Abstract
Background
Zinc oxide engineered nanoparticles (ZnO ENPs) have potential as nanomedicines due to their inherent properties. Studies have described their pulmonary impact, but less is known about the consequences of ZnO ENP interactions with the liver. This study was designed to describe the effects of ZnO ENPs on the liver and Kupffer cells after intravenous (IV) administration.
Materials and methods
First, pharmacokinetic studies were conducted to determine the tissue distribution of neutron-activated 65ZnO ENPs post-IV injection in Wistar Han rats. Then, a noninvasive in vivo method to assess Kupffer cell phagosomal motility was employed using ferromagnetic iron particles and magnetometry. We also examined whether prior IV injection of ZnO ENPs altered Kupffer cell bactericidal activity on circulating Pseudomonas aeruginosa. Serum and liver tissues were collected to assess liver-injury biomarkers and histological changes, respectively.
Results
We found that the liver was the major site of initial uptake of 65ZnO ENPs. There was a time-dependent decrease in tissue levels of 65Zn in all organs examined, refecting particle dissolution. In vivo magnetometry showed a time-dependent and transient reduction in Kupffer cell phagosomal motility. Animals challenged with P. aeruginosa 24 hours post-ZnO ENP injection showed an initial (30 minutes) delay in vascular bacterial clearance. However, by 4 hours, IV-injected bacteria were cleared from the blood, liver, spleen, lungs, and kidneys. Seven days post-ZnO ENP injection, creatine phosphokinase and aspartate aminotransferase levels in serum were significantly increased. Histological evidence of hepatocyte damage and marginated neutrophils were observed in the liver.
Conclusion
Administration of ZnO ENPs transiently inhibited Kupffer cell phagosomal motility and later induced hepatocyte injury, but did not alter bacterial clearance from the blood or killing in the liver, spleen, lungs, or kidneys. Our data show that diminished Kupffer cell organelle motion correlated with ZnO ENP-induced liver injury.
Introduction
The potential of nanomedicine has fueled the design and deployment of novel engineered nanoparticles (ENPs) for many biomedical applications. ENPs have high surface area-to-volume ratios and many characteristics providing selected electrical, magnetic, and structural properties that may prove useful in the diagnosis and treatment of diseases. Currently, various metal oxide ENPs are being explored as vehicles for cancer treatments,Citation1 tumor detection,Citation2 and gene-delivery therapies.Citation3 The size, shape, and surface characteristics of metal oxide ENPs can be modified to enable complex interactions with biomolecules, increasing their ability to traverse cellular membranes via nonendocytic mechanisms.Citation4 Therefore, when tailored correctly, the biological responses to ENPs could lead to enhanced interaction with target cells and tissues in comparison to conventional medicines.
Of particular interest in nanomedicine is ZnO ENPs. Various studies have reported the selectivity and toxicity of ZnO ENPs for cancer cells,Citation5,Citation6 which may prove useful in cancer treatments. However, the therapeutic use of ZnO ENPs may alter host immune defenses having beneficial or possibly deleterious effects. For example, ZnO ENPs have been shown to upregulate metallothionein,Citation7 an important protein involved in phagocytosis, antigen presentation by macrophages,Citation8 and inflammatory responses.Citation9 In contrast, ZnO ENPs also have the potential to reduce the viability of certain immune cells, including macrophages,Citation10 and to impair macrophage activity by downregulating the genes coding for major histocompatibility complex class II molecules.Citation11 Therefore, it is important to understand the influence of ZnO ENPs on immune cells to evaluate the potential of these nanoparticles to affect immunological responses.
Although the mechanism of action of ZnO ENPs is not completely understood, it is postulated that increased cytosolic Zn2+ and the generation of reactive oxygen species (ROS) play important roles.Citation12 The associated cytotoxicity from these mechanisms is thought to be selective for cells that produce greater levels of ROS or are dividing rapidly, such as immune cells,Citation5 bacteria,Citation13 and cancer cells.Citation6 A prime example of immune cells potentially susceptible to ENP-mediated alterations is Kupffer cells. Kupffer cells are macrophages that line the hepatic sinusoids, and represent the largest population of fixed macrophages within the body.Citation14 Their primary purpose is continuously to clear the portal blood of toxins, bacteria, macromolecular debris, and particles. As they perform their biological functions, activation often occurs. However, chronic excessive activation of Kupffer cells has been implicated in the pathogenesis of liver diseases, such as hepatitis and fibrosis.Citation15
In previous studies, we utilized ferromagnetic particles and in vivo magnetometry to monitor the motility of particle-containing phagosomes in Kupffer cells during sepsis or toxic injury.Citation16 This noninvasive technique can be used to repeatedly study the intracellular (phagosomal) movement within macrophages. It is an established method to assess cytoskeletal function within lungCitation17–Citation21 and liver macrophages in intact animals.Citation19,Citation22,Citation23 When injected intravenously (IV), ferromagnetic iron oxide particles are rapidly cleared from the circulation, and the majority of them are retained in macrophages that have access to the circulation, such as in the liver and spleen.Citation24 An external magnet is then applied over the organ of interest. This magnetizes the particles within the macrophages by orienting magnetic domains, thus producing a measurable remanent magnetic field (RMF) after removal of the external magnet. This RMF can be monitored using a fluxgate magnetometer. The RMF decays over time, due to the progressive rotation or misalignment of magnetized particle-containing phagosomes and phagolysosomes.Citation25 This decrease in the RMF or “relaxation” is the result of phagosomal motion mediated by microtubules, microfilaments, and available adenosine triphosphate.Citation26
Modifications in relaxation rates have been reported as a result of toxic exposures; generally, lower relaxation rates indicate decreases in cytoskeleton function and/or toxicity. This technique has been used as an indicator of altered macrophage function after exposure to various toxins, such as cigarette smoke,Citation27 malaria infection,Citation16 and particles.Citation28 In this study, we assessed the effects of IV-injected ZnO ENPs on Kupffer cell phagosomal motility using in vivo magnetometry and on clearance of bacteria from the blood and subsequent killing using bacteriological techniques. Serum biomarkers of liver function were measured to evaluate ENP effects on hepatocytes. In toto, we sought to evaluate the potential consequences of IV-administered ZnO ENPs on liver- and Kupffer cell function.
