Abstract
The myriad pain pathophysiology has intrigued and challenged humanity for centuries. In this regard, the traditional pain therapies such as opioids and nonsteroidal anti-inflammatory drugs have been highly successful in treating acute and chronic pain. However, their drawback includes adverse events such as psychotropic effects, addiction potential, and gastrointestinal toxicities, to mention a few. These factors combined with the likelihood of an increase in chronic pain conditions due to an aging population calls for the development of novel mechanism-based or “site-specific” agents to target novel pain pathways. In this regard, rapid progress has been made in understanding the molecular mechanisms of novel pain targets such as cannabinoid receptors, fatty acid hydrolase, voltage-gated and ligand-gated ion channels such as P2 receptors, transient receptor potential channels and glial cell modulators. Accordingly, preclinical studies indicate that the site-specific/selective agents exhibit sufficient efficacy and reduced side effects such as lack of psychotropic effects indicating their clinical potential. This review provides a brief summary of some “at-site” pain targets and their role in the pain pathophysiology, and describes the efforts in developing some small molecules as novel pain therapeutics.
Introduction
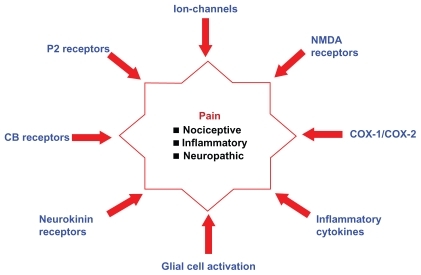
The word “pain” is simple, yet complex. The quest of humans to conquer pain by investigating the underlying cause is a challenging and ongoing process. According to the International Association for the Study of Pain, the definition of pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” indicates the subjective nature of pain.Citation1 It is the most common complaint for which patients seek medical attention, lose productivity, and incur health care costs.Citation2 The epidemic status of pain across the globe is highlighted by a recent study which showed that nearly 37.3% and 41.1% of the adult population in the developed and developing countries, respectively, experience chronic pain due to diseases or disorders or injuries.Citation3 Pain is characterized by both physical and psychological symptoms. Based on clinical characteristics, pain can be classified as nociceptive, neuropathic, or psychogenic/idiopathic.Citation2,Citation4–Citation6 The mechanical, chemical, or thermal stimulation of peripheral sensory nerves due to surgery or trauma in a well localized area is described as nociceptive pain, whereas neuropathic pain is defined as an abnormal signaling resulting from injury or dysfunction of the peripheral or central nervous system (CNS) leading to pain. In addition, the latter is less localized and can persist in the absence of visible injury or inflammation.Citation7–Citation11 Pain perception/assessment in patients with personality disorders, mood disorders, or substance abuse indicates the influence of psychiatric disorders on pain etiology.Citation12,Citation13 Traditional pain-management therapies involve analgesics such as acetaminophen and aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and indomethacin, and narcotics such as morphine.Citation14,Citation15 However, there is a growing concern on the risks of overdose, abuse, and addiction potential of these agents. For example, in the United States alone, about 30,000 hospitalizations are attributed to acetaminophen overdose, whereas NSAID therapy is associated with fatal gastrointestinal bleeding and potential cardiovascular risks.Citation16–Citation18 In addition, narcotic analgesic abuse and addiction is a serious concern.Citation19 These facts mandate the need to look beyond traditional pain targets such as cyclooxygenases and opioid receptors. The complexity in understanding the pain mechanisms listed in will go a long way in developing new therapies. Current research efforts are ongoing to discover agents with superior efficacy and safety profiles that target novel pathological routes as pain therapeutics (). Emerging pain targets include cannabinoid (CB) receptors, fatty acid amide hydrolase (FAAH), voltage- and ligand-gated ion channels (sodium channels, T-type calcium channels, N-type calcium channels, P2 receptors, transient receptor potential [TRP] channels), peptide receptor antagonists, nerve growth factor (NGF), and glial cell modulators. This review describes recent developments in the discovery of CB2 agonists, TRP vanilloid-1 (TRPV1) channel antagonists, P2 receptor antagonists, and agents that target activated glia.
