Abstract
Tanshinones, the major lipid-soluble pharmacological constituents of the Chinese medicinal herb Tanshen (Salvia miltiorrhiza), have attracted growing scientific attention because of the prospective biomedical applications of these compounds. Numerous pharmacological activities, including anti-inflammatory, anticancer, and cardio-cerebrovascular protection activities, are exhibited by the three primary bioactive constituents among the tanshinones, ie, tanshinone I (TNI), tanshinone IIA (TNIIA), and cryptotanshinone (CPT). However, due to their poor solubility and low dissolution rate, the clinical applications of TNI, TNIIA, and CPT are limited. To solve these problems, many studies have focused on loading tanshinones into liposomes, nanoparticles, microemulsions, cyclodextrin inclusions, solid dispersions, and so on. In this review, we aim to offer an updated summary of the biological activities and drug delivery systems of tanshinones to provide a reference for these constituents in clinical applications.
Introduction
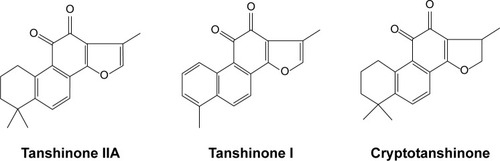
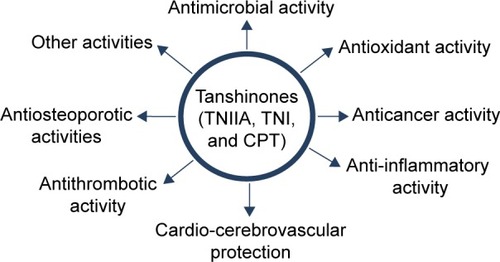
Tanshen, also known as Salvia miltiorrhiza, red sage, Chinese sage, or danshen, is widely used in traditional Chinese medicine for the treatment of cerebrovascular and cardiovascular diseases, as well as inflammatory diseases.Citation1 Tanshinones are the major liposoluble constituents of Tanshen and consist of abietane type-diterpene quinone pigments.Citation2 The primary bioactive constituents among the tanshinones are tanshinone I (TNI), tanshinone IIA (TNIIA), and cryptotanshinone (CPT) (), which have attracted special attention for possessing various pharmacological effects, including anti-inflammatory, anticancer, and cardio-cerebrovascular protection activities.Citation3,Citation4 TNIIA, which has been studied from the 1930s, has potential effects against diabetes, neurodegenerative diseases, and cardiac hypertrophy.Citation5 In addition to the anti-inflammatory and anticancer effects, TNI also enhances the ability to memorize and learn and ameliorates memory impairment.Citation6–Citation8 Recently, a number of studies have been published concerning the modification, biosynthesis, metabolism, pharmacological actions, and therapeutic applications of tanshinones.Citation5,Citation9–Citation15 However, the poor water solubility and low oral bioavailability of tanshinones have limited their clinical applications. There have been no reviews that focused on the drug delivery systems of tanshinones to date. To give summary of current research progress of tanshinones, the organization of this review is as described later. First of all, the review outlines the various pharmacological of tanshinones (), which indicates the high medicinal value of them. Then, the development of drug delivery systems for tanshinones are discussed, including liposomes, solid dispersion, and nanoparticles (NPs), which has been used to solve the poor bioavailability of tanshinones. Finally, we make a conclusion of our personal perspectives on the directions for developing tanshinone.
Biological activities
Cardio-cerebrovascular protection
Tanshinones have been investigated for the treatment of cardio-cerebrovascular diseases, such as myocardial infarction, atherosclerosis, hyperlipidemia, hypertension, and stroke.Citation4,Citation10,Citation16 TNIIA and its derivative, sodium TNIIA sulfonate (STS), were found to significantly reduce the size of a myocardial infarct and improve cardiac function via the activation of the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) signaling pathway in the myocardium.Citation10,Citation17–Citation19 TNIIA also inhibited the formation of atherosclerotic lesions and hyperlipidemia by reducing the oxidation of low-density lipoproteins, cholesterol accumulation in macrophages, platelet aggregation, and monocyte adhesion to the endothelium.Citation10,Citation20–Citation22 In addition, TNIIA and STS attenuated pulmonary hypertension by modulating calcium and potassium channels and inhibited the proliferation and migration of vascular smooth muscle cells by blocking the Akt pathway.Citation23–Citation26 TNIIA and TNI protect the brain from ischemic damage in stroke models by reducing the brain infarct volume and restoring neurological function, which might be correlated with the induced nuclear translocation of transducer of regulated cAMP response element-binding protein (CREB) 1 (TORC1) and upregulated expression of phosphorylated (pCREB), TORC1, and brain-derived neurotrophic factor.Citation27–Citation29 In a clinic study of 100 unstable angina pectoris (UAP) patients, 60 mg STS in combination with 300 mg aspirin can significantly attenuate angina pectoris attacks.Citation30 The clinical trials of S. miltiorrhiza and tanshinones in patients with ischemic conditions have been well summarized in other reviews.Citation1,Citation31
Anticancer activites
Recently, the anticancer activities of tanshinones have been systematically summarized in an informative review.Citation15 TNIIA, TNI, and CPT are potent cytotoxic agents that significantly inhibit the growth and survival of multiple types of cancer cells by inducing cell cycle arrest and apoptosis with IC50 at the micromolar level. The potential mechanisms involved include the upregulation of pro-apoptosis proteins such as p53, Bax, p21, etc, downregulation of antiapoptosis proteins, including Bcl-2, survivin, and c-Myc and activation of caspase proteins to trigger cell apoptosis.Citation15,Citation32 TNIIA was also found to induce autophagic cell death in various cancer cells by activating AMP-activated protein kinase and extracellular signal-regulated kinase (ERK) and inhibiting the mammalian target of rapamycin and 70 kDa ribosomal protein S6 kinase signaling pathways.Citation33 In addition, TNIIA and TNI are able to inhibit the migration, invasion, and metastasis of cancer cells through the alteration of matrix metalloproteinases and/or tissue inhibitor of metalloproteinases.Citation34–Citation38 Notably, TNIIA can also promote cell differentiation in several cancer cell types likely by regulating CCAAT/enhancer binding protein (C/EBP) beta and C/EBP homologous protein 10.Citation39–Citation41 Furthermore, TNIIA, TNI, and CPT exhibited promising