Abstract
Objective
Capsaicinoids (CAPs), most commonly found in chili peppers, have a multitude of pharmacological and physiological effects, such as anti-inflammation, antioxidant, and anticancer effects. In the present study, we set out to investigate the hypothesis that CAPs mitigate obesity in rats and the possible mechanisms thereof.
Materials and methods
Rats were divided into six groups, including control (±10 mg CAPs/kg body weight [BW]), low-fat–high-sucrose diet (±10 mg CAPs/kg BW), and high-fat diet (±10 mg CAPs/kg BW). Blood samples and liver and aortic tissues were taken at the end of the study.
Results
CAPs supplementation significantly reduced hyperglycemia and hyperlipidemia (P<0.001) and ameliorated oxidative damage by reducing malondialdehyde concentrations in serum and liver and by increasing total antioxidant capacity in serum induced by the low-fat–high-sucrose and high-fat diets (P<0.001 for all). CAPs also depressed levels of NFκB p65, gp91phox, and p22phox, essential components of NADPH oxidase, in the aorta of rats. However, levels of Nrf2, Sirt1, and endothelial nitric oxide synthase were significantly increased in the aorta.
Conclusion
CAPs may at least partially reduce adverse effects due to high-fat diet and sucrose consumption through regulation of energy metabolism, oxidative stress, and proteins involved in vasoprotection.
Introduction
Metabolic syndrome is a worldwide health problem with increased morbidity and comprises a group of risk factors such as insulin resistance, obesity, hyperglycemia, hypertension, and dyslipidemia.Citation1–Citation3 Metabolic syndrome has been reported to develop due to such factors as a surplus of fat and simple carbohydrates from high-fat diets (HFDs) and high-sucrose diets (HSDs).Citation2,Citation3 Experimental models have been studied in detail by our research group on the metabolic changes that result from the consumption of these diets. As a result of our and other studies, these diets have been reported to trigger insulin resistance and promote inflammatory processes, hyperinsulinemia, and hyperlipidemia, and increase blood pressure, hepatic steatosis, and vascular dysfunction.Citation3–Citation6 In addition, an association between HFDs and HSDs facilitating oxidative stress and a reduction in nitric oxide levels has been revealed.Citation7 In recent years, many studies have been conducted to determine the potential effects of phytochemicals, such as cinnamon, genistein, resveratrol, and capsaicin, on animal models induced by HFDs.Citation6,Citation8,Citation9 However, there have been no reports on the effects of the molecular mechanism of capsaicinoids (CAPs) in HFDs and HSDs in rats.
CAPs are the major pungent, naturally occurring active compounds in capsicum fruit, such as hot chili peppers (genus Capsicum), with the most abundant forms being capsaicin, dihydrocapsaicin, and nordihydrocapsaicin.Citation10 The available information indicates that CAPs possess a wide variety of biological and physiological properties, including anti-inflammatory,Citation11 antioxidant,Citation12 and anticancer.Citation13 Whiting et alCitation14 indicated that CAPs played a beneficial role as part of a weight-management program. Capsicum extract is a CAP-enriched standardized product obtained from dried red fruit of C. annuum L. CAPs are responsible for the spicy taste of the chili pepper berry.Citation11 They are hydrophobic, colorless, odorless, and crystalline waxy compounds, and their varieties are present in capsicum. The CAPs in the pepper are biosynthesized from branched-chain amino acids and phenylalanine in pepper fruit. In chili pepper, capsaicin is the primary CAP, followed by dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin.Citation11,Citation15,Citation16
Capsicum spp. have been used as a carminative, digestive irritant, stomachic, stimulant, rubefacient, and tonic.Citation16 The plants have also been used as folk remedies for dropsy, colic, diarrhea, asthma, arthritis, muscle cramps, and toothache.Citation16,Citation17 It has also been reported that consumption of capsaicin promoted fat oxidation in negative energy balance and did not increase blood pressure significantly.Citation18 Additionally, capsiate activates TRPV1 receptors in the gut.Citation19 Capsicum extract is also used in cosmetic products, where it functions as an external analgesic, flavoring agent, fragrance component, or skin-conditioning agent.Citation15,Citation16,Citation20 Therefore, the present study was undertaken in an animal model to investigate the effects of CAPs on lipid profile, metabolic health risk factors, and oxidative stress markers, and explored the possible mechanisms in the aorta of rats fed healthy or unhealthy diets.