The CB receptors
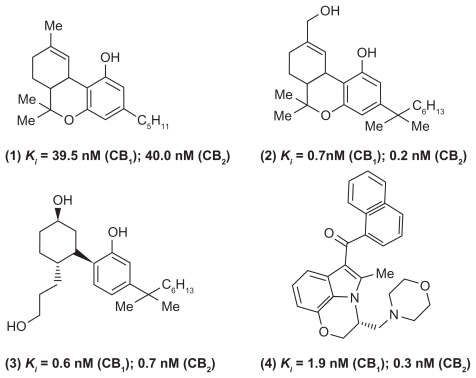
The CB receptors are part of the endocannabinoid system and are G-protein coupled receptors. Their role in the modulation of pain and inflammation is well documented.Citation20–Citation22 Mammalian tissues express two types of CB receptors: CB1 and CB2 respectively. CB1 receptors are primarily expressed in the CNS, whereas CB2 receptors are primarily located in the periphery such as immune cells, spleen, and tonsils.Citation23 In this regard, a number of CB receptor agonists were reported. The classic CBs, tetrahydrocannabinol (Δ9-THC, Marinol®, Solvay Pharmaceuticals, Ixelles, Brussels, Belgium) (, compound (1)) and dimethylheptyl tetrahydrocannabinol (HU-210) (, compound (2)) based on a tricyclic terpenoid template are nonselective CB agonists with HU-210 exhibiting a greater degree of binding affinity toward CB receptors (). The major drawback of classical CB therapy in pain management is the impairment of cognitive/motor function and altered psychological state.Citation24,Citation25 Compound CP-55,940 (, compound (3)), a nonclassical CB was developed based on the chemical structure of Δ9-THC and played a major role in the discovery of the CB1 receptor.Citation26 Consequently, the development of an aminoalkyl indole-based small molecule such as WIN55212 (, compound (4)) provided some degree of CB2 selectivity.Citation27 Eicosanoids such as anandamideCitation22,Citation28 represent endogenous CBs that exhibit greater affinity toward CB1 than CB2. Since centrally acting CB1 receptor agonists are known to produce CNS side effects such as dizziness and cognitive impairment, current focus is to develop CB2 receptor agonists that could produce minimal CNS side effects and to target CB1 receptors at the periphery.Citation22,Citation29,Citation30
Figure 2 Chemical structures of some nonselective CB receptor modulators.
A number of small-molecule CB2 receptor agonists have been developed in the past decade as potential agents to treat nociceptive, inflammatory, and neuropathic pain. The mechanism of CB2-mediated analgesia is not clearly understood. Some studies suggest that CB2 agonists could act on immune cells and prevent the associated inflammatory response. A recent investigation by Hsieh and coworkers shows that the dorsal root ganglia and spinal cord regions are the potential sites of CB2-receptor-mediated analgesia.Citation31 In this regard, one of the early CB2-receptor agonists HU-308 (, compound (5), a bicyclic derivative) exhibited high CB2 selectivity (CB2 inhibition constant [Ki] = 23 nM) and significant pain relief in the formalin model.Citation32
Figure 3 Chemical structures of some selective CB2 receptor modulators.