anticancer effects with minor side effects in many xenograft animal models.Citation15,Citation36,Citation38,Citation42,Citation43 TNIIA, TNI, and CPT have synergistic anticancer effects with chemotherapeutic drugs such as cisplatin, doxorubicin, 5-flurouracid, and arsenic trioxide.Citation44–Citation48 Moreover, TNIIA, TNI, and CPT can overcome P-glycoprotein (P-gp)-mediated multidrug resistance in cancer by acting as substrates of P-gp and suppressing its pump activity.Citation48–Citation51 The clinical use of TIIA for cancer treatment has been reported in a patient with acute promyelocytic leukemia, who had no response to all-trans retinoic acid (ATRA) (20 mg, three times per day) for 14 days, and they achieved a complete remission after treated with TIIA (30 mg, oral, two times per day) for 12 weeks.Citation52 Cancer-related clinical trials of TIIA and tanshinone-containing traditional Chinese medicine formulas were well presented in this review.Citation53
Anti-inflammatory activities
TNIIA has shown remarkable anti-inflammatory activity by inhibiting the expression of inflammatory mediators, including interleukin (IL)-1 beta, IL-6, and tumor necrosis factor (TNF)-alpha, in an estrogen receptor subtype-dependent manner in murine macrophage RAW264.7 cells pretreated with lipopolysaccharide (LPS).Citation54 Additionally, TNIIA suppresses LPS-induced nuclear factor kappaB (NF-κB) activation through the inhibition of the NF-κB-inducing kinase/IkappaB alpha kinase (NIK/IKKalpha), ERK1/2, p38, and c-Jun N-terminal kinase (JNK) pathways.Citation55 Recently, data from a protein interaction network analysis have suggested that the anti-inflammatory effect of TNIIA may result in part from activating TNF receptor-associated factor (TRAF) 2/3/6 and inhibiting the toll-like receptor (TLR) signaling pathway.Citation56 TNI significantly inhibited the activity of group IIA secretory phospholipase A2 (GIIA) to thereby block prostaglandin E2 (PGE2) formation in LPS-activated macrophages and exhibited in vivo anti-inflammatory activity in rats with adjuvant-induced arthritis and carrageenan-induced paw edema.Citation57 TNI and CPT also significantly inhibit IL-12 production in LPS-activated macrophages and interferon-γ production in lymph node cells.Citation58
Other pharmacological activities
Tanshinones are known as natural antioxidants by forming a quinone adduct of the lipid radical to form a stabilized radical.Citation2 TNIIA is able to prevent the DNA damage in liver cells resulting from lipid peroxidation by scavenging lipid free radicals and breaking the peroxidation chain reactions.Citation59 Preincubation with TNIIA significantly decreases the H2O2-induced death of ECV-304 human umbilical vein endothelial cells and J774 macrophages.Citation60,Citation61 In addition, TNI activated the NF-E2 p45-related factor 2 (Nrf2)-dependent antioxidant response by preventing ubiquitination-mediated Nrf2 degradation and protected against As (III)-induced lung damage in vitro and in vivo.Citation62 TNI inhibited peroxynitrite-induced DNA damage by diminishing the 5,5-dimethyl-1-pyrroline N-oxide-hydroxyl (DMPO-OH) radical adduct signal from peroxynitrite.Citation63 Tanshinones also have potent antiosteoporotic activities by targeting different pathways in the bone remodeling cycle.Citation64 It has been demonstrated that TNIIA, TNI, and CPT have obvious inhibitory effects on osteoclast differentiation.Citation65 It was found in further experiments that TNIIA inhibits osteoclast differentiation through blocking Akt, ERK, and NF-κB activation and downregulating the expression of c-Fos and nuclear factor of activated T-cells (NFATC1), cytoplasmic, calcineurin-dependent 1, which all are induced by receptor activator of NF-κB ligand.Citation66,Citation67 Furthermore, TNIIA also suppressed bone resorption of differentiated osteoclasts.Citation67 Notably, TNIIA and TNI have insulin-sensitizing effects and enhance the ability of insulin to promote the tyrosine phosphorylation of the insulin receptor and the activation of the downstream ERK, Akt, and glycogen synthase kinase-3 beta (GSK-3β) kinases in Chinese hamster ovary cells and 3T3-L1 adipocytes.Citation68 It has been shown that CPT has potent antibacterial activity against a wide range of Gram-positive bacteria in a reactive oxygen species (ROS)-dependent mannerCitation69 and has the potential to treat Alzheimer’s disease as an inhibitor of acetylcholinesterase.Citation70 Particularly, major tanshinones such as TNIIA, TNI, and CPT are able to competitively inhibit the metabolism of CYP1A2 substrates,Citation71 and TNIIA and CPT are efficacious pregnane X receptor (PXR) agonists that induce CYP3A4 expression, suggesting that attention should be paid when tanshinones are used in combination with drugs metabolized by CYP1A2 and CYP3A4.Citation72 Recently, TNIIA, TNI, and CPT were found to be specific and selective inhibitors for the SARS-CoV cysteine proteases 3CLpro and PLpro, but did not exert significant inhibitory effects against other proteases, including chymotrypsin, papain, and HIV protease.Citation73 Moreover, TNIIA could inhibit tat-induced HIV-1 transactivation through redox-regulated AMPK/NAMPT pathway.Citation74
Drug delivery systems
Despite the multiple pharmacological effects of TNIIA, the poor water-solubility and low dissolution rate of this compound result in low oral bioavailability and have hampered the clinical application of TNIIA.Citation75 To tackle this problem, various methods have been developed, including the preparation of STS, the water-soluble derivative of TNIIA, the preparation of TNIIA in discoidal and spherical high-density lipoproteins, and the development of drug delivery systems for TNIIA.Citation5 To date, many studies have focused on loading TNIIA into liposomes or NPs, microemulsions, cyclodextrin (CD) inclusion, and solid dispersions.Citation76–Citation80 In addition, preparations of TNI and CPT with better intestinal absorption have been studied in recent years due to the unsatisfactory clinical effects of these agents, which have been attributed to their low levels of biological utilization;Citation81,Citation82 these preparations include solid dispersion and solid lipid NPs.Citation81,Citation83 shows the various drug delivery systems of tanshinones.
