Abstract
Nanoparticles (NPs) present in the environment and in consumer products can cause immunotoxic effects. The immune system is very complex, and in vivo studies are the gold standard for evaluation. Due to the increased amount of NPs that are being developed, cellular screening assays to decrease the amount of NPs that have to be tested in vivo are highly needed. Effects on the unspecific immune system, such as effects on phagocytes, might be suitable for screening for immunotoxicity because these cells mediate unspecific and specific immune responses. They are present at epithelial barriers, in the blood, and in almost all organs. This review summarizes the effects of carbon, metal, and metal oxide NPs used in consumer and medical applications (gold, silver, titanium dioxide, silica dioxide, zinc oxide, and carbon nanotubes) and polystyrene NPs on the immune system. Effects in animal exposures through different routes are compared to the effects on isolated phagocytes. In addition, general problems in the testing of NPs, such as unknown exposure doses, as well as interference with assays are mentioned. NPs appear to induce a specific immunotoxic pattern consisting of the induction of inflammation in normal animals and aggravation of pathologies in disease models. The evaluation of particle action on several phagocyte functions in vitro may provide an indication on the potency of the particles to induce immunotoxicity in vivo. In combination with information on realistic exposure levels, in vitro studies on phagocytes may provide useful information on the health risks of NPs.
Introduction
Nanoparticles (NPs) are used in many industrial applications and consumer products, and they are also being developed for targeted drug delivery, imaging, and implants in the medical sector. In addition to cytotoxicity, NPs can act on the immune system. Potential immunotoxic effects of NPs are relevant for human health because the immune system is present at all potential portals of entry of NPs and a variety of immunomodulatory actions of NPs has been proposed.Citation1 The immunmodulatory action of a compound usually describes a desired change in the immune system – for instance, for therapeutic intervention – while “immunotoxicity” is used for adverse immunomodulation indicating nondesired effects on the immune system. Immunotoxicity includes interactions with blood (hemolysis, coagulation, and protein binding), accumulation in the mononuclear phagocyte system (MPS), adjuvant properties, binding of haptens, interference with phagocytosis, and modulation of the Th2/Th1 response to antigens. Epidemiological studies in regions with increased concentrations of ultrafine particles suggested that NPs could influence the immune system. High levels of airborne particles caused worsening of asthma and pneumonia in exposed individuals.Citation2–Citation5 Ultrafine particles in the atmosphere do not meet the size requirements of NPs because their upper size limit is usually 2.5 μm, but the reports stimulated further studies on size-related particle effects and raised the awareness that the large surface area of NPs was the reason for their high biological reactivity and toxicity.Citation6
In contrast to cytotoxicity, the role of in vitro immunotoxicity testing is not well established. This is firstly due to general problems in simulating the complexity of the immunological system in vitro, as well as in the extrapolation of in vitro and animal data to human reactions and, secondly, to NP-specific problems. The immune system is redundant and has the capacity to compensate for minor immunotoxicological effects. High interindividual variations of the immune system further complicate the identification of a link between NP exposure and immunotoxicity in humans. Due to the high proliferation rate and compensation capacity of the immune system, only extreme alterations will result in clinical symptoms. On the other hand, decreased immunosurveillance may have long-term consequences, which cannot be directly linked to immunotoxicity. One example of such effects is the three- to fourfold increase in cancer incidence by immunosuppression with cyclosporine A for 5 years.Citation7
Engineered NPs, to which humans might be exposed, comprise titanium dioxide (TiO2) and zinc oxide (ZnO) NPs in consumer products, silver (Ag) NPs in clothing, and silica (SiO2) NPs in food. For medical products, gold (Au), carbon nanotubes (CNTs), and iron oxide are likely candidates. The main exposure routes are dermal for NPs in consumer products and oral for NPs in food and intravenous for NPs in medical use. The exposure of humans to engineered NPs, due to the different use of these products, is expected to be highly variable. Site-specific composition and reaction of the immune system (lung, skin, blood, etc) affords exposure-specific models because the same NPs might cause no immune effects when applied by the oral and dermal route, but they may induce sensitization after intradermal injection.Citation8 This creates a high number of different testing scenarios and renders the testing of all variations in vivo ethically and financially problematic. In this situation, prescreening by in vitro assays, similar to cytotoxicity screening for systemic toxicity, would be helpful. Of course, in vitro testing has the limitation that only one or a few cell types can be evaluated. Data produced after exposure to high doses for a short period are not representative for the exposure to most NPs.Citation9 Furthermore, the protective mechanisms of the body – for instance, mucociliary clearance in the lung and radical scavenging by glutathione in the blood – will mitigate the toxic effect observed in vitro.
According to the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS), immunotoxicity testing of NPs should focus on macrophages, granulocytes, and dendritic cells (DCs), and the testing should use cytokines as readout parameters.Citation10 Since phagocytes are involved in the unspecific defense, as well as in the specific immune response, impairment of phagocyte function can indicate a decreased reserve of the immune system in NP-exposed individuals.
Therefore, phagocytes appear to be suitable for discriminating between NPs interfering or not interfering with the immune system. Several studies report interference with phagocyte function by iron oxide particles, but the iron oxide NPs, which have been approved for medical use (such as Ferumoxtran-10 [Sinerem®]), did not influence the different aspects of phagocyte function. The secretion of proinflammatory cytokines, oxidative burst, phagocytosis, and chemotaxis was not affected by the exposure to the particles in vitro.Citation11 The few studies in which the same NPs were assessed by animal exposure and by exposure of cells to Ag and SiO2 NPs show that impairment of phagocytes function in vitro accords with immune inflammation in vivo.Citation12,Citation13 Proinflammatory action was seen in vivo as well as in macrophages isolated from animals exposed to TiO2 and ZnO NPs.Citation14,Citation15
This review is focused on plain (not pegylated or formulated) metal and metal oxide NPs, such as SiO2, iron oxide, Ag, Au, TiO2, and ZnO NPs, and single-walled CNTs (SWCNTs) and multiwalled CNTs (MWCNTs). These NPs are relevant for humans because they are used in a variety of consumer products and as imaging reagents in medicine. Their classification as non- or low biodegradable NPs is often used to differentiate these particles from the enzymatically degradable NPs, such as liposomes, poly(lactic-co-glycolic acid), dendrimers, and so on, which can cause additional effects by their degradation products. However, it should not be forgotten that metal and metal oxide release ions which can interact with proteins and induce inflammation.Citation16 Nevertheless, the NPs mentioned in this review form a more homogeneous group than nanocarriers for drug delivery, which consist of different materials and possess different surface charges and functionalization. Polystyrene (PS) particles are included in this review because they are often used as model particles for nonbiodegradable NPs.Citation17
Role of phagocytes in the immune system
Professional phagocytes are a group of immune cells that share the feature that they can ingest 0.5–10 μm sized particles better than epithelial cells. Since they are key players in the immune defense, they are represented in almost all organs.Citation18,Citation19 Mononuclear phagocytes are derived from myeloid progenitor cells in bone marrow and develop into granulocytes and monocytes. Monocytes circulate in the blood and differentiate into macrophages (Mφ) in the tissue, where they reside as peritoneal Mφ, alveolar Mφ, mesangial phagocytes of the kidney, synovial type A cells, bone marrow stromal Mφ, splenic red pulp and splenic white pulp Mφ, osteoclasts in the bone, histiocytes in the connective tissue, and as microglia in the brain.Citation20 DCs are a specific lineage of monocytic phagocytes and are mainly present as myeloid and plasmacytoid DCs in the blood, as interstitial DCs in many organs, and as interdigitating DCs in the lymphatic organs. Based on the history of their discovery, some of them received specific names, such as the DCs in the epidermis (Langerhans cells) and Mφs in the liver (Kupffer cells). Phagocytes express different surface markers and differ in their optimum size of phagocytosis. Peritoneal macrophages and monocytes in the peripheral blood optimally phagocytose 0.3–1.1 μm particles. The optimal size for phagocytosis by alveolar macrophages is 3–6 μm particles.Citation21–Citation23 Granulocytes are classified into neutrophilic, eosinophilic, and basophilic granulocytes. The phagocytosis of invading pathogens is the main role of neutrophilic granulocytes. After self-destruction, they are the main component of pus. Compared to neutrophilic granulocytes, eosinophilic and basophilic granulocytes have only a low potential for phagocytosis and act mainly against pathogens by the release of enzymes, as well as toxic and proinflammatory substances.