Materials and methods
Animals
A total of 42 male Wistar rats (age 8 weeks, weight 180±20 g) were housed in a controlled standard laboratory environment (12:12-hour light:dark cycle at 22°C) and fed with rat chow and HFD diets, and had water ad libitum. All experiments were conducted under the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the ethics committee of Firat University, Elazig, Turkey. The composition of diets (control and HFD) is shown in .
Table 1 Composition of diets (g/kg) fed to rats
Experimental diets and design
After 1 week of acclimation to a standard rodent-chow diet, 42 rats were randomly allocated into six groups, with seven rats in each group: controls, rats fed chow diet (12% of calories as fat); CAPs, rats fed chow diet and administered CAPs (Capsimax® [OmniActive Health Technologies, Ltd., Morristown, NJ, USA]; 10 mg/kg body weight [BW], 0.2 mg CAPs); HSD, rats fed chow diet plus 20% sucrose (30% w:v) in the drinking water;Citation21,Citation22 HSD + CAPs, rats fed chow diet plus 20% sucrose (30% w:v) in the drinking water and administered CAPs (Capsimax; 10 mg/kg BW, 0.2 mg CAPs); HFD, rats fed an HFD (42% of calories as fat); and HFD + CAPs, rats fed an HFD and administered CAPs (Capsimax; 10 mg/kg BW, 0.2 mg CAPs). Rats were treated orally with CAPs (10 mg/kg BW dissolved in 5% dimethyl sulfoxide) daily by oral gavage to the end of the experiment. Capsimax consists of CAPs obtained from dried red fruit of C. annum L. The product is standardized to 2% CAPs, of which 1.2%–1.35% is capsaicin, 0.6%–0.8% dihydrocapsaicin, and 0.1%–0.2% nordihydrocapsaicin. CAP concentrate was provided by OmniActive Health Technologies, Ltd., (Morristown, NJ, USA). The dosage of CAPs chosen was based on previously reported dosage in rodents.Citation23 Animals were administered CAPs for 8 weeks. HFDs were prepared weekly in our laboratory in pellets and stored at −4°C.
Sample collection
At the end of the experimental period, rats were weighed and then killed by decapitation. Blood samples were collected and serum was prepared by centrifuging the blood at 3,000 × g for 10 minutes, aliquoted into 1.5 mL vials, frozen at −80°C, and used for biochemical parameters and malondialdehyde (MDA) analyses. Livers and aortas were carefully removed, weighed, and then stored at −80°C. Samples (1 g) were homogenized in 2 mL TBS buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) and centrifuged at 3,000 × g for 15 minutes at 4°C. Supernatants were collected and used for MDA estimations.
Laboratory analyses
Serum parameters were determined by an automated analyzer (Labgeo PT10; Samsung Electronics Co, Seoul, South Korea). Levels of insulin and leptin were analyzed with rat insulin and leptin kits (Linco Research, Inc., St Charles, MO, USA) via an enzyme-linked immunosorbent assay device (Elx-800; BioTek Instruments, Inc., Winooski, VT, USA). The assay’s sensitivity was 0.18 and 0.26 ng/mL for insulin and leptin, respectively. Inter- and intra-assay constants were 5.2% and 6.1% for insulin and 4.7% and 6.5% for leptin, respectively. MDA concentrations in livers and aortas were measured as per the method in KaratepeCitation24 via high-performance liquid chromatography with a Shimadzu ultraviolet-visible SPD-10A VP detector, a CTO-10AS VP column, and a mobile phase comprising 30 mM KH2PO4 and methanol (82.5:17.5 v:v, pH 3.6) at a flow rate of 1.2 mL/min. Tissue homogenates (10% w:v) were prepared in 10 mM phosphate buffer (pH 7.4) and centrifuged at 13,000 × g for 10 minutes at 4°C. Total antioxidant capacity (TAC) was measured using dark blue–green color reduction 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) by antioxidants to its colorless form via the antioxidants in the sample.Citation25
Western blot analysis
Levels of NFκB, Nrf2, Sirt1, endothelial nitric oxide synthase (eNOS), gp91phox, and p22phox in the aortas were analyzed via Western blotting.Citation26 Samples were analyzed in quadruplicates for each experimental situation. For this purpose, 50 μg of proteins were transferred to a nitrocellulose membrane after electrophoresis (Whatman, Maidstone, UK). The phosphorylated form of antibodies against NFκB, Sirt1, Nrf2, eNOS, gp91phox, and p22phox proteins (Abcam, Cambridge, UK) were diluted in a concentration of 1:1,000 PBS buffer containing 0.05% of Tween 20. The loading of proteins was controlled by a monoclonal mouse antibody versus β-actin (A5316; Sigma-Aldrich, St Louis, MO, USA). Bands were viewed with ImageJ, an image-analysis system (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Sample size was based on a power of 85% to obtain P<0.05. Differences among groups were evaluated by analysis of variance and Tukey’s post hoc analysis. P<0.05 was considered statistically significant. Data were analyzed in SPSS for Windows version 20 (IBM, Armonk, NY, USA).