Table 1 Various drug delivery systems of tanshinones
Liposomes
Liposomes are closed spherical vesicles composed of a lipid bilayer and have attractive properties, including biocompatibility, biodegradability, low clearance rates, and low toxicity.Citation84 To establish a highly efficient delivery system with multiple functions, more than one therapeutic drug can be entrapped within the aqueous liposomal interior or embedded into the liposomal membrane, depending on the characteristics of the drug and the process of encapsulation.Citation85 Because of these benefits, liposomes were used to encapsulate glycyrrhetinic acid (GA), salvianolic acid B (SB), and TNIIA, enhancing the bioavailability and water solubility of the compounds, which then exerted synergistic effects on the inhibition of hepatic stellate cell (HSC) proliferation.Citation76 In this study, TSIIA and GA, the hydrophobic constituents, were incorporated into phospholipid bilayers by employing the film hydration method with probe sonication, and the pH-gradient method was then used to load the hydrophilic constituent SB, which finally yielded the GA-TNIIA-SB compound liposomes (GTS-lip) with cholesterol and soybean phospholipids. Eventually, the encapsulation efficiency of these drugs was >80% with little difference among the individual compounds. This study demonstrated that GTS-lip could suppress the proliferation of HSC with a sustained-release effect more effectively than a mixed solution of GA, TNIIA, and SB, which may promote the clinical application and therapeutic activities of tanshinones.Citation76 The outstanding advantage of the liposomal drug delivery system is the ability to co-encapsulate different drugs to exert synergistic effects in a sustained-release manner, which is more efficient than treatment with unencapsulated drugs. However, a challenge still exists in controlling the ratio of different drugs to produce an improved healing efficacy.
Emulsions
Emulsions are a class of formulation consisting of two immiscible phases stabilized by a surfactantCitation86 and are widely used to enhance the stability of active constituents, thereby maintaining their effectiveness.Citation87 In previous studies, lipid emulsions,Citation88 microemulsions,Citation89 and nanoemulsionsCitation90 of soybean phospholipid, pluronic F68 (F68), glycerol, oleic acid, lecithin, and so on were used to produce TNIIA formulations with long-term stability and obvious anticancer activity.
However, nanoemulsions have proved to have higher bioaccessibility, stability, and optical clarity than conventional emulsions;Citation91,Citation92 nanoemulsions are similar to microemulsions that are monodispersed spherical droplets with thermodynamic stability as well as excellent solubility properties.Citation93,Citation94 In recent years, a series of studies have been reported regarding the preparation processes, quality control, and evaluation profiles of tanshinone microemulsions.Citation89,Citation90,Citation95–Citation97 TNIIA was encapsulated into a microemulsion, consisting of F68, phospholipid, ethyl oleate, and glycerol, which had an antitumor effect on hepatoma H22 cells and mice.Citation89 Moreover, another TNIIA microemulsion with a mean droplet size of 32.25±6.59 nm was prepared to improve the bioavailability in rat small intestine. The results of the absorption in small intestine of TNIIA microemulsion showed that TNIIA microemulsion could improve the absorption of TNIIA in rat small intestine with the influence of the water-phase ratio (TNIIA microemulsion) to the absorption coefficient.Citation97 Additionally, TNIIA nanoemulsions were formed with an average particle size of 95.6 nm and an excellent entrapment efficiency of 99.3%, which showed potent cytotoxicity with 103.4-fold greater than TNIIA alone against T24 human bladder cancer cells in a time- and dose-dependent manner.Citation90 It was also reported that a tanshinone microemulsion possessed cytotoxic effects on the human leukemia cell line K562/ADM,Citation95 and a method of establishing the high performance liquid chromatography fingerprints of tanshinone microemulsions has been developed to control the quality.Citation96
Although TNIIA emulsions have excellent long-term stability and anticancer activity, it is not convenient enough to prepare the emulsions with more than four types of materials, which may hinder the application of TNIIA emulsions with unsatisfactory bioavailability.