Materials and methods
Particle reagents
Ferromagnetic Fe2O3 nanoparticles were obtained from Alfa Aesar (Ward Hill, MA, USA). ZnO ENPs were synthesized in house at the Harvard T.H. Chan School of Public Health Center for Nanotechnology and Nanotoxicology using the versatile engineered nanomaterial-generation system, as previously described.Citation29 The primary particle size was determined for Fe2O3 and ZnO ENPs using X-ray diffraction. Prior to each experiment, the ENPs were suspended in sterile distilled injectable water at 5 mg/mL. The ENP suspension was sonicated using a protocol previously described.Citation30 The ENP suspensions were measured for hydrodynamic diameter (DH), polydispersity index (PDI), and zeta potential by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Transmission electron microscopy was performed to determine morphology.
Animal handling and ENP exposure
Eight-week-old Wistar Han rats weighing an average of 0.255 kg were purchased from Charles River Laboratories (Raleigh, NC, USA). Rats were housed in groups of two in polypropylene cages with food and water provided ad libitum, and were maintained on a 12-hour light/dark cycle. All rats were allowed to acclimate for 1 week before each experiment. The protocols used in this study were approved by the Harvard Medical Area Institutional Animal Care and Use Committee. Rats were randomly assigned to one of three different pretreatment groups: vehicle control (sterile injectable DiH2O), suspension of Fe2O3 only (n=6 rats), or suspension of ZnO and Fe2O3 ENPs (n=6 rats). IV-administered 65ZnO and Fe2O3 at a ratio of 1:1 (5 mg/kg each) were injected via the tail vein while rats were anesthetized using 2% isoflurane.
Pharmacokinetic studies
To determine the anatomic distribution of injected ZnO ENPs when combined with Fe2O3, we performed a pharmacokinetic study using neutron-activated ZnO ENPs. The ZnO ENPs were irradiated at the Massachusetts Institute of Technology Nuclear Reactor Laboratory (Cambridge, MA, USA) with thermal neutron flux of 5×1013 n/(cm s) for 120 hours. Neutron irradiation generated 65Zn, which decays with a half-life of 244.3 days and emits gamma energies of 345, 770, and 1,115 keV. The resulting specific activity for 65Zn was 41.7±7.2 kBq/mg of 65ZnO ENPs. These radioactive particles were used to determine the distribution of IV-injected ZnO ENPs. 65ZnO and Fe2O3 at a ratio of 1:1 (5 mg/kg each) were injected via the tail vein. At 30 minutes, 24 hours, 7 days, and 21 days post-IV injection, groups of three rats/group were killed, and tissue 65Zn levels were analyzed. The whole brain, spleen, kidneys, heart, liver, lungs, gastrointestinal tract, and testes, as well as samples of blood, bone marrow, skeletal muscle, and skin, were collected and weighed. Radioactivity was measured in a Wizard 1480 gamma counter (PerkinElmer Inc, Waltham, MA, USA). Disintegrations per minute were calculated from the net counts per minute and the measured counter efficiency. Data are expressed as a percentage of the administered dose retained in each organ. The radioactivity in organs and tissues not measured in their entirety was estimated from measured aliquots as a percentage of total body weight, as follows: skeletal muscle, 40%; bone marrow, 3.2%; peripheral blood, 7%; skin, 19%; and bone, 6%.Citation31,Citation32
Magnetometry measurements
During magnetic relaxation measurements, each rat was anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg), xylazine (10 mg/kg), and acepromazine (3 mg/kg). The rat was then secured on a Plexiglass platform, and a permanent magnet (0.43 Tesla, 2×2×0.5 cm) was placed on the marked area over the liver (xiphoid process) for 2 minutes and then removed. The magnetized rat was immediately placed under a fluxgate magnetometer in gradiometer mode (F 1.067; Foerster Instruments, Pittsburgh, PA, USA), which detects the RMF emanating from the aligned magnetic particles in the liver. The magnetometer is enclosed in a cylindrical Moly permalloy shield to reduce external magnetic noise. The RMF was followed for 30 minutes. WinDaq software (DATAQ Instruments, Akron, OH, USA) was used to collect the data. Since the peak initial field strength (B0) cannot be measured directly, due to an approximate 5- to 10-second delay needed to move the rat from the magnetizing field into the shielded magnetometer probe, the B0 was estimated by nonlinear regression analyses using GraphPad Prism version 5 (GraphPad Software Inc, La Jolla, CA, USA). Data are presented as a percentage of the extrapolated B0 over time.
Bacterial preparation and administration
Rats were randomly assigned to one of three different pretreatment groups: 1) control (IV injection of sterile Dulbecco’s phosphate-buffered saline, n=17 rats), 2) suspension of ZnO and Fe2O3 ENPs (n=5 rats), or 3) suspension of ZnO ENPs only (n=10 rats). Twenty-four hours after the injection of ZnO, ZnO and Fe2O3 ENPs together, or saline, we IV-injected each rat with 3.33 mL/kg (2×109 cfu/kg) of the final bacterial suspension. The IV route was chosen because it is a common method of administration for both antimicrobial and anticancer drugs. Pseudomonas aeruginosa is one of the most common nosocomial causes of Gram-negative infections and bacteremia,Citation33 and thus it is especially relevant to patients with septicemia or undergoing IV therapies. To prepare the bacterial suspension for IV injection, an aliquot of a stock of P. aeruginosa was added to tubes containing tryptic soy broth. The resulting suspension was placed in a shaking incubator at 37°C. After 18 hours of incubation, the suspension was washed and cleared in successive centrifugations and resuspended in sterile Dulbecco’s phosphate-buffered saline. After three washes, the final suspension volume was adjusted to an optical density of 1.3 at 600 nm.