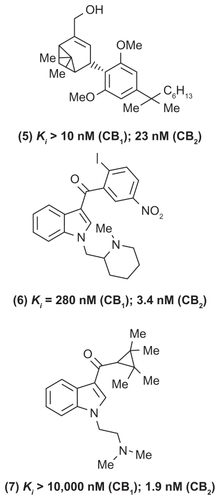
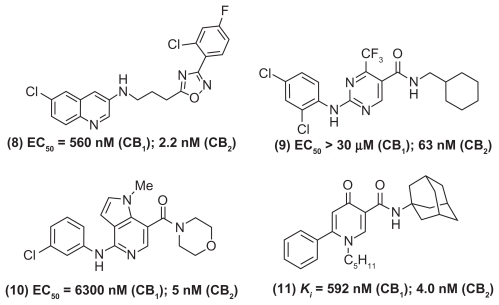
A wide range of small molecules with diverse ring templates have been developed as CB2-selective agonists (, , and ). In this regard, several aminoalkyl indole-based derivatives exhibit superior CB2 receptor binding and selectivity.Citation33–Citation36 For example, AM1241 (, compound (6)) is a highly selective CB2-agonist (CB2 Ki = 3.4 nM; CB1 Ki = 280 nM) that exhibits in vivo peripheral analgesia in inflammatory and neuropathic pain models without exhibiting CNS side effects. Furthermore, the aminoalkyl indole (, compound (7)) was a highly selective CB2 receptor agonist (CB2 Ki = 1.9 nM; CB1 Ki > 10,000 nM; CB1/CB2 selectivity > 5263). In another study, Cheng and coworkers developed a novel series of N-arylamide oxadiazoles where they identified an amide-linked quinolone derivative (, compound (8)) as a potent and selective CB2 agonist (CB2 half-maximal effective concentration [EC50] = 2.2 nM) with excellent oral bioavailability profile in rats.Citation37 In an elegant study, a research team from GlaxoSmithKline discovered compound GW842166X (, compound (9)) based on a pyrimidinecarboxamide template as a clinical candidate to treat inflammatory pain. Compound GW842166X was a selective CB2 receptor agonist (CB2 EC50 = 63 nM; CB1EC50 > 30 μM) and exhibited potent oral activity (ED50 [half-maximal effective dose] = 0.1 mg/kg) in animal models of inflammatory pain. Further lead optimization provided the 5-azaindole (, compound (10)) with superior CB2 binding affinity (CB2EC50 = 5 nM) and efficacy in both acute and chronic pain models.Citation38,Citation39 Another study showed that 4-oxo-1,4-dihydropyridines could serve as useful templates to develop selective CB2 receptor ligands. The phenyl-substituted dihydropyridine (, compound (11)) exhibited excellent CB2-binding affinity and was an inverse agonist (CB2 Ki = 4.0 nM; CB1 Ki = 592 nM).Citation40 Rapid progress has been made in the development of CB2-selective ligands based on a wide variety of ring templates, and a detailed discussion is beyond the scope of this review.Citation30 The evidence acquired to date, clearly supports targeting CB2, CB1/CB2, or CB1 receptors and to develop “peripherally restricted” CB (CB2 selective, dual CB1/CB2) agonists that exhibit reduced CNS side effects as novel agents in the pharmacotherapy of pain disorders.
The TRP channels
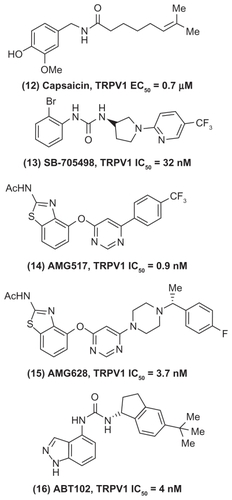
The TRP channel family belongs to ligand-gated and voltage-dependent ion channels/nociceptors that respond to chemical, mechanical, or thermal noxious stimuli at the periphery. They are divided into subfamilies. Many are located in the central and peripheral sensory neurons and are potential targets to treat neuropathic pain.Citation41–Citation43 The TRPV1 channels have been studied extensively and are known to play a critical role in peripheral sensitization of nociceptors and reduce pain threshold when activated by noxious stimuli. Its expression level is high in sensory neurons. The active ingredient of chili peppers, capsaicin (, compound (12)) is a known activator of TRPV1 and is effective as a topical agent to treat pain states. Although opioids are used to treat chronic pain, they exhibit serious side effects such as dizziness, sedation, loss of cognitive function, dependency, respiratory depression, development of tolerance, and constipation. These shortcomings support the need to target novel pathways of pain. In this regard, the role of TRPV1 in peripheral sensitization contributing to acute and chronic pain dictates the need to develop TRPV1 antagonists as potential agents to treat inflammatory and neuropathic pain.Citation44–Citation47 