Abstract
Abnormal uterine bleeding (AUB) is associated with significant direct medical costs and impacts both society and the quality of life for individual women. Heavy menstrual bleeding, a subset of AUB, also referred to as menorrhagia, is defined as menstrual blood loss greater than 80 mL or the patient’s perception of excessive blood loss. The newest treatment option available is a novel combination oral contraceptive product containing estradiol valerate (E2V) and dienogest (DNG). As with other combination oral contraceptives, E2V/DNG works primarily by preventing ovulation. However, in contrast with other combination oral contraceptives, it is the progestin component of E2V/DNG that is responsible for endometrial stabilization. Use of E2V/DNG for six months has led to significant reductions in heavy menstrual bleeding with an average 65% reduction in mean blood loss. Approximately half of the women with heavy menstrual bleeding who received E2V/DNG for six months demonstrated an 80% reduction in mean blood loss. Additionally, significant improvements in hematologic indicators (ie, ferritin, hemoglobin, and hematocrit) have been shown. Based on its chemical properties, E2V/DNG may have fewer adverse effects on lipid and glucose metabolism and reduced risk of thromboembolic complications compared with other combination oral contraceptives. This has not yet been shown in clinical trials and until then it should be assumed that E2V/DNG has a safety profile similar to other combination oral contraceptives containing 35 μg or less of ethinyl estradiol. E2V/DNG has been compared with another combination oral contraceptive in healthy women without heavy menstrual bleeding and demonstrated improved bleeding patterns. E2V/DNG has not been compared with the levonorgestrel intrauterine device or other treatments for heavy menstrual bleeding. When compared with some other treatment options for AUB, E2V/DNG provides the added advantage of effective contraception.
Introduction
Abnormal uterine bleeding (AUB) is a prevalent complaint among women of reproductive age.Citation1 Nearly one-third of outpatient gynecologist visits and over 400,000 hospitalizations a year can be attributed to AUB.Citation2,Citation3 This is associated with significant direct medical costs and impacts both society and quality of life for individual women, due to loss of work productivity and limitations to leisure activity.Citation4–Citation6 In the US alone, AUB has direct costs of $1 billion and indirect costs of $12 billion according to conservative estimates.Citation7 Women with AUB have a health-related quality of life below the 25th percentile of that for the general population of women within a similar age range.Citation7
AUB in women of reproductive age is characterized by menstrual flow outside of normal volume, duration, regularity, or frequency and can present anytime from adolescence to menopause.Citation8 The diagnosis can be further classified as heavy menstrual bleeding or intermenstrual bleeding.Citation6,Citation8 Heavy menstrual bleeding, also referred to as menorrhagia, is defined as menstrual blood loss greater than 80 mL or the patient’s perception of excessive blood loss.Citation8,Citation9
Given that up to 20% of patients presenting with heavy menstrual bleeding have a bleeding disorder, all patients should be screened for an underlying bleeding disorder with a thorough medical history, and if indicated, further hematologic testing.Citation10–Citation13 Medications such as anticoagulants and nonsteroidal anti-inflammatory drugs (NSAIDs) or herbal remedies, such as ginkgo biloba and ginseng, should also be considered as contributors to heavy menstrual bleeding. Other structural etiologies, including polyps, adenomyosis, leiomyoma, and hyperplasia/malignancy, and nonstructural etiologies, including anovulation and polycystic ovary syndrome, should be considered in the differential diagnosis.
Following appropriate gynecologic evaluation, medical, radiological (ie, uterine artery embolization), or surgical management (ie, hysterectomy) may be indicated. While hysterectomy cures heavy menstrual bleeding, it is associated with operative risk and postoperative morbidity that can be avoided with less invasive treatments when appropriate. Many etiologies of heavy menstrual bleeding can be adequately managed with uterine-preserving pharmacotherapy. Therapies include NSAIDs, antifibrinolytics (ie, tranexamic acid), or hormonal modulators (ie, danazol), including oral progestins, combination estrogen/progestin oral contraceptives, gonadotropin receptor antagonists, and the levonorgestrel-releasing intrauterine device (LNG-IUD).
The newest treatment option available is a novel combination estrogen/progestin oral contraceptive product containing estradiol valerate (E2V) and dienogest (DNG). This quadriphasic product was developed to improve upon prior E2V oral contraceptive product weaknesses. The product received US Food and Drug Administration approval for prevention of pregnancy in 2010 and for the treatment of heavy menstrual bleeding in 2012. This article will review the pharmacology and mechanism of action of E2V/DNG, as well as its efficacy in treating heavy menstrual bleeding and place in therapy.