Blood and tissue-sample collection and bacteriological analysis
We randomly assigned rats from each group to be killed at either 10 minutes or 240 minutes after injection of bacteria. Rats scheduled for death at 10 minutes postinjection had sequential blood samples collected at 1, 5, and 10 minutes postinjection. Rats killed at 240 minutes postinjection had sequential blood samples collected at 10, 15, 30, 60, and 240 minutes postinjection. Blood samples were added to previously weighed sterile Eppendorf tubes with 900 µL of sterile distilled water and their weights recorded. Tenfold serial dilutions in sterile distilled water of each blood sample were prepared. Anesthetized rats were killed by exsanguination, and the liver, lungs, kidneys, and spleen were aseptically removed and weighed. The liver and the spleen were included as they are the primary sites of uptake for circulating bacteria and ZnO ENPs. Tissue aliquots were weighed and homogenized in sterile distilled water using tissue grinders. After homogenization, tenfold serial dilutions in sterile distilled water of each tissue aliquot were made. The dilutions for each blood and tissue sample were pour-plated in melted trypticase soy agar and incubated for 24 hours.
Liver-injury enzyme assays
A separate cohort of Wistar Han rats was IV-injected with 5 (n=4), 10 (n=4), and 20 mg/kg (n=3) ZnO ENPs. Whole blood was collected in BD (Franklin Lakes, NJ, USA) Vacutainer SST tubes at 24 hours and 7 days postinjection. Samples were centrifuged, and the serum was collected and stored at −80°C until shipment to IDEXX Laboratories (Westbrook, ME, USA) for protein assays reflecting hepatic function. The following enzymes were measured: ALT, ALP, AST, CPK, GGT, total protein, total bilirubin, and albumin.
Statistical analysis
Differences among treatment groups were analyzed by oneway analysis of variance and Bonferroni multiple-comparison post hoc tests (GraphPad Prism 5). Magnetometry data, such as B30/B0, were analyzed using Student’s t-test.
Results
Nanoparticle characterization
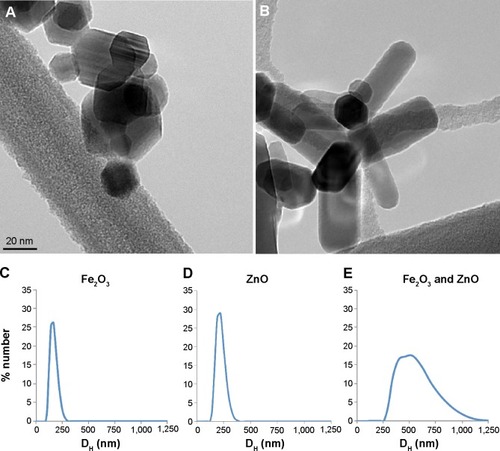
ZnO ENPs and iron oxide were characterized to determine surface charge, as well as primary particle and agglomerate size, prior to IV injections. The crystallite primary particle size of ZnO determined by X-ray diffraction was 20±3 nm. After dispersion in distilled water and sonication at 242 J/mL, we observed a mean agglomerate DH of 217±22.9 nm, PDI of 0.224, and zeta potential of 19.1±2.2 mV for ZnO ENPs. Fe2O3 ENPs had a primary particle size of 19.6±2.9 nm, and when dispersed and sonicated in sterile water had a DH of 163.1±49.0 nm, PDI of 0.257, and zeta potential of −28±0.3 mV. To ensure that the tracer particle Fe2O3 and ZnO ENP had the same uptake pattern by hepatic and splenic macrophages, they were mixed together at a ratio of 1:1. The combined suspensions had a DH of 518.9±36.5 nm, PDI of 0.606, and zeta potential of 3.31±0.5 mV. The interval between sonication of ENPs and rat injection was 1–2 minutes. Therefore, these agglomerate DHs were representative of what was injected IV. Transmission electron microscopy revealed the hexagonal and rod-shaped structure of Fe2O3 () and ZnO ENPs (), respectively. displays the size distribution of Fe2O3, ZnO, and combined suspension of Fe2O3 and ZnO, respectively.
Distribution of injected ZnO ENPs – pharmacokinetic analysis
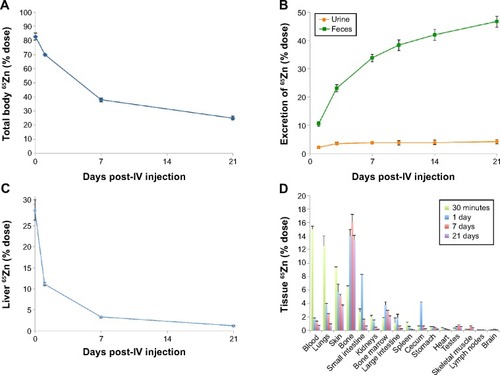
The amounts of total recovered 65Zn in rats over time are shown in . This shows that 82.7% of the injected dose was recovered at 30 minutes postinjection and declined to 38% over 7 days. After 21 days, the amount of 65Zn was 25% in the total rat carcass. Excretion of 65Zn through urine and feces is presented in . At 24 hours postinjection of 65Zn, 2.1% had been excreted in the urine and 10.5% in the feces. Cumulative urine and fecal elimination of 65Zn at 21 days was 4% and 47%, respectively. The liver was found to have the highest retention of 65Zn at 30 minutes (), which declined to 11% over a 24-hour period. After 7 days, the presence of ZnO ENPs in the liver diminished to 3.3%, and only 1.2% remained after 21 days. displays the overall tissue distribution of 65Zn at four different time points after injection of combined 65ZnO and Fe2O3. At 24 hours, we observed that 65Zn was found in the bone (14%), skin (9%), lungs (13%), blood (15%), and plasma (13%). Retained 65Zn decreased over time from the liver and other organs, most likely due to ZnO ENP dissolution and clearance of Zn through the urine and feces. However, notable accumulation of 65Zn within the bone was observed ().
