Abstract
Background
Postoperative delirium (POD) significantly impacts patient outcomes after acute type A aortic dissection (ATAAD) surgeries. This study investigates the role of Neuronal Pentraxin 2 (NPTX2) as a potential biomarker for POD in ATAAD patients.
Methods
This secondary analysis involved ATAAD patients from a prospective observational study. Serum NPTX2 levels were measured preoperatively and immediately postoperatively using Enzyme-Linked Immunosorbent Assay (ELISA). Delirium was assessed using the Confusion Assessment Method (CAM) or CAM for the ICU (CAM-ICU). Statistical analyses included the Pearson Correlation Coefficient and multivariate logistic regression to evaluate the association between NPTX2 levels and POD.
Results
Among the 62 patients included, 46.77% developed POD. Patients with POD had significantly lower preoperative and postoperative serum NPTX2 levels. The Receiver Operating Characteristic (ROC) curve analysis showed that postoperative NPTX2 had a strong predictive capability for POD (AUC = 0.895). The optimal cutoff for postoperative NPTX2 in predicting POD was less than 421.4 pg/mL. Preoperative NPTX2 also demonstrated predictive value, albeit weaker (AUC = 0.683).
Conclusion
Serum NPTX2 levels, both preoperatively and postoperatively, are promising biomarkers for predicting POD in ATAAD patients. These findings suggest that NPTX2 could be instrumental in early POD detection and intervention strategies.
Introduction
Aortic dissection, a severe cardiovascular condition, presents a significant risk of morbidity and mortality.Citation1 This condition, characterized by a tear in the aorta’s inner layer, allows blood to flow between the layers of the aortic wall, causing them to separate. Particularly, acute type A aortic dissection (ATAAD) demands immediate medical intervention due to its potential for catastrophic complications, such as aortic rupture, cerebrovascular accidents, and multi-organ dysfunction.Citation2 Surgical intervention remains the cornerstone for managing ATAAD in clinical settings. Recent advancements have been observed in the realm of surgical approaches and techniques.Citation3 Despite these developments, postoperative complications remain a significant concern,Citation4 underscoring the need for continuous exploration and management strategies in this domain.
Delirium, characterized by acute confusion and fluctuating consciousness levels, is a common complication during the postoperative period.Citation5 The occurrence of postoperative delirium (POD) profoundly impacts patient outcomes, leading to extended hospital stays, elevated mortality rates, and an increased risk of sustained cognitive deficits.Citation6,Citation7 In the context of ATAAD surgeries, the incidence of POD is usually higher than in other types of surgery which is attributed to the lengthy durations of operation and cardiopulmonary bypass, substantial surgical trauma, and the requisite implementation of deep hypothermic circulatory arrest.Citation8–10 The high incidence of POD following ATAAD surgery underscores the need for intensive postoperative surveillance and proactive intervention strategies to manage and mitigate the risks associated with this significant cardiovascular challenge. However, the early detection of POD is still challenging due to the lack of effective clinical detection methods, highlighting the urgent need for reliable and sensitive diagnostic biomarkers.
Neuronal pentraxin 2 (NPTX2), a key component of the neuronal pentraxins family, plays a crucial role in the development and plasticity of synapses between excitatory and inhibitory neurons, and this interaction is fundamental for regulating neuronal network activities.Citation11 Recent research has identified a reduction in NPTX2 levels within the cerebrospinal fluid (CSF) or serum as a promising predictive biomarker for various neurodegenerative disorders, including Alzheimer’s Disease (AD), vascular dementia, and schizophrenia.Citation12–14 Given the established connection between abnormal neuronal network activities, neurodegenerative diseases, and the onset of POD,Citation15,Citation16 it is plausible to hypothesize that NPTX2 may serve as an effective predictive biomarker for the development of POD. Additionally, a study reported that NPTX2 plays a key role in regulating functional connectivity in the salience/ventral attention network.Citation17 Higher levels of NPTX2 in the CSF correlate with greater functional connectivity in this network. Furthermore, the salience/ventral attention network plays a central role in cognitive control and externally focused attention.Citation18–20 Dysfunctions in these areas are core symptoms of POD. This evidence further suggests that NPTX2 may play a role in the occurrence of POD and may be a promising biomarker for its development. Nonetheless, the precise correlations between NPTX2 levels and the incidence of POD have yet to be thoroughly investigated. Therefore, our study is dedicated to exploring the potential relationship between NPTX2 and the development of POD in patients with ATAAD, to provide valuable insights for the prediction and management of this postoperative complication.