Literature review
In order to review the mechanism of action, pharmacology, pharmacokinetics, efficacy, safety, and place in therapy of E2V/DNG for heavy menstrual bleeding, a PubMed search restricted to English language articles citing estradiol valerate or dienogest from 1985 to January 2013 was conducted. Additional data sources were identified from the references of selected articles. Available data sources were then evaluated to summarize the mechanism of action, pharmacology, pharmacokinetics, efficacy, safety, and place in therapy of E2V/DNG when used for heavy menstrual bleeding.
Mechanism of action, pharmacology, and pharmacokinetics
As with other combination oral contraceptives, E2V/DNG works primarily by preventing ovulation.Citation14 Combination oral contraceptives provide negative feedback to the hypothalamic-pituitary-ovarian axis. This suppresses ovulation by lowering gonadotropin-releasing hormone pulsation and the pituitary response to gonadotropin-releasing hormone. The estrogen component prevents the release of follicle-stimulating hormone from the pituitary and in turn prevents follicle development. The progestin component prevents the surge of luteinizing hormone from the pituitary gland and subsequent release of the dominant follicle. DNG also increases cervical mucosa thickness, resulting in decreased sperm motility, and prevents proliferation of the endometrium. These additional effects may prevent implantation.
In most combination oral contraceptives, the estrogen component is responsible for providing cycle control and stabilizing the endometrium for an acceptable bleeding pattern. E2V, unlike ethinyl estradiol, did not provide an adequate level of endometrial stability, as evidenced by breakthrough bleeding rates in preliminary studies.Citation15 For this reason, E2V-containing combination oral contraceptives did not reach the marketplace until this novel combination of E2V and DNG was evaluated.Citation15
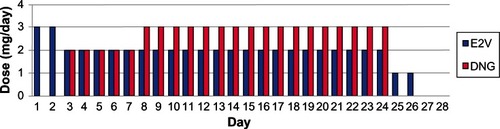
The effects of a 2 mg/day dose of E2V on the hypothalamic-pituitary-ovarian axis and impact on endometrium and ovarian function are expected to be similar to that of a 20 μg/day dose of ethinyl estradiol.Citation16 In this quadriphasic dosing regimen, DNG is increased during week 2 of the cycle while E2V is decreased on day 3 and again on day 25 ().
Figure 1 Daily doses of E2V and DNG in the quadriphasic regimen.
E2V is a novel estrogen with structural similarity to 17β-estradiol and a shorter half-life than ethinyl estradiol.Citation14 Therefore, in theory, it may have fewer adverse effects on lipid and glucose metabolism and a decreased risk for thromboembolic or cardiovascular complications. E2V is a prodrug, and upon ingestion is rapidly metabolized to 17β-estradiol and valeric acid during absorption in the small intestine and in its first pass through the liver.Citation14 A 1 mg dose of E2V is equal to 0.76 mg of 17β-estradiol.Citation16,Citation17
17β-estradiol is then metabolized to mostly inactive metabolites, ie, estrone, estrone glucuronide, or estrone sulfate, by cytochrome P450 3A enzymes in the liver and intestinal mucosa, resulting in about 5% of the ingested dose reaching the circulation intact.Citation14,Citation18 With this low bioavailability, only 3% of ingested E2V becomes available as 17β-estradiol. 17β-estradiol is highly protein-bound, with 38% bound to sex hormone binding globulin and 60% bound to albumin, leaving only 2%–3% unbound.Citation14,Citation18 It is excreted mainly in the urine, with only 10% eliminated in the feces, and has a half-life of approximately 14 hours.Citation14
DNG is a unique progestin due to its structure and pharmacologic properties. It is a C-19 nortestosterone derivative, like norethindrone, but is also similar to progesterone derivatives.Citation19 Compared with other progestins, DNG has an additional carbon-carbon double bond on its steroidal B ring that results in high activity at progesterone receptors.Citation19
DNG is rapidly absorbed, with 90% bioavailability.Citation14,Citation19 It is highly bound (90%) to albumin, but does not bind to sex hormone binding globulin or corticosteroid binding globulin.Citation14,Citation19 About 10% of DNG circulates unbound, which is a higher percentage than for other nortestosterone derivatives.Citation14,Citation19 The lack of DNG binding to sex hormone binding globulin means it does not displace testosterone, resulting in the androgenic effects observed with other progestins.Citation14,Citation19,Citation20 Receptor binding studies indicate that it may have antiandrogenic effects similar to cyproterone acetate, a C-21 progestin derivative, but to a lesser degree.Citation20 DNG is metabolized by hydroxylation and conjugation to mostly inactive metabolites that are excreted.Citation14,Citation19 Unchanged DNG is the dominant form, and steady state is reached with 2–3 days of once-daily dosing. There is no significant accumulation.Citation14,Citation19 The plasma half-life for DNG is about 10 hours, which is similar to that of other nortestosterone derivatives.Citation14,Citation19