Figure 2 Pharmacokinetics of 65Zn post-intravenous (IV) injection of 65ZnO.
Notes: (A) Total distribution of recovered 65Zn in rat tissues at 0, 7, 14, and 21 days postinjection. (B) Excretion of 65Zn post-IV injection of 65Zn ENPs. (C) Percentage of injected 65Zn dose retained in the liver diminished over time. (D) Overall organ distribution of 65Zn over time post-IV injection of 65ZnO ENPs.
Abbreviation: ENPs, engineered nanoparticles.
Organelle motility in Kupffer cells after ZnO nanoparticle exposure
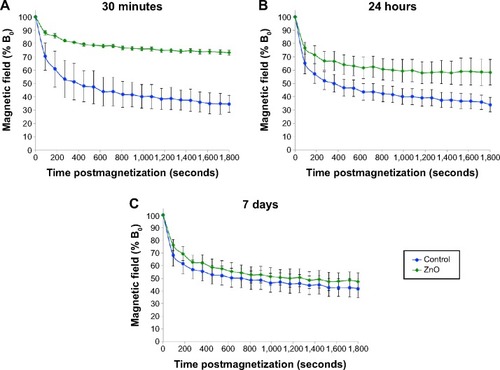
In vivo magnetometry was employed to assess the impact of ENP exposure on the phagosomal motility of liver macrophages or Kupffer cells. Magnetometric analyses were performed at three time points: 30 minutes, 24 hours, and 7 days. As shown in , 30 minutes after injecting ZnO ENPs, significant slowing in relaxation was observed compared with control (Fe2O3 only). The slowed relaxation persisted at 24 hours (). However, the motility of particle-containing phagosomes in Kupffer cells returned to the control level at 7 days ().
Figure 3 Magnetometric evaluations of Kupffer cells.
Notes: At (A) 30 minutes, (B) 24 hours, and (C) 7 days postinjection of ZnO engineered nanoparticles. rats were intravenously injected with 5 mg/kg each of ZnO engineered nanoparticles and Fe2O3 tracer nanoparticles. At each time point, magnetometric measurements were collected for 30 minutes. Significant slowing in relaxation was observed at 30 minutes and 24 hours compared to control animals (Fe2O3 only; student’s t-test was used to evaluate the B30/B0). At 7 days, relaxation returned to normal when there was a 25% reduction in 65Zn retained in the liver (n=6, P<0.01).
Abbreviation: B0, initial magnetic field strength.
Effects of ZnO ENPs on bacterial clearance and killing
To assess if the observed reduced organelle motility could impact vascular clearance of bacteria from the blood or subsequent killing, we injected a separate cohort of Wistar Han rats with P. aeruginosa (2×109 cfu/kg). Each rat had received normal saline, ZnO ENPs, or ZnO and Fe2O3 24 hours earlier. Rats were weighed prior to the pretreatment with ENPs or saline (day 1) and prior to the injection of P. aeruginosa (day 2). The original weights (day 1) among all rats were similar. The mean body weight of the rats at day 1 from the control group was 272.7±5 g, from the group treated with ZnO ENPs 271.2±3.6 g, and from the group treated with ZnO and Fe2O3 ENPs 267.7±2.6 g. On day 2, we observed an increase in weight in the control group. In contrast, the rats treated with ZnO ENPs alone and in combination with Fe2O3 showed a slight decrease in average body weight (data not shown).
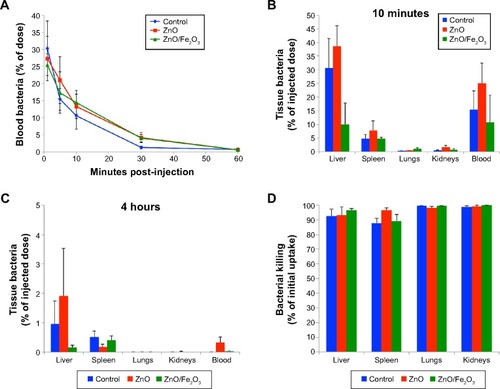
As shown in , there had been rapid clearance of bacteria from the blood at 1 hour postinjection. Although there were initially higher levels of bacteria remaining in the blood of the ZnO and ZnO/Fe2O3 groups, viable bacteria were almost completely cleared from the blood in the first hour in all three groups. The percentages of viable bacteria recovered in the liver, spleen, lungs, kidneys, and blood at 10 minutes postinjection are shown in . After 4 hours, the viable bacteria remaining in the liver, spleen, lungs, kidneys, and blood were significantly reduced, as shown in . A summary of the percentage bacterial killing within each tissue is presented in . The graph indicates that bacteria in the lungs and kidneys were inactivated by almost 100% over a 4-hour period. Bacterial killing in the liver and spleen was over 92%.
Figure 4 Effect of ZnO ENPs on the fate of IV-injected Pseudomonas aeruginosa.
Notes: (A) Bacterial clearance of Pseudomonas aeruginosa in the blood and tissues. Bacteria were rapidly cleared from the blood. Rats pretreated with ZnO engineered nanoparticles with or without Fe2O3 showed slightly slower clearance for the first 30 minutes, but by 60 minutes there were almost no viable bacteria detected in the blood. (B) At 10 minutes postinjection of bacteria, the majority of the injected dose was found in the liver, spleen, and blood. (C) At 4 hours, very low levels of viable bacteria remained in these organs. (D) Bacterial inactivation in 4 hours. Compared to 10 minutes, viable bacteria (cfu/g) in the liver, lungs, and kidneys were significantly decreased. However, pretreatment of rats with ZnO engineered nanoparticles with or without Fe2O3 did not affect bacterial killing in these organs.
