapovokRBoydRAccelerated stem cell attachment to ultrafine grained titaniumActa Biomater20117290090620887818
- WangCTGaoNGeeMGWoodRJKLangdonTGProcessing of an ultrafine-grained titanium by high-pressure torsion: an evaluation of the wear properties with and without a TiN coatingJ Mech Behav Biomed Mater201317016617523140675
- ParkJ-WKimY-JParkCHEnhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topographyActa Biomater2009583272328019426841
- PerlaVWebsterTJBetter osteoblast adhesion on nanoparticulate selenium – A promising orthopedic implant materialJ Biomed Mater Res A200575235636416059879
- TranPASarinLHurtRHWebsterTJTitanium surfaces with adherent selenium nanoclusters as a novel anticancer orthopedic materialJ Biomed Mater Res A20109341417142819918919
- TranPWebsterTJEnhanced osteoblast adhesion on nanostructured selenium compacts for anti-cancer orthopedic applicationsInt J Nanomedicine20083339118990948
- CatledgeSAFriesMDVohraYKNanostructured ceramics for biomedical implantsJ Nanosci Nanotechnol200223–429331212908255
- SimchiATamjidEPishbinFBoccacciniARecent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applicationsNanomedicine201171223921050895
- Ovid’koISheinermanAMicromechanisms for improved fracture toughness in nanoceramicsRev Adv Mater Sci2011292105125
- HafeziFHosseinnejadFFooladiAMohit MafiSAmiriANouraniMTransplantation of nano-bioglass/gelatin scaffold in a non-autogenous setting for bone regeneration in a rabbit ulnaJ Mater Sci Mater Med201223112783279222826004
- AgarwalRGarcíaAJBiomaterial strategies for engineering implants for enhanced osseointegration and bone repairAdv Drug Deliv Rev2015 Epub201548
- GalloJHolinkaMMouchaCSAntibacterial surface treatment for orthopaedic implantsInt J Mol Sci2014158138491388025116685
- ParkHTemenoffJSMikosAGBiodegradable orthopedic implantsEngineering of Functional Skeletal TissuesNew YorkSpringer20075568
- MandalBBGrinbergASeok GilEPanilaitisBKaplanDLHigh-strength silk protein scaffolds for bone repairProc Nat Acad Sci2012109207699770422552231
- SahooNGPanYZLiLHeCBNanocomposites for bone tissue regenerationNanomedicine20138463965323560413
- Sadat-ShojaiMKhorasaniM-TJamshidiA3-Dimensional cell-laden nano-hydroxyapatite/protein hydrogels for bone regeneration applicationsMater Sci Eng C2015490835843
- BaradaranSMoghaddamEBasirunWJMechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide compositeCarbon20146903245
- WuMWangQLiuXLiuHBiomimetic synthesis and characterization of carbon nanofiber/hydroxyapatite composite scaffoldsCarbon2013510335345
- DeligianniDDKatsalaNLadasSSotiropoulouDAmedeeJMissirlisYEffect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorptionBiomaterials200122111241125111336296
- KollerGCookRJThompsonIDWatsonTFDi SilvioLSurface modification of titanium implants using bioactive glasses with air abrasion technologiesJ Mater Sci Mater Med200718122291229617562133
- ChenW-CChenY-SKoC-LLinYKuoT-HKuoH-NInteraction of progenitor bone cells with different surface modifications of titanium implantMater Sci Eng C2014370305313
- VariolaFBrunskiJBOrsiniGde OliveiraPTWazenRNanciANanoscale surface modifications of medically relevant metals: state-of-the art and perspectivesNanoscale20113233535320976359
- CiganovicJStasicJGakovicBSurface modification of the titanium implant using TEA CO 2 laser pulses in controllable gas atmospheres–Comparative studyAppl Surf Sci2012258727412748
- LorenzettiMBiglinoDNovakSKobeSPhotoinduced properties of nanocrystalline TiO2-anatase coating on Ti-based bone implantsMater Sci Eng C2014370390398
- LinLWangHNiMEnhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structuresJ Orthop Translat2014213542
- LiL-HKongY-MKimH-WImproved biological performance of Ti implants due to surface modification by micro-arc oxidationBiomaterials200425142867287514962565
- OrdikhaniFTamjidESimchiACharacterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infectionsMater Sci Eng C2014410240248