Efficacy in treating heavy menstrual bleeding
Studies have specifically evaluated E2V/DNG for its efficacy in heavy and/or prolonged menstrual bleeding.Citation21–Citation23 One such multicenter, randomized, double-blind, placebo-controlled Phase III study examined the efficacy and safety of E2V/DNG for the treatment of confirmed heavy menstrual bleeding, prolonged menstrual bleeding, or heavy and prolonged menstrual bleeding.Citation21 This study was done in 47 centers across the US and Canada between December 2005 and May 2008, and included a screening phase that lasted up to 28 days, a 90-day run-in interval, a 196-day treatment period, and a 30-day follow-up phase. The study included women 18 years and older who had heavy menstrual bleeding (at least two bleeding episodes with a measured menstrual blood loss ≥ 80 mL), prolonged menstrual bleeding (at least two bleeding episodes each lasting ≥ 8 days), frequent menstrual bleeding (more than five bleeding episodes with a minimum of 20 bleeding days overall), or a combination thereof, and were confirmed by electronic diaries and hemoglobin extraction from collected sanitary protection for quantification of blood loss. Participants had to use a barrier method of contraception, use only sanitary protection items (pads and tampons) provided to them, and have a normal endometrial biopsy or, at most, mild simple endometrial hyperplasia during the six months before study entry. Women older than 40 years had to have a follicle-stimulating hormone level less than 40 mIU/mL. Women were excluded if they had an abnormal transvaginal ultrasonogram, any clinically significant abnormal laboratory value, endometrial ablation or dilatation and curettage within two months of the study, or organic pathology (eg, von Willebrand disease, chronic endometritis, adenomyosis, endometriosis) for their heavy and prolonged menstrual bleeding. Women were also excluded if they used symptomatic treatment for AUB (eg, NSAIDs), had a body mass index > 32, smoked > 10 cigarettes per day (if aged > 35 years), or met other criteria consistent with contraindications for the use of combined oral contraceptives. Iron supplementation was allowed if the attending physician deemed it necessary.
Women who met the inclusion criteria with at least one qualifying symptom were randomized (2:1) to E2V/DNG or matching placebo for 196 days (seven cycles). Data from the last 90 days and initial (run-in phase) 90 days were compared. The primary outcome was the proportion of women with a complete response to treatment (ie, a return to complete menstrual normality) defined as achieving all qualifying conditions, ie, no bleeding episodes that lasted more than seven days, not more than four bleeding episodes, no bleeding episodes that involved blood loss ≥ 80 mL, no more than one bleeding episode increase from baseline, no more than 24 days of bleeding, and no increase from baseline for an individual’s total number of bleeding days. Participants were categorized as complete, partial, or nonresponders (missed at least one of the relevant criteria for a complete response), or participants with missing data.
Data from 120 women randomized to E2V/DNG and 70 women to placebo were evaluated. The proportion of complete responders in the intention-to-treat analysis was significantly higher in the E2V/DNG group compared with the placebo group (29.2% and 2.9%, respectively; P < 0.001). For participants with evaluable data (ie, excluding those with missing data), a complete response was seen in 43.8% (35/80) of women taking E2V/DNG and 4.2% (2/48) of women taking placebo (P < 0.001). The proportion of patients experiencing resolution of heavy bleeding (ie, no episodes with ≥80 mL menstrual blood loss) was much higher in the treatment group (56.0%) than in the placebo group (26.7%). An 80% reduction in menstrual blood loss was found for 45% of women in the E2V/DNG group and 5% in the placebo group. The mean adjusted between-treatment difference for menstrual blood loss was a 252 mL reduction (95% CI −339 to −165; P < 0.001) and the mean adjusted between-treatment difference over 90 days for sanitary protection items used was 23 fewer items (95% CI −39 to −8; P < 0.001), favoring E2V/DNG. Statistically significant improvements were also seen in various measures of iron metabolism (ie, ferritin, hemoglobin, and hematocrit).
This study demonstrated the efficacy of E2V/DNG in women with heavy and prolonged menstrual bleeding without organic pathology. Strict inclusion criteria and difficult logistic requirements for adherence (eg, collecting all sanitary products used) may have limited recruitment. Further, strict criteria were applied for participants to be considered a complete responder which may not be a realistic clinical measure.