Abbreviations: ENPs, engineered nanoparticles; IV, intravenous.
ZnO ENP exposure increases liver-injury enzymes and causes histological changes
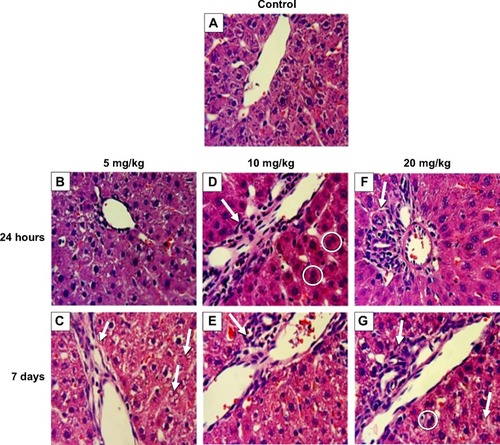
Two of eight liver-injury enzyme biomarkers that were measured showed time-dependent changes (). We observed a significant elevation in AST () at the 5 and 10 mg/kg doses after 7 days. The higher dose of 20 mg/kg incited a fivefold increase in AST after 24 hours, which decreased after 7 days. CPK levels at 5, 10, and 20 mg/kg were significantly increased at 7 days (). This elevation coincided with dose-dependent histopathological changes, including necrosis and infiltration of inflammatory cells (). Normal liver-tissue structure could be seen in control (saline-administered) rats (). Light micrographs of representative rat liver sections taken at 24 hours post-ENP exposure of 5 mg/kg show an influx of inflammatory cells (). After 1 week, noticeable areas of hepatocyte granulation and nuclear condensation were evident, along with spotty necrosis (). In , the higher dose of 10 mg/kg induced a significant influx of neutrophils into the portal triad, which persisted after 7 days (). shows inflammatory cell infiltrate near the hepatic portal triad of a rat injected with 20 mg/kg of ZnO ENPs. The inflammation was observed at 7 days, along with focal regions of necrosis and binucleated cells ().
Figure 5 Effect of ZnO engineered nanoparticles (ENPs) on liver function.
Notes: (A) Time-dependent significant increases in aspartate aminotransferase (AST) were observed in rats after intravenous administration of ZnO ENPs. At the higher dose of 20 mg/kg ZnO ENPs, a fivefold increase in AST was observed. However, it had decreased by 7 days. (B) Increases in creatine phosphokinase (CPK) were also found 7 days after ZnO ENP injection. The P-values were determined by one-way ANOVa followed by Bonferroni’s post-hoc test, where *P≤0.05, and ***P≤0.0001 versus baseline.
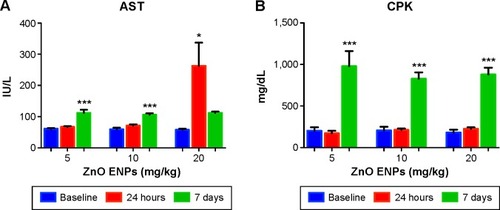
Figure 6 Effect of ZnO nanoparticles on liver histology of male Wistar Han rats.
Notes: (A) Control (intravenously injected with sterile saline). (B) At 24 hours, hepatocyte swelling was evident with an intravenous dose of 5 mg/kg of ZnO engineered nanoparticles (ENPs). (C) After 7 days, granulation and necrotic regions accompanied by neutrophil infiltration were observed; the arrow on the left indicates neutrophil infiltration; the arrows on the right indicate granulation and necrotic regions. (D) At 24 hours, 10 mg/kg ZnO ENPs caused an influx of neutrophils (arrow) and binucleated hepatocytes (circled). (E) At 7 days, inflammation and liver necrosis were evident (arrow). (F) At 24 hours, 20 mg/kg ZnO ENPs induced inflammatory cell infiltration (arrow). (G) At 7 days, inflammatory cell infiltration remained (upper arrow), along with binucleated cells (circled) and some necrosis (lower arrow).
Discussion
ZnO ENPs have been associated with various forms of toxicity in biological systems.Citation10,Citation34,Citation35 The observed toxicities have been linked to their physicochemical properties, such as dissolution into ionic Zn and their ability to generate ROS.Citation36 Consequently, these same properties have been exploited in the development of nanomedicines.Citation2,Citation37 To utilize their potential in nanomedicines fully and avoid adverse health effects, comprehensive toxicological characterization is essential. Traditional in vitro studies are limited in their ability to mimic in vivo systems, which are complex and contain multiple nano–bio interactions that influence biodistribution, clearance, and metabolism, as well as inflammatory and immune responses. Therefore, it is necessary to utilize in vivo studies to confirm in vitro observations.Citation38 In this study, we utilized a rat model to assess the effects of IV exposure to ZnO ENPs on Kupffer cell function and on the cells’ ability to clear and inactivate circulating P. aeruginosa.
To validate that the Kupffer cells were targeted with injected ZnO in the presence of other ENPs, such as iron oxide, we used neutron-activated ZnO ENPs to verify the organ localization of administered ZnO ENPs. The highest percentage of injected 65ZnO ENPs was measured in the liver. Of the 5 mg/kg injected, approximately 29% was taken up by the liver. This was lower than obtained from previous studies in our lab and other studies evaluating IV-administered gold colloids and iron oxide suspension.Citation24 However, the particles we used were far smaller, and thus the number injected was far greater at the same dose. It is therefore possible that uptake mechanisms may have been saturated. It is also true that our aggregates had a greater probability of being trapped in or adherent to capillaries in other organs, especially lungs, skin, and bone.