- Rosales-LealJIRodríguez-ValverdeMAMazzagliaGEffect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesionColloids Surf A Physicochem Eng Asp20103651–3222229
- WuSLiuXYeungKWSurface nano-architectures and their effects on the mechanical properties and corrosion behavior of Ti-based orthopedic implantsSurf Coat Technol20132331326
- ProdanaMDutaMIonitaDA new complex ceramic coating with carbon nanotubes, hydroxyapatite and TiO2 nanotubes on Ti surface for biomedical applicationsCeram Int2015415, Part A63186325
- SalouLHoornaertALouarnGLayrollePEnhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbitsActa Biomater201511049450225449926
- ProdanovLLamersEDomanskiMLuttgeRJansenJAWalboomersXFThe effect of nanometric surface texture on bone contact to titanium implants in rabbit tibiaBiomaterials201334122920292723380354
- SafonovVZykovaASmolikJRogowskaRLukyanchenkoVKolesnikovDModification of implant material surface properties by means of oxide nanostructured coatings depositionAppl Surf Sci20143100174179
- ParkSWLeeDLeeHRGeneration of functionalized polymer nanolayer on implant surface via initiated chemical vapor deposition (iCVD)J Colloid Interface Sci20154390344125463173
- OrdikhaniFSimchiALong-term antibiotic delivery by chitosan-based composite coatings with bone regenerative potentialAppl Surf Sci201431705666
- AhmedRAFekryAMFarghaliRAA study of calcium carbonate/multiwalled-carbon nanotubes/chitosan composite coatings on Ti–6Al–4V alloy for orthopedic implantsAppl Surf Sci2013285 Part B(0)309316
- OrdikhaniFRamezani FaraniMDehghaniMTamjidESimchiAPhysicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacityCarbon201584091102
- NguyenHQDeporterDAPilliarRMValiquetteNYakubovichRThe effect of sol–gel-formed calcium phosphate coatings on bone ingrowth and osteoconductivity of porous-surfaced Ti alloy implantsBiomaterials200425586587614609675
- ShimIKChungHJJungMRBiofunctional porous anodized titanium implants for enhanced bone regenerationJ Biomed Mater Res A2014102103639364824265190
- OrdikhaniFTamjidESimchiACharacterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant associated infectionsMater Sci Eng C201441240248
- RadinSDucheynePControlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate materialBiomaterials20072891721172917184835
- Mattioli-BelmonteMCometaSFerrettiCCharacterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implantsCarbohydr Polym2014110017318224906744
- ChennellPFeschet-ChassotEDeversTAwitorKODescampsSSautouVIn vitro evaluation of TiO2 nanotubes as cefuroxime carriers on orthopaedic implants for the prevention of periprosthetic joint infectionsInt J Pharm20134551–229830523892151
- YunYHEtesholaEBhattacharyaATiny medicine: nanomaterial-based biosensorsSensors (Basel)20099119275929922291565
- YangLWebsterTMonitoring tissue healing through nanosensorsWebsterTJNanotechnology Enabled In Situ Sensors for Monitoring HealthNew YorkSpringer20114159
- LinYTaylorSLiHAdvances toward bioapplications of carbon nanotubesJ Mater Chem2004144527541
- SupronowiczPRAjayanPMUllmannKRArulanandamBPMetzgerDWBiziosRNovel current-conducting composite substrates for exposing osteoblasts to alternating current stimulationJ Biomed Mater Res200259349950611774308
- SirinrathSThomasJWMultiwalled carbon nanotubes enhance electrochemical properties of titanium to determine in situ bone formationNanotechnology2008192929510121730595
- YunYBangeAHeinemanWRA nanotube array immunosensor for direct electrochemical detection of antigen–antibody bindingSens Actuators B Chem20071231177182
- JiangLGaoLFabrication and characterization of carbon nanotube–titanium nitride composites with enhanced electrical and electrochemical propertiesJ Am Ceram Soc2006891156161
- BullEMadaniSYShethRSeifalianAGreenMSeifalianAMStem cell tracking using iron oxide nanoparticlesInt J Nanomedicine201491641165324729700