Another study with very similar methodology was performed at 34 centers in Europe and Australia from February 2006 through May 2008.Citation22 Similar to the previous study, the primary efficacy outcome was the proportion of women who showed a complete response to treatment. The intention-to-treat population comprised of 231 women (149 randomized to E2V/DNG and 82 randomized to placebo). The proportion of complete responders was significantly higher in both the intention-to-treat and evaluable data populations. In the intention-to-treat population, 29.5% of women receiving E2V/DNG versus 1.2% of those receiving placebo had a complete response (P < 0.0001). In women with an evaluable response (ie, those with no missing data), 40.7% receiving E2V/DNG versus 1.6% of those receiving placebo had a complete response (P < 0.0001). More than 90% of women recruited had heavy menstrual bleeding; 63.2% of women treated with E2V/DNG responded with <80 mL menstrual blood loss during each episode compared with 14.5% of women taking placebo. Secondary findings included an 80% reduction in menstrual blood loss for 50% of women in the E2V/DNG group and 0% in the placebo group. The mean adjusted between-treatment difference for menstrual blood loss was 373 mL in favor of E2V/DNG (95% CI 255–490; P < 0.0001) and the mean adjusted between-treatment difference over 90 days for sanitary protection used was 22 fewer items (95% CI −30 to −14; P < 0.0001). Marked improvements were also seen in various measures of iron metabolism (ie, ferritin, hemoglobin, and hematocrit). Investigators and patients alike noted significantly improved bleeding symptoms with E2V/DNG compared with placebo. Given the limitations, which were similar to those of the previous study, this study also demonstrated effectiveness for E2V/DNG in women with heavy and prolonged menstrual bleeding.
In a pooled analysis of the two aforementioned studies, the efficacy of E2V/DNG in the treatment of heavy and prolonged menstrual bleeding without organic pathology was evaluated.Citation23 Women 18 years or older were randomized to E2V/DNG (n = 269) or placebo (n = 152) for 196 days. The efficacy endpoints in this study were changes in menstrual blood loss, number of sanitary protection items used, and iron metabolism parameters.
In the intention-to-treat population, the reduction in menstrual blood loss with E2V/DNG was rapid and sustained over the duration of treatment for seven cycles. After six months of treatment, median menstrual blood loss was reduced by 88% compared with a 24% reduction with placebo. The mean absolute total reduction in 90-day menstrual blood loss from baseline to the 90-day efficacy phase was 414 ± 373 mL with E2V/DNG and 109 ± 300 mL with placebo (P < 0.0001). Overall, an 80% reduction in menstrual blood loss was achieved by 46% of women in the E2V/DNG group and 2% of women in the placebo group. The mean between-treatment difference in amount of sanitary protection used significantly favored E2V/DNG, with 22.1 fewer items (95% CI−30.7 to −13.6; P < 0.0001). Markedly significant improvements in hemoglobin, hematocrit, and ferritin were observed in women receiving E2V/DNG compared with little or no improvement in women receiving placebo. Specifically, the change from baseline for hemoglobin (g/dL) was 0.64 ± 1.1 with E2V/DNG compared with 0.12 ± 1.0 for placebo (P < 0.0001); the change from baseline for hematocrit (%) was 1.48 ± 3.7 with E2V/DNG compared with 0.08 ± 3.1 for placebo (P = 0.0002); and the change from baseline for ferritin (ng/mL) was 7.1 ± 28.8 compared with 1.2 ± 12.2 for placebo(P < 0.05).
This pooled analysis was recently reanalyzed using a definition of treatment success (used in other studies) as menstrual blood loss less than 80 mL together with a reduction in menstrual blood loss of ≥50% from baseline.Citation24 This analysis was restricted to those subjects with heavy menstrual bleeding and included 220 women randomized to E2V/DNG and 135 women randomized to placebo. At study end (cycle 7 or last observation carried forward), a significantly greater proportion of women achieved successful treatment with E2V/DNG (63.6%) compared with placebo (11.9%), with a corresponding difference of 51.8% (95% CI 43.4–60.2; P < 0.001). The proportion of women with menstrual blood loss less than 80 mL was 68.2% using E2V/DNG compared with 15.6% using placebo (corresponding difference 52.6%; 95% CI 44.0–61.3; P < 0.001) and the proportion of women with a menstrual blood loss reduction ≥ 50% from baseline was 70.0% for those using E2V/DNG and 17% for those using placebo (corresponding differences 53.0%; 95% CI 44.2–61.7; P < 0.001).
These pooled analyses demonstrated that E2V/DNG rapidly reduced menstrual blood loss in women with heavy and prolonged menstrual bleeding upon treatment initiation and over treatment duration. These pooled analyses show that the decrease in menstrual blood loss is consistent across a larger and more diverse population. This decrease in menstrual blood loss was associated with use of fewer sanitary protection items and improved iron metabolism parameters. It should be noted that E2V/DNG has not yet been compared with other forms of contraception in women with heavy menstrual bleeding (eg, other combination oral contraceptives, LNG-IUD). E2V/DNG has shown significantly improved bleeding patterns compared with ethinyl estradiol/LNG in healthy women aged 18–50 years without heavy menstrual bleeding.Citation25 While effective for contraception, E2V/DNG has the added advantage of reducing menstrual blood loss and should be considered when assessing treatment options for patients with heavy and prolonged menstrual bleeding without organic pathology.