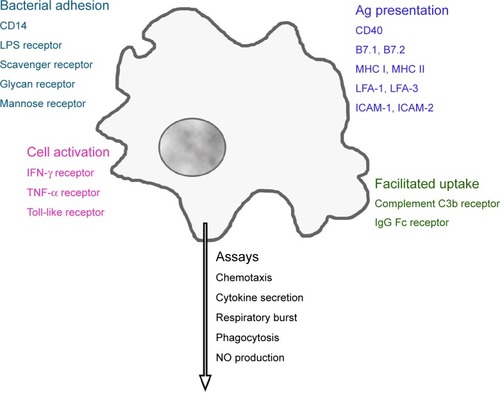
Macrophages possess a variety of receptors for the binding of bacterial constituents (). Complement C3b and the Fc fragment of immunoglobulin (Ig)G enable better uptake of opsonized particles. Distinct adhesion molecules, intercellular adhesion molecule (ICAM)-1 (CD54), ICAM-2 (CD102), lymphocyte function-associated antigen (LFA)-1 (CD11a), and LFA-3 (CD58), together with costimulatory molecules B7.1 (CD80), B7.2 (CD86), or CD40, and processed cytosolic proteins presented by major histocompatibility complex (MHC) I or extracellular proteins presented by MHC II, molecules activate T-cells.Citation24 Cytokines such as tumor necrosis factor-alpha (TNF-α) and interferon (IFN)-gamma (IFN-γ), as well as their interaction with lipopolysaccharide (LPS)-binding protein, activate macrophages. Phagocytes ingest a variety of pathogens, such as bacteria, mycobacteria, virus, fungi, and nonpathogenic particles (for instance, dyes and dust) in an unspecific way. On the other hand, they fulfill a definite function as antigen-presenting cells for the correct function of the specific immune system.
Figure 1 Receptors linked to main functions of phagocytes.
Note: Activation of these receptors regulates macrophage function, which can be evaluated by a panel of in vitro assays.
Abbreviations: CD14, lipopolysaccharide-binding protein receptor; LPS, lipopolysaccharide; IFN, interferon; TNF, tumor necrosis factor; MHC, major histocompatibility complex; LFA, lymphocyte function-associated antigen; ICAM, intercellular adhesion molecule; Ig, immunoglobulin; NO, nitric oxide.
In vitro assays to study phagocyte function
A panel of in vitro assays of different complexities can assess phagocyte function. Cytokine secretion, chemotaxis, phagocytosis, and respiratory burst can be measured in all phagocytes. Nitric oxide generation is used only for monocytes and macrophages, whereas the detection of the release of myeloperoxidase and elastase is specific for neutrophilic granulocytes.Citation25 The evaluation of DC function is more complex because it requires interactions with T-cells. Cell isolation, cell exposure, and the detection platform for the performance of the respective assays are described in the core publication and in the supplements of Current Protocols in Immunology.Citation26
Cytokine secretion
A wide spectrum of cytokines is being used in immunotoxicity studies, and phagocytes isolated from exposed animals or cultures of primary cells, and cell lines are equally suitable for these analyses.Citation27 In the presence or absence of the test substance, the release of cytokines/chemokines can be analyzed by enzyme-linked immunosorbant assays, enzyme-linked immunosorbent spot assays, antibody array assays, and bead-based assays. To identify proinflammation responses, interleukin (IL)-1, IL-6, IL-8, and TNF-α are routinely used.Citation28 The classification of allergic responses is based on the type of lymphocyte helper cells that are activated. IL-4 and IL-5 identify TH2 responses, while marker cytokines for TH1 responses are IFN-γ and TNF-β.
Chemotaxis
The migration of leukocytes from an upper chamber across a membrane to a lower chamber containing a chemoattractant is termed chemotaxis. Human serum-derived complement 5a, human lymphocyte-derived chemotactic factor, monocyte chemoattractant protein 1, or N-formyl-methionyl-leucyl-phenylalanine are commonly used attractants.Citation29 All leukocytes are able for chemotaxis, but monocytes, either as primary cells or as cell lines, are used most frequently. In conventional assays, membrane-containing inserts separating the upper from the lower chamber are used. The amount of cells that passed the membrane and reached the lower chamber is counted or quantified by viability assays. Alternatively, an impedance-based system (eg, xCELLigence and ECIS/Taxis) can be used.Citation30,Citation31
Phagocytosis
The phagocytosis assay evaluates the phagocytic activity of fluorescein-labeled bacteria (Staphylococcus aureus, Escherichia coli) in macrophages, monocytes, and polymorphonuclear neutrophils exposed to the test compound.Citation32
Respiratory burst (reactive oxygen production)
This assay can be performed in macrophages, monocytes, and polymorphonuclear neutrophils by the detection of reactive oxygen species (ROS), which is produced upon phagocytosis. For the assays, mostly unlabeled E. coli is used as the phagocytic stimulus. Either chemiluminescent detection by lucigenin or the oxidation of dyes to fluorescent products (eg, rhodamine 123) can be employed for the quantification of the produced oxygen species.Citation33
Nitric oxide (NO) generation
Murine macrophages are routinely used because, when compared to human monocytes, they possess a much higher production of NO.Citation34 An additional advantage of their use is that, in contrast to human macrophages, they do not need a differentiation step. Differentiation of monocytes with the commonly used phorbol 12-myristate 13-acetate or vitamin D3 cannot reproduce the phenotype of human macrophages in vivo, and it introduces additional variations in the assay.Citation35 The common and very reliable detection method of NO uses the Griess reagent.Citation36
Release of elastase and myeloperoxidase
These enzymes are used as indicators for neutrophilic granulocyte activation.Citation37 Assays are performed in whole blood or in neutrophilic granulocytes isolated from peripheral blood. These cells only rarely show direct effects to conventional chemicals, but they are activated by particles.Citation38,Citation39 The relevance of granulocyte activation for immunotoxicity in vivo, however, is currently unclear.
Function of dendritic cells
DCs for testing cannot be obtained directly from the blood in sufficient amounts, but they require differentiation in vitro. CD14+ mononuclear cells isolated from peripheral blood mononuclear cells (PBMCs) are treated with recombinant (rh) granulocyte macrophage colony-stimulating factor and IL-4 for 7 days. Maturation to DCs induced by LPS in the presence and absence of the test compound is verified by the surface expression of CD80, CD83, CD86, and human leukocyte antigen-DR, and by the secretion of IL-12.
DC function requires a mixed lymphocyte culture, which analyzes the ability of T-cells to recognize allogenic cells as not belonging to the organism (nonself) as a result of the presence and proliferation of different MHC class II antigens on their surface. This assay is used to identify sensitizing agents. A DC to T-cell ratio of 1:100 is sufficient to initiate vigorous and optimal responses.Citation40 Splenocytes or lymph node cells from treated animals (responder cells) with genetically dissimilar cells (stimulator cells) are cocultured. The assay is usually performed in mice, where cells from another strain can be used as stimulators.Citation41 Stimulator cells are inactivated by irradiation or treatment with a DNA intercalating agent such as mitomycin C. After incubation for several days, proliferation of the responder cells is measured using Citation3H-thymidine uptake.Citation42 The reaction can also be performed using human PBMC-derived DCs mixed with allogenic lymphocytes,Citation43 and the proliferation of the responder T-cells after contact with allogenic lymphocytes is assessed using a viability (formazan bioreduction) assay. Human myeloid leukemia-derived MUTZ-3 cells have the ability to differentiate into DCs,Citation44 and this assay is in the process of validation as an alternative to the in vivo identification of sensitizing agents.Citation45
Specific issues in the assessment of NPs
The specific nature of NPs, mainly linked to their high surface reactivity, complicates their assessment by in vitro assays. The adsorption of molecules (either bacterial proteins or macromolecules from the body to the particle surface) holds importance for the in vivo and in vitro testing of phagocyte function.
In vivo and in vitro – binding of endotoxin
NPs may bind endotoxin, an LPS and pyrogenic compound of the wall of Gram-negative bacteria. Endotoxin is a strong stimulant of the immune response and causes a pyrogenic reaction in the human body.Citation46 Endotoxin contamination of metal and metal oxide NPs and CNTs is less expected because synthesis often includes steps that kill bacteria. However, contamination is often difficult to exclude because endotoxin can be present in distilled water.Citation47 Due to the strong stimulation of endotoxin, its presence in the sample does not allow for the identification of NP effects. The detection of endotoxin is usually achieved by evaluation in the limulus amebocyte lysate assay, one of the accepted alternatives to the in vivo endotoxin detection assays.Citation48 This assay can be performed in different formats, generally as clotting tests and by colorimetric detection.Citation49 Unfortunately, several NPs interfere with this assay. While for some NPs (TiO2, Ag, CaCO3, SiO2 NPs), interference with the gel-clotting assay was more prominent,Citation50 for other particles (Au NPs), interference with the colorimetric limulus amebocyte lysate assay has been reported.Citation51 The release of inflammatory cytokines (IL-6, IL-8, IL-1) from PBMCs produced variable results and it has been suggested that NPs and endotoxin compete against each other in the induction of cytokines.Citation52
In vivo and in vitro – protein corona
High surface activity leads to the binding of macromolecules to the particle surface once they get into contact with physiological solutions. This coating consists mainly of proteins and has been termed “protein corona”.Citation53 It is hypothesized that the composition of the protein corona determines the trafficking and biological effects of NPs. For a description of the composition and variability of the protein corona, the reader is referred to reviews focusing on this topic.Citation54,Citation55 The physicochemical parameters of the NPs and the composition of the biological fluid are the main factors determining the composition of the protein corona. As a general rule, hydrophobic particles bind more proteins than do hydrophilic particles,Citation56 and abundant proteins in the incubation solution are bound faster on the NP surface than the low abundant proteins.Citation57 Dependence on size and shape, as well as surface charge, has been reported in the following way: Au and SiO2 NPs >10 nm bound more proteins than particles <10 nm; more proteins were attached to TiO2 nanospheres than to nanorods and nanotubes; and binding to positively charged Au, PS, and carbon black particles was higher than to particles without charged groups.Citation58–Citation62 While the composition of the inner coating (hard corona) appeared to be more stable, the composition of the outer part (soft corona) was dynamic and changed in its composition when the particle was transferred from one medium to the other.Citation63 The passage through various media left a fingerprint of the protein composition of the previous media on the NP.Citation64 NPs retained the protein corona during endocytosis; the coat was subsequently removed in lysosomes.Citation65
The role of the protein corona composition for biological effects is still not entirely clear. The reduction of toxic effects, such as cytotoxicity and hemolysis, by protein coating of NPs has been observed in several studies of nonphagocytic cells.Citation66–Citation70 This decreased effect was linked to reduced cellular uptake. Bovine serum albumin (BSA) bound to the surface of carboxyl-functionalized PS, quantum dot (Qdot), and Au NPs decreased cell uptake. The opposite was observed for BSA bound to these types of NPs when they were functionalized with amine groups instead of carboxyl groups.Citation71 All BSA-coated NPs displayed the same effective surface charge, but apparently the BSA structure was influenced by the binding in such a way that different groups were visible for the cells. As a result, BSA-coated carboxylated NPs bound to the albumin receptor, while BSA-coated amine-functionalized NPs were ingested after binding to the cellular scavenger receptor.