One of the early TRPV1 antagonists to enter the clinical trial was SB-705498 (, compound (13)) based on a pyrrolidine urea that exhibited excellent oral activity in animal models.Citation48 Furthermore, Amgen reported the discovery of a clinical candidate AMG517 (, compound (14)) based on a oxopyrimidine ring template. Compound AMG517 exhibited excellent TRPV1 inhibition (half-maximal inhibitory concentration [IC50] = 0.9 nM); however, it had a long half-life and low aqueous solubility. Further lead optimization provided compound AMG628 (Figure, compound (15)), the piperazinylpyrimidine derivative that exhibited good TRPV1 inhibition, in vivo half-life, and aqueous solubility and was considered as a clinical candidate.Citation49,Citation50 Recently, Abbott Laboratories reported the discovery of an orally active clinical candidate (R)-1-(5-tert-butyl-2,3-dihydro-1-H-inden-1-yl)-3-(1H-indazol-4-yl) urea (ABT102, , compound (16)) to treat chronic pain. This small molecule exhibited potent TRPV1 binding (TRPV1 IC50 = 4 nM) and was effective in various in vivo pain models such as carrageenan induced postoperative and cancer pain. In addition, this agent did not exhibit side effects such as sedation and constipation commonly seen with opiate therapy, highlighting the fact that selective targeting of TRPV1 should provide agents that lack the adverse side effects of opiates.Citation51–Citation53 Accordingly, several small-molecule candidates are being developed by the pharmaceutical companies.Citation41,Citation47,Citation54 The representative examples discussed here indicate the enormous potential of targeting TRPV1 receptors to treat both acute and chronic pain. Compared with peripherally restricted CB agonists, TRPV1 receptor modulators reduce pain by acting on both central and peripheral pain pathways, suggesting their potential to cause CNS side effects. The challenges include recognizing TRPV1 gene polymorphism in patients to predict desired therapeutic responses and identify potential side effects such as hyperthermia.Citation46
P2X receptors
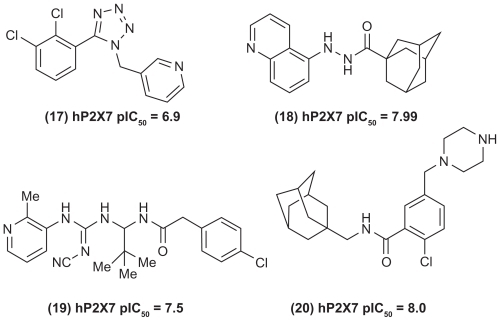
The neurotransmitter ATP (adenosine-5′-triphosphate) is known to produce pain through activating the purinergic receptors P1 and P2. The P1 receptors are known as adenosine receptors, whereas P2 receptors are further divided into P2Y (G-protein coupled receptors) and P2X (ligand-gated channels). The P2X receptors are subdivided into seven receptor subtypes (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 respectively) and have attracted widespread attention as potential targets to develop novel pain therapeutics.Citation55–Citation58 Among the P2X family, P2X7 is known to be present in immune cells such as monocytes, macrophages, mast cells, lymphocytes, and microglia, indicating their role in disorders such as pain, neurodegeneration, and inflammatory conditions.Citation59,Citation60 In this regard, novel P2X7 receptor antagonists are being developed as clinical candidates to treat acute and chronic pain.Citation61 Scientists at Abbott Laboratories reported the development of some distinct small molecules as P2X7 receptor antagonists. In this regard, the 1-benzyl-5-phenyltetrazoles (, compound (17)) exhibited potent P2X7 inhibition and were effective in a neuropathic pain model. In another study, a series of N′-acyl hydrazides was reported as a novel series of P2X7 receptor antagonists with in vivo activity. Compound (18) shown in was identified as a potent P2X7 receptor antagonist. Further studies led to the development of a novel series of cyanoguanidines with potent P2X7 inhibition, and compound (19) () was effective in a neuropathic pain model.Citation62–Citation64 In addition, researchers from AstraZeneca reported the development of adamantine-based small molecules (eg, compound (20) shown in ) as P2X7 receptor antagonists with ability to prevent the formation of the pro-inflammatory cytokine interleukin-1β.Citation65 A detailed description of several small-molecule P2X receptor modulators has been reviewed elsewhere.Citation61,Citation66