Materials and Methods
Study Overview
This study is a secondary analysis of blood samples collected from patients enrolled in a prospective observational study, which tested the prognostic value of quantitative EEG monitoring on brain function in patients with type A aortic dissection (ChiCTR2200055980). The study was conducted at Nanjing Drum Tower Hospital, an Affiliated Hospital of the Medical School at Nanjing University, and received ethical approval from the Medical Ethics Committee of Nanjing Drum Tower Hospital (2022-034-01). This study was performed following the ethical principles for medical research involving human subjects, as detailed in the Declaration of Helsinki. Subjects were enrolled from February 1 to June 30, 2022. Written informed consent was obtained from all patients before their participation.
In this secondary study, the inclusion criteria were as follows: (1) being 18 years of age or older; (2) diagnosis of ATAAD and undergoing open thoracotomy aortic surgery; and (3) provision of informed consent. The exclusion criteria included: (1) a history of neurological or psychiatric disorders; (2) cerebral infarction within the past six months; (3) pregnancy; (4) malignant tumors; (5) extremely moribund patients with an expected life expectancy of less than 24 hours; (6) inability to complete delirium testing due to preoperative or postoperative coma, stroke, or other reasons; (7) baseline delirium; and (8) lack of blood samples drawn preoperatively or immediately after ICU admission.
Delirium Assessment
Delirium was assessed using the Confusion Assessment Method (CAM) or CAM for the ICU (CAM-ICU) when the Richmond Agitation-Sedation Score (RASS) was greater than or equal to −2. This assessment was conducted twice daily at 8:00 AM and 8:00 PM for the first three days, followed by once daily at 8:00 AM for the subsequent four days after surgery, in both the ICU and the general ward. Patients who met the CAM or CAM-ICU criteria during any of the postoperative screenings were considered to have delirium.
The CAM or CAM-ICU evaluates delirium based on four clinical features: (a) acute onset of cognitive changes with a fluctuating course, (b) inattention, (c) disorganized thinking, and (d) altered level of consciousness. A diagnosis of delirium was made based on the presence of features (a) and (b), plus either (c) or (d). For the baseline assessment of delirium, participants were evaluated before undergoing surgery. All assessments were conducted by well-trained investigators.
Data Collection
For all patients, we recorded key characteristics, including age, sex, body mass index (BMI), and comorbidities (such as hypertension, diabetes, smoking, and alcoholism history). Additionally, scores from two types of risk prediction models—European System for Cardiac Operative Risk Evaluation II (EUROSCORE II) and German Registry of Acute Aortic Dissection Type A (GERAADA)—were noted. Surgical details documented encompassed the specifics of aortic root procedure, arch surgery, any combined cardiac surgery, duration of surgery, cardiopulmonary bypass (CPB) time, aorta cross-clamp time, deep hypothermic circulatory arrest (DHCA) time, and the lowest bladder temperature. Preoperative and Postoperative (immediately after ICU admission) laboratory tests were also recorded, including levels of total bilirubin, albumin, glucose, creatinine, sodium (Na+), chloride (Cl−), white blood cell count (WBC), hemoglobin (Hb), and platelets. Since preoperative C-reactive protein (CRP) tests are not routinely conducted at our center, only post-ICU admission immediate CRP levels were recorded. The selection of these recorded items was based on previous studies and the specific circumstances of our center, hypothesizing their potential relevance to the incidence of POD.