Protein-coated NPs are expected to produce more pronounced immunological effects because coating with serum increased the uptake by phagocytes.Citation72 The secretion of proinflammatory cytokines by DCs was higher for spherical-than sheet-shaped ZnO NPs, which also bound more proteins on their surface.Citation73 While increased protein binding might have caused the higher secretion of cytokines, the opposite behavior has also been observed: coating of SiO2 NPs with serum decreased cytokine secretion of murine macrophages.Citation74 The presence of complement in the protein corona plays a specific role because the binding of complement C3b and IgG increases uptake by phagocytes by binding to the complement and Fc-receptors. Responses to complement binding were variable; firstly, complement proteins could be activated or inactivated by the binding, and secondly, increased uptake could lead to the activation or inhibition of phagocytes.Citation75,Citation76 Changes in protein conformation appear to be the reason for the different effects; binding of fibrinogen to negatively charged poly(acrylic acid)-conjugated Au NPs induced activation of the Mac-1 receptor on THP-1 monocytes, resulting in a proinflammatory response.Citation77 While these studies support a specific role of the bound proteins, other studies do not support the hypothesis of a protein corona-specific effect because the composition of the protein corona did not correlate with hemocompatibility.Citation78
In vitro – cellular doses
Dose-dependent effects are more difficult to identify for NPs than for conventional compounds because cellular uptake is influenced by the diffusion and sedimentation of the single NPs and agglomerates of the NPs. Several mathematical models have been developed to calculate the deposition of particles suspended in liquids on adherent cells.Citation79,Citation80 Particle-dependent minimal deposition was seen between 50–200 nm, while larger and smaller particles deposited at higher rates.Citation79 Small changes in the dispersion factor caused considerable variations in the deposited dose.Citation80 The differences are due to the formation of agglomerates, but the extent of agglomeration and its effect on deposition are difficult to quantify by mathematical models. The measured deposition of 50–1,000 nm plain PS particles on macrophages increased over time and showed a minimum for 100 nm particles.Citation17 Carboxyl PS particles of 20–1,000 nm showed the cellular uptake of 25%–40% in macrophages with a minimum at 100 nm.Citation81 The cellular dose of the same type of particles with sizes of 20–500 nm in endothelial cells increased from 4.6% to 28.4%, demonstrating higher particle uptake by phagocytic cells, as compared to nonphagocytic cells, in general.Citation82 When adherent cells were cultured upside-down, they ingested much less NPs than the cells cultured in the standard orientation.Citation83 Further complications arise when cells are exposed to aerosolized NPs because cell contact is dependent on the used exposure system, as well as on the variations in the size and concentration of the aerosol; great variations in deposition rates between 0.037% and 30% of the applied dose per well for different particles have been reported.Citation84–Citation88 Furthermore, the influence on flow has to be considered when assessing NP uptake from the systemic blood circulation.Citation89 Endothelial cells best ingested Qdots and SiO2 NPs at a shear stress of 0.05 Pa, which corresponds to postcapillary venules and peripheral arteries.Citation89
In vitro – assay interference
The interference of NPs with several assay systems can strongly influence the results (). The absorbance of NPs could lead to false-negative results (absence of cytotoxicity is detected, although the NP is cytotoxic) because the metabolic activity (according to absorbance) is estimated to be higher than it actually is.Citation90 Enzyme inhibition by NPs could also cause false-negative results. Lactate dehydrogenase (LDH) is released into the supernatant of cells when the plasma membrane integrity is lost. Its enzymatic activity correlates to the amount of damaged cells. If LDH activity is inhibited by NPs, a lower degree of cell damage will be determined.
Table 1 Mechanisms of interference between nonbiodegradable NPs and in vitro assays
False-positive results (cytotoxicity is detected although the NP is not toxic) are detected when the fluorescent signals of dihydrofluorescein (the detection of oxidative stress) or of propidium iodide (the disruption of membrane integrity) are enhanced by NPs.Citation91–Citation95 Depending on the assays used, the masking of toxic effects and the identification of nonexistent toxicity by NPs can occur simultaneously. Increased absorbance by colored NPs will result in a higher signal of LDH (indicating more dead cells) and in the MTT assay (indicating more viable cells). The use of multiple assays, therefore, helps to reveal assay interference. The addition of protein, mostly BSA, could prevent interference, but it also could increase it. While false-negative results by the inhibition of LDH activity by Si, Au, and CdSe NPsCitation96 was avoided, the addition of BSA caused false-negative effects in protein detection via the Bradford reagent.Citation97 For the identification of potential assay interference, the incubation of NPs with the assay compounds alone (in the absence of cells) and with cells alone (in the absence of assay compounds) can be used. These controls are, however, only useful when the NPs interact with assay compounds and with the readout; assay interactions by Au NPs, which increased the detected amount of dead cells by shuttling the indicator dye, propidium iodide, into the cells, would not have been revealed.Citation98,Citation99 Similarly, the more global effects of NPs on cultured cells, such as the depletion of nutrients by SWCNTs,Citation100 would go unnoticed. Interference can show dose dependency; dye (acridine) fluorescence is increased by low concentrations of Ag NPs and quenched at high concentrations of NPs.Citation101
Some general rules may help identify and prevent the false interpretation of results. The use of low NP concentrations reduces the problem of interference, but the removal of NPs by centrifugation is generally not recommended because analytes adsorbed to the particles might be removed. Assay interference of the colored CNTs, carbon black, C60 fullerenes, and Au NPs, and of the fluorescent Qdots, may occur more frequently than interference with noncolored Si, SiO2, TiO2, and ZnO NPs. Testing of NPs with several assays based on different detection methods can reduce the risk of misinterpretation.Citation90,Citation102 In this regard, immunotoxicity testing poses more problems than cytotoxicity testing because a lower number of assays for a given immunological function are usually available. On the other hand, compared to cytotoxicity testing, NPs are usually studied at much lower NP concentrations, reducing the risk for interference.
Immunotoxicological data from NP exposure
In vivo exposure includes voluntary inhalation and oral application, forced inhalation (intranasal and intratracheal instillation, oropharyngeal administration), forced oral (intragastric/gavage) application as well as noninvasive dermal and invasive (intradermal injection) dermal applications. Parenteral applications include intravenous and intraperitoneal injection. shows the general reaction pattern of the immune system after in vivo exposure to NPs.
Table 2 Effects of NPs after inhal, IN, IG, IP, IV, oral, oroph, and SC application, and ID and IT in normal animals and in animal models (Model)
Systemic immune effects
Effects in the respiratory tract with only a thin epithelium were more pronounced than effects after dermal or oral ingestion exposure, where a horny layer or a thick mucus layer separated NPs from epithelial and immune cells.Citation103
Inflammation in the lung is one of the most frequently reported effects of respiratory exposure to NPs.Citation104 Since cytokines are produced by several cell types, it is not clear whether the reported increases in cytokine secretion and subsequent inflammation were due to specific activation of immune cells, or if they were a consequence of cytotoxic action on alveolar epithelial cells. Heavy metal-containing NPs reacted in a similar manner as PS particles.Citation105,Citation106 Given that heavy metal-containing NPs show ROS generation, and since they are expected to have greater cytotoxicity, the similarity of the reaction does not support the hypothesis of cell death (induced by more cytotoxic heavy metal-containing NPs) as a main inductor of inflammation.