Targeting glia cells
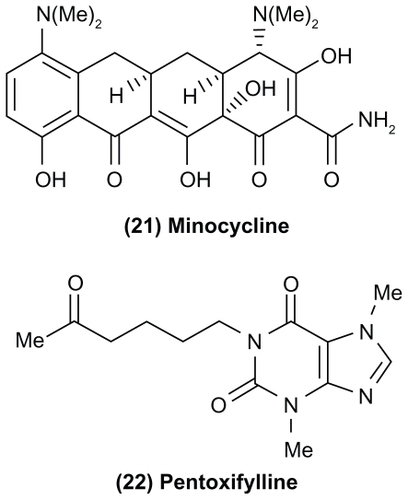
Recent studies have focused on the role of non-neuronal cells such as astrocytes and glia or glial cells in pain pathophysiology. Under pathological conditions, glial cells get activated and are known to release pro-inflammatory cytokines, chemokines, and other signaling molecules that contribute to neuropathic pain.Citation67–Citation69 Some strategies include blocking glial cell activation, prevent the biosynthesis of pro-inflammatory cytokines, block the action of pro-inflammatory cytokines, and disrupt their signaling. In this regard, the tetra-cycline antibiotic minocycline (, compound (21)) is known to selectively target microglia and could suppress the release of pro-inflammatory cytokines, whereas the xanthine derivative pentoxyfylline (Trental®, Aventis, Strasbourg, France) (, compound (22)) currently used to treat chronic occlusive arterial disease and AV411 (Ketas™, Senju Pharmaceutical Co, Osaka, Japan) are known to inhibit pro-inflammatory cytokine biosynthesis. These small molecules are able to cross the blood–brain barrier, indicating their potential to target neuropathic pain. In contrast, biological molecules such as etanercept (Enbrel®, Amgen, Thousand Oaks, CA) and the interleukin-1β antagonist anakinra (Kineret®, Amgen) are known to exhibit efficacy in neuropathic animal models, suggesting their ability to block glial cell mediated cytokine signaling.Citation68 However, these agents are injectables that exhibit poor CNS penetration. In the last decade, glial cells have emerged as attractive targets to prevent chronic pain.Citation70,Citation71 In this regard, the neuroprotective nature of glial cells during tissue injury suggests a careful approach toward developing novel glial cell modulators. The molecular mechanisms of glial cell activation and its consequences is still a work in progress.
Conclusion
The last decade has seen an unprecedented surge in understanding the complexity of pain pathology. The identification of novel pain targets such as CB receptors, FAAH, voltage- and ligand-gated ion channels (sodium channels, T-type calcium channels, P2X receptors, TRP channels), peptide receptor antagonists, NGF and glial cell modulators to treat nociceptive, inflammatory, and neuropathic pain is highly promising. Preclinical data shows that one can develop peripherally restricted agents such as CB receptor modulators and FAAH inhibitors that do not exhibit psychotropic effects indicating their superior side-effect profile compared with traditional pain therapies. The challenge is to prove the efficacy seen in preclinical data of novel agents with clinical evidence. The benefit-to-risk ratios of novel pain therapies will come under careful scrutiny of regulatory agencies. Despite the challenges ahead, it is clear that understanding the molecular mechanisms of novel pain targets will go a long way in developing selective or “site specific” agents that exhibit efficacy and superior side-effect profile as pain therapeutics.
Acknowledgments
The authors would like to thank the School of Pharmacy and Faculty of Science at the University of Waterloo for supporting the research work.
Disclosure
The authors have no conflicts of interest with the current work and received no payment for the preparation of this manuscript.