CD inclusion
CD and its derivatives, including α-CD, β-CD, γ-CD, and 2-hydroxypropyl-beta-cyclodextrin (HP-β-CD), have a cage-like supramolecular structure and the ability to form inclusion complexes with various molecules; these complexes have been used as drug carriers in a number of applications, such as nasal administration, oral drug delivery, and dermal drug delivery, to enhance the stability, solubility, and bioavailability of the drugs.Citation98 Hence, the inclusion complexes of TNIIA or TNI and CDs were obtained via coprecipitation and lyophilization to enhance the level of biological utilization, and the results proved that HP-β-CD had a greater stability than β-CD.Citation99 Furthermore, the transport mechanism of TNIIA-HP-β-CD was found by the recirculation intestinal perfusion techniqueCitation100 to be passive transport with no particular absorption site for TNIIA in vivo, and the permeability rate of TNIIA-HP-β-CD through the intestinal epithelial membrane was seven times higher than that of free TNIIA, indicating that the TNIIA-HP-β-CD complex enhanced the gastrointestinal tract absorption of TNIIA.Citation77 In addition, inclusion complexes of CPT with HP-β-CD (inclusion ratio 1:1) were prepared by the wet grinding method, which significantly improved the dissolution of CPT.Citation101
Solid dispersions
The production of solid dispersions is a technique for improving the dissolution rate and bioavailability of poorly water-soluble drugs by dispersing the drugs into solid-state hydrophilic carriers with an increased surface area.Citation102 Therefore, solid dispersions of TNIIA, TNI, and CPT have been applied to improve the dissolution, stability, and bio-availability of these tanshinones. There are various types of carriers being used to prepare solid dispersions of TNIIA via the solvent technique or spray-drying method, such as combinations of polyvinylpyrrolidone (PVP)-K30 and F68,Citation103 nano-silica and F68,Citation104 poloxamer 407 or povidone K-30,Citation81 porous silica,Citation105 copovidone (PVP-S630),Citation106 low molecular weight chitosan (LMC),Citation17 nano-CaCO3, and F68.Citation80 Among TNIIA-poloxamer407, TNIIA-HP-β-CD, and TNIIA-PVP-K30, the best dissolution rate of TNIIA solid dispersions was TNIIA-poloxamer407.Citation81 In addition, TNIIA-PVP-S630 had better dissolution rates than TNIIA-silica NPs and TNIIA-nano-silica-F68.Citation104–Citation106 Moreover, TNIIA-PVP-S630 at a proportion of 1:10 improved the solubility up to 100% at 30 minutes and could be stored for 3 months with no change in the dissolution or components and little moisture absorption, which was better than TNIIA-poloxamer407.Citation106 Moreover, LMC and TNIIA (weight ratio 9:1) were used to prepare a solid dispersion that increased the dissolution rate by 368.2% compared to free TNIIA, which could enhance the absorption rate and oral bioavailability.Citation17 It was also reported that ternary solid dispersions that were composed of F68, nano-CaCO3, and TNIIA demonstrated high dissolution rates and stability, representing a promising method to prepare solid dispersions.Citation80 Despite the benefits of solid dispersions mentioned earlier, it is necessary to obtain long-lived circulation within the human body and greater efficacy against cancer.
Nanoparticles
NPs, loosely defined as particles with 1–100 nm diameters,Citation107 can enhance the solubility and dissolution rate of drugs by decreasing the particle size and enlarging the surface area.Citation105 Therefore, a number of polymeric carriers have been used to prepare NPs of TNIIA with better bioavailability, such as poly(D,L-lactic-co-glycolic acid) (PLGA),Citation108,Citation109 polylactic acid (PLA),Citation110 F68,Citation111 and cationic bovine serum albumin (CBSA)-conjugated PEGylated PLA.Citation78 NPs of TNIIA-loaded PLGA (TNIIA-PLGA-NPs) had good biocompatibility and high absorption with preventive effects in the neointimal hyperplasia of the rabbit carotid artery after intimal denudation.Citation109 Furthermore, it is more efficient to prepare TNIIA-PLGA-NPs by the nanoprecipitation method instead of the emulsion evaporation method, resulting in particles with average diameters of 225 nm, an entrapment efficiency of 95.49%, a drug loading of 2.03%, and drug recovery rate of 38.42%.Citation108 Another type of material, PLA, was used to prepare novel NPs of TNIIA (TNIIA-PLA-NPs) by a single oil-in-water emulsion/solvent evaporation method, and the resulting particles had a significant dose- and time-dependent growth-inhibitory effect on hepatomas with sustained release in vitro and in vivo, which had 2.69-fold survival time of mice bearing hepatoma tumor than TNIIA.Citation110 Despite the enhanced bioavailability and water solubility of TNIIA with the NPs mentioned earlier, it is more efficient to modify the NPs to obtain an extended circulation within the human body. As a result, TNIIA NPs were modified with PEG (polyethylene glycol)Citation78 and F68Citation111 to enable an extended circulation within the human body with potent pharmacological activities. TNIIA PEGylated nanoparticles (PEG-TNIIA-NPs) were prepared using the double emulsion/solvent evaporation technique and then conjugated with CBSA through the maleimide function, finally forming CBSA-PEG-TNIIA-NPs with a long circulating time of 7.89 hours and better uptake efficiency in the brain than a TNIIA solution. The study also indicated that CBSA-PEG-TNIIA-NPs, which exhibited significant neuroprotective effects in ischemic stroke, could suppress microglial activation, inhibit neutrophil infiltration, and downregulate multiple pro-inflammatory cytokines via the regulation of p38 MAPK and PPAR signaling pathways.Citation78 Moreover, solid lipid NPs of CPT were prepared by an ultrasonic and high-pressure homogenization method to improve the low bioavailability with soy lecithin and Tween 80 as emulsifiers.Citation83 Although TNIIA NPs have greater bioavailability as well as water solubility and a long circulation time in the human body with excellent pharmacological effects, it would be promising to prepare polymeric NPs of TNIIA with the capability of targeted delivery, which has attracted much attention due to the enhanced cell uptake of drugs with minimal systemic side effects.Citation112
Others
In addition to the formulations of TNIIA mentioned earlier, pelletsCitation113 and micellesCitation114 have also been used to enhance the solubility and stability of TNIIA. Several materials were applied to the TNIIA pellets with sustained release, including hydroxypropyl methylcellulose (HPMC) and surelease,Citation113 PVP and F68,Citation115 polyvinyl acetate (PVAc), and poly(vinyl alcohol)-poly(ethylene glycol) (PVA-PEG) graft copolymer.Citation116 In addition, D-alpha-tocopheryl polyethylene glycol succinate-graft-PLGA (TPGS-g-PLGA) copolymer and F68 were used to form mixed micelles with improved bioavailability and prolonged circulation times against hepatocellular carcinoma.Citation114 However, neither of these formulations had a targeted delivery system for the improved cellular uptake of TNIIA.