Only a few studies have reported the absence of immunological effects, which could be due to restricted access to immune cells.Citation107 The absence of immune effects after the oral ingestion of and exposure to ZnO and TiO2 NPs could be explained by the hindered assessment of the particles to the cells by mucus.Citation8,Citation107 On the other hand, the low reactivity of intraperitoneally applied Au NPs appears to be due to their high biocompatibility given that few studies have reported on the adverse cellular effects of Au NPs.Citation108,Citation109 This statement is supported by a lack of immunological interference in the cellular assays showing no increased cytokine secretion,Citation110,Citation111 and no effect on DC maturation and activation.Citation94,Citation112
When NPs were applied to diseased animals, the pathology of the disease was aggravated. This aggravation was seen in asthma models, as well as in atopic dermatitis (). Aggravation of asthma is unlikely to be caused by cytotoxicity of the NPs because exposure by the respiratory tract and by other routes (subcutaneous, intraperitoneal), where no direct contact with the alveolar epithelium occurred, caused the same effects.Citation113–Citation115 The mechanisms for amplifying pre-existing pathologies have been proposed through the following mechanisms:Citation116 pre-existing inflammation in the respiratory tubes could be amplified by enhancing the levels of inflammatory factors or humoral immunity. Second, NPs within the size range of <100 nm were able to stimulate and enhance hypersensitivity, which is primarily mediated by Th2 cells.Citation116
In vitro and ex vivo effects
Phagocyte function after in vitro (cells exposed in wells) and ex vivo (cells harvested from exposed animals) exposure is summarized in . To evaluate the potential of screening in phagocytes, first, data obtained from ex vivo and in vitro studies have to be compared. Second, the similarity of ex vivo and in vitro exposures to in vivo exposure has to be tested. In vitro data on cytokine secretion and chemotaxis corresponded to the respective ex vivo data (). NPs showed a similar pattern of interference with phagocyte functions; proinflammatory cytokine secretion (mostly IL-6, IL-1β, and TNF-α) and respiratory burst increased, while phagocytosis and chemotaxis decreased. The degranulation of neutrophilic granulocytes has been shown for a few particles.Citation81,Citation117 The influence on DC maturation and function varied markedly between the particles. MWCNTs inhibited maturation, Au and iron oxide showed no prominent effect, and SiO2 and TiO2 activated DCs.Citation94,Citation112,Citation118,Citation119 The different results could be due to the use of different readouts (maturation and activation).
Table 3 Immune effects in isolated phagocytes, either after in vivo treatment with nanoparticles (ex vivo) or by in vitro treatment
The secretion of proinflammatory cytokines was increased by all NPs when applied by in vitro exposure, and after the ex vivo respiratory exposure, to NPs. The lower sensitivity of phagocytes by the oral route was confirmed in an ex vivo study.Citation120
Uptake of NPs by phagocytes
When NPs are coated with proteins in biological fluids, they are well ingested by phagocytes.Citation121 Phagocytosis of NPs by primary cells, cell lines, macrophages, monocytes, and monocyte-derived macrophages indicated accumulation in the MPS and showed a good correlation to the accumulation of particles in the MPS of the spleen and liver in vivo.Citation122 Due to the crucial function of macrophages and DCs in the specific immune response, the accumulation of NPs in the MPS could result in immunotoxicity. The indication of uptake by the MPS or accumulation in lymphatic organs, however, was not correlated to adverse effects on the immune system in vivo or in vitro.Citation81,Citation123 Accumulation in the spleen was only observed for 30 nm Au particles, while adverse effects on the immune system according to increases in relative spleen weight and immune cell numbers were seen for 5 nm, 10 nm, and 60 nm Au particles.Citation123 Small carboxyl PS particles were ingested in much higher numbers than 1,000 nm particles by macrophages.Citation81 While the 1,000 nm large particles induced oxidative burst and cell damage, particles in the size range between 40 nm and 500 nm were taken up without obvious interference with cell viability and function. Taken together, these data suggest that the uptake of NPs may not result in impaired phagocyte function.
Guidelines for sample preparation and exposure
Physiologically relevant testing is based on sample preparation, as well as on the use of dispersant and intended exposure routes. Most NPs form stable solutions in distilled water, which cannot be used for in vitro studies. The presence of ions and protein in the physiological solution leads to NP agglomerates, which may increase in size, but they may also disintegrate. The surface coating of NPs determines their penetration of barriers, cellular uptake, and immune response.Citation124 The Office of Economic Co-operation and Development (OECD) guidelines for sample preparation and dosimetry had advised that dose should be indicated in terms of mass, surface area, and particle number at a minimum.Citation125 To get information on the stability of the dispersion, repeated measurements are recommended with the documentation of agglomeration and ion release. The dispersants should preferentially contain macromolecules that are present in the target tissue. For exposure with aerosols, and in addition to the NP parameters, the mass median aerodynamic diameter and aerosol concentration should be determined. Guidelines for sample preparation for nanoscale TiO2 are already available,Citation126 and existing guidelines for exposure by spontaneous inhalation, oral gavage, and dermal application are applicable for NP exposure. Moreover, the additional effects of intravenous exposure (for instance, behavior in the syringe) have to be considered.
Freshly prepared solutions from stock solutions prepared in water, diluted in cell culture medium, and treated by sonification should be added to the cells. In the case that no route-specific surfactants, such as 1,2 dipalmitoyl-sn-glycero-3-phosphocholine for pulmonary exposure, are used, BSA appears to be a good choice because this zwitterionic molecule prevents the binding of protein from the solution.
Conclusion
Due to the complexity of the immune system, in vivo testing will remain the gold standard. However, intraindividual variations in the immune system, as well as its compensatory abilities, are major limitations. As has been observed in environmental studies of airborne particles, individuals with impaired immune function were affected by small particle doses, while no effects were observed in the healthy population.Citation2–Citation5
This overview on a variety of carbon, metal, and metal oxide NPs shows that these particles caused relatively similar patterns of immunotoxicity in vivo, which involved inflammation and immunosuppression in healthy animals and aggravation of the pathology in animals with pre-existing diseases. This suggests that the classification of particles as more or less immunotoxic by in vitro screening might be helpful. The extent to which such screening could lead to valid results was studied by comparing data obtained by in vivo exposure, in vitro testing and in vitro data (). This analysis showed that the results obtained in cells isolated from NP-exposed animals were similar to the data obtained of cells, which were exposed to NPs in vitro. Secondly, NPs that inhibited phagocyte functions in vitro reacted in an immunotoxic manner in vivo ( and ). The data suggest that the in vitro testing of phagocytes might predict the typical immunotoxicity pattern of NPs in vivo. Cellular assays may also be suitable to identify disease-related alterations in the immune reaction to NPs because comparison between reactions of PBMCs from healthy and allergic donors showed that the cells exhibited disease-related differences upon challenge.Citation118
Table 4 Overview of nanoparticle actions on phagocyte functions
Due to the specific composition of the immune system at different portals of entry, exposure-specific coculture models including immune cells could serve as a possibility to assess immunotoxicants in vitro. Alveolar epithelial cells and alveolar macrophages in cocultures released inflammatory cytokines at lower concentrations of TiO2 NPs than did the respective monocultures.Citation127 At the expense of greater complexity, these systems could increase the sensitivity of immunotoxicity in vitro screening and enable exposure-specific testing. However, until a correlation of these findings in these systems to data obtained in humans has been shown, their value remains elusive.
Acknowledgments
Support by the European integrated project NMP4-CT-2006-026723 and by the Austrian Science Fund grant P22576-B18 is gratefully acknowledged.
Disclosure
The author reports no conflicts of interest in this work.