Results
Body weight and biochemical parameters
The effects of CAP supplementation on final BW, lipid profile, and safety end markers for liver-function tests are shown in . HSD and HFD feeding increased final BW, total cholesterol and triglycerides by 10% and 6%, 56 and 49%, and 182% and 139% compared to control rats, respectively (P<0.001). CAP treatment decreased total cholesterol and triglycerides by 17% and 26% and 12% and 19% in HSD- and HFD-fed rats, respectively (P<0.001). No significant difference in BW (P>0.05, ) was observed in the HSD- and HSD-fed rats treated with CAPs (P>0.05, ). No significant difference was found either in BW between HFD-fed rats and HFD-fed rats treated with CAPs (P>0.05, ). No significant differences were detected in safety end markers for liver-function tests in any treatment (P>0.05, ).
Table 2 Effects of capsaicinoids (CAPs) on final BW and plasma biochemical parameters in high-sucrose diet (HSD)- and high-fat diet (HFD)-fed rats
As seen in , HSD and HFD feeding increased serum levels of glucose (87% and 70%), insulin (138% and 61%), free fatty acid (FFA; 233% and 288%) and leptin (345% and 103%) in HSD and HFD rats, respectively (P<0.001). Hypertriglyceridemia and elevated lipid indicators in HSD-and HFD-fed rats were reduced with CAP supplementation by 18% and 16% for glucose, 8% and 14% for insulin, 43% and 52% for FFA, and 44% and 40% for leptin, respectively (P<0.05). Serum and liver MDA levels increased 114% and 97% for HSD and 62% and 73% for HFD (P<0.001, ), and serum TAC decreased by 72% for HSD and 57% for HFD upon obesity induction. CAP treatment caused a 32% and 37% reduction for HSD and 16% and 18% reduction for HFD in serum and liver MDA concentrations and elevation in serum TAC by 163% and 54% in the HSD- and HFD-fed rats (P<0.001), respectively, similar to the control group (P>0.05).
Table 3 Effects of capsaicinoids (CAPs) on serum glucose, insulin, FFA, leptin, MDA, TAC, and liver MDA concentrations in high-sucrose diet (HSD)- and high-fat diet (HFD)-fed rats
Aortic protein levels
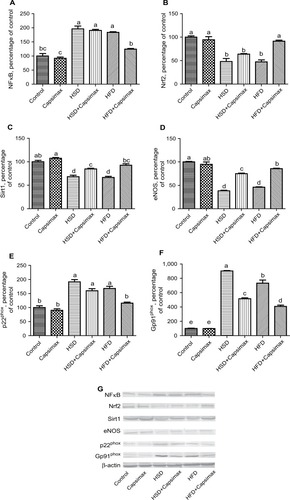
Aortic NFκB, a transcription factor, gp91phox, and p22phox (essential components of NADPH oxidase) levels were increased by HFD and HSD intake, whereas HFD- and HSD-fed rats had decreased heart Nrf2, an emerging regulator of cellular resistance to oxidants, Sirt1, an important regulator of energy metabolism, and eNOS, a vasoprotective molecule of nitric oxide expression (P<0.0001 for all). However, CAPs decreased levels of NFκB, gp91phox, and p22phox and increased levels of Sirt1, Nrf2, eNOS significantly in aortas of CAP-treatment groups (P<0.05, ).
Figure 1 Effects of capsaicinoids (CAPs) in high-sucrose diet (HSD)- and high-fat diet (HFD)-fed rats.