Safety
The safety profile of E2V/DNG is similar to that of other combination oral contraceptives containing 35 μg or less of ethinyl estradiol. With the theoretically lower adverse effect profile of E2V with regard to lipid and glucose metabolism, there may be a decrease in the rate and severity of thromboembolic complications. There is a paucity of comparative data to prove or disprove these effects. One open-label trial comparing E2V/DNG with ethinyl estradiol/LNG showed slight improvement of high-density lipoprotein in the E2V/DNG group. However, there was no significant difference in low-density lipoprotein, carbohydrate metabolism, or hemostasis between the two groups.Citation26 Other studies showed that E2V/DNG has no significant effect on coagulation factors when compared with ethinyl estradiol/LNG. This includes factor VII, VIII, antithrombin III, proteins C and S, or inactivated protein C.Citation27 E2V/DNG also had no effect on D-dimer or fibrinogen levels when compared with ethinyl estradiol/LNG.Citation28 Currently only one case each of myocardial infarction and deep vein thrombosis have been reported in the literature for patients taking E2V/DNG.Citation14 Studies currently ongoing are appropriately powered to show whether E2V/DNG has an increased or decreased risk of thromboembolic and cardiovascular events.Citation29 Until the results of these studies are available, clinicians should apply the same precautions and contraindications for use of combination oral contraceptives containing ethinyl estradiol to use of E2V. Common side effects of E2V/DNG are similar to those found with other combination oral contraceptives. In a combined report of three studies consisting of over 2200 patients, the most common adverse events were breast discomfort (4.9%), dysmenorrhea (4.9%), headache (3.1%), and acne (2.8%).Citation30 Other common adverse events reported included increased weight, amenorrhea, and abdominal pain. The most common reasons for discontinuing E2V/DNG were breakthrough bleeding (metrorrhagia), acne, and weight gain.Citation17 In a study comparing E2V/DNG and ethinyl estradiol/LNG, adverse effects were similar between the two groups.Citation25 The most commonly reported side effects in the E2V/DNG group were breast pain, headache, and vaginal infections compared with acne, headache, and nasopharyngitis in the ethinyl estradiol/LNG group. There was no statistically significant difference in the number of adverse events reported for the two groups.
Serious adverse events that could be possibly linked to treatment with E2V/DNG were rare, and included presumed ocular histoplasmosis syndrome, uterine leiomyoma, focal nodular hyperplasia of the liver, myocardial infarction, deep vein thrombosis, ruptured ovarian cyst, autonomic nervous system imbalance, and breast cancer. There was only one reported case per event and other causes could not be ruled out.Citation31
At this time, no distinct safety advantages or disadvantages can be considered when comparing E2V/DNG with other combination oral contraceptives containing ethinyl estradiol 35 μg or less. More studies are needed to characterize further the impact on lipid and glucose metabolism, and ultimately determine if there is less risk of thromboembolic or cardiovascular events.
Place in therapy
Several options exist for the treatment of heavy menstrual bleeding. The best option for a patient depends on etiology, severity of bleeding (interference with daily activities), associated symptoms (ie, pelvic pain), contraindications to hormonal or other medications, contraceptive needs, future pregnancy plans, comorbidities, and patient preference for length of treatment. Finally, treatment cost must be considered. Treatment options may include surgery or medications, such as NSAIDs, antifibrinolytics (ie, tranexamic acid), or hormonal modulators (ie, danazol), including oral progestins, combination oral contraceptives, gonadotropin receptor antagonists, and LNG-IUD. Only primary and secondary medical treatment options for chronic heavy menstrual bleeding will be discussed. E2V/DNG will be compared with other treatment options for heavy menstrual bleeding. Antifibrinolytics and hormonal modulators are not preferred agents and are not discussed here.