References
- DobrovolskaiaMAMcNeilSEImmunological properties of engineered nanomaterialsNat Nanotechnol20072846947818654343
- D’AmatoGOutdoor air pollution in urban areas and allergic respiratory diseasesMonaldi Arch Chest Dis199954647047410695313
- DelfinoRJZeigerRSSeltzerJMStreetDHMcLarenCEAssociation of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication useEnviron Health Perspect200211010A607A61712361942
- LiNXiaTNelAEThe role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticlesFree Radic Biol Med20084491689169918313407
- PenttinenPTimonenKLTiittanenPMirmeARuuskanenJPekkanenJUltrafine particles in urban air and respiratory health among adult asthmaticsEur Respir J200117342843511405521
- BuzeaCPachecoIIRobbieKNanomaterials and nanoparticles: sources and toxicityBiointerphases200724MR177120419892
- SodemannUBistrupCMarckmannPCancer rates after kidney transplantationDan Med Bull20115812A434222142571
- AuttachoatWMcLoughlinCEWhiteKLJrSmithMJRoute-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticlesJ Immunotoxicol201411327328224134492
- WarheitDBSayesCMReedKLSwainKAHealth effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risksPharmacol Ther20081201354218703086
- ClaudeJRDomenjoudLFattalERecommendations for Toxicological Evaluation of Nanoparticle Medicinal ProductsParis, FranceAgence Française de Sécurité Sanitaire des Produits de Santé2011 Available from: http://ansm.sante.fr/var/ansm_site/storage/original/application/2968a90b774b563b03405379b7d4f4e6.pdfAccessed April 24, 2014
- MüllerKSkepperJNPosfaiMEffect of ultrasmall superpara-magnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitroBiomaterials20072891629164217178155
- LiuHYangDYangHComparative study of respiratory tract immune toxicity induced by three sterilisation nanoparticles: silver, zinc oxide and titanium dioxideJ Hazard Mater2013248–249478486
- ParkEJParkKOxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitroToxicol Lett20091841182519022359
- NogueiraCMde AzevedoWMDagliMLTitanium dioxide induced inflammation in the small intestineWorld J Gastroenterol201218344729473523002342
- ChenJKHoCCChangHParticulate nature of inhaled zinc oxide nanoparticles determines systemic effects and mechanisms of pulmonary inflammation in miceNanotoxicology20159435324559390
- KarlssonHLCronholmPHedbergYTornbergMDe BatticeLSvedhemSWallinderIOCell membrane damage and protein interaction induced by copper containing nanoparticles–importance of the metal release processToxicology20133131596923891735
- Ahmad KhanbeigiRKumarASadoukiFThe delivered dose: Applying particokinetics to in vitro investigations of nanoparticle internalization by macrophagesJ Control Release2012162225926622824784
- RoittIDelvesPRoitt’s Essential Immunology10th edOxford, UKWiley2001
- JanewayCJrTraversPWalportMShlomchikMImmunobiology: The Immune System in Health and Disease6th edNew York, NYGarland Science Pubishing2005
- GordonSPluddemannAMartinez EstradaFMacrophage heterogeneity in tissues: phenotypic diversity and functionsImmunol Rev20142621365525319326
- HirotaKHasegawaTHinataHOptimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophagesJ Control Release20071191697617335927
- KawaguchiHKoiwaiNOhtsukaYMiyamotoMSasakawaSPhagocytosis of latex particles by leucocytes. I. Dependence of phagocytosis on the size and surface potential of particlesBiomaterials19867161663955160
- SeymourLSchachtEDuncanRThe effect of size of polystyrene particles on their retention within the rat peritoneal compartment, and on their interaction with rat peritoneal macrophages in vitroCell Biol Int Rep19911542772861878974
- AbbasAKLichtmanHPallaiSCellular and Molecular Immunology6th edPhiladelphia, PASaunders2007
- DaleDCBoxerLLilesWCThe phagocytes: neutrophils and monocytesBlood2008112493594518684880
- ColiganJEBiererBMarguliesDShevachEStroberWKruisbeekACurrent Protocols in ImmunologyNew York, NYJohn Wiley and Sons1991
- KrebsFCMillerSRCataloneBJFichorovaRAndersonDMalamudDHowettMKWigdahlBComparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfateAntimicrob Agents Chemother20024672292229812069993
- BorishLCSteinkeJW2Cytokines and chemokinesJ Allergy Clin Immunol20031112 SupplS460S47512592293
- FalkWGoodwinRHJrLeonardEJA 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migrationJ Immunol Methods19803332392476989919
- PietrosimoneKMYinXKnechtDALynesMAMeasurement of cellular chemotaxis with ECIS/TaxisJ Vis Exp201262pii:3840
- IqbalAJRegan-KomitoDChristouIA real time chemotaxis assay unveils unique migratory profiles amongst different primary murine macrophagesPLoS One201383e5874423516549
- GilleCSpringBTewesLPoetsCFOrlikowskyTA new method to quantify phagocytosis and intracellular degradation using green fluorescent protein-labeled Escherichia coli: comparison of cord blood macrophages and peripheral blood macrophages of healthy adultsCytometry A200669315215416479601
- ElsnerJKappAReactive Oxygen ReleaseProudfootAWellsTPowerCMethods in Molecular Biology: Chemokine ProtocolsHuman PressTotowa2000153157
- SchneemannMSchoedenGMacrophage biology and immunology: man is not a mouseJ Leukoc Biol2007813579 discussion 58017332373
- DaigneaultMPrestonJAMarriottHMWhyteMKDockrellDHThe identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophagesPLoS One201051e866820084270
- MosserDMEdwardsJPExploring the full spectrum of macrophage activationNat Rev Immunol200881295896919029990
- MannBSChungKFBlood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapyRespir Res200675916600024
- JovanovićBAnastasovaLRoweEWPalićDHydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820)Aquat Toxicol2011101247448221122929
- VesninaLÉMamontovaTVMikitiukMVEffect of fullerene C60 on functional activity of phagocytic cellsEksp Klin Farmakol20117462629 Russian21870772
- SteinmanRMWitmerMDLymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in miceProc Natl Acad Sci U S A1978751051325136154105
- KangHGLeeJEYangSHDonor-strain-derived immature dendritic cell pre-treatment induced hyporesponsiveness against allogeneic antigensImmunology2010129456757720102412
- HouseRVThomasPTBhargavaHNIn vitro evaluation of fentanyl and meperidine for immunomodulatory activityImmunol Lett1995461–21171247590906
- LiYLiXLiZGaoHSurface-structure-regulated penetration of nanoparticles across a cell membraneNanoscale20124123768377522609866
- MastersonAJSombroekCCDe GruijlTDMUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursorsBlood2002100270170312091369
- NelissenISelderslaghsIHeuvelRVWittersHVerheyenGRSchoetersGMUTZ-3-derived dendritic cells as an in vitro alternative model to CD34+ progenitor-derived dendritic cells for testing of chemical sensitizersToxicol In Vitro20092381477148119732821
- MagalhãesPOLopesAMMazzolaPGRangel-YaguiCPennaTCPessoaAJrMethods of endotoxin removal from biological preparations: a reviewJ Pharm Pharm Sci200710338840417727802
- AndersonWBHuckPMDixonDGMayfieldCIEndotoxin inactivation in water by using medium-pressure UV lampsAppl Environ Microbiol20036953002300412732578
- TsujiKSteindlerKAHarrisonSJLimulus amoebocyte lysate assay for detection and quantitation of endotoxin in a small-volume parenteral productAppl Environ Microbiol19804035335386448582
- HofmanJBacterial endotoxinsShierWMebsDHandbook of ToxicologyNew York, NYMarcel Dekker Inc1990655682
- SmuldersSKaiserJPZuinSContamination of nanoparticles by endotoxin: evaluation of different test methodsPart Fibre Toxicol201294123140310
- OostinghGJCasalsEItalianiPProblems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effectsPart Fibre Toxicol201181821306632
- DobrovolskaiaMAMcNeilSEEndotoxin and Engineered Nano-materialsDobrovolskaiaMAMcNeilSEHandbook of Immunological Properties of Engineered NanomaterialsRepublic of SingaporeWorld Scientific Pub Co Inc201277116
- CedervallTLynchIFoyMDetailed identification of plasma proteins adsorbed on copolymer nanoparticlesAngew Chem Int Ed Engl200746305754575617591736
- MonopoliMPAbergCSalvatiADawsonKABiomolecular coronas provide the biological identity of nanosized materialsNat Nanotechnol201271277978623212421
- RahmanMLaurentSTawilNYahiaLHMahmoudiMNanoparticle and protein coronaRahmanMLaurentSTawilNYahiaLHMahmoudiMProtein-Nanoparticle Interactions: The Bio-Nano Interface SpringerSeries in Biophysics, Vol. 15Berlin, GermanySpringer20132144
- DingHMMaYQComputer simulation of the role of protein corona in cellular delivery of nanoparticlesBiomaterials201435308703871025005681
- Izak-NauEVoetzMEidenSDuschlAPuntesVFAltered characteristics of silica nanoparticles in bovine and human serum: the importance of nanomaterial characterization prior to its toxicological evaluationPart Fibre Toxicol20131015624206572
- DengZJLiangMTothIMonteiroMJMinchinRFMolecular interaction of poly(acrylic acid) gold nanoparticles with human fibrinogenACS Nano20126108962896922998416
- DengZJMortimerGSchillerTMusumeciAMartinDMinchinRFDifferential plasma protein binding to metal oxide nanoparticlesNanotechnology2009204545510119822937
- Fertsch-GappSSemmler-BehnkeMWenkAKreylingWGBinding of polystyrene and carbon black nanoparticles to blood serum proteinsInhal Toxicol201123846847521689008
- LundqvistMSethsonIJonssonBHProtein adsorption onto silica nanoparticles: conformational changes depend on the particles’ curvature and the protein stabilityLangmuir20042024106391064715544396