Notes: Aortic NFκB (A); Nrf2 (B); Sirt1 (C); endothelial nitric oxide synthase (eNOS) (D); p22phox (E); gp91phox (F). The intensity of the bands was quantified by densitometric analysis. Data expressed as ratio of normal control value (set to 100%). Data are expressed as percentage of control value. Each bar represents the mean and standard error of mean. The intensity of the bands (G) was quantified by densitometric analysis. Blots were repeated at least four times, and representative blots are shown. β-Actin was included to ensure equal protein loading. Different superscripts denote significant differences between groups not sharing the same superscript (P<0.05). Capsimax®; OmniActive Health Technologies, Ltd., Morristown, NJ, USA.
Discussion
In this study, we report that hyperglycemia, hypertriglyceridemia, p22phox, and gp91phox levels, the major components of NADPH oxidase in rats fed HFD and HSD, were increased in the aorta and that this rise was linked to increased lipid peroxidation and NFκB activities and reduction of Nrf2, Sirt1, and eNOS activities. Supplemental CAPs alleviate the adverse effects of HFD and HSD.
Capsicum has been shown to help improve metabolism and hormone function,Citation27 stabilize blood glucose,Citation28 and reduce insulin and leptin resistance.Citation29 Capsicum and CAPs have also been linked to cardiovascular health, by improving endothelial function,Citation30 and inhibiting low-density-lipoprotein cholesterol oxidation.Citation31 Capsicum may also help prevent cancer, likely due to its antioxidant activity.Citation32,Citation33 The burning sensation associated with capsaicin results from its chemical interaction with sensory neurons. Capsaicin binds selectively to TRPV1, which resides on the membranes of pain- and heat-sensing neurons.Citation34 Capsaicin causes an ion channel to open below body temperature, which is why capsaicin is linked to the sensation of heat. Prolonged activation of these neurons by capsaicin depletes presynaptic substance P, one of the body’s neurotransmitters for pain and heat. The changes observed in this study may have been due to its selective binding to TRPV1; however, further studies need to be carried out in humans. In the current study, it was observed that CAPs reduced total cholesterol, triglycerides, glucose, and insulin in both the HSD and HFD groups. In addition, we observed HSD and HFD enhanced all metabolic health risk factors and increased oxidative stress markers.
CAPs are potent free radical scavengers and prevent membrane stability.Citation35 Kogure et alCitation36 showed that capsaicin hindered MDA levels and ROS production in rat peritoneal macrophages, as well as inflammatory reactions in sepsis. In addition, chronic exposure to capsaicin depletes neurons of neurotransmitters, reduces pain sensation, and blocks inflammation.Citation37,Citation38 In the current study, CAPs increased potential antioxidant properties and demonstrated their ability to protect tissue from damage or inflammation. Similarly, in a previous study, we reported that CAP supplementation elevated the activity of SOD, CAT, and glutathione peroxidase and significantly decreased MDA levels in serum and ovaries.Citation5 Kempaiah and SrinivasanCitation39 reported that reduced liver antioxidant enzymes, including SOD, CAT, and glutathione peroxidase, in hypercholesterolemic rats were efficiently resisted by capsaicin supplementation (0.015%). Lee et alCitation40 reported that capsaicin administration for three days reduced MDA concentration in liver, kidney, muscle, and lung in rats. It is also known that CAPs stimulate energy consumption by activation of the sympathetic nervous system, which induces catecholamine secretion from the adrenal medulla.Citation10,Citation41 This thermogenic influence has the advantage of weight management. CAPs have also been reported to decrease appetite,Citation42–Citation44 increase thermogenesis,Citation28,Citation45 and increase lipolysis, and FFA.Citation10 Associations among consumption of CAP-containing food and low incidence of obesity have been reported earlier.Citation10,Citation46 CAPs reduce metabolic health risk factors causing obesity and increased energy metabolism. Sirt1 decreases in the HSD and HFD groups indicate that these diets increase the risk of obesity and cardiometabolic syndrome.