References
- AnandKJSCraigKDNew perspectives on the definition of painPain1996671368895225
- MelnikovaIPain marketNat Rev Drug Dis201098589590
- TsangAVon KorffMLeeSCommon chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disordersJ Pain200891088389118602869
- GouckeRCThe management of persistent painMed J Aust2003178944444712720511
- HelmsJEBaroneCPPhysiology and treatment of painCrit Care Nurse2008286384919047694
- ReichlingDBLevineJDPain and death: neurodegenerative disease mechanisms in the nociceptorAnn Neurol2011691132121280072
- BaronRNeuropathic pain: a clinical perspectiveHandb Exp Pharmacol200919433019655103
- VoscopoulosCLemaMWhen does acute pain become chronic?Br J Anaesth2010105Suppl 1i69i8521148657
- MilanMJThe induction of pain: an integrative reviewProg Neurobiol199957111649987804
- SchaibleHGPeripheral and central mechanisms of pain generationHandb Exp Pharmacol200717732817087118
- ScholzJWoolfCJCan we conquer pain?Nat Neurosci20025Suppl1062106712403987
- BrasMDordevicVGrequrekRBulajicMNeurobiological and clinical relationship between psychiatric disorders and chronic painPsychiatr Danub201022222122620562750
- MarazzitiDMungaiFVivarelliLPrestaSDell’OssoBPain and psychiatry: a critical analysis and pharmacological reviewClin Pract Epidemiol Ment Health200623117087832
- LynchMEWatsonCPNThe pharmacotherapy of chronic pain: a reviewPain Res Manage20061111138
- KroenkeKKrebsEEBairMJPharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviewsGen Hosp Psychiatry200931320621919410099
- Centers for Disease Control and PreventionNational Hospital Ambulatory Medical Care Survey (NHAMCS)Atlanta, GACenters for Disease Control and Prevention Available from: http://www.cdc.gov/nchs/ahcd.htmAccessed June 18, 2011
- WoodcockJA difficult balance – pain management, drug safety, and the FDAN Eng J Med20093612221052107
- TrelleSReichenbachSWandelSCardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysisBMJ2011342c708621224324
- MendelsonJFlowerKPletcherMJGallowayGPAddiction to prescription opioids: characteristics of the emerging epidemic and treatment with buprenorphineExp Clin Psychopharmacol200816543544118837640
- FoxABevanSTherapeutic potential of cannabinoid receptor agonists as analgesic agentsExpert Opin Investig Drugs2005146695703
- PertweeRGCannabinoid receptors and painProg Neurobiol200163556961111164622
- ChengYHitchcockSATargeting cannabinoid agonists for inflammatory and neuropathic painExpert Opin Investig Drugs2007167951965
- GaliequeSMarySMarchandJExpression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulationsEur J Biochem1995232154617556170
- CurranHVBrignellCFletcherSMiddletonPHenryJCognitive, subjective dose–response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis usersPsychopharmacology20021641617012373420
- RobinsonLGoonawardenaAVPertweeRGHampsonRERiedelGThe synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in ratsBr J Pharmacol2007151568870017502849
- HerkenhamMLynnABJohnsonMRMelvinLSde CostaBRRiceKCCharacterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic studyJ Neurosci19911125635831992016
- DevaneWAHanusLBreuerAIsolation and structure of a brain constituent that binds to the cannabinoid receptorScience19922585090194619491470919
- PertweeRGPharmacological actions of cannabinoidsHandb Exp Pharmacol200516815116596770
- RahnEJHohmanAGCannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedsideNeurotheraptics200964713737
- ThakurGATichkuleRBajajSMakriyannisALatest advances in cannabinoid receptor agonistsExpert Opin Ther Patents2009191216471673
- HsiehGCPaiMChandranPCentral and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in ratsBr J Pharmacol2011162242844020880025
- HanusLBreuerATchillibonSHU-308: a specific agonist for CB2, a peripheral cannabinoid receptorProc Natl Acad Sci U S A19999625142281423310588688
- YaoBBMukherjeeSFanYIn vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor?Br J Pharmacol2006149214515416894349
- IbrahimMMDengHZvonokAActivation of CB2-cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNSProc Natl Acad Sci U S A200310018105291053312917492
- FrostMDartMJTietjeKRIndol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activityJ Med Chem201053129531519921781
- FrostJMDartMJTietjeKRIndol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activityJ Med Chem20085161904191218311894
- ChengYAlbrechtBKBrownBDiscovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonistsJ Med Chem200851165019503418680277
- GiblinGMPO’ShaughnessyCTNaylorADiscovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory painJ Med Chem200750112597260017477516