- DengZJLiangMTothIMonteiroMMinchinRFPlasma protein binding of positively and negatively charged polymer-coated gold nanoparticles elicits different biological responsesNanotoxicology20137331432222394123
- MonopoliMPWalczykDCampbellAPhysical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticlesJ Am Chem Soc201113382525253421288025
- LundqvistMStiglerJCedervallTThe evolution of the protein corona around nanoparticles: a test studyACS Nano2011597503750921861491
- WangFYuLMonopoliMPThe biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomesNanomedicine2013981159116823660460
- BaierGCostaCZellerABSA adsorption on differently charged polystyrene nanoparticles using isothermal titration calorimetry and the influence on cellular uptakeMacromol Biosci201111562863821384550
- De AngelisIBaroneFZijnoAComparative study of ZnO and TiO2 nanoparticles: physicochemical characterisation and toxicological effects on human colon carcinoma cellsNanotoxicology2013781361137223078188
- HsiaoILHuangYJEffects of serum on cytotoxicity of nano- and micro-sized ZnO particlesJ Nanopart Res201315182924078789
- VidicJHaqueFGuignerJMVidyAChevalierCStankicSEffects of water and cell culture media on the physicochemical properties of ZnMgO nanoparticles and their toxicity toward mammalian cellsLangmuir20143038113661137425184703
- SahaKMoyanoDFRotelloVMProtein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticlesMater Horiz20142014110210524535933
- FleischerCCPayneCKNanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomesAcc Chem Res20144782651265925014679
- RugeCAKirchJCañadasOUptake of nanoparticles by alveolar macrophages is triggered by surfactant protein ANanomedicine20117669069321839052
- HengBCZhaoXTanECEvaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticlesArch Toxicol201185121517152821656222
- PanasAMarquardtCNalcaciOScreening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophagesNanotoxicology20137325927322276741
- PondmanKMSobikMNayakAComplement activation by carbon nanotubes and its influence on the phagocytosis and cytokine response by macrophagesNanomedicine20141061287129924607938
- LingWLBiroABallyIProteins of the innate immune system crystallize on carbon nanotubes but are not activatedACS Nano20115273073721214219
- DengZJLiangMMonteiroMTothIMinchinRFNanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammationNat Nanotechnol201161394421170037
- DobrovolskaiaMANeunBWManSProtein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticlesNanomedicine20141071453146324512761
- HinderliterPMMinardKROrrGISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studiesPart Fibre Toxicol2010713621118529
- CárdenasWHMamaniJBSibovTTCaousCAAmaroEJrGamarraLFParticokinetics: computational analysis of the superpara-magnetic iron oxide nanoparticles deposition processInt J Nanomedicine201272699271222745539
- PrietlBMeindlCRobleggEPieberTRLanzerGFröhlichENano-sized and micro-sized polystyrene particles affect phagocyte functionCell Biol Toxicol201430111624292270
- FröhlichEMeindlCRobleggEEbnerBAbsengerMPieberTRAction of polystyrene nanoparticles of different sizes on lysosomal function and integrityPart Fibre Toxicol201292622789069
- ChoECZhangQXiaYThe effect of sedimentation and diffusion on cellular uptake of gold nanoparticlesNat Nanotechnol20116638539121516092
- FröhlichEBonstinglGHöflerAComparison of two in vitro systems to assess cellular effects of nanoparticles-containing aerosolsToxicol In Vitro201327140941722906573
- LenzAGKargELentnerBA dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticlesPart Fibre Toxicol200963220015351
- MertesPPraplanAPKünziLA compact and portable deposition chamber to study nanoparticles in air-exposed tissueJ Aerosol Med Pulm Drug Deliv201326422823523421898
- SaviMKalbererMLangDA novel exposure system for the efficient and controlled deposition of aerosol particles onto cell culturesEnviron Sci Technol200842155667567418754491
- TippeAHeinzmannURothCDeposition of fine and ultrafine aerosol particles during exposure at the air/cell interfaceJ Aerosol Sci2002332207218
- SamuelSPJainNO’DowdFMultifactorial determinants that govern nanoparticle uptake by human endothelial cells under flowInt J Nanomedicine201272943295622745555
- Monteiro-RiviereNAInmanAOZhangLWLimitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell lineToxicol Appl Pharmacol2009234222223518983864
- BergJMHoSHwangWInternalization of carbon black and maghemite iron oxide nanoparticle mixtures leads to oxidant productionChem Res Toxicol201023121874188221067130
- DoakSHGriffithsSMManshianBConfounding experimental considerations in nanogenotoxicologyMutagenesis200924428529319351890
- KrollAPillukatMHHahnDSchnekenburgerJCurrent in vitro methods in nanoparticle risk assessment: limitations and challengesEur J Pharm Biopharm200972237037718775492
- PfallerTColognatoRNelissenIThe suitability of different cellular in vitro immunotoxicity and genotoxicity methods for the analysis of nanoparticle-induced eventsNanotoxicology201041527220795902
- StoneVJohnstonHSchinsRPDevelopment of in vitro systems for nanotoxicology: methodological considerationsCrit Rev Toxicol200939761362619650720
- MaccormackTJClarkRJDangMKInhibition of enzyme activity by nanomaterials: potential mechanisms and implications for nanotoxicity testingNanotoxicology20126551452521639725
- OngKJMacCormackTJClarkRJWidespread nanoparticle-assay interference: implications for nanotoxicity testingPLoS One201493e9065024618833
- KeeneAMTynerKMAnalytical characterization of gold nanoparticle primary particles, aggregates, agglomerates, and agglomerated aggregatesJournal of Nanoparticle Research201113834653481
- ShuklaSPriscillaABanerjeeMPorous gold nanospheres by controlled transmetalation reaction: a novel material for application in cell imagingChem Mater2005172050005005
- CaseyAHerzogELyngFMByrneHJChambersGDavorenMSingle walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cellsToxicol Lett20081792788418502058
- SabatiniCAPereiraRVGehlenMHFluorescence modulation of acridine and coumarin dyes by silver nanoparticlesJ Fluoresc200717437738217549612
- FröhlichEMeindlCPieberTRImportant issues in the cytotoxicity screening of nano-sized materialsEURO-NanoTox Letters2010116
- SCENIHR working group [webpage on the Internet]The appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies2006 Available from: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_003b.pdfAccessed January 1, 2015
- BrashersVAlterations of pulmonary functionHuetherSMcCanceKUnderstanding Pathophysiology5th edSt Louis, MOElsevier2012678699
- FröhlichESambergerCKueznikTCytotoxicity of nanoparticles independent from oxidative stressJ Toxicol Sci200934436337519652459
- XiaTKovochichMLiongMZinkJINelAECationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathwaysACS Nano200821859619206551
- MatsumuraMTakasuNNagataMNakamuraKKawaiMYoshinoSEffect of ultrafine zinc oxide (ZnO) nanoparticles on induction of oral tolerance in miceJ Immunotoxicol20107323223720507255
- ChenHDorriganASaadSHareDJCortieMBValenzuelaSMIn vivo study of spherical gold nanoparticles: inflammatory effects and distribution in micePLoS One201382e5820823469154
- SumbayevVVYasinskaIMGarciaCPGold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responsesSmall20139347247723112137
- ZhangQHitchinsVMSchrandAMHussainSMGoeringPLUptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of pro-inflammatory mediatorsNanotoxicology20115328429520849214
- ShuklaRBansalVChaudharyMBasuABhondeRRSastryMBiocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overviewLangmuir20052123106441065416262332
- VilliersCFreitasHCoudercRVilliersMBMarchePAnalysis of the toxicity of gold nano particles on the immune system: effect on dendritic cell functionsJ Nanopart Res2010121556021841911
- NygaardUCHansenJSSamuelsenMAlbergTMarioaraCDLøvikMSingle-walled and multi-walled carbon nanotubes promote allergic immune responses in miceToxicol Sci2009109111312319293371
- MatsumuraMNagataMNakamuraKAdjuvant effect of zinc oxide on Th2 but not Th1 immune responses in miceImmunopharmacol Immunotoxicol2010321566219811107
- RoyRKumarSVermaAKZinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a Th2 response in Balb/c miceInt Immunol201426315917224225181
- LiYZhangYYanBNanotoxicity overview: nano-threat to susceptible populationsInt J Mol Sci20141533671369724590128
- HaaseHFahmiAMahltigBImpact of silver nanoparticles and silver ions on innate immune cellsJ Biomed Nanotechnol20141061146115624749409
- LavernyGCassetAPurohitAImmunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patientsToxicol Lett201321729110123266719
- WinterMBeerHDHornungVKrämerUSchinsRPFörsterIActivation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cellsNanotoxicology20115332634020846021
- KimCSNguyenHDIgnacioRMImmunotoxicity of zinc oxide nanoparticles with different size and electrostatic chargeInt J Nanomedicine20149Suppl 219520525565837
- FadeelBClear and present danger? Engineered nanoparticles and the immune systemSwiss Med Wkly2012142w1360922736064
- CaronWPLayJCFongAMTranslational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacologyJ Pharmacol Exp Ther2013347359960624042160
- ZhangXDWuDShenXSize-dependent in vivo toxicity of PEG-coated gold nanoparticlesInt J Nanomedicine201162071208121976982
- RemediosSerranoLopezDLalatsaAActive TargetingUchegbuISchätzleinAChengWLalatsaAFundamentals of Pharmaceutical NanoscienceSpringerNew York2013337374