The current study revealed different regulatory pathways to prevent oxidative stress, improve antioxidant potential, and enhance energy metabolism (). NFκB influenced inflammatory proteins, and CAPs decreased NFκB levels and enhanced Nrf2 activity. In addition, Sirt1 modulates various cellular processes that directly regulate glucose metabolism and stress metabolism, increases fat mobilization and energy metabolism, thereby controlling the direct chromatin structure and stimulating brown remodeling of the white fat in white adipose tissue, regulates pancreatic insulin secretion, detects nutrient availability in the hypothalamus, impairs obesity-induced inflammation in macrophages, and modulates circadian time activity in tissues.Citation47 In the current study, CAPs increasing SIRT1 suggest its metabolic role in activating energy metabolism. Further studies are needed to explore this pathway. The thermogenic effect of CAPs is mediated at least in part by a CAP-sensitive structure located in the rostral ventrolateral medulla.Citation48 Increased eNOS protein levels in CAP treatment suggest vasoprotection and assistance in reducing cardiovascular risk. CAP treatment may also stimulate vasodilation,Citation30 which may indirectly impact thermogenesis, as any resultant loss of heat may necessitate an increase in metabolism. A previous study showed that capsaicin upregulated HO1 expression by Nrf2 stimulation in HEPG2 cells.Citation49
Many studies have shown that obesity, HFD intake, hyperglycemia, high tissue lipids, excessive angiotensin II production, and hyperleptinemia can cause vascular damage through numerous metabolic pathways, such as free-radical generation and inflammation.Citation50,Citation51 The inflammatory condition of obesity increases leukocyte tissue infiltration and free-radical production, while cytokines are known to upregulate the activity of redox enzymes, including NADPH oxidase (NOX).Citation52 NOX is an enzyme system that contains membrane (gp91phox, p22phox) and cytosolic (p47phox, p67phox, p40phox) components and produces superoxide, which combines on the plasma membrane to form active oxidase.Citation53 Previous studies have suggested that obesity-linked vascular dysfunction is facilitated by NOX-induced oxidative stress.Citation51,Citation54 Upregulation of NOX has been related to elevated ROS induced by angiotensin II and TNFα.Citation55,Citation56 In previous studies, it has been reported that high glucose incubation upregulates expression of the NADPH subunits p22phox and p67phox in bovine aortic endothelial cellsCitation51 and in microvascular endothelial cells.Citation57 In the current study, the upregulated NADPH oxidase subunits p22phox and p67phox stimulated by HSD or HFD were decreased by CAP supplementation. The results of this study showed that CAPs might enhance the increased NADPH oxidase levels that result in increased ROS generation, whereas the increase in ROS generation is mostly responsible for the HFD- or HSD-induced endothelial hyperpermeability. Therefore, CAPs inhibited endothelial overpermeability by inhibiting NOX. In addition, Zuo et alCitation51 reported that greentea polyphenols protected against overexpression of high glucose-stimulated p22phox and p67phox. Akar et alCitation54 reported that decreased relaxation to acetylcholine and intensified contractions to phenylephrine and angiotensin II were linked with downregulated eNOS and Sirt1, whereas gp91phox and p22phox proteins were upregulated and superoxide production triggered in aortas from high-fructose corn syrup-treated rats. They also reported that resveratrol supplementation efficiently restored high-fructose corn syrup-induced deteriorations.
Several proteins are altered by CAPs, many of which suggest antioxidant properties, reducing oxidative stress and increased metabolism. The results suggest that thermogenesis- and lipid metabolism-associated proteins were significantly changed upon capsaicin administration, suggesting that capsaicin may be a beneficial phytochemical for attenuation of obesity.Citation23 CAPs also provide antioxidant activity, which may help protect against inflammatory diseases. In hamsters, CAPs support heart health by lowering cholesterol and increasing blood flow, presumably due to antioxidant activity.Citation58 In another study, chili peppers not only reduced total and non-high-density cholesterol and triglycerides but were also related to reduced levels of compounds connected to inflammation, such as Cox2.Citation59
This study further suggests that HSD and HFD diets increase cardiometabolic health risk factors, inflammation, and oxidative stress and reduce potential antioxidant capacity. Therefore, adding CAPs to these diets may help to regulate metabolism and improve antioxidant properties, including reducing metabolic and cardiometabolic health risk factors. Further long-term clinical studies are required to show and explore similar findings in humans.
Acknowledgments
This work was supported by a grant from OmniActive Health Technologies Ltd., India. This work was also supported in part by the Turkish Academy of Sciences.
This paper was presented at the Experimental Biology annual meeting, April 2–6, 2016 in San Diego, CA, USA. Its abstract was published in the FASEB Journal, volume 30, issue 1, supplement 428.7, April 2016.
Disclosure
VJ is an employee of OmniActive Health Technologies, Inc., NJ, USA. The authors report no other conflicts of interest in this work.