Heavy menstrual bleeding in sexually active women with anovulatory or ovulatory cycles
The American College of Obstetrics and Gynecology recommends that patients with anovulatory or ovulatory bleeding be treated with a combination oral contraceptive if they require contraception.Citation32,Citation33 The LNG-IUD has shown superior efficacy to combination oral contraceptives and for this reason may be considered first-line treatment.Citation34,Citation35 LNG-IUD reduces heavy menstrual bleeding by 95% after three months.Citation36 E2V/DNG is an appropriate first choice for most premenopausal women who have a need for contraception, do not have contraindications to estrogen therapy, and do not desire insertion of a LNG-IUD. No comparative trials of E2V/DNG versus LNG-IUD have been conducted; until these studies are completed, the LNG-IUD is considered better than E2V/DNG given the superiority of the LNG-IUD data compared with those for other combination oral contraceptives used to treat long-term heavy menstrual bleeding.Citation34,45
As discussed earlier, E2V/DNG was more effective than placebo in patients with heavy menstrual bleeding in reducing bleeding volume and has been given an indication for this condition.Citation21,Citation22 These trials were six months in duration, and long-term comparative trials versus LNG-IUD are needed. To compare combination oral contraceptives in healthy women seeking contraception, one study performed in 798 women aged 18–50 years demonstrated improved bleeding patterns with seven cycles of E2V/DNG (n = 399) compared with a combination oral contraceptive containing ethinyl estradiol/LNG (n = 399).Citation25 There were significantly fewer bleeding/spotting days reported by women receiving E2V/DNG compared with ethinyl estradiol/LNG (17.3 ± 10.4 versus 21.5 ± 8.6, respectively, P < 0.0001) and better cycle control (more cycles with absent withdrawal bleeding in the E2V/DNG group, P < 0.001). Scheduled withdrawal bleeding episodes were shorter (median 4.0 versus 5.0 days, P < 0.05) and lighter in women treated with E2V/DNG versus ethinyl estradiol/LNG. While this study was not performed in women specifically with heavy menstrual bleeding, it provides evidence that E2V/DNG may be a good oral contraceptive option for women with menstrual disturbances.
Premenopausal ovulatory patients not desiring pregnancy within the upcoming year who have contraindications to estrogen therapy may benefit most from LNG-IUD. Patients not planning a pregnancy who are anovulatory may benefit from E2V/DNG as a first-line option. Alternatively, a cyclic oral progestin or LNG-IUD could be used.Citation32
Heavy menstrual bleeding in nonsexually active women with anovulatory or ovulatory cycles
Anovulatory bleeding in women who are not sexually active may be treated with an oral contraceptive, cyclic progestin, or an NSAID.Citation32 A combination oral contraceptive such as E2V/DNG is an appropriate option for women experiencing ovulatory or anovulatory cycles, given the data from studies mentioned above. A progestin such as micronized progesterone or medroxyprogesterone acetate given cyclically (for 14–16 days of the month prior to the anticipated start of the menstrual cycle) or the LNG-IUD are also first-line options.
Cost and daily compliance may be considered in patients who are not sexually active. A significant difference in treatment costs exists (). Cost differences may limit treatment with E2V/DNG, other combination oral contraceptive, or progestin if not covered by insurance. An NSAID may be the best treatment option for those with ovulatory heavy menstrual bleeding, who do not want surgery, cannot receive hormone therapy, or have insurance difficulties. Ibuprofen and meclofenamic acid are commonly used, but no single NSAID has shown superior efficacy.Citation37,Citation38 NSAID treatments typically last 5 days or until menses ends. NSAIDS reduce blood loss by 20%–40% and much greater reductions have been shown in patients with excessive bleeding.Citation39,Citation40 Patients using E2V/DNG average around a 65% reduction in mean blood loss while nearly half experience up to an 80% reduction in mean blood loss.Citation21 Thus, E2V/DNG may be cost-effective compared with an NSAID if it successfully controls heavy menstrual bleeding. Patients with compliance issues may benefit most from the LNG-IUD.
Table 1 Cost comparison of treatments for heavy menstrual bleedingCitation36
Heavy menstrual bleeding in women who plan to conceive in the upcoming year
E2V/DNG is not an appropriate choice for patients with ovulatory cycles who are attempting conception within a year. These women may want to avoid E2V/DNG, along with other combination oral contraceptives, and instead use an NSAID or an oral cyclic progestin, such as micronized progestin or medroxyprogesterone acetate.Citation32
Heavy menstrual bleeding in women with a concomitant condition
Patients with fibroids and heavy menstrual bleeding are best treated with gonadotropin-releasing hormone agonists or NSAIDs if drug therapy is chosen over surgical treatment.Citation38,Citation41,Citation42 Although E2V/DNG has not been studied in these patients, other combination oral contraceptives have been used successfully to decrease the volume and duration of blood loss to the levels seen in patients without fibroids. Thus, E2V/DNG may be an option for these patients.