- OECD Environment, Health and Safety Publications. [webpage on the Internet]Guidance on sample preparation and dosimetry for the safety testing of manufactured nanomaterialsParis, FranceParis Environment Directorate organisation for economic co-operation and development2012 Available from: http://www.oecd.org/official-documents/publicdisplaydocumentpdf/?cote=ENV/JM/MONO%282012%2940&docLanguage=EnAcessed January 1, 2015
- TaurozziJHackleyVWiesnerMPreparation of a nanoscale TiO2 aqueous dispersion for toxicological or environmental testingVersion 1.2. NanoEHS Protocols(NIST Special Publication 1200-3)2012 Available from: http://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.1200-3.pdfAccessed November 1st, 2014
- TaoFKobzikLLung macrophage-epithelial cell interactions amplify particle-mediated cytokine releaseAm J Respir Cell Mol Biol200226449950511919087
- DobrovolskaiaMAAggarwalPHallJBMcNeilSEPreclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistributionMol Pharm20085448749518510338
- HallJBDobrovolskaiaMAPatriAKMcNeilSECharacterization of nanoparticles for therapeuticsNanomedicine (Lond)20072678980318095846
- MurthyRPathakYDrug delivery nanoparticles: formulation and characterizationPathakYThassuDIn Vitro Blood Interaction and Pharmacological and Toxicological Characterization of NanosystemsNew York, NYInforma Health Care2009190218
- DobrovolskaiaMAGermolecDRWeaverJLEvaluation of nanoparticle immunotoxicityNat Nanotechnol20094741141419581891
- MaluginAGhandehariHArnidaCellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheresJ Appl Toxicol201030321221719902477
- CliftMJRothen-RutishauserBBrownDMThe impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell lineToxicol Appl Pharmacol2008232341842718708083
- PujaltéIPassagneIBrouillaudBCytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cellsPart Fibre Toxicol201181021371295
- KrollAPillukatMHHahnDSchnekenburgerJInterference of engineered nanoparticles with in vitro toxicity assaysArch Toxicol20128671123113622407301
- ZhangLWZengLBarronARMonteiro-RiviereNABiological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytesInt J Toxicol200726210311317454250
- YingEHwangHMIn vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytesSci Total Environ2010408204475448120673962
- Braydich-StolleLHussainSSchlagerJJHofmannMCIn vitro cytotoxicity of nanoparticles in mammalian germline stem cellsToxicol Sci200588241241916014736
- WahlBWahlBDaumNOhremHLLehrCMNovel luminescence assay offers new possibilities for the risk assessment of silica nanoparticlesNanotoxicology200824243251
- MurdockRCBraydich-StolleLSchrandAMSchlagerJJHussainSMCharacterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering techniqueToxicol Sci2008101223925317872897
- Monteiro-RiviereNAInmanAOChallenges for assessing carbon nanomaterial toxicity to the skinCarbon200644610701078
- Monteiro-RiviereNAOldenburgSJInmanAOInteractions of aluminum nanoparticles with human epidermal keratinocytesJ Appl Toxicol201030327628520013751
- Wörle-KnirschJMPulskampKKrugHFOops they did it again! Carbon nanotubes hoax scientists in viability assaysNano Lett2006661261126816771591
- GonzalesMMitsumoriLMKushleikaJVRosenfeldMEKrishnanKMCytotoxicity of iron oxide nanoparticles made from the thermal decomposition of organometallics and aqueous phase transfer with Pluronic F127Contrast Media Mol Imaging20105528629320623517
- CaseyAHerzogEDavorenMLyngFMByrneHJChambersGSpectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicityCarbon200745714251432
- DavorenMHerzogECaseyAIn vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cellsToxicol In Vitro200721343844817125965
- L’azouBJorlyJOnDIn vitro effects of nanoparticles on renal cellsPart Fibre Toxicol200852219099552
- LowSPWilliamsKACanhamLTVoelckerNHEvaluation of mammalian cell adhesion on surface-modified porous siliconBiomaterials200627264538454616707158
- HanXGeleinRCorsonNValidation of an LDH assay for assessing nanoparticle toxicityToxicology20112871–39910421722700
- OhSJKimHLiuYIncompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generationToxicol Lett2014225342243224463055
- StuekerOOrtegaVAGossGGStepanovaMUnderstanding interactions of functionalized nanoparticles with proteins: a case study on lactate dehydrogenaseSmall201410102006202124591162
- HolderALGoth-GoldsteinRLucasDKoshlandCPParticle-induced artifacts in the MTT and LDH viability assaysChem Res Toxicol20122591885189222799765
- SuskaFGretzerCEspositoMIn vivo cytokine secretion and NF-kappaB activation around titanium and copper implantsBiomaterials200526551952715276360
- BelyanskayaLManserPSpohnPBruininkAWickPThe reliability and limits of the MTT reduction assay for carbon nanotubes–cell interactionCarbon2007451326432648
- LaaksonenTSantosHViholaHFailure of MTT as a toxicity testing agent for mesoporous silicon microparticlesChem Res Toxicol200720121913191817990852
- BrownDMDicksonCDuncanPAl-AttiliFStoneVInteraction between nanoparticles and cytokine proteins: impact on protein and particle functionalityNanotechnology2010212121510420431193
- KocbachATotlandsdalAILågMRefsnesMSchwarzePEDifferential binding of cytokines to environmentally relevant particles: a possible source for misinterpretation of in vitro results?Toxicol Lett2008176213113718079072
- ValSHussainSBolandSHamelRBaeza-SquibanAMaranoFCarbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial epithelial cells: need for multiparametric evaluation due to adsorption artifactsInhal Toxicol200921Suppl 111512219558243
- VeranthJMKaserEGVeranthMMKochMYostGSCytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dustsPart Fibre Toxicol20074217326846
- LinMHHsuTSYangPMTsaiMYPerngTPLinLYComparison of organic and inorganic germanium compounds in cellular radiosensitivity and preparation of germanium nanoparticles as a radiosensitizerInt J Radiat Biol200985321422619296338
- AamBBFonnumFCarbon black particles increase reactive oxygen species formation in rat alveolar macrophages in vitroArch Toxicol200781644144617119925
- HoetPHNemeryBNapierskaDIntracellular oxidative stress caused by nanoparticles: what do we measure with the dichlorofluorescein assay?Nano Today201383223227
- KarlssonHLThe comet assay in nanotoxicology researchAnal Bioanal Chem2010398265166620640410
- InoueKTakanoHYanagisawaRKoikeEShimadaASize effects of latex nanomaterials on lung inflammation in miceToxicol Appl Pharmacol20092341687618938192
- van ZijverdenMGranumBAdjuvant activity of particulate pollutants in different mouse modelsToxicology20001521–3697711090941
- FifisTGamvrellisACrimeen-IrwinBSize-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumorsJ Immunol200417353148315415322175
- NygaardUCSamuelsenMAaseALøvikMThe capacity of particles to increase allergic sensitization is predicted by particle number and surface area, not by particle massToxicol Sci200482251552415456925
- NygaardUCOrmstadHAaseALøvikMThe IgE adjuvant effect of particles: characterisation of the primary cellular response in the draining lymph nodeToxicology2005206218119315588912
- YanagisawaRTakanoHInoueKIKoikeESadakaneKIchinoseTSize effects of polystyrene nanoparticles on atopic dermatitislike skin lesions in NC/NGA miceInt J Immunopathol Pharmacol201023113114120378001
- ShvedovaAAFabisiakJPKisinERSequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivityAm J Respir Cell Mol Biol200838557959018096873
- ParkEJRohJKimSNA single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in miceArch Toxicol20118591121113121472445
- InoueKYanagisawaRKoikeENishikawaMTakanoHRepeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stressFree Radic Biol Med201048792493420093178
- InoueKTakanoHKoikeEEffects of pulmonary exposure to carbon nanotubes on lung and systemic inflammation with coagulatory disturbance induced by lipopolysaccharide in miceExp Biol Med (Maywood)2008233121583159018849540
- CrouzierDFollotSGentilhommeECarbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lungToxicology20102721–3394520381574
- MengJYangMJiaFXuZKongHXuHImmune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubesNanotoxicology20115458359121034373
- MitchellLAGaoJWalRVGigliottiABurchielSWMcDonaldJDPulmonary and systemic immune response to inhaled multiwalled carbon nanotubesToxicol Sci2007100120321417660506
- TkachAVShurinGVShurinMRDirect effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposureACS Nano2011575755576221657201
- ParkEJChoWSJeongJYiJChoiKParkKPro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillationToxicology2009259311312119428951
- WangXPodilaRShannahanJHRaoAMBrownJMIntravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axisInt J Nanomedicine201381733174823662055
- YamaguchiAFujitaniTOhyamaKEffects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in miceJ Toxicol Sci201237117718922293422
- InoueKKoikeEYanagisawaRHiranoSNishikawaMTakanoHEffects of multi-walled carbon nanotubes on a murine allergic airway inflammation modelToxicol Appl Pharmacol2009237330631619371758
- Ryman-RasmussenJPTewksburyEWMossORCestaMFWongBABonnerJCInhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthmaAm J Respir Cell Mol Biol200940334935818787175
- CestaMFRyman-RasmussenJPWallaceDGBacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubesAm J Respir Cell Mol Biol201043214215119738159
- SungJHJiJHParkJDSubchronic inhalation toxicity of silver nanoparticlesToxicol Sci2009108245246119033393
- HanYHKimSHKimSZParkWHIntracellular GSH levels rather than ROS levels are tightly related to AMA-induced HeLa cell deathChem Biol Interact20081711677817935707
- ParkEJBaeEYiJRepeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticlesEnviron Toxicol Pharmacol201030216216821787647
- VandebrielRJTonkECde la Fonteyne-BlankestijnLJImmunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in ratsPart Fibre Toxicol2014112124885556
- SuCLChenTTChangCCTaiwan CardioPulmonary Research Group (T-CPR)Comparative proteomics of inhaled silver nanoparticles in healthy and allergen provoked miceInt J Nanomedicine201382783279923946650
- GosensIPostJAde la FonteyneLJImpact of agglomeration state of nano- and submicron sized gold particles on pulmonary inflammationPart Fibre Toxicol2010713721126342
- KhanHAAbdelhalimMAAlhomidaASAl-AyedMSEffects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidneyBiomed Res Int2013201359073023781503
- HussainSVanoirbeekJALuytsKLung exposure to nanoparticles modulates an asthmatic response in a mouse modelEur Respir J201137229930920530043
- ChoWSChoMKimSRPulmonary toxicity and kinetic study of Cy5.5-conjugated superparamagnetic iron oxide nanoparticles by optical imagingToxicol Appl Pharmacol2009239110611519520096
- ParkEJKimHKimYYiJChoiKParkKInflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in miceToxicology20102751–3657120540983
- ChenBAJinNWangJThe effect of magnetic nanoparticles of Fe(3)O(4) on immune function in normal ICR miceInt J Nanomedicine2010559359920856834
- ShenCCWangCCLiaoMHJanTRA single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c miceInt J Nanomedicine201161229123521753874
- ShenCCLiangHJWangCCLiaoMHJanTRIron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivityInt J Nanomedicine201272729273722701318
- ZhuMLiYShiJFengWNieGZhaoYExosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activationSmall20128340441222144073
- ZhuMTianXSongXNanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activationSmall20128182841284822674628
- BanMLangonnéIHuguetNGoutetMEffect of submicron and nano-iron oxide particles on pulmonary immunity in miceToxicol Lett2012210326727522343040
- ChoWSDuffinRPolandCAMetal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testingEnviron Health Perspect2010118121699170620729176
- MorishigeTYoshiokaYInakuraHCytotoxicity of amorphous silica particles against macrophage-like THP-1 cells depends on particle-size and surface propertiesPharmazie201065859659920824960
- ChenQXueYSunJKupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivoInt J Nanomedicine201381129114023515466
- NishimoriHKondohMIsodaKTsunodaSTsutsumiYYagiKSilica nanoparticles as hepatotoxicantsEur J Pharm Biopharm200972349650119232391
- HiraiTYoshikawaTNabeshiHAmorphous silica nano-particles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injectionPart Fibre Toxicol20129322296706
- HanBGuoJAbrahaleyTAdverse effect of nano-silicon dioxide on lung function of rats with or without ovalbumin immunizationPLoS One201162e1723621359146
- AmbalavananNStanishevskyABulgerATitanium oxide nanoparticle instillation induces inflammation and inhibits lung development in miceAm J Physiol Lung Cell Mol Physiol20133043L152L16123220372
- GrassianVHO’shaughnessyPTAdamcakova-DoddAPettiboneJMThornePSInhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nmEnviron Health Perspect2007115339740217431489
- OberdörsterGFerinJLehnertBECorrelation between particle size, in vivo particle persistence, and lung injuryEnviron Health Perspect1994102Suppl 51731797882925
- OberdörsterGFinkelsteinJNJohnstonCAcute pulmonary effects of ultrafine particles in rats and miceRes Rep Health Eff Inst200096574 disc. 75–8611205815
- RoursgaardMJensenKAPoulsenSSAcute and subchronic airway inflammation after intratracheal instillation of quartz and titanium dioxide agglomerates in miceScientific World Journal20111180182521479351
- GustafssonÅLindstedtEElfsmarkLSBuchtALung exposure of titanium dioxide nanoparticles induces innate immune activation and long-lasting lymphocyte response in the Dark Agouti ratJ Immuno-toxicol201182111121
- KobayashiNNayaMEndohSMaruJYamamotoKNakanishiJComparative pulmonary toxicity study of nano-TiO(2) particles of different sizes and agglomerations in rats: different short- and long-term post-instillation resultsToxicology20092641–211011819666077
- ShengLWangLSangXNano-sized titanium dioxide-induced splenic toxicity: a biological pathway explored using microarray technologyJ Hazard Mater201427818018824968254
- SangXFeiMShengLImmunomodulatory effects in the spleen-injured mice following exposure to titanium dioxide nanoparticlesJ Biomed Mater Res A2014102103562357224243549
- MaLZhaoJWangJThe acute liver injury in mice Caused by nanoanatase TiO2Nanoscale Res Lett20094111275128520628458
- LarsenSTRoursgaardMJensenKANielsenGDNano titanium dioxide particles promote allergic sensitization and lung inflammation in miceBasic Clin Pharmacol Toxicol2010106211411719874288
- de HaarCHassingIBolMBleuminkRPietersRUltrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in miceClin Exp Allergy200636111469147917083358
- MoonCParkHJChoiYHParkEMCastranovaVKangJLPulmonary inflammation after intraperitoneal administration of ultrafine titanium dioxide (TiO2) at rest or in lungs primed with lipopolysaccharideJ Toxicol Environ Health A201073539640920155581
- Adamcakova-DoddAStebounovaLVKimJSToxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation modelsPart Fibre Toxicol2014111524684892
- XiaTHamiltonRFBonnerJCInterlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nano-materials: the NIEHS Nano GO ConsortiumEnviron Health Perspect2013121668369023649538
- WitaspEShvedovaAAKaganVEFadeelBSingle-walled carbon nanotubes impair human macrophage engulfment of apoptotic cell corpsesInhal Toxicol200921Suppl 113113619558245
- GreulichCKittlerSEppleMMuhrGKöllerMStudies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs)Langenbecks Arch Surg2009394349550219280220
- ShinSHYeMKKimHSKangHSThe effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cellsInt Immunopharmacol20077131813181817996693
- CarlsonCHussainSMSchrandAMUnique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen speciesJ Phys Chem B200811243136081361918831567
- StebounovaLVAdamcakova-DoddAKimJSNanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation modelPart Fibre Toxicol201181521266073
- SmuldersSLuytsKBrabantsGToxicity of nanoparticles embedded in paints compared with pristine nanoparticles in miceToxicol Sci2014141113214024924400
- YangEJKimSKimJSChoiIHInflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticlesBiomaterials201233286858686722770526
- ShavandiZGhazanfariTMoghaddamKNIn vitro toxicity of silver nanoparticles on murine peritoneal macrophagesImmunopharmacol Immunotoxicol201133113514020507217
- HutterEBoridySLabrecqueSMicroglial response to gold nanoparticlesACS Nano2010452595260620329742
- YenHJHsuSHTsaiCLCytotoxicity and immunological response of gold and silver nanoparticles of different sizesSmall20095131553156119326357
- SrinivasARaoPJSelvamGGoparajuAMurthyPBReddyPNOxidative stress and inflammatory responses of rat following acute inhalation exposure to iron oxide nanoparticlesHum Exp Toxicol201231111113113122699116
- SettlesMEtzrodtMKosankeKDifferent capacity of monocyte subsets to phagocytose iron-oxide nanoparticlesPLoS One2011610e2519721984904
- HsiaoJKWengTITaiMFCellular behavior change of macrophage after exposure to nanoparticlesJ Nanosci Nanotechnol2009921388139319441531
- HsiaoJKChuHHWangYHMacrophage physiological function after superparamagnetic iron oxide labelingNMR Biomed200821882082918470957
- LiuRZhangXPuYSmall-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivoJ Nanosci Nanotechnol20101085161516921125865
- YangEJChoiIHImmunostimulatory effects of silica nanoparticles in human monocytesImmune Netw20131339410123885223
- WatersKMMasielloLMZangarRCMacrophage responses to silica nanoparticles are highly conserved across particle sizesToxicol Sci2009107255356919073995
- SunQTanDZeYPulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in miceJ Hazard Mater2012235–2364753
- LiuRYinLHPuYPThe immune toxicity of titanium dioxide on primary pulmonary alveolar macrophages relies on their surface area and crystal structureJ Nanosci Nanotechnol201010128491849921121358
- ScherbartAMLangerJBushmelevAContrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanismsPart Fibre Toxicol201183121995556
- Lozano-FernándezTBallester-AntxordokiLPérez-TempranoNPotential impact of metal oxide nanoparticles on the immune system: The role of integrins, L-selectin and the chemokine receptor CXCR4Nanomedicine20141061301131024650882
- HedenborgMTitanium dioxide induced chemiluminescence of human polymorphonuclear leukocytesInt Arch Occup Environ Health1988611–2163198275
- SahuDKannanGMVijayaraghavanRSize-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytesJ Toxicol Environ Health A201477417719124555677
- RoyRTripathiADasMDwivediPDCytotoxicity and uptake of zinc oxide nanoparticles leading to enhanced inflammatory cytokines levels in murine macrophages: comparison with bulk zinc oxideJ Biomed Nanotechnol20117111011121485828
- FeltisBNOKeefeSJHarfordAJPivaTJTurneyTWWrightPFIndependent cytotoxic and inflammatory responses to zinc oxide nanoparticles in human monocytes and macrophagesNanotoxicology20126775776522087559
- WilhelmiVFischerUWeighardtHZinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent mannerPLoS One201386e6570423755271