Major goals of this study were to explore whether: 1) ZnO ENP exposure would reduce organelle motility or 2) reduce the ability of Kupffer cells and other reticuloendothelial system (RES) macrophages to clear P. aeruginosa. Using in vivo magnetometry, we observed a transient diminished motility (relaxation) of Kupffer cells due to ZnO ENPs at 30 minutes and 24 hours postexposure. These data correlate with other findings, such as a study that reported a delay in relaxation due to exposure to silicon carbide whiskers in the alveolar macrophages of Syrian golden hamsters.Citation39 Some in vitro studies have yielded similar results. For instance, a study of alveolar macrophages recovered from bronchoalveolar lavage fluid from Syrian golden hamsters showed reduced relaxation and toxicity due to cadmium oxide exposures.Citation40 TiO2 ENPs, which are extensively used in cosmetics and pharmaceuticals, have also been found to inhibit relaxation and induce membrane damage.Citation41 Furthermore, another study showed impairment of cytoskeletal function and early necrotic changes due to in vitro exposure to chrysotile asbestos.Citation42 Importantly, cytoskeletal impairment is thought to be the primary mechanism of action that affects relaxation. In previous magnetometric studies, magnetic relaxation was found to be the result of cytoskeletal machinery and ATP.Citation43,Citation44 Principal components of the cytoskeleton, the actin microfilaments are highly susceptible to oxidative damage and resulting deformations, such as cross-linking, may be enhanced by the presence of ZnO ENPs or dissolved Zn ions.Citation45
Our data also explored whether IV exposure to ZnO ENPs may impair Kupffer cell phagosomal motion and activity, and in turn may increase the risk of infection following bacteremia. This possibility is suggested by the fact that ZnO ENPs can generate ROS in different ways. They can indirectly increase ROS generation by saturating oxidative defense compounds, which are then unavailable to bind other transition metal ions, such as Fe and Cu. These transition metals are then free to catalyze Fenton-type reactions.Citation46,Citation47 Moreover, ZnO ENPs can directly generate ROS, because they present a significant number of electron–hole pairs (e−–h+). The electrons and holes can react with the oxygen and hydroxyl ions, respectively, in the ZnO ENPs’ surrounding aqueous environment. This produces highly reactive free radical compounds, including the superoxide anion (from electrons) and hydroxyl radicals (from holes).Citation37 Importantly, ZnO ENPs are highly soluble, especially when in the acidic compartments of phagolysosomes. This has been shown to cause membrane destabilization, leading to the release of toxic amounts of Zn within intra- and extracellular environments.Citation48 Whether by direct or indirect oxidation, we surmised that ZnO ENPs might affect the bactericidal activity of Kupffer cells within the liver and other RES macrophages, such as splenic and bone marrow macrophages. However, our data indicate that ZnO ENPs did not impair the ability of RES macrophages to clear and inactivate bacteria. By 4 hours, virtually all injected bacteria were eliminated from the blood, liver, spleen, lung, and kidneys. Our results are consistent with other studies. For example, pretreatment with IV injection of crystalline silica was shown not to inhibit the uptake of Salmonella typhimurium in a rat liver-perfusion model.Citation49 Additionally, only minor reductions in the clearance of Trypanosoma musculi were observed after silica exposure in mice.Citation50
A potential contributing factor in the elimination of bacteria from the blood and tissues is the recruitment of neutrophils during sepsis. This possibility is supported by studies showing that in the early stages of infection, recruited neutrophils contribute to the removal of bacteria along with Kupffer cells.Citation51 Although neutrophils comprise only a small portion of the nonparenchymal liver cells in normal murine tissue, Gregory and Wing found that 10- to 20-fold increases in neutrophils can occur 2 hours after Listeria monocytogenes infection.Citation52 In certain instances, the elimination of bacteria from the liver may also be enhanced by the regulatory activity of Kupffer cells over neutrophils, in addition to their own bactericidal activity.Citation52 Consistent with this idea, we observed neutrophilia in whole blood samples of rats preexposed to ZnO ENPs then challenged with P. aeruginosa 24 hours later (data not shown). This suggests recruitment of neutrophils from the blood to other organs and possibly the liver. Therefore, impaired Kupffer cells may rely on neutrophil recruitment to help eliminate bacteria. However, neutrophils and other aspects of inflammation might induce liver injury over time.
Of the eight liver-injury enzymes analyzed, AST and CPK were both increased at 7 days after exposure to ZnO ENPs, suggesting liver injury. The highest dose of 20 mg/kg induced a fivefold increase in AST after 24 hours. AST is a known serum biomarker of liver injury that is elevated due to hepatocellular injury.Citation53 Similarly, in a study investigating the effects of nanoceria on rat liver, AST was significantly elevated in response to IV injections.Citation54 It is important to note that the elevation of AST in tandem with CPK could also be related to extrahepatic tissue trauma, such as muscle or heart injury.Citation55 Our experimental design and results differed from a study by Wang et al.Citation56 A principal difference is that we administered our reagents IV, while Wang et al exposed the animals by direct spraying of dry powder into both nasal passages using a dry-powder sprayer. The elevated serum biomarkers found within our work may have differed from this study because of the different route of administration and especially far-higher dose. The authors found severe liver and lung histopathological changes after 3 days of exposure (twice daily) to iron oxide or ZnO nanomaterials, but no increases in serum biomarkers of the liver.Citation56
Histological analysis of liver sections showed areas of necrosis accompanied by significant inflammatory cell infiltration near the hepatic portal triad at day 7 of ENP exposure. By this time, the engulfed ZnO ENPs would have dissolved, releasing zinc ions, which may have caused toxicity to the surrounding hepatocytes. This “Trojan horse” scenario is consistent with a study investigating the effect of silica ENPs on Kupffer cell-mediated hepatic injury.Citation57 The authors found significant histological changes in the liver due to IV administered silica ENPs (5 mg/kg) to Sprague Dawley rats. They surmised that the significant toxicological and histological changes were induced by Kupffer cell activation, the release of ROS, and inflammation in response to the silica particles. The same study also utilized an in vitro validation study where rat liver cells were exposed to the culture media from Kupffer cells treated with silica ENPs. It was concluded that Kupffer cell metabolites released in response to silica were toxic to hepatocytes. The involvement of Kupffer cells in the pathogenesis of liver injury mediated by chemicals is well documented,Citation58–Citation60 and might be important in ENP-mediated liver damage as well.