Conclusion
To overcome the low bioavailability of tanshinones, many methods have been used to improve the solubility and dissolution rates as well as prolong the circulating times of these compounds; in particular, solid dispersion and NPs have shown better results than other formulations. In recent research, most of the formulations have had no tissue specificity. To enhance the pharmacological effects of TNIIA, it is necessary to design a type of drug preparation with components that actively target tumor tissue or other parts of the body. Despite the high dissolution rates of tanshinones obtained by these novel techniques, there has been little development in the clinical application of these formulations due to the complex process to produce the formulations and the lack of further research of TNIIA formulations on clinical trials, which is the ultimate goal in pharmaceutical research. In addition, it is necessary to clarify the light sensitivity of TNIIA in future research, and the development of TNIIA preparations may in part be driven by this lack of information. In conclusion, it is expected that with more and more effort for developing drug delivery systems of tanshinones and their clinical application, formulations of tanshinones can make a breakthrough and better therapy in the future.
Acknowledgments
This study was supported by the Macau Science and Technology Development Fund (102/2012/A3), the Research Fund of the University of Macau (MYRG2014-00033-ICMS-QRCM, MYRG2014-00051-ICMS-QRCM, MRG005/CMW/2014/ICMS), the National Natural Science Foundation of China (grant numbers 31271444 and 81201726), and the Guangdong Natural Science Funds for Distinguished Young Scholar (grant number 2014A030306001).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhouLZuoZChowMSDanshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical useJ Clin Pharmacol200545121345135916291709
- WangXHMorris-NatschkeSLLeeKHNew developments in the chemistry and biology of the bioactive constituents of tanshenMed Res Rev200727113314816888751
- TseAKChowKYCaoHHThe herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligandJ Biol Chem201328841299232993323986445
- ChengTOCardiovascular effects of DanshenInt J Cardiol2007121192217363091
- XuSLiuPTanshinone II-A: new perspectives for old remediesExpert Opin Ther Pat201323214915323231009
- ParkJHParkOkChoJHAnti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampusNeurochem Res20143971300131224760430
- SuCCChenGWLinJGGrowth inhibition and apoptosis induction by tanshinone I in human colon cancer colo 205 cellsInt J Mol Med200822561361818949381
- KimDHKimSJeonSJTanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathwayBr J Pharmacol200915841131114219775283
- TianXHWuJHTanshinone derivatives: a patent review (January 2006–September 2012)Expert Opin Ther Pat2013231192923094864
- GaoSLiuZLiHLittlePJLiuPXuSCardiovascular actions and therapeutic potential of tanshinone IIAAtherosclerosis2012220131021774934
- WangJWWuJYTanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue culturesAppl Microbiol Biotechnol201088243744920694462
- WuWYWangYPPharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active componentsActa Pharmacol Sin20123391119113022941285
- ZhouXChanKYeungJHHerb-drug interactions with Danshen (Salvia miltiorrhiza): a review on the role of cytochrome P450 enzymesDrug Metabol Drug Interact201227191822718621
- MaPLiuJZhangCLiangZRegulation of water-soluble phenolic acid biosynthesis in Salvia miltiorrhiza BungeAppl Biochem Biotechnol201317061253126223673485
- WuWYYanHWangXBSodium tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinasePLoS One201494e9495724739942
- ShangQXuHHuangLTanshinone IIA: a promising natural cardio-protective agentEvid Based Complement Alternat Med2012201271645922454677
- LiuQYZhangZHJinXJiangYRJiaXBEnhanced dissolution and oral bioavailability of tanshinone IIA base by solid dispersion system with low-molecular-weight chitosanJ Pharm Pharmacol201365683984623647677
- ZhangYWeiLSunDTanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic ratsDiabetes Obes Metab201012431632220380652
- YuanXJingSWuLChenLFangJPharmacological postconditioning with tanshinone IIA attenuates myocardial ischemia-reperfusion injury in rats by activating the phosphatidylinositol 3-kinase pathwayExp Ther Med20148397397725120632
- LiuZWangJHuangETanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1J Lipid Res201455220121324302760
- XuSLiuZHuangYTanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-kappaB activationTrans Res20121602114124
- ChenWTangFXieBChenSHuangHLiuPAmelioration of atherosclerosis by tanshinone IIA in hyperlipidemic rabbits through attenuation of oxidative stressEur J Pharmacol20126742–335936422088276
- JinUHSuhSJChangHWTanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathwayJ Cell Biochem20081041152617979138