- GiblinGMPBillintonABriggsMDiscovery of 1-[4-(3-chlorophenylamino)-1-methyl-1H-pyrrolo[3,2-c]pyridin-7-yl]-1-morpholin-4-ylmethanone (GSK554418A), a brain penetrant 5-azaindole CB2 agonist for the treatment of chronic painJ Med Chem200952195785578819743867
- El BakaliJMuccioliGGRenaultN4-Oxo-1,4-dihydropyridines as selective CB2 Cannabinoid receptor ligands: structural insights into the design of a novel inverse agonist seriesJ Med Chem201053227918793120979417
- PatapoutianATateSWoolfCJTransient receptor potential channels: targeting pain at the sourceNat Rev Drug Dis2009815568
- RomanovskyAAAlmeidaMCGaramiAThe transient receptor potential vanilloid-1 channel in thermoregulation: thermosensor it is notPharmacol Rev200961322826119749171
- VenkatachalamKMontellCTRP channelsAnnu Rev Biochem20077638741717579562
- LevineJDHaberNATRP channels: targets for the relief of painBiochim Biophys Acta200717728989100317321113
- PremkumarLSTargeting TRPV1 as an alternative approach to narcotic analgesic to treat chronic pain conditionsAAPS J201012336137020440589
- JoshiNKSzallasiATRPV1 antagonists: the challenges for therapeutic targetingTrends Mol Med2009151142219097938
- GunthorpeMJChizhBAClinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathwayDrug Discov Today2009141–2566719063991
- RamiHKThompsonMStempGDiscovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical developmentBioorg Med Chem Lett200616123287329116580202
- DohertyEMBannonAWBoYNovel vanilloid receptor-1 antagonists: 2. Structure-activity relationship of 4-oxopyrimidines leading to the selection of a clinical candidateJ Med Chem200750153515352717585750
- WangHLKatonJBalanCNovel vanilloid receptor-1 antagonists: 3. The identification of a second-generation clinical candidate with improved physicochemical and pharmacokinetic propertiesJ Med Chem200750153528353917585751
- GomtsyanABayburtEKSchmidtRGIdentification of (R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl) urea (ABT-102) as a potent TRPV1 antagonist for pain managementJ Med Chem200851339239518183945
- HonorePChandranPHernandezGRepeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist-induced hyperthermiaPain20091421–2273519135797
- RowbothamMCNothaftWDuanWROral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trialPain201115251192200021377273
- WestawaySMThe potential of transient receptor potential vanilloid type 1 channel modulators for the treatment of painJ Med Chem200750112589259617489570
- ChizhBAIllesPP2X receptors and nociceptionPharmacol Rev200153455356811734618
- KennedyCAssisTSCurrieJRowanEGCrossing the pain barrier: P2 receptors as targets for novel analgesicsJ Physiol2003553Pt 368369414514872
- KennedyCP2X receptors: targets for novel analgesics?Neuroscientist200511434535616061521
- BurnstockGPurinergic signaling and disorders of the central nervous systemNat Rev Drug Dis200878575590
- SurprenantANorthRASignaling at purinergic P2X receptorsAnnu Rev Physiol20097133335918851707
- HughesJPHatcherJPChessellIPThe role of P2X7 in pain and inflammationPurinergic Signal200731–216316918404430
- GunosewoyoHKassiouMP2X purinergic receptor ligands: recently patented compoundsExpert Opin Ther Pat201020562564620205618
- NelsonDWGreggRJKortMEStructure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonistsJ Med Chem200649123659366616759108
- NelsonDWSarrisKKalvinDMStructure-activity relationship studies on N′-aryl carbohydrazide P2X7 antagonistsJ Med Chem200851103030303418438986
- MedranoAPDonnelly-RobertsDLHonorePDiscovery and biological evaluation of novel cyanoguanidine P2X7 antagonists with analgesic activity in a rat model of neuropathic painJ Med Chem200952103366337619397270
- FurberMAlcarazLBentJEDiscovery of potent and selective adamantane-based small-molecule P2X7 receptor antagonists/interleukin-1β inhibitorsJ Med Chem200750245882588517963373
- GuileSDAlcarazLBirkinshawTNAntagonists of the P2X7 receptor. From lead identification to drug developmentJ Med Chem200952103123314119191585
- WatkinsLRMaierSFTargeting glia to control clinical pain: an idea whose time has comeDrug Discov Today2004118388
- MilliganEDWatkinsLRPathological and protective roles of glia in chronic painNat Rev Neurosci2009101233619096368
- McMahonSBMalcangioMCurrent challenges in glia-pain biologyNeuron2009641465419840548
- SvenssonCIBrodinESpinal astrocytes in pain processing: non-neuronal cells as therapeutic targetsMol Interv2010101253820124561
- BeggsSSalterMWMicroglia-neuronal signaling in neuropathic pain hypersensitivity 2.0Curr Opin Neurobiol201020447448020817512