In summary, the results presented here show that ZnO ENPs at 5 mg/kg could alter Kupffer cell phagosomal motility. However, this reduction in phagosomal motility of Kupffer cells did not significantly inhibit the bacterial clearance from the blood or alter the ability of the liver and other organs to kill P. aeruginosa. This may have been due in part to possible margination of neutrophils from the blood into the liver and their subsequent participation in bacterial inactivation. Over time, liver intracellular enzymes were elevated, and histological changes were observed at 5, 10, and 20 mg/kg doses of ZnO ENPs, which might have been due to released Zn ions. Interestingly, these toxicological changes in hepatocytes coincided with the restoration of phagosomal motility in Kupffer cells at 7 days. These data indicate the potential of Kupffer cells to mediate liver injury and of the utility of magnetometry as indicators of ENP-mediated liver toxicity.
Conclusion
We showed that injected ZnO ENPs cause an early and reversible reduction in Kupffer cell phagosomal motion, and later cause hepatocellular injury. However, the capacity of the liver, spleen, lungs, and kidneys to clear and inactivate circulating bacteria were not affected, probably in part due to compensatory mechanisms, such as neutrophil margination in the liver. The release of bioactive metabolites or Zn ions from Kupffer cells may cause adverse effects on the hepatocytes over time, as indicated by histological evidence of liver inflammation, and by increased serum CPK and AST levels. We conclude that ZnO ENPs may not significantly reduce the bactericidal capacity of the liver, but can alter Kupffer cells and the liver microenvironment, leading to hepatotoxicity over time. These risks must be balanced against the potential benefits, in order to utilize ZnO ENPs as nanomedicines. Nanomedicines utilizing ZnO ENPs will have a range of applications, depending on the desired target, the outcome, and the nature of the formulation. Since almost all medicines have side effects, the key criteria remain efficacy and safety. Continued exploration of potential side effects is warranted.
Acknowledgments
The authors thank Melissa Curran for her help with edits. Also, special thanks to Phil Demokritou for supplying the ZnO ENPs. The authors acknowledge the financial support from NIH (grant P30ES000002). CYW was funded by an NIH NHLBI Ruth L Kirschstein T32 training grant (NIH HL007118).
Disclosure
The authors report no conflicts of interest in this work.
References
- Brannon-PeppasLBlanchetteJONanoparticle and targeted systems for cancer therapyAdv Drug Deliv Rev200456111649165915350294
- YangSCShenYCLuTCYangTLHuangJJTumor detection strategy using ZnO light-emitting nanoprobesNanotechnology201223505520222238275
- ZhangPLiuWZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimagingBiomaterials201031113087309420096454
- GeiserMRothen-RutishauserBKappNUltrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cellsEnviron Health Perspect2005113111555156016263511
- HanleyCLayneJPunnooseAPreferential killing of cancer cells and activated human T cells using ZnO nanoparticlesNanotechnology2008192929510318836572
- PremanathanMKarthikeyanKJeyasubramanianKManivannanGSelective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidationNanomedicine2011721841921034861
- MoosPJOlszewskiKHoneggarMResponses of human cells to ZnO nanoparticles: a gene transcription studyMetallomics20113111199121121769377
- SugiuraTKurodaEYamashitaUDysfunction of macrophages in metallothionein-knock out miceJ UOEH200426219320515244072
- RoyRKumarSVermaAKZinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a Th2 response in Balb/c miceInt Immunol201426315917224225181
- WilhelmiVFischerUWeighardtHZinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent mannerPLoS One201386e6570423755271
- TuomelaSAutioRBuerki-ThurnherrTGene expression profiling of immune-competent human cells exposed to engineered zinc oxide or titanium dioxide nanoparticlesPLoS One201387e6841523894303
- SahuDKannanGMVijayaraghavanRAnandTKhanumFNanosized zinc oxide induces toxicity in human lung cellsISRN Toxicol2013201331607523997968
- KairyteKKadysALuksieneZAntibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspensionJ Photochem Photobiol B2013128788424035847
- ThomasPKupffer cellsSchwabMEncyclopedia of CancerHeidelbergSpringer201219631965
- KoliosGValatasVKouroumalisERole of Kupffer cells in the pathogenesis of liver diseaseWorld J Gastroenterol200612467413742017167827
- BellowsCFMolinaRMBrainJDDiminished organelle motion in murine Kupffer cells during the erythrocytic stage of malariaJ R Soc Interface201185871171921068031
- MolinaRMBrainJDIn vivo comparison of cat alveolar and pulmonary intravascular macrophages: phagocytosis, particle clearance, and cytoplasmic motilityExp Lung Res2007332537017454102
- MollerWBarthWKohlhäuflMHäussingerKStahlhofenWHeyderJHuman alveolar long-term clearance of ferromagnetic iron-oxide microparticles in healthy and diseased subjectsExp Lung Res200127754756811597117
- WeinstockSBBrainJDComparison of particle clearance and macrophage phagosomal motion in liver and lungs of ratsJ Appl Physiol (1985)1988654181118203182541
- BrainJDBloomSBValbergPAGehrPCorrelation between the behavior of magnetic iron oxide particles in the lungs of rabbits and phagocytosisExp Lung Res1984621151316745211
- BrainJDGodleskiJKreylingWIn vivo evaluation of chemical biopersistence of nonfibrous inorganic particlesEnviron Health Perspect1994102Suppl 51191257882915
- GehrPBrainJDBloomSBNoninvasive studies of Kupffer cells in situ by magnetometryJ Leukoc Biol198435119306584511