- WangJDongMQLiuMLTanshinone IIA modulates pulmonary vascular response to agonist and hypoxia primarily via inhibiting Ca2+ influx and release in normal and hypoxic pulmonary hypertension ratsEur J Pharmacol20106401–312913820460121
- LuoYXuDQDongHYTanshinone IIA inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via Akt/Skp2/p27-associated pathwayPLoS One201382e5677423437233
- ZhengLLiuMWeiMTanshinone IIA attenuates hypoxic pulmonary hypertension via modulating K currentsRespir Physiol Neurobiol201420512012825305099
- LiuLZhangXWangLThe neuroprotective effects of tanshinone IIA are associated with induced nuclear translocation of TORC1 and upregulated expression of TORC1, pCREB and BDNF in the acute stage of ischemic strokeBrain Res Bull2010823–422823320417695
- LeeJCParkJHParkOKNeuroprotective effects of tanshinone I from Danshen extract in a mouse model of hypoxia-ischemiaAnat Cell Biol201346318319024179693
- LamBYLoACSunXLuoHWChungSKSucherNJNeuroprotective effects of tanshinones in transient focal cerebral ischemia in micePhytomedicine200310428629112809358
- YanFFLiuYFLiuYZhaoYXSulfotanshinone sodium injection could decrease fibrinogen level and improve clinical outcomes in patients with unstable angina pectorisInt J Cardiol2009135225425518790543
- AdamsJDWangRYangJLienEJPreclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditionsChin Med20061317302964
- YuanSLWangXJWeiYQ丹參酮抗腫瘤作用及其機理的研究 [Anticancer effect of tanshinone and its mechanisms]Ai Zheng2003221213631366 Chinese14693071
- YunSMJungJHJeongSJSohnEJKimBKimSHTanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cellsPhytother Res201428345846423813779
- ZhangYWeiRXZhuXBCaiLJinWHuHTanshinone IIA induces apoptosis and inhibits the proliferation, migration, and invasion of the osteosarcoma MG-63 cell line in vitroAnticancer Drugs201223221221922126901
- TsaiMYYangRCWuHTPangJHHuangSTAnti-angiogenic effect of tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cellsCancer Lett2011310219820621788102
- YuxianXFengTRenLZhengcaiLTanshinone II-A inhibits invasion and metastasis of human hepatocellular carcinoma cells in vitro and in vivoTumori200995678979520210245
- ShanYFShenXXieYKInhibitory effects of tanshinone II-A on invasion and metastasis of human colon carcinoma cellsActa Pharmacol Sin200930111537154219820721
- NizamutdinovaITLeeGWLeeJSTanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion moleculesCarcinogenesis200829101885189218586687
- ZhangKLiJMengWXingHYangYC/EBPbeta and CHOP participate in tanshinone IIA-induced differentiation and apoptosis of acute promyelocytic leukemia cells in vitroInt J Hematol201092457157820981511
- WangJWangXJiangSGrowth inhibition and induction of apoptosis and differentiation of tanshinone IIA in human glioma cellsJ Neurooncol2007821112116955220
- LiangYYangYYuanS丹參酮IIA誘導原代培養人急性早幼粒細胞白血病細胞分化 [Terminal differentiation of human acute promyelocytic leukemia (APL) cells induced by tanshinone II A in primary culture]Hua Xi Yi Ke Da Xue Xue Bao2000312207210 Chinese12515138
- ChiuSCHuangSYChenSPSuCCChiuTLPangCYTanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivoProstate Cancer Prostatic Dis201316431532224042854
- GongYLiYLuYBioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in miceInt J Cancer201112951042105220848589
- HouLLXuQJHuGQXieSQ丹參酮IIA增強順鉑抗前列腺癌作用及分子機制研究 [Synergistic antitumor effects of tanshinone II A in combination with cisplatin via apoptosis in the prostate cancer cells]Yao Xue Xue Bao2013485675679 Chinese23888689
- ParkIJKimMJParkOJCryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulationCancer Lett20102981889820638780
- ZhangGYangYMMengWTZhouJ丹參酮IIA與三氧化二砷協同誘導急性早幼粒白血病細胞凋亡的研究 [Apoptosis of NB4 cells induced by tanshinone II A combined with arsenic trioxide]Sichuan Da Xue Xue Bao Yi Xue Ban20104115761 Chinese20369471
- KanSCheungWMZhouYHoWSEnhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2 human hepatoma cellsPlanta Med2014801707624414309
- SuCCTanshinone IIA potentiates the efficacy of 5-FU in Colo205 colon cancer cells in vivo through downregulation of P-gp and LC3-IIExp Ther Med20123355555922969929
- FangZYLinRYuanBXYangGDLiuYZhangHTanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbitJ Ethnopharmacol2008115221722217997063
- ChenXZhouZWXueCCLiXXZhouSFRole of P-glycoprotein in restricting the brain penetration of tanshinone IIA, a major active constituent from the root of Salvia miltiorrhiza Bunge, across the blood-brain barrierXenobiotica200737663567817614009
- HuTToKKWangLReversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhizaPhytomedicine201421111264127225172788
- YangYMLiuT丹參酮IIA治療耐全反式維甲酸的急性早幼粒細胞白血病1例報道 [Complete remission of acute promyelocytic leukemia resisting all-trans retinoic acid of one case treated by tanshinone II A]Sichuan Da Xue Xue Bao Yi Xue Ban2006376965967 Chinese17236602
- ZhangYJiangPYeMKimSHJiangCLuJTanshinones: sources, pharmacokinetics and anti-cancer activitiesInt J Mol Sci20121310136211366623202971
- FanGWGaoXMWangHThe anti-inflammatory activities of tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOSJ Steroid Biochem Mol Biol20091133–527528019429433