NPTX2 Measurement
Blood samples from the included patients were drawn preoperatively and immediately upon admission to the ICU. Following collection, the serum was separated and stored at −80°C for subsequent analysis. The serum level of NPTX2 was then measured using an enzyme-linked immunosorbent assay (ELISA) kit (MBS924168, MyBioSource, San Diego, USA).
Statistical Analysis
Continuous variables were assessed for normality using the Shapiro–Wilk test. Those conforming to a normal distribution were represented using the mean ± standard deviation (SD), while non-normally distributed variables were represented by the median (Q1, Q3). For data following a normal distribution, independent t-tests were employed. Conversely, non-normally distributed data were analyzed using the Mann–Whitney U-test. Categorical variables were examined by calculating the count and percentage in each group and were analyzed using either the Chi-square test or Fisher’s exact test, as appropriate. After univariate analyses, the risk factors for POD were evaluated using multivariate logistic regression analysis. In this analysis, variables with univariate P values less than 0.05 were included. The predictive ability of the variables for POD was analyzed using the receiver operating characteristic (ROC) curve. The Youden Index was used to identify the optimal cutoff point for the variables. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Characteristics of Patients with or Without POD
The study flow is illustrated in . In total, 135 patients were screened. Of these, 73 patients were excluded for various reasons: 1 patient had neurological conditions, 1 patient had cerebral infarction within half a year, 2 patients’ life expectancy few than 24 h, 14 patients were unable to complete delirium testing, and 55 patients lacked the necessary blood samples. Consequently, 62 patients were ultimately included in the analysis. Among these, 29 patients (46.77%) were diagnosed with POD.
Figure 1 The study flowchart, analysis of the association between serum NPTX2 levels and the occurrence of POD, a correlation matrix of variables, and ROC curve analysis. (A) Flow chart of the study. (B) The preoperative serum NPTX2 level was significantly lower in the POD group compared to the Non-POD group. (C) Incidence of POD in relation to quartiles of preoperative serum NPTX2 level. (D) The postoperative serum NPTX2 level was significantly lower in the POD group compared to the Non-POD group. (E) Incidence of POD in relation to quartiles of postoperative serum NPTX2 levels. (F) Correlation matrix of the variables. (G) ROC curve analysis of preoperative and postoperative serum NPTX2 levels for distinguishing POD (n=62). **P < 0.01, ***P < 0.001.
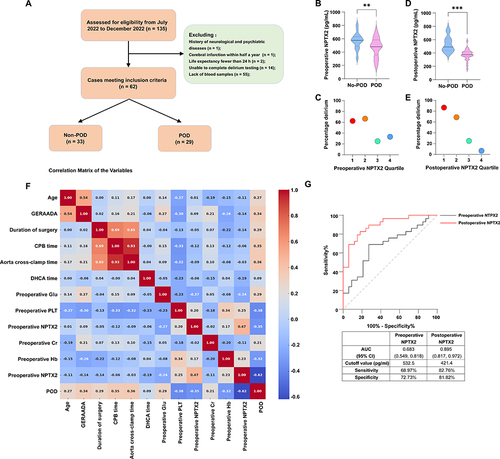
Patients with POD exhibited several distinct characteristics compared to those without POD. They were of advanced age (P = 0.036) and had higher GERAADA scores (P = 0.001). Additionally, the duration of surgery (P = 0.008), CPB time (P = 0.005), aorta cross-clamp time (P = 0.008), and DHCA time (P = 0.047) were longer. These patients also had higher preoperative glucose (P = 0.036) and postoperative creatinine (P = 0.034) levels. Moreover, compared to non-POD patients, those with POD had significantly lower levels of preoperative platelets (P = 0.002) and NPTX2 (P = 0.006), postoperative Hb (P = 0.011) and NPTX2 (P < 0.001), as detailed in .