The US Medical Eligibility Criteria for Contraceptive Use or the World Health Organization Medical Eligibility Criteria for Contraceptive Use can be used to determine whether women with concurrent conditions are candidates for heavy menstrual bleeding therapies that also function as contraceptives.Citation43,Citation44 Women with heavy menstrual bleeding and a high risk of cardiovascular disease require nonestrogen-based treatment.Citation43 Risks include older age, smoking, hypertension, recent major surgery, history of arterial or venous thrombosis, or medical conditions, such as inherited thrombophilia, active cancer, or diabetes.Citation43 E2V/DNG should be used with caution in obese patients since the safety and efficacy have not been adequately evaluated in women with a body mass index (BMI) > 30 kg/m2 (average BMI 24.6 to 26 kg/m2 among study participants, up to 32 kg/m2).Citation14 LNG-IUD, cyclic progestin, and NSAID are alternative options for women with heavy menstrual bleeding and a concurrent condition.Citation32,Citation33
Duration of therapy preferences
Patients must be informed of the efficacy of the different treatment options for heavy menstrual bleeding. Given these options, clinicians need to provide guidance according to the length of treatment desired by the patient. Daily, short-term, or long-term options are available. Patients who do not want a daily oral regimen and are not planning to conceive in the next year may be better suited for insertion of an LNG-IUD. Patients without contraindications who prefer a daily regimen may benefit most from E2V/DNG or other combination oral contraceptives. Those who prefer a monthly two-week course may be best suited to cyclic progestin. Patients who desire a very short course (five days or less) may consider an NSAID.
Conclusion
E2V/DNG is a novel combination oral contraceptive recently approved for heavy menstrual bleeding. E2V/DNG has been found to be more efficacious than placebo in reducing heavy menstrual bleeding and improving hematologic indices. In healthy women without heavy menstrual bleeding, E2V/DNG was found to decrease the bleeding duration and induce amenorrhea more effectively than ethinyl estradiol/LNG. E2V/DNG has a safety profile similar to that of other combination oral contraceptives. This product is best suited to women with heavy menstrual bleeding who are anovulatory or ovulatory and require contraception. LNG-IUD may be the most appropriate choice in patients not desiring conception for at least one year or who do not want a daily or cyclic pill burden. NSAIDS are an option for ovulating patients who are not sexually active but require a cost-effective or short-course treatment. Cyclic progestins may be best suited to anovulatory or ovulatory patients with contraindications to estrogen who plan for pregnancy within a year. When compared with alternative treatment options, E2V/DNG has the added benefit of providing effective contraception. Further studies are needed to compare the efficacy of E2V/LNG with that of other combination oral contraceptives, the LNG-IUD, and other standard therapies in women with heavy menstrual bleeding.
Disclosure
The authors have no conflicts of interest to report in this work.
References
- CoteIJacobsPCummingDCUse of health services associated with increased menstrual loss in the United StatesAm J Obstet Gynecol200318834334812592237
- SpencerCPWhiteheadMIEndometrial assessment re-visitedBr J Obstet Gynaecol199910662363210428515
- ThompsonBLPonce de LeonRKiekeBVelebilPWingoPATrends in hospitalizations for abnormal uterine bleeding in the United States: 1980–1992J Women’s Health1997673819065376
- ShapleyMJordanKCroftPRIncreased vaginal bleeding and psychological distress: a longitudinal study of their relationship in the communityBJOG200311054855412798470
- CoteIJacobsPCummingDWork loss associated with increased menstrual loss in the United StatesObstet Gynecol200210068368712383534
- MunroMGCritchleyHOBroderMSFraserISFIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive ageInt J Gynaecol Obstet201111331321345435
- LiuZDoanQVBlumenthalPDuboisRWA systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in abnormal uterine bleedingValue Health20071018319417532811
- American College of Obstetricians and GynecologistsPractice bulletin no 128: diagnosis of abnormal uterine bleeding in reproductive-aged womenObstet Gynecol201212019720622914421
- FraserISCritchleyHOMunroHGBroderMA process designed to lead to international agreement on terminologies and definitions to be used to describe abnormalities of menstrual bleedingFertil Steril20078746647617362717
- JamesAHMore than menorrhagia: a review of the obstetric and gynaecological manifestations of bleeding disordersHaemophilia20051129530716011580
- ShankarMLeeCASabinCAEconomidesDLKadirRAvon Willebrand disease in women with menorrhagia: a systematic reviewBJOG200473474015198765
- DilleyADrewsCMillerCvon Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagiaObstet Gynecol20019763063611275041
- KadirRAEconomidesDLSabinCAOwensDLeeCAFrequency of inherited bleeding disorders in women with menorrhagiaLancet19983514854899482440