- JangSIKimHJKimYJJeongSIYouYOTanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathwaysEur J Pharmacol20065421–31716797002
- ZhengSRenZZhangYQiaoYAnti-inflammatory mechanism research of tanshinone II A by module-based network analysisBiomed Mater Eng20142463815382425227098
- KimSYMoonTCChangHWSonKHKangSSKimHPEffects of tanshinone I isolated from Salvia miltiorrhiza bunge on arachidonic acid metabolism and in vivo inflammatory responsesPhytother Res200216761662012410540
- KangBYChungSWKimSHRyuSYKimTSInhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhizaImmunopharmacology200049335536110996033
- CaoEHLiuXQWangJJXuNFEffect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cellsFree Radic Biol Med19962068018068728027
- LinRWangWRLiuJTYangGDHanCJProtective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanismJ Ethnopharmacol2006108221722216797899
- LiYIElmerGLeboeufRCTanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidaseLife Sci20088315–1655756218762198
- TaoSZhengYLauATanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivoAntioxid Redox Signal201319141647166123394605
- ZhouSChenWSuHZhengXProtective properties of tanshinone I against oxidative DNA damage and cytotoxicityFood Chem Toxicol20136240741224021569
- GuoYLiYXueLSalvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugsJ Ethnopharmacol201415531401141625109459
- LeeSYChoiDYWooERInhibition of osteoclast differentiation by tanshinones from the root of Salvia miltiorrhiza bungeArch Pharm Res200528890991316178416
- KwakHBYangDHaHTanshinone IIA inhibits osteoclast differentiation through down-regulation of c-Fos and NFATc1Exp Mol Med200638325626416819284
- KimHHKimJHKwakHBInhibition of osteoclast differentiation and bone resorption by tanshinone IIA isolated from Salvia miltiorrhiza BungeBiochem Pharmacol20046791647165615081864
- JungSHSeolHJJeonSJSonKHLeeJRInsulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza BungePhytomedicine200916432733519200697
- LeeDSLeeSHNohJGHongSDAntibacterial activities of cryptotanshinone and dihydrotanshinone I from a medicinal herb, Salvia miltiorrhiza BungeBiosci Biotechnol Biochem199963122236223910664860
- RenYHoughtonPJHiderRCHowesMJNovel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorrhizaPlanta Med200470320120415114495
- WangXLeeWYOrPMYeungJHEffects of major tanshinones isolated from Danshen (Salvia miltiorrhiza) on rat CYP1A2 expression and metabolism of model CYP1A2 probe substratesPhytomedicine200916871272519403289
- YuCYeSSunHPXR-mediated transcriptional activation of CYP3A4 by cryptotanshinone and tanshinone IIAChem Biol Interact20091771586418805405
- ParkJYKimJHKimYMTanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteasesBioorg Med Chem201220195928593522884354
- ZhangHSChenXYWuTCZhangFJTanshinone II A inhibits tat-induced HIV-1 transactivation through redox-regulated AMPK/Nampt pathwayJ Cell Physiol201422991193120124414799
- YuXYLinSGZhouZWRole of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza BungeCurr Drug Metab20078432534017504222
- FanLCTengHWShiauCWSHP-1 is a target of regorafenib in colorectal cancerOncotarget20145156243625125071018
- WangLLaiYLiCJiangXStudy on the intestinal absorption profiles of tanshinone IIA and its inclusion complex with cyclodextrin in ratsPDA J Pharm Sci Technol200963539040020158045
- LiuXAnCJinPLiuXWangLProtective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemiaBiomaterials201334381783023111336
- MaHFanQYuJXinJZhangCAnticancer activities of tanshinone microemulsion against hepatocellular carcinoma in vitro and in vivoMol Med Rep201371596423064251
- YanHMZhangZHJiangYRDingDMSunEJiaXBAn attempt to stabilize tanshinone IIA solid dispersion by the use of ternary systems with nano-CaCO3 and poloxamer 188Pharmacogn Mag201410suppl 2S311S31724991109
- YuHSubediRKNepalPRKimYGChoiHKEnhancement of solubility and dissolution rate of cryptotanshinone, tanshinone I and tanshinone IIA extracted from Salvia miltiorrhizaArch Pharm Res20123581457146422941489
- ZhangJHuangMGuanSA mechanistic study of the intestinal absorption of cryptotanshinone, the major active constituent of Salvia miltiorrhizaJ Pharmacol Exp Ther200631731285129416497784
- HuLXingQMengJShangCPreparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticlesAAPS Pharm Sci Tech2010112582587
- MalamYLoizidouMSeifalianAMLiposomes and nanoparticles: nanosized vehicles for drug delivery in cancerTrends Pharmacol Sci2009301159259919837467
- ElbayoumiTATorchilinVPCurrent trends in liposome researchMethods Mol Biol201060512720072870
- IyerRReview of surfactant evaluation methods and perturbations of components in phases to predict onset of emulsion instabilityParticul Sci Technol2008264306317
- MadeneAJacquotMScherJDesobrySFlavour encapsulation and controlled release – a reviewInt J Food Sci Technol2006411121