Table 1 Characteristics of Patients with or Without POD
A Decrease in Serum NPTX2 Levels, Both Preoperatively and Postoperatively, Was Associated with the Occurrence of POD
We utilized Enzyme-Linked Immunosorbent Assay (ELISA) to measure these serum NPTX2 levels. The results indicated that NPTX2 levels were significantly lower in the POD group compared to the Non-POD group, evident both before surgery (P = 0.006) () and after surgery (P < 0.001) (). Additionally, patients in the lower quartile for serum NPTX2 levels exhibited a higher incidence of POD, particularly in postoperative measurements ( and ).
A Lower Serum Level of NPTX2 Noted Both Before and After Surgery, Was Identified as an Independent Risk Factor for the Development of POD
Our initial analysis incorporated variables from that exhibited a P value less than 0.05. Subsequent analysis employed the Pearson Correlation Coefficient to evaluate the correlation between pairs of variables, as depicted in . This examination uncovered a significant correlation among the duration of surgery, cardiopulmonary bypass (CPB) time, and aortic cross-clamp time, with an especially strong correlation observed between CPB time and aortic cross-clamp time. To mitigate multicollinearity in the ensuing multivariate logistic regression analysis, we excluded CPB time and aorta cross-clamp time. The refined multivariate logistic regression, which adjusted for age, GERAADA score, duration of surgery, DHCA time, preoperative glucose level, preoperative platelet count, postoperative creatinine level, and postoperative Hb level, affirmed that low serum NPTX2 levels, both preoperative (OR = 0.991, 95% CI: 0.984 −0.999, P = 0.022) and postoperative (OR = 0.916, 95% CI: 0.856 −0.981, P = 0.012), are independent risk factors for POD, as detailed in .
Table 2 Multivariate Logistic Regression Analysis of POD Risk Factors
The Predictive Performance of Preoperative and Postoperative Serum NPTX2 Levels for POD
This performance was evaluated using the ROC curve. As depicted in , the postoperative NPTX2 level showed strong predictive capability for POD, with an area under the ROC curve (AUC) of 0.895 and a 95% CI ranging from 0.817 to 0.972 (P < 0.001). The optimal cutoff point for postoperative NPTX2 in predicting POD was identified as less than 421.4 pg/mL, demonstrating a sensitivity of 82.76% and a specificity of 81.82%. Conversely, the predictive performance of preoperative NPTX2 was comparatively weaker, with an AUC of 0.683 and a 95% CI from 0.549 to 0.818 (P = 0.013). The optimal cutoff value for preoperative NPTX2 in predicting POD was determined to be less than 532.5 pg/mL, with a sensitivity of 68.97% and a specificity of 72.73%.
Discussion
Our study showed that preoperative and postoperative serum level of NPTX2 was closely associated with the development of POD in patients with ATAAD. Thus, the NTPX2 could serve as a new serum biomarker to identify patients at risk for developing POD. To the best of our knowledge, this is the first study to investigate serum NPTX2 levels with POD.
Our study observed a 46.77% occurrence rate of postoperative delirium (POD) in acute type A aortic dissection (ATAAD) patients, aligning with findings from previous research in this area.Citation21 The emergence of POD is commonly attributed to a complex interplay of multiple predisposing and precipitating factors.Citation15 Notably, advanced age stands out as a prominent and well-documented predisposing factor for POD, a finding corroborated by our study. The presence of multiple predisposing factors reduces the number of precipitating factors needed to trigger POD.Citation22 Consequently, older, more vulnerable adults are at a higher risk of developing delirium from triggers that would not typically affect younger individuals.
The EuroSCORE system, known for its simplicity, objectivity, and user-friendliness, is a standard tool for evaluating adult heart surgery risks.Citation23 Earlier research has indicated that higher EuroSCORE II scores correlate with an increased risk of delirium following coronary artery bypass grafting (CABG) surgery.Citation24 However, our study did not observe significant differences in EuroSCORE II scores between the non-POD and POD groups in ATAAD surgeries, despite its reputed efficacy in predicting operative mortality in these cases.Citation25 On the other hand, the GERAADA score, a straightforward and effective metric for predicting 30-day mortality in patients undergoing ATAAD surgery,Citation26 displayed notable differences. This finding suggests that the GERAADA score may be more indicative of delirium risk than the EuroSCORE II in these patients.