References
- GrundySMMetabolic syndrome pandemicArterioscler Thromb Vasc Biol200828462963618174459
- AlbertiKGEckelRHGrundySMHarmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of ObesityCirculation2009120161640164519805654
- Molinar-ToribioEFuguetERamos-RomeroSA high-fat high-sucrose diet affects the long-term metabolic fate of grape proanthocyanidins in ratsEur J Nutr Epub20161012
- IshimotoTLanspaMARivardCJHigh-fat and high-sucrose (Western) diet induces steatohepatitis that is dependent on fructokinaseHepatology20135851632164323813872
- SahinKOrhanCAkdemirFβ-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodentsFood Chem Toxicol201710727027928689061
- TuzcuZOrhanCSahinNJuturuVSahinKCinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed ratsOxid Med Cell Longev20172017158309828396714
- YamamotoYOueEAntihypertensive effect of quercetin in rats fed with a high-fat high-sucrose dietBiosci Biotechnol Biochem200670493393916636461
- LiuHZhongHYinYJiangZGenistein has beneficial effects on hepatic steatosis in high fat-high sucrose diet-treated ratsBiomed Pharmacother20179196496928514835
- Milton-LaskibarIAguirreLFernández-QuintelaALack of additive effects of resveratrol and energy restriction in the treatment of hepatic steatosis in ratsNutrients201797E73728696376
- BloomerRJCanaleREShastriSSuvarnapathkiSEffect of oral intake of capsaicinoid beadlets on catecholamine secretion and blood markers of lipolysis in healthy adults: a randomized, placebo controlled, double-blind, cross-over studyLipids Health Dis201097220633266
- Reyes-EscogidodeMLGonzalez-MondragonEGVazquez-TzompantziEChemical and pharmacological aspects of capsaicinMolecules20111621253127021278678
- HenningSMZhangYSeeramNPAntioxidant capacity and phytochemical content of herbs and spices in dry, fresh and blended herb paste formInt J Food Sci Nutr201162321922521118053
- LuHFChenYLYangJSAntitumor activity of capsaicin on human colon cancer cells in vitro and Colo 205 tumor xenografts in vivoJ Agric Food Chem20105824129991300521082859
- WhitingSDerbyshireETiwariBKCapsaicinoids and capsinoids: a potential role for weight management? A systematic review of the evidenceAppetite201259234134822634197
- GannonNPLambalotELVaughanRAThe effects of capsaicin and capsaicinoid analogs on metabolic molecular targets in highly energetic tissues and cell typesBiofactors201642322924626945685
- JuturuVCapsaicinoids modulating cardiometabolic syndrome risk factors: current perspectivesJ Nutr Metab20162016498693727313880
- GovindarajanVSSathyanarayanaMNCapsicum: production, technology, chemistry, and quality – part V: impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequencesCrit Rev Food Sci Nutr19912964354742039598
- JanssensPLHurselRMartensEAWesterterp-PlantengaMSAcute effects of capsaicin on energy expenditure and fat oxidation in negative energy balancePLoS One201387e6778623844093
- LudyMJMooreGEMattesRDThe effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humansChem Senses201237210312122038945
- JohnsonWFinal report on the safety assessment of Capsicum annuum extract, Capsicum annuum fruit extract, Capsicum annuum resin, Capsicum annuum fruit powder, Capsicum frutescens fruit, Capsicum frutescens fruit extract, Capsicum frutescens resin, and capsaicinInt J Toxicol20072613106
- LarquéCVelascoMNavarro-TablerosVEarly endocrine and molecular changes in metabolic syndrome modelsIUBMB Life2011631083183921905198
- Torres-VillalobosGHamdan-PérezNTovarARCombined high-fat diet and sustained high sucrose consumption promotes NAFLD in a murine modelAnn Hepatol201514454054626019041
- JooJIKimDHChoiJWYunJWProteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat dietJ Proteome Res2010962977298720359164
- KaratepeMSimultaneous determination of ascorbic acid and free malondialdehyde in human serum by HPLC-UVLC GC N Am2004224362365
- ErelOA novel automated method to measure total antioxidant response against potent free radical reactionsClin Biochem200437211211914725941
- SahinKTuzcuMOrhanCAnti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocinBr J Nutr2013110219720523211098
- KangJHTsuyoshiGLe NgocHDietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic miceJ Med Food201114331031521332406
- LejeuneMPKovacsEMWesterterp-PlantengaMSEffect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjectsBr J Nutr200390365165913129472