- ChuTZhangQLiHDevelopment of intravenous lipid emulsion of tanshinone IIA and evaluation of its anti-hepatoma activity in vitroInt J Pharm20124241–2768822226873
- MaHFanQYuJXinJZhangCNovel microemulsion of tanshinone IIA, isolated from Salvia miltiorrhiza Bunge, exerts anticancer activity through inducing apoptosis in hepatoma cellsAm J Chin Med201341119721023336516
- ChangLCWuCLLiuCWChuoWHLiPCTsaiTRPreparation, characterization and cytotoxicity evaluation of tanshinone IIA nanoemulsionsJ Biomed Nanotechnol20117455856721870460
- McClementsDJNanoemulsion-based oral delivery systems for lipophilic bioactive components: nutraceuticals and pharmaceuticalsTher Deliv20134784185723883127
- LiangZMaoSJYinZNJinHLiHChuT丹參酮IIA靜脈乳劑的制備與質量評價 [Preparation and quality evaluation of intravenous tanshinone II (A) emulsion]Zhongguo Zhong Yao Za Zhi2008331112491252 Chinese18831197
- SolankiJNMurthyZVPControlled size silver nanoparticles synthesis with water-in-oil microemulsion method: a topical reviewInd Eng Chem Res201150221231112323
- ShakeelFRamadanWFaisalMSTransdermal and topical delivery of anti-inflammatory agents using nanoemulsion/microemulsion: an updated reviewCurr Nanosci201062184198
- FanQFanGJYangPMZhaoJY丹參酮微乳逆轉腫瘤細胞多藥耐藥的研究 [Effect of tanshinone microemulsion on reversing MDR in human tumor cells]Zhongguo Zhong Yao Za Zhi2004291110791081 Chinese15656144
- LvHYFanQZhangNLiBY丹參酮微乳制劑的HPLC指紋圖譜研究 [Studies on HPLC fingerprints of tanshinone microemulsion]Zhongguo Zhong Yao Za Zhi2004291110471049 Chinese15656134
- LiHLZhangZYMaLLChenXY丹參酮微乳的制備及大鼠在體腸吸收 [Preparation of tanshinone microemulsion and its absorption in rat intestine in situ]Zhongguo Zhong Yao Za Zhi2007321110241027 Chinese17672333
- Laza-KnoerrALGrefRCouvreurPCyclodextrins for drug deliveryJ Drug Target201018964565620497090
- YuexianFJunfenLChuanDPreparation and study on the inclusion complexes of two tanshinone compounds with beta-cyclodextrinSpectrochim Acta A Mol Biomol Spectrosc2005611–213514015556431
- LingWRuiLCHuaJXIn situ intestinal absorption behaviors of tanshinone IIA from its inclusion complex with hydroxypropyl-beta-cyclodextrinBiol Pharm Bull200730101918192217917262
- LuoXXuYHChenBGuLQHuangMLiuPQ羥丙基-β-環糊精對隱丹參酮的增溶作用及其包合物的制備 [Solubilization on cryptotanshinone by hydroxypropyl-beta-cyclodextrin and preparation of their inclusion compound]Zhongguo Zhong Yao Za Zhi2005301713281331 Chinese16323539
- ZhaoXLiuXGanLZhouCMoJPreparation and physicochemical characterizations of tanshinone IIA solid dispersionArch Pharm Res201134694995921725816
- YuanJMaoSShenQHouSHeY載體聯用固體分散技術對丹參酮IIA體外溶出的影響 [Influence of solid dispersion technique combination on dissolution of tanshinone IIA]Zhongguo Zhong Yao Za Zhi2009346685689 Chinese19624004
- JiangYZhangZLuYTangJMaTJiaX丹參酮IIA二元載體固 體分散體的研究 [Study on solid dispersion of binary vector of tanshinone II A]Zhongguo Zhong Yao Za Zhi2012371013831387 Chinese22860446
- JiangYRZhangZHLiuQYHuSYChenXYJiaXBPreparation, characterization, and in vivo evaluation of tanshinone IIA solid dispersions with silica nanoparticlesInt J Nanomedicine201382285229323836971
- JiangYRZhangZHXiaHJJiaXB基于共聚維酮的丹參酮IIA固體分散體的研究 [Study on solid dispersion of copovidone-based tanshinone II(A)]Zhongguo Zhong Yao Za Zhi2013382174178 Chinese23672037
- ChowJCBiswasPEatoughDNanoparticles and the environment – introductionJ Air Waste Manage2005556706707
- GanLCHouSXBiYQWangCGWangXCChenQX2種方法制備丹參酮IIA-PLGA納米粒的比較 [Comparison of two preparation methods applied in tanshinone II(A)-loaded PLGA nanoparticles]Zhongguo Zhong Yao Za Zhi2007327578581 Chinese17583194
- LiangLChenYXiongSZengZSunMZhangH丹參酮IIA納米球對兔頸動脈球囊損傷后內膜增殖的抑制 [The inhibitive effect produced by local perfusion of tanshinone IIA nanoparticle on neointimal hyperplasia of rabbit carotid artery following intimal denudation]Sheng Wu Yi Xue Gong Cheng Xue Za Zhi2007244812816 Chinese17899751
- LiQWangYFengNFanZSunJNanYNovel polymeric nanoparticles containing tanshinone IIA for the treatment of hepatomaJ Drug Target2008161072573219005937
- ZhangWLLiuJPLiuXXChenZQStealth tanshinone IIA-loaded solid lipid nanoparticles: effects of poloxamer 188 coating on in vitro phagocytosis and in vivo pharmacokinetics in ratsYao Xue Xue Bao200944121421142821348419
- PrabhuRHPatravaleVBJoshiMDPolymeric nanoparticles for targeted treatment in oncology: current insightsInt J Nanomedicine2015101001101825678788
- LiangXDZhengYFanTY丹參酮IIA脈沖微丸的制備與體外質 量評價 [Preparation and in vitro evaluation of tanshinone IIA pulsatile release pellets]Beijing Da Xue Xue Bao2010425559564 Chinese20957015
- ZhangJLiYFangXZhouDWangYChenMTPGS-g-PLGA/pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapyInt J Pharm20144761–218519825223472
- LiJLiuPLiuJPZhangWLYangJKFanYQNovel tanshinone II A ternary solid dispersion pellets prepared by a single-step technique: in vitro and in vivo evaluationEur J Pharm Biopharm201280242643222119664
- LiuPLiJLiuJYangJFanYRelease behavior of tanshinone IIA sustained-release pellets based on crack formation theoryJ Pharm Sci201210182811282022610467