Extended surgical durations, exposure to CPB, and prolonged aortic cross-clamp times are established risk factors for POD in cardiac surgery patients.Citation27–29 Additionally, DHCA is a distinctive feature of aortic surgery compared to general cardiac procedures.Citation30 Despite the significant advancements DHCA has brought to ATAAD surgeries, it also poses a risk for POD.Citation31 Our findings align with these previously mentioned studies.
Contrary to common perception among many clinicians, only about 25% of delirium cases are hyperactive, characterized by agitation; the majority are hypoactive, often linked to worse outcomes, possibly due to its under-recognition.Citation32 Over the past decades, more than 40 clinical assessment tools for delirium have been developed, yet the condition remains largely undetected. Although these tools demonstrate high reliability and validity in research settings, their effectiveness is considerably less satisfactory in clinical practice.Citation33 Therefore, establishing early, objective, and accurate assessment methods for delirium is essential.
In recent years, the core of delirium has been increasingly understood as aberrant neural network activities.Citation34,Citation35 Consequently, molecules influencing these neural networks are considered potential biomarkers. Despite this, research specifically focusing on these molecules, particularly in ATAAD patients, is scarce. Recent studies are increasingly recognizing NPTX2 as a critical molecule in regulating neural network activities.Citation36 This led us to investigate the association between NPTX2 levels and POD. Our findings indicate that a decrease in serum NPTX2 levels, both preoperatively and postoperatively, serves as a significant predictor for the onset of POD, particularly the postoperative levels. Furthermore, our study did not identify significant predictive value in perioperative serum levels of glucose, platelets, creatinine, and hemoglobin for POD. These discoveries open up new possibilities for early detection and preemptive alert systems for POD using serum NPTX2 levels.
NPTX2 has been verified as being intricately linked to the pathogenesis of AD. Notably, a previous study indicated that cerebrospinal fluid (CSF) levels of the synaptic protein NPTX2 are markedly reduced in AD patients, positioning NPTX2 as a potential biomarker for tracking cognitive deterioration and overall disease progression.Citation37 This protein’s predictive capabilities have been found to surpass those of traditional biomarkers like Aβ1-42 and Tau. Moreover, evidence suggests that higher baseline levels of NPTX2 in AD may offer neuroprotective effects, correlating with lesser medial temporal lobe atrophy and slower cognitive decline.Citation38 Given the established role of AD as a predisposing factor for POD and the increased likelihood of AD following POD, NPTX2 appears to be a crucial molecular mechanism in both the onset of delirium and its progression to AD. This connection not only enhances our understanding of these conditions but also suggests that modulating NPTX2 expression levels could be a novel clinical intervention strategy to prevent delirium and potentially hinder the progression to AD.
Our study, while insightful, presents several limitations that merit consideration. First, being a single-center clinical study with a relatively small sample size, there’s an inherent risk of biases that might not be fully representative of a larger, more diverse population. This is particularly relevant as our focus was on identifying biomarkers. Additionally, our research is a derivative of a prior prospective observational study. Owing to the absence of a comprehensive blood collection protocol in the original study, a significant number of patients were excluded due to the unavailability of blood samples, potentially leading to selection bias. Furthermore, the correlation between serum levels of NPTX2 and its levels in the brain or CSF remains unclear. Future studies aiming to elucidate this relationship could deepen our understanding of NPTX2’s role in predicting and influencing the development of POD, thereby paving the way to comprehend the mechanisms of POD occurrence and identifying new targets for its prevention. Lastly, our investigation was exclusively concentrated on ATAAD surgeries. Consequently, whether our findings regarding NPTX2’s effectiveness as a biomarker extend to other surgical contexts remains unclear.