- Natazia (estradiol valerate/dienogest) [package insert]Wayne, NJBayer HealthCare Pharmaceuticals2012 Available from: http://labeling.bayerhealthcare.com/html/products/pi/natazia_pi.pdfAccessed April 9, 2013
- FruzzettiFBitzerJReview of clinical experience with estradiol in combined oral contraceptivesContraception20108181520004267
- KileyJWShulmanLPEstradiol valerate and dienogest: a new approach to oral contraceptionInt J Womens Health2011328128621892339
- PalaciosSWildtLParkeSMachlittARömerTBitzerJEfficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a Phase III trialEur J Obstet Gynecol Reprod Biol2010149576219969409
- ZeunSLuMUddinAPharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogestEur J Contracept Reprod Health Care20091422123219565420
- RuanXSeegerHMueckAOThe pharmacology of dienogestMaturitas20127133734422364708
- SasagawaSShimizuYKamiHDienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profileSteroids20087322223118061638
- JensenJTParkeSMellingerUMachlittAFraserISEffective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trialObstet Gynecol201111777778721422847
- FraserISRomerTParkeSEffective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: a randomized, double-blind Phase III trialHum Reprod2011262698270821784734
- FraserISParkeSMellingerUMachlittASerraniMJensenJEffective treatment of heavy and/or prolonged menstrual bleeding without organic cause: pooled analysis of two multinational, randomized, double-blind, placebo-controlled trials of oestradiol valerate and dienogestEur J Contracept Reprod Health Care20111625826921774563
- FraserISJensenJSchaefersMMellingerUParkeSSerraniMNormalization of blood loss in women with heavy menstrual bleeding treated with an oral contraceptive containing estradiol valerate/dienogestContraception2012869610122240178
- AhrendtHJMakalovaDParkeSMellingerUMansourDBleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven cycle, randomized, comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrelContraception20098043644419835717
- ParkeSNahumGMellingerUJungeWMetabolic effect of a new four-phasic oral contraceptive containing estradiol valerate and dienogestObstet Gynecol2008111Suppl 4S12S13
- HoySMScottLJEstradiol valerate/dienogest: in oral contraceptionDrugs2009691635164619678714
- ParkeSJungeWMellingerUDujkersIKlippingCComparative effects of a four-phasic regimen of estradiol valerate/dienogest versus ethinyl estradiol/levonorgestrel on haemostatic parametersHum Reprod200823Suppl 1i78i79
- International Active Surveillance Study-Safety of Contraceptives: Role of Estrogens (INAS-SCORE) Available from: from: http://clinicaltrials.gov/Accessed January 31, 2013
- NelsonASampson-LandersCParkeSJensenJTEfficacy of estradiol valerate/dienogest OC: results of 3 large studies in North America and EuropeAbstract presented at the 57th Annual Clinical Meeting of the American Congress of Obstetricians and GynecologistsMay 2–6, 2009Chicago, IL
- BorgeltLMMartellCWEstradiol valerate/dienogest: a novel combined oral contraceptiveClin Ther201234375522169052
- American College of Obstetricians and GynecologistsACOG practice bulletin: management of anovulatory bleedingInt J Gynaecol Obstet20017226327111296797
- [No authors listed]ACOG practice bulletin: Noncontraceptive uses of hormonal contraceptivesObstet Gynecol2010110206218
- ShaabanMMZakherahMSEl-NasharSALevonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trialContraception201183485421134503
- GuptaJKaiJMiddletonLPattisonHGrayRDanielsJLevonorgestrel intrauterine system versus medical therapy for menorrhagiaN Engl J Med201336812813723301731
- LethabyAECookeIReesMProgesterone or progestogen-releasing intrauterine systems for heavy menstrual bleedingCochrane Database Syst Rev200519CD00212616235297
- FraserISMcCarronGRandomized trial of 2 hormonal and 2 prostaglandin-inhibiting agents in women with a complaint of menorrhagiaAust N Z J Obstet Gynaecol19913166701872778
- MäkäräinenLYlikorkalaOPrimary and myoma-associated menorrhagia: role of prostaglandins and effects of ibuprofenBr J Obstet Gynaecol1986939749783533137
- HallPMaclachlanNThornNNuddMWTaylorCGGarriochDBControl of menorrhagia by the cyclo-oxygenase inhibitors naproxen sodium and mefenamic acidBr J Obstet Gynaecol1987945545583304401
- LethabyAAugoodCDuckittKNonsteroidal anti-inflammatory drugs for heavy menstrual bleedingCochrane Database Syst Rev20021CD00040011869575
- CarrBRMarshburnPBWeatherallPTAn evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trialJ Clin Endocrinol Metab199376121712238496313
- MarshallLMSpiegelmanDGoldmanMBA prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomataFertil Steril1998704324399757871
- Centers for Disease Control and PreventionUS medical eligibility criteria for contraceptive use, 2010MMWR Available from: http://www.cdc.gov/mmwr/pdf/rr/rr59e0528.pdfAccessed April 9, 2013
- World Health OrganizationMedical Eligibility Criteria for Contraceptive Use4th edGeneva, SwitzerlandWorld Health Organization2009 Available from: http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/index.htmlAccessed April 9, 2013