- KangJHGotoTHanISKawadaTKimYMYuRDietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat dietObesity (Silver Spring)201018478078719798065
- ChularojmontriLSuwatronnakornMWattanapitayakulSKInfluence of capsicum extract and capsaicin on endothelial healthJ Med Assoc Thai2010932S92101
- AhujaKDBallMJEffects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and womenBr J Nutr200696223924216923216
- MoriALehmannSO’KellyJCapsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cellsCancer Res20066663222322916540674
- JankovicBLoblawDANamRCapsaicin may slow PSA doubling time: case report and literature reviewCan Urol Assoc J201041E9E1120174488
- CaterinaMJSchumacherMATominagaMRosenTALevineJDJuliusDThe capsaicin receptor: a heat-activated ion channel in the pain pathwayNature199738966538168249349813
- SrinivasanKBiological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a reviewCrit Rev Food Sci Nutr20165691488150025675368
- KogureKGotoSNishimuraMMechanism of potent antiperoxidative effect of capsaicinBiochim Biophys Acta200215731849212383946
- GeppettiPNassiniRMaterazziSBenemeiSThe concept of neurogenic inflammationBJU Int2008101326
- KissinIVanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicityAnesth Analg2008107127128118635498
- KempaiahRKSrinivasanKAntioxidant status of red blood cells and liver in hypercholesterolemic rats fed hypolipidemic spicesInt J Vitam Nutr Res200474319920815296079
- LeeCYKimMYoonSWLeeCHShort-term control of capsaicin on blood and oxidative stress of rats in vivoPhytother Res200317545445812748978
- BelzaAFrandsenEKondrupJBody fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: a placebo-controlled, double-blind 8-week intervention in obese subjectsInt J Obes (Lond)200731112113016652130
- YoshiokaMSt-PierreSDrapeauVEffects of red pepper on appetite and energy intakeBr J Nutr199982211512310743483
- YoshiokaMDoucetEDrapeauVDionneITremblayACombined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foodsBr J Nutr200185220321111242488
- Westerterp-PlantengaMSSmeetsALejeuneMPSensory and gastrointestinal satiety effects of capsaicin on food intakeInt J Obes (Lond)200529668268815611784
- YoshiokaMSt-PierreSSuzukiMTremblayAEffects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese womenBr J Nutr199880650351010211048
- WahlqvistMLWattanapenpaiboonNHot foods: unexpected help with energy balance?Lancet2001358927934834911502310
- LiXSIRT1 and energy metabolismActa Biochim Biophys Sin (Shanghai)2013451516023257294
- OsakaTLeeTHKobayashiAInoueSKimuraSThermogenesis mediated by a capsaicin-sensitive area in the ventrolateral medullaNeuroreport200011112425242810943697
- JoungEJLiMHLeeHGCapsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential targetAntioxid Redox Signal20079122087209817979524
- VincentHKTaylorAGBiomarkers and potential mechanisms of obesity-induced oxidant stress in humansInt J Obes (Lond)200630340041816302012
- ZuoXTianCZhaoNTea polyphenols alleviate high fat and high glucose-induced endothelial hyperpermeability by attenuating ROS production via NADPH oxidase pathwayBMC Res Notes201427120
- CaveACBrewerACNarayanapanickerANADPH oxidases in cardiovascular health and diseaseAntioxid Redox Signal200685–669172816771662
- DeleoFRQuinnMTAssembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteinsJ Leukoc Biol19966066776918975869
- AkarFUludağOAydınAHigh-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats: protective effect of resveratrolFood Chem Toxicol20125062135214122465803
- YingCJXuJWIkedaKTakahashiKNaraYYamoriYTea poly-phenols regulate nicotinamide adenine dinucleotide phosphate oxidase subunit expression and ameliorate angiotensin II-induced hyperpermeability in endothelial cellsHypertens Res2003261082382814621186
- ZhangWFXuYYXuKPInhibitory effect of selaginellin on high glucose-induced apoptosis in differentiated PC12 cells: role of NADPH oxidase and LOX-1Eur J Pharmacol20126941–3606822964466
- DingHAljofanMTriggleCROxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cellsJ Cell Physiol2007212368268917443690
- ChenJLiLLiYActivation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influxCardiovasc Diabetol2015142225849380
- LiangYTTianXYChenJNCapsaicinoids lower plasma cholesterol and improve endothelial function in hamstersEur J Nutr201352137938822466858