Conclusion
Our study demonstrated that Serum NPTX2 levels, measured both preoperatively and postoperatively, are a promising biomarker for predicting the occurrence of POD in patients with ATAAD. Additionally, this finding could offer a potential target for interventions aimed at preventing POD. However, to fully understand the mechanisms and the impact of NPTX2 on the development and prevention of POD, further research, encompassing both preclinical and clinical studies, is necessary.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study received approval from the Medical Ethics Committee of Nanjing Drum Tower Hospital (Approval Number: 2022-034-01). This research was conducted in accordance with the ethical principles for medical research involving human subjects as outlined in the Declaration of Helsinki. Written informed consent was obtained from each patient for their participation in this study.
Disclosure
The authors declare no competing interests in this work.
Additional information
Funding
References
- Hameed I, Cifu AS, Vallabhajosyula P. Management of Thoracic Aortic Dissection. JAMA. 2023;329(9):756–757. doi:10.1001/jama.2023.0265
- Zhu Y, Lingala B, Baiocchi M, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2020;76(14):1703–1713. doi:10.1016/j.jacc.2020.07.061
- Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18(5):331–348. doi:10.1038/s41569-020-00472-6
- Song Y, Liu L, Jiang B, Wang Y. Risk factors of cerebral complications after Stanford type A aortic dissection undergoing arch surgery. Asian J Surg. 2022;45(1):456–460. doi:10.1016/j.asjsur.2021.07.071
- Li T, Li J, Yuan L, et al. Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: the RAGA Randomized Trial. JAMA. 2022;327(1):50–58. doi:10.1001/jama.2021.22647
- Goldberg TE, Chen C, Wang Y, et al. Association of Delirium With Long-term Cognitive Decline: a Meta-analysis. JAMA Neurol. 2020;77(11):1373–1381. doi:10.1001/jamaneurol.2020.2273
- Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. 2009;87(5):1469–1474. doi:10.1016/j.athoracsur.2009.02.080
- Cai S, Zhang X, Pan W, et al. Prevalence, Predictors, and Early Outcomes of Post-operative Delirium in Patients With Type A Aortic Dissection During Intensive Care Unit Stay. Front Med Lausanne. 2020;7:572581. doi:10.3389/fmed.2020.572581
- Liu J, Yang F, Luo S, et al. Incidence, Predictors and Outcomes of Delirium in Complicated Type B Aortic Dissection Patients After Thoracic Endovascular Aortic Repair. Clin Interv Aging. 2021;16:1581–1589. doi:10.2147/cia.S328657
- Liu Z, Pang X, Zhang X, Cao G, Fang C, Wu S. Incidence and Risk Factors of Delirium in Patients After Type-A Aortic Dissection Surgery. J Cardiothorac Vasc Anesth. 2017;31(6):1996–1999. doi:10.1053/j.jvca.2016.11.011
- Xiao M-F, Xu D, Craig MT, et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. Elife. 2017;6:798. doi:10.7554/eLife.23798
- Libiger O, Shaw LM, Watson MH, et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimers Dement. 2021;17(12):1976–1987. doi:10.1002/alz.12353
- Shao K, Shan S, Ru W, Ma C. Association between serum NPTX2 and cognitive function in patients with vascular dementia. Brain Behav. 2020;10(10):e01779. doi:10.1002/brb3.1779
- Xiao MF, Roh SE, Zhou J, et al. A biomarker-authenticated model of schizophrenia implicating NPTX2 loss of function. Sci Adv. 2021;7(48):eabf6935. doi:10.1126/sciadv.abf6935
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/s0140-6736(13)60688-1
- Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. doi:10.1016/j.jagp.2013.09.005
- Soldan A, Moghekar A, Walker KA, et al. Resting-State Functional Connectivity Is Associated With Cerebrospinal Fluid Levels of the Synaptic Protein NPTX2 in Non-demented Older Adults. Front Aging Neurosci. 2019;11:132. doi:10.3389/fnagi.2019.00132
- Segal A, Parkes L, Aquino K, et al. Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders. Nat Neurosci. 2023;26(9):1613–1629. doi:10.1038/s41593-023-01404-6
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013;33(16):7091–7098. doi:10.1523/jneurosci.4692-12.2013
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi:10.1007/s00429-010-0262-0
- Shi Q, Mu X, Zhang C, Wang S, Hong L, Chen X. Risk Factors for Postoperative Delirium in Type A Aortic Dissection Patients: a Retrospective Study. Med Sci Monit. 2019;25:3692–3699. doi:10.12659/msm.913774
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857.
- Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–744. doi:10.1093/ejcts/ezs043
- Greaves D, Psaltis PJ, Davis DHJ, et al. Risk Factors for Delirium and Cognitive Decline Following Coronary Artery Bypass Grafting Surgery: a Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9(22):e017275. doi:10.1161/jaha.120.017275
- Nežić DG, Živković IS, Miličić MD, et al. On-line risk prediction models for acute type A aortic dissection surgery: validation of the German Registry of Acute Aortic Dissection Type A score and the European System for Cardiac Operative Risk Evaluation II. Eur J Cardiothorac Surg. 2022;61(5):1068–1075. doi:10.1093/ejcts/ezab517
- Luehr M, Merkle-Storms J, Gerfer S, et al. Evaluation of the GERAADA score for prediction of 30-day mortality in patients with acute type A aortic dissection. Eur J Cardiothorac Surg. 2021;59(5):1109–1114. doi:10.1093/ejcts/ezaa455
- O’Neal JB, Billings F, Liu X, et al. Risk factors for delirium after cardiac surgery: a historical cohort study outlining the influence of cardiopulmonary bypass. Can J Anaesth. 2017;64(11):1129–1137. doi:10.1007/s12630-017-0938-5
- Habeeb-Allah A, Alshraideh JA. Delirium post-cardiac surgery: incidence and associated factors. Nurs Crit Care. 2021;26(3):150–155. doi:10.1111/nicc.12492
- Salem M, Salib M, Friedrich C, et al. Influence of Age on Postoperative Neurological Outcomes after Surgery of Acute Type A Aortic Dissection. J Clin Med. 2021;10(8):546.
- Pupovac SS, Hemli JM, Bavaria JE, et al. Moderate Versus Deep Hypothermia in Type A Acute Aortic Dissection Repair: insights from the International Registry of Acute Aortic Dissection. Ann Thorac Surg. 2021;112(6):1893–1899. doi:10.1016/j.athoracsur.2021.01.027
- Fleck TM, Czerny M, Hutschala D, Koinig H, Wolner E, Grabenwoger M. The incidence of transient neurologic dysfunction after ascending aortic replacement with circulatory arrest. Ann Thorac Surg. 2003;76(4):1198–1202. doi:10.1016/s0003-4975(03)00832-4
- Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377(15):1456–1466. doi:10.1056/NEJMcp1605501
- Mulkey MA, Roberson DW, Everhart DE, Hardin SR. Choosing the Right Delirium Assessment Tool. J Neurosci Nursing. 2018;50(6):343–348. doi:10.1097/jnn.0000000000000403
- Boukrina O, Kowalczyk M, Koush Y, Kong Y, Barrett AM. Brain Network Dysfunction in Poststroke Delirium and Spatial Neglect: an fMRI Study. Stroke. 2022;53(3):930–938. doi:10.1161/strokeaha.121.035733
- Choi SH, Lee H, Chung TS, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169(5):498–507. doi:10.1176/appi.ajp.2012.11060976
- Pelkey KA, Barksdale E, Craig MT, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85(6):1257–1272. doi:10.1016/j.neuron.2015.02.020
- Galasko D, Xiao M, Xu D, et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement. 2019;5:871–882. doi:10.1016/j.trci.2019.11.002
- Swanson A, Willette AA. Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain Behav Immun. 2016;58:201–208. doi:10.1016/j.bbi.2016.07.148