- GehrPBrainJDBloomSBValbergPAMagnetic particles in the liver: a probe for intracellular movementNature198330259063363386835369
- BrainJDMolinaRMDeCampMMWarnerAEPulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 speciesAm J Physiol19992761 Pt 1L146L1549887067
- ValbergPAAlbertiniDFCytoplasmic motions, rheology, and structure probed by a novel magnetic particle methodJ Cell Biol198510111301404040136
- ValbergPFeldmanHMagnetic particle motions within living cells. Measurement of cytoplasmic viscosity and motile activityBiophys J19875245515613676436
- BrainJDMacrophage damage in relation to the pathogenesis of lung diseasesEnviron Health Perspect19803521286997029
- MollerWBrownDKreylingWStoneVUltrafine particles cause cytoskeletal dysfunctions in macrophages: role of intracellular calciumPart Fibre Toxicol20052716202162
- DemokritouPBüchelRMolinaRMDeloidGMBrainJDPratsinisSEDevelopment and characterization of a versatile engineered nanomaterial generation system (VENGES) suitable for toxicological studiesInhal Toxicol201022Suppl 210711620701428
- CohenJDeloidGPyrgiotakisGDemokritouPInteractions of engineered nanomaterials in physiological media and implications for in vitro dosimetryNanotoxicology2013741743122393878
- BrownRPDelpMDLindstedtSLRhombergLRBelilesRPPhysiological parameter values for physiologically based pharmacokinetic modelsToxicol Ind Health19971344074849249929
- SchoeffnerDJWarrenDAMuralidaraSBrucknerJVSimmonsJEOrgan weights and fat volume in rats as a function of strain and ageJ Toxicol Environ Health A199956744946210201633
- PereiraCAMarraARCamargoLFNosocomial bloodstream infections in Brazilian pediatric patients: microbiology, epidemiology, and clinical featuresPLoS One201387e6814423861860
- AhamedMAkhtarMJRajaMZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: role of oxidative stressNanomedicine20117690491321664489
- KaoYChenYChengTChiungYLiuPZinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicityToxicol Sci2011125246247222112499
- XiaTZhaoYSagerTDecreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryosACS Nano2011521223123521250651
- RasmussenJWMartinezELoukaPWingettDGZinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applicationsExpert Opin Drug Deliv2010791063107720716019
- SharmaVSinghPPandeyADhawanAInduction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticlesMutat Res20117451–2849122198329
- WatanabeMOkadaMAizawaYSakaiYYamashinaSKotaniMMagnetometric evaluation for the effects of silicon carbide whiskers on alveolar macrophagesInd Health200038223924510812849
- NiitsuyaMWatanabeMOkadaMMagnetometric evaluation of cadmium oxide-induced toxicity to pulmonary alveolar macrophages of Syrian golden hamstersJ Toxicol Environ Health A200366436537812554542
- WatanabeMOkadaMKudoYDifferences in the effects of fibrous and particulate titanium dioxide on alveolar macrophages of Fischer 344 ratsJ Toxicol Environ Health A200265151047106012167218
- KeiraTOkadaMKatagiriHAizawaYOkayasuIKotaniMMagnetometric evaluation for the effect of chrysotile on alveolar macrophagesTohoku J Exp Med19981862879810223613
- MöllerWTakenakaSRustMStahlhofenWHeyderJProbing mechanical properties of living cells by magnetopneumographyJ Aerosol Med199710317318610174196
- MöllerWNemotoIMatsuzakiTHoferTHeyderJMagnetic phagosome motion in J774A.1 macrophages: influence of cytoskeletal drugsBiophys J200079272073010920006
- MöllerWTakenakaSBuskeNFeltenKHeyderJRelaxation of ferromagnetic nanoparticles in macrophages: in vitro and in vivo studiesJ Magn Magn Mater20052931245251
- KrezelAHaoQMaretWThe zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signalingArch Biochem Biophys2007463218820017391643
- ChevionMA site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metalsFree Radic Biol Med19885127373075945
- ChoWSDuffinRHowieSEProgressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomesPart Fibre Toxicol201182721896169
- FriedmanRLMoonRJRole of Kupffer cells, complement, and specific antibody in the bactericidal activities of perfused liversInfect Immun19802911521576995334
- KongshavnPAShawKGhadirianEUlczakOFailure to demonstrate a major role for Kupffer cells and radiosensitive leukocytes in immunoglobulin-mediated elimination of Trypanosoma musculiInfect Immun1990586197119782160436
- GregorySHSagnimeniAJWingEJBacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophilsJ Immunol19961576251425208805652
- GregorySWingENeutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infectionsJ Leukoc Biol200272223924812149414
- AlvarezAMMukherjeeDLiver abnormalities in cardiac diseases and heart failureInt J Angiol201120313514222942628
- TsengMTLuXDuanXAlteration of hepatic structure and oxidative stress induced by intravenous nanoceriaToxicol Appl Pharmacol2012260217318222373796
- LofthusDMStevensSRArmstrongPWGrangerCBMahaffeyKWPattern of liver enzyme elevations in acute ST-elevation myocardial infarctionCoron Artery Dis2012231223022113063
- WangLWangLDingWZhangFAcute toxicity of ferric oxide and zinc oxide nanoparticles in ratsJ Nanosci Nanotechnol201010128617862421121374
- ChenQXueYSunJKupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivoInt J Nanomedicine201381129114023515466
- RobertsRAGaneyPEJuCKamendulisLMRusynIKlaunigJERole of the Kupffer cell in mediating hepatic toxicity and carcinogenesisToxicol Sci200796121517122412
- ItoYBetheaNWAbrilERMcCuskeyRSEarly hepatic microvascular injury in response to acetaminophen toxicityMicrocirculation200310539140014557822
- SteibCGerbesASignaling pathways in liver diseases Kupffer cellsDufourJFClavienPASignaling Pathways in Liver DiseasesHeidelbergSpringer20106978