- WimpennyIMarkidesHEl HajAJOrthopaedic applications of nanoparticle-based stem cell therapiesStem Cell Res Ther2012321322520594
- HsiehMFLiJKLinCATracking of cellular uptake of hydrophilic CdSe/ZnS quantum dots/hydroxyapatite composites nanoparticles in MC3T3-E1 osteoblast cellsJ Nanosci Nanotechnol2009942758276219438032
- XiDDongSMengXLuQMengLYeJGold nanoparticles as computerized tomography (CT) contrast agentsRSC Adv20122331251512524
- PopovtzerRAgrawalAKotovNATargeted gold nanoparticles enable molecular CT imaging of cancerNano Lett20088124593459619367807
- AhnSJungSLeeSGold nanoparticle contrast agents in advanced X-ray imaging technologiesMolecules20131855858589023685939
- GaneshNAshokanARajeshkannanRChennazhiKKoyakuttyMNairSVMagnetic resonance functional nano-hydroxyapatite incorporated poly(caprolactone) composite scaffolds for in situ monitoring of bone tissue regeneration by MRITissue Eng A20142019–2027832794
- PotapovaIFunctional imaging in diagnostic of orthopedic implant-associated infectionsDiagnostics201334356
- RutherCLohrengelAKluessDCurrent Possibilities for Detection of Loosening of Total Hip Replacements and How Intelligent Implants Could Improve Diagnostic AccuracyRijeka, CroatiaINTECH Open Access Publisher2012
- GouPKrautNDFeigelIMCarbon nanotube chemiresistor for wireless pH sensingSci Rep20144446824667793
- KansaraMThomasDMMolecular pathogenesis of osteosarcomaDNA Cell Biol200726111817263592
- SuvaLJWashamCNicholasRWGriffinRJBone metastasis: mechanisms and therapeutic opportunitiesNat Rev Endocrinol20117420821821200394
- MohamedMBorchardGJordanOIn situ forming implants for local chemotherapy and hyperthermia of bone tumorsJ Drug Deliv Sci Technol201222393408
- LiBJiaDZhouYHuQCaiWIn situ hybridization to chitosan/magnetite nanocomposite induced by the magnetic fieldJ Magn Magn Mater20063062223227
- HuQChenFLiBShenJPreparation of three-dimensional nanomagnetite/chitosan rodMater Lett2006603368370
- MurakamiSHosonoTJeyadevanBKamitakaharaMIokuKHydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancerJ Ceram Soc Jpn20081161357950954
- ThomasKSayrePResearch strategies for safety evaluation of nanomaterials, part I: evaluating the human health implications of exposure to nanoscale materialsToxicol Sci200587231632116049265
- OberdorsterGOberdorsterEOberdorsterJNanotoxicology: an emerging discipline evolving from studies of ultrafine particlesEnviron Health Perspect2005113782383916002369
- MajesticBJErdakosGBLewandowskiMA review of selected engineered nanoparticles in the atmosphere: sources, transformations, and techniques for sampling and analysisInt J Occup Environ Health201016448850721222392
- MadlAKKovochichMLiongMFinleyBLPaustenbachDJOberdörsterGToxicology of wear particles of cobalt-chromium alloy metal-on-metal hip implants Part II: Importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessmentNanomedicine20151151285129825735266
- WebsterTJAhnESNanostructured biomaterials for tissue engineering boneAdv Biochem Eng Biotechnol200710327530817195467
- ChengL-CJiangXWangJChenCLiuR-SNanobio effects: interaction of nanomaterials with cellsNanoscale2013593547356923532468
- LiXWangLFanYFengQCuiF-ZBiocompatibility and toxicity of nanoparticles and nanotubesJ Nanomater2012201219
- BakandSHayesADechsakulthornFNanoparticles: a review of particle toxicology following inhalation exposureInhal Toxicol201224212513522260506
- FanAMAlexeeffGNanotechnology and nanomaterials: toxicology, risk assessment, and regulationsJ Nanosci Nanotechnol201010128646865721121378
- FischerHCChanWCNanotoxicity: the growing need for in vivo studyCurr Opin Biotechnol200718656557118160274
- WebsterTJNanomedicine: real commercial potential or just hype?Int J Nanomedicine20061437337417722271
- MorigiVTocchioABellavite PellegriniCSakamotoJHArnoneMTasciottiENanotechnology in medicine: from inception to market dominationJ Drug Deliv201220127